Abstract
The hypervariable region 1 (HVR-1) of the putative envelope encoding E2 region of hepatitis C virus (HCV) RNA was analyzed in sequential samples from three patients with acute type C hepatitis infected from different sources to address (i) the dynamics of intrahost HCV variability during the primary infection and (ii) the role of host selective pressure in driving viral genetic evolution. HVR-1 sequences from 20 clones per each point in time were analyzed after amplification, cloning, and purification of plasmid DNA from single colonies of transformed cells. The intrasample evolutionary analysis (nonsynonymous mutations per nonsynonymous site [Ka], synonymous mutations per synonymous site [Ks], Ka/Ks ratio, and genetic distances [gd]) documented low gd in early samples (ranging from 2.11 to 7.79%) and a further decrease after seroconversion (from 0 to 4.80%), suggesting that primary HCV infection is an oligoclonal event, and found different levels and dynamics of host pressure in the three cases. The intersample analysis (pairwise comparisons of intrapatient sequences; rKa, rKs, rKa/rKs ratio, and gd) confirmed the individual features of HCV genetic evolution in the three subjects and pointed to the relative contribution of either neutral evolution or selective forces in driving viral variability, documenting that adaptation of HCV for persistence in vivo follows different routes, probably representing the molecular counterpart of the viral fitness for individual environments.
Hepatitis C virus (HCV), a positive-strand RNA virus of about 9.4 kb (3, 4, 9) included in the family Flaviviridae (21), is the major causative agent of non-A, non-B acute and chronic hepatitis. After primary HCV infection, progression to viral persistence is observed in most patients (1, 28). The HCV genome in persistently infected hosts is described as a dynamic population of heterogeneous, closely related variants designated quasispecies (11, 15, 20). Recent biological and molecular data strongly suggest that HCV variability plays a crucial role in escaping the host immune surveillance and establishing the chronic carrier state (8, 11, 12, 29–31). A high degree of variability has been revealed in two discrete sequences of the putative envelope-encoding E2 region of HCV RNA, which have been designated hypervariable regions 1 and 2 (HVR-1 and HVR-2). Notably, the 27-amino-acid HVR-1, located in the N-terminal portion of the HCV envelope protein, bears major neutralizing epitopes (25, 34). More recently, the role of the humoral immune response to HCV in driving viral genetic variability has been studied in vivo by analyzing chronically infected, immunocompromised patients (14, 16, 23); the results support the general hypothesis that the immune response acts as a major selective force during HCV persistence, even though the involvement of different, nonimmunological mechanisms, as observed in other RNA viruses (5), cannot be excluded.
The error-prone nature of viral RNA polymerases provides the biochemical basis for the variability observed in most RNA viruses. However, conflicting hypotheses have been advanced to explain the mechanisms of viral evolution in infected hosts; these include mutation-driven evolution (27), neutral evolution (7), and viral fitness for a selective environment. One method for the evaluation of the selective pressure envisages the analysis of Ka and Ks values and the Ka/Ks ratio, where Ka is the frequency of nonsynonymous (antonymous) substitutions per nonsynonymous site and Ks is the frequency of synonymous substitutions per synonymous site (17, 18); the higher the Ka/Ks ratio, the stronger the selective pressure for amino acid changes.
In this study, we addressed the intrahost variability of HCV HVR-1 in three patients infected with acute type C hepatitis from different sources, aiming at evaluating the relevant features and dynamics of virus variability before and after a specific immune response is elicited. The three Italian patients (indicated here as A, B, and C) included in this study at the onset of acute type C hepatitis were observed over a period of 12 months (patient A), 9 months (patient B), and 12 months (patient C). Patient A (female, 14 years old) was infected by a blood transfusion (2 units); patient B (female, 40 years old), a medical doctor, was infected via a transmucosal (conjunctival) blood splash from an infected subject; and patient C (male, 32 years old) was infected after a surgical intervention (gastrectomy).
Antibodies to HCV were detected by a third-generation enzyme-linked immunosorbent assay (HCV 3.0 ELISA; Ortho Diagnostic Systems, Raritan, N.J.) and a recombinant immunoblot assay (Chiron RIBA 3; Ortho Diagnostic Systems). For molecular analysis, total RNA was purified from EDTA-treated plasma by the guanidinium thiocyanate method (2); HCV genotyping was performed on all plasma samples by nested PCR of the core region as described by Okamoto et al. (24), with minor modifications for better detection of Italian HCV types (26); while quantitation of HCV RNA molecules in plasma samples was performed by quantitative competitive reverse transcription-PCR (cRT-PCR), as previously described (19).
Acute type C hepatitis was diagnosed on the basis of (i) the presence of clinical and biochemical signs of overt liver injury, (ii) detection of HCV RNA in the first available plasma sample, and (iii) absence of specific anti-HCV antibodies (by ELISA) in the first sample and compatible dynamics of anti-HCV antibodies in subsequent sequential samples. Other markers of viral hepatitis (types A, B, D, and E) and antibodies to human immunodeficiency virus type 1 tested negative in commercially available ELISAs; absence of anti-nuclear antibodies, mitochondrial antibodies, smooth muscle antibodies, and liver kidney microsomal antibodies was documented by indirect immunofluorescence assays. Other causes of liver disease (i.e., Wilson’s disease, hemochromatosis, α1-antitrypsin deficiency) were excluded by specific laboratory tests. Hepatotoxic drug intake and intravenous drug use were excluded by personal and familial anamnestic data. Liver tests, including alanine aminotransferase (ALT) levels in serum, were performed at the first clinical examination and repeated monthly during follow-up.
For sequence analysis, amplified sequences of the N terminus of the E2/NS1 region (HVR-1) were obtained by cRT-PCR with primer sets specific for this region. Two primers encompassing a 612-bp sequence of the E1/E2 HCV region were used (from nucleotides 1278 to 1889; sense primer 5′-ATA ACG GGT CAC CGA TGG CAT GGG ATA T; antisense primer 5′-CAC CAC CAC GGG GCT GGG AGT GAA GCA AT). The amplified product was directly ligated to pCR-Script SK(+) plasmid vector (Stratagene, La Jolla, Calif.). After transformation, transformant colonies were streaked onto a fresh dish; plasmid DNA from single colonies was extracted and purified from overnight-cultured minipreps by the Wizard DNA purification system (Promega, Madison, Wis.). The sequences of the inserted DNAs were determined in 20 independent clones derived from each region by fluorescence-labeled dideoxynucleotides with an automated sequencer (model 373A; Perkin-Elmer, Norwalk, Conn.), using the sequencing conditions specified in the protocol for the DyeDeoxy Terminator Cycle Sequencing Kit (Perkin-Elmer), and with Ampli-Taq DNA polymerase FS (Perkin Elmer), and the above-mentioned HCV-specific oligonucleotides (both sense and antisense) as bidirectional sequencing primers. Nucleotide and amino acid sequences were aligned with the MEGALIGN program (DNASTAR, Madison, Wis.), and analysis of the selective pressure was performed for all sequences by the method described by Nei and Gojobori (22). In the absence of any reliable ancestor, a general consensus sequence was computed for all sequences from each patient, and each clone sequence was compared with this consensus. The Ka and Ks values and the Ka/Ks ratio were calculated with the MEGA program for all sequences (13); the mean Ka/Ks ratio for all the clones from each sample was taken as representative of the sample. The distance matrix was generated by Kimura’s two-parameter model (6). Phylogenetic trees were constructed by the neighbor-joining method, and their reliability was assessed by bootstrap resampling (1,000 data sets). These methods were implemented with software from the MEGA and PHYLIP 3.5c packages.
Infection with type 1b HCV was documented in patients A and B, while patient C had been infected with a type 2c virus (Table 1). Table 1 shows that the dynamics of viral load during acute infection were quite different in the three cases. In patient A, HCV viremia was high only during the first 6 months of observation (8 × 106 to 11 × 106 HCV RNA molecules per ml of plasma [samples A1 to A3]) and then dropped to undetectable levels (below 10 copy numbers per ml by cRT-PCR [samples An′ and An" [collected 8 and 9 months from time zero]), paralleling the normalization of the ALT level. After 12 months of observation, the ALT level in serum rose and the amount of circulating cell-free virus increased again to detectable levels (2.5 × 104 molecules per ml of plasma [sample A4]). In patient B, cell-free HCV viremia was detectable throughout the period of observation (albeit at low levels, ranging from 1 × 103 to 5 × 105 HCV RNA molecules per ml of plasma), accompanied by high ALT levels (three- to fourfold higher than the normal level). After 9 months of follow-up, patient B underwent a liver biopsy; the grading necroinflammatory injury and the architectural damage (i.e., fibrosis) were scored separately by the criteria Ishak et al. (10), to avoid the disadvantage of combined grading and staging; scores of 6 and 1 were assigned, respectively. Subsequently, treatment with recombinant alpha interferon (3 MU three times a week) was initiated; HCV RNA copy numbers and ALT levels became negative and normal, respectively, within a few weeks of the beginning of therapy. Finally, in patient C, the number of HCV RNA molecules in plasma ranged from 2 × 104 to 1.5 × 106 per ml; ALT levels were high in the first samples and fluctuated in subsequent ones.
TABLE 1.
Molecular, biochemical, and serological characterization of the three HCV primary infections
| Patient | Causative event | Age (yr)/sex | HCV genotype | HCV viremiaa | ALT level (IU/ml)b | Results ofc:
|
|
|---|---|---|---|---|---|---|---|
| ELISA | RIBA3 | ||||||
| A | Transfusion (whole blood, 2 units) | 14/F | 1b | A1 (time zero): 1.1 × 107 | 800 | Neg | 0/1/0/0 |
| A2 (2 mo): 1.0 × 107 | 780 | Pos | 1/4/1/0 | ||||
| A3 (6 mo): 8.0 × 106 | 78 | Pos | 2/4/1/1 | ||||
| An′ (8 mo): <1 × 101 | 38 | Pos | 2/4/1/1 | ||||
| An" (9 mo): <1 × 101 | 25 | Pos | 2/4/2/2 | ||||
| A4 (12 mo): 2.5 × 104 | 97 | Pos | 3/4/3/2 | ||||
| B | Transmucosal infection (conjunctival blood splash) | 40/F | 1b | B1 (time zero): 5.0 × 105 | 165 | Neg | 0/1/0/0 |
| B2 (3 mo): 1.0 × 105 | 128 | Pos | 1/3/1/0 | ||||
| B3 (6 mo): 1.0 × 103 | 112 | Pos | 3/4/1/1 | ||||
| B4 (9 mo): 2.5 × 105 | 98 | Pos | 4/4/3/3 | ||||
| C | Surgical intervention (gastrectomy) | 32/M | 2c | C1 (time zero): 1.5 × 106 | 1185 | Neg | 0/0/0/0 |
| C2 (6 mo): 2.0 × 104 | 218 | Pos | 2/3/4/1 | ||||
| C3 (12 mo): 2.0 × 105 | 105 | Pos | 3/4/3/2 | ||||
Number of HCV RNA molecules per ml plasma. Time zero is the time where the first sample was available; other times are times after time zero. An′ and An" identify two consecutive samples that tested negative for HCV RNA.
Normal value, <40 IU/ml.
RIBA3 scores range negative (0) to strongly reactive (4) for c100/c33c/c22/NS5. For ELISA: neg, negative; pos, positive.
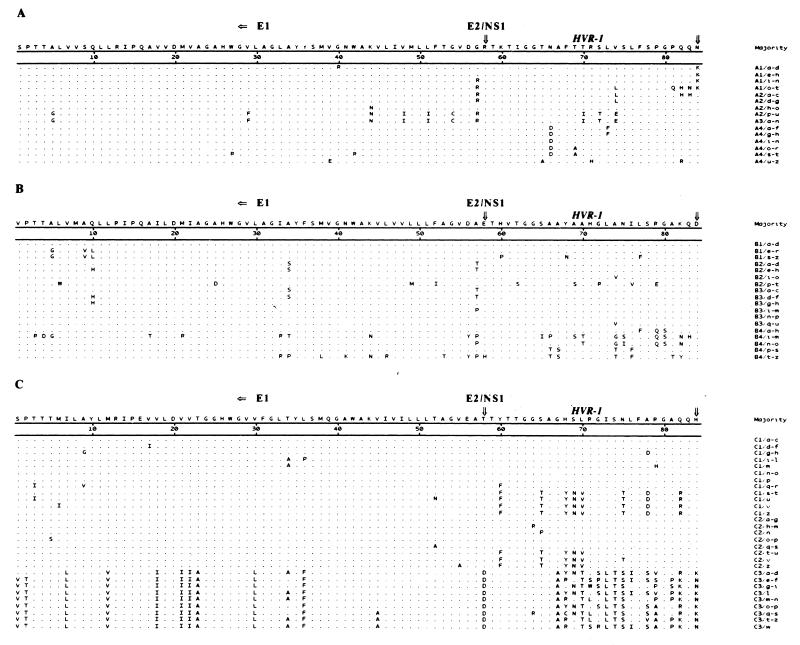
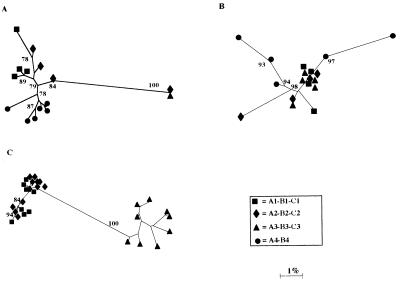
Figure 1 shows the amino acid alignments of the sequences derived from the cloned HVR-1 and the flanking E1 subfragment obtained from the three patients and highlights the nonsynonymous changes. A phylogenetic study of these sequences (Fig. 2) revealed that early (time zero) sequences substantially clustered around the origin of the trees in all three cases whereas divergent profiles were observed in later samples. For patient A, the sequences from samples A1 to A4 maintained a low mean intrasample genetic distance (gd): 3.67% (A1), 7.56% (A2), 0% (A3), and 2.47% (A4), respectively (Table 2). In this patient, the clones derived from sample A3 (collected at 6 months) accounted for 100% of the sequences detected at that time (A3/a-n [Fig. 1 and 2]); this sequence was identical to the major prevalent clone (prevalence, 90%) detected at 2 months (A2/p-u [Fig. 1 and 2]). These results were confirmed by analyzing sample A3 again. The sequences found in the samples collected from patients B and C displayed a greater intrasample distance (Fig. 2; Table 2), with late samples (B4 [collected 9 months after B1] and C3 [collected 12 months after C1]) documenting a sharp increase. For the samples from patient B, the mean intrasample gd (Table 2) was 2.11% (sequences from B1), 5.88% (B2), 0.35% (B3), and 13.78% (B4); for those from patient C, it was 7.79% (C1), 4.8% (C2), and 10.83% (C3). An additional intrasample parameter evaluated in this study was the Ka/Ks ratio (Table 2); Ka/Ks ratios higher than 1.0 were observed in samples collected at all time points in patients B and C but not in the first three samples from patient A. This indicates that a positive selection of HCV mutants had been active in two patients; by contrast, in patient A the Ka/Ks ratio shifted from less than 1.0 to 2.47 in the last sample (A4) (Table 2). Overall, intrasample analysis principally documents that (i) early sequences have a low genetic divergence (oligoclonal profile), (ii) a homogeneous viral population may be detected in parallel with (or soon after) seroconversion (samples A3, B3, and C2), and (iii) the dynamics and extent of the host selective pressure may differ among HCV-infected patients, thus indicating that the process of HCV adaptation for persistence follows different routes in different persons.
FIG. 1.
Amino acid sequences of HVR-1 and the flanking E1 region in samples obtained from patients A, B, and C are aligned with the majority sequence derived from each clone at different time points. Dots represent amino acid identity to the majority, and letters indicate amino acid substitutions. For each patient, numbers indicate different samplings (see Materials and Methods), and lowercase letters indicate the different clones obtained.
FIG. 2.
Phylogenetic tree analysis of HVR-1 sequences in samples obtained from patients A, B, and C. Branch lengths are drawn to scale. Bootstrap values (indicating the reproducibility of the particular bifurcation point) obtained with 1,000 replications of bootstrap sampling are shown only when they are greater than 75%.
TABLE 2.
Intra- and intersample variability of HVR-1 during HCV primary infection
| Sample | Intrasample variability
|
Samples | Intersample variability
|
||
|---|---|---|---|---|---|
| Ka/Ksa | gdb (mean) | rKa/rKsa | gdb (mean ± SD) | ||
| A1 | 0.87 | 3.67 | |||
| A2 | 0.66 | 7.56 | A1-A2 | 0.68 | 6.51 ± 2.66 |
| A3 | 0.00 | 0.00 | A1-A3 | 0.56 | 6.90 ± 2.45 |
| A4 | 2.47 | 2.47 | A1-A4 | 0.69 | 5.14 ± 2.92 |
| B1 | 2.80 | 2.11 | |||
| B2 | 1.26 | 5.88 | B1-B2 | 3.00 | 1.47 ± 0.89 |
| B3 | 4.90 | 0.35 | B1-B3 | 2.02 | 4.40 ± 1.92 |
| B4 | 2.16 | 13.78 | B1-B4 | 2.88 | 8.24 ± 2.96 |
| C1 | 1.42 | 7.79 | |||
| C2 | 1.69 | 4.80 | C1-C2 | 1.57 | 6.06 ± 2.58 |
| C3 | 1.11 | 10.83 | C1-C3 | 0.51 | 32.53 ± 11.65 |
The average number of nucleotide substitutions per nonsynonymous site and per synonymous site for all pairwise comparisons within each sampling point (Ka, Ks) and of sequences obtained at different time points (rKa, rKs) were calculated by using the Jukes-Cantor model of molecular evolution implemented in the MEGA program (see Materials and Methods).
The intra- and intersample gd were calculated with DNADIST software implemented in the PHYLIP package (version 3.5c).
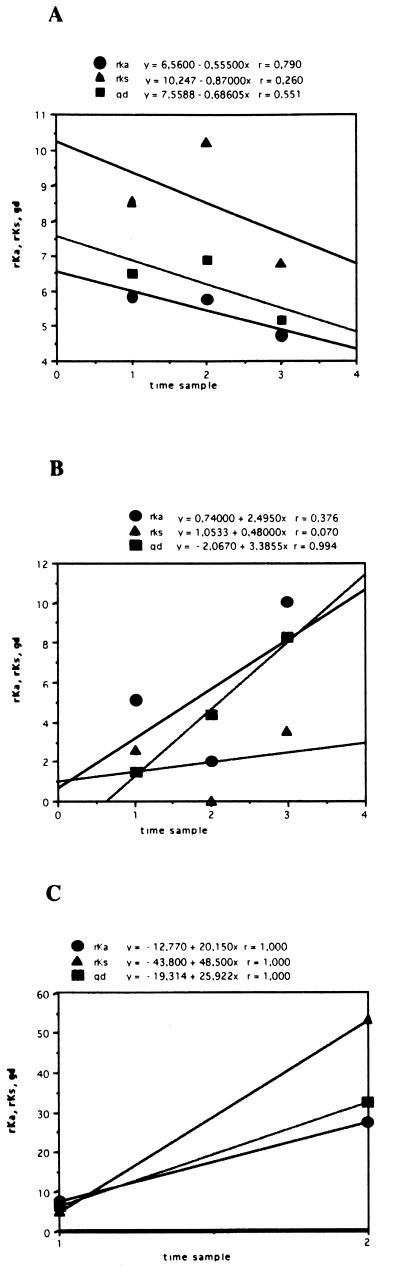
Pairwise comparisons were performed between sequences obtained at different time points; regression curves were constructed with DeltaGraph software (Deltapoint, Inc., Monterey, Calif.), and correlation coefficients (r values) were calculated by the least-squares method. The values were expressed as average nucleotide substitutions per nonsynonymous site (rKa) or per synonymous site (rKs). The Jukes-Cantor model of molecular evolution (22) was used to calculate the intersample gd among sequences at different times. Table 2 lists the intersample rKa/rKs ratios and the gd calculated for different time points for the three patients; by using this approach, the relative contributions of nonsynonymous and synonymous changes can be efficiently estimated. The results are shown graphically in Fig. 3, where rKa, rKs, and gd values are plotted against time. In the samples from patients B and C, all parameters increased with time (Table 2; Fig. 3); in the samples from patient B, the intersample rKa/rKs ratios always exceeded 1.0, indicating that rKa was the dominant contributor to the overall gd. By contrast, this ratio was consistently below 1.0 in the samples from patient A (documenting low selective forces, at least in the early phases of acute infection [samples A1 to A3]) and fluctuated in patient C. In patient C, rKs was the dominant contributor to gd in sample C3. Overall, the analysis of intersample parameters of molecular evolution yielded three different profiles in the cases of acute type C hepatitis evolving into chronic infection. These differences in viral genetic evolution parallel differences in viral load (Table 1).
FIG. 3.
Average values of frequencies of rKa, rKs, and gd are plotted against the time sampling points for each patient (A, B, and C, respectively). Each symbol represents the average value of pairwise comparisons between the sequences from the first sample and those from each of the subsequent samples. For each regression line, the r values are reported above the graphs.
A better characterization of HCV variability in patients with acute infection has important implications for the understanding of the natural history of this infection. Either because of the high evolutionary potential of HCV HVR-1 compared with other viral sequences or because this region includes neutralizing epitopes, the analysis of its intrahost evolution may provide crucial information on virus-host relationships at any point in time. The low intrasample genetic divergence observed in the first samples collected from these three patients, before a specific humoral immune response was elicited, suggests that in its early phase HCV infection is an oligoclonal event. This finding may reflect either selective amplification or selective transmission, as hypothesized for human immunodeficiency virus type 1 infection (32, 33). In the former case, viral selection from a heterogeneous inoculum would occur even though multiple HCV variants penetrate into the new host (because of its biomolecular characteristics only one would be amplified, becoming the dominant population); in the latter, a single viral variant would be transmitted efficiently, due either to its high concentration in specific compartments or biological fluids or to its ability to penetrate the mucosal barriers of the new host, such as the conjunctiva of patient B. Independent of the underlying biomolecular mechanism and even though our evidence is largely indirect, since we could not analyze any sample from the transmitters, the hypothesis that in its early phase HCV infection could be an oligoclonal event may have important implications for planning efficient prevention strategies.
At 2 to 4 months after collection of the first sample and soon after seroconversion, the intrasample gd decreased further. In patient A, this reduction reached its peak 6 months after collection of the first sample (A3). The finding of a single dominant HVR-1 variant among 20 clones in sample A3 suggests a process of selection of variants within the incoming viral population in this phase. Nonetheless, a substantial increase in the intrasample gd of actively replicating viruses was subsequently observed (in patients B and C in the third samples, in patient A only 1 year later and to a lesser extent [Fig. 2; Table 2]). These data suggest that when HCV becomes persistent after primary infection, viral genetic evolution enters an adaptive phase in most cases, albeit to different extents. From this point of view, HCV persistence (although not always the primary infection) may be compatible with an ideal Darwinian system, as also documented by Ka/Ks ratios higher than 1.0 (in patient A only in the fourth sample [A4]), since synonymous substitutions (Ks) are not subjected to selection. The heterogeneous features of acute HCV infection were confirmed by the dynamics of intersample gd and rKa and rKs values. In other words, our data documenting the absence of a precise common pattern during primary infection indicate that the adaptation of HCV to persistence shows clear differences in different subjects who are infected with different HCV genotypes and have different exposures, clinical features, and outcomes.
Our study does not directly address the issue of the nature of the host forces for viral selection. Theoretically, two major components of these forces may be active in infected patients: immune response and acquisition of a wider cellular host range for the virus. Whether (as presently believed) or not the major components of the selective pressure are related to immune selection, it would be important to account for the absence of a clear-cut correlation between the gd, rKa, and rKs and the time, at least in acute infection.
In conclusion, the present molecular study of primary HCV infection and early viral persistence documents different dynamics of viral genetic evolution (as well as of viral replication) in three patients with acute type C hepatitis and suggests that these features are the molecular counterpart of a differential dynamics of viral fitness for individual environments.
Nucleotide sequence accession numbers.
The sequences described in this paper have been submitted to EMBL and assigned accession no. AJ225271 to AJ225286 (patient A), AJ225287 to AJ225303 (patient B), and AJ225304 to AJ225331 (patient C).
Acknowledgments
This work was supported in part by grants from the Italian Istituto Superiore di Sanità (target project “Epatiti Virali”) and Consiglio Nazionale delle Ricerche (target project “Biotechnology”) to M.C.
REFERENCES
- 1.Alter J H, Purcell R H, Shih J W, Melpolder J C, Houghton M, Choo Q-L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 2.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;161:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 3.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q L, Richman K H, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr P J, Weiner A J, Bradley D W, Kuo G, Houghton M. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domingo E, Diez J, Martinez M A, Hernandez J, Holguin A, Borrego B, Mateau M G. New observations on antigenic diversification of RNA viruses. Antigenic variation is not dependent on immune selection. J Gen Virol. 1993;74:2039–2045. doi: 10.1099/0022-1317-74-10-2039. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein J. Phylogeny interference package, version 3.5. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 7.Gojobori T, Morijama E N, Kimura M. Molecular clock of viral evolution, and the neutral theory. Proc Natl Acad Sci USA. 1990;87:10015–10018. doi: 10.1073/pnas.87.24.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashi Y, Kakumu S, Yoshioka K, Wakita T, Mizokami M, Ohba K, Ito Y, Ishikawa T, Takayanagi M, Nagai Y. Dynamics of genome change in the E2/NS1 region of hepatitis C virus in vivo. Virology. 1993;197:659–668. doi: 10.1006/viro.1993.1641. [DOI] [PubMed] [Google Scholar]
- 9.Houghton M, Weiner A, Han J, Choo Q-L. Molecular biology of hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991;14:381–388. [PubMed] [Google Scholar]
- 10.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween R N, Phillips M J, Portmann B G, Poulsen H, Scheuer P J, Schmid M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 11.Kao J H, Chen P-J, Lai M-J, Wang T H, Chen D S. Quasispecies of hepatitis C virus and genetic drift of the hypervariable region in chronic type C hepatitis. J Infect Dis. 1995;172:261–264. doi: 10.1093/infdis/172.1.261. [DOI] [PubMed] [Google Scholar]
- 12.Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetic analysis for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Kumar U, Monjardino J, Thomas H C. Hypervariable region of hepatitis C virus envelope glycoprotein (E2/NS1) in an agammaglobulinemic patient. Gastroenterology. 1994;106:1072–1075. doi: 10.1016/0016-5085(94)90770-6. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki M, Enomoto N, Marumo F, Sato C. Evolution and selection of hepatitis C virus variants in patients with chronic hepatitis C. Virology. 1994;205:161–169. doi: 10.1006/viro.1994.1631. [DOI] [PubMed] [Google Scholar]
- 16.Lawal Z, Petrik J, Wong V-S, Alexander G J M, Allain J P. Hepatitis C virus genomic variability in untreated and immunocompromised patients. Virology. 1997;228:107–111. doi: 10.1006/viro.1996.8359. [DOI] [PubMed] [Google Scholar]
- 17.Li W H, Graur D. Fundamentals of molecular evolution. Sunderland, Mass: Sinauer Associates Inc.; 1991. [Google Scholar]
- 18.Li W H, Wu C I, Luo C C. A new method for estimating synonimous and nonsynonimous rates of nucleotide substitutions considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985;2:150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- 19.Manzin A, Bagnarelli P, Menzo S, Giostra F, Brugia M, Francesconi R, Bianchi F B, Clementi M. Quantitation of hepatitis C virus genome molecules in plasma samples. J Clin Microbiol. 1994;32:1939–1944. doi: 10.1128/jcm.32.8.1939-1944.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martell M, Esteban J I, Quer J, Genesca J, Weiner A, Esteban R, Guardia J, Gomez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–2062. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nei M, Gojobori T. Simple methods for estimating the numbers of synonimous and nonsynonimous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 23.Odeberg J, Yun Z, Sönnerborg A, Bijøro K, Uhlén M, Lundeberg J. Variation of hepatitis C hypervariable region 1 in immunocompromised patients. J Infect Dis. 1997;175:938–943. doi: 10.1086/513995. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y, Tanaka T, Sato K, Tsuda F, Miyakawa Y, Mayumi M. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Purcell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silini E, Bono F, Cerino A, Piazza V, Solcia E, Mondelli M U. Virological features of hepatitis C virus infections in hemodialysis patients. J Clin Microbiol. 1993;31:2913–2917. doi: 10.1128/jcm.31.11.2913-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temin H W. Is HIV unique or merely different? J Acquired Immune Defic Syndr. 1989;2:1–29. [PubMed] [Google Scholar]
- 28.Tong M J, El-Farra N S, Reikes A S, Co R L. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 29.Van Doorn L-J, Capriles I, Maertens G, DeLeys R, Murray K, Kos T, Schellekens H, Quint W. Sequence evolution of the hypervariable region in the putative envelope region E2/NS1 of hepatitis C virus is correlated with specific humoral immune responses. J Virol. 1995;69:773–778. doi: 10.1128/jvi.69.2.773-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Tanaka E, Higashi K, Kiyosawa K, Matsumoto A, Furuta S, Hasegawa A, Tanaka S, Kohara M. Adaptation of hepatitis C virus for persistent infection in patients with acute hepatitis. Gastroenterology. 1994;106:1344–1348. doi: 10.1016/0016-5085(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Diaz R S, Ho D D, Mosley J W, Busch M P, Mayer A. Host-specific driving force in human immunodeficiency virus type 1 evolution in vivo. J Virol. 1997;71:2555–2561. doi: 10.1128/jvi.71.3.2555-2561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu T, Mo H, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 34.Zibert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]