Abstract
The Impella 5.5® (Abiomed, Danvers, MA, USA) is a microaxial flow pump that promotes left ventricular unloading and improves end-organ perfusion before durable left ventricular assist device (LVAD) implantation. Thrombus formation after Impella 5.5 insertion can occur and represents a significant challenge to device explantation. Durable LVAD implantation is typically performed without aortic cross-clamping, so a dislodged thrombus can potentially embolize and lead to catastrophic events. We describe our technique to safely explant an Impella 5.5 in patients who develop a thrombus on the inflow portion of the device before surgical LVAD implantation.
Keywords: heart failure, mechanical circulatory support, Impella, thrombus, left ventricular assist device
Visual abstract.
Central Message.
Our technique highlights a practical solution to minimize the risk of embolization in patients with left ventricular thrombus at the time of switching from Impella to durable LVAD support.
Introduction
The Impella 5.5® device (Abiomed, Danvers, MA, USA) is a microaxial pump that shunts blood from the left ventricle (LV) to the aorta; it improves LV unloading in patients with cardiogenic shock. 1 In the United States, the use of Impella devices is increasing for multiple indications.2,3 An LV thrombus is a contraindication for inserting any Impella device. 4 However, thrombus formation can occur after Impella support is initiated by a combination of profound impairment of LV contractility, a poor flow state at the time of Impella 5.5 insertion, turbulent flow in the LV, or the thrombogenic nature of the device. 5 A patient with an LV and/or inflow site thrombus presents a challenging dilemma, as the clot could lead to systemic emboli at the time of device explantation.
The removal of Impella devices is usually done by pulling the device out of the LV through the original insertion site (typically the axillary artery), followed by vascular repair of the access site. Device removal in the presence of an LV or inflow site thrombus could result in systemic embolization. 6 There is little information regarding the surgical approach to managing a thrombus at the time of durable left ventricular assist device (LVAD) implantation. We describe our step-by-step technique of how to safely explant the Impella 5.5 in the presence of an inflow site thrombus in patients undergoing definitive surgical LVAD implantation.
Surgical Technique
The patient in this report is a 62-year-old man with chronic systolic heart failure and a dilated ventricle with an end-diastolic diameter of 6.7 cm. He was transferred to our center with acute decompensation requiring dual inotropic support. In addition, he was deconditioned with impaired nutrition. The decision was to proceed with the implantation of the Impella 5.5 as a bridge to durable LVAD. The patient received Impella 5.5 support for 16 days. He was on systemic heparin with a partial thromboplastin time goal of 60 to 70 s; there was no evidence of hemolysis.
The patient was taken to the operating room, placed in a supine position, and placed under general anesthesia. A transesophageal echocardiogram (TEE) probe was inserted before surgical incision to check the Impella device position, assess the presence of a thrombus, and determine the exact location and configuration of the clot (Fig. 1). A conventional median sternotomy was completed, and systemic heparin was given. Cannulas for cardiopulmonary bypass (CPB) support were placed in standard fashion. CPB was initiated, and the Impella flow was decreased. The CPB flow was increased while the Impella was turned off to avoid ventricular ejection. The padded aorta cross-clamp was placed across the aorta and the Impella catheter, and the heart was arrested using cardioplegia (Fig. 2).
Fig. 1.
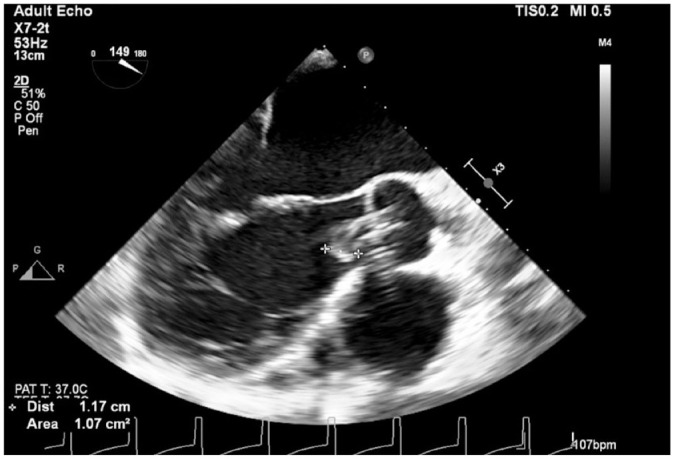
Transesophageal echocardiogram (long-axis aortic view) showing the Impella cannula (Abiomed, Danvers, MA, USA) in the left ventricle with a 1 cm clot on the cannula toward the inflow portion.
Fig. 2.
Cardiac diastolic arrest using paddle cross-clamp and antegrade cardioplegia needle.
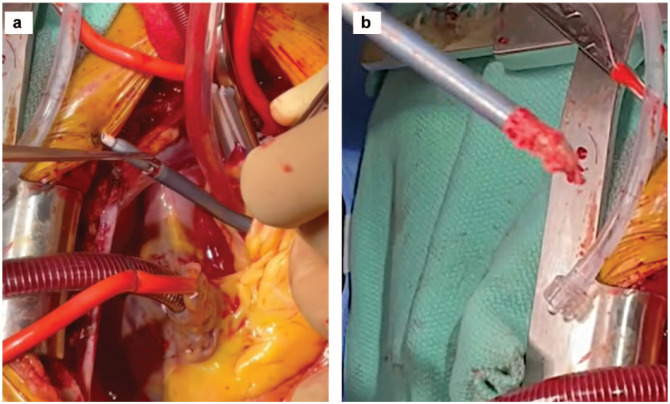
The heart was gently lifted, and a HeartMate 3 coring knife (Abbott Laboratories, Chicago, IL, USA) was used to perform a full-thickness apical left ventriculotomy (Fig. 3). A vertical aortotomy was made at the planned site for outflow graft anastomosis (Fig. 4). The Impella catheter was identified and transected using a wire cutter. The Impella was carefully pulled out from the aorta (Fig. 5).
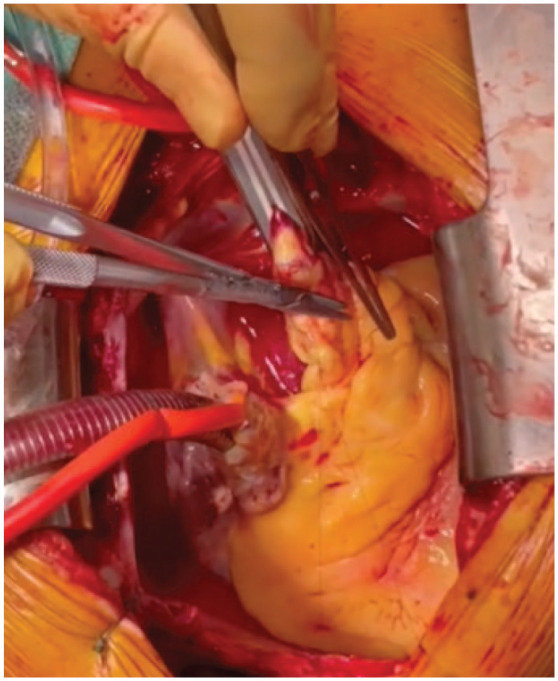
Fig. 3.

Left ventriculostomy with coring knife before suturing a sewing ring to allow better ventricular inspection.
Fig. 4.

Aortotomy at the planned aorta-outflow graft anastomosis to expose the Impella (Abiomed, Danvers, MA, USA).
Fig. 5.
(a) Transaortic Impella (Abiomed, Danvers, MA, USA) transection and transaortic removal. (b) Impella removal with clot.
The LV was inspected through the left ventriculotomy and freed from residual clots. The aortotomy was temporarily closed using a nonabsorbable monofilament suture in a running fashion (Fig. 6). After making sure no thrombi were seen in the LV cavity, the cross-clamp was released (Fig. 7); the rest of the operation was done on a beating heart to minimize the total ischemic time and right ventricular dysfunction.
Fig. 6.
Temporary closure of aortotomy site and cross-clamp removal.
Fig. 7.
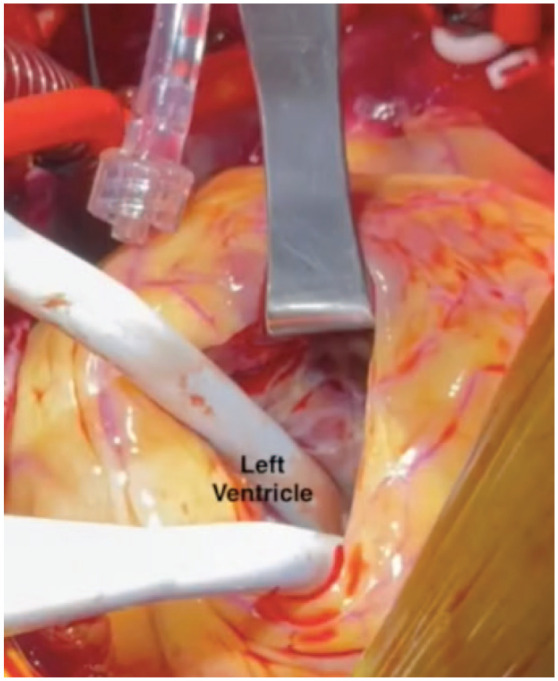
Left ventricular endocardial examination for residual clot through ventriculostomy.
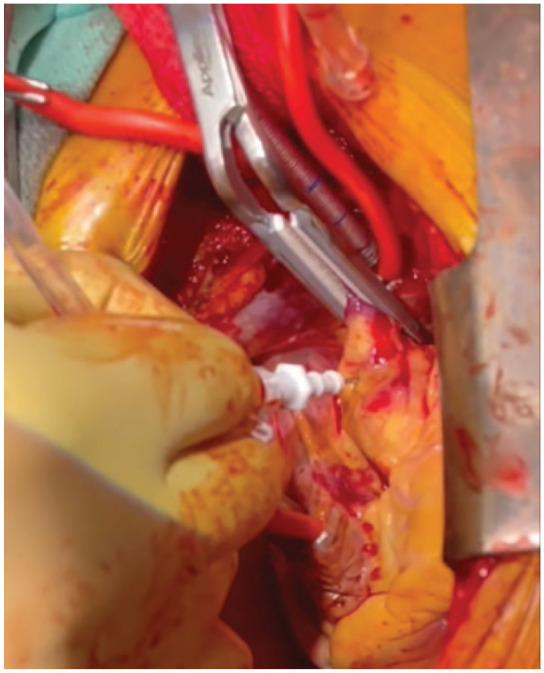
The sewing ring was sutured to the apex of the LV, the pump was secured to the sewing ring (Fig. 8), and the LV was de-aired in standard fashion. 7 The side-biting clamp was placed under the previously sutured aortotomy. The suture was removed, and an anastomosis to the outflow graft was completed using a running Prolene suture. The rest of the operation was performed in standard fashion.
Fig. 8.
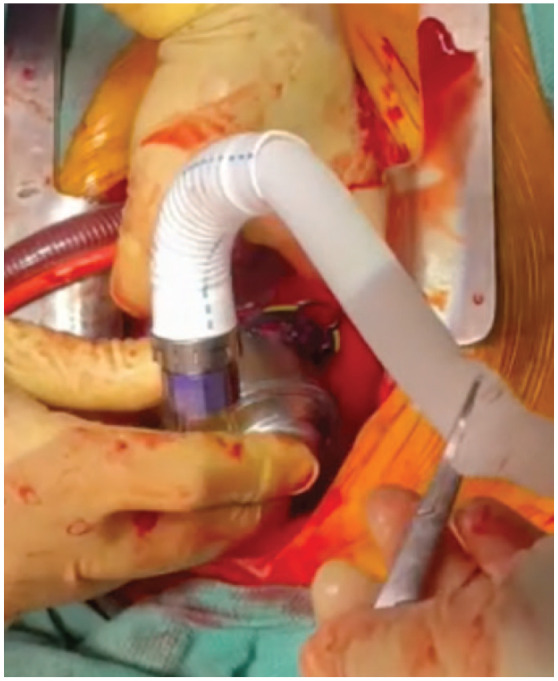
Placement of the sewing ring and inflow portion.
Discussion
Intraoperative TEE or transthoracic echocardiogram are valuable tools for detecting LV and Impella inflow thrombi during durable LVAD implantation. 8 When an LV or inflow site thrombus is visualized before the insertion of a durable LVAD, the evacuation of the entire clot should be performed via a ventriculotomy either with a beating or arrested heart.7,9
In patients with Impella 5.5 support as a bridge to durable LVAD, the device is usually stopped before CPB initiation, and the device is retracted into the insertion sheath. However, this sequence cannot be applied if there is a thrombus in the inflow portion of the device due to the risk of clot dislodgement and systemic embolization. Minimal manipulation of the heart before clot evacuation and early aortic cross-clamp application to decrease the chances of emboli showers are necessary. A padded aortic cross-clamp must be applied to avoid fracturing the shaft of the device and prevent aortic intimal injury. Some clinicians report not using an aortic cross-clamp, but we view using the cross-clamp in the presence of a thrombus located on the left side chambers (even when attached to a device) as a safety measure to minimize the risk for systemic embolization. Although not considered at the time, we recognize an alternative strategy is to remove the Impella through the ventriculotomy to avoid passing the clot through the aortic valve and detaching into the ventricle.
Right ventricular dysfunction is associated with prolonged aortic cross-clamp time; therefore, the aortic cross-clamp should be released as soon as the inflow site thrombus and Impella device have been removed. 10 Thus, closing the aortotomy after Impella explantation to reuse it later will allow for cross-clamp removal and avoid major bleeding. Coring the LV before suturing the sewing ring provides better visualization of the ventricle and enables assessment for additional thrombi. Once the area is cleared of thrombi, the cross-clamp is released. The outflow graft is appropriately trimmed. The side-bite aortic clamp is placed under the temporarily closed aortotomy so that an end-to-side anastomosis between the outflow graft and the ascending aorta can be completed.
Conclusions
With the increasing use of Impella devices, our technique highlights a practical solution to minimize the risk of embolization in patients with an LV or inflow site thrombus at the time of switching from Impella to durable LVAD support.
Acknowledgments
The authors acknowledge R. Michelle Sauer, PhD, ELS, and Jessica Moody, PhD, for their editorial and manuscript submission assistance.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IAS is a consultant for Abiomed, Inc. (Danvers, MA, USA).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement: Patient consent was attained for the images used in the article.
ORCID iD: Igor D. Gregoric  https://orcid.org/0000-0003-2290-2964
https://orcid.org/0000-0003-2290-2964
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino America. J Am Coll Cardiol 2015; 65: e7–e26. [DOI] [PubMed] [Google Scholar]
- 2. Roka-Moiia Y, Li M, Ivich A, et al. Impella 5.5 versus Centrimag: a head-to-head comparison of device hemocompatibility. ASAIO J 2020; 66: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imaoka S, Kainuma S, Toda K, et al. Impella support as a bridge to surgery for severe mitral regurgitation with cardiogenic shock. Circ Rep 2021; 3: 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamarche Y, Cheung A, Ignaszewski A, et al. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg 2011; 142: 60–65. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen D, Ellison D, Ngo C, et al. Intraventricular free-floating thrombus in an Impella-supported patient: damage control in a no-win scenario. JACC Case Rep 2020; 2: 886–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asadourian M, Sharma AV, Kiel R, et al. Removal of Impella in the setting of left ventricular thrombus: a potential indication for cerebral embolic protection devices. VAD J 2021; 7: e2021714. [Google Scholar]
- 7. Salas De, Armas IA, Patel JA, Akay MH, et al. Off-pump continuous-flow left ventricular assist device implantation. Texas Hear Inst J 2021; 48: 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gregoric ID, Poredos P, Jezovnik MK, et al. Use of transthoracic echocardiogram to detect left ventricular thrombi. Ann Thorac Surg 2021; 111: 556–560. [DOI] [PubMed] [Google Scholar]
- 9. Whitson BA. Surgical implant techniques of left ventricular assist devices: an overview of acute and durable devices. J Thorac Dis 2015; 7: 2097–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin A. The cardiovascular effects of aortic clamping and unclamping. South African J Anaesth Analg 2010; 16: 62–71. [Google Scholar]