Abstract
Theiler’s murine encephalomyelitis virus is a neurotropic murine picornavirus which replicates permissively and causes a cytopathic effect in the BHK-21 cell line. We examined the interactions between the GDVII and DA strains of Theiler’s virus and BHK-21 host cell proteins in a virus overlay assay. We observed binding of the virions to two proteins of approximately 60 kDa. These proteins were microsequenced and identified as desmin and vimentin, two main components of the intermediate filament network. The association between desmin or vimentin and virions was demonstrated by immunoprecipitation. Anti-desmin and anti-vimentin monoclonal antibodies precipitated GDVII or DA virions from extracts of infected BHK-21 cells. The intracellular distributions of virions and of the desmin and vimentin intermediate filaments of BHK-21 cells were investigated by two-color immunofluorescence confocal microscopy. Following infection, the intermediate filament network was rearranged into a shell-like structure which surrounded a viral inclusion. Finally, close contact between GDVII virus particles and 10-nm intermediate filaments was observed by electron microscopy.
Theiler’s murine encephalomyelitis virus (TMEV) is a murine picornavirus that is responsible for inapparent enteric infections and, on occasion, neurological diseases (23, 28). Depending on the viral strain, the neurological disease can be a fatal encephalomyelitis or a chronic demyelinating disease that is studied as a model for multiple sclerosis. The GDVII strain causes the former, while the DA and BeAn strains are responsible for the latter. All strains of TMEV can be readily adapted to grow in the BHK-21 cell line, in which they replicate permissively and cause a characteristic cytopathic effect with rounding up of the cell followed by lysis.
The capsid of picornaviruses interacts with host cell components at many different steps of the viral life cycle. The interaction with the receptor and other components important for entry has been studied most extensively. However, at later times such as maturation, assembly, and release of virions, the capsid interacts with other, mostly uncharacterized intracellular components. For example, the work of Doedens et al. (4) suggested that the capsid of poliovirus interacts with and rearranges the network of intermediate filaments. The structures of the DA, BeAn, and GDVII capsids have been solved at the atomic level by X-ray crystallography (11, 21, 26). The main differences between these capsids are located at the surface of the particle and could be responsible for differences in the way the virion interacts with its environment, including the viral receptor and other cellular components (30). In the present work, we investigated the nature of the proteins of BHK-21 cells to which the virions of the DA and GDVII strains of TMEV bind. We report that both viruses bind specifically to desmin and vimentin, two components of the intermediate filament network, and that this network undergoes considerable reorganization in the course of infection of BHK-21 cells. To our knowledge, this is the first demonstration of the existence of direct interactions between picornavirus particles and intermediate filaments.
MATERIALS AND METHODS
Cell lines and viruses.
The RAW264.7 monocyte-macrophage cell line was purchased from the American Type Culture Collection. The M1 cell line was kindly provided by Alain Israël (Institut Pasteur, Paris France). All cell lines, including BHK-21 and L929, were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (4,500 mg of glucose per liter) containing 100 μg of streptomycin per ml, 100 U of penicillin per ml, and 10% fetal calf serum. In some experiments, BHK-21 cells were incubated for 1 h with DMEM containing 5 μg of cytochalasin D (Sigma) per ml and 10 μg of nocodazole (Sigma) per ml before being infected with either the GDVII or the DA strain (10 PFU/cell). Working stocks of the DA and GDVII strains of TMEV were prepared on BHK-21 cells. The techniques for growing and assaying both strains have been described previously (20).
Radiolabeling of virus.
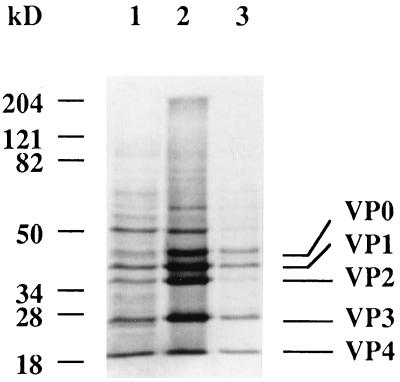
Confluent BHK-21 cells were infected with 5 PFU of either the GDVII or DA virus per cell in DMEM without serum. The medium was removed after 90 min and replaced by the same medium containing 1 μg of actinomycin D (Sigma) per ml. After 3 h, the medium was replaced by DMEM without methionine and cysteine but containing 1 μg of actinomycin D per ml. At 5 h later, 1 mCi of [35S]methionine plus [35S]cysteine (Pro-mixt; Amersham) was added, and the cells were incubated overnight at 34°C. The cells were collected by centrifugation at 8,000 × g and treated with 1% sodium dodecyl sulfate (SDS). The solubilized pellet was centrifuged at 8,000 × g, and the supernatant was centrifuged at 140,000 × g for 3 h at 21°C. The supernatant of this centrifugation contained 107 PFU of TMEV per ml and gave an SDS-polyacrylamide gel electrophoresis (PAGE) profile which corresponded to virtually pure virus proteins (see Fig. 1).
FIG. 1.
Characterization of radioactive GDVII virus. The virus was grown overnight in BHK-21 cells in the presence of [35S]Met-[35S]Cys. Infected cells and cell debris were collected by centrifugation at 8,000 × g, treated with 1% SDS, and centrifuged at 140,000 × g for 3 h. Various fractions were analyzed by SDS-PAGE (10% polyacrylamide) and exposed to a PhosporImager screen. Lanes 1: 8,000 × g supernatant; 2, 1% SDS-treated pellet; 3, 140,000 × x supernatant.
Extraction of cellular proteins by a solid-phase assay for virus binding.
The solid-phase assay is essentially as described by Kilpatrick and Lipton (15). Cell lines (BHK-21, RAW 264.7, M1, and L929) were lysed with Nonidet P-40 (NP-40) buffer (50 mM Tris HCl [pH 8.0], 150 mM NaCl, 1% NP-40) and centrifuged for 10 min at 13,000 × g and 4°C. Phenylmethylsulfonyl fluoride (1 mM) was added, and the supernatant, containing mainly cytoplasmic proteins, was stored at −80°C. Known amounts of extracted proteins were bound to a sheet of nitrocellulose (Hybond-C extra; Amersham) by filtration in a 96-well minifold apparatus. All subsequent steps were performed at 25°C in shallow plastic boxes with gentle rocking. The filters were incubated for 30 min in blocking buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20, 5% milk) and then for 1 h with radioactive virus. The filters were washed three times for 5 min each with washing buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and exposed to the screen of a PhosphorImager.
Virus overlay assay.
Crude protein extracts of BHK-21, RAW 264.7, M1, and L929 cells (100 μg of protein per lane) were separated by SDS-PAGE (10% polyacrylamide). The gels were electroblotted onto nitrocellulose filters with a semidry Multiphor II NovaBlot Unit (Pharmacia Biotech). The transfer buffer contained 50 mM Tris HCl (pH 8.6), 40 mM glycine, 0.04% SDS, and 20% ethanol. Transfer was carried out for 2 h at 100 mA. The nitrocellulose sheets were incubated at 25°C in blocking buffer and then for 1 h with radioactive virus. The sheets were washed three times for 5 min each with washing buffer and exposed to the screen of a PhosphorImager. The molecular weights of proteins were determined with a series of prestained markers (Bio-Rad).
2D gel electrophoresis and microsequencing.
Cultured cells were washed twice in phosphate-buffered saline (PBS). They were lysed by incubation for 3 min at 100°C in a solution containing 50 mM Tris HCl (pH 8.0), 1% β-mercaptoethanol, and 0.3% SDS and cooled on ice. The lysates were treated for 5 min with DNase (100 μg/ml) and RNase (50 μg/ml). These extracts were quickly frozen in liquid nitrogen and lyophilized. They were then resuspended in a buffer containing 9.95 M urea, 4% NP-40, 2% ampholytes, and 100 mM dithiothreitol and stored at −80°C until used. Two-dimensional (2D) electrophoresis was performed as previously described (10, 16) with a few modifications. Typically, a 10-μl sample containing 100 μg of proteins was analyzed in an isoelectrofocusing gel (pH range, 4 to 8; Millipore Corp.), and the second dimension was analyzed in a 10% acrylamide slab gel. The relative molecular masses of the proteins were determined according to the molecular weight markers applied to a slot of the same gel. The relative isoelectric points (pI) were determined by running a carbamylated muscle creatine phosphokinase standard (BDH) in parallel. Spots of interest were excised from four 2D gels previously stained for 2 h with naphthol blue black (0.003% in a solution consisting of 45% methanol, 10% acetic acid, and 45% MilliQ water) and rinsed extensively in water. The spots were subsequently digested in situ with trypsin (0.01% in Tween 20). Peptides were separated by high-performance liquid chromatography in a 2 to 55% acetonitrile gradient (0.1% trifluoroacetic acid). Individual peptides were collected and applied to a Sequenator apparatus (Applied Biosystems model 470).
Immunoprecipitation and Western blotting.
BHK-21 cells were infected with 10 PFU of GDVII or DA virus per cell and incubated overnight at 34°C. The medium was harvested and centrifuged at 2,000 × g for 10 min, and 500 μl of supernatant was used for each immunoprecipitation. Immunoprecipitation was performed for 1 h at 4°C in a solution containing 50 mM Tris HCl (pH 7.4), 150 mM NaCl, and 50 mM EDTA–0.05% Tween 20. The following antibodies were used: anti-VP1 monoclonal antibody (MAb) (1a), anti-desmin MAb (DE-B-5; Boehringer Mannheim), anti-vimentin MAb (V9; Boehringer Mannheim), or anti-α-actin MAb (asm-1; Boehringer Mannheim). The mixture was then incubated for 1 h at 4°C with protein A-agarose (GIBCO BRL) to collect the immune complexes as already described (12). The immune complexes were separated by SDS-PAGE (10% polyacrylamide) and transferred onto nitrocellulose filters as described above for the virus overlay assay. The nitrocellulose sheets were incubated in blocking buffer (50 mM Tris HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20, 5% milk) for 30 min at 25°C with gentle rocking and then a 1:100 dilution of the anti-VP1 MAb in blocking buffer for 1 h at 25°C. The sheets were washed twice for 10 min with blocking buffer and then incubated for 1 h in blocking buffer containing a 1:1,000 dilution of anti-mouse immunoglobulin (Ig) coupled to horseradish peroxidase (Amersham). After two washes in washing buffer, the peroxidase activity was detected with the ECL Western blotting system (Amersham).
Confocal microscopy.
BHK-21 cells were grown to semiconfluence on glass coverslips, infected with the GDVII or DA strain of TMEV (10 PFU/cell), and incubated at 37°C for 5, 8, or 10 h. The cells were then washed twice in PBS, fixed for 6 min with cold methanol-acetone (7:3, vol/vol), and washed twice in PBS.
The viral capsid was detected with an anti-VP1 MAb (1a). VP1/desmin and VP1/vimentin double immunofluorescence was carried out with a 1:100 dilution of the anti-VP1 MAb and either a polyclonal anti-desmin D8281 antibody (1:6 dilution) obtained in rabbits (Boehringer) or a polyclonal anti-vimentin antibody (1:200 dilution) obtained in goats (a gift from D. Paulin). After incubation for 1 h at room temperature, the cells were washed three times with PBS. For VP1/desmin double labeling, the cells were incubated for 30 min with a mixture of a 1:200 dilution of a goat anti-mouse Ig tetramethylrhodamine-5-isothiocyanate (TRITC)-labeled antibody (Biosys) and a 1:300 dilution of a goat anti-rabbit fluorescein isothiocyanate (FITC)-labeled antibody (Biosys). For VP1/vimentin double labeling, the cells were incubated for 30 min with a mixture of a 1:100 dilution of a TRITC-labeled rabbit anti-mouse Ig antibody (Sigma) and a 1:200 dilution of FITC-labeled rabbit anti-goat Ig antibody (Nordic).
Single immunofluorescence was carried out with the anti-VP1 MAb or the polyclonal anti-desmin and anti-vimentin antibodies at the dilution indicated above.
After incubation with the secondary antibody and washing, the coverslips were washed three times in PBS and mounted upside down onto a drop of Mowiol (Hoechst) placed on a microscope slide. Double immunofluorescence microscopy was performed with a Wild Leitz confocal microscope.
Electron microscopy.
BHK-21 cells were infected with the GDVII or DA strain of TMEV (10 PFU/cell) and incubated for 8 or 10 h at 37°C. Infected cells were treated as previously described (29). In brief, cell pellets were fixed for 40 min at 4°C in a solution containing 1% glutaraldehyde, 1% OsO4, and 0.05 M phosphate buffer. The cell pellets were rinsed three times with distilled water and stained overnight in 0.5% uranyl acetate. The cells were dehydrated in increasing concentrations of alcohol and embedded in Epon. Ultrathin sections were cut on a Reichert Ultracut E microtome. The sections were examined in a JEOL 1200 EX electron microscope.
RESULTS
Biochemical analysis of the binding of TMEV to cellular proteins.
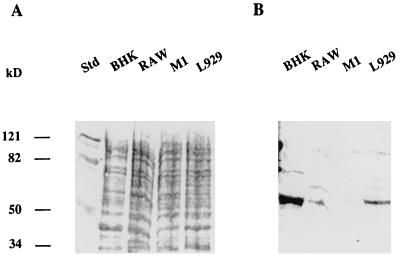
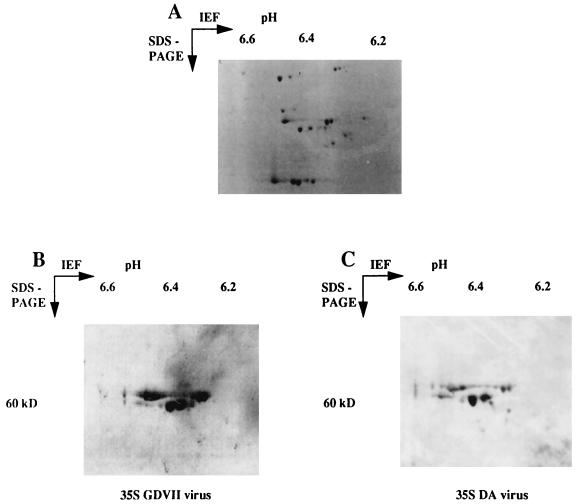
The binding of radioactive GDVII virus to a crude extract of cellular proteins was tested by the solid-phase assay described by Kilpatrick and Lipton (15). As shown in Fig. 1, the radioactivity of the probe was associated almost exclusively with viral capsid proteins. The cell lines used to prepare the extracts were either permissive (BHK-21, RAW264.7, and L929) or nonpermissive (M1) for virus replication (13). An example of the results obtained by the solid-phase assay is shown in Fig. 2. The amount of virus that bound to the cellular extract depended on the cell line. The largest amount was observed with the BHK-21 line, whereas there was no binding to extracts of the M1 line. Proteins from the different cell lines were separated by SDS-PAGE and probed with radioactive virus by the virus overlay assay described in Materials and Methods. The virus bound to a major protein of 60 kDa. The amount of virus bound depended on the cell line in the same way as in the assay in Fig. 2. The strongest binding was observed with the BHK-21 line (Fig. 3) whereas there was no binding to a 60-kDa protein in M1 cell extracts. To characterize the 60-kDa protein further, the virus overlay assay was repeated after separation of BHK-21 cellular proteins by 2D gel electrophoresis (Fig. 4). As shown in Fig. 4, the radioactive virus bound two proteins of 59 and 60 kDa, each with various isoelectric isoforms. No binding was observed for proteins extracted from the M1 cell line (data not shown). Furthermore, no proteins in the range of 60 kDa and with an isoelectric point between 6.37 and 6.31 were observed when a 2D gel of M1 cell proteins was stained with Coomassie blue (results not shown). These results indicated that the GDVII and DA strains of TMEV bind to two different cellular proteins, each with several isoforms. To identify these proteins, the spots containing one isoform of each protein were cut out. The proteins were digested in situ with trypsin and the resulting peptides were separated by HPLC. The sequence of one peptide from each protein (LLEGEESRINLIQT and QESNEYRRQVQSLTC) identified them as, respectively, desmin and vimentin, two components of the intermediate filament network (19, 25).
FIG. 2.
Binding of 35S-labeled GDVII virus to crude cell protein extracts. Cell extracts (1, 10, and 100 μg of proteins) from the BHK-21, RAW264.7, and M1 cell lines were blotted onto a nitrocellulose membrane. The BHK-21 and RAW264.7 lines are permissive for TMEV replication, and the M1 line is nonpermissive. After blocking, the membrane was incubated for 1 h with 106 cpm of radiolabeled virus, washed, and exposed for a few hours to the screen of a PhosphorImager. H2O: control without proteins.
FIG. 3.
Binding of 35S-labeled GDVII virus to proteins of different cell lines. The proteins (100 μg) were separated by SDS-PAGE (10% polyacrylamide) and transferred to a nitrocellulose membrane. (A) The membrane was stained with Ponceau red. (B) The membrane was incubated in blocking buffer and then with radiolabeled GDVII virus (106 cpm) for 1 h and washed three times in blocking buffer without milk. Std, Protein molecular mass standards.
FIG. 4.
Binding of 35S-labeled GDVII or DA virus to proteins extracted from BHK-21 cells. The proteins (100 μg) were separated by 2D gel electrophoresis. (A) Staining with Coomassie blue. (B and C) The proteins from polyacrylamide gels run in parallel were transferred onto nitrocellulose membranes. The membranes were incubated in blocking buffer containing 5% milk powder and then incubated for 1 h with 106 cpm of radioactive GDVII (B) or DA (C) virus. After three washes with blocking buffer without milk, the membranes were exposed for a few hours to the screen of a PhosphorImager.
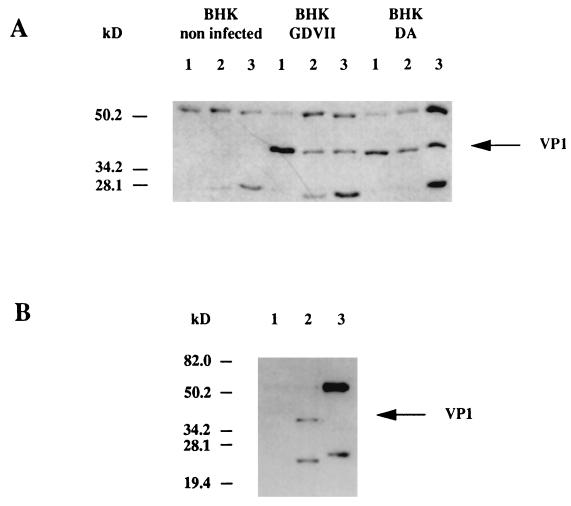
Immunoprecipitation experiments were used to confirm that TMEV binds desmin and vimentin. BHK-21 cells were infected with either the GDVII or the DA strain of TMEV and lysed, and the proteins were immunoprecipitated with either anti-desmin or anti-vimentin MAb as described in Materials and Methods. The precipitates were analyzed by PAGE followed by Western blotting with an anti-VP1 MAb. As shown in Fig. 5A, anti-desmin and anti-vimentin MAb were able to immunoprecipitate VP1 of the GDVII and DA strains of TMEV. On the other hand, an anti-actin MAb did not immunoprecipitate VP1 (Fig. 5B). Heavy and light Ig chains (60 and 28 kDa, respectively) are seen on the Western blots because the secondary anti-mouse Ig used to detect the primary anti-VP1 MAb also reacted with the anti-desmin and anti-vimentin MAb contained in the precipitates (see Materials and Methods). Because desmin and vimentin have similar molecular weights to that of the Ig heavy chain, it was not possible to analyze the converse immunoprecipitation and to show that the anti-VP1 MAb could precipitate desmin and vimentin. In summary, both the virus overlay experiments and the immunoprecipitations suggested an association between TMEV, desmin, and vimentin in infected cells.
FIG. 5.
(A) Immunoprecipitation of BHK-21 cell lysates. The cells were either not infected or were infected with the GDVII or DA virus. The lysates were immunoprecipitated with the anti-VP1 MAb (lanes 1), the anti-desmin MAb (lanes 2) or the anti-vimentin MAb (lanes 3). Immunoprecipitated proteins were separated by SDS-PAGE (10% polyacrylamide), transferred to a nitrocellulose membrane, and analyzed by Western blotting with the anti-VP1 MAb as described in Materials and Methods. (B) Immunoprecipitation of BHK-21 cell lysates. The cells were either not infected (lane 1) or infected with the GDVII virus (lanes 2 and 3). The lysates were immunoprecipitated with the anti-VP1 MAb (lanes 1 and 2) or an anti-α-actin MAb (lane 3). Immunoprecipitated proteins were separated by SDS-PAGE (10% polyacrylamide), transferred to a nitrocellulose membrane, and analyzed by Western blotting with the anti-VP1 MAb as described in Materials and Methods.
Cytochemical analysis of the interaction between TMEV and intermediate filaments.
We used double-immunofluorescence confocal microscopy to examine the organization of the desmin and vimentin cytoskeletal network in BHK-21 cells infected with TMEV. The cells were infected with either the GDVII or the DA strain of TMEV and harvested 5, 8, or 10 h later. Noninfected BHK-21 cells were used as controls. They showed a diffuse intermediate filament network which emanated from the perinuclear region and extended toward the cell membrane (Fig. 6). This network was altered extensively during infection by TMEV, unlike the microtubules and the actin filaments (results not shown). We will describe these alterations as a function of the time postinfection (p.i.) (5, 8, and 10 h) and for both strains of TMEV.
FIG. 6.
Organization of the intermediate filament network in uninfected BHK-21 cells. Uninfected cells were stained with an anti-desmin MAb (A) or anti-vimentin MAb (B) followed by an FITC-labeled anti mouse Ig antibody, as described in Materials and Methods.
No change in the network of desmin filaments was apparent 5 h after infection with the DA strain (Fig. 7A). At this time, viral antigens were present throughout the cytoplasm in most cases. On occasion, they tended to accumulated in a juxtanuclear location. In GDVII virus-infected cells, on the other hand, viral capsid antigen was often observed in patches located at the periphery of the cell, just below the cytoplasmic membrane (Fig. 7B). This location was demonstrated by the fact that staining disappeared as the optical sectioning was moved downward into the cells (results not shown).
FIG. 7.
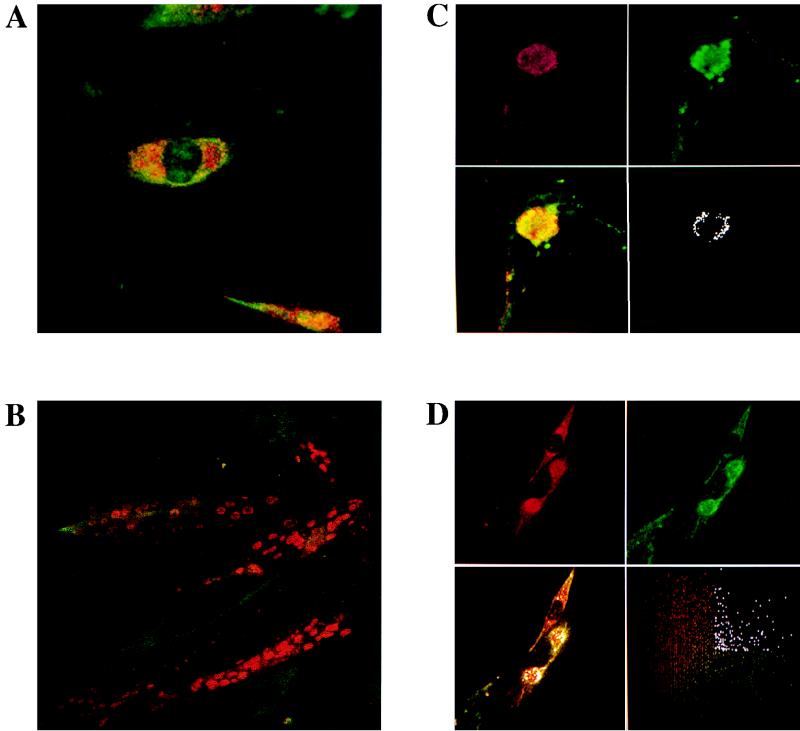
Detection of the desmin filament network and viral capsid antigens in infected BHK-21 cells by double-immunofluorescence confocal microscopy. Infected cells were incubated with the anti-VP1 MAb and a polyclonal anti-desmin antibody, D8281. After being washed, the cells were incubated with a TRITC-labeled rabbit anti-mouse Ig antibody and with an FITC-labeled rabbit anti-goat Ig antibody as described in Materials and Methods. (A) DA-infected cells, 5 h p.i. (B) GDVII-infected cells, 5 h p.i. (C) DA-infected cells, 8 h p.i. Upper panels: virus (red) and desmin (green). Lower left panel: combined image of red and green fluorescence. Lower right panel: pixels of high intensity for both colors. (D) GDVII-infected cells, 8 h p.i. Upper panels: virus (red) and desmin (green). Lower left panel: combined image of red and green fluorescence superimposed on the pixels of high intensity for both colors (white). Lower right panel: cytofluorogram analysis.
By 8 h after infection with the DA strain, the desmin network had collapsed almost entirely. Desmin was found only in one or two juxtanuclear locations, where it formed a shell at the periphery of a viral inclusion which contained most of the intracellular viral antigen (Fig. 7C). The colocalization of virus and desmin is shown clearly in Fig. 7C, lower right panel, in which, following cytofluorogram analysis, all pixels of high intensity for both the red (virus) and green (desmin) fluorescence are shown in white. As shown, colocalization occurred mainly at the periphery of the juxtanuclear inclusion. The findings with the GDVII strain at this time were very similar (Fig. 7D). The lower right panel of Fig. 7D shows the distribution of pixels for each color, according to their intensity. The pixels which were both red and green and of high intensity are shown in white. The results obtained when studying the vimentin network after 8 h of infection with the GDVII or DA strain of TMEV were identical to those reported above for the desmin network (results not shown).
With both strains of TMEV, the entire network of intermediate filaments had disappeared by 10 h p.i. At this time point, all the desmin and vimentin antigens formed shell-like structures surrounding viral capsid antigens (results not shown).
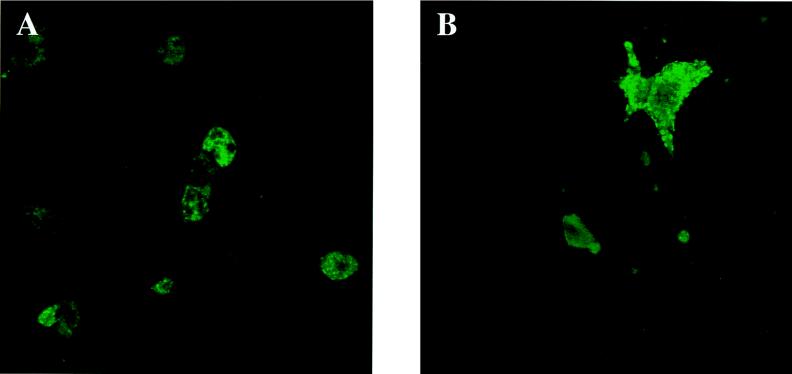
We tested the effect of disrupting the cytoskeleton with cytochalasin D and nocodazole on the distribution of viral antigens. BHK-21 cells were treated with 5 μg of cytochalasin D per ml and 10 μg of nocodazole per ml for 1 h, infected with the GDVII or DA strain of TMEV, and harvested 8 h later. The viral yield, measured by a plaque assay, was the same for cells treated or not treated with the drugs. This was true for both TMEV strains (results not shown). As expected, the distributions of desmin and vimentin were considerably altered by the treatment. Instead of forming an organized network, desmin and vimentin were distributed diffusely throughout the cytoplasm at all times after treatment with the drugs. Interestingly, viral capsid antigens were also distributed diffusely throughout the cytoplasm and did not form juxtanuclear inclusions in drug-treated cells (Fig. 8). On the other hand, the patches of GDVII capsid antigen observed under the cytoplasmic membrane 5 h p.i. were still present, in spite of the treatment (results not shown).
FIG. 8.
Detection of viral capsid antigens in infected BHK-21 cells (8 h p.i.) treated with cytochalasin D and nocodazole. (A) DA virus-infected cells; (B) GDVII virus-infected cells. The cells were incubated with the anti-VP1 MAb and then with FITC-labeled goat anti-mouse antibody as described in Materials and Methods.
Finally, we used transmission electron microscopy to examine BHK-21 cells infected with the GDVII or DA strain of TMEV. At 8 h p.i., cells infected with the GDVII strain showed a proliferation of intracytoplasmic vesicles characteristic of picornavirus infections (1). Viral particles were observed throughout the cytoplasm, often in association with typical 10-nm intermediate filaments (Fig. 9A), particularly near the nucleus (Fig. 9B). Later (10 h p.i.), viral particles formed crystals which were often close to the nucleus (results not shown). It was difficult to identify viral particles in cells which had been infected with the DA strain for 8 to 10 h, although the proliferation of intracytoplasmic vesicles was conspicuous. DA viral particles, however, could be seen after 14 to 16 h of infection, although association of particles with intermediate filaments was not observed. On the other hand, viral particles were sometimes associated with membranes, as already described by Friedmann and coworkers (5, 6).
FIG. 9.
Transmission electron micrographs of ultrathin sections of BHK-21 cells infected with the GDVII virus. The cells were harvested 8 h p.i. and fixed in 1% glutaraldehyde–1% OsO4–0.05 M phosphate buffer. Arrows point to regions of apposition between the viral particle and intermediate filaments (10 nm). Bar, 200 nm.
DISCUSSION
Intermediate filaments are composed of at least 40 different proteins which belong to five different types (7). Types I to IV are cell specific and cytoplasmic. Types I and II are the acidic and basic keratin expressed in epithelial cells. Type III includes vimentin, desmin, glial fibrillary acidic protein, and peripherin, which are found, respectively, in mesenchymal cells, muscle cells, astrocytes, and some neurons. Type IV corresponds to the neurofilament proteins and to α-internexin, which are found in most neurons, and to nestin, which is found in neuroepithelial cells and muscle precursors. Finally, type V corresponds to the ubiquitous nuclear A, B, and C laminins, which form a network underlying the nuclear membrane. The function of intermediate filaments has recently been addressed by gene knockout experiments. These experiments provided the first evidence that intermediate filaments are involved in cell resilience and maintenance of tissue integrity (8). Knockout of the gene encoding desmin causes the rupture of skeletal and cardiac muscle and the collapse of blood vessel walls (18, 22). The vimentin-deprived mice appear to have no abnormality during embryonic development, are fertile, and can live to adulthood (3). However, a subpopulation of astrocytes in the white matter of the corpus callosum of these mice is no longer able to form a normal network of glial fibrillary acidic protein GFAP filaments (8). The BHK-21 cells, which we used in this study, have a network of intermediate filaments made only of desmin and vimentin (27). In a series of experiments described in this paper, we demonstrated that the capsid of TMEV binds to both proteins. This led us to study the changes occurring in the intermediate-filament network of BHK-21 cells following infection by these viruses. We demonstrated that the desmin and vimentin networks are disrupted and that by the end of the viral cycle, these proteins surround juxtanuclear inclusions which contain progeny virions. A direct interaction of GDVII virions with intermediate filaments was strongly suggested by observations made with the electron microscope.
Many viruses reorganize the cell architecture during their replication (2, 17, 24). For example, in cells infected with picornaviruses, there is a proliferation of intracytoplasmic membranes, which form vesicles that play an important role in viral RNA replication (1), and a collapse of the intermediate filaments around the nucleus (4). The mechanism of this collapse is unknown. It could result from the loss of inner membrane anchorage or from a specific depolymerization-repolymerization process. Its function is not clear either, since it is not required for a full yield of progeny poliovirus by HeLa cells (4). However, these studies were conducted in vitro in highly permissive cells, and reorganization of the cytoskeleton could have essential functions in vivo. Several other viruses besides picornaviruses cause a collapse of the intermediate filaments. This is the case for human respiratory syncytial virus (9), human immunodeficiency virus (14), frog virus 3 (24), and vaccinia virus (17). With respiratory syncytial virus and human immunodeficiency virus, this collapse seems to be due to proteolysis (9, 14). We observed that cells treated with drugs which depolymerize microtubules and actin microfilaments, and by consequence all type of filament networks (7), did not contain TMEV capsid inclusions. Instead, viral capsid antigens were evenly distributed in the cytoplasm. This suggests that reorganization of intermediate filaments may be involved in the formation of juxtanuclear viral inclusions. It is interesting that the M1 macrophage cell line, which is resistant to infection by TMEV, does not express desmin or vimentin. However, the resistance of this cell line is probably explained by the absence of a viral receptor (13).
It has been known for some time that before killing the cell, the GDVII strain accumulates in intracytoplasmic crystalline arrays whereas the DA strain does so only rarely. Instead, the DA strain is often found associated with intracytoplasmic membrane structures (5, 6). Our present data confirm these conclusions. This difference of behavior suggests that, depending on the strain, the viral capsid interacts differently with cell components and with other capsids. This was one reason for comparing the interactions with cellular proteins of the DA and GDVII strains. Our results showed no difference in the behavior of the two strains in the virus overlay assay or the immunoprecipitation assay. The apposition of virions and intermediate filaments was observed by electron microscopy for the GDVII strain (Fig. 9) but not for the DA strain. However, viral particles were not visible in DA virus-infected cells before 14 h p.i., and at such a late time the intermediate filament network had entirely disappeared. Therefore, it is impossible to draw conclusions about a difference of behavior at this level of analysis.
Some years ago, Kilpatrick and Lipton demonstrated an interaction between the BeAn strain of TMEV and a 34-kDa membrane protein (15). The fact that we did not observe this interaction could be due to a difference in the viral strains or, more probably, to a difference in the methods used to prepare cellular proteins. Kilpatrick and Lipton were interested in the identification of the viral receptor and therefore used membrane proteins, whereas we used a total-cell protein extract that was composed mainly of cytoplasmic proteins.
The reorganization of intermediate filaments was observed by confocal microscopy for both strains of TMEV during the first 5 to 8 h of infection. However, since DA viral particles were not seen in the cells at this time, it is most likely that this reorganization did not depend on the association of mature viral particle with the intermediate filaments. The role of the interactions between the capsid of TMEV and the intermediate filament network in pathogenesis remains to be examined. Mice in which the genes for vimentin or desmin have been inactivated (3, 8) should provide important tools for these studies.
ACKNOWLEDGMENTS
We thank M. Gau for secretarial help, D. Paulin for providing the polyclonal anti-vimentin antibody, and A. Israël for giving us the M1 cell line.
P. Nédellec was a recipient of an EMBO long-term fellowship. This work was supported by grants from the Centre National de la Recherche Scientifique, the Institut Pasteur Foundation, and the EC Human Capital and Mobility program (contract CHRX-CT94-0670).
REFERENCES
- 1.Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- 1a.Chamorro, M. Unpublished data.
- 2.Ciampor F. The role of the cytoskeleton and nuclear matrix in virus replication. Acta Virol. 1988;32:168–189. [PubMed] [Google Scholar]
- 3.Colluci-Guyon E, Portier M, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 4.Doedens J, Maynell L A, Klymkowsky M W, Kirkegaard K. Secretory pathway function, but not cytoskeletal integrity, is required in poliovirus infection. Arch Virol Suppl. 1994;9:159–172. doi: 10.1007/978-3-7091-9326-6_16. [DOI] [PubMed] [Google Scholar]
- 5.Frankel G, Lorch Y, Karlik P, Friedmann A. Fractionation of Theiler’s virus-infected BHK21 cell homogenates: isolation of virus-induced membranes. Virology. 1987;158:452–455. doi: 10.1016/0042-6822(87)90220-0. [DOI] [PubMed] [Google Scholar]
- 6.Friedmann A, Lipton H L. Replication of Theiler’s murine encephalomyelitis viruses in BHK21 cells: an electron microscopic study. Virology. 1980;101:389–398. doi: 10.1016/0042-6822(80)90452-3. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 8.Galou M, Gao J, Humbert J, Mericskay M, Li Z, Paulin D, Vicart P. The importance of intermediate filaments in the adaptation of tissues to mechanical stress: evidence from gene knockout studies. Biol Cell. 1997;89:85–97. [PubMed] [Google Scholar]
- 9.Garcia-Barreno B, Jorcano J L, Aukenbauer T, Lopez-Galindez C, Melero J A. Participation of cytoskeletal intermediate filaments in the infectious cycle of human respiratory syncytial virus (RSV) Virus Res. 1988;9:307–322. doi: 10.1016/0168-1702(88)90090-1. [DOI] [PubMed] [Google Scholar]
- 10.Garrels J I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- 11.Grant R A, Filman D J, Fujinami R S, Icenogle J P, Hogle J M. Three-dimensional structure of Theiler’s virus. Proc Natl Acad Sci USA. 1992;89:2061–2065. doi: 10.1073/pnas.89.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 13.Jelachich M L, Bandyopadhyay P, Blum K, Lipton H L. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 14.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick D R, Lipton H L. Predominant binding of Theiler’s viruses to a 34-kilodalton receptor protein on susceptible cell lines. J Virol. 1991;65:5244–5249. doi: 10.1128/jvi.65.10.5244-5249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent-Winter C, Fougère-Deschatrette C, Weiss M C. Identification of polypeptides whose presence correlates with retention or loss of an albumin extinguisher chromosome in rat hepatoma-mouse L cell fibroblast microcell hybrids. Differentiation. 1994;55:225–232. doi: 10.1046/j.1432-0436.1994.5530225.x. [DOI] [PubMed] [Google Scholar]
- 17.Leao Ferria L R, Moussatché N, Moura Neto V. Rearrangement of intermediate filament network of BHK-21 cell infected with vaccinia virus. Arch Virol. 1994;138:273–285. doi: 10.1007/BF01379131. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Colucci-Guyon E, Pinçon-Raymond M, Mericskay S, Pournin S, Paulin D, Babinet C. Cardiac lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Lilienbaum A, Buttler-Browne G, Paulin D. Human desmin gene: complete nucleotide sequence, characterization and regulation of expression during myogenesis development. Gene. 1989;78:243–254. doi: 10.1016/0378-1119(89)90227-8. [DOI] [PubMed] [Google Scholar]
- 20.Lipton H L. Persistent Theiler’s murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980;46:169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- 21.Luo M, He C, Toth K S, Zhang C X, Lipton H L. Three-dimensional structure of Theiler murine encephalomyelitis virus (BeAn strain) Proc Natl Acad Sci USA. 1992;89:2409–2413. doi: 10.1073/pnas.89.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milner D J, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteyne P, Bureau J-F, Brahic M. The infection of mouse by Theiler’s virus: from genetics to immunology. Immunol Rev. 1997;159:163–176. doi: 10.1111/j.1600-065x.1997.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 24.Murti K G, Goorha R, Klymkowsky M W. A functional role for intermediate filaments in the formation of frog virus 3 assembly sites. Virology. 1988;162:264–269. doi: 10.1016/0042-6822(88)90420-5. [DOI] [PubMed] [Google Scholar]
- 25.Perreau J, Lilienbaum A, Vasseur M, Paulin D. Nucleotide sequence of the human vimentin gene and regulation of its transcription in tissues and cultured cells. Gene. 1988;62:7–11. doi: 10.1016/0378-1119(88)90575-6. [DOI] [PubMed] [Google Scholar]
- 26.Pevear D C, Luo M, Lipton H L. Three-dimensional model of the capsid of two biologically different Theiler’s virus strains: clustering of amino acid differences identifies possible locations of immunogenic sites on the virion. Proc Natl Acad Sci USA. 1988;85:4496–4500. doi: 10.1073/pnas.85.12.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starger J M, Brown W E, Goldman A E, Goldman R D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978;78:93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;55:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Lin X, Green T J, Lipton H L, Luo M. Role of sialyloligosaccharide binding in Theiler’s virus persistence. J Virol. 1997;71:9701–9712. doi: 10.1128/jvi.71.12.9701-9712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]