Background
Monocytes and macrophages are essential components of innate immune system and have versatile roles in homeostasis and immunity. These phenotypically distinguishable mononuclear phagocytes play distinct roles in different stages, contributing to the pathophysiology in various forms making them a potentially attractive therapeutic target in inflammatory conditions. Several pieces of evidence have supported the role of different cell surface receptors expressed on these cells and their downstream signaling molecules in initiating and perpetuating the inflammatory response. In this review, we discuss the current understanding of the monocyte and macrophage biology in inflammation, highlighting the role of chemoattractants, inflammasomes, and integrins in the function of monocytes and macrophages during events of inflammation. This review also covers the recent therapeutic interventions targeting these mononuclear phagocytes at the cellular and molecular levels.
Keywords: Monocytes, Macrophages, Inflammation, Integrins, Inflammasomes, Metabolic reprogramming
Abbreviations
- BM
Bone marrow
- CCR2
C–C chemokine receptor 2
- CCL2
C–C chemokine ligand 2
- CNS
Central Nervous System
- COX-2
Cyclooxygenase-2
- CXCR4
C-X-C chemokine receptor 4
- CX3CR1
CX3C chemokine receptor 1
- DC
Dendritic cells
- ECM
Extracellular matrix
- FAO
Fatty acid β-oxidation
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- Ly6C
Lymphocyte antigen 6C
- MCP-1
Monocyte chemoattractant protein-1
- MDP
Monocyte/Dendritic cell progenitors
- NFkB
Nuclear factor kappa-Β
- NLRP3
NLR family pyrin domain containing 3
- PPAR
Peroxisome proliferator-activated receptor
- RA
Rheumatoid arthritis
- RGD
Arginylglycylaspartic acid
- TNF
Tumor necrosis factor
- T2D
Type-2 diabetes
- VCAM-1
Vascular cell adhesion molecule-1
- WISP1
WNT1-inducible-signaling pathway protein 1
- 2DG
2-Deoxy-d-glucose
1. Introduction
Monocytes and macrophages are the key innate immune cells which play crucial role in inflammatory processes and are important part of the mononuclear phagocyte system (MPS) [1]. They are a heterogeneous population of evolutionarily conserved phagocytes known for their versatile ability to respond to various stimuli [[2], [3], [4]], with remarkably distinct roles in homeostasis, immunity, and the pathophysiology of different diseases [5]. Monocytes originate in a well-characterized differentiation program of the Monocyte/Dendritic cell progenitors (MDP) in the bone marrow (BM). The BM-derived monocytes were believed to be the precursor of the tissue-resident macrophages (TRMs) until the reports from the last decade suggested the origin of most of the TRMs to be yolk sacs or fetal liver-derived progenitors [6]. However, in inflammatory conditions, the circulating monocytes originating from the BM invade the inflamed tissue and differentiate into macrophages. The TRMs are primarily responsible for tissue homeostasis, whereas the monocyte-derived macrophages play a pivotal role in the host-defense mechanisms [7]. The TRMs have been named differently in different tissues, and their range of function varies in different compartments. The macrophages are present in the form of Langerhans cells in the epidermis, alveolar macrophages in the lungs, microglia in the CNS, osteoclasts in the bone, and Kupffer cells in the liver [8].
In response to environmental cues, phenotypic plasticity in macrophages plays crucial roles in modulating tissue-specific homeostatic and inflammatory responses [9]. The recruitment of these mononuclear phagocytes at the site of inflammation, where the stereotypical alternations in the expression of several surface markers and response to different stimulation lead to a functional polarization into two phenotypically distinct sub-populations of macrophages- M1 and M2 that have dynamic roles in induction and repair/resolution of inflammation. The broadly varied spectrum of macrophages is the M1 and M2 continuum, where the M1 macrophages are classically activated and pro-inflammatory end of the spectrum, which mediates tissue destruction and resistance to invasion. In contrast, the M2 macrophages are anti-inflammatory, alternatively activated, and steer the regulation and modeling of inflamed or injured tissue [[10], [11], [12]]. The re-balancing between the pro-inflammatory and pro-resolution responses in macrophages vividly affects the sustaining of inflammation in acute and chronic inflammatory disorders, including but not limited to sepsis, renal inflammation, colitis, Diabetes, etc. [13,14].
2. Monocytes and macrophages in human and mice
Monocytes are a heterogeneous population of innate immune cells which can be discriminated by the differential expression of cell surface markers in both mice and humans [15]. There are broadly two subsets of monocytes identified in mice based on the relative expression of Ly6C, a glycosylphosphatidylinositol-anchored glycoprotein. The monocytes expressing a higher level of Ly6C are termed as Ly6Chi monocytes, which mainly participate in the pro-inflammatory functions. They usually express a higher level of CCR2 and a lower level of CX3CR1. The Ly6Clo subset participates in patrolling, initial events of inflammation, and tissue repair. They express lower levels of CCR2 and higher levels of CX3CR1 [16]. In some inflammatory conditions, an intermediate phenotype has also been observed, which is often termed as Ly6Cint monocyte or inflammatory intermediate monocyte and is believed to be differentiated from Ly6Chi monocyte. The two major subsets of monocytes in mice can be identified as Ly6ChiCCR2hiCX3CR1lo (classical phenotype) and Ly6CloCCR2loCX3CR1hi (non-classical phenotype) [17,18]. Similar to mice, there are three subsets of human monocytes: classical, intermediate, and non-classical, which can be discriminated against based on the variability in the expression of CD14 and CD16. The classical monocyte, identical to the murine Ly6Chi monocytes, is the most prevalent among the three and can be identified as CD14++CD16−. The non-classical monocytes are identical to the murine Ly6Clo patrolling monocytes and can be identified as CD14+CD16++. The intermediate monocytes (CD14++CD16+) show phagocytic and anti-inflammatory functions and are believed to be in transition from classical to non-classical phenotype [19,20].
Reinvestigating through fate mapping approaches, transcriptional and surface marker studies have shown the diversity of macrophages in terms of functionality and lineage. Macrophage localization in tissues facilitates monitoring of pathogens or so called ‘danger signals’, which are sensed through diverse receptors expressed on it. In common epithelia of lymph and capillaries, macrophages are placed in the basement membrane. Langerhans cells are populated in the epidermis and are shown to have a self-renewing capacity. They are a prototypic representation of dendritic cell lineage; however, like most tissue macrophages, they self-maintain locally. Osteoclasts in bones can be derived from mature peripheral monocytes and have receptors for macrophage colony-stimulating factor or CSF1, depending on it for development, as was seen in osteopetrotic mice (mutation in M-CSF gene) with defects in osteoclast development. Red pulp macrophages reside in venous sinuses of the spleen and screen the blood for pathogens and degradation of aged and damaged erythrocytes. Further, highly specialized macrophages of the lungs (alveolar macrophages), CNS (meningeal macrophages, choroid plexus macrophages, perivascular macrophages, and microglial cells), liver (Kupffer cells) are essential for the repair, remodeling and to maintain the integrity of tissue homeostasis [[21], [22], [23], [24]].
3. Differentiation of monocytes and macrophages from myeloid cells
As briefly discussed in the previous section, monocytes originate in the bone marrow from the MDP through a well-characterized differentiation program [25]. Conventionally, the classical monocytes (Ly6Chi in mice and CD14++CD16− monocytes in humans) are derived from the progenitors and subsequently enter circulation [26]. However, Swirski et al. demonstrated that spleen serve as a critical reservoir of monocytes in mice with similar phenotype to blood-derived monocytes [27].
A study by Chong et al. reported the heterogeneity of the BM Ly6Chi monocytes concerning CXCR4 expression in both mice and humans, where the CXCR4 regulates the monocyte replenishment into the circulation as well as peripheral cellular activities [28]. Transcriptome profiling of the progenitors, CXCR4lo, and CXCR4hi BM monocytes showed the CXCR4hi monocyte to be functionally more mature than the progenitors but less mature than CXCR4lo monocytes. The CXCR4hi monocytes are immobilized in the BM before being functionally mature to replenish the CXCR4lo classical monocytes in circulation (Fig. 1) [29,30]. Several common transcription factors such as PU.1 are indispensable for the proper development of monocytes and macrophages [31]. A very recent study in zebrafish showed the role of ZBTB14, a transcription repressor, in regulating monocyte and macrophage development by negatively regulating PU.1 [32]. The well-established CCR2-CCL2 axis plays central role in monocyte egression from the BM. The involvement of different chemokine/chemoattractants responsible for monocyte/macrophage mobilization is discussed further in the next section. Under a steady state, the Ly6Chi classical monocytes, which have a concise shelf life in circulation, get converted into the Ly6Clo monocytes, which patrol the vasculature. Under inflammatory conditions, the Ly6Chi monocytes are rapidly recruited to the site of inflammation [33]. In the study by Lessard et al., the NOD2 receptor by muramyl dipeptide has been shown to convert inflammatory Ly6Chi monocytes into patrolling Ly6Clo monocytes [34].
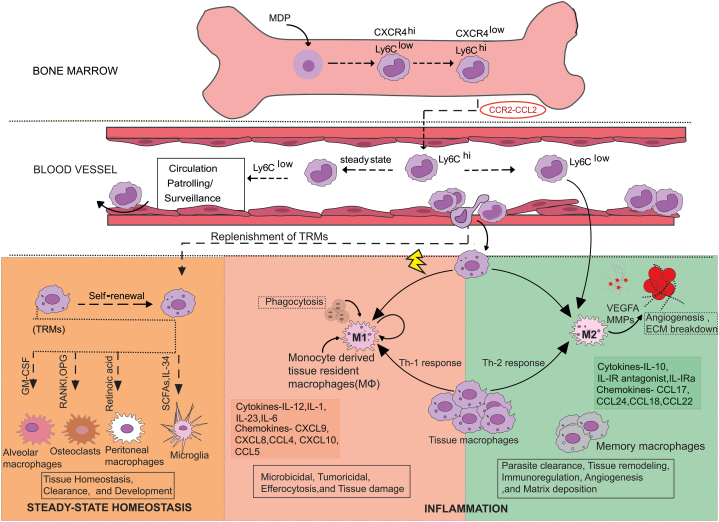
Fig. 1.
The mononuclear phagocyte system-trafficking, recruitment, and differentiation. CXCR4hi/Ly6Clo (precursor of CXCR4lo/Ly6Chi monocytes) is derived from Monocyte-Macrophage and Dendritic cell common precursor (MDPs) in the bone marrow. CCR2-CCL2 dependent extravasation of Ly6Chi, CX3CR1-CX3CL1 dependent extravasation of Ly6Clo; monocytes into blood perform different functions to maintain homeostasis in steady-state conditions. Ly6Clo 'patrol' the endothelial cell supported vessel and can differentiate into pro-resolving/alternatively activated M2 macrophages. In response to injury, inflammation, or neoplasia and infection, inflammatory monocytes (Ly6Chi) mature into inflammatory macrophages, which participate in an inflammatory response by secreting chemokines and TNF-α. In such inflammatory niches, monocyte-derived macrophages undergo maturation towards the resolution phase or M2 subset until the tissue returns to normal or homeostasis. The development of TRMs in response to niche signals gives them unique functional and morphological identities. They can also differentiate into M1 and M2 subtypes depending on Th1/Th2 cell-mediated inflammation, respectively. Monocyte-dendritic cell progenitors (MDPs), Tissue-resident macrophages (TRMs), Ly6C (lymphocyte antigen six complexes, locus C1), C-X-C motif chemokine receptor 4 (CXCR4), Interleukin (IL) Chemokine (C-X-C) motif ligand (CXCL), Chemokine (C-C) motif ligand (CCL), Vascular endothelial growth factor A (VEGFA), Matrix metalloproteinase (MMP).
Tissue-resident macrophages (TRMs) have important roles in clearance (senescent erythrocytes, pulmonary surfactant, and apoptotic cells), development (degradation of bone and angiogenesis), and regulation of metabolism [35]. They also play a fundamental role as sentinels of the immune system, initiating inflammatory responses, clearing debris, and restoring homeostatic tissue environments after responses of inflammation. Although their origins and maintenance are debatable, TRMs play critical roles in tissue homeostasis, inflammation, and remodeling [36]. When macrophages were first hypothesized, monocytes in circulation were thought to be the source. However, more recent research has challenged this model, leading to a significant conceptual change in the area. Extensive research is being conducted to determine the precise role that adult definitive hematopoiesis plays in TRMs. Hashimoto et al. used parabiosis and genetic fate-mapping techniques to demonstrate how some TRMs, such as peritoneal macrophages, red pulp macrophages, and lung alveolar macrophages (AMs), self-maintain locally until adulthood with little contribution from circulating monocytes. These heterogeneous populations of immune cells fulfill tissue and niche-specific functions [37]. Experimental ablation of resident macrophages has shown bone marrow-derived cells' potential to replace self-renewing resident cells that self-maintain locally throughout adult life. A study of tissue-specific transcriptional signatures would allow a more refined assessment of whether they recapitulate the function or replace the pre-natal versus monocyte-derived cells where fate mapping studies and single-cell analysis identified tissue macrophages such as Langerhans cells and microglia to be derived from the yolk sac during early embryogenesis, the contribution of bone marrow-derived inflammatory macrophages to these resident pools remains unclear. The recovery of the resident population during homeostasis has raised questions about the fate of inflammatory monocytes. However, recent observations have shown in situ phenotypic conversion to become tissue macrophages [38]. Previously, the BM-derived monocytes were believed to be the precursor of the TRMs, but different investigations have shown most of the TRMs to be prenatally established, which are maintained independently from the BM monocytes; the precursor being the yolk sac or fetal liver-derived progenitors [39]. Under inflammatory conditions, the Ly6Chi or classical monocytes are recruited into the site of inflammation, where the local milieu decides the fate of these pro-inflammatory cells. The monocytes differentiate into macrophages and may either acquire a pro-inflammatory phenotype to eliminate pathogens or an immunosuppressive or regulatory phenotype to aid in the resolution of inflammation [40] (Fig. 1).
4. Monocytes and macrophages recruitment and associated molecules
4.1. Monocyte and macrophages chemokines
Chemokines/chemoattractants play a central role in the recruitment of immune cells. The egress of classical monocytes is mainly dependent on CCR2, which binds to MCP1 (CCL-2) and MCP-3 (CCL-7) (Fig. 1) [41]. Upon binding, CCR2-CCL2 dimerizes with ECM (Extracellular Matrix) Glycosaminoglycan, leading to monocyte recruitment in infected lungs and the production of reactive oxygen species and up-regulated integrin expression [42]. The lack of expression in Ccr2−/− mice resulted in reduced numbers of circulating monocytes and impaired recruitment to various tissues during inflammation. In addition to bone marrow, siRNA-mediated knockdown of CCR2 in splenic monocytes prevented recruitment at the sites of injury/inflammation [33]. Immune regulatory molecules like PGE2 (Prostaglandin E2) secreted by Ly6Chi monocytes upregulates the CCR7 expression in monocytes. CCR7 is known to retain activated T cells in the Tcell area and the migration of dendritic cells (DC) to lymph nodes. Other regulators like glucocorticoids increase migration and up-regulated expression of anti-inflammatory cytokines in monocytes [43]. Monocytes and monocyte-derived dendritic cells are the maximum populations in the antigen-presenting cell (APC) pool in the secondary lymphoid Organs. Monocytes and monocyte-derived dendritic cells are known to initiate the CD8+ T cell responses by antigen presentation; their roles have also been investigated, and contradictory results have been obtained in various animal models. For example, tumor-infiltrating monocyte-derived dendritic cells have been shown to prime CD8+ T cells and induce anti-tumor immunity. In contrast, monocytic cells in chronic infections abolish the induction of anti-infectious CD8+ T cell responses. Therefore, the specific contribution of monocytes and monocyte-derived dendritic cells to the differentiation of CD8+ T cells still needs to be better understood [44].
The dual nature of chemokines, inflammatory (controls recruitment of effector immune cells at the site of injury, tumor) and homeostatic (navigates leukocytes during hematopoiesis), is attributed to the "four Cys residues'' fingerprint arrangement and chemoattractant activity of these assigned chemotactic proteins. CXCL8 is a chemokine released by macrophages/monocytes and is functionally important for the recruitment and activation of neutrophils at the site of inflammation [45,46]. CXCL10 is also considered a critical chemokine involved in TLR4-TRIF downstream signaling. It attracts monocytes and natural killer cells and activates cell-mediated immune responses, which leads to oxidative bursts and exacerbation of lung inflammation [47]. CCL5 is a chemotactic protein for monocytes and DC, and CCL5/CCR5 axis is important for preventing apoptosis in macrophages by bilateral activation of Gαi/MEK/ERK and Gαi/PI3K/AKT signaling pathways in mice. In the same study absence of CCL5 was shown to cause excessive airway inflammation and respiratory death [48]. CX3CL1/Fractalkine -CX3CR1 signaling is reported to be important for monocyte survival and myeloid turnover in the bone marrow [49]. Several other chemokines, such as CXCL6, CCL2, CCL4, CCL7, CCL8, CXCL11, and CCL20, are reported to constitute the majority of the chemoattractant significant in lung inflammation in COVID-19 patients [50,51]. Monocytes play an important role in antimicrobial immune defense and tissue repair. However, like a double-edged sword, they can also contribute to tissue death during various infections and inflammatory disorders. Recent research has helped us comprehend monocyte heterogeneity and the processes that regulate their trafficking with tight regulation of the recruitment and termination of monocyte-driven inflammatory and anti-inflammatory events may lead to new interventions to treat several inflammatory immunopathologies.
4.2. Integrin signaling in macrophages
Integrins are transmembrane glycoproteins essential for bi-directional (outside-in and inside-out) signaling across cell membranes. The non-covalently attached combinations of 18α and 8β subunits relay sequential and dynamic signals in homing and trafficking of immune cells as well as other cellular functions [52,53]. Genetic studies have strengthened the role of these adhesion receptors in inflammation, as is evident in patients with leukocyte adhesion deficiency (LAD), a heritable immune deficiency involving mutations in the β2-subunit [54]. The members of the β1, β2, and β3 integrin families play a vital role in leukocyte recruitment in remote sites of inflammation in tissues. The initial infiltration of inflammatory monocytes in inflammation can be attributed to α4, with reports in endotoxemic and CLP models of murine sepsis showing higher expression of α4β1 in inflammatory monocytes [55]. The α4β1 integrin is also involved in an important step of leukocyte extravasation by the interaction of the integrin to another adhesion molecule VCAM-1. A recent study also reported higher α4β1, α5β1 and αvβ3 expressions in the macrophages during murine sepsis [56]. While the α4β1 and α5β1 integrins were more associated with the inflammatory phenotype, the αvβ3 expression was prominent on the immunosuppressive phenotype of macrophages in the lungs [57]. RAGE/PS-mediated efferocytosis can be suppressed by High mobility group box-1 protein (HMGB1), a classical DAMP that binds to integrin αvβ3 in macrophages. Conversely, HMGB1-deficient macrophages effectively phagocytose apoptotic neutrophils and thymocytes, translocating HMGB1 into the cytoplasm and its secretion into the extracellular milieu [58].
Duarte et al. reported the interaction of α4β1-fibronectin to be involved in the inflammatory response of monocytes/macrophages in Hepatic Cold Ischemia and Reperfusion injury. Blocking of fibronectin- α4β1 interaction by CS1 therapy reduced the local release of TNF-α, cyclooxygenase-2 (COX-2), and inducible Nitric oxide synthase (iNOS) [59]. Similarly, studies in murine models of spinal cord injury and traumatic brain injury showed the role of CD11d integrin in the development of systemic inflammatory response syndrome post-injury. The blockade of the CD11d integrin with an anti-integrin antibody reduced the influx of macrophages and neutrophils with dampened NF-kB and oxidative enzyme expression [60]. Another report by Fondevila et al. showed the role of α5β1 integrin in the inflammatory function of monocytes and macrophages in Steatotic liver cold ischemia and reperfusion injury. Cyclic-RGD therapy against the integrin inhibited the recruitment of monocytes and macrophages with a significant reduction in the level of inflammatory mediators such as IFN-γ and iNOS [61]. Another study showed the involvement of CD11b integrin in the accumulation of monocytes in the L. monocytogenes-infected liver where blockade of CD11b by administration of mAb diminished the localization of monocytes to the hepatic foci [62]. αvβ3 is also involved in the differentiation and outside-in signaling of macrophages. Studies have reported that substantial ligation of these heterodimers on monocyte-derived macrophages results in PI3 kinase/Akt-dependent activation of NF-κB transcription factor, which enhances the mRNA levels of many pro-inflammatory cytokines (TNF-α, IL-1β, IL-8, and IL-10) at the site of inflammation [63,64]. In recent findings of polymicrobial sepsis in lungs, a matricellular accessory protein, WISP1, was found to positively regulate the TLR4-mediated inflammatory response of peritoneal macrophages in WISP1-αvβ3 integrin signaling, suggesting a novel pathway for therapy [65]. Integrins have been identified as a mediator of internalization of different pathogen-based ligands, with reports on macrophages showing short hairpin RNA knockdown of α3β1 integrin, reduced endocytosis of TLR2/1 receptor complex, indicating that α3β1 might have a role in phagocytosis in inflammatory macrophages [66]. Research findings have also shown that αM (CD11b) signaling negatively regulates TLR-mediated responses in macrophages by activating Src and Syk kinases, which regulate downstream degradation of MyD88 and TRIF, the critical effectors of TLR signaling [67]. Integrin β2 (CD18) governs GMP (Granulocyte/Macrophage Progenitor) proliferation with its deficiency upregulates GATA2/FcεRIα, thus besides mediating cell adhesion to endothelial cells and stromal cells, β2 integrin and GATA2/FcεRIα/JNK axis regulates proliferation of GMP [68]. Macrophage retention is a critical step during inflammation, initiating the progression of diseases. CD11d/CD18 (αDβ2) upregulation contributes to the retention of macrophages at inflammatory sites during atherogenesis. CD11d adhesive mechanism contributes to mesenchymal migration and extravasation; however, less is known about the bi-directional signaling that contributes to the harmful accumulation in various pathophysiological states [69]. The involvement of integrins in the inflammatory response of monocytes and macrophages during different inflammatory conditions makes it a potential target for developing anti-inflammatory therapeutics. However, the comparison and critical validation of the findings of animal studies in human cells or systems is absolutely necessary. Expression of different integrins on monocytes and macrophages studies in different pre-clinical models have been described in Table 1.
Table 1.
Overview of different Integrin expressions in various inflammatory conditions-.
| Integrin | Inflammatory Disorders, Model organism, and Cell Types | Main Expression Features and signaling pathways involved | References |
|---|---|---|---|
| α4β1 |
|
|
[59] |
| CD11d/CD18 |
|
|
[60] |
| α5β1 |
|
|
[61] |
| αvβ3 |
|
|
[65] |
| αvβ5 |
|
|
[70] |
| α1β1 |
|
|
[71] |
| α4β1, α5β1 and αvβ3 |
|
|
[55] |
5. Functional regulation of macrophage polarization
Macrophages are fully differentiated sentinels with a wired sensory array to sense extramural or danger signals through a plethora of receptors such as pattern recognition receptors (PRRs), integrins, etc. As discussed in the previous section, the tissue microenvironment is central to the resultant phenotype of macrophages [72,73]. Different factors present in the tissue microenvironment, including cytokines, chemokines, ECM, microbial components, and damage associated molecular patterns (DAMPs), are responsible for the functional polarization of macrophages [73,74]. Reactive oxygen species (ROS), Reactive Nitrogen Species (RNS), and redox modifications are also reported to be involved in deciding the macrophage phenotype [75]. Th1 cytokines, such as TNF-α and IFN-γ, or ligands of TLR or microbial components, such as LPS, usually activate M1 macrophages. In resting conditions, macrophages release very low inflammatory cytokines. Upon inflammatory stimuli, the macrophage first switches to the M1 phenotype displaying enhanced cytotoxic capacity with high pro-inflammatory cytokines such as IL-6, IL-12, IL-1α, IL-1β, IL-23, and TNF- α production, and low IL-10 production. The level of cyclooxygenase-2 has also been shown to be elevated in the M1 subtype [76]. The characteristic surface markers expressed on M1 macrophages include CD80, CD86, CD14, and high MHC-II [77]. M2 phenotypic macrophages are more functionally diverse with several subtypes- M2a (Arg1, CD163, MHCII), M2b (CD86, MHCII), M2c (CD163, TLR1, TLR8) and M2d (VEGF). They can be stimulated by several cytokines such as IL-4 and 1L-13 (Fig. 2). Various stimulants induce the different subtypes of M2 macrophages, and their functions range from wound healing and tissue remodeling to promoting infection and tumor progression [78]. M2a macrophages are stimulated by IL-4 or IL-13, which in turn increases the production of TGF-β, CCL17, CCL18, CCL22, and IL-10. These macrophages support tissue healing, cell proliferation, and endocytic activity. TLR ligands, immune complexes, and IL-1β activate M2b macrophages, which then secrete pro- and anti-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-10. M2b macrophages control the intensity and range of immune responses as well as inflammatory reactions based on the expression profiles of cytokines and chemokines. IL-10, TGF-β, and glucocorticoids produce M2c macrophages, also referred to as inactivated macrophages. These cells are essential for the process of phagocytosis of apoptotic cells because they release IL-10, TGF-β, CCL16, and CCL18. M2d macrophages are stimulated by TLR antagonists, which causes the release of VEGF and IL-10, which in turn promotes angiogenesis and the growth of tumors.
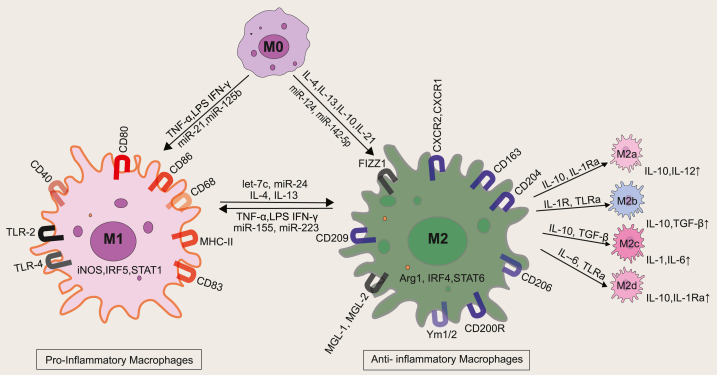
Fig. 2.
The two extremes of macrophage phenotype. M0 macrophages are non-activated macrophages with the potential to polarize into pro-inflammatory M1 macrophages (upon stimulation with TNF-α, LPS, and IFN-γ, which have a role in the production of pro-inflammatory cytokines). M0 on stimulation with IL-4, IL-13, IL-10, IL-21 matures into M2 macrophages. M2 macrophages can further differentiate into M2a (upon stimulation with IL-10, IL-1RA, which upregulates expression of IL-10 and TGF-β, responsible for tissue regeneration), M2b (upon stimulation with IL-1R, TLRa, which produces IL-10, TNF-α which leads to an anti-inflammatory activity), M2c (upon stimulation with IL-10, TGF-β, glucocorticoids, responsible for the suppression of immune response) and M2d (upon stimulation with IL-6, TLRa, these macrophages are also referred to as TAM or Tumor-associated macrophages. Different regulatory molecules like non-coding micro-RNAs also regulate the inflammatory/non-inflammatory signaling post-transcriptionally, with more details in the main text. MicroRNA (miRNA), Toll-like receptors (TLRs), Arginase 1 (Arg1), Interleukin-1 receptor antagonist (IL-1RA) Interferon regulatory factor (IRF), Signal transducer and activator of transcription (STAT).
The re-balancing between the pro-inflammatory and pro-resolution responses in macrophages vividly affects the sustaining of inflammation in acute and chronic inflammatory disorders. In systemic inflammatory condition such as sepsis, in the early stage, macrophages differentiate into the M1 subtype through TLR-ligand binding. This leads to releasing large amounts of pro-inflammatory cytokines such as IL-1 and IL-6 to eliminate the pathogens. However, during later stages, the polarization to the M2-like phenotype by suppressing the NF-kB pathway reduces the pro-inflammatory response [79,80]. A recent report showed an adaptation of the anti-inflammatory phenotype of macrophages correlated with the relatively immunosuppressive microenvironment in the lungs during murine sepsis [55] Similarly, a link between TLR and FcγR with Th17 polarizing macrophage phenotype has been linked to activation and inflammation in the human gut. The FcγR co-stimulation cross-linking in vitro induces a potent macrophage inflammatory response by producing IL-6, TNF-α, IL-10, IL-1β, IL-12, and chemokines, including CXCL8 [81]. Numerous reports suggest that failing to regulate the switch between M1 and M2 macrophages can also lead to renal inflammation. M1, the first responder to kidney damage, mediates leukocyte migration and releases cytokines and cytotoxic agents that lead to renal damage. Reports also suggest the contributory role of M2 macrophages showing immunosuppressive effects by inducing regulatory T-cells that promote angiogenesis and endothelial repair in inflamed kidneys [82]. Chronic tissue inflammation is the primary factor responsible for developing type 2 diabetes. A study showed that human monocytes undergo M1 polarization when kept in a high glucose environment with up-regulated CD11c and iNOS. Reports have also shown that dysregulated wound healing in diabetic mice is due to improper transition from M1 to M2 phenotype in vivo [83].
5.1. Inflammasomes
Inflammasomes are multimeric protein complexes responsible for inflammatory caspase activation and the maturation of pro-inflammatory cytokines and pyroptosis in different immune cells, including macrophages [84,85]. Monocytes and macrophages are important innate immune cells expressing inflammasome genes, and several studies have established the inflammasome-dependent function of these cells [86]. Our current understanding has shown that upon sensing PAMPs and DAMPs, the canonical inflammasome recruits inactive pro-caspase1. Oligomerized pro-caspase 1 leads to auto cleavage into active caspase-1, which induces inflammatory cytokines (pro-IL-1β to IL-1β) and pyroptosis, an inflammatory form of cell death. The well-described inflammasome, NLRP3, reacts to external cues like ATP, cholesterol, and β-amyloid, and there are reports to show that proportionately the inflammatory profile decreases in mice treated with caspase-1 and NLRP3 inhibitors [87]. Hypoxia-inducible factor-1α (HIF-1α) promotes IL-1β secretion through the activation of the NF-kB pathway, the first signal for NLRP3 activation [88]. Recent reports suggest the second signaling in which HIF-2α deficiency enhances FAO with metabolic contributions from palmitic acid and oxidized phospholipids which promotes/plays a decisive role in NLRP3 activation. Ultimately, Tschopp et al. have shown that inhibition of NLRP3 leads to an immunosuppressive behavior of type1 interferons [89]. Numerous recent investigations have also established the contribution of inflammasome activation in SARS-CoV-2 infected macrophages in driving COVID-19 pathology. Rodrigues et al. proposed the role of NLRP3 inflammasome in COVID-19 severity when they found active NLRP3 inflammasome in PBMC and tissues collected during the autopsy. The inflammasome was activated in the infected monocytes and macrophages in the lungs leading to an exacerbated inflammatory response in moderate and severe COVID-19 patients [90]. A very recent report by Sefik et al. used a SARS-CoV-2-infected MISTRG6-hACE2 humanized mouse model, which comprises a human immune system [91]. The activation of inflammasomes in infected macrophages mediated IL-1 and IL-18 release and the pyroptosis of macrophages, resulting in the lungs' hyperinflammation [87]. Inflammasome activation, when blocked, leads to the release of the virus from the infected cells showing the role of inflammasomes in preventing a productive viral cycle through inflammatory cytokine production by infected macrophages and its suicide by pyroptosis [92]. Similarly, the major inflammasome pathways in sensing bacterial infections include NLRP3, NLRP1b, NLRC4, AIM2, Pyrin, caspase-11/4, and NLRP6. A wide range of bacterial products induces the formation of these inflammasome complexes and the downstream signaling cascades. However, it has also been well-established that numerous bacterial pathogens have developed strategies to evade inflammasome activation [93]. The contributory role of inflammasomes, particularly NLRP3 in the monocyte and macrophage mediated inflammation in driving the pathophysiology of inflammatory conditions makes it a potential therapeutic target for various inflammatory diseases, including COVID-19. We have discussed various inflammasome targeting molecules in modulating macrophage mediated inflammatory response in later section.
5.2. Transcriptional regulation of functional genes
Upon activation, many transcription factors in the cytoplasm are transferred to the nucleus, which regulates gene expression. Sometimes, they get regulated by small non-coding endogenous RNAs called microRNAs (miRNAs) [94]. Recent studies have identified essential transcription factors like KLF6, IRF9, IRF5, C/EBP-α, STAT1, and NF-κB involved in the polarization of M1 macrophages; in contrast, STAT3, GATA3, c-MYC, IRF4, PPARs, and STAT6 are involved in the polarization of M2 macrophages [95,96]. Peroxisome proliferator-activated receptors (PPARs) have been reported to be associated with polarization of the M2 spectrum with enhanced M2 specific markers like Arg-1, Mrc1, and TGF-β through inhibition of inflammatory cytokines and nitric oxide synthase 2 in the macrophages of T. cruzi infected mice [97]. STAT protein family members have been reported to regulate M1/M2 polarization and promote an inflammatory response in inflammatory bowel disease and atherosclerosis [98]. In IFN-γ-induced macrophages, STAT1 homodimer acts as a crucial mediator by binding to cis elements known as IFN-γ-activated sites in the promoter region of M1-specific marker genes IL-12 and NOS2 [99]. Upon activation, STAT3 plays an essential role in the progression and polarization of macrophages in the tumor microenvironment. IL-6 and IL-10-induce STAT3 mediated M2 phenotype-specific genes like Mrc1 [100]. As opposed to 2 % of protein-coding transcripts, nearly 98 % of the genome accounts for non-coding RNAs [101]. Genome-wide sequencing has identified a concomitant increase in the numbers of short (20–24 nucleotides in length) ssRNA molecules represented by microRNAs (miRNAs) that undergo epigenetic regulatory mechanisms (histone modifications and promoter methylation) at the expression level like other protein-coding genes [102]. Nearly 109 miRNAs were identified to have differential expression profiles in human and murine M1 and M2 subsets of macrophages [103]. miRNAs cause gene silencing through regulation at the post-transcriptional level, specifically in immune cells such as monocytes and macrophages, by binding to the 3′-untranslated region (3′UTR) of target mRNA. miRNA-24, miRNA-142-3p, miRNA-30b, and miRNA-199a-5p suppress monocyte-to-macrophage differentiation, with several miRNAs found abundant in polarised macrophages. miRNA-125, miRNA-146, miRNA-155, miRNA-let-7a/f, and miRNA-378 are required for M1 macrophage polarization, whereas miRNA-let-7c/e, miRNA-9, miRNA-21, miRNA-146, miRNA-147, miRNA-187, and miRNA-223 are required for M2 polarization [96]. Subsequent studies have identified miR-21, miR-127, miR-720, miR- 451, miR- 181 expressions to be up-regulated in M0 to M1 polarization of classically activated macrophages in response to bacterial products and several pro-inflammatory cytokines; miR-125a, miR-511-5p/3p, miR- 92a expression in M0 to M2 polarization of alternatively activated macrophages in response to several anti-inflammatory cytokines and glucocorticoids. Different reports also suggest the microRNA-mediated regulation of PPAR and STAT signaling, which regulates the functional phenotype of immune cells. miR-9 has been reported to regulate the expression of PPAR-δ in inflammatory monocytes [104]. The most extensively studied miRNA, miR-155, drives polarization of the M1 phenotype with decreased levels in M2 macrophages, and over-expression of miR-155 drives repolarization towards the M1 phenotype of M2 phenotypic mononuclear cells [105]. miR-155 regulates the expression of nearly 650 genes, but recent pieces of evidence have shown that its interference in STAT6 signaling alters the expression of M2 polarization-related genes such as SERPINE, CCL18, and CD23 [106]. Therefore, miRNAs play essential roles in macrophage biology, and targeting them may be an effective therapeutic strategy for macrophage-mediated immune diseases. Unfortunately, the miRNA-specific network is poorly understood, making drug development difficult. Therefore, developing macrophage-specific carriers could significantly boost miRNA-based therapeutic strategy.
5.3. Macrophage metabolic reprogramming during inflammation
Along with the known inflammatory and anti-inflammatory regulators of functional plasticity carried out by mononuclear phagocytes, recent shreds of evidence depict the role of sugar, amino acid, and lipid metabolic pathways reprogramming in modulating their immune response under diverse tissue microenvironments [77,107,108]. The M1 subtype is marked by a high glycolysis rate, fatty acid synthesis, and truncated TCA cycle, with flux through the pentose phosphate pathway [109,110]. Along with this, inflammatory functions of this phenotype are also mediated by certain intermediates. For example, LPS/IFN-γ stimulation act on succinate dehydrogenase (SDH) and isocitrate dehydrogenase (IDH) enzymes (an enzyme responsible for the conversion of isocitrate to alpha-ketoglutarate) of the TCA cycle due to which citrate and succinate accumulate (TCA break) in these mononuclear cells having inflammatory roles [111]. This accumulated citrate converts to itaconic acid, inhibiting SDH and promoting succinate accumulation during inflammation [112].
Two possible mechanisms have been reported to ensure carbon entry (for enhanced energy demand) in the TCA cycle: repressed PDH kinase 1, which leads to sustained conversion of pyruvate to acetyl-CoA, and glutamine-dependent anaplerosis, which replenishes succinate through the GABA shunt pathway [113]. During inflammatory conditions, these phenotypes also show up-regulated iNOS for NO production by converting arginine to l-citrulline and NO in the presence of the iNOS enzyme [114,115]. NO suppresses the mitochondrial electron transport system (ETS) and orchestrates the metabolic reprogramming of macrophages to the M1 subtype [[115], [116], [117]]. In contrast, the M2 subtype shows a threshold rate of glycolysis and TCA cycle, high oxidative phosphorylation, and fatty acid oxidation [118]. Upon IL-4 stimulation, they generate ATP through β-oxidation and glutamine metabolism, rely on LDL and VLDL conveyed through scavenger receptors, CD36, and catabolized in lysosomes and catalyze the conversion of l-arginine to urea and l-ornithine (via overexpression of Arginase 1), which serve as precursors of proline required for the biosynthesis of collagen hence, repair [115,119] (Fig. 3).
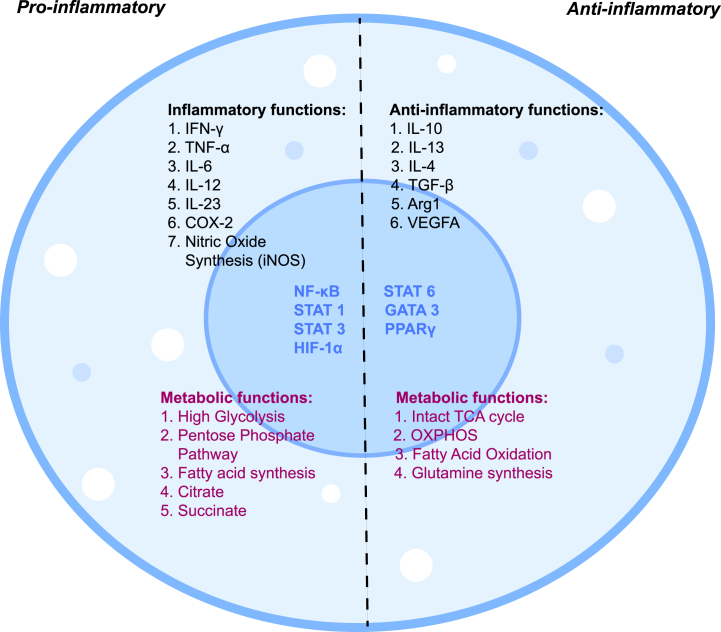
Fig. 3.
Metabolic signatures of macrophage activation.
Pro-inflammatory stimulation acts via specific transcription factors such as STAT 1, STAT 3, NF-κB, and HIF-1α which activate iNOS, and COX-2 production. Interestingly, these mononuclear phagocytes also undergo metabolic reprogramming such as high glycolysis rate, pentose phosphate pathway, and fatty acid synthesis. Even certain TCA metabolites such as succinate and citrate get accumulated which are reported to have inflammatory roles. On the other hand, anti-inflammatory macrophages are well known for the expression of Arg1, TGF-β, IL-10, IL-4, and VEGFA production. They have upregulated STAT6 and GATA3 transcription factors which regulate its functional properties. Metabolically they have high OXPHOS, enhanced fatty acid oxidation, glutaminolysis for energy production, and anti-inflammatory functions. Arginase 1 (Arg1), cyclooxygenase-2 (COX-2); inducible Nitric Oxide Synthase (iNOS), GATA binding protein 3 (GATA 3), Hypoxia Inducible Factor-1α (HIF-1α), Interferon-γ (IFN-γ), Nuclear Factor kappa-light chain (NF-κB), Signal Transducer and activator of transcription (STAT), Vascular endothelial growth factor A (VEGFA).
The shift in macrophage phenotypic, metabolic, and functional properties can lead to specific disease conditions including inflammation. For example, acute bacterial infections such as Listeria monocytogenes cause inflammation in immune-compromised individuals and pregnant woman enhancing M1 reprogramming of macrophages and leading to in vitro and in vivo intracellular bacterial lysis [120]. However, the metabolic influence of such bacterial diseases needs more exploration. Additionally, chronic inflammatory states such as type-2 diabetes (T2D) can be divided into metabolic or inflammatory types, and the extent of abnormal functionality defines their severity by tissue-specific macrophages [121,122]. Even metabolically, certain TCA intermediates were found to play a critical role in governing the disease condition. For example, in the body of T2D patients, succinate level is high in plasma, and M1 markers are more expressive in islet's micro-organ patches, which release pro-inflammatory cytokines such as TNF-α and IL-1β [122]. Along with this, during diabetic retinopathy also, this succinate binds its receptor (SUCNR1), resulting in inflammation [[123], [124], [125], [126]].Therefore, a complex array and interconnected regulation of metabolic reprogramming in the macrophages are responsible for their inflammatory or immunosuppressive states in the presence of specific stimuli or factors present in the tissue microenvironment and these can be specifically targeted for improvement of inflammatory condition as discussed in the next section.
6. Monocytes and macrophage targeting therapeutics in inflammatory diseases
The ubiquity, plasticity and extensive roles of macrophages in different stages of inflammatory conditions make them an intriguing subset that can be targeted for the treatment of inflammatory diseases. Macrophage migration inhibitory factor (MIF) emerged as an essential protein for the recruitment of monocytes and macrophages to tissues. The antagonistic effect of MIF on glucocorticoids used therapeutically in broad-spectrum anti-inflammatory conditions establishes MIF as a potent target in chronic inflammatory diseases. 4-IPP and ISO-1 were designed to inhibit MIF-mediated tumor-promoting and inflammatory effects by targeting specific MIF/CD74 signaling involved in the upregulation of pro-inflammatory cytokines in macrophages [[127], [128], [129]]. An inhibitor of NLRP3, Dapansutrile, is validated to reduce inflammation and interleukin levels in murine models [130]. Several other groundbreaking molecular inhibitors identified include MCC950, a diarylsulfonylurea, a particular inhibitor of caspase1-dependent processing of IL1-b and ketone metabolite β-hydroxybutyrate, which has been reported to decrease IL-18 and IL-1β production in human monocytes [131,132]. A recent study by Camilli et al. has shown the potential of β-glucan in attenuating NLRP3-dependent inflammation. Specifically, the naturally occurring polysaccharide acted upstream of the NLRP3 and prevented the K+ efflux, generation of mitochondrial ROS, ASC oligomerization, speck formation, and ultimately the β-glucan-induced memory in macrophages of cryopyrin-associated periodic syndromes patients showed dampened IL-1β secretion and caspase-1 activation [133]. These NLRP3 targetting molecules showing promising results can be further evaluated for clinical applications in alleviating the severity of macrophage mediated inflammatory disorders.
As discussed in the previous section, several integrins have been observed to be associated with the macrophage functions participating in pathophysiology of inflammatory diseases. Thus, the integrin-based therapeutics have hoarded hefty interest in an attempt of the bench to bedside-based translational research in adverse cases of inflammation. An anti-integrin therapy for treating sepsis uses antibodies and cyclic or linear peptides as antagonists designed to target integrin's natural ligand binding site. Antibodies like natalizumab and VDZ have shown few systemic adverse effects; however, newer drugs like PF-00547659, etrolizumab, and AJM300, to name a few, are under clinical trials to demonstrate their efficacy towards genuinely personalized treatment [134]. In an attempt at ligand-targeted anti-Integrin therapy, anti-M7, a monoclonal antibody, was reported to block specific interactions between MAC1 and CD40L (pro-inflammatory ligand), reducing in vitro and in vivo recruitment of leukocyte without potentiating inhibition of other integrin functionality [135]. αVβ3 plays a voluntary role in macrophage-dependent inflammation, development, migration, and inflammatory angiogenesis. MEDI-522 or vitaxin humanized monoclonal antibody, was studied in 2002 as an antagonist of αVβ3 receptors in rheumatoid arthritis (RA) patients [136]. Cilengitide, a cyclic peptide and an identified inhibitor of αVβ3 used in the treatment of glioblastoma, has been reported to reduce E. coli interaction with endothelium in an experimental model of sepsis [137,138]. As integrin is known to be associated with a variety of macrophage function ranging from recruitment to inflammatory cytokine production, differentiation and polarization, the molecules showing potential therapeutic efficacy can further be evaluated in clinical settings. Myeloid plasticity is the hallmark of the innate response to the Toll-like receptor (TLR) activation. A technique by which adoptive transfer for monocyte cell fate tracking in systemic inflammation is brought on by activation of TLR7, the primary innate immune receptor in mice that detects viral RNA. Through cell sorting, defined monocyte subsets are extracted from donor mice's bone marrow and adoptively injected into the systemic circulation of congenic hosts. This can be done with or without concurrent TLR7 activation through topical application of imiquimod, a small molecule agonist, in a cream formulation that triggers a systemic inflammatory response. Benefits include the ability to define donor cell populations precisely and the destiny of those cells without requiring host conditioning in a model that accurately reflects important elements of the systemic inflammatory response to TLR7 stimulation [139].
Lactic acid produced as a by-product of the LPS-induced shift from OXPHOS to the aerobic glycolysis pathway in inflammatory macrophages is known to create negative feedback to regulate the function of inflammatory macrophages. An increase in the congregated levels of lactic acid reduces the extracellular acidification rate and increases the oxygen consumption rate. In vitro studies have shown that macrophages inhibit the TLR-mediated activation of macrophages with downregulated expression of cytokines and chemokines such as IL-23, TNF-α, and CCL7, respectively [140,141]. The above observation can be used in the treatments of inflammatory diseases as it is reported in a study that hypertonic levels of sodium lactate can alleviate cardiac inflammation in sepsis. Small molecular targets of lactic acid agonist G1/O protein-coupled receptor (GPR81) present on the cell surface can also be used as a promising target for the programmed metabolic pathway of macrophages [142,143]. In addition to this, well-known TCA metabolites can be targeted, for example, succinate, which gets accumulated at high concentration in diabetic patients and is known to act via the SUCNR1(GPR91) receptor and regulates macrophage functions such as enhancing pro-inflammatory cytokine production, ROS formation, stabilizes HIF-1α and decreases anti-inflammatory cytokine synthesis [112,123,[144], [145], [146], [147], [148], [149]]. Therefore, drugs can target SUCNR1 and inhibit its function during inflammation.
Plasticity of macrophages provides legitimate strategies to target specific molecules for therapeutic interventions. TSG-6 (TNF-α-induced protein 6), an anti-inflammatory protein secreted by activated macrophages, was studied as a target to alleviate IBD in DSS-Induced mice [145]. Fibrinogen-like protein 2 (Fgl2) expressed on membranes of macrophages promotes polarization and inhibits the worsening of enteritis in colitis mice [150]. Yes-associated protein (YAP) has been known to regulate the conversion of M1 and M2 macrophages, and YAP conditional knockouts in macrophages relieved colitis in mice [151]. Thus, this accumulating evidence supports new potential targets in macrophage-based therapy by regulating its phenotype in inflammatory diseases. Besides varying phenotypes, macrophage ontogeny from marrow-derived macrophages to yolk-sac/fetal liver-derived tissue macrophages is a governing concern in developing therapies. The recent development of CAR on macrophages as a bridge between innate and adaptive immunity might add an advantage in tissue regions with poor trafficking, such as inflamed joints in RA [152,153]. Human trials using ex vivo macrophages (IFNγ/LPS) have shown safety but not efficacy. This lays the foundation stone for using macrophage-modulating technology in the future. Nanomedicine in macrophage therapeutics was explored by You et al., where metabolic glycoengineered exosomes were targeted against membrane-based scavenger receptors (class A), which are up-regulated in RA-inflamed joints [154]. Metabolic regulators of macrophages, such as 2DG, were shown to reduce the inflammatory response in the nephritis model by inhibiting glycolysis in macrophages in the kidneys [113,155]. These advances in macrophage manipulation have been tested for years to attain homeostasis with valid and flexible cellular platforms against several pathophysiologies. As macrophages are the bridge between the innate and adaptive immune system, the therapeutic approaches against disease-causing active macrophages with specific dose selection and reduced risk of resistance are a profound challenge, particularly without compromising immunity [156].
7. Conclusion
Monocytes and macrophages are essential to the immune system due to their ubiquity, plasticity, and versatile roles in tissue remodeling, inflammatory response, and homeostasis. The increasing complexity of embryonic and monocyte-derived macrophages and their change in transcriptional profile, established by reference datasets using high throughput techniques like single-cell RNA sequencing, have enabled us to identify heterogeneity in activation pathways (phagocytic/oxidative stress/inflammatory/tissue remodeling) of all predicted clusters of macrophages. Future studies and validation of the vast atlas of transcriptional factors and their regulators will introduce new targeted therapies against inflammatory diseases. The redundancy in integrin functions and the overlapping roles of diversified integrin-ligand interactions have enhanced our understanding of the different regulatory mechanisms of this heterodimeric molecule. Further in this review, aberrant metabolic rewiring in macrophages integrates our knowledge of metabolic networks regulating cellular responses to external perturbations. The interconnected positive and negative feedback loops with altered metabolic signatures could help develop new promising, conducive approaches targeting "meta-inflammation" or "metabolic dysfunction" [157]. To date, extensive investigation has been done in animal models to decipher the molecular events involving monocytes and macrophages, their distribution, and phenotypical changes in the events of inflammatory conditions. At the same time, the study of the human system is comparatively less. Validation and re-investigation of the findings of in vivo studies in the human system or patients will help enumerate the potential therapeutic targets involving monocytes and macrophages.
CRediT authorship contribution statement
Shiba Prasad Dash: Writing – review & editing, Writing – original draft. Saloni Gupta: Writing – original draft. Pranita P. Sarangi: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The corresponding author Dr. Pranita P Sarangi is an Associate Editor of this journal.r.
Acknowledgements
This work is funded by the Department of Biotechnology, Govt. of India (BT/010/IYBA/2017/04) and Science Engineering Research Board, Govt. of India (CRG-2020-0025), to PPS; UGC fellowship to SPD, and CSIR fellowship to SG.
References
- 1.Guilliams M., et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper M.D., Alder M.N. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Tauber A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- 4.Artyomov M.N., et al. Integrating immunometabolism and macrophage diversity. Semin. Immunol. 2016;28:417–424. doi: 10.1016/j.smim.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A., et al. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Chen S., et al. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020;17:36–49. doi: 10.1038/s41423-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiani P., Boraschi D. New insights into tissue macrophages: from their origin to the development of memory. Immune network. 2015;15:167–176. doi: 10.4110/in.2015.15.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasheed A., Rayner K.J. Macrophage responses to environmental stimuli during homeostasis and disease. Endocr. Rev. 2021;42:407–435. doi: 10.1210/endrev/bnab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 11.Ross E.A., et al. Macrophages: the good, the bad, and the gluttony. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.708186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L.X., et al. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019;106:345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomão R., et al. TLR signaling pathway in patients with sepsis. Shock. 2008;30(Suppl 1):73–77. doi: 10.1097/SHK.0b013e318181af2a. [DOI] [PubMed] [Google Scholar]
- 14.Porta C., et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meghraoui-Kheddar A., et al. Revising CX3CR1 expression on murine classical and non-classical monocytes. Front. Immunol. 2020;11:1117. doi: 10.3389/fimmu.2020.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jian Z., et al. The involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 2019;10:2167. doi: 10.3389/fimmu.2019.02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang P., et al. Immunological feature and transcriptional signaling of Ly6C monocyte subsets from transcriptome analysis in control and hyperhomocysteinemic mice. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.632333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapellos T.S., et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A.A., et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017;214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 22.Franken L., et al. Macrophages: sentinels and regulators of the immune system. Cell Microbiol. 2016;18:475–487. doi: 10.1111/cmi.12580. [DOI] [PubMed] [Google Scholar]
- 23.Epelman S., et al. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy J.M., et al. Tissue-dependent adaptations and functions of innate lymphoid cells. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.836999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coillard A., Segura E. In vivo differentiation of human monocytes. Front. Immunol. 2019;10:1907. doi: 10.3389/fimmu.2019.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilliams M., et al. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Swirski F.K., et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science (New York, N.Y.) 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong S.Z., et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J. Exp. Med. 2016;213:2293–2314. doi: 10.1084/jem.20160800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balabanian K., et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012;119:5722–5730. doi: 10.1182/blood-2012-01-403378. [DOI] [PubMed] [Google Scholar]
- 30.Bachelerie F. CXCL12/CXCR4-axis dysfunctions: markers of the rare immunodeficiency disorder WHIM syndrome. Dis. Markers. 2010;29:189–198. doi: 10.3233/DMA-2010-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurotaki D., et al. Transcriptional control of monocyte and macrophage development. Int. Immunol. 2017;29:97–107. doi: 10.1093/intimm/dxx016. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y., et al. Zbtb14 regulates monocyte and macrophage development through inhibiting pu.1 expression in zebrafish. Elife. 2022;11 doi: 10.7554/eLife.80760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kratofil R.M., et al. Monocyte conversion during inflammation and injury. Arterioscler. Thromb. Vasc. Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 34.Lessard A.J., et al. Triggering of NOD2 receptor converts inflammatory Ly6C(high) into Ly6C(low) monocytes with patrolling properties. Cell Rep. 2017;20:1830–1843. doi: 10.1016/j.celrep.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z., et al. Fate mapping via ms4a3-expression history traces monocyte-derived cells. Cell. 2019;178:1509–1525. doi: 10.1016/j.cell.2019.08.009. e1519. [DOI] [PubMed] [Google Scholar]
- 36.Davies L.C., et al. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y., Hirschi K.K. Tissue-resident macrophage development and function. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.617879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeffel G., Ginhoux F. Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 2018;330:5–15. doi: 10.1016/j.cellimm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingersoll M.A., et al. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsou C.L., et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomino D.C., Marti L.C. Chemokines and immunity. Einstein (Sao Paulo, Brazil) 2015;13:469–473. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L., et al. The immune function of Ly6Chi inflammatory monocytes during infection and inflammation. Curr. Mol. Med. 2017;17:4–12. doi: 10.2174/1566524017666170220102732. [DOI] [PubMed] [Google Scholar]
- 44.Sung S.S. Monocyte-derived dendritic cells as antigen-presenting cells in T-cell proliferation and cytokine production. Methods Mol. Med. 2008;138:97–106. doi: 10.1007/978-1-59745-366-0_9. [DOI] [PubMed] [Google Scholar]
- 45.Das S.T., et al. Monomeric and dimeric CXCL8 are both essential for in vivo neutrophil recruitment. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ichikawa A., et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am. J. Respir. Crit. Care Med. 2013;187:65–77. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI insight. 2020;5 doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalil B.A., et al. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021;19:976–988. doi: 10.1016/j.csbj.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cormican S., Griffin M.D. Fractalkine (CX3CL1) and its receptor CX3CR1: a promising therapeutic target in chronic kidney disease? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.664202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamilloux Y., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mezu-Ndubuisi O.J., Maheshwari A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021;89:1619–1626. doi: 10.1038/s41390-020-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mrugacz M., et al. Integrins: an important link between angiogenesis, inflammation and eye diseases. Cells. 2021;10 doi: 10.3390/cells10071703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kishimoto T.K., et al. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 55.Dash S.P., et al. Inflammatory monocytes and subsets of macrophages with distinct surface phenotype correlate with specific integrin expression profile during murine sepsis. J. Immunol. 2021;207:2841–2855. doi: 10.4049/jimmunol.2000821. [DOI] [PubMed] [Google Scholar]
- 56.Nader D., et al. A new perspective in sepsis treatment: could RGD-dependent integrins be novel targets? Drug Discov. Today. 2020;25:2317–2325. doi: 10.1016/j.drudis.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sloan E.K., et al. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge Y., et al. Efferocytosis and its role in inflammatory disorders. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.839248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duarte S., et al. Fibronectin-α4β1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am. J. Transplant. : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:2689–2699. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver L.C., et al. CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats. Exp. Neurol. 2015;271:409–422. doi: 10.1016/j.expneurol.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fondevila C., et al. Cytoprotective effects of a cyclic RGD peptide in steatotic liver cold ischemia and reperfusion injury. Am. J. Transplant. : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2240–2250. doi: 10.1111/j.1600-6143.2009.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serbina N.V., et al. Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Adv. Immunol. 2012;113:119–134. doi: 10.1016/B978-0-12-394590-7.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonov A.S., et al. αVβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-κB activation. J. Cell. Physiol. 2011;226:469–476. doi: 10.1002/jcp.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cam A., de Mejia E.G. RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin. Mol. Nutr. Food Res. 2012;56:1569–1581. doi: 10.1002/mnfr.201200301. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z., et al. WISP1-αvβ3 integrin signaling positively regulates TLR-triggered inflammation response in sepsis induced lung injury. Sci. Rep. 2016;6 doi: 10.1038/srep28841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marre M.L., et al. Human integrin α(3)β(1) regulates TLR2 recognition of lipopeptides from endosomal compartments. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han C., et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L.J., et al. The impact of integrin β2 on granulocyte/macrophage progenitor proliferation. Stem cells (Dayton, Ohio) 2019;37:430–440. doi: 10.1002/stem.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blythe E.N., et al. β2 integrin CD11d/CD18: from expression to an emerging role in staged leukocyte migration. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.775447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding X., et al. Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: role of WISP1-αvβ5 integrin pathway in TLR4-mediated inflammation and injury. Crit. Care. 2018;22:302. doi: 10.1186/s13054-018-2237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker H.M., et al. α1β1 integrin-mediated adhesion inhibits macrophage exit from a peripheral inflammatory lesion. J. Immunol. 2013;190:4305–4314. doi: 10.4049/jimmunol.1202097. [DOI] [PubMed] [Google Scholar]
- 72.Amarante-Mendes G.P., et al. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X., Mosser D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santoni G., et al. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflammation. 2015;12:21. doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mittal M., et al. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yunna C., et al. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 77.Atri C., et al. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orecchioni M., et al. Macrophage polarization: different gene signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vergadi E., et al. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 80.Wang N., et al. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castro-Dopico T., Clatworthy M.R. IgG and fcγ receptors in intestinal immunity and inflammation. Front. Immunol. 2019;10:805. doi: 10.3389/fimmu.2019.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xing L., et al. Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berbudi A., et al. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020;16:442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo H., et al. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Zoete M.R., et al. Inflammasomes. Cold Spring Harbor Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abderrazak A., et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sefik E., et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606:585–593. doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X., et al. Macrophage HIF-2α suppresses NLRP3 inflammasome activation and alleviates insulin resistance. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109607. [DOI] [PubMed] [Google Scholar]
- 89.Dagenais M., et al. The inflammasome: in memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodrigues T.S., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sefik E., et al., A humanized mouse model of chronic COVID-19. Nature biotechnology. 2022;40(6):906–920. doi: 10.1038/s41587-021-01155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Junqueira C., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606(7914):576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ta A., Vanaja S.K. Inflammasome activation and evasion by bacterial pathogens. Curr. Opin. Immunol. 2021;68:125–133. doi: 10.1016/j.coi.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., et al. Transcriptional regulation of macrophages polarization by MicroRNAs. Front. Immunol. 2018;9:1175. doi: 10.3389/fimmu.2018.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molawi K., Sieweke M.H. Transcriptional control of macrophage identity, self-renewal, and function. Adv. Immunol. 2013;120:269–300. doi: 10.1016/B978-0-12-417028-5.00010-7. [DOI] [PubMed] [Google Scholar]
- 96.Shapouri-Moghaddam A., et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 97.Wagner N., Wagner K.D. The role of PPARs in disease. Cells. 2020;9 doi: 10.3390/cells9112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giles E.M., et al. Regulation of human intestinal T-cell responses by type 1 interferon-STAT1 signaling is disrupted in inflammatory bowel disease. Mucosal Immunol. 2017;10:184–193. doi: 10.1038/mi.2016.44. [DOI] [PubMed] [Google Scholar]
- 99.Takaishi K., et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101:2128–2136. doi: 10.1111/j.1349-7006.2010.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nejad C., et al. A guide to miRNAs in inflammation and innate immune responses. FEBS J. 2018;285:3695–3716. doi: 10.1111/febs.14482. [DOI] [PubMed] [Google Scholar]
- 101.Ashrafizadeh M., et al. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022;59 doi: 10.1016/j.smim.2022.101606. [DOI] [PubMed] [Google Scholar]
- 102.Sonkoly E., Pivarcsi A. microRNAs in inflammation. Int. Rev. Immunol. 2009;28:535–561. doi: 10.3109/08830180903208303. [DOI] [PubMed] [Google Scholar]
- 103.Singh R.P., et al. The role of miRNA in inflammation and autoimmunity. Autoimmun. Rev. 2013;12:1160–1165. doi: 10.1016/j.autrev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Thulin P., et al. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response. Int. J. Mol. Med. 2013;31:1003–1010. doi: 10.3892/ijmm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahesh G., Biswas R. MicroRNA-155: a master regulator of inflammation. J. Interferon Cytokine Res. : the official journal of the International Society for Interferon and Cytokine Research. 2019;39:321–330. doi: 10.1089/jir.2018.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Connell R.M., et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diskin C., Pålsson-McDermott E.M. Metabolic modulation in macrophage effector function. Front. Immunol. 2018;9:270. doi: 10.3389/fimmu.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murray P.J., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freemerman A.J., et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hard G.C. Some biochemical aspects of the immune macrophage. Br. J. Exp. Pathol. 1970;51:97–105. [PMC free article] [PubMed] [Google Scholar]
- 111.Krawczyk C.M., et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zasłona Z., O'Neill L.A.J. Cytokine-like roles for metabolites in immunity. Mol. Cell. 2020;78:814–823. doi: 10.1016/j.molcel.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y., et al. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021;9:1. doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MacMicking J., et al. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 115.Palmieri E.M., et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020;11:698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Granger D.L., Lehninger A.L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J. Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koo S.J., Garg N.J. Metabolic programming of macrophage functions and pathogens control. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ren W., et al. Glutamine metabolism in macrophages: a novel target for obesity/type 2 diabetes. Adv. Nutr. 2019;10:321–330. doi: 10.1093/advances/nmy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfeffer K., et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 121.Orliaguet L., et al. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Front. Endocrinol. 2020;11:62. doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Diepen J.A., et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia. 2017;60:1304–1313. doi: 10.1007/s00125-017-4261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He W., et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 124.Peti-Peterdi J., et al. Activation of the renal renin-angiotensin system in diabetes--new concepts. Nephrol. Dial. Transplant. : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:3047–3049. doi: 10.1093/ndt/gfn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sadagopan N., et al. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007;20:1209–1215. doi: 10.1016/j.amjhyper.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 126.Toma I., et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Invest. 2008;118:2526–2534. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoi A.Y., et al. Macrophage migration inhibitory factor: a therapeutic target across inflammatory diseases. Inflamm. Allergy - Drug Targets. 2007;6:183–190. doi: 10.2174/187152807781696455. [DOI] [PubMed] [Google Scholar]
- 128.Kim K.W., Kim H.R. Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis. Kor. J. Intern. Med. 2016;31:634–642. doi: 10.3904/kjim.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Q., et al. MIF as a biomarker and therapeutic target for overcoming resistance to proteasome inhibitors in human myeloma. Blood. 2020;136:2557–2573. doi: 10.1182/blood.2020005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marchetti C., et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 2018;20:169. doi: 10.1186/s13075-018-1664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coll R.C., et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shao B.Z., et al. NLRP3 inflammasome and its inhibitors: a review. Front. Pharmacol. 2015;6:262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Camilli G., et al. β-Glucan-induced reprogramming of human macrophages inhibits NLRP3 inflammasome activation in cryopyrinopathies. J. Clin. Invest. 2020;130:4561–4573. doi: 10.1172/JCI134778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park S.C., Jeen Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018;24:1868–1880. doi: 10.3748/wjg.v24.i17.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolf D., et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat. Commun. 2018;9:525. doi: 10.1038/s41467-018-02896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilder R.L. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 2002;61(Suppl 2):ii96–99. doi: 10.1136/ard.61.suppl_2.ii96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mas-Moruno C., et al. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anti Cancer Agents Med. Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McHale T.M., et al. Inhibition of vascular endothelial cell leak following Escherichia coli attachment in an experimental model of sepsis. Crit. Care Med. 2018;46:e805–e810. doi: 10.1097/CCM.0000000000003219. [DOI] [PubMed] [Google Scholar]
- 139.Okun E., et al. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohashi T., et al. M2-like macrophage polarization in high lactic acid-producing head and neck cancer. Cancer Sci. 2017;108:1128–1134. doi: 10.1111/cas.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou H.C., et al. Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Int. Rev. Immunol. 2022;41:4–18. doi: 10.1080/08830185.2021.1955876. [DOI] [PubMed] [Google Scholar]
- 142.Besnier E., et al. Hypertonic sodium lactate improves microcirculation, cardiac function, and inflammation in a rat model of sepsis. Crit. Care. 2020;24:354. doi: 10.1186/s13054-020-03083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duburcq T., et al. Hypertonic sodium lactate improves fluid balance and hemodynamics in porcine endotoxic shock. Crit. Care. 2014;18:467. doi: 10.1186/s13054-014-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dang E.V., et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Day A.J., Milner C.M. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. : journal of the International Society for Matrix Biology. 2019;78–79:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 146.Littlewood-Evans A., et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016;213:1655–1662. doi: 10.1084/jem.20160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rubic T., et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 2008;9:1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 148.Semenza G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda, Md. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 149.Tannahill G.M., et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hu J., et al. The duality of Fgl2 - secreted immune checkpoint regulator versus membrane-associated procoagulant: therapeutic potential and implications. Int. Rev. Immunol. 2016;35:325–339. doi: 10.3109/08830185.2014.956360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou X., et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176–1189. doi: 10.1016/j.celrep.2019.03.028. e1175. [DOI] [PubMed] [Google Scholar]
- 152.Klichinsky M., et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang S., et al. CAR-macrophage: an extensive immune enhancer to fight cancer. EBioMedicine. 2022;76 doi: 10.1016/j.ebiom.2022.103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.You D.G., et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jing C., et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc. Natl. Acad. Sci. U.S.A. 2020;117:15160–15171. doi: 10.1073/pnas.2000943117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seyedizade S.S., et al. Current status of M1 and M2 macrophages pathway as drug targets for inflammatory bowel disease. Arch. Immunol. Ther. Exp. 2020;68:10. doi: 10.1007/s00005-020-00576-4. [DOI] [PubMed] [Google Scholar]
- 157.Russo S., et al. Meta-inflammation and metabolic reprogramming of macrophages in diabetes and obesity: the importance of metabolites. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.746151. [DOI] [PMC free article] [PubMed] [Google Scholar]