Abstract
Background: Robotic systems have successfully been introduced into other surgical fields in the past. First attempts with different setups are made in the field of microsurgery. The Symani® Surgical System, a flexible platform consisting of two robotic arms, features motion scaling with tremor filtration to address the demands and complexity of microsurgery. Symani’s NanoWrist Instruments are the world’s smallest, wristed surgical instruments, intended to improve a surgeon’s range of motion beyond the capability of the human hand. This combination allows surgeons to scale their hand movements while seamlessly articulating the robotic micro instruments.
Purpose: We report on our experience in extremity reconstruction with this novel system.
Research Design: The Symani Surgical System® was used for 6 cases of extremity reconstruction. The surgeon controlled the manipulators along with the footswitch while either sitting away from the operating table relying on 3D visualization with an exoscope or sitting at the operating table using a standard microscope.
Data Collection: Microsurgical anastomoses were performed in 4 patients (3 end-to-end arterial anastomoses and one end-to-side arterial anastomosis) and nerve grafting was performed in 2 patients.
Results: Microvascular anastomoses were slower vs conventional microsurgery, but all anastomoses were patent. Epineural coaptation showed proper fascicle alignment and tissue manipulation could be kept to a minimum. The platform’s motion scaling allows the surgeon to perform precise micro-movements with only minimal tissue manipulation and hard-to-reach anatomy becomes accessible more easily.
Conclusions: Robotic microsurgery might gain importance in the nearer future but more data will need to be collected.
Keywords: robotic microsurgery, extremity reconstruction, reconstructive microsurgery
Introduction
In the last two decades robot-assisted surgery has been used for several procedures in different surgical specialties. 1 It is still fairly new in the field of plastic and reconstructive surgery, but it was already demonstrated that it can be used with many advantages, particularly in intra-oral operations. 2 Parallel to other robotics based surgical innovations, robot-assisted microsurgery has been developed for both vascular 3 and peripheral nerve surgery. 4 Currently, there are two dedicated robotic platforms for microsurgery: MUSA by Microsure 5 and Symani® Surgical System by MMI. 6 The Symani® Surgical System uses wristed micro instruments and motion scaling to enhance microsurgical precision. 7 Its successful use for lymphatic surgery and free flap surgery has been already reported on by our group.8,9 At our department, the Symani® Surgical Systemwas used for microsurgical reconstruction of the extremities in 6 consecutive cases, both for nerve coaptation and for microvascular anastomoses in free flaps. Based on this we aim to share our experiences and report about indications, advantages and limits of the Symani® Surgical System in extremity reconstruction.
Material and Methods
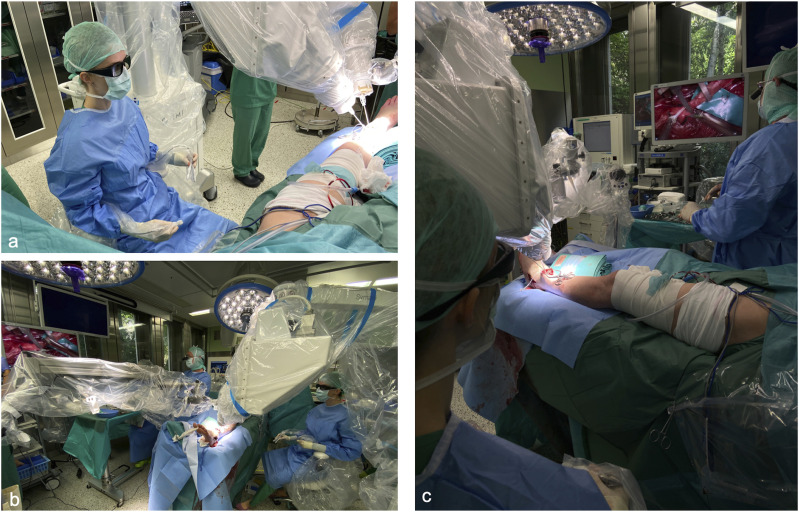
The study was approved by the Cantonal Ethics Committee of Zurich, Switzerland (ethical approval number 2021-02351). All patients provided written informed consent. The Symani® Surgical System (Medical Micro Instruments [MMI], Calci, Italy) is based on two robotic arms and a console with an ergonomic chair, a footswitch controller, and forceps shaped hand pieces (manipulators) (Figure 1). 10 Setup and manipulator handling have been already described in detail before. 8 The Symani® Surgical System was used in a hybrid setup and visualization was achieved with either a Pentero 900 microscope (Carl Zeiss Meditec AG, Jena, Germany) or the VITOM 3D system (Karl Storz SE & Co KG, Tuttlingen, Germany) (Figure 2). While the operating surgeon was sitting on the console chair using the robot’s manipulators an assisting microsurgeon provided manual assistance with conventional microsurgery instruments. The Symani® Surgical System converts the hands’ movements into motion scaled movements of the robotic arms which are equipped with a dilator and a needle holder. 10 The motion scaling is available in a range between 7-20×, that means that the computer reduces the speed of the robot instruments to a slower speed according to the chosen ratio. All anastomoses/epineural coaptations were performed by the same surgeon (IB) with 10× motion scaling, with the needle holder on the right robotic arm and the dilator on the left with a 9-0 nylon suture (Video 1). The system does not provide a haptic feedback therefore the lack of a touch sensation leads to the necessity of developing a “see-feel” with the eyes to reliably perform micro sutures.
Figure 1.
The Symani surgical system® is a flexible platform consisting of two robotic arms that can be easily positioned to facilitate surgical procedures across any anatomical region. 10
Figure 2.
The Symani® surgical system is used with an exoscope for visualization. The surgeon uses the manipulators to operate the robotic arms without the necessity of a close proximity to the surgical field (A) via a screen the entire operating team can closely follow the surgery (B) If manual assistance was needed this could be easily provided by an assisting surgeon from the side of the operating table opposite to the Symani® surgical system (C).
Patient selection was not specifically tailored to the system. Instead, it consisted of typical clinical challenges. Notably, cases involving sclerotic vessels are quite common, especially in lower extremity procedures. Thus, we assessed the feasibility of utilizing the Symani® Surgical System in such cases.
Furthermore, in extremity reconstruction, end-to-side anastomosis procedures are routinely conducted to maintain vascular continuity distal to the flap. As a result, we performed these anastomoses as part of our evaluation. Finally, epineural coaptation is a standard procedure in extremity reconstruction. While it aligns with the principles of microsurgery, it’s important to note that nerves differ significantly in texture and handling from blood vessels. Therefore, we also assesed the feasibility of employing the Symani® Surgical System in these nerve-related procedures.
Results
A total of 6 patients were operated on with the Symani® Surgical System, 4 males and two females (Table 1). Two arterial end-end anastomoses were performed in two medial femoral condyle free flaps (vessel size 1.5 mm) and one arterial anastomosis in an ALT free flap (vessel size 2 mm). Even though a sclerotic vessel was encountered in the recipient leg the robot-assisted anastomosis could be performed without problems. One end-to side anastomosis was performed in a lateral arm free flap (vessel size 1.5 mm) and epineural coaptations were executed for two nerve grafting cases using a nerve allograft. Anastomoses and epineural coaptations in these first cases took significantly longer than hand-sewn ones, primarily because of poor position (robot and microscope) and some instrument surface problems (stickiness) (Table 1). The patency rate of anastomoses was 100% and was checked by direct observation in the surgical field of pulsation, flap perfusion, and by a positive milking test. All flaps survived. Epineural coaptation showed proper fascicle alignment and tissue manipulation could be kept to a minimum.
Table 1.
Illustrating Patient Characteristics.
| Gender | Age | Type of Flap/Nerve Reconstruction | Number/Type of Anastomosis/Coaptation Performed With Symani | Duration of Robot - Assisted Suture (min) | Visualization |
|---|---|---|---|---|---|
| Male | 59 | Medial femoral condyle free flap for subtalar revision fusion. | 1 arterial anastomosis end-to-end to anterior tibial artery. | 49 | VITOM |
| Male | 49 | Medial femoral condyle free flap for talonavicular revision fusion. | 1 arterial anastomosis end-to-end to dorsalis pedis artery. | 42 | VITOM |
| Male | 47 | Nerve allograft for reconstruction of common palmar digital nerve ad digits III/IV. | 2 epineural coaptations on either side of the allograft. | 21 | VITOM |
| Female | 62 | Nerve allograft for reconstruction of palmar branch of the median nerve. | 2 epineural coaptations on either side of the allograft. | 23 | VITOM |
| Female | 35 | Lateral arm free flap for reconstruction of dorsoulnar soft tissue defect on the hand. | 1 arterial anastomosis end-to-side on ulnar artery just proximal to the wrist. | 29 | Pentero |
| Male | 42 | ALT free flap for reconstruction of a soft tissue defect over the medial ankle. | 1 arterial anastomosis end-to-end to posterior tibial artery. | 34 | Pentero |
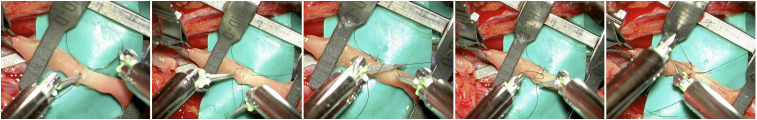
For vascular anastomoses the Pentero 900 microscope was preferred, because of the higher resolution and contrast. While the combination with the VITOM 3D system allowed for a more comfortable positioning when a close proximity to the operating table was not needed, switching from conventional to robot-assisted microsurgery and back again was slightly less smooth. The Symani® Surgical System provided an exceptional accuracy in stitch placement (Figure 3). The lack of a haptic feedback was not perceived as a disadvantage as a see-feel could easily be developed.
Figure 3.
The Symani® surgical system microdilator and micro needle holder allow for delicate atraumatic vessels manipulation and precise stitch placement.
Discussion
Functional reconstruction of extremity injuries often involves the repair of both nerves and soft tissue 11 and therefore microvascular anastomoses and epineural coaptations are among the key surgical techniques of this subspecialty of plastic surgery. Our experience support the safety of the Symani® Surgical System in these challenging cases where microsurgical skills are central for a successful nerve repair and free flap transfer.
In extremity reconstructions involving free tissue transfer, it is crucial to supply the transferred tissue with a sturdy blood flow while maintaining adequate blood supply distal to the reconstructed area. This is why end-to-side vessel anastomoses are commonly employed in extremity reconstruction, especially where vessel might be lost due to trauma or vascular occlusive disease. 12 Aditionally vessel size mismatches are commonly encountered in extremity reconstruction, end-to-side anastomosis is the technique of choice in this situation. 13 The Symani® Surgical Systemin was successfully used in this scenario.
The improvement of dexterity and precision together with the reduction or possibly complete suppression of any tremor are the expected features of a robotic system for microsurgery. The wide range of motion scaling and the miniaturization of robotic instrumentation available in the Symani® Surgical System make it an optimal tool to fulfill these requirements. 14 In our cases a 9-0 Nylon suture was used without problems.
In the first cases, the robot-assisted anastomosis or epineural coaptation required more time compared with the manual microsurgery technique (Table 1). Motion scaling was set to 10× in all cases. This allowed for precise but still reasonably fast needle handling. After some time using the robot, speed improved progressively, even thoughhe motion scaling itself remained a reason for slower movements. We have shown in a previous study, that the learning curve on the Symani® Surgical System is steep and the time needed to perform the anastomosis decreased consistently over time, to a point where robotic anastomosis time was comparable to that of hand sewn anastomosis. 9
Microsurgery is a technique that traditionally is trained and mastered only over years of experience. Plastic surgery trainees today have less surgical exposure due to working time regulations than in the past making it difficult to reach a high level of technical skill. For other types of robotic surgery, previous video game experience >6 h/week was supposed to give an advantage in simulated robotic surgery. 15 Future studies will show whether this applies also for the Symani® Surgical System with its special microsurgery application.
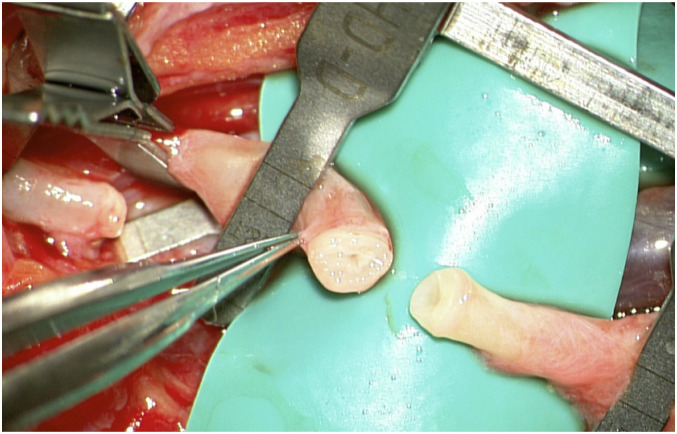
In reconstructive microsurgery, flap survival is the most important primary outcome and it provides proof of anastomosis patency. In our case series all flaps survived proving that all anastomoses remained patent. Minimal intimal injury, good apposition of the vessel walls, and equidistant sutures along the anastomosis are known factors for a successful microvascular anastomosis 16 and the optimal patency rate suggests that all these can be fulfilled with the Symani® Surgical System instruments. These factors are even more important in vessels affected by peripheral arterial disease as often encountered in the lower extremities (Figure 4), which will be particularly unforgiving in case of poor suture technique
Figure 4.
Especially in lower extremity reconstruction vessels may be affected by peripheral artery disease. Precise placement of microsurgical sutures is of paramount importance for a competent microvascular anastomosis in these cases in particular.
In epineural nerve coaptation the needle is driven through the epineurium from the outside inward. 17 This requires a precise needle placement in order to avoid damage to the fascicles. This level of precision is easy to reach with the Symani® Surgical System that allows for ideal nerve coaptation also of the smaller nerves like the two sensory nerves (palmar branch of the median nerve and a common digital nerve) included in this series to demonstrate the usability of this machine. However, the benefit of the Symani® Surgical System might be even more noticeable in deeper surgical fields with limited access like nerve repair in the neck or axilla.
Lower extremity peripheral arterial disease (PAD) affects approximately 10% of the American population. 18 Due to the significant number of affected patients, it is a common occurrence in lower extremity reconstruction. Atherosclerosis, as the primary cause of PAD, leads to intima thickening and narrowing of the vessel lumen. 19 Atraumatic vessel handling and perfect vessel alignment are of paramount important in these cases. The Symani® Surgical System was successfully used in this clinical scenario.
At present time the system has a narrow field of application. While it can effectively suture vessels and nerves, it cannot currently be used for dissecting these structures. Surgeons must still rely on established microsurgical techniques for surgical approaches and vessel and nerve preparation. Further development of the system will need to address this current limitation.
These first cases prove the effectiveness of the Symani® Surgical System for microvascular anastomoses and epineural coaptations in reconstructive microsurgery of the extremities. Further studies are needed to better understand in which cases the use of a microsurgery robot can significantly improve the outcomes and represent an added value that justifies the high costs of the system. A further interesting aspect to be analyzed is its potential use in microsurgical training in order to shorten the learning curve of younger surgeons and improving at the same time the quality of the surgery and the outcomes.
Supplemental Material
Video 1.
Author Contributions: All authors made substantial contributions to all of the following: (1) the conception and design of the study, analysis and interpretation of data, (2) drafting the article or revising it critically, (3) and approval of the final version.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Nicole Lindenblatt acts as a consultant and scientific advisor for Medical Microinstruments.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Inga S. Besmens https://orcid.org/0000-0002-3821-7163
Nicole Lindenblatt https://orcid.org/0000-0003-0293-1004
References
- 1.Innocenti M. Back to the future: robotic microsurgery. Arch Plast Surg. 2022;49(03):287-288. doi: 10.1055/s-0042-1748020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao AS, Sandberg LJM. Incorporating robotics into a plastic surgery practice. In: Selber JC, ed. Robotics in Plastic and Reconstructive Surgery. Berlin, Germany: Springer International Publishing; 2021:113-124. doi: 10.1007/978-3-030-74244-7_10. [DOI] [Google Scholar]
- 3.Saleh DB, Syed M, Kulendren D, Ramakrishnan V, Liverneaux PA. Plastic and reconstructive robotic microsurgery--a review of current practices. Ann Chir Plast Esthet. 2015;60(4):305-312. doi: 10.1016/j.anplas.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Nectoux E, Taleb C, Liverneaux P. Nerve repair in telemicrosurgery: an experimental study. J Reconstr Microsurg. 2009;25(04):261-265. doi: 10.1055/s-0028-1104562. [DOI] [PubMed] [Google Scholar]
- 5.MUSA . Developing robots for microsurgery. https://microsure.nl/musa/. Accessed September 3, 2022.
- 6.MMI . Mmi - Medical Micro Instruments. Medical Microinstruments (MMI). https://www.mmimicro.com. Accessed September 3, 2022. [Google Scholar]
- 7.Ballestín A, Malzone G, Menichini G, Lucattelli E, Innocenti M. New robotic system with wristed microinstruments allows precise reconstructive microsurgery: preclinical study. Ann Surg Oncol. 2022;29:7859-7867. doi: 10.1245/s10434-022-12033-x. [DOI] [PubMed] [Google Scholar]
- 8.Lindenblatt N, Grünherz L, Wang A, et al. Early experience using a new robotic microsurgical system for lymphatic surgery. Plast Reconstr Surg Glob Open. 2022;10(1):e4013. doi: 10.1097/GOX.0000000000004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbon C, Grünherz L, Uyulmaz S, Giovanoli P, Lindenblatt N. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. 2022;34:126-133. doi: 10.1016/j.jpra.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MMI . Symani System Overview. Medical Microinstruments (MMI). Accessed September 14, 2022. https://www.mmimicro.com/symani-system-overview [Google Scholar]
- 11.Qiu CS, Hanwright PJ, Khavanin N, Tuffaha SH. Functional reconstruction of lower extremity nerve injuries. Plast Aesthet Res. 2022;9(3):19. doi: 10.20517/2347-9264.2021.126. [DOI] [Google Scholar]
- 12.Broer PN, Moellhoff N, Mayer JM, Heidekrueger PI, Ninkovic M, Ehrl D. Comparison of outcomes of end-to-end versus end-to-side anastomoses in lower extremity free flap reconstructions. J Reconstr Microsurg. 2020;36(6):432-437. [DOI] [PubMed] [Google Scholar]
- 13.Sabapathy SR, Venkatramani H, Bhardwaj P. Reconstruction of the thumb amputation at the carpometacarpal joint level by groin flap and second toe transfer. Injury. 2013;44(3):370-375. [DOI] [PubMed] [Google Scholar]
- 14.Teichmann H, Innocenti M. Development of a new robotic platform for microsurgery. In: Selber JC, ed. Robotics in Plastic and Reconstructive Surgery. Berlin, Germany: Springer International Publishing; 2021:127-137. doi: 10.1007/978-3-030-74244-7_11. [DOI] [Google Scholar]
- 15.Hvolbek AP, Nilsson PM, Sanguedolce F, Lund L. A prospective study of the effect of video games on robotic surgery skills using the high-fidelity virtual reality robotiX simulator. Adv Med Educ Pract. 2019;10:627-634. doi: 10.2147/AMEP.S199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohil D, Mohammed N, Mahadevan A, Pruthi N. Histopathology of microvascular anastomosis—comparison of patent and nonpatent anastomosis: an experimental study. Indian J Neurosurg. 2022;11(01):007-012. doi: 10.1055/s-0040-1719237. [DOI] [Google Scholar]
- 17.Khachatryan A, Tevosyan A, Novoselskiy D, Arakelyan G, Yushkevich A, Nazaretovich Nazarian D. Microsurgical suture technique: nerve coaptation. In: Microsurgery Manual for Medical Students and Residents. Berlin, Germany: Springer International Publishing; 2021:49-55. doi: 10.1007/978-3-030-73531-9_6. [DOI] [Google Scholar]
- 18.Dhaliwal G, Mukherjee D. Peripheral arterial disease: epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16(02):36-44. doi: 10.1055/s-0031-1278244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandaglio-Collados D, Marín F, Rivera-Caravaca JM. Peripheral artery disease: update on etiology, pathophysiology, diagnosis and treatment. Med Clin. 2023;161(8):344-350. doi: 10.1016/j.medcli.2023.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1.