To the Editor: Spontaneous preterm labor (PTL) causes infant morbidity and mortality. Regular uterine contractions were the common characteristics in spontaneous labor at term or PTL. Thus, effective decrease or suppression of myometrial contraction could prevent preterm delivery. We discovered that microRNA-206 (miR-206) was a myometrium contraction-associated miRNA based on the transcriptome data of human laboring myometrium and indicated that miR-206 suppressed myometrial contractility by targeting junction protein alpha 1 (GJA1) in the myometrium and would be a new potential therapeutic for preterm birth. Administration of miR-206 in RU486-induced PTL decreased myometrial contractility, prolonged pregnancy, reversed PTL to term labor, and improved perinatal outcomes.
This research was approved by the Ethics Committee of Guangzhou Women and Children Medical Center (No. 2018041701). Written informed consent was obtained from all participants. The animal study was approved by the Medical Ethics Committee of the Guangzhou Medical University (No. GY2022-059).
A total of 25 lower uterine segment samples were collected from singleton, nulliparous women undergoing cesarean deliveries. The samples were divided into term in labor (n = 10) and term non-labor (n = 15) groups. All patients had no pregnancy complications, placenta previa, or uterine fibroids. Labor was defined as regular palpable contractions and cervical dilation. Preterm labor was defined when regular contractions result in the opening of the cervix after week 28 and before week 37 of pregnancy.
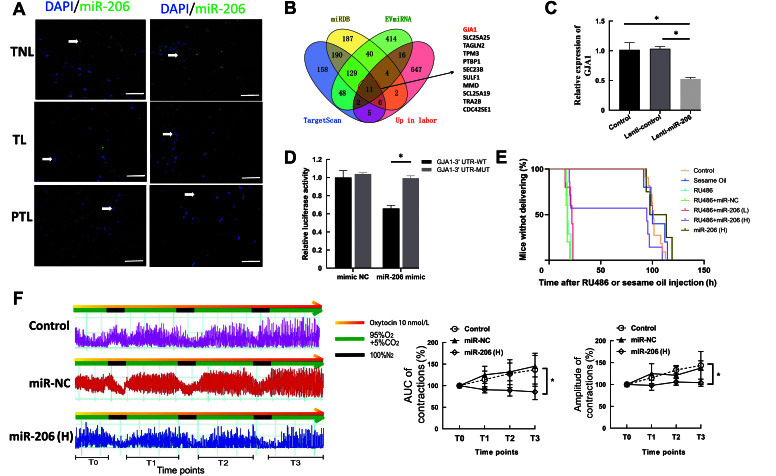
MicroRNA (miRNA)-sequencing was performed to analyze miRNA expressions of samples in two groups. miR-206 was the most differentially expressed miRNA with the biggest fold change and lowest adjusted P-value (log2 fold change = -4.031, adjusted P-value = 1.76E-07) [Supplementary Figure 1A, http://links.lww.com/CM9/B758, Supplementary Table 1, http://links.lww.com/CM9/B757]. Fluorescence in situ hybridization (FISH) was conducted to detect miR-206 in human myometrium in PTL, labor and non-labor groups. The results showed that miR-206 was mainly located in the cell nucleus of myometrial smooth muscle cells [Figure 1A].
Figure 1.
MicroRNA-206 (miR-206) targets GJA1 functions as a potential oligonucleotide therapeutics in preterm birth. (A) FISH of miR-206 in TNL, TL, and PTL myometrium. Bar=50 μm. (B) Venn diagram of the overlap of putative target of miR-206 and up-regulated mRNAs in laboring myometrium. (C) The expression of GJA1 after overexpressing miR-206 in HMSMCs (n = 3). (D) GJA1 binding to miR-206 in HEK293T cells (n = 3). (E) Survival analysis for hours after injection of RU486 or sesame oil among all seven groups (left) and the average pregnancy length of all groups (right). (F) Tension measurement of myometrial strips collected from three mice groups (left) and AUC and amplitude of contractions of myometrial strips (n = 3). *P <0.01. AUC: Area under the curve; DAPI: 4, 6-diamino-2-phenylindole; FISH: Fluorescence in situ hybridization; GJA1: Junction protein alpha 1; HMSMCs: Human myometrial smooth muscle cells; MUT: mutation; NC: Normal control group; PTL: Preterm labor; TL: Term labor; TNL: Term non-labor; UTR: untranslated region; WT: Wild type.
To explore the target genes involved in contraction, downstream message RNAs (mRNAs) were predicted by combining three miRNA databases and an upregulated mRNA list during labor in our previous research (GSE181348).[1] The results showed that 11 mRNAs had putative binding with miR-206 and could be targets of miR-206 [Figure 1B and Supplementary Table 2, http://links.lww.com/CM9/B757]. Specifically, a key contraction-associated protein GJA1, also known as connexin 43 (CX43), was found in the list of 11 genes. GJA1 was confirmed widely as a key contraction-associated protein that can enhance the gap junction formation between myometrial cells, stimulating intensity and coordinated myometrial contractions.[2] The mRNA and protein expression of GJA1 were upregulated in laboring myometrium compared to that in non-laboring myometrium, and negatively associated with the expression level of miR-206 [Supplementary Figures 1B–E, http://links.lww.com/CM9/B758].
To confirm the regulation of miR-206 on the expression of GJA1, miR-206 was overexpressed in human myometrial smooth muscle cells (HMSMCs) through lentivirus [Supplementary Figure 1F, http://links.lww.com/CM9/B758] and the mRNA and protein expression of GJA1 was decreased [Figure 1C and Supplementary Figure 1G, http://links.lww.com/CM9/B758]. Based on the predicted target sites of miR-206 in the 3΄ untranslated regions (UTRs) of GJA1, reporter plasmid vectors containing wild type (WT) or mutation (MUT) sequences of the 3΄ UTR of GJA1 were used, and a dual-luciferase reporter assay was performed in 293T cells. The luciferase activity of the wild type vector was decreased in the cells co-transfected with miR-206 mimics [Figure 1D]. Combined with bioinformatics and in vitro experiments, miR-206 directly targeted the 3΄ UTR of GJA1 and decreased its expression, confirming GJA1 as a target of miR-206.
Since miR-206 suppresses the expression of contraction-associated protein GJA1, we tried to determine the therapeutic role of miR-206 in RU486-induced PTL mice model by injecting RU486 or miR-206 agomir. The experimental methods and grouping were shown in Supplementary Figures 2A and 2B, http://links.lww.com/CM9/B758. A total of 47 ICR/CD1 pregnant mice were randomly divided into 7 groups: Control group, Sesame oil group, RU486 group, RU486 + miR-NC group, RU486 + miR-206(L) group, RU486 + miR-206(H) group, and miR-206(H) group (L: low; H: High). RU486 successfully induced PTL within 16.50–24.00 h and no associated maternal mortality was found. The high dose of miR-206 administration significantly delayed PTL time compared with that in the RU486 + miR-NC group. The percentage of preterm births was decreased to 43% in the RU486 + miR-206(H) group. However, this delayed delivery was not observed in the RU486 + miR-206(L) [Figure 1E, Supplementary Figure 2C, http://links.lww.com/CM9/B758 and Supplementary Table 3, http://links.lww.com/CM9/B757].
As for the outcomes of pups, the RU486 + miR-206(H) group had bigger length and weight compared with that in the RU486 + miR-NC group and RU486 + miR-206(L), the survival rate of pups was also increased significantly in the RU486 + miR-206(H) group [Supplementary Figures 2D–F, http://links.lww.com/CM9/B758]. Pups was monitored for 8 days after birth in control and miR-206(H) groups. The pups from both groups were alive, and their body weights increased steadily with no statistical difference. The appearance of pups between day 1 and day 8 after birth in the control and RU486 + miR-206(H) group showed no differences. Specifically, on day 8, no significant differences were observed in the timing of eye-opening, hair growth, and nail and tooth eruptions of the offspring in the two groups [Supplementary Figure 2G, http://links.lww.com/CM9/B758]. The results showed that miR-206 delayed the onset of preterm birth to a certain extent. No effect on the short-term growth of the offspring was observed in association with miR-206 administration.
The mice myometrium was collected to investigate the effect of miR-206 on the expression of GJA1 in vivo. The results showed that the expression of GJA1 was upregulated in the RU486 and RU486 + miR-NC groups, while downregulated in RU486 + miR-206 group especially in RU486 + miR-206(H) group [Supplementary Figures 2H, 2I, http://links.lww.com/CM9/B758]. These findings indicated that overexpression of miR-206 reduced the expression of GJA1 in the myometrium.
To explore the effect of miR-206 on myometrial contractility, in vitro myometrial tension measurement was performed on the myometrium from the mice treated with miR-206 or miR-NC. Under the condition of the hypoxia-reoxygenation cycle, two indexes of the myometrial contractility, the area under the curve (AUC) and amplitude of contractions, in the control and miR-NC group gradually increased at each hypoxia-reoxygenation cycle. While both the AUC and amplitude of contractions in T2 and T3 periods in the miR-206(H) group were significantly lower than that in the control and miR-NC group [Supplementary Figures 2F, http://links.lww.com/CM9/B758]. These results validated the suppression function of miR-206 on myometrial contractility.
Unlike protein, miRNA is a better therapeutic agent for various diseases. Administration of miR-206 agomir significantly prolonged pregnancy in all mice, reversed four of seven-to-term labor, and decreased myometrium contractility. These results showed that miR-206 prevents PTL by targeting GJA1 to inhibit myometrial contraction. In addition, maternal mortality was not associated with miR-206 administration, and most pups were alive and healthy after miR-206 therapy. Improvements in perinatal outcomes indicated the effect of miR-206 treatment for non-infectious PTL. Although miR-206 showed a positive effect and safety toward PTL prevention in animals, tail vein injection is a systemic administration method; hence, more precise delivery and dosage, as posterior vault administration, should be explored extensively.
In clinical settings, early detection of the risk factors of PTL and individual prevention management, such as cervical cerclage, progesterone supplementation, and tobacco control, are important strategies for reducing PTL.[3] Many attempts have been made to explore the treatment of PTL, but only a few have succeeded in reversing it to term delivery. Recently, experimental and clinical evidence[4] suggested that antibiotic administration in infection-induced PTL prolonged pregnancy and reduced adverse neonatal outcomes, which was an exciting achievement. However, additional interventions need to be developed to address other non-infectious mechanisms responsible for PTL. RU486-induced PTL model is an advanced and recognized model, which was used in this study to explore the functions of miR-206 on myometrium contraction and PTL prevention.[5]
In summary, miR-206 is a myometrium contraction-associated miRNA that directly targets GJA1. Administration of miR-206 in the RU486-induced PTL mice model decreased myometrial contractility, leading to prolonged pregnancy, reversion of PTL to term labor, and improved perinatal outcomes. The present study showed that miR-206 was a promising molecular therapeutic target for non-infectious preterm birth.
Acknowledgements
We thank Shiyu Li and Weihan Zheng for animal technical support. We thank Xiuyu Pan, Lanlan Guo, Yanmin Jiang, Xueya Qian, and Lei Liu for the clinical sample collection. Wei Li and Chao Liu are appreciated for the revised suggestion.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81871181) and the High-tech Major Featured Technology Program of Guangzhou Municipal Health Commission (No. 2019GX07).
Conflicts of interest
None.
Supplementary Material
Footnotes
Wenfeng Deng, Lina Chen, and Bolun Wen contributed equally to this work.
How to cite this article: Deng WF, Chen LN, Wen BL, Wang XD, Wang LL, Yang F, Chen YS, Bao JJ, Zhang GZ, Ji KY, Liu HS. MicroRNA-206 functions as a potential oligonucleotide therapeutics in preterm birth. Chin Med J 2024;137:1000–1002. doi: 10.1097/CM9.0000000000002876
References
- 1.Chen L Wang L Luo Y Huang Q Ji K Bao J, et al. Integrated proteotranscriptomics of human myometrium in labor landscape reveals the increased molecular associated with inflammation under hypoxia stress. Front Immunol 2021;12: 722816. doi: 10.3389/fimmu.2021.722816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, He X, Ding Y. Effect of corticotrophin-releasing hormone on connexin-43 phosphorylation and gap junction intercellular communication in human myometrial smooth muscle cells (in Chinese). J Central South Univ (Med Ed) 2013;38: 155–161. doi: 10.3969/j.issn.1672-7347.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Romero R. Spontaneous preterm labor can be predicted and prevented. Ultrasound Obstet Gynecol 2021;57: 19–21. doi: 10.1002/uog.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh KJ Romero R Park JY Lee J Conde-Agudelo A Hong JS, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol 2019;221: 140.e1–140.e18. doi: 10.1016/j.ajog.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol 1987;157: 1281–1285. doi: 10.1016/s0002-9378(87)80315-0. [DOI] [PubMed] [Google Scholar]