Abstract
Background
Nivolumab plus cabozantinib (NIVO + CABO) was approved for first-line treatment of advanced renal cell carcinoma (aRCC) based on superiority versus sunitinib (SUN) in the phase III CheckMate 9ER trial (18.1 months median survival follow-up per database lock date); efficacy benefit was maintained with an extended 32.9 months of median survival follow-up. We report updated efficacy and safety after 44.0 months of median survival follow-up in intent-to-treat (ITT) patients and additional subgroup analyses, including outcomes by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic risk score.
Patients and methods
Patients with treatment-naïve aRCC received NIVO 240 mg every 2 weeks plus CABO 40 mg once daily or SUN 50 mg for 4 weeks (6-week cycles), until disease progression/unacceptable toxicity (maximum NIVO treatment, 2 years). Primary endpoint was progression-free survival (PFS) per blinded independent central review (BICR). Secondary endpoints were overall survival (OS), objective response rate (ORR) per BICR, and safety and tolerability.
Results
Overall, 323 patients were randomised to NIVO + CABO and 328 to SUN. Median PFS was improved with NIVO + CABO versus SUN [16.6 versus 8.4 months; hazard ratio (HR) 0.59; 95% confidence interval (CI) 0.49-0.71]; median OS favoured NIVO + CABO versus SUN (49.5 versus 35.5 months; HR 0.70; 95% CI 0.56-0.87). ORR (95% CI) was higher with NIVO + CABO versus SUN [56% (50% to 62%) versus 28% (23% to 33%)]; 13% versus 5% of patients achieved complete response, and median duration of response was 22.1 months versus 16.1 months, respectively. PFS and OS favoured NIVO + CABO over SUN across intermediate, poor and intermediate/poor IMDC risk subgroups; higher ORR and complete response rates were seen with NIVO + CABO versus SUN regardless of IMDC risk subgroup. Any-grade (grade ≥3) treatment-related adverse events occurred in 97% (67%) versus 93% (55%) of patients treated with NIVO + CABO versus SUN.
Conclusions
After extended follow-up, NIVO + CABO maintained survival and response benefits; safety remained consistent with previous follow-ups. These results continue to support NIVO + CABO as a first-line treatment for aRCC.
Trial registration
ClinicalTrials.gov, NCT03141177.
Key words: renal cell carcinoma, nivolumab, cabozantinib, IMDC, immunotherapy, phase III
Highlights
-
•
With 44.0 months of median follow-up for OS, long-term efficacy outcomes with NIVO + CABO over SUN were maintained.
-
•
Median OS (95% CI) in ITT patients: 49.5 months (40.3 months-NE) with NIVO + CABO vs 35.5 months (29.2-42.3 months) with SUN.
-
•
Survival outcomes favoured NIVO + CABO over SUN across intermediate, poor and combined intermediate/poor IMDC risk subgroups.
-
•
Objective responses were durable with NIVO + CABO; response rates were higher versus SUN regardless of IMDC risk group.
-
•
No new safety signals emerged with additional follow-up in either treatment arm.
Introduction
There has been substantial development in the first-line setting of advanced renal cell carcinoma (aRCC) with the advent of immunotherapy-based combination therapies.1,2 Treatment modalities combining immune checkpoint inhibitors (ICIs) and vascular endothelial growth factor receptor-directed tyrosine kinase inhibitors (VEGFR-TKIs) have led to improved efficacy and survival outcomes in this patient population.3, 4, 5, 6, 7, 8, 9, 10 As ICI combination therapy is known to be associated with durable response in this disease setting, longer follow-ups are important to assess the durability of clinical benefit observed with ICI plus VEGFR-TKI combinations.4,6,9, 10, 11
In the phase III CheckMate 9ER trial (NCT03141177), first-line nivolumab plus cabozantinib (NIVO + CABO) demonstrated efficacy over sunitinib (SUN) in patients with advanced or metastatic renal cell carcinoma (RCC) across all three efficacy endpoints of progression-free survival (PFS), overall survival (OS) and objective response rate (ORR) at primary analysis with median follow-up for OS of 18.1 months per database lock of 30 March 2020 (clinical data cut-off date, 12 February 2020).3 On the basis of these results, NIVO + CABO is now a standard of care for first-line aRCC.12,13 Improved efficacy outcomes with NIVO + CABO versus SUN were maintained at extended median follow-up for OS of 32.9 months per database lock of 24 June 2021 (clinical data cut-off date, 26 April 2021).4
Trials of ICI plus VEGFR-TKI combinations have examined efficacy by the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk category, with efficacy benefits observed mostly in the intermediate- and poor-risk groups.4,6,9,10 Long-term follow-up is needed to determine the durable benefits of ICI plus VEGFR-TKI combinations among IMDC risk groups, particularly among favourable-risk patients who have more indolent disease.14
Furthermore, NIVO treatment in the CheckMate 9ER trial was not to exceed a maximum of 2 years per study design. As such, data regarding the extent of survival benefit after immunotherapy discontinuation as well as the impact of subsequent therapy from the patient population who completed per-protocol 2 years of NIVO treatment have not yet been reported, and would provide insights into the durability of NIVO efficacy after per-protocol discontinuation of NIVO treatment.
Here, we report updated results after an extended median follow-up for OS of 44.0 months. We also report exploratory subgroup analyses of efficacy by IMDC risk category and subsequent therapy in patients who completed the per-protocol 2 years of NIVO treatment.
Patients and methods
Study design and patients
CheckMate 9ER is an open-label, randomised phase III trial. Trial design and methods have been reported in detail previously.3,4 Briefly, adults with treatment-naïve histologically confirmed advanced or metastatic RCC with a clear cell component, any IMDC prognostic risk category and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 were recruited from 125 hospitals and cancer centres across 18 countries. Patients were randomised 1 : 1 to intravenous NIVO 240 mg every 2 weeks plus oral CABO 40 mg per day, or to oral SUN 50 mg per day monotherapy for 4 weeks in 6-week cycles. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent or end of study, whichever occurred first, with a maximum duration of 2 years of NIVO treatment. According to the protocol, dose delays for adverse events (AEs) were allowed for all study drugs, but dose reductions were not permitted for NIVO. Randomisation was carried out via permuted blocks within each stratum using a block size of four and stratified by IMDC prognostic risk score [0 (favourable) versus 1-2 (intermediate) versus 3-6 (poor)], tumour programmed death ligand 1 (PD-L1) expression (≥1% versus <1% or indeterminate) and geographical region (United States, Canada and Europe versus rest of the world). The Bristol Myers Squibb (Princeton, NJ) interactive response technology team generated the allocation sequence, which was transferred to a third-party vendor for patient enrolment and assignment to trial groups in collaboration with the investigators at their respective study sites.
Endpoints and assessments
The primary endpoint was RECIST v1.1-defined PFS per blinded independent central review (BICR). Secondary endpoints were OS, ORR by RECIST v1.1 per BICR (including time to and duration of response), and safety and tolerability (including treatment-related events and AEs leading to discontinuation).
Disease progression and objective response were defined per RECIST v1.1, as assessed by the investigator and confirmed by BICR. Best overall response of complete response or partial response were verified by a confirmatory tumour assessment.
Duration of study therapy was defined as the time from the first dose to last dose of study treatment or, for patients who were still on study treatment, the clinical data cut-off date. Time to treatment discontinuation was based on Kaplan–Meier analysis. The last dose date was the event date for patients off study treatment (patients in the NIVO + CABO arm were considered off treatment if both NIVO and CABO were discontinued); patients who were still on study treatment at the clinical data cut-off date were censored.
AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0) and are reported between first dose and 30 days after the last dose of study therapy. A post hoc analysis was conducted on incidence of any-grade and grade 3-4 treatment-related AEs in all treated patients by a 6-month interval. Immune-mediated AEs were reported and defined as specific events occurring within 100 days of the last dose of study drug regardless of causality, treated with immune-modulating medication, with no clear alternate aetiology (based on investigator assessment) or had an immune-mediated component. The use of corticosteroids (≥40 mg prednisone daily or equivalent) to manage immune-mediated AEs was also reported.
Several pre-specified or post hoc exploratory subgroup analyses were carried out by individual IMDC prognostic risk subgroups and in the intermediate/poor combined subgroup for efficacy (pre-specified); exposure (time to treatment discontinuation and duration of therapy; post hoc); and subsequent therapy (post hoc and pre-specified, respectively). A post hoc analysis of subsequent therapy in patients who completed the planned 2 years of NIVO treatment was also carried out. Time to subsequent therapy was defined as the time from the last dose of study treatment to the start of subsequent therapy or death; patients who never received subsequent anticancer therapy were censored at the date the patient was last known alive.
Statistical analysis
Statistical methods have been previously reported.3,4 Assuming a 25% screen failure rate, ∼850 patients were to be enrolled to randomise 638 patients. The planned overall alpha for this trial was 0.05 (two-sided) for the primary and secondary endpoints, and a hierarchical testing procedure was used. PFS, OS, time to response, duration of response, time to treatment discontinuation and time to subsequent therapy were estimated using Kaplan–Meier methods.15 A stratified Cox proportional hazards model, based on stratification factors used in randomisation, was used for between-treatment arm comparisons of PFS and OS for the intent-to-treat (ITT) population (all randomised patients). An unstratified Cox proportional hazards model was used for IMDC risk groups. For ORR, the exact two-sided 95% confidence intervals (CIs) were computed using the Clopper–Pearson method.16
Efficacy endpoints and subsequent therapy were analysed in the ITT population, and in IMDC risk subgroups. Exposure, safety and tolerability were analysed in all treated patients (patients who received at least one dose of any study drug). The time to subsequent therapy was evaluated in all treated patients and in patients who completed the per-protocol 2 years of NIVO treatment. Temporal analysis of treatment-related AEs by a 6-month interval was done using the total number of new events out of the total number of patients at risk at the beginning of the interval and evaluated in all treated patients.
A data-monitoring committee provided oversight of efficacy, safety and study conduct. All statistical analyses were carried out using SAS (v9.2 and v9.4; SAS Institute Inc, Cary, NC).
Trial oversight
The study protocol and its amendments were approved by the institutional review board or an ethics committee at each site, and the study was conducted in accordance with Good Clinical Practice guidelines defined by the International Council for Harmonisation. Patients provided written informed consent before enrolment. Protocol amendments that were made on 18 December 2017 and 3 May 2019 affected the design of the study and recruitment, which included terminating enrolment into the NIVO plus ipilimumab plus CABO triplet arm, increasing the number of randomly assigned patients, including patients with IMDC favourable-risk disease in the primary data analysis, and adjustment to interim analyses and the overall α level of endpoints.
Results
Patients
At the time of database lock (27 May 2022), the median follow-up for OS was 44.0 months [range 36.5-56.5 months; median (range) follow-up for OS at clinical cut-off (12 April 2022), 42.5 months (35.0-55.0 months)] in all randomised patients (NIVO + CABO, n = 323; SUN, n = 328). Of all treated patients, 57 of 320 (17.8%) in the NIVO + CABO arm versus 32 of 320 (10.0%) in the SUN arm remained on treatment; the two most common reasons for discontinuation were disease progression [154 (48.1%) versus 201 (62.8%)] and study drug toxicity [32 (10.0%) versus 37 (11.6%)], respectively (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102994). Baseline characteristics in the ITT population and patient subgroups of interest are summarised in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.102994.
Efficacy outcomes
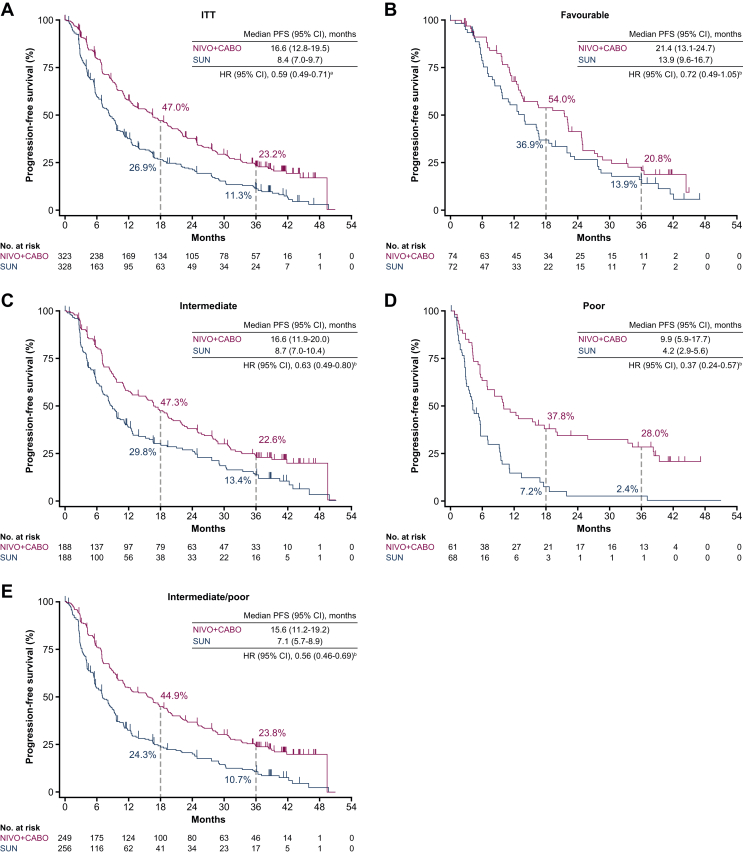
PFS was improved with NIVO + CABO [median PFS 16.6 months (95% CI 12.8-19.5 months)] versus SUN [median PFS 8.4 months (95% CI 7.0-9.7 months)], with a hazard ratio (HR) (95% CI) of 0.59 (0.49-0.71) in the ITT population. PFS probability (95% CI) at 36 months favoured NIVO + CABO [23.2% (18.4% to 28.3%)] versus SUN [11.3% (7.7% to 15.7%)]. An improvement in PFS outcomes was also seen across intermediate [HR 0.63 (95% CI 0.49-0.80)], poor [HR 0.37 (95% CI 0.24-0.57)] and intermediate/poor [HR 0.56 (95% CI 0.46-0.69)] combined IMDC risk groups, with a trend towards favouring NIVO + CABO with the favourable-risk group [HR 0.72 (95% CI 0.49-1.05); Figure 1].
Figure 1.
PFS per BICR in the (A) ITT population and by IMDC favourable (B), intermediate (C), poor (D) and intermediate/poor combined (E) risk group (IRT). BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IRT, interactive response technology; ITT, intent-to-treat; NIVO + CABO, nivolumab plus cabozantinib; PFS, progression-free survival; SUN, sunitinib.aStratified Cox proportional hazard model used for HR. bUnstratified Cox proportional hazard model used for HR.
ORR (95% CI) was improved with NIVO + CABO [56.0% (50.4% to 61.5%)] versus SUN [28.0% (23.3% to 33.2%)] in the ITT population, and across all IMDC risk subgroups, particularly in the combined intermediate/poor-risk group (Table 1). Higher complete response rates were observed with NIVO + CABO versus SUN in the ITT population (13.3% versus 4.9%) and across all IMDC risk subgroups, including the favourable-risk group (16.2% versus 9.7%) and the combined intermediate/poor-risk group (12.4% versus 3.5%). Progressive disease rates were lower with NIVO + CABO versus SUN in the ITT population (6.5% versus 14.0%) and across almost all IMDC risk subgroups, including the combined intermediate/poor-risk group (7.6% versus 17.2%); similar progressive disease rates were seen in the favourable-risk group (2.7% versus 2.8%). Median (95% CI) duration of response in the ITT population was 22.1 months (18.0-26.0 months) with NIVO + CABO versus 16.1 months (11.1-19.4 months) with SUN, with similar trends across all IMDC risk subgroups (Table 1).
Table 1.
Confirmed ORR, confirmed best overall response and time to and duration of response in the ITT population and by IMDC risk group (IRT)
| Outcome | ITT population |
IMDC risk category |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Favourable |
Intermediate |
Poor |
Intermediate/poor |
|||||||
| NIVO + CABO (N = 323) | SUN (N = 328) | NIVO + CABO (n = 74) | SUN (n = 72) | NIVO + CABO (n = 188) | SUN (n = 188) | NIVO + CABO (n = 61) | SUN (n = 68) | NIVO + CABO (n = 249) | SUN (n = 256) | |
| Confirmed ORR (95% CI), % | 56.0 (50.4-61.5) | 28.0 (23.3-33.2) | 67.6 (55.7-78.0) | 45.8 (34.0-58.0) | 56.4 (49.0-63.6) | 27.7 (21.4-34.6) | 41.0 (28.6-54.3) | 10.3 (4.2-20.1) | 52.6 (46.2-58.9) | 23.0 (18.0-28.7) |
| Complete response | 43 (13.3) | 16 (4.9) | 12 (16.2) | 7 (9.7) | 28 (14.9) | 8 (4.3) | 3 (4.9) | 1 (1.5) | 31 (12.4) | 9 (3.5) |
| Partial response | 138 (42.7) | 76 (23.2) | 38 (51.4) | 26 (36.1) | 78 (41.5) | 44 (23.4) | 22 (36.1) | 6 (8.8) | 100 (40.2) | 50 (19.5) |
| Stable disease | 103 (31.9) | 135 (41.2) | 22 (29.7) | 28 (38.9) | 56 (29.8) | 80 (42.6) | 25 (41.0) | 27 (39.7) | 81 (32.5) | 107 (41.8) |
| Progressive disease | 21 (6.5) | 46 (14.0) | 2 (2.7) | 2 (2.8) | 15 (8.0) | 29 (15.4) | 4 (6.6) | 15 (22.1) | 19 (7.6) | 44 (17.2) |
| Unable to determine or not reported | 18 (5.6) | 55 (16.8) | 0 | 9 (12.5) | 11 (5.9) | 27 (14.4) | 7 (11.5) | 19 (27.9) | 18 (7.2) | 46 (18.0) |
| Median time to response (range), monthsa,b | 2.8 (1.0-24.4) | 4.3 (1.7-30.4) | 2.8 (1.5-24.4) | 4.2 (1.7-30.4) | 2.8 (1.0-22.2) | 5.4 (1.7-18.1) | 3.3 (2.4-11.4) | 3.1 (2.8-12.4) | 2.8 (1.0-22.2) | 4.4 (1.7-18.1) |
| Median duration of response (95% CI), monthsa,b,c | 22.1 (18.0-26.0) | 16.1 (11.1-19.4) | 19.8 (16.7-23.2) | 17.8 (11.1-19.4) | 22.9 (16.6-28.2) | 19.3 (8.7-27.8) | 35.9 (12.8-NE) | 4.9 (2.8-9.5) | 24.6 (17.5-31.3) | 13.8 (7.1-24.7) |
All values presented as no. of patients (%) unless otherwise specified.
CI, confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IRT, interactive response technology; ITT, intent-to-treat; NE, not estimable; NIVO + CABO, nivolumab plus cabozantinib; ORR, objective response rate; SUN, sunitinib.
Calculated only for patients with a complete response or partial response.
Six confirmed responders have <36 months follow-up.
Median computed using the Kaplan–Meier method.
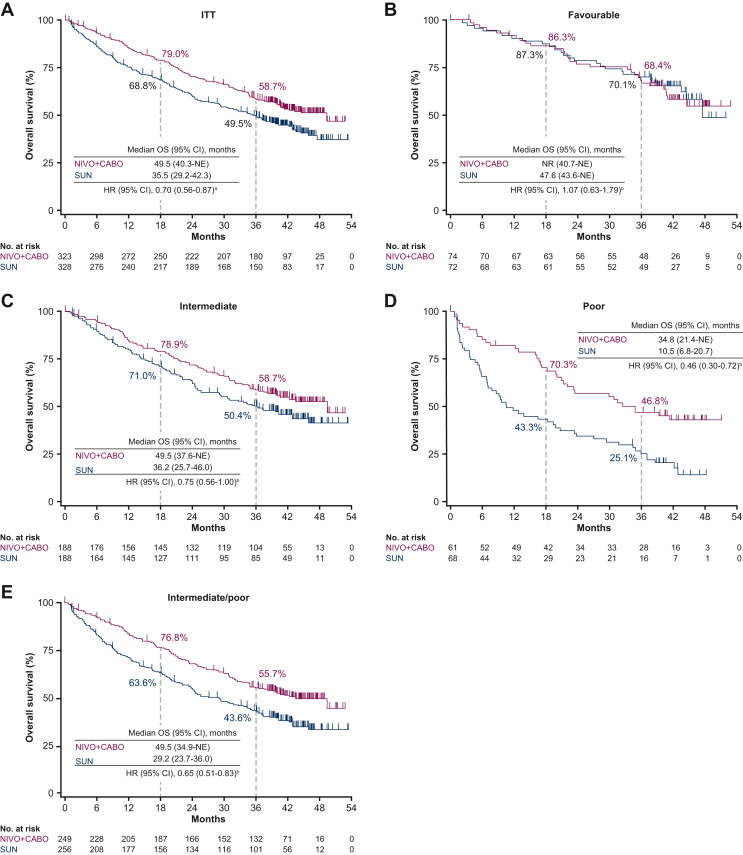
Similar to PFS, OS outcomes favoured NIVO + CABO [median OS 49.5 months (95% CI 40.3 months-not estimable)] versus SUN [median OS 35.5 months (95% CI 29.2-42.3 months)] in the ITT population [HR (95% CI) 0.70 (0.56-0.87)]. OS probability (95% CI) at 36 months was 58.7% (53.0% to 63.9%) with NIVO + CABO versus 49.5% (43.9% to 54.9%) with SUN. An improvement in OS was also seen across intermediate [HR 0.75 (0.56-1.00)], poor [HR 0.46 (0.30-0.72)] and combined intermediate/poor [HR 0.65 (0.51-0.83)] IMDC risk groups; no difference in OS outcomes was observed in the favourable-risk group [HR 1.07 (0.63-1.79); Figure 2].
Figure 2.
OS in the (A) ITT population and by IMDC favourable (B), intermediate (C), poor (D) and intermediate/poor combined (E) risk group (IRT). CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IRT, interactive response technology; ITT, intent-to-treat; NE, not estimable; NIVO + CABO, nivolumab plus cabozantinib; NR, not reached; OS, overall survival; SUN, sunitinib.aStratified Cox proportional hazard model used for HR. bUnstratified Cox proportional hazard model used for HR.
Exposure and subsequent therapy
Among all treated patients, median time to treatment discontinuation (95% CI) was 21.8 months (18.0-23.7 months) in the NIVO + CABO arm (patients were considered off treatment if both NIVO and CABO were discontinued) versus 8.4 months (7.0-10.5 months) in the SUN arm. In patient subgroups by IMDC risk category, median time to treatment discontinuation (95% CI) was 23.6 months (19.3-27.6 months) versus 13.4 months (9.5-20.9 months) in favourable-risk patients, 23.4 months (18.0-24.4 months) versus 9.9 months (7.3-11.8 months) in intermediate-risk patients, 14.0 months (9.0-18.4 months) versus 3.4 months (2.3-4.8 months) in poor-risk patients and 19.6 months (16.3-23.5 months) versus 7.1 months (6.1-9.2 months) in intermediate/poor-risk patients, respectively.
The median duration of therapy (quartile 1-quartile 3) in all treated patients was 21.8 months (8.8-34.0 months) in the NIVO + CABO arm (where either NIVO or CABO were continued) versus 8.9 months (2.9-20.7 months) in the SUN arm. In patient subgroups by IMDC risk category, median duration of therapy (quartile 1-quartile 3) was 23.6 months (12.7-37.7 months) versus 13.9 months (6.5-32.7 months) in favourable-risk patients, 23.4 months (8.7-35.5 months) versus 10.4 months (3.8-21.2 months) in intermediate-risk patients, 14.0 months (5.8-23.8 months) versus 3.8 months (2.5-7.4 months) in poor-risk patients and 19.6 months (8.3-33.2 months) versus 7.6 months (2.8-16.8 months) in intermediate/poor-risk patients, respectively.
Among the ITT population, a higher proportion of patients in the SUN arm (133/328, 40.5%) received any subsequent systemic anticancer therapy compared with the NIVO + CABO arm (81/323, 25.1%; Table 2). In the NIVO + CABO arm, a VEGF-targeted or VEGFR-targeted therapy (69/323, 21.4%) was the most common subsequent systemic therapy, with axitinib most commonly used in this class (29/323, 9.0%); followed by programmed death 1 (PD-1) or PD-L1 inhibitor-based therapy (21/323, 6.5%), with NIVO most commonly used in this class (17/323, 5.3%). The most common subsequent systemic therapy in the SUN arm was a PD-1 or PD-L1 inhibitor-based therapy (101/328, 30.8%), with NIVO most commonly used in this class (93/328, 28.4%); followed by a VEGF-targeted or VEGFR-targeted therapy, with cabozantinib most commonly used in this class (30/328, 9.1%). The trends in subsequent anticancer therapy were generally consistent between the ITT population and each IMDC risk group. In the NIVO + CABO arm, axitinib use among IMDC favourable-risk patients tended to be higher versus other IMDC risk groups (Table 2).
Table 2.
Summary of subsequent anticancer therapy in the ITT population and in patients by IMDC risk group
| Therapya | ITT population |
IMDC risk category |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Favourable |
Intermediate |
Poor |
Intermediate/poor |
|||||||
| NIVO + CABO (N = 323) | SUN (N = 328) | NIVO + CABO (n = 74) | SUN (n = 72) | NIVO + CABO (n = 188) | SUN (n = 188) | NIVO + CABO (n = 61) | SUN (n = 68) | NIVO + CABO (n = 249) | SUN (n = 256) | |
| Any subsequent therapyb | 116 (35.9) | 148 (45.1) | 26 (35.1) | 30 (41.7) | 71 (37.8) | 89 (47.3) | 19 (31.1) | 29 (42.6) | 90 (36.1) | 118 (46.1) |
| Any subsequent systemic therapy | 81 (25.1) | 133 (40.5) | 20 (27.0) | 29 (40.3) | 47 (25.0) | 78 (41.5) | 14 (23.0) | 26 (38.2) | 61 (24.5) | 104 (40.6) |
| Any PD-(L)1 inhibitor | 21 (6.5) | 101 (30.8) | 5 (6.8) | 21 (29.2) | 13 (6.9) | 58 (30.9) | 3 (4.9) | 22 (32.4) | 16 (6.4) | 80 (31.3) |
| Nivolumab | 17 (5.3) | 93 (28.4) | 5 (6.8) | 20 (27.8) | 10 (5.3) | 52 (27.7) | 2 (3.3) | 21 (30.9) | 12 (4.8) | 73 (28.5) |
| Pembrolizumab | 7 (2.2) | 7 (2.1) | 3 (4.1) | 2 (2.8) | 3 (1.6) | 4 (2.1) | 1 (1.6) | 1 (1.5) | 4 (1.6) | 5 (2.0) |
| Atezolizumab | 0 | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Durvalumab | 0 | 4 (1.2) | 0 | 2 (2.8) | 0 | 2 (1.1) | 0 | 0 | 0 | 2 (0.8) |
| Any CTLA-4 inhibitor | 8 (2.5) | 20 (6.1) | 3 (4.1) | 6 (8.3) | 5 (2.7) | 10 (5.3) | 0 | 4 (5.9) | 5 (2.0) | 14 (5.5) |
| Ipilimumab | 8 (2.5) | 19 (5.8) | 3 (4.1) | 5 (6.9) | 5 (2.7) | 10 (5.3) | 0 | 4 (5.9) | 5 (2.0) | 14 (5.5) |
| Tremelimumab | 0 | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Any VEGF(R) inhibitor | 69 (21.4) | 63 (19.2) | 18 (24.3) | 17 (23.6) | 38 (20.2) | 36 (19.1) | 13 (21.3) | 10 (14.7) | 51 (20.5) | 46 (18.0) |
| Axitinib | 29 (9.0) | 20 (6.1) | 11 (14.9) | 3 (4.2) | 15 (8.0) | 12 (6.4) | 3 (4.9) | 5 (7.4) | 18 (7.2) | 17 (6.6) |
| Sunitinib | 21 (6.5) | 8 (2.4) | 4 (5.4) | 2 (2.8) | 12 (6.4) | 6 (3.2) | 5 (8.2) | 0 | 17 (6.8) | 6 (2.3) |
| Pazopanib | 13 (4.0) | 8 (2.4) | 3 (4.1) | 1 (1.4) | 6 (3.2) | 7 (3.7) | 4 (6.6) | 0 | 10 (4.0) | 7 (2.7) |
| Lenvatinib | 10 (3.1) | 3 (0.9) | 3 (4.1) | 3 (4.2) | 5 (2.7) | 0 | 2 (3.3) | 0 | 7 (2.8) | 0 |
| Cabozantinib | 7 (2.2) | 30 (9.1) | 2 (2.7) | 9 (12.5) | 4 (2.1) | 15 (8.0) | 1 (1.6) | 6 (8.8) | 5 (2.0) | 21 (8.2) |
| Sorafenib | 2 (0.6) | 7 (2.1) | 0 | 1 (1.4) | 1 (0.5) | 5 (2.7) | 1 (1.6) | 1 (1.5) | 2 (0.8) | 6 (2.3) |
| Tivozanib | 2 (0.6) | 0 | 1 (1.4) | 0 | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) | 0 |
| Sorafenib tosylate | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 1 (1.6) | 0 | 1 (0.4) | 0 |
| Tivozanib hydrochloride monohydrate | 0 | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 20 (6.2) | 18 (5.5) | 7 (9.5) | 6 (8.3) | 9 (4.8) | 9 (4.8) | 4 (6.6) | 3 (4.4) | 13 (5.2) | 12 (4.7) |
| Everolimus | 12 (3.7) | 10 (3.0) | 5 (6.8) | 4 (5.6) | 3 (1.6) | 5 (2.7) | 4 (6.6) | 1 (1.5) | 7 (2.8) | 6 (2.3) |
| Investigational antineoplastic drugs | 4 (1.2) | 4 (1.2) | 0 | 1 (1.4) | 4 (2.1) | 2 (1.1) | 0 | 1 (1.5) | 4 (1.6) | 3 (1.2) |
| Belzutifan | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BMS 986179 | 1 (0.3) | 0 | 0 | 0 | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) | 0 |
| Gimeracil; oteracil potassium; tegafur | 1 (0.3) | 0 | 0 | 0 | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) | 0 |
| MK 4280 | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 1 (1.6) | 0 | 1 (0.4) | 0 |
| Talazoparib | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Investigational drug | 0 | 1 (0.3) | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoclonal antibodies and antibody–drug conjugates | 0 | 1 (0.3) | 0 | 0 | 0 | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) |
| Savolitinib | 0 | 2 (0.6) | 0 | 1 (1.4) | 0 | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) |
| Temsirolimus | 0 | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 | 1 (0.4) |
All values presented as no. of patients (%).
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; ITT, intent-to-treat; NIVO + CABO, nivolumab plus cabozantinib; PD-(L)1, programmed death-(ligand) 1; SUN, sunitinib; VEGF(R), vascular endothelial growth factor (receptor).
Patients may have received more than one type of subsequent therapy. Subsequent therapy was defined as therapy started on or after the date of first study dose (date of randomisation if patient was never treated).
Includes patients who received subsequent radiotherapy, surgery or systemic therapy.
Among patients who completed the per-protocol 2 years of NIVO treatment, 10.4% (12/115) received any subsequent systemic anticancer therapy; the most common was a VEGF-targeted or VEGFR-targeted therapy (7/115, 6.1%), followed by a PD-1 or PD-L1 inhibitor-based therapy (5/115, 4.3%, Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102994). NIVO (5/115, 4.3%) and everolimus (4/115, 3.5%) were the most common individual subsequent therapies received in this patient subgroup.
The median time to subsequent therapy (95% CI) in all treated patients was 4.0 months (2.6-6.8 months) in the NIVO + CABO arm and 2.1 months (1.4-2.8 months) in the SUN arm (after discontinuation of all study drugs; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.102994) and 20.6 months (7.9 months-not estimable) in patients who completed per-protocol 2 years of NIVO treatment (irrespective of continuing or completing CABO treatment) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.102994).
Safety
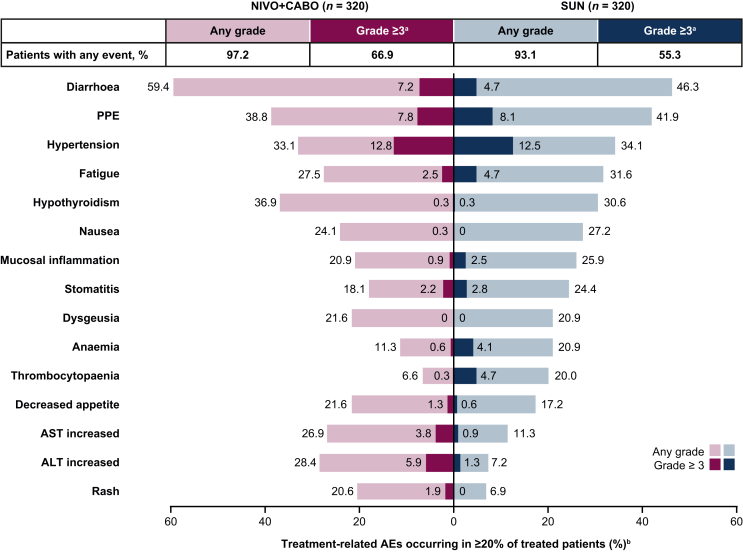
Among all treated patients, the incidence of any-grade and grade ≥3 treatment-related AEs was 97.2% (311/320) and 66.9% (214/320) in the NIVO + CABO arm and 93.1% (298/320) and 55.3% (177/320) in the SUN arm, respectively; similar to previous reporting.4 The most common any-grade treatment-related AEs were diarrhoea [59.4% (190/320)], palmar–plantar erythrodysaesthesia [38.8% (124/320)] and hypothyroidism [36.9% (118/320)] in the NIVO + CABO arm, and diarrhoea [46.3% (148/320)], palmar–plantar erythrodysaesthesia [41.9% (134/320)] and hypertension [34.1% (109/320)] in the SUN arm (Figure 3). The most common grade ≥3 treatment-related AEs were hypertension [12.8% (41/320)], palmar–plantar erythrodysaesthesia [7.8% (25/320)] and diarrhoea [7.2% (23/320)] in the NIVO + CABO arm, and hypertension [12.5% (40/320)] and palmar–plantar erythrodysaesthesia [8.1% (26/320)] in the SUN arm.
Figure 3.
Treatment-related AEs in ≥20% of all treated patients. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NIVO + CABO, nivolumab plus cabozantinib; PPE; palmar–plantar erythrodysaesthesia; SUN, sunitinib.aNo patients had a grade 5 event with NIVO + CABO and one patient had a grade 5 event with SUN. bTotal bar represents treatment-related AEs of any grade in either treatment arm reported between first dose and 30 days after last dose of study therapy; of these events, none were grade 5.
The incidence of any-grade and grade 3-4 treatment-related AEs in all treated patients by a 6-month interval decreased over time in both arms and was generally higher with NIVO + CABO versus SUN (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.102994).
Any-grade treatment-related AEs led to discontinuation of either therapy in 27.5% (88/320) of patients in the NIVO + CABO arm [6.6% (21/320) discontinued NIVO and CABO simultaneously; 1.6% (5/320) discontinued NIVO and CABO sequentially; while 9.7% (31/320) discontinued NIVO only; and 9.7% (31/320) discontinued CABO only]; 10.6% (34/320) of patients discontinued SUN.
The incidence of immune-mediated AEs was similar to that reported previously, with most as low-grade events.4 The most common any-grade immune-mediated AEs were hypothyroidism [27.8% (89/320)], hyperthyroidism [9.4% (30/320)] and rash [8.4% (27/320)] in the NIVO + CABO arm, and hypothyroidism [9.7% (31/320)] and hepatotoxicity [1.9% (6/320)] in the SUN arm. The most common grade ≥3 immune-mediated AEs were alanine aminotransferase increased [2.8% (9/320)], diarrhoea [2.5% (8/320)] and hepatotoxicity [2.2% (7/320)] in the NIVO + CABO arm, and hypothyroidism, hepatotoxicity and hyperbilirubineamia [each 0.3% (1/320)] in the SUN arm.
Overall, 21.9% (70/320) of patients treated with NIVO + CABO required continuous corticosteroids (≥40 mg prednisone daily or equivalent) for any duration of time to manage immune-mediated AEs; 12.8% (41/320) and 5.3% (17/32) received corticosteroids (≥40 mg prednisone daily or equivalent) continuously for ≥14 days and ≥30 days, respectively.
No new treatment-related deaths were reported since the previous database lock (14 June 2021).4
Discussion
With extended follow-up (44.0 months median survival follow-up, per database lock date), first-line NIVO + CABO in patients with previously untreated aRCC continued to maintain clinical benefits over SUN in all three efficacy endpoints of PFS per BICR, OS, and ORR per BICR. Notably, median OS in the NIVO + CABO arm increased by 11.8 months in the ITT population since the previous database lock.4 With the longer follow-up reported here, the safety profile of NIVO + CABO remains consistent with previous follow-up for this trial,4 with no new safety signals emerging.
Responses with NIVO + CABO were durable, with higher complete response rates with NIVO + CABO versus SUN observed within the ITT population and across all IMDC risk groups. OS and PFS outcomes favoured NIVO + CABO versus SUN across most IMDC risk groups except for patients in the favourable-risk group; however, there was a trend toward favouring NIVO + CABO for PFS. Outcomes observed by individual IMDC risk categories are consistent with results from previous CheckMate 9ER follow-ups.3,4 Other phase III aRCC trials comparing first-line ICI plus VEGFR-TKI combination regimens with SUN have demonstrated generally similar outcomes in subgroup analyses by individual IMDC risk category, including KEYNOTE-426 (pembrolizumab plus axitinib), CLEAR (pembrolizumab plus lenvatinib) and JAVELIN Renal 101 (avelumab plus axitinib).6,9,10 In this analysis, median PFS and objective response with NIVO + CABO versus SUN were doubled (PFS 15.6 months versus 7.1 months; ORR 52.6% versus 23.0%) in the combined IMDC intermediate/poor-risk group. Generally similar efficacy outcomes for PFS and ORR in the combined intermediate/poor-risk group were observed with the first-line anti-PD-1 plus VEGFR-TKI combination, pembrolizumab plus axitinib.10 In this extended follow-up, median OS also favoured NIVO + CABO versus SUN (49.5 versus 29.2 months) in the combined intermediate/poor-risk group. These OS outcomes are generally similar with those previously reported in long-term follow-up analyses of this subpopulation in trials evaluating first-line ICI plus VEGFR-TKI or dual ICI regimens.9, 10, 11 Controversy surrounding the use of anti-PD-1 plus VEGFR-TKI combination therapy in patients with IMDC favourable-risk remains, as notable improvements in PFS and ORR, but not OS, have been observed in this patient population.6,10
Subsequent systemic cancer therapy in the ITT population followed trends similar to those previously reported, with VEGF-targeted therapy in the NIVO + CABO group and PD-(L)1 inhibitor-based regimens in the SUN group as the most common subsequent systemic anticancer therapy3,4; these trends were also seen across all IMDC risk groups and in patients who completed 2 years of NIVO treatment. In addition, patients who completed 2 years of NIVO treatment experienced an extended time to initiation of subsequent therapy (median 20.6 months), suggesting the continued durability of efficacy with NIVO.
In this updated analysis, a post hoc assessment of treatment-related AEs by a 6-month interval in all treated patients was conducted to evaluate safety over time. Interestingly, this assessment showed that the highest incidence of most treatment-related AEs occurred during the treatment initiation period with NIVO + CABO and decreased over time. These data suggest that tolerability with NIVO + CABO may improve over time.
Some limitations should be considered when evaluating outcomes in this report. This open-label trial is limited due to the lack of blinding. Exploratory subgroup analyses (i.e. exposure and subsequent therapy by IMDC prognostic risk category, and subsequent therapy in patients who completed the per-protocol 2 years of NIVO treatment), together with the analysis of incidence of treatment-related AEs over time by a 6-month interval, were limited by their post hoc exploratory nature. Additionally, this trial was not powered to detect differences between treatment arms in subsets of patients stratified by IMDC risk. Finally, the Kaplan–Meier curve for time to subsequent therapy in patients who completed the per-protocol 2 years of NIVO flattened after 12 months post-NIVO discontinuation until a single event led to crossing of the median and contributed to the wide CI, with the upper bound not estimable.
In conclusion, NIVO + CABO combination continues to demonstrate prolonged survival and durable antitumour activity versus SUN after a median follow-up of 44.0 months in patients with previously untreated aRCC, including among the IMDC intermediate, poor and intermediate/poor-risk subgroups. These results continue to support first-line NIVO + CABO as a standard of care for this population.
Acknowledgements
We thank the patients who participated in this study; the clinical study teams; and the staff of Dako, and Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay (Santa Clara, CA, USA). All authors contributed to and approved the manuscript; professional medical writing and editorial assistance was provided by Erika Young, PharmD, of Parexel, funded by Bristol Myers Squibb.
Funding
This work was supported by Bristol Myers Squibb (Princeton, NJ, USA) in collaboration with Ono Pharmaceutical Company Ltd (Osaka, Japan). Exelixis, Ipsen Pharma and Takeda Pharmaceutical provided indirect funding. The funders contributed to the study design, data analysis and data interpretation in collaboration with the authors. The funders did not have a role in data collection. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant number P30 CA016672). Patients treated at the Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (core grant number P30 CA008748).
Disclosure
TP reports consulting fees from AstraZeneca, Bristol Myers Squibb (BMS), Exelixis, Incyte, Ipsen, Merck, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai, Roche and MSD; travel reimbursement from Roche, Pfizer, MSD, AstraZeneca and Ipsen; sponsorship from Mashup Ltd.; and research grants (to institution) from AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck, MSD, Seattle Genetics, Novartis, Pfizer, Merck Serono, Astellas, Johnson & Johnson and Eisai. MB reports honoraria from BMS, Roche, Merck and AstraZeneca. BE reports consulting fees from Pfizer, BMS, Ipsen, AVEO and Oncorena; travel expenses from BMS, Ipsen and MSD; honoraria from Pfizer, BMS, Ipsen and Oncorena; and research funding (to institution) from BMS. MTB reports consulting fees from BMS, Asofarma, Eisai, MSD Oncology, Janssen Oncology, Novartis, Bayer, Ferring, Pfizer and MSD; leadership role from BMS; speakers’ bureau from Asofarma, MSD Oncology, BMS, Bayer, Eisai, Janssen Oncology, Ipsen, Pfizer, Merck, Ferring, Tecnofarma, Medicamenta, AstraZeneca and Astellas Pharma; travel reimbursement from Asofarma, Janssen-Cilag, MSD Oncology, BMS (Mexico) and Pfizer; expert testimony from Asofarma; honoraria from Tecnofarma and BMS; other relationship from Sanofi; and research funding from Pfizer. AYS reports consulting fees from Exelixis, BMS and Pfizer/EMD Serono; honoraria from Eisai; research grants from BMS, Eisai and EMD Serono; and research funding (to institution) from 4D Pharma. CS reports advisory board and honoraria from Astellas Pharma, Bayer, BMS, Ipsen, Pfizer S.L.U., Sanofi-Aventis, Hoffmann-LaRoche Ltd., Merck Sharp and Dohme, and Merck; and research funding (to institution) from Ipsen. CP reports consulting fees from Angelini Pharma, AstraZeneca, BMS, Eisai, EUSA Pharma, Ipsen, Merck Serono and MSD; honoraria from BMS, EUSA Pharma, General Electric, Ipsen and MSD; expert testimony from EUSA Pharma and Pfizer; and travel reimbursement from Roche. CHB reports consulting fees from Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical and Lilly; travel reimbursement from Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology and Lilly; stock and stock ownership with MEDSIR and Tummi; honoraria from Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca and Zodiac Pharma; and research funding (to institution) from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, BioMarin, BMS, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana BioSciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Clinica Atlantis, INC Research, Halozyme, Covance, Celgene, inVentiv Health, Merck KGaA, Shanghai Henlius Biotech, Polyphor and PharmaMar. MR reports consulting fees from BMS, Boehringer Ingelheim, AstraZeneca, Pfizer, MSD and Merck; honoraria from BMS, Merck, AstraZeneca, Boehringer Ingelheim, MSD and Pfizer; expert testimony payment from BMS, Merck, AstraZeneca, Pfizer and MSD; and travel reimbursement from Pfizer, Roche, MSD and Varifarma. HG reports consulting fees from BMS, Ipsen, Merck Sharp & Dohme, AstraZeneca, Janssen-Cilag, Pfizer, Roche and Merck Serono; speakers’ bureau from Merck Serono; and travel reimbursement from AstraZeneca. ERK reports stock and ownership of stock with Johnson & Johnson; research funding from Medivation/Astellas; and research funding (to institution) from BMS, Genentech/Roche, Pfizer and Merck Serono. YT reports consulting fees from Astellas, BMS, Merck and Ono Pharmaceutical; advisory roles with Eisai and Ono Pharmaceutical; and research grants (to institution) from Chugai and Ono Pharmaceutical. JB reports consulting fees from AstraZeneca, Astellas, BMS, Eisai, Ipsen, MSD, Merck Serono, Pfizer, Roche and Janssen; consulting fees (to institution) from BMS and Pfizer; honoraria (to institution) from AstraZeneca, Astellas, BMS, Eisai, MSD, Ipsen, Novartis, Nektar, Pfizer, Roche and Seagen; and is a member of the Renal Cell Carcinoma Group of the European Association of Urology. SG reports consulting fees from BMS, Bayer, Pfizer, Exelixis, Corvus Pharmaceuticals, Sanofi, EMD Serono, Seattle Genetics/Astellas, Eisai, Merck, AVEO and QED Therapeutics; and research funding (to institution) from Pfizer, Merck, Agensys, Novartis, BMS, Bayer, Eisai, Seattle Genetics/Astellas, Calithera Biosciences, Corvus Pharmaceuticals, Surface Oncology, Exelixis, Aravive, AVEO and Gilead Sciences. CS reports employment with and ownership of stock with Exelixis. PW reports employment with and ownership of stock with BMS; and stock ownership with Intercept Pharmaceuticals. VF reports employment with and ownership of stock with BMS. RJM reports consulting fees from Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Pfizer, AstraZeneca, EMD Serono, Calithera Biosciences and AVEO; travel reimbursement from BMS; and research funding (to institution) from Pfizer, BMS, Eisai, Novartis, Genentech/Roche, Exelixis, Merck and AVEO. TKC reports consulting fees from Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, BMS, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Infinity Pharmaceuticals, Aravive, Tempest Therapeutics, Nuscan Diagnostics and Arcus Biosciences; leadership role with Dana Farber Cancer Hospital, NCCN, KidneyCan, ASCO and ESMO; travel reimbursement from Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, BMS, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly and ESMO; royalties received (to institution) from two international patent applications and from ctDNA technologies; medical writing and editorial assistance support funded by communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies and Parexel; stock and ownership of stock with Pionyr, Tempest Therapeutics, Osel and Precede Bio. Honoraria from UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Bayer, BMS, Genentech/Roche, Merck, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Kidney Cancer Association, Exelixis, EMD Serono, Lilly, Janssen Oncology, IQVIA, AVEO and NCI Genitourinary Cancers Steering Committee; and research funding (to institution) from Pfizer, Novartis, Merck, Exelixis, TRACON Pharma, GlaxoSmithKline, BMS, AstraZeneca, Peloton Therapeutics, Roche/Genentech, Celldex, Agensys, Eisai, Takeda, Prometheus, Ipsen, Corvus Pharmaceuticals, Cerulean Pharma, Seattle Genetics/Astellas, Bayer, Foundation Medicine, Roche, Calithera Biosciences, Analysis Group, NCI, GATEWAY for Cancer and Congressionally Directed Medical Research Programs (DOD). ABA has declared no conflicts of interest.
Data sharing
Data are available upon reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplementary data
References
- 1.Gulati S., Labaki C., Karachaliou G.S., Choueiri T.K., Zhang T. First-line treatments for metastatic clear cell renal cell carcinoma: an ever-enlarging landscape. Oncologist. 2022;27(2):125–134. doi: 10.1093/oncolo/oyab056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer R.J., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(7):888–898. doi: 10.1016/S1470-2045(22)00290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri T.K., Eto M., Motzer R., et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023;24(3):228–238. doi: 10.1016/S1470-2045(23)00049-9. [DOI] [PubMed] [Google Scholar]
- 7.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haanen J., Larkin J., Choueiri T.K., et al. Extended follow-up from JAVELIN Renal 101: subgroup analysis of avelumab plus axitinib versus sunitinib by the International Metastatic Renal Cell Carcinoma Database Consortium risk group in patients with advanced renal cell carcinoma. ESMO Open. 2023;8(3) doi: 10.1016/j.esmoop.2023.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plimack E.R., Powles T., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur Urol. 2023;84(5):449–454. doi: 10.1016/j.eururo.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Motzer R.J., McDermott D.F., Escudier B., et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085–2097. doi: 10.1002/cncr.34180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):422–423. doi: 10.1016/j.annonc.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network Kidney cancer (v.4.2023) https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf Available at. Accessed June 16, 2023.
- 14.Lalani A.A., Heng D.Y.C., Basappa N.S., et al. Evolving landscape of first-line combination therapy in advanced renal cancer: a systematic review. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221108685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1938;53:457–481. [Google Scholar]
- 16.Clopper C.P., Pearson E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.