Abstract
Herpesviruses enter cells by a yet poorly understood mechanism. We visualized the crucial steps of the entry pathway of bovine herpesvirus 1 (BHV-1) and BHV-5 by transmission and scanning electron microscopy, employing cryotechniques that include time monitoring, ultrarapid freezing, and freeze substitution of cultured cells inoculated with virus. A key step in the entry pathway of both BHV-1 and BHV-5 is a unique fusion of the outer phospholipid layer of the viral envelope with the inner layer of the plasma membrane and vice versa resulting in “crossing” of the fused membranes and in partial insertion of the viral envelope into the plasma membrane. The fusion area is proposed to function as an axis for driving the virus particle into an invagination that is concomitantly formed close to the fusion site. The virus particle enters the cytoplasm through the opened tip of the invagination, and the viral envelope defuses from the plasma membrane. There is strong evidence that the intact virus particle is then transported to the nuclear region.
Cell entry of herpesviruses is mediated by various glycoproteins of the viral envelope (29, 31). Herpesviruses are generally believed to enter cells via fusion of the viral envelope with the plasma membrane, whereby the nucleocapsid and the tegument proteins gain access to the cytoplasm (6, 8, 21, 30) or by endocytosis (8). The entry pathway and fusion mechanism of other enveloped viruses such as human immunodeficiency virus (HIV), Sendai virus, and influenza virus are well documented (for reviews, see references 11, 13, 26, and 40). HIV and influenza viruses need only one glycoprotein that mediates both cell binding and fusion. Sendai viruses require two glycoproteins for cell entry; one mediates binding, the other mediates fusion. Influenza viruses enter cells by endocytosis. They are released into the cytoplasm by fusion of the viral envelope with the membrane of the endocytotic vacuole. Sendai virus and HIV enter cells by fusion of the viral envelope with the plasma membrane. Herpesviruses require a not clearly defined number of glycoproteins (29, 31) that mediate binding (4, 19, 20, 39, 43), fusion (27, 35), and penetration (7, 27). Recently, it was shown that four glycoproteins of herpes simplex virus type 1 (HSV-1) are necessary and essential for membrane fusion (35). The involvement of more than two glycoproteins suggests a complicated mechanism for binding, fusion, and/or penetration. To our knowledge, fusion of the herpesvirus envelope with the plasma membrane per se has never been demonstrated. The hypothesis is based on electron microscopy of specimens obtained by conventional preparation protocols that revealed enveloped virus particles in close apposition to the outer cell surface and naked nucleocapsids within the cytoplasm close to the plasma membrane shortly after incubation of cells with pseudorabies virus (8), HSV-1 (6, 21, 24, 30), or human cytomegalovirus (33) at 37°C.
Conventional protocols for electron microscopy include fixation by glutaraldehyde (and formaldehyde) and osmium tetroxide at 4 to 24°C, dehydration with ethanol or acetone, embedding in epoxy resins at room temperature, and polymerization at 60 or 80°C. Glutaraldehyde reacts more rapidly with proteins than does formaldehyde (5, 9), but it is far too slow considering the time range of milliseconds that membranes need to fuse (14, 18). Further, glutaraldehyde, which is commonly used as primary fixative for ultrastructural research, does not react with lipids. A substantial amount of lipids (1, 38) and even membranous compartments (41) are lost during further processing, despite the postfixation with osmium tetroxide.
During the last decade low-temperature methods were established that allow immediate stopping of cellular processes by rapid freezing and further processing such as freeze substitution without a substantial loss of material (12, 15, 25, 28, 36, 37). Biological material may be frozen by plunging it into liquid propane (3). Ultrarapid freezing followed by freeze etching allowed the study of fusion events of influenza virus (14). To visualize the entry pathway of herpesviruses, we adapted a methodology that allowed us (i) to monitor the incubation of cells with virus in the range of seconds, (ii) to arrest the entry process within milliseconds at any desired time by plunging the incubated cells into liquid propane, and (iii) to keep cellular material in place (42) for visualizing various stages of the entry process after freeze substitution by both transmission and scanning electron microscopy. We will show that both bovine herpesvirus 1 (BHV-1) and BHV-5 enter cells by a unique fusion of the viral envelope with the plasma membrane followed by movement into the cytoplasm through a perforation of the plasma membrane.
MATERIALS AND METHODS
Cells and viruses.
Madin-Darby bovine kidney (MDBK) cells were grown in minimum essential medium (Gibco, Bethesda, Md.) containing Hank’s salts and 10% fetal calf serum (Gibco). BHV-1 and BHV-5 were propagated in MDBK cells and concentrated by centrifugation at 1,000 × g for 15 min in Centricon Plus-20 centrifugal devices (100 kDa; Millipore Corp., Bedford, Mass.).
Incubation and electron microscopy.
MDBK cells were grown for 2 days on sapphire disks 3 mm in diameter (Bruegger, Minusio, Switzerland) covered with a 9- to 10-nm-thick layer of carbon. Cells were inoculated with BHV-1 or BHV-5 at a multiplicity of infection of 150 to 200 and kept at 4°C for 1.5 h to admit adsorption. For incubation at 37°C, the sapphire disks covered with inoculated cells were rapidly transferred one by one to a guillotine within a humid chamber placed directly above a container filled with liquid propane. They were then plunged into the liquid propane after a delay of 1 to 90 s. Alternatively, the sapphire disks were first placed in medium at 37°C for 1 to 30 min prior to being transferred to the guillotine for freezing. Once frozen, the cells were substituted with acetone in the presence of 0.25% glutaraldehyde and 0.5% osmium tetroxide at −90°C for at least 4 h. Then, the temperature was slowly raised (5°/h) to 0°C and kept there for 1 h.
For transmission electron microscopy, cells were washed briefly with pure acetone at 4°C, embedded in Epon at 4°C within 6 h, and polymerized at 60°C for 2 days. Ultrathin sections were stained with uranyl acetate and lead citrate and then examined in a Philips CM12 electron microscope (Eindhoven, The Netherlands) equipped with a slow-scan closed coupled device camera (Gatan, Pleasanton, Calif.).
For scanning electron microscopy, freeze-substituted cells were washed with acetone, placed in hexamethyl-disilazane (Fluka, Buchs, Switzerland) for 2 to 5 min, transferred into a vacuum unit for coating with gold-palladium (at an angle of 60°), and immediately examined in a Philips CM12 scanning transmission electron microscope utilizing the secondary electrons. Digital images were recorded by the digital aquisition system Digiscan (Gatan). Uninfected cells were used as controls.
RESULTS
Cryoimmobilization followed by freeze-substitution resulted in a distinct appearance of the membrane bilayers that enabled us to investigate the cell entry pathway despite the occasionally perturbed cytoplasmic architecture due to ice crystal formation. BHV-1 and BHV-5 follow similar pathways of cell entry (Fig. 1 to 3). The process, verified by about 180 observations, may be subdivided into four phases: attachment, fusion, perforation and penetration, and defusion and intracellular transport.
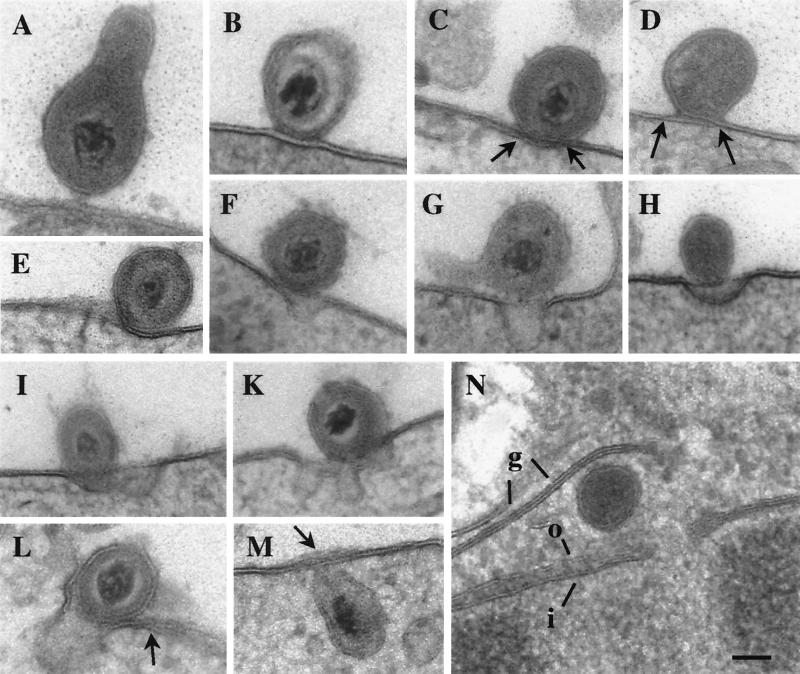
FIG. 1.
Transmission electron micrographs of BHV-1 entering MDBK cells. Panels A to E show attachment (A); fusion of the outer layer of the viral envelope with the outer layer of the plasma membrane (B); and fusion of the outer layer of the viral envelope with the inner layer of the plasma membrane and vice versa, resulting in a crossing (arrows) of the membranes (C to E). Panels E to L show various stages of invagination and perforation of the plasma membrane, including loss of membrane integrity close to the fusion site (E); virus particles just above a small invagination (F and G); the viral envelope being connected to the plasma membrane above an invagination (H [thick tangential section]), at the edge of an invagination (I), above a wide complicated invagination (K), and at the entrance of an invagination (L); the integrity of the plasma membrane is lost at the tip of the invagination but is crossed (panel L, arrow) just beside the virus particle. In panels M and N, virions are within the cytoplasm, and the envelope is associated with the plasma membrane, of which more than two layers are visible (arrow), indicating folds (M); a location close to a nuclear pore is shown (N [tangential section]), with the outer (o) and inner (i) nuclear membranes and Golgi membranes (g) indicated. Bar, 150 nm.
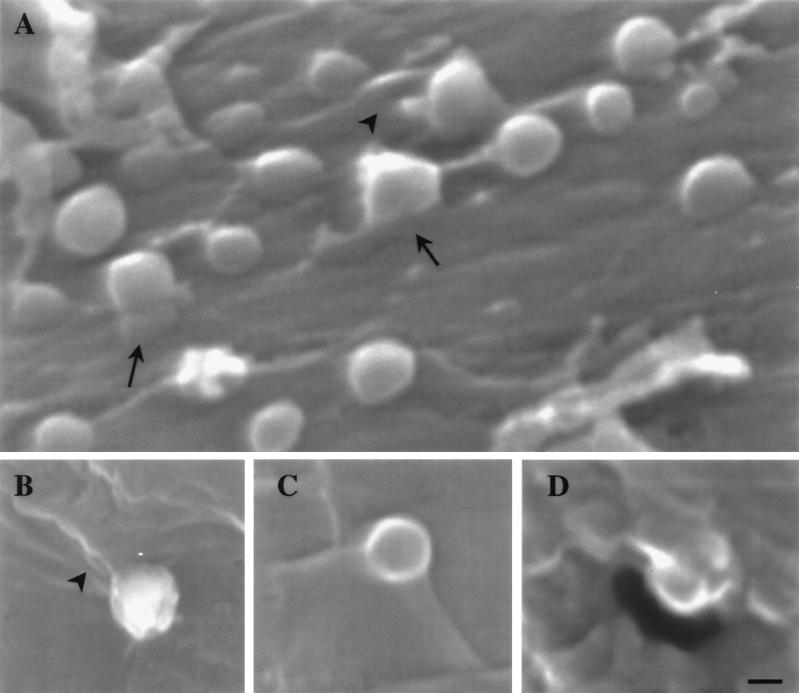
FIG. 3.
Scanning electron micrographs of BHV-1 (A and B) and BHV-5 (C and D). Viruslike particles with diameters of 240 to 290 nm are connected to the surface of MDBK cells by bands which run continuously from the particle surface to the cell surface (arrows) or by folds of the cell surface that are inserted into the particles. Some of the folds (arrowheads) suggest that the viruslike particles had rotated. Indentations or holes may be seen close to the particles (A and D). Bar, 150 nm.
Attachment.
Virions observed between 0 and 30 s after incubation at 37°C were all extracellular; some were in close proximity to the plasma membrane (Fig. 1A; Fig. 2A). These virions were considered to be in the attachment phase.
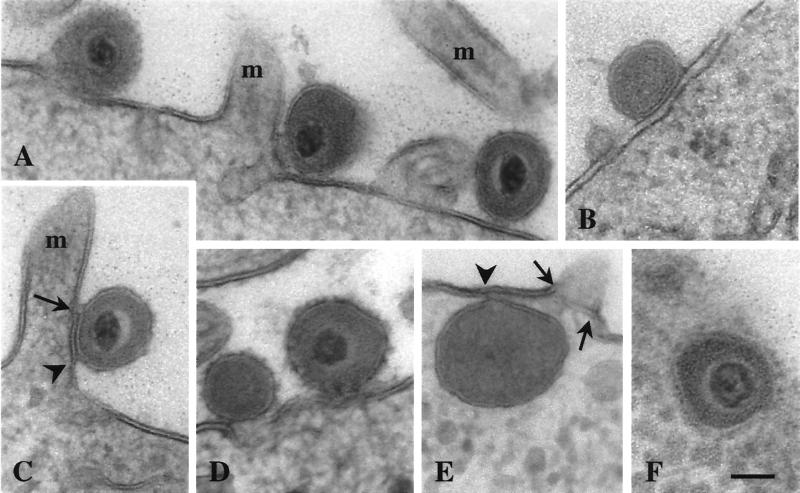
FIG. 2.
Transmission electron micrographs of BHV-5 entering MDBK cells. Virus particles attached to the plasma membrane (A, right), associated with the plasma membrane (left), and located above a small invagination of the plasma membrane beside a microvillus (m) are shown. Panels B to D show fusion of the outer layer of the viral envelope with the outer layer of the plasma membrane (B [tangential section]); complete fusion of the viral envelope with the plasma membrane forming a short crossing (arrow) and a “trilayer” (between the arrow and the arrowhead), loss of membrane integrity, and the early signs of perforation at the base of a microvillus (C); and virus particles above a small invagination (D [tangential section]) and in association with the undulated plasma membrane (right). Panel E (tangential section) shows a virion within the cytoplasm; the envelope is fused (arrowhead) with the plasma membrane, and, apart from the fusion site, the cytoplasmic matrix seems to leak through the perforated plasma membrane (arrows). In panel F, an enveloped virus particle within the cytoplasm can be seen. Bar, 150 nm.
Fusion.
After 30 to 40 s of incubation at 37°C the envelope of some virions was found to be partially fused with the outer plasma membrane, so that only three layers of the two bilayers were visible (Fig. 1B; Fig. 2B and C), suggesting that only the outer layer of the viral envelope had fused with the outer layer of the plasma membrane. Complete fusion was found as early as 45 s after the start of incubation. Complete fusion was always seen as fusion of the outer layer of the viral envelope with the inner layer of the plasma membrane and vice versa (Fig. 1C to E; Fig. 2C) that resulted in a “crossing” of the membranes at and beside the fusion site. This unique fusion resulted in a partial insertion of the viral envelope and in the formation of plasma membrane folds, as verified by scanning electron microscopy (Fig. 3).
Perforation and penetration.
Invagination and/or perforation of the plasma membrane close to the fusion site was observed to occur after 40 s of incubation (Fig. 2C). Later, virions were found either at the edges (Fig. 1F, I, and K), above (Fig. 1G, H, and K; Fig. 2A, D, and E), or within invaginations (Fig. 1L). The envelope was often seen to be associated with the plasma membrane (Fig. 1F to K; Fig. 2A, D, and E). Scanning electron microscopy of cells frozen after 50 to 70 s of temperature shift revealed viruslike particles (250 to 290 nm), whose surfaces continuously ran into the cell surfaces at the sites of bands and folds (Fig. 3). There were indentations or holes beside the viruslike particles. Some of these particles were situated at the edge of the holes, suggesting that these particles were entering the cell by rotation around the folds that originated by fusion of the viral envelope with the plasma membrane (Fig. 3D). In uninfected cells no roundish particles of this size were observed.
Defusion.
Enveloped virions were found within the cytoplasm after 50 s of incubation. The envelope was associated (Fig. 1M) or fused (Fig. 2E) with the plasma membrane. Later, enveloped virions were found within the cytoplasmic matrix (Fig. 2F); occasionally, they were close to the nucleus in front of a nuclear pore (Fig. 1N).
DISCUSSION
The high spatial and temporal resolution yielded data that indicate a novel entry pathway of BHV-1 and BHV-5 into MDBK cells, including a unique fusion of the viral envelope with the plasma membrane, invagination and perforation of the plasma membrane, and entry of the whole enveloped virus particle into the cytoplasm.
The complex entry pathway of herpesviruses starts by attachment of the virus particle to the cell surface via the binding of glycoproteins (19, 20, 43, 44) to receptors of the plasma membrane (4, 10, 34, 39). The findings obtained by cryotechnique-based electron microscopy suggest that, after BHV-1 and BHV-5 are bound, the outer layer of the viral envelope fuses with the outer layer of the plasma membrane (Fig. 1B and 2B). Whether the fusion of the two outer layers equals hemifusion (16, 23) is doubtful, since hemifusion proceeds in pore formation (22, 32). During the entry of BHV-1 and BHV-5, the initial fusion proceeds in a fusion of the outer layer of the viral envelope with the inner (cytoplasmic) layer of the plasma membrane and vice versa, resulting in formation of a “crossing” (Fig. 1C and D; Fig. 2C). Figure 4 schematically outlines the fusion area. The hydrophilic and hydrophobic nature of phospholipids demands that the phospholipids of the outer layer of the viral envelope must be driven into the outer phospholipid layer of the plasma membrane and finally through it, a process possibly mediated by one or more of the glycoproteins that are assumed to act as fusion proteins (6, 27). The splitting of the phospholipid layers for subsequent fusion may require specific domains at both sites, the plasma membrane and the viral envelope, and a specific mechanism for triggering and accomplishing fusion.
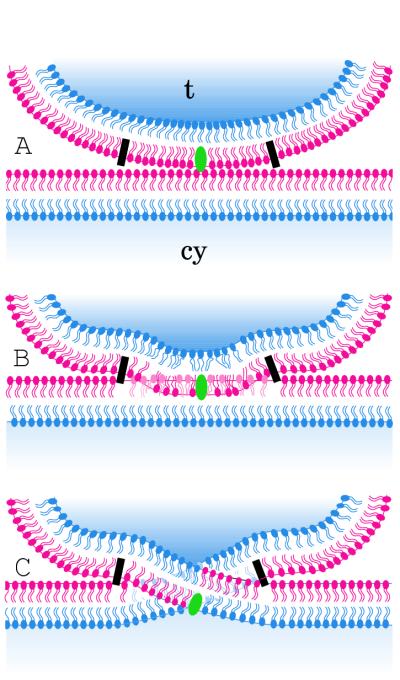
FIG. 4.
Schematic drawing of the fusion events of the viral envelope with the plasma membrane. The panels show attachment of the virion to the plasma membrane mediated by glycoprotein C and/or D (green) (A), fusion of the outer layer of the viral envelope with the outer layer of the plasma membrane (B), and fusion of the outer layer of the viral envelope with the cytoplasmic layer of the plasma membrane and vice versa (C). This type of fusion implies a crossing of the membranes. Fusion requires regulatory mechanisms that are indicated by a simple black rectangle that represents the probable involvement of glycoproteins. cy = cytoplasm; t = tegument.
The envelope of herpesviruses is believed to originate by wrapping of the Golgi membranes (reference 8 and unpublished data). If so, the viral envelope is an inside-out particle. It is principally able to fuse with the plasma membrane, maintaining transmembrane asymmetry (2), e.g., as shown previously for the fusion of influenza virus with liposomes (17). Fusion of the herpesvirus envelope with the plasma membrane results in a partial insertion of the viral envelope into the plasma membrane. For geometric reasons, folds of the plasma membrane must arise, as was clearly shown by transmission (Fig. 1C, D, and L; Fig. 2D) and scanning electron microscopy (Fig. 3), and/or the integrity of both the plasma membrane and the viral envelope must be lost in a circumscribed area. The meaning of this type of membrane fusion is unclear. It certainly does not enable the virus to penetrate the plasma membrane. Rather, the virus gains access to the cytoplasm through an invagination (Fig. 1F to L; Fig. 2A and C to E; Fig. 3A to D) that develops close to the fusion area and that opens toward the cytoplasm. Whether this complicated way of cell entrance is mediated by glycoproteins assumed to function as penetration proteins (7, 27) still needs to be clarified. Folds of the plasma membrane seem to form ridges, which may function as axes for rotating the virion into the nearby invagination (Fig. 3) or may generate forces that drive the virus particle into the cytoplasm. The mechanisms for the special type of membrane fusion, the invagination process, the perforation of the plasma membrane, and the way the virion is driven into the cytoplasm are not yet understood. The data presented here may help to provide a basis for elucidating the functions of the various glycoproteins that are essential for herpesvirus cell entry.
After herpesviruses have gained access to the cytoplasm, the viral envelope probably defuses soon from the plasma membrane, which in turn must be restored to maintain cellular integrity. It is assumed that nucleocapsids are transported along microtubules to the microtubule organizing center (30), which is situated close to the nucleus. We found enveloped viruses close to the nucleus by as early as 15 min after start of incubation. Work to further clarify this route is in progress.
The entry process of BHV-1 and BHV-5 was found to be completed in less than 60 s after a temperature shift from 4 to 37°C. About 30 s were required for the binding and fusion of the membranes and for the invagination process. The complicated cell entry mechanism of these two members of the herpesvirus family into MDBK cells requires subtle mechanisms, including binding to the cell surface, fusion of membranes, signaling for and completion of the invagination process, perforation of the plasma membrane without a substantial loss of cytoplasmic material, generation of the energy to drive the virus particle into the cytoplasm, and defusion and restoration of the plasma membrane after virus entry is completed.
ACKNOWLEDGMENTS
We thank M. Ackermann, H. Adler, E. Kellenberger, and R. Wyler for helpful suggestions and critical reading of the manuscript and M. Balushev for assistance in preparing the manuscript.
This study was supported by Stiftung für wissenschaftliche Forschung an der Universität Zürich.
REFERENCES
- 1.Cope G H, Williams M A. Quantitative studies of the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. J Microsc. 1969;90:31–46. doi: 10.1111/j.1365-2818.1969.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 2.Devaux P F. Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- 3.Elder H Y, Gray C C, Jardine A G, Chapman J N, Biddlecombe W H. Optimum conditions for cryoquenching of small tissue blocks in liquid coolants. J Microsc. 1982;126:45–61. doi: 10.1111/j.1365-2818.1982.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 4.Flynn S J, Ryan P. A heterologous heparin-binding domain can promote functional attachment of a pseudorabies virus gC mutant to cell surfaces. J Virol. 1995;69:834–839. doi: 10.1128/jvi.69.2.834-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox C H, Johnson F B, Whiting J, Roller P P. Formaldehyde fixation. J Histochem Cytochem. 1985;8:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 6.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller A O, Santos R E, Spear P G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granzow H, Weiland F, Jöns A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayat M A. Fixation for electron microscopy. New York, N.Y: Academic Press, Inc.; 1981. [Google Scholar]
- 10.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Verdun D, Quintana C, Masson C, Gautier T, Arnoult J. Cryofixation, cryosubstitution, cryo-embedding for visualizing of nuclear ultrastructure and for immunodetection in HeLa cells. Biol Cell. 1991;72:121–132. [Google Scholar]
- 13.Hughson F M. Enveloped viruses: a common mode of membrane fusion? Curr Biol. 1997;7:R565–R569. doi: 10.1016/s0960-9822(06)00283-1. [DOI] [PubMed] [Google Scholar]
- 14.Kanaseki T, Kawasaki K, Murata M, Ikeuchi Y, Ohnishi S. Structural features of membrane fusion between influenza virus and liposome as revealed by quick-freezing electron microscopy. J Cell Biol. 1997;137:1041–1056. doi: 10.1083/jcb.137.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellenberger E. The potential of cryofixation and freeze substitution: observations and theoretical considerations. J Microsc. 1991;161:183–203. doi: 10.1111/j.1365-2818.1991.tb03083.x. [DOI] [PubMed] [Google Scholar]
- 16.Kemble G W, Danieli T, White J M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 17.Klotz K-H, Bartoldus I, Stegman T. Membrane asymmetry is maintained during influenza-induced fusion. J Biol Chem. 1996;271:2383–2386. doi: 10.1074/jbc.271.5.2383. [DOI] [PubMed] [Google Scholar]
- 18.Knoll G, Braun C, Plattner H. Quenched flow analysis of exocytosis in paramecium cells: time course, changes in membrane structure, and calcium requirements revealed after rapid mixing and rapid freezing of intact cells. J Cell Biol. 1991;113:1295–1304. doi: 10.1083/jcb.113.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, van Drunen Littel-van den Hurk S, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang X, Babiuk L A, van Drunel Littel-van den Hurk S, Fitzpatrick D R, Zamb T J. Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J Virol. 1991;65:1124–1132. doi: 10.1128/jvi.65.3.1124-1132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101:87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- 22.Melikyan G B, Brener S A, Ok D C, Cohen F S. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J Cell Biol. 1997;136:995–1005. doi: 10.1083/jcb.136.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melikyan G B, White J M, Cohen F S. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan C H, Rose M, Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968;2:507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas G. Advantages of fast-freeze fixation followed by freeze-substitution for the preservation of cell integrity. J Electron Microsc Tech. 1991;18:395–405. doi: 10.1002/jemt.1060180408. [DOI] [PubMed] [Google Scholar]
- 26.Nir S, Düzgünes N, Pedroso de Lima M C, Hoekstra D. Fusion of enveloped viruses with cells and liposomes. Cell Biophys. 1990;17:181–201. doi: 10.1007/BF02990496. [DOI] [PubMed] [Google Scholar]
- 27.Peeters B, De Wind N, Hooisma M, Wagenaar F, Gielkens A, Moorman R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintana C. Cryofixation, cryosubstitution, cryoembedding for ultrastructural, immunocytochemical and microanalytical studies. Micron. 1994;25:63–99. doi: 10.1016/0968-4328(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 29.Schwyzer M, Ackermann M. Molecular virology of ruminant herpesviruses. Vet Microbiol. 1996;53:17–29. doi: 10.1016/s0378-1135(96)01231-x. [DOI] [PubMed] [Google Scholar]
- 30.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 32.Stegmann T, White J M, Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topilko A, Michelson S. Hyperimmediate entry of human cytomegalovirus virions and dense bodies into human fibroblasts. Res Virol. 1994;145:75–82. doi: 10.1016/s0923-2516(07)80009-4. [DOI] [PubMed] [Google Scholar]
- 34.Tufaro F. Virus entry: two receptors are better than one. Trends Microbiol. 1997;7:257–259. doi: 10.1016/S0966-842X(97)01057-3. [DOI] [PubMed] [Google Scholar]
- 35.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Schack M L, Fakan S, Villiger W, Müller M. Cryofixation and cryosubstitution: a useful alternative in the analyses of cellular fine structure. Eur J Histochem. 1993;37:5–18. [PubMed] [Google Scholar]
- 37.Weibull C, Christiansson A. Extraction of proteins and membrane lipids during low temperature embedding of biological material for electron microscopy. J Microsc. 1986;142:79–86. doi: 10.1111/j.1365-2818.1986.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 38.Weibull C, Christiansson A, Carlemalm E. Extraction of membrane lipids during fixation, dehydration and embedding of Acholeoplasma laidlawii cells for electron microscopy. J Microsc. 1983;129:201–207. doi: 10.1111/j.1365-2818.1983.tb04174.x. [DOI] [PubMed] [Google Scholar]
- 39.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White J M. Membrane fusion. Science. 1992;258:917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- 41.Wild P, Bertoni G, Schraner E M, Beglinger R. Influence of calcium and magnesium containing fixatives on the ultrastructure of parathyroids. Micron Microsc Acta. 1987;18:259–271. [Google Scholar]
- 42.Wild P, Gabrieli A, Schraner E M, Pellegrini A, Thomas U, Frederik P M, Stuart M C A, von Fellenberg R. Reevaluation of the effect of lysozyme on Escherichia coli employing ultrarapid freezing followed by cryoelectronmicroscopy or freeze substitution. Microsc Res Tech. 1997;39:297–304. doi: 10.1002/(SICI)1097-0029(19971101)39:3<297::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 43.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zsak L, Sugg N, Ben-Porat T, Robbins A K, Whealy M E, Enquist L W. The gIII glycoprotein of pseudorabies virus is involved in two distinct steps of virus attachment. J Virol. 1991;65:4317–4324. doi: 10.1128/jvi.65.8.4317-4324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]