Abstract
Atrial fibrillation (AF) is a common condition with a global estimated prevalence of 60 million cases, and the most common cardiac complication of hyperthyroidism, occurring in 5–15% of overtly hyperthyroid patients. Additionally, subclinical hyperthyroidism and high-normal free T4 have been associated with an increased risk in the development of AF. Hyperthyroidism-related AF is a reversible cause of AF, and the majority of patients spontaneously revert to sinus rhythm in 4–6 months during or after restoration of euthyroidism. Therefore, restoring thyroid function is an indispensable element in hyperthyroidism-related AF management. Rate control with beta-blockers consists another first-line therapy, reserving rhythm control in cases of persistent hyperthyroidism-related AF. It is still controversial whether hyperthyroidism is an independent risk factor of stroke in nonvalvular AF. As a result, initiating anticoagulation should be guided by the clinical thromboembolic risk score CHA2DS2-VASc score in the same way it is applied in patients with non-hyperthyroidism-related AF. Treatment with the novel direct oral anticoagulants appears to be as beneficial and may be safer than warfarin in patients with hyperthyroidism-related AF. In this review, we address the epidemiology, prognosis, and diagnosis of hyperthyroidism-related AF, and we discuss the management strategies and controversies in patients with hyperthyroidism-related AF.
Keywords: hyperthyroidism, thyrotoxicosis, atrial fibrillation, stroke, anticoagulation
Introduction
Atrial fibrillation (AF) is the most common sustained heart rhythm disorder, becoming a worldwide public health problem due to its increased incidence and prevalence, healthcare costs, morbidity, and mortality. The absolute global prevalence of AF has more than doubled between 1990 and 2019, from worldwide estimates of 28 million AF cases in 1990 to 60 million cases in 2019 (1). AF patients have a significantly increased risk of serious complications and death (risk of stroke, heart failure, and death four to five times, three times, up to two times compared with people without AF, respectively) (2). The most important risk factors for AF are increasing age and male sex. Smoking, obesity, diabetes, hypertension, myocardial infarction, heart failure, and stroke are other common risk factors for AF (3). Today, the increased knowledge of the genetic causes of AF has revealed around 140 genetic loci associated with AF (4).
Hyperthyroidism is also a common condition with an estimated global prevalence of 0.2–1.3% in its overt form (i.e. elevated thyroxine (T4), triiodothyronine (T3), or both) in iodine-sufficient populations, and an even higher prevalence in iodine-deficient areas (5). The thyroid hormone excess impacts cardiac energy homeostasis, cardiac function, cardiovascular hemodynamics, and the cardiac electrical conduction system (6). Of the clinical effects of hyperthyroidism with respect to cardiac complications, sinus tachycardia, and atrial fibrillation are the most frequent.
In this review, we aim to provide an overview of the latest relevant literature on the association between hyperthyroidism and AF and to discuss the management of hyperthyroidism-related AF (Box 1).
Box 1 Search strategy and selection criteria.
A comprehensive systematic review was beyond the scope of this paper. We searched MEDLINE from inception to December 1, 2023, for articles published in English using the terms ‘hyperthyroidism’, ‘thyrotoxicosis’, ‘Graves’ Disease’, ‘toxic adenoma’, ‘toxic multinodular goiter’ in combination with ‘atrial fibrillation’ or ‘arrhythmia’. We also search the European Society of Cardiology, European Heart Rhythm Association, American Heart Association, European Thyroid Association and American Thyroid Association for publication of relevant guidelines. The relevant references cited in the resultant articles were also reviewed and selected.
Epidemiology
The bidirectional association between AF and hyperthyroidism
The connection between an enlarged thyroid gland and palpitations was already described over 200 years ago, and the presence of auricular fibrillation was a common electrocardiographic finding in hyperthyroid patients over 100 years ago (7). A review of published literature from 1916 to 1943 showed that the prevalence of AF in hyperthyroid patients ranged from 4.7% to 25% in various studies with a number of patients ranging between 22 and 2400 (8). More recent studies estimated that AF occurs in 5–15% of the hyperthyroid patients, depending on the study population, compared with the 1–3% in the general population (9, 10). According to data from two Danish population registry-based studies, AF or atrial flutter occurred in 8.3% of more than 40,000 hospitalized hyperthyroid patients within ±30 days from the diagnosis of hyperthyroidism (11) and in 4.6% of 3966 hyperthyroid patients followed for a period of 5.5 years in comparison with the 2.9% of the 586,460 euthyroid subjects (12). Two recent studies from the UK and the USA demonstrated that 5.7% and 10.1% of the 4189 and 1371 Graves’ disease patients, respectively, developed AF during a long-term follow-up (13, 14). The British study suggests that Graves’ disease patients have over twice the risk of incident AF compared with population controls (13). Other retrospective and smaller studies reported the prevalence of AF in hyperthyroidism (15, 16, 17, 18) (Table 1).
Table 1.
Prevalence and risk of AF in overt and subclinical hyperthyroidism and FT4 within the normal reference range.
| Study | Year | Country | Design | Subjects, n | Age, years | FU, years | Thyroid function | AF, % | Risk (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Overt hyperthyroidism | |||||||||
| Selmer et al. (12) | 2012 | Denmark | CS | 586,460 | 5.5 | ||||
| Euthyroid | 562,461 | 2.9% | IRR 1.41 (1.22–1.63)a | ||||||
| oHT | 3966 | 4.6% | |||||||
| Frost et al. (11) | 2004 | Denmark | CS | 40,628 | 30 days | oHT | 8.3% | ||
| Naser et al. (14) | 2023 | USA | RS | 1371 | 3.3 | GD | 10.1%b | ||
| Okosieme et al. (13) | 2019 | UK | RS | 20,945 | 0.5–16.8 | ||||
| Controls | 16,756 | 2.3% | HR 2.67 (2.25–3.15)c | ||||||
| GD | 4189 | 5.7% | |||||||
| Auer et al. (17) | 2001 | Austria | RS | 23,638 | – | ||||
| Euthyroid | 22,300 | 2.3% | |||||||
| oHT | 725 | Elderly | 13.8% | RR 5.8 (2.7–8.5) | |||||
| Osman et al. (38) | 2007 | UK | P/CCS | 786 | 35–63 | – | |||
| Euthyroid | 393 | 7.3%d | |||||||
| HT | 393 | ||||||||
| Mohasci et al. (16) | 1990 | Hungary | 219e | 17–84 | HT | 16.4% | |||
| 50–84 years | 35% | ||||||||
| 17–49 years | 1.6% | ||||||||
| Iwasaki et al. (15) | 1989 | Japan | RS | 92 | 7–71 | GD | 21% | ||
| >40 years | 31% | ||||||||
| <40 years | 0% | ||||||||
| Wong et al. (18) | 2017 | Hong Kong | RS | 1918 | 28–90 | HT | 6.9% | ||
| Subclinical hyperthyroidism | |||||||||
| Selmer et al. (12) | 2012 | Denmark | CS | 586,460 | 5.5 | ||||
| Euthyroid | 562,461 | 2.9% | |||||||
| sHT | 6276 | 6.9% | IRR 1.30 (1.18–1.43)a | ||||||
| Collet et al. (27) | MA | 52,674 | 8.8 | ||||||
| Euthyroid | 7901 | - | |||||||
| sHT | 810 | 4.2% | HR 1.68 (1.16–2.43)f | ||||||
| Cappola et al. (26) | 2006 | USA | CS | 3233 | 13 | ||||
| Euthyroid | 2639 | 5.2% | |||||||
| sHT | 47 | ≥65 | 8.5% | HR 1.98 (1.29–3.03)f | |||||
| Auer et al. (17) | 2001 | Austria | RS | 23,638 | – | ||||
| Euthyroid | 22,300 | 2.3% | |||||||
| sHT | 613 | Elderly | 12.7% | RR 5.2 (2.1–8.7) | |||||
| Gammage et al. (31) | 2007 | UK | CS | 5860 | – | ||||
| Euthyroid | 5519 | 4.7% | |||||||
| sHT | 126 | ≥65 | 9.5% | OR 1.87 (1.01–3.57)g | |||||
| Reduced TSH levels | |||||||||
| Selmer et al. (12) | 2012 | Denmark | CS | 586,460 | 5.5 | ||||
| Reduced TSH, mIU/L | |||||||||
| 0.2–0.4 | IRR 1.12 (1.03–1.21)a | ||||||||
| 0.1–0.2 | – | IRR 1.16 (0.99–1.36)a | |||||||
| <0.1 | – | IRR 1.41 (1.35–1.89)a | |||||||
| Sawin et al. (25) | 1994 | USA | CS | 2007 | >60 | 10 | Euthyroid | ||
| Euthyroid | 1576 | 8.4% | |||||||
| Reduced TSH, mIU/L | |||||||||
| 0.1–0.4 | 187 | 12.2% | RR 1.6 (1.0–2.5)h | ||||||
| <0.1 | 61 | 21.3% | RR 3.8 (1.7–8.3)h | ||||||
| Collet et al. (27) | MA | 52,674 | 8.8 | HR 1.63 (1.10–2.41)f | |||||
| TSH, mIU/L | |||||||||
| 0.1–0.44 | |||||||||
| <0.1 mU/L | HR 2.54 (1.08–5.99)f | ||||||||
| High-normal FT4 | |||||||||
| Chaker et al. (28) | 2015 | Netherlands | LS | 9166 | 6.8 | ||||
| FT4, quartiles | |||||||||
| First (lowest) | 3.4% | 1 | |||||||
| Second | ≥45 | 4.5% | HR 1.22 (0.88–1.70)a | ||||||
| Third | 4.3% | HR 1.18 (0.85–1.63)a | |||||||
| Fourth (highest) | 5.8% | HR 1.56 (1.14–2.12) a | |||||||
| Cappola et al. (26) | 2015 | USA | CS | 2843 | 17 | 30.3% | HR 1.06 (1.03–1.10)i | ||
| 2673j | ≥65 | Risk of FT4 within the ref range | |||||||
| Gammage et al. (31) | 2007 | UK | CS | 5860 | – | FT4 | – | OR 1.08 (1.02–1.15)k | |
| ≥65 | (per 1 pmol/L fT4 change) | ||||||||
| Baumgartner et al. (30) | 2017 | MA | 20,921 | ||||||
| FT4, quartiles | |||||||||
| First (lowest) | 6.6% | 1 | |||||||
| Second | 7.8% | HR 1.17 (1.02–1.35)f | |||||||
| Third | 8.3% | HR 1.25 (1.09–1.43)f | |||||||
| Fourth (highest) | 9.4% | HR 1.45 (1.26–1.66)f |
aAdjusted for age, sex, race, calendar year, Charlson comorbidity index, and socioeconomic status; b77% within/23% beyond 90 days from GD diagnosis; cAdjusted for age, sex, and comorbidity; d5.3% newly diagnosed AF; e94% females; fAdjusted for age and sex; gAdjusted for male, age >70, diabetes mellitus, heart failure, and hypertension; hAdjusted for age, sex, smoking, diabetes mellitus, hypertension, left ventricular hypertrophy, myocardial infarction, congestive heart failure, and cardiac murmur; iAdjusted for age, sex, race, and initiation of thyroid hormone replacement; jNormal fT4; kEuthyroid subjects TSH 0.4–4 mIU/L; lAdjusted for age, sex, cardiovascular disease, thyroid medication use, atrial size, systolic blood pressure, fasting glucose. valvular heart disease, b-blockers, and diuretics use.
FT4, free thyroxine; GD, Graves’ disease; HR, hazard ratio; HT, hyperthyroidism; IRR, incidence rate ratio; oHT, overt hyperthyroidism; OR, odds ratio; RR, relative risk; sHT, subclinical hyperthyroidism; TSH, thyroid-stimulating hormone; y, years; CS, cohort study; RS, retrospective study; P/CCS, prospective/case–control study; MA, meta-analysis; LS, longitudinal study.
The risk factors contributing to AF in hyperthyroid patients do not differ from those in the general population. The age-dependent distribution of AF in the general population has also been observed in hyperthyroidism-related AF (11, 12, 14, 16), with the adjusted odds ratio for hyperthyroidism-related AF increasing by 1.7 per 10-year increment in age (11). However, the relative risk of AF is highest among the younger and lower in elderly hyperthyroid subjects, most likely due to the cardiovascular risk factors (hypertension, heart failure, myocardial infarction, valvular heart disease, diabetes) that contribute to the AF risk, which increase with advancing age (12). In addition, men had double the odds than women (14), and the odds ratios for hyperthyroidism-related AF were 1.8, 3.9, and 2.6 in the presence of ischemic heart disease, congestive heart failure, or heart valve disease, respectively (11). Finally, patients with toxic multinodular Goiter have a higher risk of persistent AF compared to those with Graves’ disease, as toxic multinodular Goiter tends to occur at more advanced ages and to develop long-term subclinical hyperthyroidism, conditions that are associated with a higher frequency of cardiovascular risk factors, cardiac disease, and other comorbidities (19).
The incidence of thyroid disease in patients with new-onset AF has been studied as well. Lower than normal TSH levels have been found to be present in 4.8% of the 707 patients with new-onset AF from the Canadian Registry of Atrial Fibrillation (0.7% having a TSH < 0.1 mU/L) and in 3.1% of the patients with new-onset AF in a large Danish registry-based study (0.8% having overt disease or TSH < 0.02 mIU/L) (20, 21). A higher frequency of low TSH was reported in patients presenting to the Emergency Department with new-onset AF (10.8% of whom 6.2% with TSH <0.1 mU/L) (22). In one series, 13% of patients with AF and no obvious cardiovascular cause were found to have hyperthyroidism (23). In addition, the Danish study showed that new-onset AF doubled the risk of later occurrence of hyperthyroidism during the study period (mean 3.53 years, up to 13 years) in comparison with the general population, suggesting that evaluation of thyroid function may well be repeated at a later time following the AF diagnosis (21). These results must be interpreted with caution as detection bias (thyroid function is evaluated more often in patients with AF) and protopathic bias (all amiodarone treated patients may not have been identified and excluded from the analysis) are likely. Additionally, there is not a clear underlying pathophysiological mechanism of how AF may cause hyperthyroidism. In any case, because hyperthyroidism is a modifiable risk factor for AF, thyroid screening is mandatory in the diagnostic assessment of AF (24).
Subclinical hyperthyroidism and high-normal free T4 and atrial fibrillation
Previous studies have established the association between subclinical hyperthyroidism (defined as low TSH in the setting of a normal free T4 (FT4) and free T3 (FT3) concentration) and AF (Table 1). The risk of AF in patients with subclinical hyperthyroidism over the age of 60 has been reported to be 2.8-fold higher compared to euthyroid patients (25). Participants from the Framingham Heart Study older than 60 years with low TSH, including those taking thyroxine, had an increased risk of incident AF compared to euthyroid participants (RR: 3.8; 95% CI: 1.7–8.3 when TSH ≤0.1 mIU/L and RR: 1.6; 95% CI: 1.0–2.5, when TSH 0.1–0.4 mIU/L) (25). Similar results were found in the Cardiovascular Health Study in individuals aged ≥65 years with endogenous subclinical hyperthyroidism (adjusted HR: 1.98; 95% CI: 1.29–3.03, for those with TSH 0.1–0.44 mU/L: 1.85 (95% CI: 1.14–3.00) (26). According to a meta-analysis of individual data on 8711 participants from five cohorts with different definitions of AF, endogenous subclinical hyperthyroidism was associated with increased AF events (HR: 1.68; 95% CI: 1.16–2.43), and incident AF was significantly greater in participants with lower TSH levels (HR: 2.54; 95% CI: 1.08–5.99) (27). A Danish registry-based study showed that not only overt hyperthyroidism but also subclinical hyperthyroidism has an increased incidence among patients with new-onset AF (3% of patients with new-onset AF developed subclinical hyperthyroidism compared to 1% in the general population) (21).
The association between thyroid function within the normal range and AF has also been investigated (Table 1). The Rotterdam study found that higher FT4 levels within the normal range are associated with an increased risk of AF (risk of AF in the highest quartile vs lowest quartile of FT4: HR adjusted for age and sex: 1.56; 95% CI: 1.14–2.12). This association was stronger among subjects younger than 65 years of age (HR: 2.23; 95% CI: 1.18–4.22 and 1.45; 95% CI: 1.01–2.08 in <65 and ≥65 years of age, respectively) (28). A population-based study of participants older than 65 years found an increased risk of AF with higher FT4 levels within the normal range (29). An analysis of individual-level data from 11 prospective cohort studies in over 28,000 euthyroid participants (mean age: 64.4 years; 50.8% women) followed the findings of the two previous studies. Eight percent of the 21,000 included in the analysis of the association between FT4 and AF developed AF and a gradually increased risk among euthyroid individuals with higher serum FT4 concentrations was found. FT4 levels in the fourth quartile of the reference range was associated with a 1.45-fold increased risk of incident AF in comparison with the first (lowest) quartile of FT4 levels (P for trend <0.001 across all quartiles) (30). Both the latter and the Rotterdam study failed to find an association between serum TSH concentrations within the reference range and incident AF (28, 30). Finally, a population-based study revealed higher FT4 concentrations in the 260 euthyroid subjects with AF compared with the 5259 euthyroid subjects without AF. A difference in serum FT4 concentration of 1 pmol/L (0.08 ng/dL) was associated with an odds ratio for the finding of AF of 1.08 (95% CI: 1.02–1.15). The larger the difference in FT4 concentrations, the larger the odds ratio for the finding of AF (differences in free T4 concentrations of 2.5 pmol/L, 5.0 pmol/L, and 10.0 pmol/L were associated with 1.21, 1.47, and 2.17 OR, respectively) (31).
The association between AF and high-normal FT4 levels needs further clarification, especially in the setting of thyroid hormone replacement therapy and TSH suppression therapy in patients with thyroid cancer (32). In addition, patients with resistance to thyroid hormone due to variants in the thyroid hormone receptor beta gene (resistance to thyroid hormone β – RTHβ) are more susceptible to AF due to lifelong exposure to thyroid hormones. Cardiomyocytes predominately express thyroid receptor alpha, and therefore patients with RTHβ preserve sensitivity in cardiac tissue. A retrospective cohort study from the UK found that 55 individuals with RTHβ were more susceptible to AF (HR: 10.56; 95% CI: 4.72–23.6) than the 2750 controls (33). According to the Reference Centre for Rare Thyroid Diseases in France, AF was present in 20% of 302 RTHβ patients (34). The much lower median age of the development of cardiac complications in patients with RTHβ highlights the importance of early screening, regular electrocardiographic follow-ups, and symptom management with beta-blockers in these patients. Finally, Mendelian Randomization studies found that genetically predicted decreased TSH levels and increased FT3:FT4 ratios were associated with AF (35, 36). The same was not true for genetically predicted increased FT4, as the polygenic FT4 risk score was not associated with AF (OR: 1.01; 95% CI: 0.89–1.14), suggesting that circulating FT4 levels are not directly involved in the development of AF (35). More powered Mendelian Randomization studies are expected in the future with the identification of novel genetic variants associated with TSH and FT4 levels to help enlighten the causality between thyroid function and AF.
Prognosis of hyperthyroidism-related AF
Most studies associated AF with hyperthyroidism when AF occurred within 30 days before or after the diagnosis of hyperthyroidism (11). However, a recent retrospective study in 1371 patients with Graves’ disease followed for up to 10 years showed that 77% developed AF within 90 days of Graves’ disease diagnosis. Of these, 71.2% developed AF by 10 days after Graves’ disease diagnosis, and the remaining 23% developed AF more than 90 days after the Graves’ disease diagnosis, of which 50% had attained euthyroidism at the time of AF diagnosis (14). The observed incidence of ‘late-onset AF’ was higher in patients with Graves’ disease than expected in the general population, although it did not achieve statistical significance (P = 0.06).
Control of hyperthyroidism results in a spontaneous return to sinus rhythm in approximately two-thirds of patients. Full restoration of normal thyroid status is not always required for conversion to sinus rhythm. In a study from the 1980s, 62% of the hyperthyroidism-related AF patients spontaneously reverted to sinus rhythm in a period of 4 months, of whom >40% reverted prior to or at the same week of restoration of euthyroidism and the remainders after the achievement of the euthyroid status (37). Similarly, in a recent study, two-thirds of the hyperthyroidism-related AF patients followed for a median period of almost 4 years had a spontaneous conversion to sinus rhythm. Of those, 66% had spontaneous sinus conversion before attaining euthyroidism, another 30% did so within 6 months of having achieved a euthyroid state, and the remaining reversals were observed within a maximum of 1 year after achieving euthyroidism (18). However, the rate of spontaneous conversion to sinus rhythm was lower (34.6%) in the study of the Graves’ disease-related AF, in which patients were followed for a longer time and assessed for both AF and Graves’ disease recurrence (14). Age, duration of hyperthyroidism, pre-treatment duration of AF, and the presence of heart disease have been reported as the main determinants of reversion of AF (14, 38, 39).
Clinical presentation/diagnosis
AF is the most common cardiac complication in patients with hyperthyroidism and may be the initial presentation of hyperthyroidism. Thus, routine laboratory assessment of thyroid function is recommended in all patients with new-onset AF (24, 40). The clinical presentation of AF varies depending on individual characteristics, such as age, sex, and comorbidities (5, 19). Typically, patients older than 60 years are oligo- or asymptomatic but are at a higher risk of AF occurrence (5, 41). Notably, rare cases of AF in children with hyperthyroidism and the absence of structural cardiovascular abnormalities have also been reported (42).
Current evidence does not support the existence of specific signs in hyperthyroidism-related AF; however, differences in clinical presentation or disease severity between individuals with hyperthyroidism-related AF and those with hyperthyroidism without AF or with non-hyperthyroidism-related AF may be documented (24). Patients with hyperthyroidism and AF may experience more pronounced symptoms than patients with hyperthyroidism without AF, such as fatigue, dyspnea, or reduced exercise capacity; hence, close monitoring and early intervention are warranted (43). Additionally, a retrospective cohort study incorporating data from a large inpatient care database in the USA highlighted that patients with AF and hyperthyroidism have a lower disease burden than patients with AF alone (44). In fact, patients with AF and hyperthyroidism had lower mortality, median duration, and hospitalization expenses. These favorable outcomes may be attributed to the transient thyroid dysfunction and the subsequent spontaneous conversion to sinus rhythm. The prognosis of hyperthyroidism-related AF has not been studied; however, the recurrence rate of hyperthyroidism-related AF has been reported to be lower than non-hyperthyroidism-related AF (24, 45). Therefore, it is reasonable to assume that the overall prognosis of patients with hyperthyroidism-related AF is not worse than those with non-hyperthyroidism-related AF when matched for other determinants.
Prognostic models of AF
Early recognition of AF could potentially prevent future thromboembolic events and subsequent mortality (46). However, AF diagnosis can be challenging because some cases may be asymptomatic or paroxysmal (24). Hence, recent European Society of Cardiology (ESC) Guidelines recommend screening for AF based on age (opportunistic screening in patients aged ≥65 years and systematic screening in those aged ≥75 years) (24). However, this screening approach may underestimate the risk of future AF in the general population, as a substantial number of newly diagnosed AF cases (20–50%) occur at a younger age (47, 48). Risk stratification for future AF with reliable and practical prognostic models incorporating traditional AF risk factors may be a reasonable and attractive alternative. However, the majority of the published prognostic models for incident AF in the general population have poor performance or include variables not routinely available. Apart from that, there is a paucity of robust evidence regarding their clinical utility and hence have low clinical uptake (48, 49, 50, 51). Specifically, ESC and the recent 2023 American College of Cardiology/American Heart Association/American College of Clinical Pharmacy/Heart Rhythm Society (ACC/AHA/ACCP/HRS) guidelines do not advocate the use of any published prognostic model for AF risk stratification (24, 52). In contrast, the European Heart Rhythm Association consensus suggests the use of the C2HEST model with low to moderate certainty in evidence (Class 2 recommendation) (53).
Despite their promising performance, the aforementioned prognostic models may not be applicable to patients with hyperthyroidism-related AF. Most of these models were developed for the general population and have not been validated in patients with hyperthyroidism. It should also be considered that the majority of prognostic models have a long prediction horizon (5–10 years), which may be inappropriate in patients with hyperthyroidism as AF manifests in the short term (54). Of the identified prognostic models, only C2HEST (coronary artery disease or chronic obstructive pulmonary disease (1 point each), hypertension (1 point), elderly (age ≥75 years, 2 points), systolic heart failure (2 points), and thyroid disease (hyperthyroidism, 1 point)) includes hyperthyroidism as a predictive factor (55). C2HEST was initially derived from a Chinese general population cohort, including almost 500,000 individuals, and was later validated in multiple settings, demonstrating adequate performance (49, 51, 56, 57). Furthermore, C2HEST predicts 1-year AF and may be applicable to patients with hyperthyroidism. Before implementation in clinical practice, external validation of C2HEST model in patients with hyperthyroidism is of cardinal importance. Of note, a machine-learning model for hyperthyroidism-related AF was developed from a small retrospective cohort, including ten hyperthyroidism-related predictors, such as the duration of hyperthyroidism and the number of relapses (58). Nevertheless, its performance needs evaluation in larger independent databases.
In summary, evidence for prognostic models of hyperthyroidism-related AF is limited. Until new development studies of hyperthyroidism-specific prognostic models or validation studies of already existing ones are conducted, C2HEST may be apt for clinical use in hyperthyroid patients for AF screening.
Management
Over the past decade, there has been a shift toward a holistic and patient-centered approach in the management of patients with AF. Current guidelines support the Atrial Fibrillation Better Care (ABC) pathway, a four-pillar approach based on an integrated care, focusing on ‘A’ Avoid stroke/thromboembolism prevention with anticoagulants drugs, ‘B’ Better rate control with beta-blockers and rhythm control with pharmacological and nonpharmacological interventions, and ‘C’ Cardiovascular risk-factor and comorbidities management (primary and secondary AF prevention) (59, 60). Recent publications have highlighted the potential benefits of this approach on AF patient outcomes and quality of life (61, 62, 63, 64).
General principles – management of hyperthyroidism in hyperthyroid-related AF
Current guidelines and position papers for the management of hyperthyroidism-related AF are based on evidence from studies of low methodological quality (e.g. observational, real-world), as hyperthyroidism was an exclusion criterion in the majority of clinical trials, and their findings may not be extrapolated to this subgroup of AF patients (24, 45, 65). Hence, the level of evidence is low, and the recommendations should be applied with caution. Consequently, the management of hyperthyroidism-related AF should follow, with some exceptions, the same principles as those for the general AF population (in accordance with the ABC pathway).
The first dimension to consider is that hyperthyroidism is one of the modifiable risk factors for AF, and thus, quick control and restoration of euthyroidism is the sine qua non of treatment. As mentioned before, up to two-thirds of patients with hyperthyroidism convert instantly to sinus rhythm after achieving and maintaining normal thyroid function in the long term (18, 37). Appropriate therapeutic options are mainly disease-specific, which in the case of thyrotoxicosis with hyperthyroidism include antithyroid drugs, radioiodine ablation, or thyroidectomy and depend on individual patient characteristics and preferences (5, 66). However, there is limited evidence regarding the optimal TSH targets for patients with hyperthyroidism and AF; a TSH within the age-specific normal reference range seems reasonable. Collaboration between cardiologists and endocrinologists might be required in the management of hyperthyroidism-related AF.
Another consideration is subclinical hyperthyroidism. The management goals of patients with AF and subclinical hyperthyroidism are similar to those of patients with overt hyperthyroidism (67, 68). However, evidence regarding treatment initiation in patients with subclinical hyperthyroidism and AF remains scarce (69). The decision to treat subclinical hyperthyroidism is based primarily on age-, sex-, and comorbidities-related factors (69, 70). As a general rule, early treatment of subclinical hyperthyroidism is recommended in patients aged 65 or older and/or in the presence of cardiovascular or osteoporosis risk factors (69, 70). Younger patients, with persistent TSH levels <0.1 mU/L, may also be candidates for treatment initiation. It is noteworthy that these recommendations are based on observational data (69, 70). In addition, owing to the paucity of robust evidence, it remains unclear whether treatment of subclinical hyperthyroidism actually mitigates the risk of developing AF, leading to a meaningful clinical benefit (69). Hence, large randomized controlled trials are necessary to clarify this question and to provide stronger evidence.
Antithyroid drugs (methimazole (MMI) and propylthiouracil (PTU)) are widely used in the management of hyperthyroidism due to their inhibitory effect on thyroid hormone synthesis (71). MMI is still favored over propylthiouracil because of its better efficacy and safety profile (71). In contrast, PTU is the preferred option in the first trimester of pregnancy and in acute settings, as it has fewer serious teratogenic effects and a more profound reduction in circulating T3 levels (inhibition of peripheral conversion of T4 to T3) (71). Notwithstanding the latter, recent evidence suggests that methimazole may have comparable effectiveness and adverse reactions to PTU in patients with thyroid storm, posing dilemmas regarding the choice of the most appropriate agent (72).
The Atrial Fibrillation Better Care pathway in the management of hyperthyroidism-related AF
The following section describes the management of hyperthyroidism-related AF by adopting the recommended ABC pathway (Fig. 1). As a general rule, most approaches described for the general AF population are also applicable to hyperthyroidism-related AF.
Figure 1.
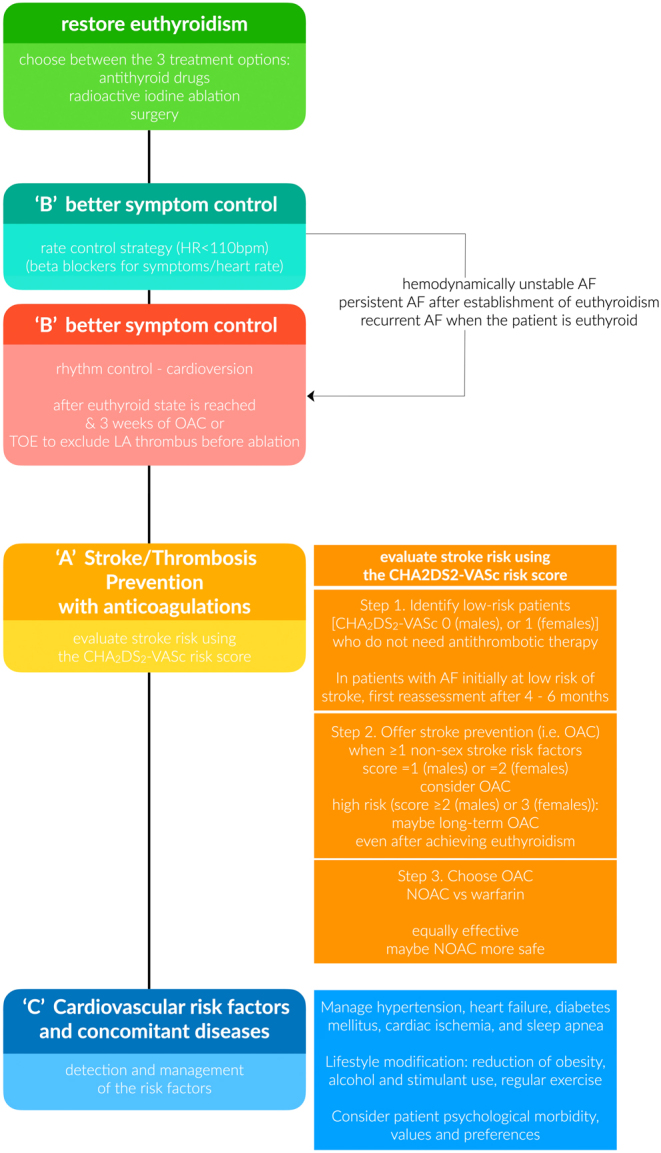
Flowchart of clinical management of patients with new-onset atrial fibrillation and hyperthyroidism. CHA2DS2-VASc score (one point for the presence of congestive heart failure, hypertension, age between 65 and 74 years, female sex, diabetes, and vascular disease, and two points for being older than 75 years and previous stroke). HR, heart rate; OAC, oral anticoagulation; TOE, trans-esophageal echocardiography; LA, left atrium; AF, atrial fibrillation; NOAC, non-vitamin-K-antagonist oral anticoagulants (novel direct oral anticoagulants).
Better symptom control (rate vs rhythm control)
Increasing evidence highlights the central role of rhythm control in the contemporary management of AF (73). Traditionally, rate control has been favored in patients with AF, whereas rhythm control is reserved for cases with refractory AF symptoms (24, 74). Given the current collection of evidence from large contemporary RCTs, early rhythm control with antiarrhythmic drugs or ablation and subsequent restoration of sinus rhythm is associated with improved survival compared to rate control (73, 75). Moreover, multiple studies have shown that rhythm control strategies improve the quality of life in patients with AF (52). However, thus far, the specific correlation between hyperthyroidism-related AF and quality of life has not been studied.
A major difference in the management of hyperthyroidism-related AF is that rhythm control has a secondary role, as hyperthyroidism-related AF is self-limiting in the majority of cases, and it is reserved for cases of persistent AF (24, 45). On the contrary, rate control in combination with antithyroid drugs is the mainstay of initial treatment (66). Beta-blockers are usually required as first-choice options to control the ventricular response (optimal target <110 bpm) and symptoms of hyperthyroidism caused by increased beta-adrenergic tone, and to prevent subsequent cardiomyopathy (76). Nonselective beta-blockers, such as propranolol, are widely used in hyperthyroid patients as they also inhibit the peripheral conversion of T4 to T3 (77, 78). The latter may be of interest in states of acute hyperthyroidism, such as thyroid storm because rapid and aggressive therapies are preferred. Propranolol is a short-acting beta-blocker that should be administered at 10–40 mg three to four times per day, and it is the preferred agent for pregnant women. Its once-daily extended-release formulation can also be used, providing clinically significant sustained beta-adrenoceptor blockade and offering the potential for better compliance (52). In case of contradictions (e.g. asthma and chronic obstructive pulmonary disease), other agents can be used instead (calcium-channel blockers, digoxin) (76).
Hyperthyroidism-related AF usually has a lower recurrence rate than non-hyperthyroidism-related AF, as previously reported (24, 45). Predictors of persistent AF include older age, longer AF duration, and uncontrolled hyperthyroidism (39, 45). In patients with persistent AF for at least 4–6 months after euthyroidism is achieved, rhythm control may be a reasonable option, especially if conversion to sinus rhythm is desired (45, 79). Pharmacological or electrical cardioversion restores sinus rhythm in approximately 90% of the patients with hyperthyroidism-related AF (80). Notably, observational data suggests that sinus rhythm is maintained in the majority of patients even after a 6-year interval (80). Α minimum 4-month duration of euthyroidism and a minimum 3-week treatment of anticoagulation due to the concern of atrial stunning (unless transesophageal echocardiography excludes the presence of thrombus in the left atrium) are prerequisites for successful cardioversion (45). Urgent cardioversion may also be considered in cases of hemodynamic instability. Furthermore, surgical or catheter ablation is also recommended for AF refractory to aggressive rate and rhythm control, as long as normal thyroid function is established and the stimulus to induce AF is no longer present (45). However, the evidence regarding its efficacy is limited and conflicting. Few studies reported that ablation in patients with hyperthyroidism-induced AF is as effective as that in non-hyperthyroidism-related AF, while others suggested that the recurrence rate was higher in patients with hyperthyroidism and AF than those without hyperthyroidism (79, 81, 82, 83, 84, 85). Potential contributors to inconsistency include the small sample size, the observational study design, and the different etiology of hyperthyroidism. The main predictor of AF recurrence after successful cardioversion is long duration of the revious AF.
Class IA, IC, and III antiarrhythmic drugs (e.g. flecainide, propafenone, ibutilide, and vernakalant) are commonly used in clinical practice (24). Nevertheless, definite evidence regarding their efficacy in hyperthyroidism-related AF per se is lacking, as individuals with secondary AF were excluded from the respective clinical trials (45). Amiodarone, an iodine-rich potent antiarrhythmic drug, is commonly used for rhythm control. However, it is also associated with an increased risk of thyroid dysfunction (subclinical and overt hypo/hyperthyroidism) (5, 86). The occurrence of either hypothyroidism or hyperthyroidism usually depends on the underlying thyroid dysfunction and iodine status of an individual (5, 24, 45). Amiodarone-induced thyrotoxicosis is further subdivided into type 1 (underlying autoimmune hyperthyroidism or multinodular goiter) and type 2 (destructive thyroiditis) thyrotoxicosis (5, 24, 45). The development of amiodarone-induced thyrotoxicosis is difficult to predict, and baseline and periodic monitoring of thyroid function is usually warranted (5, 24, 45). Due to the paucity of data, the prescription of amiodarone for hyperthyroidism-related AF is discouraged and should be discontinued if hyperthyroidism develops, if the cardiological status allows (24, 45). In contrast, amiodarone may have a place in the therapeutic armamentarium of thyroid storm, along with antithyroid drugs and beta-blockers (24, 45).
Stroke/thrombosis prevention with anticoagulations
There is still no consensus on the necessity for thrombosis prevention in patients with hyperthyroidism-related AF, as reflected in the current guidelines (24, 45). First, data from epidemiological studies do not provide clear evidence of the association between hyperthyroidism, stroke, and systemic thrombosis. Hyperthyroidism is associated with a hypercoagulable state, as elevated levels of different clotting factors have been reported in individuals with hyperthyroidism (87). Thus, it is reasonable to speculate that hyperthyroidism per se may be implicated in the occurrence of stroke/systemic embolism irrespective of AF. However, a retrospective cohort study failed to find an increased risk of stroke in hyperthyroid subjects (88) and the findings regarding specifically the hyperthyroidism-related AF in the existing literature are inconsistent (Table 2). In fact, three studies demonstrated that hyperthyroidism-related AF is associated with an increased risk of stroke within months since diagnosis compared to AF without hyperthyroidism (89, 90, 91). On the contrary, a prospective observational study from Sweden including nearly 180,000 patients from a large nationwide registry reported that hyperthyroidism was not an independent risk factor for stroke, which was also documented in a more recent, but relatively smaller, retrospective study (92, 93). Similarly, three real-world studies found that patients with hyperthyroidism-related AF had a lower risk of stroke than those with non-hyperthyroidism-related AF (94, 95, 96). These discrepancies may be attributed to the different study design, sample size, and baseline risk of the included cohorts, as well as the different proportions of patients receiving anticoagulation therapy. Another possible explanation may be that, in most studies, TSH was evaluated only at one-time point, which may not have captured the possible resolution of hyperthyroidism during follow-up. Consequently, current clinical guidelines do not incorporate hyperthyroidism as an independent stroke risk factor in nonvalvular AF (24, 45).
Table 2.
Stroke/systemic embolism risk in hyperthyroidism-related AF.
| Study | Design | Subjects, n | Age(years) | HTN/DM/CVD(%) | CHA2DS2VASc(mean) | FU, years | OAC | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | F (%) | Stroke | SSE | Bleeding | |||||||
| Increased risk | |||||||||||
| Siu et al. (79) | PS | 63 | 64.7 | 1 | WF aHR: 0.17 (0.04–0.79) | ||||||
| HT-AF | 160 | 23/16/- | 0.7 | WF: 29% | 9.4% | ||||||
| Non HT-AF | 160a | 52/16/- | 1.0 | WF: 36% | 3.1% | ||||||
| Zhang et al. (91) | RS | 2 | N/A | aHR: 1.13 (1.08–1.19); 1st yr: 1.20 (1.12–1.29) |
|||||||
| HT-AF | 32,400 | 62 | 77.0 | 60/21/21 | 3.6 | IR 2.6%/year | 5.1% | ||||
| Non HT-AF | 2,388,687 | 47 | 77.2 | 60/21/24 | 3.5 | IR 2.3%/year | 5.4%c | ||||
| Kim et al. (90) | RS | 61 (48–72) | 2.0 | 5.9 | None | aHR: 1.13 (1.07–1.19); 1st yr 1.36 (1.24–1.50) | |||||
| HT-AF | 20,773 | 52 | 55/17/10 | IR 1.83/100 py | |||||||
| Non HT-AF | 62,319 | 52 | 55/16/10 | IR 1.62/100 py | |||||||
| Comparable risk | |||||||||||
| Friberg et al. (92) | RS | 90,490 NV-AF | 47 | 76.2 | N/A | N/A | 1.5 | None | |||
| ΗΤ | 55 | HR:0.92 (0.70 -1.19) | HR:1.11 (0.81–1.54)d | ||||||||
| Bruere et al. (93) | PS | 8,962 | 2.5 | ||||||||
| EU | 8,271 | 37 | 71±14 | 41/15/30 | 3.1 | 57% | |||||
| HT | 141 | 49 | 68±13 | 41/13/25 | 3.1 | 73% | aHR: 0.85 (0.41–1.76) | aHR: 1.26 (0.70–2.24) | |||
| Lower risk | |||||||||||
| Chan et al. (65) | RS | 642 NV-HT-AF | 68 | 71.9±14.6 | 45/20/13 | 3.3 | 2 | WF: 21%, AS: 38%, None: 41% | WF aHR:0.33(0.12-0.91)h | No bleeding events | |
| HT-AF | 0: 0%f ; 1–2: 3.17%f; ≥3: 8.11%f | ||||||||||
| Non HT-AF | 0: 2.56%f; 1–2: 7.02%f; ≥3: 10.54f | ||||||||||
| Lin et al. (96) | RS | 4.3 | HR: 0.73 (0.64–0.92); HR: 0.41 (0.35–0.48)g |
||||||||
| HT-AF | 3,880 | 65 | 60.1 | 45/14/23 | 2.2 | 14.2% | 1.6/100 py | ||||
| Non HT-AF | 17,871 | 43 | 71.9 | 56/18/36 | 3.2 | 13.2 % | 2.2/100 py | ||||
| Chen et al. (95) | RS | 66 | N/A | 27/12/10 | |||||||
| HT-AF | 1,868 | 0: 20%, 1: 34%, 2:18%, ≥3: 28% | WF: 24%, AS: 62%, NOAC: 0.3% | aHR: 0.73 (0.64-0.84)e | aHR: 0.90 (0.77–1.07)e | ||||||
| Non HT-AF | 7,472b | 0: 19%, 1: 33%, 2:19%, ≥3: 28% | WF 23%, AS 59%, NOAC 0.3% | ||||||||
aAge and sex-matched; bMatched by age, sex, and comorbidities; c(p<0.001); dMajor bleeding; eAdjusted for age, gender, comorbidities, CHA2DS2VASc score and comedications; f Annual risk by CHA2DS2VASc score; g AF pts without OAC at CHA2DS2-VASc ≤ 4; hWarfarin group vs. no OAC
AF, atrial fibrillation; aHR, adjusted hazard ratio; AS, aspirin; CVD, cardiovascular disease; DM, diabetes mellitus; EU, euthyroid; F, female; FU, follow-up; HR, hazard ratio; HT, hyperthyroidism; HTN, hypertension; IR, incidence rate; N/A, not available; NOAC; novel oral anticoagulation; NV-HT-AF, non-valvular HT-AF; OAC, oral anticoagulation; PS, prospective study; pts, patients; py, person-years; RS, retrospective study; SE, systemic embolism; SSE,stroke and systemic embolism; WF, warfarin.
Second, most cases of hyperthyroidism-related AF are transient, with spontaneous conversion to sinus rhythm after normal thyroid function is restored. Furthermore, hyperthyroidism-related AF has a lower recurrence rate than that of non-hyperthyroidism-related AF (79). Hence, long-term anticoagulation therapy may be unnecessary and represents a matter of discussion among experts. In fact, no RCT has investigated the potential benefits and harms of long-term anticoagulation therapy in this subgroup (Table 2). Notwithstanding the latter, conducting such an RCT may raise ethical considerations, considering the proven benefit of this intervention in the general AF population.
Given the clinical uncertainty regarding the benefits and harms of anticoagulation in patients with hyperthyroidism-related AF, a recommendation on whether to initiate anticoagulation appears warranted. As in other patients with AF, CHA2DS2-VASc and HAS-BLED scores are recommended to guide therapeutic decisions in hyperthyroidism-related AF. The CHA2DS2-VASc score (one point for the presence of congestive heart failure, hypertension, age between 65 and 74 years, female sex, diabetes, and vascular disease, and two points for being older than 75 years and previous stroke – Fig. 2) is a multiple-validated risk stratification score for estimating stroke risk for nonvalvular atrial fibrillation in adults (97, 98). CHA2DS2-VASc can accurately identify low- and high-cardioembolic risk patients and thus prioritize thromboembolic prevention in those with higher risk (≥1 men or ≥2 women) (24, 45). Taking into account that all anticoagulant drugs that reduce thrombotic risk simultaneously increase the risk of bleeding, this risk should be evaluated before initiating oral anticoagulants. A number of bleeding risk scores have been developed and validated. HAS-BLED (hypertension >160, abnormal renal function; creatinine >2.26 mg/dL, abnormal liver function; cirrhosis or bilirubin >2× normal with AST/ALT/AP >3× normal, stroke, bleeding or predisposition to bleeding, labile INR, age >65, medications predisposing to bleeding, alcohol use) is a widely used prognostic model for bleeding risk assessment of patients with AF (99, 100). A score ≥3 indicates a high bleeding risk; hence, the initiation of anticoagulation should be reconsidered in high-risk individuals (24, 45). These recommendations are consistent with the findings of a recent meta-analysis of observational studies in patients with hyperthyroidism-related AF (101). Tng et al. reported that anticoagulation mitigates the risk of ischemic stroke and systemic thromboembolism by 3% (95% CI: 1–6%) in patients with hyperthyroidism-related AF, CHA2DS2-VASc ≥1, and persistent AF compared to no anticoagulation (101). Nevertheless, the certainty of evidence is very low according to the GRADE framework. No study included in the meta-analysis compared the bleeding risk between anticoagulation versus no anticoagulation (Table 2).
Figure 2.
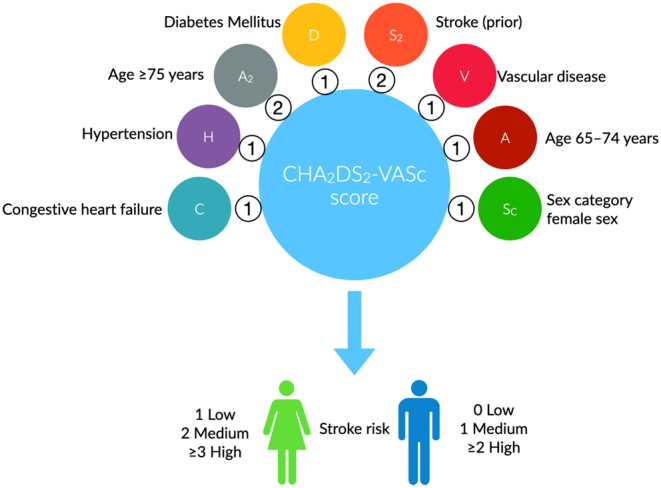
The CHA2DS2-VASc score for AF stroke risk.
It should be acknowledged, though, that these scores were not developed in patients with hyperthyroidism-related AF, and consequently, they may overestimate or underestimate the risk of stroke/bleeding. Concerns about their applicability in hyperthyroidism-related AF have been raised in a small number of external validation studies (102, 103). Thus, their performance and generalizability should be evaluated in future real-world studies, enhancing our confidence in predictions.
The uncertainty regarding the potential clinical benefit of anticoagulation on patients with hyperthyroidism-related AF is depicted among different published guidelines. The ESC 2020 guidelines recommend that patients with hyperthyroidism-related AF should be managed following the same principles as in the general AF population by calculating the thromboembolic risk with CHA2DS2-VASc (24). Along the same line, the recent 2023 American College of Cardiology/American Heart Association/American College of Clinical Pharmacy/Heart Rhythm Society guideline recommended anticoagulation for patients in a hyperthyroid state, based on the estimated annual thromboembolic risk (CHA2DS2-VASc risk factors) (52). In contrast, the Canadian Cardiovascular Society/Canadian Heart Rhythm Society favors anticoagulating hyperthyroid patients with warfarin until the euthyroid state is restored (weak recommendation/low quality) (104).
There are different therapeutic options for thrombosis prevention in patients with AF and hyperthyroidism. Warfarin, a vitamin-K antagonist, maybe a reasonable first-line treatment for stroke prevention when indicated. According to the observational study by Chan et al., warfarin therapy was associated with an almost 70% lower risk for stroke in patients with hyperthyroidism-related AF, CHA2DS2-VASc score >2, and persistent AF compared to no treatment (HR: 0.33; 95% CI: 0.12, 0.91) (94). In contrast, another retrospective study found no benefit of warfarin in stroke prevention (adjusted HR: 1.16; 95% CI: 0.52–2.56) (105). However, the patients had new-onset AF and a lower CHA2DS2-VASc score (mean score: 2) than the study by Chan et al., including a population with less disease severity and lower thromboembolic risk. Physicians should be aware that hyperthyroidism enhances the degradation of vitamin K-related clotting factors, and thus the response to warfarin (106, 107). In other words, patients with hyperthyroidism may require lower doses to achieve and maintain INR within the therapeutic range (107). Hence, periodic monitoring of warfarin treatment and subsequent dose adjustments based on the INR are of cardinal importance. Additionally, evidence regarding the use of the novel direct oral anticoagulants (DOACs) is scarce and inconsistent. Real-world data suggest that treatment with DOACs is at least as beneficial as warfarin in patients with hyperthyroidism and AF (HR: 1.25; 95% CI: 0.88, 178) but with a lower bleeding risk (HR: 0.65; 95% CI: 0.44–0.96) (65). On the contrary, a post hoc analysis of ARISTOTLE including only 321 participants with new-onset AF and a history of hyperthyroidism found no benefit of apixaban on bleeding risk compared to warfarin (HR: 0.47; 95% CI: 0.14–1.57) (108). In conclusion, there is clinical uncertainty regarding the benefit/harm ratio of DOACs in patients with hyperthyroidism-related AF (101).
Cardiovascular risk factors and concomitant diseases: detection and management
The holistic approach recommended for the general AF population may also be applicable to hyperthyroidism-related AF (24, 45, 109). Thus, optimal management of comorbidities (hypertension, heart failure, diabetes, obesity) and modifiable risk factors (smoking and alcohol consumption), adopting a healthier lifestyle, and appropriate treatment with the goal of mitigating residual cardiovascular risk is of paramount importance (24, 45, 109).
Conclusion (Box 2)
Box 2 Key points.
AF is the most common cardiac condition associated with hyperthyroidism.
The prevalence of AF among patients with HT is 5–15%, compared with 1–3% of the general population.
Risk factors of AF in hyperthyroid patients are age, male sex, diabetes, heart failure, and valve disease.
Hyperthyroidism is a reversible cause of AF. Approximately two-thirds of hyperthyroid patients will spontaneously return to sinus rhythm after controlling the hyperthyroid status. Return to sinus rhythm occurs in 4–6 months.
Achieving euthyroidism is the most crucial step in the management of hyperthyroidism-related AF.
Rate control is the mainstay of initial symptomatic treatment, while rhythm control has a secondary role in hyperthyroidism-related AF (reserved in cases of persistent and recurrent AF and in hemodynamically unstable AF).
There is no clear evidence whether the risk of stroke/thromboembolism in case of hyperthyroidism-related AF is higher compared with non-hyperthyroidism-related AF.
The decision whether or not to use anticoagulant therapy to prevent stroke in patients with hyperthyroidism-related AF should be guided by the CHA2DS2-VASc and HAS-BLED scores, as in other patients with AF.
Novel direct oral anticoagulants (DOAC) are equally effective as warfarin while they may have a safer profile.
Clinical evidence shows that not only overt hyperthyroidism but also subclinical hyperthyroidism and high-normal FT4 are associated with a significant risk of atrial fibrillation. The prevalence of AF among hyperthyroid patients is between 5% and 15%.
Hyperthyroidism is a reversible cause of AF, and the majority of patients with hyperthyroidism-related AF are expected to revert spontaneously to sinus rhythm in 4–6 months after restoration of euthyroidism is achieved. Therefore, quick restoration of euthyroidism is fundamental in the management of hyperthyroidism-related AF. In addition, rate control with beta-blockers plays a primary role in better symptom management, reserving rhythm control in cases of persistent AF, as opposed to the management of non-hyperthyroidism-related AF. Given that the findings in the existing literature regarding the risk of stroke and systemic embolism are inconsistent, the decision on whether to start anticoagulation therapy follows the same recommendations as in other AF patients and is based on the CHA2DS2-VASc and HAS-BLED scores, which assess the risk of systemic embolization, stroke, and bleeding in patients suffering from AF. Recent data suggest that warfarin and DOACs are equally beneficial, but DOACs might be associated with a lower incidence of bleeding.
More research is needed regarding risk factors and prevention of hyperthyroidism-related AF, as well as of AF in individuals with high-normal FT4. Future studies on unresolved issues like the risk of stroke and systemic embolism in hyperthyroidism-related AF and the comparison between warfarin and DOACs are necessary to establish optimal management strategies to prevent these conditions.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
All authors have been involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
References
- 1.Ohlrogge AH Brederecke J & Schnabel RB. Global burden of atrial fibrillation and flutter by national income: results from the global burden of disease 2019 database. Journal of the American Heart Association 202312e030438. ( 10.1161/JAHA.123.030438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorenek B, Benjamin EJ, Boriani G, Crijns HJ, Vogel RI, Van Gelter IC, Halle M, Kudaiberdieva G, Lane DA, Larsen TB, et al.European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 201719190–225. ( 10.1093/europace/euw242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott AD Middeldorp ME Van Gelder IC Albert CM & Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nature Reviews. Cardiology 202320404–417. ( 10.1038/s41569-022-00820-8) [DOI] [PubMed] [Google Scholar]
- 4.Kim JA Chelu MG & Li N. Genetics of atrial fibrillation. Current Opinion in Cardiology 202136281–287. ( 10.1097/HCO.0000000000000840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga WM Poppe KG & Effraimidis G. Hyperthyroidism: aetiology, pathogenesis, diagnosis, management, complications, and prognosis. The Lancet Diabetes and Endocrinology 202311282–298. ( 10.1016/S2213-8587(2300005-0) [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, Fishman GI, Goldman S, Cooper DS, Mora S, et al.Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Circulation 20191392892–2909. ( 10.1161/CIRCULATIONAHA.118.036859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sgarbi JA. Thyroid and the heart: a historical perspective. In Thyroid and Heart : A Comprehensive Translational Essay, pp. 3–11. Iervasi G Pingitore A Gerdes AM & Razvi S, eds. Cham: Springer International Publishing; 2020. ( 10.1007/978-3-030-36871-5) [DOI] [Google Scholar]
- 8.Hertz J.On Goitre and Allied Diseases. Copenhagen, Denmark: Munksgaard Publishers; 1943. [Google Scholar]
- 9.Zoni-Berisso M Lercari F Carazza T & Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clinical Epidemiology 20146213–220. ( 10.2147/CLEP.S47385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornej J Borschel CS Benjamin EJ & Schnabel RB. Epidemiology of atrial fibrillation in the 21st Century: novel Methods and New Insights. Circulation Research 20201274–20. ( 10.1161/CIRCRESAHA.120.316340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost L Vestergaard P & Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Archives of Internal Medicine 20041641675–1678. ( 10.1001/archinte.164.15.1675) [DOI] [PubMed] [Google Scholar]
- 12.Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, Faber J, Hansen PR, Pedersen OD, Torp-Pedersen C, et al.The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ 2012345e7895. ( 10.1136/bmj.e7895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okosieme OE, Taylor PN, Evans C, Thayer D, Chai A, Khan I, Draman MS, Tennant B, Geen J, Sayers A, et al.Primary therapy of Graves' disease and cardiovascular morbidity and mortality: a linked-record cohort study. The Lancet Diabetes and Endocrinology 20197278–287. ( 10.1016/S2213-8587(1930059-2) [DOI] [PubMed] [Google Scholar]
- 14.Naser JA Pislaru SV Stan MN & Lin G. Incidence, risk factors, and outcomes of incident atrial fibrillation in patients with Graves disease. Mayo Clinic Proceedings 202398883–891. ( 10.1016/j.mayocp.2022.12.013) [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki T Naka M Hiramatsu K Yamada T Niwa A Aizawa T Murakami M Ishihara M & Miyahara Y. Echocardiographic studies on the relationship between atrial fibrillation and atrial enlargement in patients with hyperthyroidism of Graves' disease. Cardiology 19897610–17. ( 10.1159/000174467) [DOI] [PubMed] [Google Scholar]
- 16.Mohacsi A Worum F Lorincz I Nagy E & Leovey A. Incidence of rhythm disorders in hyperthyrosis with special respect of old age form. Acta Medica Hungarica 19904721–29. [PubMed] [Google Scholar]
- 17.Auer J Scheibner P Mische T Langsteger W Eber O & Eber B. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. American Heart Journal 2001142838–842. ( 10.1067/mhj.2001.119370) [DOI] [PubMed] [Google Scholar]
- 18.Wong CL Tam HV Fok CV Lam PE & Fung LM. Thyrotoxic atrial fibrillation: factors associated with persistence and risk of ischemic stroke. Journal of Thyroid Research 201720174259183. ( 10.1155/2017/4259183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biondi B & Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nature Reviews. Endocrinology 20106431–443. ( 10.1038/nrendo.2010.105) [DOI] [PubMed] [Google Scholar]
- 20.Krahn AD Klein GJ Kerr CR Boone J Sheldon R Green M Talajic M Wang X & Connolly S. How useful is thyroid function testing in patients with recent-onset atrial fibrillation? The Canadian registry of atrial fibrillation investigators. Archives of Internal Medicine 19961562221–2224. ( 10.1001/archinte.156.19.2221) [DOI] [PubMed] [Google Scholar]
- 21.Selmer C, Hansen ML, Olesen JB, Merie C, Lindhardsen J, Olsen AM, Madsen JC, Schmidt U, Faber J, Hansen PR, et al.New-onset atrial fibrillation is a predictor of subsequent hyperthyroidism: a nationwide cohort study. PLoS One 20138e57893. ( 10.1371/journal.pone.0057893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buccelletti F Carroccia A Marsiliani D Gilardi E Silveri NG & Franceschi F. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. American Journal of Emergency Medicine 2011291158–1162. ( 10.1016/j.ajem.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 23.Forfar JC Miller HC & Toft AD. Occult thyrotoxicosis: a correctable cause of "idiopathic" atrial fibrillation. American Journal of Cardiology 1979449–12. ( 10.1016/0002-9149(7990243-1) [DOI] [PubMed] [Google Scholar]
- 24.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, et al.ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. European Heart Journal 202042373–498. ( 10.1093/eurheartj/ehaa612) [DOI] [PubMed] [Google Scholar]
- 25.Sawin CT Geller A Wolf PA Belanger AJ Baker E Bacharach P Wilson PW Benjamin EJ & D’Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New England Journal of Medicine 19943311249–1252. ( 10.1056/NEJM199411103311901) [DOI] [PubMed] [Google Scholar]
- 26.Cappola AR Fried LP Arnold AM Danese MD Kuller LH Burke GL Tracy RP & Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 20062951033–1041. ( 10.1001/jama.295.9.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Åsvold BO, Sgarbi JA, Völzke H, et al.Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Archives of Internal Medicine 2012172799–809. ( 10.1001/archinternmed.2012.402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaker L Heeringa J Dehghan A Medici M Visser WE Baumgartner C Hofman A Rodondi N Peeters RP & Franco OH. Normal thyroid function and the risk of atrial fibrillation: the Rotterdam study. Journal of Clinical Endocrinology and Metabolism 20151003718–3724. ( 10.1210/jc.2015-2480) [DOI] [PubMed] [Google Scholar]
- 29.Cappola AR Arnold AM Wulczyn K Carlson M Robbins J & Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. Journal of Clinical Endocrinology and Metabolism 20151001088–1096. ( 10.1210/jc.2014-3586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, Cappola AR, Heckbert SR, Ceresini G, Gussekloo J, et al.Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation 20171362100–2116. ( 10.1161/CIRCULATIONAHA.117.028753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gammage MD Parle JV Holder RL Roberts LM Hobbs FD Wilson S Sheppard MC & Franklyn JA. Association between serum free thyroxine concentration and atrial fibrillation. Archives of Internal Medicine 2007167928–934. ( 10.1001/archinte.167.9.928) [DOI] [PubMed] [Google Scholar]
- 32.Kostopoulos G Doundoulakis I Antza C Bouras E Nirantharakumar K Tsiachris D Thomas GN Lip GYH & Toulis KA. Incident atrial fibrillation in patients with differentiated thyroid cancer: a meta-analysis. Endocrine-Related Cancer 202128325–335. ( 10.1530/ERC-20-0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okosieme OE Usman D Taylor PN Dayan CM Lyons G Moran C Chatterjee K & Rees DA. Cardiovascular morbidity and mortality in patients in Wales, UK with resistance to thyroid hormone beta (RTHbeta): a linked-record cohort study. Lancet. Diabetes and Endocrinology 202311657–666. ( 10.1016/S2213-8587(2300155-9) [DOI] [PubMed] [Google Scholar]
- 34.Illouz F Briet C Mirebeau-Prunier D Bouhours-Nouet N Coutant R Sibilia P & Rodien P. Cardiac complications of thyroid hormone resistance syndromes. Annales d’Endocrinologie 202182167–169. ( 10.1016/j.ando.2020.03.008) [DOI] [PubMed] [Google Scholar]
- 35.Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CM, Trompet S, Arking DE, Geelhoed B, Guo X, et al.Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiology 20194144–152. ( 10.1001/jamacardio.2018.4635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salem JE, Shoemaker MB, Bastarache L, Shaffer CM, Glazer AM, Kroncke B, Wells QS, Shi M, Straub P, Jarvik GP, et al.Association of thyroid function genetic predictors with atrial fibrillation: a phenome-wide association study and inverse-variance weighted average meta-analysis. JAMA Cardiology 20194136–143. ( 10.1001/jamacardio.2018.4615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakazawa HK Sakurai K Hamada N Momotani N & Ito K. Management of atrial fibrillation in the post-thyrotoxic state. American Journal of Medicine 198272903–906. ( 10.1016/0002-9343(8290850-6) [DOI] [PubMed] [Google Scholar]
- 38.Osman F Franklyn JA Holder RL Sheppard MC & Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. Journal of the American College of Cardiology 20074971–81. ( 10.1016/j.jacc.2006.08.042) [DOI] [PubMed] [Google Scholar]
- 39.Zhou ZH Ma LL & Wang LX. Risk factors for persistent atrial fibrillation following successful hyperthyroidism treatment with radioiodine therapy. Internal Medicine 2011502947–2951. ( 10.2169/internalmedicine.50.6135) [DOI] [PubMed] [Google Scholar]
- 40.Ali H Sarfraz S Hassan L & Ali H. Atrial fibrillation as an initial presentation of apathetic thyroid storm. Cureus 202113e17786. ( 10.7759/cureus.17786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boelaert K Torlinska B Holder RL & Franklyn JA. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. Journal of Clinical Endocrinology and Metabolism 2010952715–2726. ( 10.1210/jc.2009-2495) [DOI] [PubMed] [Google Scholar]
- 42.Subramonian D Wu YJ Amed S & Sanatani S. Hyperthyroidism with atrial fibrillation in children: a case report and review of the literature. Frontiers in Endocrinology 202112689497. ( 10.3389/fendo.2021.689497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadiq AM & Chamba NG. Challenges in the management of thyrotoxicosis associated with atrial fibrillation and heart failure: two case reports. Clinical Medicine Insights 2021141179547621994573. ( 10.1177/1179547621994573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zubair Khan M, Gupta A, Hodge J, Patel K, Patel K, Zarak MS, Franklin S, Patel H, Jesani S, Savani S, et al.Clinical outcomes of atrial fibrillation with hyperthyroidism. Journal of Arrhythmia 202137942–948. ( 10.1002/joa3.12550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorenek B, Boriani G, Dan GA, Fauchier L, Fenelon G, Huang H, Kudaiberdieva G, Lip GYH, Mahajan R, Potpara T, et al.European Heart Rhythm Association (EHRA) position paper on arrhythmia management and device therapies in endocrine disorders, endorsed by Asia Pacific Heart Rhythm Society (APHRS) and Latin American Heart Rhythm Society (LAHRS). Europace 201820895–896. ( 10.1093/europace/euy051) [DOI] [PubMed] [Google Scholar]
- 46.Proietti M & Boriani G. Screening for atrial fibrillation in relation to stroke and mortality risk. Thrombosis and Haemostasis 2022122171–175. ( 10.1055/a-1562-0747) [DOI] [PubMed] [Google Scholar]
- 47.Proietti M, Mairesse GH, Goethals P, Scavee C, Vijgen J, Blankoff I, Vandekerckhove Y, Lip GY. & Belgian Heart Rhythm Week Investigators. A population screening programme for atrial fibrillation: a report from the Belgian Heart Rhythm Week screening programme. Europace 2016181779–1786. ( 10.1093/europace/euw069) [DOI] [PubMed] [Google Scholar]
- 48.Nadarajah R, Wu J, Hogg D, Raveendra K, Nakao YM, Nakao K, Arbel R, Haim M, Zahger D, Parry J, et al.Prediction of short-term atrial fibrillation risk using primary care electronic health records. Heart 20231091072–1079. ( 10.1136/heartjnl-2022-322076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himmelreich JCL Veelers L Lucassen WAM Schnabel RB Rienstra M van Weert HCPM & Harskamp RE. Prediction models for atrial fibrillation applicable in the community: a systematic review and meta-analysis. Europace 202022684–694. ( 10.1093/europace/euaa005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poorthuis MHF Jones NR Sherliker P Clack R de Borst GJ Clarke R Lewington S Halliday A & Bulbulia R. Utility of risk prediction models to detect atrial fibrillation in screened participants. European Journal of Preventive Cardiology 202128586–595. ( 10.1093/eurjpc/zwaa082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadarajah R Alsaeed E Hurdus B Aktaa S Hogg D Bates MGD Cowan C Wu J & Gale CP. Prediction of incident atrial fibrillation in community-based electronic health records: a systematic review with meta-analysis. Heart 20221081020–1029. ( 10.1136/heartjnl-2021-320036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Writing Committee Members, Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberrger ZD, et al.20 23 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology 20248310, 9–2. ( 10.1016/j.jacc.2023.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalarus Z, Mairesse GH, Sokal A, Boriani G, Sredniawa B, Casado-Arroyo R, Wachter R, Frommeyer G, Traykov V, Dagres N, et al.Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. Europace 202325185–198. ( 10.1093/europace/euac144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Effraimidis G Strieder TG Tijssen JG & Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. European Journal of Endocrinology 2011164107–113. ( 10.1530/EJE-10-0785) [DOI] [PubMed] [Google Scholar]
- 55.Li YG Bisson A Bodin A Herbert J Grammatico-Guillon L Joung B Wang YT Lip GYH & Fauchier L. C(2) HEST score and prediction of incident atrial fibrillation in poststroke patients: a French nationwide study. Journal of the American Heart Association 20198e012546. ( 10.1161/JAHA.119.012546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haybar H Shirbandi K & Rahim F. C(2)HEST score for atrial fibrillation risk prediction models: a Diagnostic Accuracy Tests meta-analysis. Egyptian Heart Journal 202173104. ( 10.1186/s43044-021-00230-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imberti JF Boriani G & Lip GYH. Updating a simple clinical score predicting incident atrial fibrillation: the C(2)HEST score or more (mC(2)HEST)? European Journal of Internal Medicine 20219027–29. ( 10.1016/j.ejim.2021.06.014) [DOI] [PubMed] [Google Scholar]
- 58.Ponomartseva DA Derevitskii IV Kovalchuk SV & Babenko AY. Prediction model for thyrotoxic atrial fibrillation: a retrospective study. BMC Endocrine Disorders 202121150. ( 10.1186/s12902-021-00809-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens D Harrison SL Kolamunnage-Dona R Lip GYH & Lane DA. The atrial fibrillation Better Care pathway for managing atrial fibrillation: a review. Europace 2021231511–1527. ( 10.1093/europace/euab092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM, Camm AJ, Casadei B, Chua W, Dagres N, et al.Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023256–27. ( 10.1093/europace/euac062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Proietti M Romiti GF Olshansky B Lane DA & Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. American Journal of Medicine 20181311359–1366.e6. ( 10.1016/j.amjmed.2018.06.012) [DOI] [PubMed] [Google Scholar]
- 62.Proietti M Vitolo M & Lip GYH. Integrated care and outcomes in patients with atrial fibrillation and comorbidities. European Journal of Clinical Investigation 202151e13498. ( 10.1111/eci.13498) [DOI] [PubMed] [Google Scholar]
- 63.Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Kozieł M, Yang PS, Guo Y, et al.Adherence to the 'atrial fibrillation better care' pathway in patients with atrial fibrillation: impact on clinical outcomes-A systematic review and meta-analysis of 285,000 patients. Thrombosis and Haemostasis 2022122406–414. ( 10.1055/a-1515-9630) [DOI] [PubMed] [Google Scholar]
- 64.Romiti GF, Proietti M, Bonini N, Ding WY, Boriani G, Huisman MV, Lip GYH. & GLORIA-AF Investigators. Adherence to the Atrial Fibrillation Better Care (ABC) pathway and the risk of major outcomes in patients with atrial fibrillation: a post-hoc analysis from the prospective Gloria-AF Registry. EClinicalmedicine 202355101757. ( 10.1016/j.eclinm.2022.101757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan YH Wu LS See LC Liu JR Chang SH Chao TF Yeh YH Kuo CT Lee HF & Lip GYH. Direct oral anticoagulants in atrial fibrillation patients with concomitant hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 2020105. ( 10.1210/clinem/dgaa050) [DOI] [PubMed] [Google Scholar]
- 66.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al.2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016261343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 67.Tsai K & Leung AM. Subclinical hyperthyroidism: a review of the clinical literature. Endocrine Practice 202127254–260. ( 10.1016/j.eprac.2021.02.002) [DOI] [PubMed] [Google Scholar]
- 68.Gencer B Cappola AR Rodondi N & Collet T-H. Challenges in the management of atrial fibrillation with subclinical hyperthyroidism. Frontiers in Endocrinology 202212795492. ( 10.3389/fendo.2021.795492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Praw SS & Brent GA. Approach to the patient with a suppressed TSH. Journal of Clinical Endocrinology and Metabolism 2023108472–482. ( 10.1210/clinem/dgac635) [DOI] [PubMed] [Google Scholar]
- 70.Biondi B Bartalena L Cooper DS Hegedüs L Laurberg P & Kahaly GJ. The 2015 European Thyroid Association guidelines on diagnosis and treatment of endogenous subclinical hyperthyroidism. European Thyroid Journal 20154149–163. ( 10.1159/000438750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan S Chen L Jin L & Fu X. The efficiency and safety of methimazole and propylthiouracil in hyperthyroidism: a meta-analysis of randomized controlled trials. Medicine 2021100e26707. ( 10.1097/MD.0000000000026707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SY Modzelewski KL Law AC Walkey AJ Pearce EN & Bosch NA. Comparison of propylthiouracil vs methimazole for thyroid storm in critically ill patients. JAMA Network Open 20236e238655. ( 10.1001/jamanetworkopen.2023.8655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camm AJ Naccarelli GV Mittal S Crijns HJGM Hohnloser SH Ma CS Natale A Turakhia MP & Kirchhof P. The increasing role of rhythm control in patients with atrial fibrillation: JACC state-of-the-art review. Journal of the American College of Cardiology 2022791932–1948. ( 10.1016/j.jacc.2022.03.337) [DOI] [PubMed] [Google Scholar]
- 74.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al.2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019140e125–e151. ( 10.1161/CIR.0000000000000665) [DOI] [PubMed] [Google Scholar]
- 75.Han S Jia R Cen Z Guo R Zhao S Bai Y Xie M & Cui K. Early rhythm control vs. rate control in atrial fibrillation: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 202310978637. ( 10.3389/fcvm.2023.978637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tankeu AT Azabji-Kenfack M Nganou CN Ngassam E Kuate-Mfeukeu L Mba C Dehayem MY Mbanya JC & Sobngwi E. Effect of propranolol on heart rate variability in hyperthyroidism. BMC Research Notes 201811151. ( 10.1186/s13104-018-3224-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiersinga WM & Touber JL. The influence of beta-adrenoceptor blocking agents on plasma thyroxine and triiodothyronine. Journal of Clinical Endocrinology and Metabolism 197745293–298. ( 10.1210/jcem-45-2-293) [DOI] [PubMed] [Google Scholar]
- 78.Perrild H Hansen JM Skovsted L & Christensen LK. Different effects of propranolol, alprenolol, sotalol, atenolol and metoprolol on serum T3 and serum rT3 in hyperthyroidism. Clinical Endocrinology 198318139–142. ( 10.1111/j.1365-2265.1983.tb03196.x) [DOI] [PubMed] [Google Scholar]
- 79.Siu CW Jim MH Zhang X Chan YH Pong V Kwok J Kung AW Lau CP & Tse HF. Comparison of atrial fibrillation recurrence rates after successful electrical cardioversion in patients with hyperthyroidism-induced versus non-hyperthyroidism-induced persistent atrial fibrillation. American Journal of Cardiology 2009103540–543. ( 10.1016/j.amjcard.2008.10.019) [DOI] [PubMed] [Google Scholar]
- 80.Nakazawa H Lythall DA Noh J Ishikawa N Sugino K Ito K & Hardman SM. Is there a place for the late cardioversion of atrial fibrillation? A long-term follow-up study of patients with post-thyrotoxic atrial fibrillation. European Heart Journal 200021327–333. ( 10.1053/euhj.1999.1956) [DOI] [PubMed] [Google Scholar]
- 81.Ma CS, Liu X, Hu FL, Dong JZ, Liu XP, Wang XH, Long DY, Tang RB, Yu RH, Lu CS, et al.Catheter ablation of atrial fibrillation in patients with hyperthyroidism. Journal of Interventional Cardiac Electrophysiology 200718137–142. ( 10.1007/s10840-007-9088-y) [DOI] [PubMed] [Google Scholar]
- 82.Machino T, Tada H, Sekiguchi Y, Yamasaki H, Kuroki K, Igarashi M, Naruse Y, Nakano E, Ito Y, Kaneshiro T, et al.Prevalence and influence of hyperthyroidism on the long-term outcome of catheter ablation for drug-refractory atrial fibrillation. Circulation Journal 2012762546–2551. ( 10.1253/circj.cj-12-0340) [DOI] [PubMed] [Google Scholar]
- 83.Mikhaylov EN Orshanskaya VS Lebedev AD Szili-Torok T & Lebedev DS. Catheter ablation of paroxysmal atrial fibrillation in patients with previous amiodarone-induced hyperthyroidism: a case–control study. Journal of Cardiovascular Electrophysiology 201324888–893. ( 10.1111/jce.12140) [DOI] [PubMed] [Google Scholar]
- 84.Wongcharoen W, Lin YJ, Chang SL, Lo LW, Hu YF, Chung FP, Chong E, Chao TF, Tuan TC, Chang YT, et al.History of hyperthyroidism and long-term outcome of catheter ablation of drug-refractory atrial fibrillation. Heart Rhythm 2015121956–1962. ( 10.1016/j.hrthm.2015.06.004) [DOI] [PubMed] [Google Scholar]
- 85.Wang M Cai S Sun L Zhao Q & Feng W. Safety and efficacy of early radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation complicated with amiodarone-induced thyrotoxicosis. Cardiology Journal 201623416–421. ( 10.5603/CJ.a2016.0029) [DOI] [PubMed] [Google Scholar]
- 86.Bartalena L Bogazzi F Chiovato L Hubalewska-Dydejczyk A Links TP & Vanderpump M. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. European Thyroid Journal 2018755–66. ( 10.1159/000486957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Squizzato A Romualdi E Büller HR & Gerdes VE. Clinical review: thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. Journal of Clinical Endocrinology and Metabolism 2007922415–2420. ( 10.1210/jc.2007-0199) [DOI] [PubMed] [Google Scholar]
- 88.Selmer C Olesen JB Hansen ML von Kappelgaard LM Madsen JC Hansen PR Pedersen OD Faber J Torp-Pedersen C & Gislason GH. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. Journal of Clinical Endocrinology and Metabolism 2014992372–2382. ( 10.1210/jc.2013-4184) [DOI] [PubMed] [Google Scholar]
- 89.Siu CW Pong V Zhang X Chan YH Jim MH Liu S Yiu KH Kung AW Lau CP & Tse HF. Risk of ischemic stroke after new-onset atrial fibrillation in patients with hyperthyroidism. Heart Rhythm 20096169–173. ( 10.1016/j.hrthm.2008.10.023) [DOI] [PubMed] [Google Scholar]
- 90.Kim K, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Sung JH, Pak HN, Lee MH, et al.Increased risk of ischemic stroke and systemic embolism in hyperthyroidism-related atrial fibrillation: a nationwide cohort study. American Heart Journal 2021242123–131. ( 10.1016/j.ahj.2021.08.018) [DOI] [PubMed] [Google Scholar]
- 91.Zhang J Bisson A Fauchier G Bodin A Herbert J Ducluzeau P Lip G & Fauchier L. Risk of ischemic stroke in patients with atrial fibrillation and concomitant hyperthyroidism: a nationwide cohort study. European Heart Journal 202142(Supplement 1). ( 10.1093/eurheartj/ehab724.0436) [DOI] [Google Scholar]
- 92.Friberg L Rosenqvist M & Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. European Heart Journal 2012331500–1510. ( 10.1093/eurheartj/ehr488) [DOI] [PubMed] [Google Scholar]
- 93.Bruere H Fauchier L Bernard Brunet A Pierre B Simeon E Babuty D & Clementy N.. History of thyroid disorders in relation to clinical outcomes in atrial fibrillation. American Journal of Medicine 201512830–37. ( 10.1016/j.amjmed.2014.07.014) [DOI] [PubMed] [Google Scholar]
- 94.Chan PH Hai J Yeung CY Lip GY Lam KS Tse HF & Siu CW. Benefit of anticoagulation therapy in hyperthyroidism-related atrial fibrillation. Clinical Cardiology 201538476–482. ( 10.1002/clc.22427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen ZC Wu NC Chang CL Ho CH Liao CT Chiang CY & Chang WT. Risk of ischaemic stroke in thyrotoxic atrial fibrillation. Clinical Endocrinology 201991561–570. ( 10.1111/cen.14061) [DOI] [PubMed] [Google Scholar]
- 96.Lin YS Tsai HY Lin CY Wu VC Chen TH Yang TY Aboyans V & Chen MC. Risk of thromboembolism in non-valvular atrial fibrillation with or without clinical hyperthyroidism. Global Heart 20211645. ( 10.5334/gh.871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lip GY Nieuwlaat R Pisters R Lane DA & Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010137263–272. ( 10.1378/chest.09-1584) [DOI] [PubMed] [Google Scholar]
- 98.Habboushe J Altman C & Lip GYH. Time trends in use of the CHADS2 and CHA2 DS2 VASc scores, and the geographical and specialty uptake of these scores from a popular online clinical decision tool and medical reference. International Journal of Clinical Practice 201973e13280. ( 10.1111/ijcp.13280) [DOI] [PubMed] [Google Scholar]
- 99.Pisters R Lane DA Nieuwlaat R de Vos CB Crijns HJ & Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the euro Heart Survey. Chest 20101381093–1100. ( 10.1378/chest.10-0134) [DOI] [PubMed] [Google Scholar]
- 100.Gao X Cai X Yang Y Zhou Y & Zhu W. Diagnostic accuracy of the HAS-BLED bleeding score in VKA- or DOAC-treated patients with atrial fibrillation: a systematic review and meta-analysis. Frontiers in Cardiovascular Medicine 20218757087. ( 10.3389/fcvm.2021.757087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tng EL Tiong YS Aung AT Chong NYY & Wang Z. Efficacy and safety of anticoagulation in thyrotoxic atrial fibrillation: a systematic review and meta-analysis. Endocrine Connections 202211. ( 10.1530/EC-22-0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Souza MV de Fatima Dos Santos Teixeira P Vaisman M & Xavier SS. Is CHA(2)DS(2)-VASc appropriate for hyperthyroid patients with atrial fibrillation? Implications of adding a transesophageal echocardiography evaluation. International Journal of Cardiology 2017228919–925. ( 10.1016/j.ijcard.2016.11.053) [DOI] [PubMed] [Google Scholar]
- 103.Zhang J Bisson A Fauchier G Bodin A Herbert J Ducluzeau PH Lip GYH & Fauchier L. Yearly incidence of stroke and bleeding in atrial fibrillation with concomitant hyperthyroidism: a national discharge database study. Journal of Clinical Medicine 2022111342. ( 10.3390/jcm11051342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al.The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Canadian Journal of Cardiology 2020361847–1948. ( 10.1016/j.cjca.2020.09.001) [DOI] [PubMed] [Google Scholar]
- 105.Liu SD Lin SJ Ray CY Lin FT Lin WC & Wang LH. Associations of warfarin use with risks of ischemic cerebrovascular events and major bleeding in patients with hyperthyroidism-related atrial fibrillation. Biomedicines 202210. ( 10.3390/biomedicines10112670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kellett HA Sawers JS Boulton FE Cholerton S Park BK & Toft AD. Problems of anticoagulation with warfarin in hyperthyroidism. Quarterly Journal of Medicine 19865843–51. [PubMed] [Google Scholar]
- 107.Chute JP Ryan CP Sladek G & Shakir KM. Exacerbation of warfarin-induced anticoagulation by hyperthyroidism. Endocrine Practice 1997377–79. ( 10.4158/EP.3.2.77) [DOI] [PubMed] [Google Scholar]
- 108.Goldstein SA Green J Huber K Wojdyla DM Lopes RD Alexander JH Vinereanu D Wallentin L Granger CB & Al-Khatib SM. Characteristics and Outcomes of atrial fibrillation in Patients with thyroid Disease (from the Aristotle Trial). American Journal of Cardiology 20191241406–1412. ( 10.1016/j.amjcard.2019.07.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nature Reviews. Cardiology 201714627–628. ( 10.1038/nrcardio.2017.153) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a