Key Points
Question
Is abatacept exposure associated with clinical outcomes in patients with severe COVID-19?
Findings
In this secondary analysis of abatacept pharmacokinetics and exposure-response data for 395 hospitalized patients in the ACTIV-1 IM randomized clinical trial, those who achieved higher projected abatacept exposure had significantly reduced mortality, a higher probability of recovery, and fewer composite safety events. Abatacept clearance and exposure were related to total body weight and baseline disease severity.
Meaning
In this study, abatacept was shown to be efficacious in patients hospitalized with severe COVID-19, although some patients may require higher dosing.
This secondary analysis of a randomized clinical trial examines the pharmacokinetics of abatacept and the association between abatacept exposure and outcomes in patients hospitalized with severe COVID-19.
Abstract
Importance
The pharmacokinetics of abatacept and the association between abatacept exposure and outcomes in patients with severe COVID-19 are unknown.
Objective
To characterize abatacept pharmacokinetics, relate drug exposure with clinical outcomes, and evaluate the need for dosage adjustments.
Design, Setting, and Participants
This study is a secondary analysis of data from the ACTIV-1 (Accelerating COVID-19 Therapeutic Interventions and Vaccines) Immune Modulator (IM) randomized clinical trial conducted between October 16, 2020, and December 31, 2021. The trial included hospitalized adults who received abatacept in addition to standard of care for treatment of COVID-19 pneumonia. Data analysis was performed between September 2022 and February 2024.
Exposure
Single intravenous infusion of abatacept (10 mg/kg with a maximum dose of 1000 mg).
Main Outcomes and Measures
Mortality at day 28 was the primary outcome of interest, and time to recovery at day 28 was the secondary outcome. Drug exposure was assessed using the projected area under the serum concentration time curve over 28 days (AUC0-28). Logistic regression modeling was used to analyze the association between drug exposure and 28-day mortality, adjusted for age, sex, and disease severity. The association between time to recovery and abatacept exposure was examined using Fine-Gray modeling with death as a competing risk, and was adjusted for age, sex, and disease severity.
Results
Of the 509 patients who received abatacept, 395 patients with 848 serum samples were included in the population pharmacokinetic analysis. Their median age was 55 (range, 19-89) years and most (250 [63.3%]) were men. Abatacept clearance increased with body weight and more severe disease activity at baseline. Drug exposure was higher in patients who survived vs those who died, with a median AUC0-28 of 21 428 (range, 8462-43 378) mg × h/L vs 18 262 (range, 9628-27 507) mg × h/L (P < .001). Controlling for age, sex, and disease severity, an increase of 5000 units in AUC0-28 was associated with lower odds of mortality at day 28 (OR, 0.52 [95% CI, 0.35-0.79]; P = .002). For an AUC0-28 of 19 400 mg × h/L or less, there was a higher probability of recovery at day 28 (hazard ratio, 2.63 [95% CI, 1.70-4.08] for every 5000-unit increase; P < .001). Controlling for age, sex, and disease severity, every 5000-unit increase in AUC0-28 was also associated with lower odds of a composite safety event at 28 days (OR, 0.46 [95% CI, 0.33-0.63]; P < .001). Using the dosing regimen studied in the ACTIV-1 IM trial, 121 of the 395 patients (30.6%) would not achieve an abatacept exposure of at least 19 400 mg × h/L, particularly at the extremes of body weight. Using a modified, higher-dose regimen, only 12 patients (3.0%) would not achieve the hypothesized target abatacept exposure.
Conclusions and Relevance
In this study, patients who were hospitalized with severe COVID-19 and achieved higher projected abatacept exposure had reduced mortality and a higher probability of recovery with fewer safety events. However, abatacept clearance was high in this population, and the current abatacept dosing (10 mg/kg intravenously with a maximum of 1000 mg) may not achieve optimal exposure in all patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT04593940
Introduction
Infection with COVID-19 can result in a clinical spectrum ranging from asymptomatic illness to hospitalization and even death.1 Mortality in patients with severe COVID-19 often occurs secondary to a heightened systemic inflammatory response known as a cytokine storm.2,3 This cytokine storm is characterized by significant elevations of multiple inflammatory cytokines.2,3 Due to the strong link between the dysregulated immune system and outcomes in COVID-19, multiple immunomodulatory drugs have been studied in the treatment of severe COVID-19.4,5
Abatacept (Orencia; Bristol Myers Squibb) is a recombinant fusion protein that inhibits T-cell activation, thereby reducing multiple inflammatory cytokines, including interleukin 6 and tumor necrosis factor α, that are part of the COVID-19 cytokine storm.6,7 In the ACTIV-1 (Accelerating COVID-19 Therapeutic Interventions and Vaccines) Immune Modulator (IM) multicenter randomized clinical trial, abatacept, combined with standard of care that often included remdesivir and corticosteroids, decreased mortality in patients hospitalized with moderate to severe COVID-19, but the primary end point of time to recovery was not met.8 However, the pharmacokinetics of abatacept and optimal dosing in this patient population are unknown. Because increased body weight is a risk factor for severe COVID-19 and is associated with increased abatacept clearance (CL),9 it is possible that the pharmacokinetics of abatacept may be different in hospitalized patients with COVID-19. Accordingly, we conducted a planned secondary analysis of the ACTIV-1 IM trial with the goals to (1) characterize abatacept pharmacokinetics, (2) relate exposure with clinical outcomes, and (3) determine the need for dosage adjustments to reach target drug exposure for COVID-19.
Methods
Study Design
This is a secondary analysis of abatacept pharmacokinetics and exposure-response data collected from the ACTIV-1 IM randomized clinical trial. The ACTIV-1 IM methods and full eligibility criteria were previously published.8 Briefly, the ACTIV-1 IM multinational trial was conducted between October 16, 2020, and December 31, 2021, using the ACTIV-1 IM master protocol (Supplement 1) that randomized adults hospitalized with moderate to severe COVID-19 to 1 of 3 immune modulators or placebo plus standard of care. All patients also received remdesivir if eligible, and most received corticosteroids. Patients self-reported race and ethnicity, which were considered important covariates due to potential genetic polymorphisms that could affect drug pharmacokinetics and outcomes. Race was reported as American Indian or Alaska Native, Asian, Black or African American (hereinafter, Black), White, other race (further categorization was not available in the pharmacokinetics dataset), or unknown race; ethnicity was reported as Hispanic or Latino (hereinafter, Hispanic), not Hispanic or Latino, or unknown ethnicity. The protocol was approved by institutional review boards at each site or a centralized institutional review board, and informed consent was obtained from all participants or their authorized representative. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients were eligible for this pharmacokinetics and exposure-response analysis if they were enrolled in the ACTIV-1 IM trial, received abatacept, and had pharmacokinetic samples with detectable concentration from venous blood. eFigure 1 in Supplement 2 presents the overall CONSORT diagram.
Abatacept was administered on day 1 as a single 10-mg/kg intravenous infusion over approximately 30 minutes, with a maximum dose of 1000 mg. Pharmacokinetic sample collection and assay methods are presented in the eMethods in Supplement 2.
Pharmacokinetic Model Development and Simulations
Abatacept serum pharmacokinetic data were analyzed using nonlinear mixed-effects modeling with Phoenix NLME software, version 8.4 (Certara). We employed a standardized population pharmacokinetics approach as outlined in the eMethods in Supplement 2.
Once a final pharmacokinetic model was selected, we used the following equations to derive individual, model-projected pharmacokinetic parameters and simulate a concentration every 0.5 hour for 28 days:
| CL = tvCL × (WT/70 kg)dClwt × exp(ηCL) |
| V1 = tvV1 × (WT/70 kg)dVwt |
| V2 = tvV2 |
| Q = tvQ |
where CL is the clearance from the central compartment, tv is the typical population value of a parameter, WT is total body weight (in kilograms), dClwt is the exponent describing the power relationship between weight and CL, η is the deviation from the average population pharmacokinetic parameter value, V1 is the volume of distribution in the central compartment, dVwt is the exponent describing the power relationship between weight and V1, V2 is the volume of distribution in the peripheral compartment, and Q is the intercompartmental CL.
Using this rich time vs concentration profile, we then conducted a noncompartmental analysis to derive each patient’s simulated area under the serum concentration time curve over 28 days (AUC0-28) and maximum and minimum concentrations (Cmax and Cmin) over 28 days.
Exposure-Response Analysis
Based on results from the ACTIV-1 IM trial, we used mortality at day 28 as our primary outcome of interest and time to recovery at day 28 as our secondary outcome. Recovery was defined as the first day a participant attained category 6, 7, or 8 on the 8-point ordinal scale of disease severity, generally meaning not requiring continuous oxygen and ongoing medical care (a score of 1 indicates death, whereas a score of 8 indicates not hospitalized and no limitations on activities). We used AUC0-28 as our primary exposure of interest, because this represents the total amount of drug in the body over the study period. Additionally, we explored Cmax and Cmin as exposure metrics. We also evaluated the association between AUC0-28 and the composite safety outcome at day 28, defined as the occurrence of death, a serious adverse event, or a grade 3 or 4 adverse event within 28 days.
Dosage Simulations
The optimal abatacept exposure was determined based on the observed data for the primary and secondary outcomes. Using the final population pharmacokinetic model estimates and each patient’s actual covariates and estimated interindividual variability (IIV), we then conducted dosing simulations (1 replicate) to determine the number of patients projected to achieve the target abatacept exposure derived from the exposure-response analysis (eMethods in Supplement 2).
Statistical Analysis
We used descriptive statistics to summarize baseline demographics, clinical characteristics, medication usage, and outcomes. Differences in abatacept exposure (AUC0-28, Cmax, and Cmin) across mortality status and recovery groups were compared using the Kruskal-Wallis test. Additionally, differences in abatacept AUC0-28 across the composite safety outcome were analyzed using the Kruskal-Wallis test.
We used adjusted and unadjusted logistic regression modeling to analyze the association between abatacept exposure (AUC0-28, Cmax, and Cmin) and 28-day mortality. The logistic regression model was adjusted for age, sex, and disease severity; additional modeling assumptions and sensitivity analyses are noted in the eMethods in Supplement 2.
The association between time to recovery and abatacept exposure (AUC0-28, Cmax, and Cmin) was examined using Fine-Gray modeling10 with death as a competing risk and was adjusted for age, sex, and disease severity. Linearity of the exposure variables was tested prior to inclusion in all models, and splines or linear transformations were used when linearity was violated.11
All statistical procedures were conducted in SAS, version 9.4 TS1M7 (SAS Institute Inc); R, version 4.1.1 (R Project for Statistical Computing); and RStudio, version 1.4.1717 (RStudio Inc). Figures 1 and 2 were generated using the Box and Whisker function and the exclusive quartile calculation in Microsoft Excel 2016 (Microsoft Corp). All tests were 2 tailed and statistical significance was declared at α < .05 unless otherwise noted. Data analysis was performed between September 2022 and February 2024.
Results
Baseline Demographics, Clinical Characteristics, and Samples
Altogether, 509 participants received abatacept in the ACTIV-1 IM trial. A total of 414 participants had pharmacokinetic samples collected, but 1 patient had only pharmacokinetic samples from the extracorporeal membrane oxygenation (ECMO) circuit and was excluded from the analysis. Of the remaining 413 patients, there were a total of 897 pharmacokinetic samples from venous blood. The ECMO samples were not used in the analysis. We excluded 49 samples (5.5%) as outlined in eFigure 1 in Supplement 2, resulting in a final population of 395 patients with 848 serum samples. Their median age was 55 (range, 19-89) years; 145 (36.7%) were women and 250 (63.3%) were men. Patients reported being American Indian or Alaska Native (5 [1.3%]), Asian (15 [3.8%]), Black (48 [12.2%]), White (239 [60.5%]), or of other race (64 [16.2%]); race was unknown for 24 (6.1%). Patients also reported being Hispanic (163 [41.3%]) or not Hispanic or Latino (216 [54.7%]); ethnicity was unknown for 16 (4.1%). Additional demographic and clinical characteristics for the 395 patients are noted in Table 1. The median number of samples per patient was 2 (range, 1-4). Patients received a single abatacept infusion at a median weight-based dose of 10 (range, 4.1-12.9) mg/kg and an absolute dose of 910 (range, 386-1000) mg.
Table 1. Baseline Demographics and Clinical Characteristicsa.
| Characteristic | Values (N = 395) |
|---|---|
| Sex | |
| Female | 145 (36.7) |
| Male | 250 (63.3) |
| Race | |
| American Indian or Alaska Native | 5 (1.3) |
| Asian | 15 (3.8) |
| Black or African American | 48 (12.2) |
| White | 239 (60.5) |
| Otherb | 64 (16.2) |
| Unknown | 24 (6.1) |
| Ethnicity | |
| Hispanic or Latino | 163 (41.3) |
| Not Hispanic or Latino | 216 (54.7) |
| Unknown | 16 (4.1) |
| Age, median (range), y | 55 (19-89) |
| Weight, median (range), kg | 91 (38.6-243.6) |
| Creatinine, median (range), mg/dL (n = 391)c | 0.8 (0.25-13) |
| BMI, median (range) (n = 391)c | 31.1 (14.6-75.8) |
| Obesity at baseline (n = 391)c | 226 (57.8) |
| Hypertension at baseline | 160 (40.5) |
| Disease severity at baseline (8-point ordinal scale) | |
| Death (1) | 0 |
| Hospitalized, invasive ventilation or ECMO (2) | 34 (8.6) |
| Hospitalized, noninvasive ventilation or high-flow oxygen devices (3) | 131 (33.2) |
| Hospitalized, requiring supplemental oxygen (4) | 212 (53.7) |
| Hospitalized, not requiring oxygen, requiring ongoing medical care (5) | 18 (4.6) |
| Hospitalized, not requiring oxygen, not requiring ongoing medical care (6) | 0 |
| Not hospitalized, limitations in activity or requiring home oxygen (7) | 0 |
| Not hospitalized, no limitations on activities (8) | 0 |
| Any tocilizumab use | 11 (2.8) |
| Any baricitinib use | 5 (1.3) |
| Any dexamethasone use | 340 (86.1) |
| ECMO (ever) | 8 (2.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECMO, extracorporeal membrane oxygenation.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Unless indicated otherwise, values are presented as the No. (%) of participants.
Further categorization was not available in the dataset.
Data were missing for 4 patients.
Population Pharmacokinetic Model Development
The base model that best characterized the observed data was a 2-compartment structural model with linear elimination, multiplicative error, and estimates of IIV on abatacept CL (eTable in Supplement 2). After covariate selection, the best statistical model included an effect of baseline disease severity (ordinal), weight, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) on CL and weight and BMI on V1. In this model, post hoc empirical bayesian estimates of pharmacokinetic parameters suggested that patients who ever received ECMO had higher abatacept CL, with a median of 0.07 (range, 0.02-0.08) L/h compared with 0.04 (range, 0.01-0.08) L/h. However, this model had less physiologic plausibility because the effect of disease severity was not consistent across categories, and there was collinearity between weight and BMI. Moreover, a reduced model (including only weight on CL and V1) had overall similar performance, which allows for direct comparison with parameter estimates from published abatacept pharmacokinetic models9 and is easier for clinicians to interpret. Accordingly, we selected the reduced model as the final population pharmacokinetic model. A sensitivity analysis comparing these models is presented in the eResults in Supplement 2.
Final Population Pharmacokinetic Model and Model Evaluation
The final pharmacokinetic model was a 2-compartment model with linear elimination and multiplicative error, IIV on CL, and a power relationship for weight normalized to a 70-kg adult on V1 and CL. Parameter estimates are presented in Table 2, and diagnostic plots and the prediction-corrected visual predictive check for the final pharmacokinetic model are presented in eFigures 2 and 3 in Supplement 2. Overall, the final model had good parameter precision with no obvious model misspecification, and the majority of observed concentrations fell within the 90% projection interval. Abatacept CL appeared higher in patients with more severe disease activity at baseline (eFigure 4 in Supplement 2).
Table 2. Parameters for the Final Abatacept Pharmacokinetic Model.
| Parameter | Estimate | RSE, % | 2.5th percentile | Bootstrap median | 97.5th percentile |
|---|---|---|---|---|---|
| V1, L/70 kg | 4.40 | 3.70 | 4.05 | 4.35 | 4.66 |
| V2, L | 4.49 | 8.46 | 4.01 | 4.58 | 5.55 |
| CL, L/h/70 kg | 0.031 | 3.76 | 0.028 | 0.031 | 0.033 |
| Q, L/h | 0.031 | 32.17 | 0.021 | 0.032 | 0.054 |
| Exponential scaling of weight/70 kg on CL | 0.62 | 13.21 | 0.45 | 0.62 | 0.79 |
| Exponential scaling of weight/70 kg on V1 | 0.50 | 26.38 | 0.24 | 0.52 | 0.75 |
| Interindividual variability (CV%) | |||||
| CL | 32.08 | 20.01 | NC | NC | NC |
| Residual error | |||||
| Multiplicative error (%) | 27.66 | 7.23 | 23.67 | 27.17 | 31.10 |
Abbreviations: CL, clearance from the central compartment; CV, coefficient of variation; NC, not calculated; Q, intercompartmental clearance; RSE, relative standard error; V1, volume of distribution in the central compartment; V2, volume of distribution in the peripheral compartment.
Noncompartmental Analysis
Using simulated time vs concentration profiles from the final population pharmacokinetic model, a noncompartmental analysis was conducted to derive individual patient exposures. Across all patients, the median AUC0-28 was 21 185 (IQR, 18 662-23 534) mg × h/L, Cmax was 172.9 (IQR, 161.5-181.3) mg/L, and Cmin was 6.9 (IQR, 5.2-8.9) mg/L (Table 3).
Table 3. Projected and Target Exposures From Dosing Simulationsa.
| Dosing | Projected exposure | Optimal exposure | ||||
|---|---|---|---|---|---|---|
| AUC0-28, mg × h/L | Cmin, mg/L | Cmax, mg/L | AUC0-28, mg × h/L | Cmin, mg/L | Cmax, mg/L | |
| ACTIV-1 IM trial (10 mg/kg, maximum 1000 mg) | 21 185 (18 662-23 534) | 6.9 (5.2-8.9) | 172.9 (161.5-181.3) | 19 400-21 428b | 7.1c | Efficacy: not established; safety: 175-427d |
| High dose (<60 kg = 1000 mg; 60-100 kg = 1250 mg; >100 kg = 1500 mg) | 32 285 (28 500-35 869) | 10.7 (7.7-13.6) | 259.4 (247.5-273.0) | |||
Abbreviations: ACTIV-1 IM, Accelerating COVID-19 Therapeutic Interventions and Vaccines Immune Modulator; AUC0-28, area under the serum concentration time curve over 28 days; Cmax, maximum concentration; Cmin, minimum concentration.
Data represent the median (25th-75th percentiles) derived from Phoenix NLME software, version 8.4 (Certara).
Lower end of target derived from the inflection point of the time-to-event analysis and higher end derived from median exposure in the survival group.
Target derived from median exposure in the survival group.
Range of exposure observed in healthy volunteers receiving a single dose of abatacept at 10 mg/kg.
Exposure Response
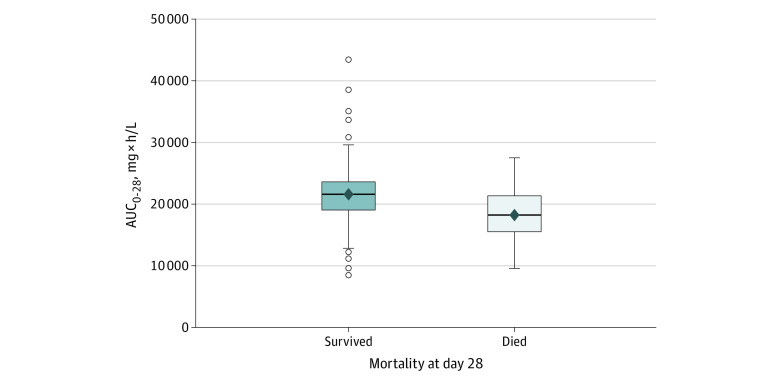
Overall, 349 patients (88.4%) were alive and 46 (11.6%) had died by day 28. The AUC0-28 was significantly higher in patients who survived vs those who died (Figure 1), with a median of 21 428 (range, 8462-43 378) mg × h/L vs 18 262 (range, 9628-27 507) mg × h/L (P < .001). Similarly, Cmin was significantly higher in those who survived, with a median of 7.1 (range, 1.2-38.7) mg/L vs 4.8 (range, 0.7-11.5) mg/L (P < .001), whereas there was no significant difference with Cmax.
Figure 1. Abatacept Exposure and Mortality at 28 Days.
The solid line in each box plot represents the median; the diamond represents the mean. Open circles represent data beyond 1.5 times the IQR; P < .001. AUC0-28 indicates the area under the serum concentration time curve over 28 days. Not all outlier data points can be seen owing to overlapping symbols.
In logistic regression modeling, no linearity violations were observed for abatacept exposure variables and 28-day mortality. Controlling for age, sex, and disease severity, a 5000-unit increase in AUC0-28 was associated with lower odds of mortality at day 28 (OR, 0.52 [95% CI, 0.35-0.79]; P = .002). Similarly, there was an association between Cmin and 28-day mortality with an odds ratio of 0.80 (95% CI, 0.70-0.92; P = .002); however, there was no association with Cmax and mortality. Besides abatacept AUC0-28 and Cmin, the only other statistically significant covariates in the multivariable model of survival at day 28 were age and disease severity at baseline. In a sensitivity analysis, addition of concomitant medications to the model did not significantly alter the association between AUC0-28 and 28-day mortality. There was no significant interaction of disease severity with drug exposure and mortality.
In time-to-recovery analyses, 320 patients (81.0%) recovered over the 28-day period, 32 (8.1%) did not recover, and 43 (10.9%) died. Three patients initially recovered but later died; these patients were treated as recovered in the time-to-recovery analysis and counted as deceased in the mortality analysis. The unadjusted association between AUC0-28 and outcome violated the linearity assumption and was best characterized using 2 linear pieces with an inflection point at 19 400 mg × h/L. Controlling for age, sex, and disease severity, every 5000-unit increase in an AUC0-28 of 19 400 mg × h/L or less was associated with a higher probability of recovery at day 28, with a hazard ratio of 2.63 (95% CI, 1.70-4.08; P < .001). In the adjusted setting, AUC values greater than 19 400 mg × h/L did not increase the likelihood of recovery (hazard ratio, 1.10 [95% CI, 0.90-1.33]; P = .36). The probability of 28-day recovery across AUC0-28 levels is displayed in eFigure 5 in Supplement 2. Additionally, higher Cmin was associated with a higher probability of recovery at day 28, but the association was nonlinear. There was no association with Cmax.
Exposure Safety
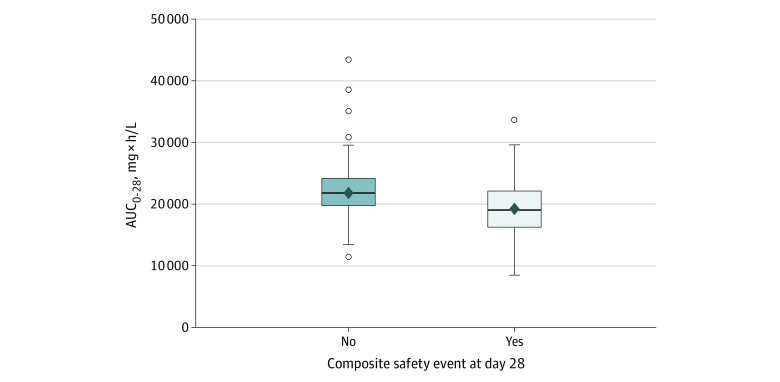
Altogether, 128 of 395 patients (32.4%) experienced a composite safety event through day 28. Of these 128 patients, 46 (35.9%) died. Abatacept AUC0-28 was significantly higher in patients who did not have a safety event through day 28 (median [range], 21 838 [11 505-43 378] vs 19 167 [8462-33 625] mg × h/L; P < .001; Figure 2). In logistic regression modeling and controlling for age, sex, and disease severity, every 5000-unit increase in AUC0-28 was associated with lower odds of a composite safety event at 28 days (OR, 0.46 [95% CI, 0.33-0.63]; P < .001). When the 46 patients who died were excluded, the AUC0-28 remained associated with lower odds of the composite safety event (odds ratio, 0.51 [95% CI, 0.36-0.72]; P < .001), suggesting the association was not entirely driven by a reduction in mortality.
Figure 2. Abatacept Exposure and Safety.
The solid line in each box plot represents the median; the diamond represents the mean. Open circles represent data beyond 1.5 times the IQR; P < .001. AUC0-28 indicates the area under the serum concentration time curve over 28 days. Not all outlier data points can be seen owing to overlapping symbols.
Dosage Simulations
The proposed minimum therapeutic target for abatacept AUC0-28 was determined to be approximately 19 400 to 21 428 mg × h/L based on the median AUC0-28 in the survival group as well as the inflection point observed in the time-to-recovery analysis. Using the dosing regimen studied in the ACTIV-1 IM trial, 121 of 395 patients (30.6%) would not achieve an abatacept exposure of at least 19 400 mg × h/L, particularly at the extremes of body weight (eFigure 6A in Supplement 2). Using the modified rheumatoid arthritis regimen, only 12 patients (3.0%) would not achieve the proposed target abatacept AUC0-28 (eFigure 6B in Supplement 2). In addition, the individual projected Cmax for the modified rheumatoid arthritis regimen approximated that observed in abatacept phase 1 clinical trials (eFigures 7 and 8 in Supplement 2).12 A summary of exposures for the dosing simulations is provided in Table 3.
Discussion
In this study, we observed that patients hospitalized with moderate to severe COVID-19 who achieved higher abatacept exposure had improved outcomes with fewer safety events. Additionally, we found that the current abatacept dosing (10 mg/kg intravenously with a maximum of 1000 mg) may not achieve the proposed optimal exposure in this population, particularly those at the extremes for body weight or who are critically ill. These results highlight that drug pharmacokinetics and dosing cannot simply be extrapolated from one population (eg, patients with rheumatoid arthritis) to another.
The US Food and Drug Administration has highlighted that exposure-response data derived from well-controlled studies can contribute substantial evidence of effectiveness and support dosing.13 Accordingly, our analyses add to the preponderance of evidence supporting abatacept efficacy in hospitalized patients with severe COVID-19. Our results broadly support the current National Institutes of Health guidelines for the treatment of COVID-19, which recommend abatacept administration for hospitalized patients who require oxygen, including noninvasive ventilation.14 However, we found that the exposure-response relationship for mortality was not dependent on baseline disease severity, suggesting that mechanically ventilated patients may also benefit from abatacept. Although time to recovery was not statistically significant compared with placebo in the overall trial, our analysis used a different exposure metric whereby we quantified drug concentrations only in the group receiving abatacept. Because approximately a third of patients who received abatacept in the ACTIV-1 IM trial may have had suboptimal exposure, it is possible that too few patients achieved sufficient abatacept concentrations compared with placebo to detect a difference in the primary study’s time-to-recovery analysis.
We made several important observations regarding abatacept pharmacokinetics in this population. Overall, CL appeared to be higher in patients hospitalized with severe COVID-19 compared with other populations, resulting in lower abatacept concentrations. For example, after administration of a single abatacept dose (10 mg/kg intravenously), the mean Cmax was 292 (range, 175-427) mg/L in 13 healthy volunteers and the mean (SD) Cmax was 202 (7.7) mg/L in patients with hematologic malignancies in a previous study compared with a mean (range) of 170 (94-215) mg/L in the ACTIV-1 IM trial.12,15 Additionally, the systemic CL of abatacept in patients with rheumatoid arthritis was approximately half (15.4 vs 31 mL/h/70 kg) of the CL observed in our study.12 The mechanism for higher CL in this population is unclear but could be due to higher body weights, with more than half of our population being obese, or to the underlying inflammatory state and hospitalization leading to increased protein catabolism or target-mediated drug disposition. Because we observed higher abatacept CL in patients receiving ECMO or mechanical ventilation at baseline, it is also possible that the lack of mortality benefit observed in the overall clinical trial in this subgroup8 was attributable to subtherapeutic exposure.
Due to the higher CL of abatacept in patients hospitalized with severe COVID-19, we conducted dosing simulations and found that a higher-dose abatacept regimen would be necessary for most patients to achieve the exposure that resulted in optimal benefit derived from this ACTIV-1 IM cohort. Additionally, we showed that most patients would not experience maximum abatacept concentrations exceeding those of healthy volunteers. This finding, combined with the observation that safety events occurred more frequently at lower (not higher) abatacept exposures, and the linear pharmacokinetics for abatacept9 all provide reassurance that higher doses could be studied in future clinical trials. However, it is important to note that these target abatacept exposures may not be representative in other patient populations.
We observed that drug exposure was higher in patients who did not experience the composite safety events. The reason for higher drug exposure in this subgroup is not entirely clear but may be partially due to the reduction in mortality observed in patients with higher drug exposure. Additionally, patients with low drug exposures were more likely to have critical illness, obesity, or both, which may be independent risk factors for adverse events.
This study was conducted between October 2020 and December 2021, before the predominant Omicron variant and subvariants. Although mortality has decreased with new variants,16 cytokine storms are believed to be a final common pathway caused by a variety of disorders2 for which abatacept may improve mortality.17 Accordingly, we would expect similar efficacy of abatacept when COVID-19 results in a hyperinflammatory state, despite continued evolution of the virus.
Limitations
This study has some limitations. Because of the sparse pharmacokinetic sampling in the trial, we were unable to compute drug exposures using actual (observed) drug concentrations and instead derived individual projected concentrations. Accordingly, the data rely heavily on the performance of the pharmacokinetic model. Due to approximately 30% residual variability in the model and high IIV in abatacept CL, it is expected that actual drug concentrations may vary from those projected by our model. Additionally, the range of abatacept exposures was limited by a dosing cap of 1000 mg and both the safety and efficacy of higher doses of abatacept require confirmation. In addition, mortality in patients with COVID-19 pneumonia is often the result of complex interactions among underlying comorbidities, heterogeneity in the disease process, and treatment effects; and therapeutic abatacept concentrations alone do not guarantee certain outcomes. Finally, the efficacy of abatacept as monotherapy cannot be determined from this analysis.
Conclusions
In this secondary analysis of the ACTIV-1 IM multinational randomized clinical trial, patients who were hospitalized with severe COVID-19 and achieved higher projected abatacept exposure had reduced mortality and a higher probability of recovery with fewer safety events. However, abatacept CL was high in this population, and the current abatacept dosing (10 mg/kg intravenously with a maximum of 1000 mg) may not achieve optimal exposure in all patients, particularly those at the extremes for body weight or those who are critically ill. Clinical trials in future pandemics could be optimized by evaluating exposure-response relationships during the study and leveraging an adaptive design to adjust dosing.
Trial Protocol
eMethods.
eResults.
eTable. Pharmacokinetic Parameters for the Base Model
eFigure 1. CONSORT Diagram
eFigure 2. Diagnostic Plots for the Final Abatacept Model
eFigure 3. Prediction-Corrected Visual Prediction Check for the Final Population Pharmacokinetic Model
eFigure 4. Abatacept Clearance and Baseline Disease Severity
eFigure 5. Abatacept Exposure and Probability of Recovery at 28 Days
eFigure 6. Target Abatacept Attainment From Dosage Simulations
eFigure 7. Sensitivity Analysis for Maximum Abatacept Concentration Using IPRED
eFigure 8. Maximum Abatacept Concentration Using DV
eReferences.
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Adjei S, Hong K, Molinari NM, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods—United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1182-1189. doi: 10.15585/mmwr.mm7137a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255-2273. doi: 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan LY, Komarasamy TV, Balasubramaniam VRMT. Hyperinflammatory immune response and COVID-19: a double edged sword. Front Immunol. 2021;12:742941. doi: 10.3389/fimmu.2021.742941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795-807. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi: 10.1056/NEJMoa2028700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis PM, Nadler SG, Stetsko DK, Suchard SJ. Abatacept modulates human dendritic cell-stimulated T-cell proliferation and effector function independent of IDO induction. Clin Immunol. 2008;126(1):38-47. doi: 10.1016/j.clim.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 7.Weisman MH, Durez P, Hallegua D, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33(11):2162-2166. [PubMed] [Google Scholar]
- 8.O’Halloran JA, Ko ER, Anstrom KJ, et al. ; ACTIV-1 IM Study Group Members . Abatacept, cenicriviroc, or infliximab for treatment of adults hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA. 2023;330(4):328-339. doi: 10.1001/jama.2023.11043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Roy A, Murthy B. Population pharmacokinetics and exposure-response relationship of intravenous and subcutaneous abatacept in patients with rheumatoid arthritis. J Clin Pharmacol. 2019;59(2):245-257. doi: 10.1002/jcph.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 11.Stone C, Koo C. Additive splines in statistics. In: Proceedings of the American Statistical Association. American Statistical Association; 1985:45-48. [Google Scholar]
- 12.Orencia (abatacept). Package insert. Bristol Myers Squibb Co; 2013. Accessed July 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125118s171lbl.pdf
- 13.US Food and Drug Administration . Guidance for industry: exposure-response relationships—study design, data analysis, and regulatory applications. May 2003. Accessed July 1, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exposure-response-relationships-study-design-data-analysis-and-regulatory-applications
- 14.National Institutes of Health . COVID-19 treatment guidelines: abatacept. Updated October 10, 2023. Accessed December 14, 2023. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/abatacept/?utm_source=site&utm_medium=home&utm_campaign=highlights
- 15.Koura DT, Horan JT, Langston AA, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant. 2013;19(11):1638-1649. doi: 10.1016/j.bbmt.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Strasser ZH, Greifer N, Hadavand A, Murphy SN, Estiri H. Estimates of SARS-CoV-2 Omicron BA.2 subvariant severity in New England. JAMA Netw Open. 2022;5(10):e2238354. doi: 10.1001/jamanetworkopen.2022.38354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voelker R. Drug approved to prevent graft-vs-host disease. JAMA. 2022;327(5):417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eResults.
eTable. Pharmacokinetic Parameters for the Base Model
eFigure 1. CONSORT Diagram
eFigure 2. Diagnostic Plots for the Final Abatacept Model
eFigure 3. Prediction-Corrected Visual Prediction Check for the Final Population Pharmacokinetic Model
eFigure 4. Abatacept Clearance and Baseline Disease Severity
eFigure 5. Abatacept Exposure and Probability of Recovery at 28 Days
eFigure 6. Target Abatacept Attainment From Dosage Simulations
eFigure 7. Sensitivity Analysis for Maximum Abatacept Concentration Using IPRED
eFigure 8. Maximum Abatacept Concentration Using DV
eReferences.
Nonauthor Collaborators
Data Sharing Statement