Abstract
Water pollution and scarcity of clean water are major issues of concern globally. In this study, titanium dioxide (TiO2) photocatalyst doped with ferric oxide (Fe2O3) was used to degrade reactive blue dye (171) using sunlight irradiation. Two approaches were employed to synthesize the photocatalyst: synthesis of ferric oxide and titanium precursor through ultrasonic-assisted sol-gel method and using iron (III) nitrate nonahydrate with commercial titanium dioxide. The photocatalysts were characterized using FTIR Spectroscopy, SEM, XRD analyses, and UVDRS to determine their chemical composition, morphology, crystallinity, and light absorption, respectively. The effect of contaminant concentration (1–3 ppm), solution pH and photocatalyst type on the degradation efficiency was studied. Doping enabled visible light absorption as confirmed by the UVDRS analysis. Solar photocatalytic degradation resulted in complete (100 % removal) of the dye within 2 h under solar irradiation for all concentrations of the dye studied. Furthermore, the photocatalysts exhibited superior performance in both neutral and acidic solutions compared to basic ones. After four cycles, the dye removal efficiency has decreased by less than 15 % for all the photocatalysts confirming the significant activity and high stability of the nanocomposite. The increased dye photodegradation efficacy of Fe2O3 doped TiO2 under sunlight irradiation is attributed to the narrowing of the photocatalyst's bandgap from 3.76 eV (in pure TiO2) to 2.83 eV. This narrowing of the bandgap enhances the absorption of visible light from sunlight, thus making this photocatalyst effective under sunlight and eliminating the use of electricity which is a requirement for ultraviolet photocatalysis.
Keywords: Photocatalysis, Reactive blue dye, Visible light, Sunlight, Titanium dioxide, Ferric oxide
1. Introduction
There has been significant focus on reactive dyes used for dyeing and printing of cellulose fibers due to their extensive use and the need to management water effluent, so that it is discarded after pretreatment. This attention stems from the widespread detection of these dyes including reactive dyes in water discharged from the dyeing and printing factories [1]. Consequently, it is imperative to develop effective strategies for mitigating the release of these dyes into accessible water sources. Addressing this concern is vital for environmental preservation and ensuring the sustainability of water resources. Various methods, such as adsorption, biological treatment, advanced oxidation processes, and membrane technologies, have been explored for their potential to remove reactive dyes and minimize their impact on water quality [[2], [3], [4]]. Photodegradation involves the decomposition of organic molecules through interaction with a photocatalyst material and exposure to UV or sun light, ultimately resulting in the production of carbon dioxide (CO2) and water (H2O) [2]. TiO2 nanoparticles stand out as one of the most widely recognized photocatalysts for the breakdown of organic contaminants [5]. When exposed to UV or sun light, TiO2 catalyzes the photodegradation process, facilitating the conversion of organic compounds into environmentally benign by-products, such as CO2 and H2O. This property makes TiO2 nanoparticles highly effective in the field of photocatalysis for the removal of organic pollutants [6,7].
When the TiO2 surface is exposed to sufficient energy that surpass its band gap, it initiates redox reactions [8]. Subsequently, electron-hole pairs are generated, potentially instigating redox processes on the TiO2 surface. However, the economic viability is constrained by suboptimal photoreaction rates and the limited spectral alignment between the TiO2 absorption spectrum and solar emission spectrum. Additionally, the photocatalytic activity is hampered by rapid charge carrier recombination and sluggish charge carrier interfacial transfer rates [[9], [10], [11]].
Nanoparticles, including zero-valence metals, semiconductors, and certain bimetallic varieties, play a crucial role in the remediation of environmental pollutants like azo dyes, Chlorpyrifos, organochlorine pesticides, nitroaromatics, and more. Notably, metal oxide nano photocatalysts such as SiO2, ZnO, TiO2, Al2O3, Fe2O3 among others, are widely employed for their effectiveness. Titanium dioxide is particularly distinguished as an outstanding photocatalyst, attributed to its cost-effectiveness, non-toxicity, chemical stability, and abundance on earth [[12], [13], [14], [15]]. A variety of nanomaterials have demonstrated success in removing heavy metals, organic contaminants, inorganic anions, and microorganisms. The use of nanomaterials and nanoparticles (NPs) has recently gained traction in addressing environmental challenges such as water contaminant treatment and environmental monitoring/sensing. These materials are considered advantageous due to their reactive nanostructures, which have the potential to efficiently convert and remove hazardous/toxic pollutants, transforming them into non-toxic substances [16].
Metal oxides typically exhibit a higher bandgap, rendering them particularly effective as substrate materials, especially in the UV region. This characteristic makes them well-suited for serving as a foundation in various applications. Conversely, materials with narrower bandgaps are often employed as secondary components. Notably, oxides like ZnO and TiO2 display heightened photocatalytic activity in the UV region, making them popular choices as substrate materials in diverse applications [17]. Hematite (α-Fe2O3), recognized as a type band gap semiconductor with a value of 2.1 eV, has garnered significant interest due to its versatile applications. It is widely employed in various fields such as gas sensors, electrochemistry, pigments, drug carriers, and wastewater treatment. The semiconductor's distinctive properties make it valuable in sensing applications, electrochemical processes, imparting color as a pigment, serving as a carrier for pharmaceutical compounds, and effectively participating in the treatment of wastewater. The diverse range of applications which includes and not limited to dye degradation [18] and removal of organic pollutants from municipal waste water (Mecha et al., 2016) underscores hematite's importance in multiple scientific and industrial domains.
This study introduces an innovative strategy to tackle the challenge of eliminating the resilient environmental pollutant, reactive blue dye, employing advanced photocatalytic degradation processes. In contrast to traditional treatment methods that prove ineffective against emerging organic contaminants, photocatalysis effectively degrades and mineralizes the dye. The novelty of this study lies in the comparative synthesis and performance evaluation of two distinct approaches used to prepare Fe2O3–TiO2 photocatalysts. Furthermore, the study contributes to knowledge by developing visible light active photocatalysts that eliminate the use of electricity which is a requirement for ultraviolet photocatalysis using pure TiO2. It therefore makes the process environment friendly and less costly, both of which are necessary for large scale deployment of this technology in addressing water pollution.
2. Materials and methods
2.1. Materials
Titanium (IV) tetraisopropoxide 97 % (TTIP) (supplied by Aldrich), titanium dioxide (TiO2) powder and iron (III) nitrate nonahydrate (Fe (NO3)3·9H2O) (supplied by DLA company), isopropanol ((CH3)2CHOH, (supplied by Gelsup company), absolute ethanol (C2H5OH) (supplied by Eldo lab), reactive navy blue HER dyes for textile industry (supplied by Rift valley textiles (Rivatex) Ltd). The physicochemical properties of the blue dye are reported in Table 1. All the chemical reagents employed in this study were of analytical grade, necessitating no additional purification.
Table 1.
Physicochemical properties of Reactive Blue.
| Name | Molecular Structure | Chemical Formula and structure |
Molecular Formula | MW (g.mol-1) | λ max (nm) (pH = 6.0) |
|---|---|---|---|---|---|
| Reactive Blue 171 | Double azo class | 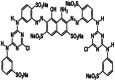 |
C40H23Cl2N15Na6O19S6 | 1418.93 | 600 |
2.2. Photocatalysts synthesis
2.2.1. Synthesis of Fe2O3
The method reported by Ref. [19] was used with modification. Initially, pure α-Fe2O3 powder was synthesized through a two-step process. Firstly, Iron III nitrate nonahydrate was dehydrated at 120 °C for 2 h, yielding the pristine material. Subsequently, this dehydrated substance underwent a controlled heat treatment at 350 °C, resulting in the production of α-Fe2O3 powders. To achieve the desired fine powder form, the resulting agglomerates were delicately ground using a mortar and pestle, ultimately yielding the desired α-Fe2O3 powder.
2.2.2. Synthesis of α-Fe2O3–TiO2 (TFT) from synthesized α-Fe2O3 and titanium precursor
Ultrasonic-assisted sol-gel method [20] with some modification was used. The procedure involved the dispersion of 0.2 g (equivalent to 1.25 mill moles) of α-Fe2O3 (synthesized in 2.2.1) in 25 mL of isopropanol through 15 min of sonication. Subsequently, this suspended solution underwent mechanical stirring, while TTIP, dissolved in 15 mL of isopropanol, was added dropwise. Following an hour of stirring, a specified amount of water was introduced, and stirring continued for an additional 2 h to ensure complete hydrolysis of TTIP. To further optimize the reaction, the mixture was subjected to 30 min of sonication in an ultrasonic cleaner. The isopropanol solvent was then removed by evaporation under reduced pressure at 50 °C using a rotary evaporator, leaving behind a precipitate, which was subsequently dried at 80 °C for 12 h and finally subjected to calcination at 350 °C for 2 h.
2.2.3. Synthesis of α-Fe2O3–TiO2 (TFC) from Fe (NO3)3 .9H2O and commercial TiO2
According to Ref. [21] methods, an initial solution was prepared by dissolving iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O) in ethanol, resulting in a solution with a concentration of 0.6 M iron nitrate. To this solution, titanium dioxide (TiO2) powder was added while applying magnetic stirring, with the beaker covered to prevent ethanol evaporation. The solution was first stirred for 30 min, and then subjected to sonication at 35 kHz for 15 min, followed by 15 min at 130 kHz. After removing the covering film, the ethanol was evaporated overnight on a hot plate at 50 °C. The dried sample was then calcined at 300 °C for 10 min, crushed into a powder, and further heated for 6 h at 300 °C in a furnace.
2.2.4. Characterization of photocatalysts
The surface morphology of the photocatalysts was measured by the Scanning electron microscopy (SEM: Zeiss, Ultra55). The chemical composition was investigated by Fourier transform-infrared Spectroscopy (FTIR: PerkinElmer, Frontier), the samples analyzed in ATR mode (4000-650cm-1). The structure of the photocatalysts was determined using X-ray Diffraction (Model: Smartlab X-Ray Diffractometer, Tool capability: PXRD, HRXRD, XRR). Energy-dispersive X-ray analysis, EDX, was also used for the chemical analysis. The optical characteristics were examined utilizing a Shimadzu UV-3600 UV–visible spectrophotometer. To ensure accuracy, baseline correction was conducted employing a calibrated reference sample of barium sulfate. The determination of band gap (Eg) values for the photocatalysts was accomplished through the application of Equation (1) [22,23].
| (1) |
where: λ is the cut-off wavelength (nm) of the UV–vis absorption spectra.
2.3. Degradation of reactive blue dye under visible light irradiation
Photodegradation studies were conducted using three distinct concentrations of Reactive Navy Blue HER dye (1, 2, 3 ppm) and pH = 7. In individual 20 ml test tubes, 0.02 mg of Fe2O3–TiO2 was introduced, followed by the addition of the respective concentrations of the blue dye. Subsequently, the test tubes were positioned under direct sunlight irradiation. Samples were collected within a period of 2 h and the concentration of the dye was determined using UV–visible spectroscopy at λ max = 600 nm to see the effect of the initial concentration of the dye and irradiation time.
To observe the impact of pH variations, samples of a 1 ppm dye solution were prepared under different pH conditions— acidic at pH 3, neutral at pH 7, and alkaline at pH 11. Subsequently, all samples were subjected to identical conditions for analysis.
The photocatalyst degradation efficiency was evaluated as the removal percentage of Reactive blue dye, equation (2)
| (2) |
where: Co and Ct represent the initial and final concentrations of Reactive blue dye by (ppm) respectively.
2.4. The effect of the pH
The dye removal rate is significantly influenced by the pH of the test solution. In this investigation, 20 ml of a blue dye solution with a concentration of 1 mg/L was utilized. To manipulate the pH within the range of 3–11, precise amounts of HCl and NaOH 0.1 M solutions were added. Subsequently, 0.004 g/L g of each photocatalyst was introduced separately into each container. The solutions were then exposed to a sun light irradiation for 60 min for half of them and 120 min for the remainder. Afterward, the specimens were separated and filtered, and the absorbance of each solution was measured using a spectrophotometer. This absorbance data was then compared with the initial absorption levels before undergoing the photocatalytic process.
2.5. Photocatalyst reusability and stability
The evaluation of nanophotocatalyst performance in extensive applications and the economic efficiency of the photo degradation process rely on key factors like robust stability and a simple recovery process. This specific investigation focuses on the Fe2O3–TiO2 nanophotocatalyst, with a notable feature being the absence of any chemical treatment for removing pollutants from its surface. Instead, the methodology involves allowing the solution to settle for several hours, followed by water separation and concluding with a drying process at 60 °C, as reported by Ref. [24] methods.
3. Results and discussion
3.1. Photocatalysts characterization
3.1.1. XRD analysis
The X-ray diffraction (XRD) analysis results are shown in Fig. 1. A total of eleven well-defined peaks, all of which can be attributed to the α-Fe2O3 phase, commonly known as hematite were observed. The prominent peaks were detected at angles of 24.9, 33.27, 35.39, 41.103, 48.2, 53.9, 57.4, 62.2, 63.8, 71.82, and 75.9°, corresponding to the respective diffraction planes (012), (104), (110), (113), (024), (116), (122), (214), (300), (1010), and (220) of hematite [25,26]. Furthermore, the presence of two additional peaks at 25.1 and 48.2°, corresponding to the diffraction planes (101) and (200), indicated the presence of the TiO2 anatase phase within the sample [20,27].
Fig. 1.
XRD curves of α-Fe2O3 nanopowder, TiO2 photocalyst, doping TFT and TFC photocatalysts.
The average crystallite size of the photocatalysts was determined using the Debye–Scherrer equation as reported in Table 2. In this equation, d represents the crystallite size, λ stands for the wavelength of the incident X-ray, β is the full width at half maximum (FWHM) of the diffraction formula, K is the Scherrer constant (a typical value is 0.94), and θ represents the scattering angle.
Table 2.
Crystals size of the photocatalysts.
| Photocatalyst type | TiO2 | Fe2O3 | TFC | TFT |
|---|---|---|---|---|
| Crystal size | 14.83 | 22.54 | 38.2 | 32.3 |
The Debye–Scherrer equation is given by:
3.1.2. FTIR analysis
The FTIR spectra of the synthesized TiO2–Fe2O3 photocatalysts from the commercial and synthesized TiO2 and the Fe2O3, between 400 and 4000 cm−1 is shown in Fig. 2. The spectra exhibited distinctive peak patterns at 1074 cm−1, 1630 cm−1, and 3439 cm−1, which are indicative of the presence of TiO2–Fe2O3 in the material. The broader peaks observed at 3439 cm−1 and 1620 cm−1 are attributed to –OH stretching and bending vibrations and are likely to play a significant role in phase formation and stabilization across all nanocomposites. The peak at 1074 cm−1 can be attributed to C–O stretching vibration, while the strong band below 700 cm−1 is associated with the Fe–O stretching mode. Specifically, the Fe–O stretching mode is observed at 556 cm−1 and 471 cm−1 in pure Fe2O3 and in commercial TiO2 doped with Fe2O3 [28,29], but in the TiO2–Fe2O3 nanocomposite synthesized from precursor materials, it appears as a single stretching peak. Furthermore, a broad band in the 500-600 cm−1 range is linked to the vibration of the Ti–O bonds [30].
Fig. 2.
FTIR curves of α-Fe2O3 nanopowder, TiO2 photocatalyst, doping TFT and TFC photocatalysts.
3.1.3. SEM analysis
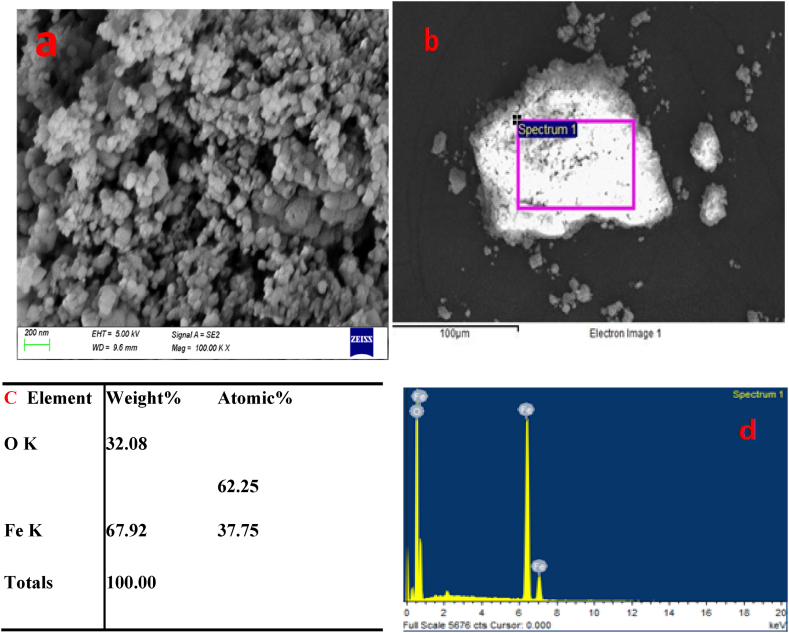
The surface morphology of iron oxide analyzed using SEM is depicted in Fig. 3 The SEM images reveal an agglomerated shape morphology, indicating that the nanoparticles exhibit a tendency to cluster and form larger assemblies due to their elevated surface energies [10]. The EDX analysis confirms the precise composition of the iron oxide, with 67.92 % iron content and 32.8 % oxygen content, highlighting the successful preparation of the material.
Fig. 3.
(a) SEM images 100.00 K X magnification and (b, c, and d) EDX images of synthesis Fe2O3 nonoparticles
Fig. 4 displays SEM images of the photocatalyst synthesized from the combination of Fe2O3 and a titanium precursor. The images reveal a somewhat porous and agglomerated morphology in the sample, suggesting potential advantages for property enhancement. Notably, both large and small particles of nearly equal sizes were observed in the images [28]. The confirmation of elemental composition in the composites was carried out through EDX measurements, providing a comprehensive understanding of the synthesized photocatalyst's constituent elements.
Fig. 4.
(a) SEM images 100.00 K X magnification and (b, c, and d) EDX images of α-Fe2O3–TiO2 (TFT) from Synthesis Fe2O3 and titanium precursor.
Surface morphology analysis was conducted on commercially available TiO2 nanoparticles doped with synthesized iron oxide. The SEM images in Fig. 5 of the Fe2O3-doped TiO2, revealing a distinctive spherical shape morphology on the nanoparticle surface. At a magnification of 100,000 times, a denser distribution of spherical shapes with minimal empty spaces is evident, Furthermore, Fig. 5 illustrates the occurrence of agglomeration of these spherical shapes, forming clusters within the samples [31].
Fig. 5.
(a) SEM images 100.00 K X magnification and (b, c, and d) EDX images of α-Fe2O3–TiO2 (TFC) from Fe (NO3)3 9H2O and commercial TiO2.
The findings indicate that the particle size of the photocatalyst synthesized from the Titanium precursor is smaller compared to that of commercially available Titanium, as depicted in Figs. 4 and 5. The results suggest that an escalation in calcination time leads to heightened material crystallinity. However, it is noteworthy that elevating the calcination time might concurrently result in an increase in crystal grain size.
3.1.4. UV-DRS analysis
Fig. 6 illustrates the UV–visible diffuse reflectance spectra (DRS) for α-Fe2O3, TiO2, and the two synthesized α-Fe2O3–TiO2 powders. The pristine α-Fe2O3 powder displays broad absorption across the entire UV–visible spectrum, displays photo-absorption about 527 nm, corresponding to band-gap energy of 2.35 eV. In contrast, TiO2/Fe2O3 photocatalysts exhibit extensive optical absorption in both the UV and visible ranges, displays photo-absorption about 438.8 nm, corresponding to band-gap energy of 2.83 eV. While pristine TiO2 powder only demonstrates limited absorption in the UV range. TiO2 specifically displays photo-absorption in the UV-light region below 400 nm, corresponding to band-gap energy of 3.76 eV. Notably, the optical absorptions of the α-Fe2O3/TiO2 composites exhibit a noticeable red-shift in the visible light region compared to TiO2, attributed to the presence of α-Fe2O3 in the composite powders. This observation suggests that α-Fe2O3/TiO2 composites could serve as effective photocatalysts for utilizing sunlight as an energy source for contaminant reduction [19].
Fig. 6.
UV DRS curves of α-Fe2O3 nanopowder, TiO2 photocatalyst, doping TFT and TFC photocatalysts.
3.2. Degradation of reactive blue dye under visible light irradiation
3.2.1. Degradation mechanism
When the blue dye solution is exposed to the sun light, photon energy (hv) higher than the bandgap is absorbed by the materials (typically with the ultraviolet illumination). Excited electrons move to the conduction band. Holes are the positively charge carriers that remain in the valence band after it has been filled as shown in Eq. (1).a &1.b [32].
The division of photogenerated electrons and holes is displayed in the following phase (Eq (2)) [33].
The optimum end products of a photocatalytic reaction, like those of other AOPs, are CO2 and water. Electron acceptors use the electrons in the conduction band as countercharge carriers, while hydroxyl groups or organic compounds consume the holes. Given that it results in the creation of additional reactive oxygen species, dissolved oxygen (DO) serves as a primary electron acceptor. Its presence is essential to maintain the electron-hole separation and to facilitate photocatalysis as DO reacts with electrons. As illustrated in Eq. 3–9 [32].
3.2.2. The effect of initial concentration of the dye with the time of irradiation
The evaluation of the photocatalytic organic degradation of the blue dye using several concentration of the dye (1, 2 and 3 ppm) on different irradiation time was conducted and the results are illustrated in Table S1(supplementary materials), Fig. 7, Fig. 8, Fig. 9 The doped TiO2 exhibited superior removal efficiency in eliminating the blue dye compared to pure TiO2, achieving a 100 % removal percentage after 2 h of sun light irradiation for both photocatlysts.
Fig. 7.
Removal efficiency % of blue dye using TiO2 photocatalyst: Concentration of dye Vs. Time. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8.
Removal efficiency % of blue dye using α-Fe2O3–TiO2 (TFC) photocatalyst: Concentration of dye Vs. Time. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 9.
Removal efficiency %/hr of blue dye using α-Fe2O3–TiO2 (TFT) photocatalyst: Concentration of dye Vs. Time. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.3. The effect of pH
The resulting removal percentages were plotted as a function of pH in the 3–11 range, as illustrated in Fig. 10, Fig. 11, Fig. 12, and Table S2(supplementary materials).
Fig. 10.
Removal Efficiency % of TiO2 Vs. pH within 1 and 2 h irradiation time.
Fig. 11.
Removal Efficiency % of TFC Vs. pH within 1 and 2 h irradiation time.
Fig. 12.
Removal Efficiency % of TFT Vs. pH within 1 and 2 h irradiation time.
The degradation of Reactive Blue 171 dye was found to be more effective in acidic and neutral pH ranges when utilizing bare TiO2 or codoped photocatalysts (TiO2–Fe2O3). This observation can be attributed to the point of zero net charge (PZC) of the photocatalysts, which is at pH 6.8. In an acidic solution (pH < 6.8), the photocatalysts carry a positive charge, while Reactive Blue 171 exhibits a negative charge due to the sulfonic group present. This favorable electrostatic interaction promotes the adsorption of the anionic Reactive Blue 171 dye onto the surface of the photocatalyst.
In this scenario, the positively charged the photocatalysts actively attract and adsorb more dye molecules onto its active sites, leading to an enhancement in the degradation efficiency of Reactive Blue 171. Previous literature has also linked photocatalytic activity with the adsorption of dye molecules onto the surface of photocatalysts, as reported by Refs. [3,24,34,35].
At elevated pH levels, photocatalysts assume a negative charge as the pH surpasses the point of zero net charge (PZC). The repulsion between the negatively charged photocatalysts and the similarly negatively charged dye molecules impedes the adhesion of dye molecules to the catalyst surface. Consequently, the photodegradation process becomes more challenging, as the degradation facilitated by electron-hole pairs (hv B+) and conduction-band electrons (e CB−) is obstructed. The hydroxyl radicals generated by the photocatalysts play a crucial role in degrading dye molecules. However, in alkaline conditions, the presence of OH− interrupts the photodegradation process. This interruption occurs because OH− induces the formation of intramolecular hydrogen bonds in electron-donating groups, such as −NH2 located in the α-position of the carbonyl group of Reactive Blue 171. This leads to increased chemical stability of the dye, making it more resistant to attack by hydroxyl radicals. Consequently, a decrease in photocatalytic activity is observed in alkaline solutions due to these hindrances in the degradation process. This effect has been previously documented, as reported by Ref. [3].
3.2.4. The stability & Reusability of nanophotocatalysis
The findings of the study, presented in Fig. 11, provide insights into the stability and activity of the photocatalyst over four consecutive cycles. This emphasizes the practical importance of understanding how well the catalyst maintains stability and efficacy across multiple usage instances, offering valuable insights into its suitability for large-scale applications with economic and environmental considerations. Notably, the results in Fig. 13 indicate that after the fourth cycle, the reactive blue dye removal efficiency has only decreased by about 13.06 % for bare TiO2 and 11.1 % for Fe2O3–TiO2 (TFC), and 9.7 % for Fe2O3–TiO2 (TFT) a confirming the significant activity and high stability of the Fe2O3–TiO2 nanocomposite.
Fig. 13.
The obtained results from the evaluation of the stability and activity of 1 ppm initial dye concentration in TiO2, TFC, and TFT in 20 ml solution up to 4 consecutive cycles.
3.2.5. Comparison of bare and doped TiO2 nanophotocatalysts with other nanophotocatalysts
The TiO2 nanophotocatalyst stands out as a unique semiconductor with excellent photodegradation activity. While TiO2 typically requires a UV source or doping with metal or metal oxide to effectively absorb sunlight irradiation as reported by Ref. [36], even a concentration of 0.02 g/L of TiO2 demonstrates significant efficiency, removing 96.26 % of a 1 mg/L dye solution under sunlight irradiation. Moreover, the introduction of Fe2O3 through doping enhances its performance, achieving 100 % removal efficiency after 120 min of sunlight irradiation. Table .3 compiles findings from various studies exploring nanoparticle applications for organic dye removal in wastewater. Notably, codoped TiO2 emerges as the most promising option, offering the highest removal efficiency. Its ability to operate under sunlight irradiation is a key advantage, contributing to cost reduction and utilizing clean energy sources.
Table 3.
Comparison of various nanophotocatalysts for the removal of organic dyes considering the source of light and experimental conditions.
| Nanophotocatalytic type | Photocatalytic Dosage (g L−1) |
t (min) | Pollutant | Source of light | Removal/efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Anatase Nano-TiO2 | 1 | 40 | Reactive Blue 4 | 125 W UV lamp/sun light | 100 | [3] |
| N–Cu, N–Fe and N codoped TiO2 and Bare TiO2 | 0.06 | 5 | RB 4 dye | 100 W halogen lamp | 100, 84, 80 and 75 | [37] |
| TiO2 | 1 | 120 | Procion Navy H-EXL (PN) | UV-LED | 100 | [38] |
| CoAl2O4 | 0.25 | 150 | navy blue | UVA lamps 75Watts | 42–67 | [39] |
| ZnO | 0.01–0.06 | 20 | reactive blue 203 | 8W UV lamp | 99.1 | [2] |
| TiO2, Fe2O3 and TiO2–Fe2O3 | 60 | 60 | Titan Yellow and Methyl Orange | UV lamp | 92.98, 71.67 and 62.89 % for TY | [28] |
| Cu2SnS3 + GO | 0.4 | 240 | Navy Blue ME2RL | 300 W xenon lamp | 88 | [40] |
| Bare TiO2 and TiO2–Fe2O3 | 0.004 | 120 | Navy Blue 171 | Sun light | 96.26 and 100 | This work |
4. Conclusion
In this study α-Fe2O3 and α-Fe2O3–TiO2 from commercial TiO2 and from syntheses TiO2 were synthesis successfully as the characterization result proved. The hematite iron oxide nanoparticles, synthesized through the Sol-Gel method, were identified as such through X-ray diffraction (XRD) analysis. Scanning electron microscopy (SEM) revealed flowered structures, confirming the presence of iron oxide. Further confirmation of iron oxide and identification of functional groups were established through Fourier-transform infrared spectroscopy (FTIR). Additionally, the optical properties of the nanoparticles were investigated using UV diffuse reflectance spectroscopy (UV DRS). Metal doping enhances the optical properties of TiO2 thus enabling utilization of solar light for photocatalysis as it appear in the UV DRS curves. There were no significant changes in the characteristics of the photcatlysts preparing by the commercial TiO2 than the synthesis TiO2 in the FTIR and the XRD results, and they absorbed in the same wavelength in the UV DRS. While their morphology was little bit different as the SEM images showed, the photcatalytic synthesis from the titanium precursor had a smaller grains size and it tended to agglomerate and make clusters more than the one created from the commercial TiO2, and also the percentage of titanium in the α-Fe2O3–TiO2 photocatalysts from TiO2 precursor more than the titanium in the α-Fe2O3–TiO2 photocatalysts from commercial TiO2 confirmed by the EDX, The two photocatalysts exhibited impressive efficiency, achieving a complete elimination of the blue dye after 2 h of exposure to sunlight across different concentrations. Notably, these photocatalysts outperformed in both neutral (96.26 %, 100 %, and 100 %) and acidic (94 %, 100 %, and 100 %) solutions compared to basic conditions (30 %, 36.3 %, and 37 %) for TiO2, commercially doped TiO2, and synthesized doped TiO2. Even after four cycles, the reduction in dye removal efficiency was less than 15 % for all photocatalysts, confirming the substantial activity and enduring stability of the nanocomposite.
Data availability statement
Data will be made available on request.
Additional information
Additional information is available on the supplementary materials.
CRediT authorship contribution statement
Zeinab A. Suliman: Writing – review & editing, Writing – original draft, Visualization, Validation, Formal analysis, Data curation. Achisa C. Mecha: Writing – review & editing, Visualization, Supervision, Conceptualization. Josphat I. Mwasiagi: Writing – review & editing, Visualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Zeinab A. Suliman reports financial support was provided by European Commission. None If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the European commission (EU) for funding this study through Strengthening mobility and promoting Regional Integration in Engineering Education in Africa (SPREE) program (Grant numbers 614586-mobaf-20191-1).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29648.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siddique M., Farooq R., Shaheen A. Removal of Reactive Blue 19 from wastewaters by physicochemical and biological processes-a review. J. Chem. Soc. Pakistan. 2011;33 [Google Scholar]
- 2.Bagheri M., Najafabadi N.R., Borna E. Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J. King Saud Univ. Sci. 2020;32(1):799–804. [Google Scholar]
- 3.Samsudin E.M., et al. Evaluation on the photocatalytic degradation activity of reactive blue 4 using pure anatase nano-TiO2. Sains Malays. 2015;44(7):1011–1019. [Google Scholar]
- 4.Behera M., et al. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: an integrated system design approach. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 5.Wang C., et al. Improved photocatalytic oxidation performance of gaseous acetaldehyde by ternary g-C3N4/Ag-TiO2 composites under visible light. J. Colloid Interface Sci. 2021;602:699–711. doi: 10.1016/j.jcis.2021.05.186. [DOI] [PubMed] [Google Scholar]
- 6.Jagadeesan D., et al. Hollow spheres to nanocups: tuning the morphology and magnetic properties of single‐crystalline α‐Fe2O3 nanostructures. Angew. Chem. 2008;120(40):7799–7802. doi: 10.1002/anie.200802626. [DOI] [PubMed] [Google Scholar]
- 7.Bee A., Massart R., Neveu S. Synthesis of very fine maghemite particles. J. Magn. Magn Mater. 1995;149(1–2):6–9. [Google Scholar]
- 8.Arghavan F.S., et al. Complete degradation of tamoxifen using FeNi3@ SiO2@ ZnO as a photocatalyst with UV light irradiation: a study on the degradation process and sensitivity analysis using ANN tool. Mater. Sci. Semicond. Process. 2021;128 [Google Scholar]
- 9.Mohsenzadeh M., Mirbagheri S.A., Sabbaghi S. Photocatalytic degradation of 1, 2-dichloroethane using immobilized PAni-TiO2 nanocomposite in a pilot-scale packed bed reactor. Desalination Water Treat. 2019;155:72–83. [Google Scholar]
- 10.Gurlhosur S.H., Sreekanth B. Synthesis, characterization of iron oxide (α-Fe2O3) nanoparticles and its application in photocatalytic reduction of chromium (VI) Rasāyan Journal of Chemistry. 2018;11(4):1678–1685. [Google Scholar]
- 11.Peiris S., et al. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021;68(5):738–769. [Google Scholar]
- 12.Yaqoob A.A., et al. Role of nanomaterials in the treatment of wastewater: a review. Water. 2020;12(2):495. [Google Scholar]
- 13.Umar K., et al. Synthesis, characterization of Mo and Mn doped ZnO and their photocatalytic activity for the decolorization of two different chromophoric dyes. Appl. Catal. Gen. 2015;505:507–514. [Google Scholar]
- 14.Bhanvase B., Shende T., Sonawane S. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environmental Technology Reviews. 2017;6(1):1–14. [Google Scholar]
- 15.Seneviratne K.L., et al. Recent progress in visible-light active (VLA) TiO2 nano-structures for enhanced photocatalytic activity (PCA) and antibacterial properties: a review. Iran. J. Catal. 2021;11(3):217–245. [Google Scholar]
- 16.Nasrollahzadeh M., et al. Green-synthesized nanocatalysts and nanomaterials for water treatment: current challenges and future perspectives. 2021;401 doi: 10.1016/j.jhazmat.2020.123401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satti U.Q., et al. Simple two-step development of TiO2/Fe2O3 nanocomposite for oxygen evolution reaction (OER) and photo-bio active applications. Colloids Surf. A Physicochem. Eng. Asp. 2023;671 [Google Scholar]
- 18.Abbasi A., et al. Photo-degradation of methylene blue: photocatalyst and magnetic investigation of Fe 2 O 3–TiO 2 nanoparticles and nanocomposites. J. Mater. Sci. Mater. Electron. 2016;27:4800–4809. [Google Scholar]
- 19.Bouziani A., Park J., Ozturk A. Synthesis of α-Fe2O3/TiO2 heterogeneous composites by the sol-gel process and their photocatalytic activity. J. Photochem. Photobiol. Chem. 2020;400 [Google Scholar]
- 20.Abdel-Wahab A.-M., et al. Photocatalytic degradation of paracetamol over magnetic flower-like TiO 2/Fe 2 O 3 core-shell nanostructures. J. Photochem. Photobiol. Chem. 2017;347:186–198. [Google Scholar]
- 21.Ahmadi E., et al. Synergistic effects of α-Fe2O3-TiO2 and Na2S2O8 on the performance of a non-thermal plasma reactor as a novel catalytic oxidation process for dimethyl phthalate degradation. Separ. Purif. Technol. 2020;250 [Google Scholar]
- 22.Jasso-Salcedo A.B., Palestino G., Escobar-Barrios V.A. Effect of Ag, pH, and time on the preparation of Ag-functionalized zinc oxide nanoagglomerates as photocatalysts. J. Catal. 2014;318:170–178. [Google Scholar]
- 23.Mecha A.C., et al. UV and solar light photocatalytic removal of organic contaminants in municipal wastewater. Separ. Sci. Technol. 2016;51(10):1765–1778. [Google Scholar]
- 24.Najafi M., et al. Sono-sorption versus adsorption for the removal of Congo red from aqueous solution using NiFeLDH/Au nanocomposite: kinetics, thermodynamics, isotherm studies, and optimization of process parameters. J. Ind. Eng. Chem. 2022;116:489–503. [Google Scholar]
- 25.Asoufi H.M., Al-Antary T.M., Awwad A.M. Green route for synthesis hematite (α-Fe2O3) nanoparticles: toxicity effect on the green peach aphid, Myzus persicae (Sulzer) Environ. Nanotechnol. Monit. Manag. 2018;9:107–111. [Google Scholar]
- 26.Lassoued A., et al. Synthesis, photoluminescence and Magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys. E Low-dimens. Syst. Nanostruct. 2018;101:212–219. [Google Scholar]
- 27.Ijadpanah-Saravy H., et al. Synthesis of titanium dioxide nanoparticles for photocatalytic degradation of cyanide in wastewater. Anal. Lett. 2014;47(10):1772–1782. [Google Scholar]
- 28.Kumar M.A., et al. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020;10(1):1249. doi: 10.1038/s41598-020-58110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehbi A., et al. Hematite iron oxide nanoparticles (α-Fe2O3): synthesis and modelling adsorption of malachite green. J. Environ. Chem. Eng. 2020;8(1) [Google Scholar]
- 30.Talebi S., Chaibakhsh N., Moradi-Shoeili Z. Application of nanoscale ZnS/TiO2 composite for optimized photocatalytic decolorization of a textile dye. J. Appl. Res. Technol. 2017;15(4):378–385. [Google Scholar]
- 31.Gareso P., et al. Journal of Physics: Conference Series. IOP Publishing; 2021. Synthesis and material characterization of TiO2 nanoparticles doped with iron (Fe) [Google Scholar]
- 32.Le H.N. Hanoi University of Science and Technology; 2018. A Concept for Nanoparticle-Based Photocatalytic Treatment of Wastewater from Textile Industry. [Google Scholar]
- 33.Sharma S., Mehta S.K., Kansal S.K. N doped ZnO/C-dots nanoflowers as visible light driven photocatalyst for the degradation of malachite green dye in aqueous phase. J. Alloys Compd. 2017;699:323–333. [Google Scholar]
- 34.Daneshvar N., Salari D., Khataee A. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J. Photochem. Photobiol. Chem. 2003;157(1):111–116. [Google Scholar]
- 35.Aguedach A., Brosillon S., Morvan J. Photocatalytic degradation of azo-dyes reactive black 5 and reactive yellow 145 in water over a newly deposited titanium dioxide. Appl. Catal. B Environ. 2005;57(1):55–62. [Google Scholar]
- 36.Mahmoodi V., Bastami T.R., Ahmadpour A. Solar energy harvesting by magnetic-semiconductor nanoheterostructure in water treatment technology. Environ. Sci. Pollut. Control Ser. 2018;25:8268–8285. doi: 10.1007/s11356-018-1224-y. [DOI] [PubMed] [Google Scholar]
- 37.Kaur N., Shahi S.K., Singh V. Anomalous behavior of visible light active TiO 2 for the photocatalytic degradation of different Reactive dyes. Photochem. Photobiol. Sci. 2015;14:2024–2034. doi: 10.1039/c5pp00165j. [DOI] [PubMed] [Google Scholar]
- 38.Tapia-Tlatelpa T., Trull J., Romeral L. In situ decolorization monitoring of textile dyes for an optimized UV-LED/TiO2 reactor. Catalysts. 2019;9(8):669. [Google Scholar]
- 39.El Jabbar Y., et al. Photocatalytic degradation of navy blue textile dye by nanoscale cobalt aluminate prepared by polymeric precursor method. Environ. Nanotechnol. Monit. Manag. 2019;12 [Google Scholar]
- 40.Shelke H.D., et al. Enhanced photocatalytic activity of the Cu2SnS3+ GO composite for the degradation of navy blue ME2RL industrial dye. Coatings. 2023;13(3):522. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.