Abstract
Objectives:
This study examined the degree to which breast cancer patients’ psychological well-being is facilitated through empathic provider communication. We explored symptom/prognostic uncertainty reduction as a mechanism through which provider communication influences patient psychological adjustment. Additionally, we tested if treatment status moderates this relationship.
Methods:
Informed by uncertainty in illness theory, current (n = 121) and former (n = 187) breast cancer patients completed questionnaires about perceptions of their oncologists’ empathy and their symptom burden, uncertainty, and adjustment to their diagnosis. Structural equation modeling (SEM) was conducted to test hypothesized relationships between perceived provider empathic communication, uncertainty, symptom burden, and psychological adjustment.
Results:
SEM supported the following: (1) higher symptom burden was associated with increased uncertainty and reduced psychological adjustment, (2) lower uncertainty was associated with increased adjustment, and (3) increased empathic communication was associated with lower symptom burden and uncertainty for all patients (χ2(139) = 307.33, p < .001; RMSEA = .063 (CI .053, .072); CFI = .966; SRMR = .057). Treatment status moderated these relationships (Δχ2 = 264.07, Δdf = 138, p < .001) such that the strength of the relationship between uncertainty and psychological adjustment was stronger for former patients than for current patients.
Conclusions:
Results of this study reinforce the importance of perceptions of provider empathic communication as well as the potential benefits of eliciting and addressing patient uncertainty about treatment and prognosis throughout the cancer care continuum.
Practice Implications:
Patient uncertainty should be a priority for cancer-care providers both throughout and post-treatment for breast cancer patients.
Keywords: Uncertainty, Psychological adjustment, Breast cancer, Patient-provider communication, Empathic communication
1. Introduction
Being diagnosed with breast cancer is an extremely stressful life event. Beginning with the initial diagnostic process (detection of a lump, biopsy), patients demonstrate levels of emotional distress that persist throughout treatment [1]. Beyond the initial diagnosis, there are a multitude of routine cancer care events that can elicit strong stress responses. From decision-making about treatment options to coping with symptoms post-treatment, patients throughout the cancer trajectory are at high-risk to develop psychological comorbidities including depression, anxiety, and general psychological distress [2–5]. Moreover, patients whose psychological well-being needs remain unmet can have significant health consequences [6,7]. Thus, supporting breast cancer patients’ psychological well-being is crucial to facilitating holistic cancer care. This study aims to explore the extent to which breast cancer patients’ psychological well-being can be facilitated through perceived effective, empathic provider communication and addressing patients’ uncertainty about cancer treatment and/or prognosis. Additionally, this study explores how the relationship between provider communication, patient uncertainty, and psychological adjustment is moderated by treatment status (undergoing treatment versus treatment completed).1
1.1. Illness uncertainty, symptom burden, and cancer
Illness uncertainty, defined as the inability to make meaning of events related to illness, is a significant source of psychological distress for breast cancer patients throughout the cancer continuum [8]. Uncertainty about cancer-related symptoms, symptom management, diagnosis, prognosis, and treatment all contribute to reports of worse quality of life [9–12]. Further, uncertainty persists post-treatment as former breast cancer patients move into a health maintenance and surveillance stage. Despite having completed treatment, former breast cancer patients experience uncertainty around new or changing symptoms (i.e., whether new symptoms are related to side effects of treatment versus cancer recurrence or a new health concern) and about cancer recurrence risk [13,14]. The consequence of uncertainty about one’s illness might include decisional regret about a treatment plan and rumination, both of which can cause significant psychological distress [15]. For example, higher uncertainty has been associated with fewer self-care behaviors for current patients [16] and with worse mood and more thoughts about recurrence for former patients [17]. In addition to symptom uncertainty, the burden of physical symptoms also contributes to worse psychological adjustment. Patients with higher levels of symptom burden also report higher levels of symptom uncertainty, highlighting the relationship between uncertainty and adjustment described above [17]. Thus, it is critical to better understand the relationship between uncertainty, symptom burden, and patients’ psychological well-being and how the relationship between uncertainty and adjustment might function across the illness trajectory to inform patient care.
1.2. The role of provider communication
One way to address patients’ uncertainty is through effective provider communication, including patient-centered behaviors such as displays of empathy [18,19]. Empathic provider communication is defined as provider communication behaviors that are attuned to, recognize, and support patient emotions and foster a relationship in which patients feel cared for holistically [20,21]. Empathic communication can be facilitated through both verbal (open-ended questions, responding explicitly to patient emotions) and nonverbal (nodding, eye contact, posture) communication. Through empathic communication and explicit conversations about treatment, prognosis, and overall illness uncertainty, it may be possible to alleviate some degree of breast cancer patients’ psychological distress. Effective empathic provider communication has been associated with better psychological adjustment [22,23]. For example, when providers are perceived as more empathic during the initial breast cancer diagnosis, the associated psychological benefits have been seen at least three months later [22].
Perceptions of provider communication have also been associated with patient-reported symptom burden. Among lung cancer patients with low understanding about their illness (i.e., high illness uncertainty) and who perceive their provider as having poor communication skills, higher symptom burden is associated with more psychological distress [24]. By contrast, among patients with high uncertainty, perceiving their provider as having good communication skills has a buffering effect. For these patients, symptom burden does not predict psychological distress. Although effective communication has been linked to psychological well-being and to a buffering effect on symptom burden and distress, what remains unclear is the intermediary psychological processes that connect provider communication with these distal mental health outcomes for cancer patients.
One possible mechanism for how empathic provider communication influences patient psychological health in the breast cancer context is through uncertainty reduction. Patients who feel their provider is communicating empathically may feel that their psychological needs are being addressed [25]. Feeling supported in this way may encourage a richer dialogue in which patient uncertainty can be explored. Without this exploration, patients’ fears and worries may remain unaddressed, leading to further psychological stress (i.e., a patient may feel their provider has dismissed their worry and not bring it up again even if they still feel distress about whether the symptom could be indicative of recurrence). On the other hand, when patients are satisfied with their provider’s communication related to uncertainty, they report less uncertainty and more perceived control over their health [26,27]. With this in mind, the following hypothesis was proposed:
H1. : Current and former breast cancer patients who perceive their oncologist as having more empathic communication will report (a) less uncertainty about their cancer and (b) less symptom burden.
1.3. Uncertainty in illness theory
A relevant framework for considering how uncertainty is both elicited and managed for patients with breast cancer is the uncertainty in illness theory [28]. This theory posits that uncertainty about illness is influenced by a patient’s perceptions of a health illness-related stimuli or the stimuli frame, such as symptom pattern or familiarity with the health event. Interpretation of these stimuli can be influenced by external structure providers, including perceptions of provider credibility. The theory also posits that structure providers can directly influence the stimuli frame, with better structure providers (i.e., better perceptions of the provider) posited to reduce perceived symptom burden. Further, the theory suggests that levels of uncertainty influence illness appraisal and, subsequently, coping strategies and adaptation to illness. Informed by this theory, the current study sought to test how symptom uncertainty was influenced by provider empathic communication (a structure provider) and symptom burden (a stimuli frame) and how uncertainty and burden influenced psychological adjustment in breast cancer patients. Given the relationships reviewed above, the following hypotheses were proposed:
H2. : Current and former breast cancer patients who have lower symptom burden will report (a) less uncertainty and (b) better psychological adjustment to their cancer diagnosis.
H3. : Current and former breast cancer patients who have less illness uncertainty will report better psychological adjustment to their cancer diagnosis.
1.4. The role of treatment status
Finally, we explored the differences in treatment status (current versus former patients) on the variables described. Current and former patients experience different appointment types (i.e., treatment decision-making for current patients, watchful waiting for former patients), have had different lengths of time to adjust to diagnoses, and, potentially, have different relationships with their provider (i.e., more or less time to develop a relationship). Because these differences have not been directly assessed for their effect on the hypothesized relationships in this study, we proposed the following research question:
RQ1. : Does treatment status (current versus former patient) moderate the proposed relationships between perception of oncologist empathy, uncertainty, symptom burden, and psychological adjustment?
2. Methods
2.1. Participants and procedure
This study utilized data from a larger cross-sectional online survey collected between June 2020 and December 2022 aimed at characterizing experiences of cancer patients and their communication with their oncology care team. Current (n = 121) and former (n = 187) cancer patients were recruited through the Love Research Army, a research registry hosted by the Dr. Susan Love Foundation for Breast Cancer Research, a national advocacy organization for breast cancer patients, survivors, and at-risk family members. All cancer patients are considered cancer survivors from the time of diagnosis. The labels of current and former patients used in this study reflect the stage of cancer treatment most relevant to the current investigation. Participants self-selected into current and former patient designations. Current breast cancer patients were those currently undergoing cancer treatment and were less than two years post-diagnosis. Former patients were those who had completed treatment (surgery, chemotherapy, and/or radiation therapy) and were two to five years post-diagnosis. Potential participants were emailed a link to access an approximately 30-minute online survey directly by the Love Research Army.
Additional eligibility criteria included participant age (18 years of age or older), ability to read English and provide informed consent, and have access to a computer or device with Internet access for survey completion. Additionally, as part of a larger study aim, eligible participants were required to indicate that they regularly brought a support person with them to their oncology visits. Demographic information including participant age, race, ethnicity, education, and marital status were collected, and significant differences were not observed across the current and former patient groups on these variables (see Table 1). Participants had the option to enter a drawing for one of three $50 gift cards after survey completion. This study was approved by an Institutional Review Board.
Table 1.
Demographic information and descriptive statistics.
| Total | Current patients | Former patients | p-value | |
|---|---|---|---|---|
|
| ||||
| Age – Mean (SD) | 57.01 (12.06) | 57.14 (12.60) | 56.92 (11.73) | .88 |
| NSa | ||||
| Education – frequency | .79 | |||
| NSb | ||||
| High school graduate | 3 | 1 | 2 | |
| Vocational, technical, business, or trade school certificate or diploma | 17 | 6 | 11 | |
| Some college | 53 | 24 | 29 | |
| Bachelor’s degree | 101 | 41 | 60 | |
| Master’s, professional, or doctoral degree | 129 | 46 | 83 | |
| Race – frequency | .42 | |||
| NSb | ||||
| White/Caucasian | 256 | 105 | 150 | |
| Black/African American | 10 | 3 | 7 | |
| American Indian/Alaska Native | 1 | 0 | 1 | |
| Asian | 4 | 0 | 4 | |
| Multiracial | 7 | 3 | 4 | |
| Ethnicity – frequency | .36 | |||
| NSb | ||||
| Hispanic/Latino | 37 | 17 | 20 | |
| Marital status – frequency | .95 | |||
| NSb | ||||
| Single | 26 | 10 | 16 | |
| Married/living as | 217 | 89 | 127 | |
| Divorced | 20 | 7 | 13 | |
| Widowed | 9 | 3 | 6 | |
| Separated | 2 | 1 | 1 | |
| Dating | 4 | 1 | 3 | |
Two-tailed t-test.
χ2 test.
2.2. Measures
For each of the following measures, initial analyses included: (1) confirmatory factor analyses (CFA) to confirm scale unidimensionality, (2) reliability assessment using Cronbach’s alpha for scale composite scores, and (3) t-tests to compare participant composite responses for current versus former patients. Additional information is included by variable in the Appendix. CFA was performed for each measure using combined data from both current and former patients. Individual items were retained and/or removed from each scale based on a combination of face validity, theoretical relevance, factor loadings, and overall model fit. Model fit was assessed using a combination of χ2, RMSEA, CFI, and SRMR [29]. Good fit was considered at RMSEA < .060, CFI > .950, and SRMR < .080. Adequate fit was considered at RMSEA < .080, CFI > .900, and SRMR < .100 [30]. Because each group of participants (current and former patients) individually were regarded as a smaller N (defined as < 200), χ2 was considered adequate if χ2/df < 3 [31–33].
2.2.1. Perceived symptom burden
Perceived symptom burden (H1a, H2a, H2b) was measured using one scale from the M. D. Anderson Symptom Inventory [34]. Participants were asked to rate how often their symptoms interfered with six aspects of daily life on a 10-point scale (did not interfere to interfered completely). An example item is, “Enjoyment of life?” Based on factor loadings and model fit, 1 item was not included (see Appendix, Table A1). The five retained items were averaged for a final composite score that could range from 0 to 10. Higher scores indicated increased symptom burden. The final scale was unidimensional and achieved high reliability (α = .92). Modification indices supported three covariations between error terms, improving overall model fit (see Appendix, Fig. A1 and A2). The final factor structure supported good data fit (χ2(2) = 4.89, p = .096; RMSEA = .066 (CI <.001, .146); CFI = .998; SRMR = .012).
2.2.2. Perceived symptom uncertainty
Symptom uncertainty (H1b, H2a, H3) was measured using a modified 3-item scale derived from the Uncertainty in Illness Scales [35]. Participants rated the degree to which they agreed with statements about their cancer-related uncertainty on a 5-point scale (strongly disagree to strongly agree). An example item is, “Because of the unpredictability of my cancer, I cannot plan for the future” (see Appendix, Table A2). All items were retained and averaged for a composite score that could range from 1 to 5. Higher scores indicated increased symptom uncertainty. The final scale was unidimensional and achieved acceptable reliability (α = .73). Given the saturated model, no further covariations were made based on modification indices and model fit was not assessed.
2.2.3. Perception of empathic communication
Perceptions of the oncologist’s empathic communication (H1a, H1b) were assessed using the Consultation and Relational Empathy (CARE) questionnaire [36]. Items were adapted to reflect a cancer setting, replacing “doctor” with “oncologist.” The 10-item measure asked participants to rate statements related to their oncologist’s empathic communication on a 6-point scale (really poor to excellent) based on the prompt, “How was the oncologist at…”. An example item from this measure includes, “Showing care and compassion.” Based on theoretical relatedness and factor loadings, 4 items were not included resulting in a final scale with 6 items (see Appendix, Table A3). Retained items were averaged, and the final composite score ranged from 0 to 5. Higher scores indicated increased empathic provider communication. The final scale was unidimensional and achieved high reliability (α = .97). Modification indices supported two covariations between error terms, improving overall model fit (see Appendix, Figs. A4 and A5). The final factor structure supported good data fit (χ2(7) = 14.16, p = .048; RMSEA = .058 (CI .005, .101); CFI = .997; SRMR = .008).
2.2.4. Adjustment to the cancer diagnosis
Participants’ psychological adjustment to their cancer diagnosis (H2b, H3) was measured using a modified form of the Mini-Mental Adjustment to Cancer scale [37]. Eight items from four of the five subscales (two items each for fighting spirit, helplessness-hopelessness, anxious preoccupation, and cognitive avoidance) were included based on factor loadings in a previous study in a breast cancer population and face validity [38]. An example item is, “I suffer great anxiety about having cancer.” All items were on a 5-point scale (does not apply to me to very strongly applies to me). Based on factor loadings and model fit, 3 items were not included resulting in a final scale with 5 items (see Appendix, Table A4). The final scale was unidimensional and achieved good reliability (α = .83). Retained items were averaged, and final composite scores ranged from 1 to 5. Higher scores indicated better psychological adjustment. Modification indices supported two covariations between error terms, improving overall model fit (see Appendix, Figs. A6 and A7). The final factor structure supported a good fit for the data (χ2(3) = 5.31, p = .150; RMSEA = .050 (CI <.001, .119); CFI = .996; SRMR = .014).
2.3. Analyses
Data were cleaned and screened at the univariate level. Mean scale replacement was used for individual items if missing two or fewer items per scale for a total of 16 individual mean replacements (< 0.20% of all items replaced). Bivariate correlations with Bonferroni-adjusted significance levels (two-tailed) were completed across variables to assess initial correlation between constructs (see Table 3). Correlation coefficients were compared to assess the strength of correlations across current and former patients (see Table 3). Demographic variables were compared between current and former patients using χ2 and two-tailed t-tests (see Table 1). Hypothesized relationships among model variables were compared across current and former patients using one-tailed t-tests (see Table 2). Three structural equation models (SEM) were run to assess whether the hypothesized model was supported by the data and to test the moderation effect of treatment status on the hypothesized variable relationships (RQ1). Model 1 was conducted using combined data from both current and former patients. Model 2 was conducted using only current patient data and model 3 was conducted with only former patient data. Overall model moderation was assessed through a multiple groups analysis. Model 1 was run twice: once with no constraints and once with all structural elements constrained. Consistent with multiple groups analysis, the change in χ2 and degrees of freedom between the restricted and unrestricted models were compared. Model fit was assessed as above. Data were analyzed using STATA/MP (version 17.0).
Table 3.
Correlation between variables included in structural equation models.
| Current patients | Former patients | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Variable | (1) | (2) | (3) | (4) | (1) | (2) | (3) | (4) |
|
| ||||||||
| Symptom burden | 1 | 1 | ||||||
| Illness uncertainty | .51 ***a | 1 | .39 ***a | 1 | ||||
| Perceived provider empathic communication | −.30 ** | −.47 *** | 1 | −.22 * | −.44 *** | 1 | ||
| Psychological adjustment to diagnosis | −.58 *** | −.62 *** | .31 ** | 1 | −.55 *** | −.65 *** | .36 *** | 1 |
Note. Two-tailed Bonferroni-adjusted significance levels. Degrees of freedom = 118 for current patients and 187 for former patients across all variables.
Comparisons across treatment status using z-tests of Fisher-transformed correlation coefficients revealed only one statistically different correlation: the strength of the correlation between illness uncertainty and symptom burden was stronger for current patients than for former (p = .026).
p < .05
p < .01
p < .001
Comparisons across treatment status using z-tests of Fisher-transformed correlation coefficients revealed only one statistically different correlation: the strength of the correlation between illness uncertainty and symptom burden was stronger for current patients than for former (p = .026).
Table 2.
Descriptive statistics for study variables.
| Confirmatory factor analysis | Current patientsa | Former patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Variable | Potential range | α | χ2 (df)b | RMSEA | CFI | SRMR | M | SD | M | SD |
|
| ||||||||||
| Symptom burden | 0–10 | .92 | 1.51 (2) | < .001 | 1.00 | .007 | 4.52 | 2.60 | 3.66 | 2.40 |
| Illness uncertainty | 1–5 | .73 | – | – | – | – | 2.35 | .99 | 2.09 | .86 |
| Perceived empathic communication | 0–5 | .97 | 13.96 (7) | .057 | .997 | .008 | 3.66 | 1.28 | 4.06 | 1.06 |
| Psychological adjustment | 1–5 | .83 | 5.18 (3) | .049 | .997 | .014 | 3.57 | .90 | 3.89 | .79 |
Note. α = Cronbach’s alpha, composite scales for combined current and former patients; M = mean; SD = standard deviation; RMSEA = root mean square error of approximation; CFI = confirmatory fit index; SRMR = standardized root mean square residual.
Current patients’ mean scores are significantly less favorable than former patients based on one-tailed t-tests at a level p < .01 across all measures (e.g., current patients report more symptom burden, more uncertainty, less empathic communication, worse adjustment).
All χ2 values non-significant at a level p > .05.
3. Results
3.1. Participants, demographics, and descriptive statistics
In total, 332 survey responses were collected. Of these, participants were excluded if they did not report their cancer type (n = 4), reported non-breast cancer diagnoses (n = 9), identified as male2 (n = 3), or did not provide responses for more than two items per scale (n = 8). After the above exclusions, a total of 309 participants were retained for analyses (current patients, n = 121; former patients, n = 187). Model variables were compared across current and former patients, and, in all cases, current patients reported less favorable ratings than former patients (see Table 2). Comparisons of the strength of the correlations revealed only one significantly different correlation across patient groups: the strength of the correlation between illness uncertainty and symptom burden was stronger for current patients than for former (p = .026). In other words, patients who reported more symptom burden tended to also report more illness uncertainty, and this association was stronger for current patients than for former (r = .51 versus r = .39).
3.2. Structural equation modeling
3.2.1. Model one: combined current and former patient data
SEM was performed to test whether the theoretical model in Fig. 1 was supported for the combined sample using the measurement structures established through CFA. Overall, the hypothesized model was well-supported with the combined current and former patient data (χ2(139) = 307.33, p < .001; RMSEA = .063 (CI .053, .072); CFI = .966; SRMR = .057) (see Fig. 2). All structural paths in the model were statistically significant (p <.001) except for the path from symptom burden to adjustment (p = .074) and directionality supported the hypothesized relationships (H1a/b, H2a, and H3 supported). In other words, people who reported their provider as having more empathic communication reported less burden of their cancer symptoms (H1a) and less uncertainty about their illness (H1b). Participants who reported more impact of their symptoms reported more uncertainty about their cancer (H2a), and participants who reported more uncertainty reported worse psychological well-being (H3).
Fig. 1.
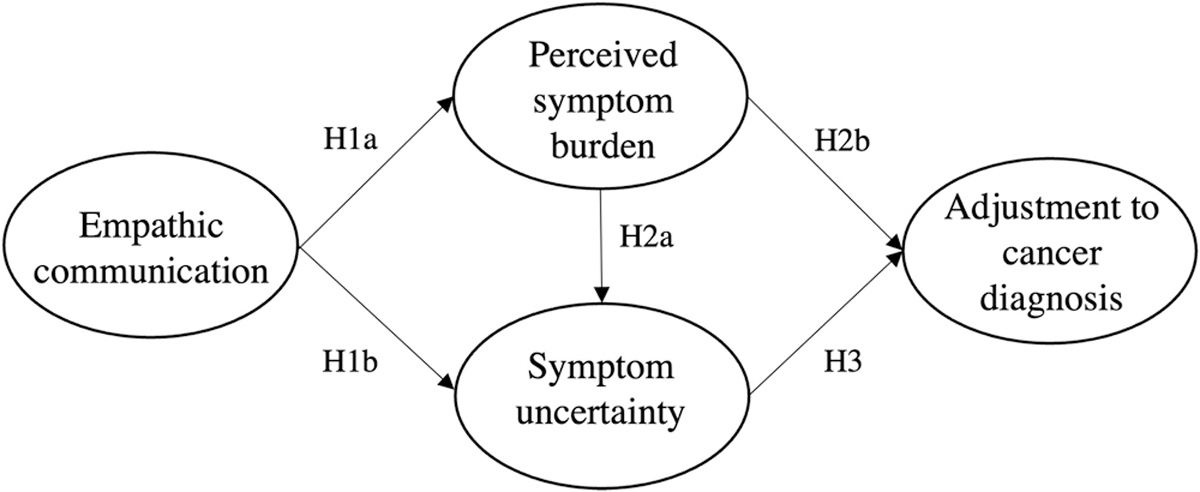
Hypothesized psychological adjustment to uncertainty models.
Fig. 2.
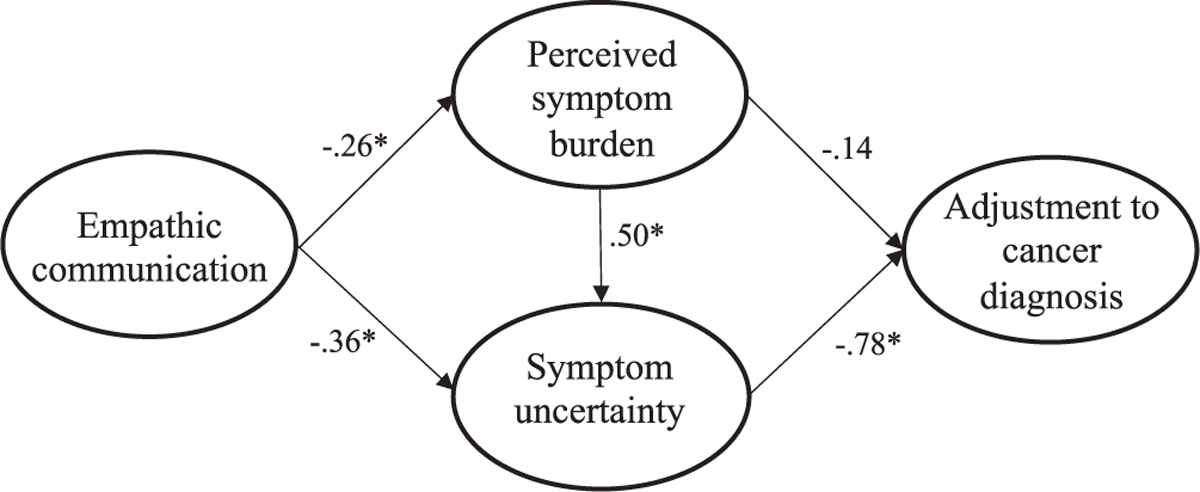
Model 1: Uncertainty models for all patients’ (combined current and former) psychological adjustment. Note: Parameter estimates are standardized. Model fit indices were χ2(139) = 307.33, p < .001; RMSEA = .063 (CI .053,.072); CFI = .966; SRMR = .057. * p < .001.
3.2.2. Models two and three: moderation effect of treatment status
Separate SEM models were performed for current and former patients to test the moderation effect of treatment status using the established measurement structures. All hypothesized paths were retained in both models. A single additional covariance was added between error terms of two illness uncertainty items as indicated by modification indices for the former patient model (see Appendix, Figs. A8, A9, A10 and A11). This modification was theoretically supported based on the item wording (see Appendix, Table A2) and applied for both current and former patient models. The covariance was added to both models to allow for direct comparison of the models. Both the current (χ2(139) = 216.84, p < .001; RMSEA = .068 (CI .050, .085); CFI = .961; SRMR = .067) and former (χ2(139) = 345.46, p < .001; RMSEA = .089 (CI .078, .101); CFI = .934; SRMR = .071) patient models were adequately supported by the data (see Fig. 3). Additionally, the addition of the covaried error term improved the fit for both current (Δχ2 = 15.03, Δdf = 1, p < .001) and former (Δχ2 = 25.45, Δdf = 1, p < .001) patient models. Consistent with model 1, all paths in the model were statistically significant (p < .001) except for the path between symptom burden and psychological adjustment (H1a/b, H2a, and H3 supported). This path was non-significant for models 2 and 3. Model moderation was assessed by comparing the unconstrained model 1 (unconstrained; χ2 (139) = 307.33) to a constrained model (all structural elements hypothesized to be equal; χ2 (285) = 571.41). These results supported a moderation effect (Δχ2 = 264.076, Δdf = 138, p<.001) of treatment status (RQ1).3 Although no statistical differences were seen for individual paths (p > .05 by Wald test of invariance), the largest structural difference between the models of current and former patients was the strength of the relationship between symptom uncertainty and psychological adjustment (−.66 and −.91, respectively). In other words, when patients reported uncertainty about their cancer, former patients had more negative reports of psychological well-being than current patients.
Fig. 3.
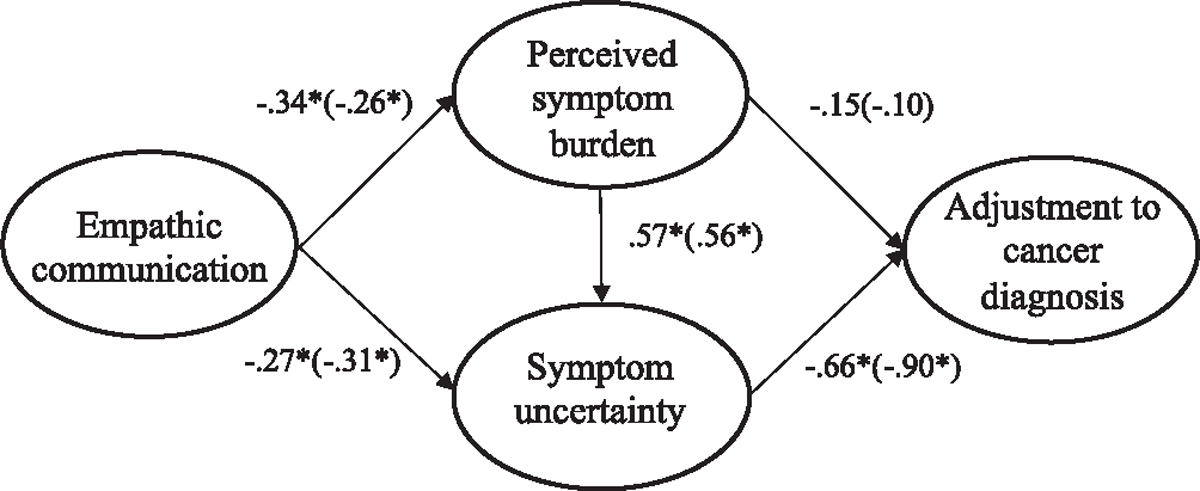
Models 2 and 3: Uncertainty models for current and former (within parentheses) patients’ psychological adjustment.Note: Parameter estimates are standardized. Model fit indices for current cancer patients (paths outside of parentheses) were χ2(139) = 216.84, p < .001; RMSEA = .068 (CI .050, .085); CFI = .961; SRMR = .067. Model fit indices for former cancer patients (paths inside of parentheses) were χ2(139) = 345.463, p < .001; RMSEA = .089 (CI .078, .101); CFI = .934; SRMR = .071. A significant moderation effect was seen across treatment groups (Δχ2 = 257.60, Δdf = 147, p<.001). * p < .01.
3.2.3. Alternative models
Although our hypothesized model (Fig. 1) was grounded in theory, cross-sectional data limit the extent to which causal relationships can be inferred. To consider possible alternative relationships among the constructs in our model, we tested a series of alternative models. The first alternative model reversed the hypothesized direction between perceived symptom burden and symptom uncertainty (H2a) such that symptom uncertainty predicted symptom burden in the model. This model demonstrated a good fit to the data, with no difference in fit from the original model 1 (χ2(139) = 307.33, p < .001; RMSEA = .063 (CI .053, .072); CFI = .966; SRMR = .057). Compared to model 1 above, the path from provider empathy to symptom burden (H1a) was no longer significant in this alternative model (p = .610). Additionally, the path from symptom burden to psychological adjustment was significant in this alternative model (p = .040).
The second alternative model hypothesized a correlated, rather than predictive, relationship between symptom uncertainty and symptom burden. This model also demonstrated good data fit, with the exact same goodness-of-fit parameters as model 1 and the first alternative model (χ2(139) = 307.33, p < .001; RMSEA = .063 (CI .053, .072); CFI = .966; SRMR = .057). All structural parameters in the second alternative model were statistically significant.
4. Discussion and conclusion
4.1. Discussion
This study examined (1) the extent to which female breast cancer patients’ psychological well-being is associated with perceptions of empathic patient-provider communication and uncertainty about cancer treatment and/or prognosis and (2) whether treatment status (undergoing treatment versus treatment completed) moderated the relationship between provider communication and patient uncertainty and psychological adjustment. The hypothesized relationships between empathic provider communication and patient symptom burden, uncertainty, and psychological adjustment were supported for both current and former patients, with a significant moderation effect observed across these two phases in the cancer experience (RQ1). In all cases, we found that more empathic communication was significantly associated with lower ratings of symptom burden (H1a, B < −.28 across models) and symptom uncertainty (H1b, B < −.31 across models). We also found that higher symptom burden was associated with higher uncertainty (H2a, B >.47 across models) and worse adjustment to the diagnosis (H2b, B < −.14 across models). However, the association between symptom burden and psychological adjustment (H2b) was not a statistically significant path in models 1–3 (p > .05) and was only supported by the two alternate models. Finally, higher uncertainty was associated with worse psychological adjustment among current and former patients (H3, B < −.66 across models).
Overall, these findings align with previous research examining uncertainty and health outcomes. Aligned with prior work, provider communication that is perceived as responsive to cancer patients’ uncertainty was associated with better reports of mental and physical well- being [37]. Additionally, in other chronic illnesses, patient uncertainty about their illness trajectory is associated with higher levels of anxiety and overall psychological distress [40]. Taken together with the results of the present study, patient uncertainty has a clear influence on well-being, and provider communication is a critical point for potential intervention. This study expanded the literature by identifying patient uncertainty concerns related to (1) having questions about their cancer that were unanswered, (2) receiving medical explanations about their cancer that were unclear, and (3) feeling as if they could not plan for their future because of the unpredictability of their cancer. Given the strong relationship between uncertainty and poor psychological adjustment [15–17,41,42], we recommend cancer providers continue to actively probe for patient understanding of their prognosis, treatment, and follow-up plans with attention to uncertainty throughout the cancer continuum.
We explored whether treatment status (current versus former patients) would moderate the relationship among the study variables (RQ1). Results indicated that the levels of symptom burden, illness uncertainty, perceived empathic communication, and psychological adjustment varied by treatment status such that current patients reported worse functioning across these outcomes. Results also supported a moderation effect of treatment status on the relationships between these factors (p < .001). The largest difference between current and former patient models was the strength of the relationships between symptom uncertainty and adjustment such that the relationship was stronger for former patients (H3; B = −.66 versus −.91). In other words, patients who reported more uncertainty also reported worse psychological adjustment, and this relationship was stronger for former patients. Given the cross-sectional design of this study, we cannot determine whether these differences are due to improvement in these outcomes because cancer treatment is over or whether patient recall becomes more positive following treatment and with time. Patient retrospective recall of appointment characteristics and affective memories does not always reflect the actual content of those appointments [43]. Future research should explore patient uncertainty directly after oncology appointments for both current and former breast cancer patients to better understand the themes most salient to patients at different points in the illness trajectory. Longitudinal data are needed to determine how these factors change as survivors transition from active treatment into long-term survivorship. Although levels of uncertainty differed depending on treatment status, results highlighted symptom uncertainty as an important contributing factor to psychological well-being across the breast cancer trajectory.
Although the hypothesized model (Fig. 1) was based in theory, it is possible that the directionality of the relationships in the model are not the only potential explanatory relationships. Thus, we ran models with H2a in the opposite direction (symptom uncertainty predicting symptom burden) as well as models with uncertainty and symptom burden correlated, rather than predictive. Both alternate models fit the data as well as the final model presented in Fig. 3 but are not aligned with prior research or theory that framed the present research. Longitudinal research is needed to overcome limitations of cross-sectional data and definitively untangle this relationship. Overall, despite the limitations, this study contributes to the broader literature in empathic communication, illness uncertainty, and psychological adjustment after a cancer diagnosis.
4.2. Strengths and limitations
The results from this study have several strengths. First, our sample represents patients both on and off treatment, which has been an understudied moderator of psychological adjustment and illness uncertainty. Additionally, the results from this study can make theoretical contributions, extending our understanding of uncertainty in illness theory by providing a potential mechanistic connection between provider communication behaviors and patient outcomes. Although connections between empathic and/or patient-centered communication have been shown to improve patient outcomes [23,39,44,45], our results are one of the first to identify intermediate processes through which this is accomplished.
This study also has several limitations. First, the demographics of participants in this study sample do not represent the broader breast cancer patient population in the United States (i.e., participants were majority white, highly educated, married, and at least somewhat digitally literate). This sample was all within five years of a breast cancer diagnosis, providing a mix of treatment status but not representing long- term survivors. Future efforts need to continue to address the experiences of a broader range of cancer patients. Additionally, the cross-sectional nature of the survey design limits the extent to which patient perceptions of provider empathic communication may represent actual provider communication behaviors. Knowing how patient perceptions align (or fail to align) with actual provider behaviors (i.e., through observation or video analysis of the medical interaction) is one key element for creating guidance for new standards of medical communication. Finally, there are limitations with our analytic strategy worth noting. We completed CFA on the combined population of participants, rather than separately for current and former patients. Although this method allows for a more direct comparison of model fit across groups because factor structures are the same, it is possible that subtle differences exist across populations and are not represented in these analyses. Additionally, the correlation of error terms within individual constructs based on modification indices may increase the chance of overfitting our data [46].4 However, the included appendices shed light on the full measurement structures and decision-making process and can be used to inform future survey design utilizing these measures in breast cancer patient populations.
4.3. Conclusion
The results of this study supported all proposed hypotheses. Participants in this study reported lower symptom burden and less symptom uncertainty when they perceived their cancer provider as having more empathic communication. Moreover, lower symptom burden and less uncertainty were associated with better overall adjustment to the cancer diagnosis. These data support the need for continued attention to breast cancer patients’ psychological well-being and level of uncertainty beyond the initial diagnosis and treatment phases into the post- treatment survivorship phase.
4.4. Practice implications
The findings of this study highlight the importance of both eliciting and addressing breast cancer patients’ uncertainty throughout the cancer trajectory to facilitate psychological adjustment. Our results suggest that provider communication is a key component to reducing uncertainty, and thus providers have a key role in helping to facilitate psychological well-being. Future efforts should focus on skills training for providers and medical trainees to more efficiently recognize and empathically address patient uncertainty throughout the breast cancer trajectory.
Acknowledgements
The authors would like to thank the participants who spent their time and energy to provide these data. We would like to acknowledge the Rutgers Cancer Institute of New Jersey’s Cancer Survivorship & Outcomes Center.
Funding
This study was supported in part by Rutgers School of Communication and Information’s Small Grants for Individual Faculty Research (GIFR) and the Rutgers Cancer Institute of New Jersey (CINJ) and RWJBarnabas Health Mission Support, Cancer Survivorship and Outcomes Center (CSOC) Award.
Appendix
This appendix provides a detailed overview of how each variable measured in this study was treated in the measurement models (and thus in the conceptual models). For each measure, the appendix includes (1) all items, (2) factor loadings, (3) item retention decisions, (4) means and standard deviations for each item, (5) initial confirmatory factor analysis (CFA) results and goodness-of-fit parameters, (6) final factor structures, including the modification indices that supported any covaried error terms. The measured variables are:
A.1 Perceived Symptom Burden.
A.2 Perceived Symptom Uncertainty.
A.3 Perceived Empathic Communication.
A.4 Adjustment to the Cancer Diagnosis.
In addition, Section A.5 provides the initial and final full structural equation models for current and former patients, including goodness-of- fit measures and relevant modification indices.
A.1. Perceived symptom burden
Table A1.
Means, standard deviations, and items of the M. D. Anderson Symptom Inventory.
| Current patients | Former patients | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| MDAnd_1 | General activity? | .849 | Y | 4.85 | 3.01 | 3.80 | 2.84 |
| MDAnd_2 | Mood? | .851 | Y | 4.92 | 3.05 | 3.94 | 2.71 |
| MDAnd_3 | Work (including work around the house)? | .845 | Y | 4.40 | 2.86 | 3.79 | 2.85 |
| MDAnd_4 | Relations with other people? | .817 | Y | 3.97 | 3.02 | 3.22 | 2.71 |
| MDAnd_5 | Walking? | .685 | N | 4.19 | 3.18 | 2.88 | 2.65 |
| MDAnd_6 | Enjoyment of life? | .840 | Y | 4.48 | 2.94 | 3.56 | 2.66 |
| Mean composite score | 4.47 | 2.59 | 3.53 | 2.27 | |||
Note. All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. The low loading item (item 5) was not retained in subsequent analyses.
Fig. A1.
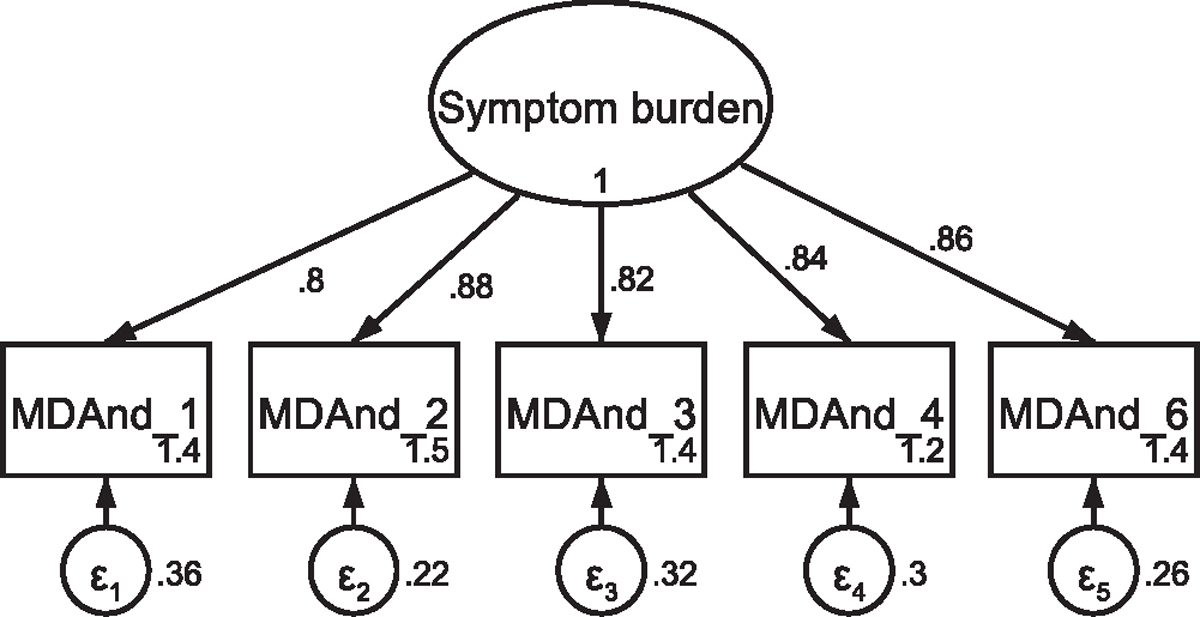
CFA of retained items in the M. D. Anderson Symptom Inventory without covaried error terms Note: Parameter estimates are standardized. Model fit indices were χ2(5) = 83.73, p < .001; RMSEA = .226 (CI .185, .270); CFI = .934; SRMR = .039.
Fig. A2.
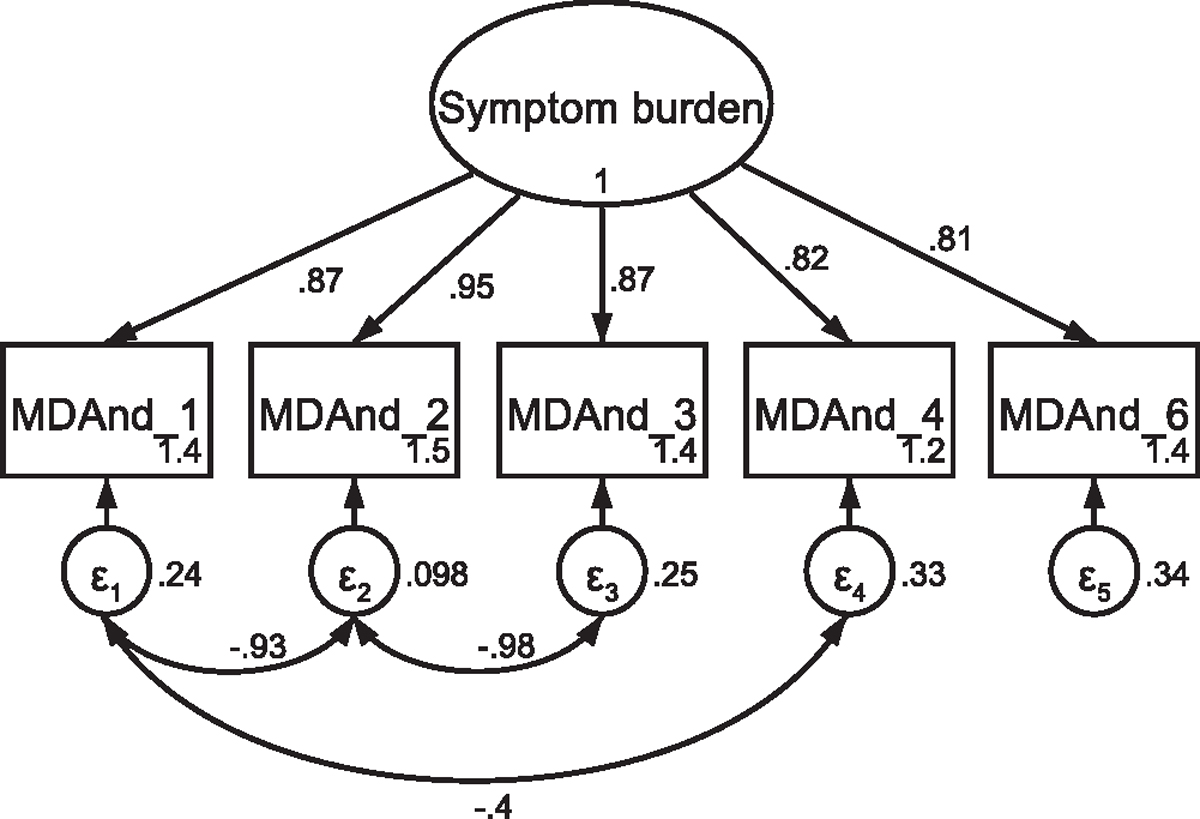
CFA of retained items in the M. D. Anderson Symptom Inventory with covaried error terms Note: Parameter estimates are standardized. Model fit indices were χ2(2) = 4.89, p = .096; RMSEA = .066 (CI <.001, .146); CFI = .998; SRMR = .012. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 48.96 Δχ2 improvement of fit for the covariation of items one and three (χ2(4) = 77.80, p < .001; RMSEA = .245 (CI .119, .294); CFI = .938; SRMR = .038). Modification indices next supported a 42.73 Δχ2 improvement of fit for the covariation of items two and three (χ2(3) = 22.66, p < .001; RMSEA = .146 (CI .094, .205); CFI = .983; SRMR = .023). Finally, modification indices supported a 15.88 Δχ2 improvement of fit for the covariation of items one and four, resulting in the above utilized model.
A.2. Perceived symptom uncertainty
Table A2.
Means, standard deviations, and items of the Uncertainty in Illness Scales.
| Current patients | Former patients | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Item wording | Rotated factor loadings | Retained? | M | SD | M | SD | |
|
| |||||||
| IllUnc_1 | I have a lot of questions about my cancer without answers. | .724 | Y | 2.36 | 1.12 | 2.22 | 1.08 |
| IllUnc_2 | I received medical explanations about my cancer that are unclear to me. | .709 | Y | 2.13 | 1.10 | 1.99 | 1.09 |
| IllUnc_3 | Because of the unpredictability of my cancer, I cannot plan for the future. | .553 | Y | 2.56 | 1.29 | 2.06 | 1.12 |
| Mean composite score | 2.35 | .98 | 2.09 | .86 | |||
Note. All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained.
Fig. A3.
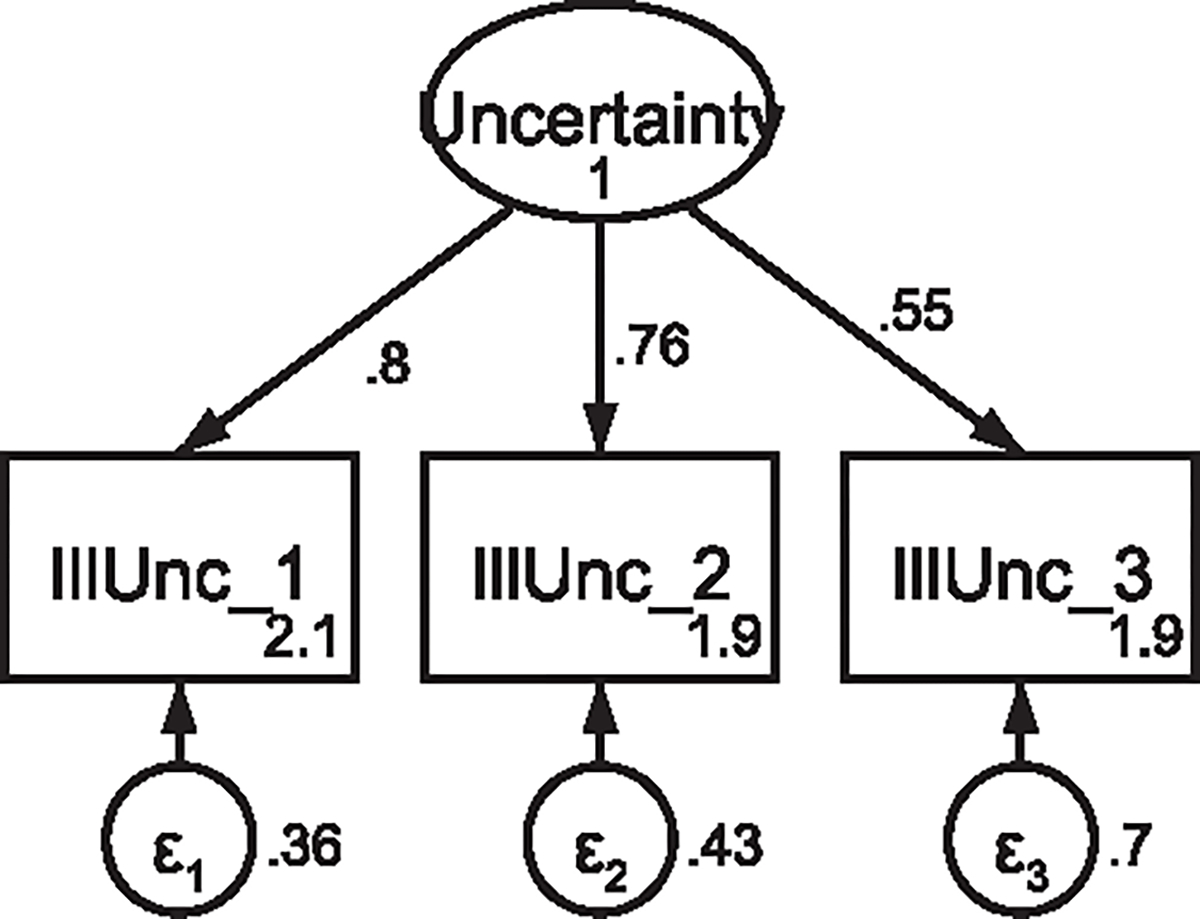
CFA of retained items in the Uncertainty in Illness Note: Parameter estimates are standardized. Model fit indices not indicated as the model is fully saturated.
A.3. Perceived empathic communication
Table A3.
Means, standard deviations, and items of the Consultation and Relational Empathy (CARE) questionnaire.
| Current patients | Former patients | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| CARE_1 | Making me feel at ease? | .912 | N | 3.75 | 1.36 | 4.21 | 1.07 |
| CARE_2 | Letting me tell me story? | .926 | Y | 3.67 | 1.33 | 4.08 | 1.11 |
| CARE_3 | Really listening to me? | .938 | Y | 3.65 | 1.43 | 4.08 | 1.18 |
| CARE_4 | Being interested in me as a whole person? | .918 | Y | 3.44 | 1.46 | 3.93 | 1.20 |
| CARE_5 | Fully understanding my concerns? | .920 | Y | 3.58 | 1.38 | 3.90 | 1.24 |
| CARE_6 | Showing care and compassion? | .908 | Y | 3.77 | 1.40 | 4.17 | 1.08 |
| CARE_7 | Remaining hopeful? | .799 | N | 4.07 | 1.14 | 4.34 | .96 |
| CARE_8 | Explaining things clearly? | .905 | Y | 3.87 | 1.23 | 4.24 | 1.03 |
| CARE_9 | Helping me to take control? | .917 | N | 3.52 | 1.51 | 3.96 | 1.15 |
| CARE_10 | Making a plan of action with me? | .821 | N | 3.63 | 1.46 | 3.89 | 1.32 |
| Mean composite score | 3.69 | 1.24 | 4.08 | 1.03 | |||
Note. All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Items that were more global statements about how the provider made them feel (item 1) or planning for the future (items 9 and 10) were not retained, despite high factor loading, to focus on specific provider behaviors. Items with loadings < .80 (item 7) were not retained.
Fig. A4.
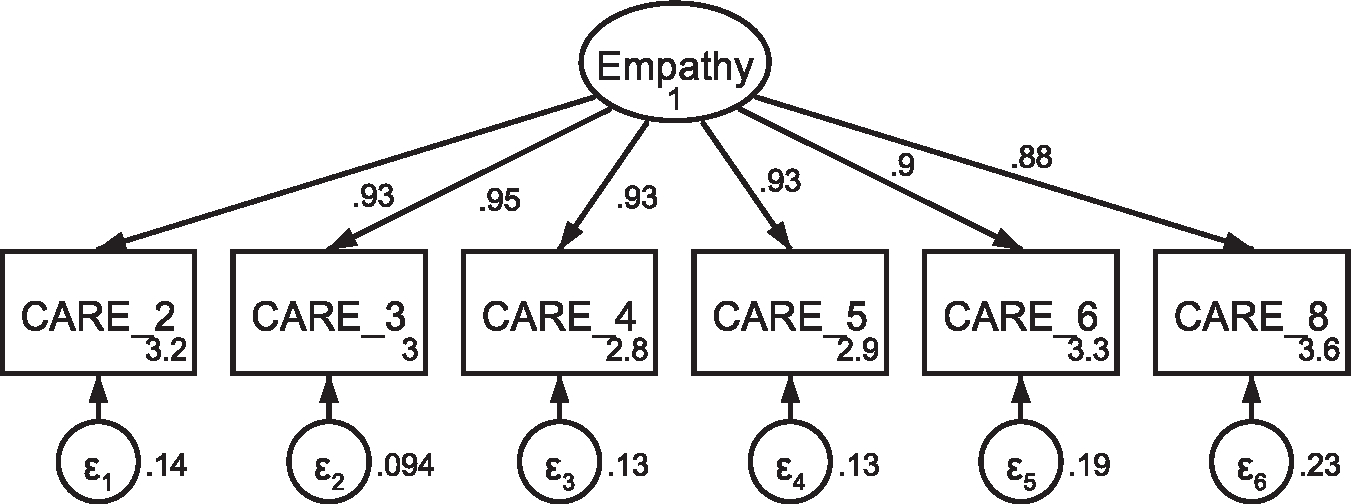
CFA of retained items in the Consultation and Relational Empathy (CARE) questionnaire without covaried error termsNote: Parameter estimates are standardized. Model fit indices were χ2(9) = 45.89, p < .001; RMSEA = .116 (CI .084, .150); CFI = .985; SRMR = .013.
Fig. A5.
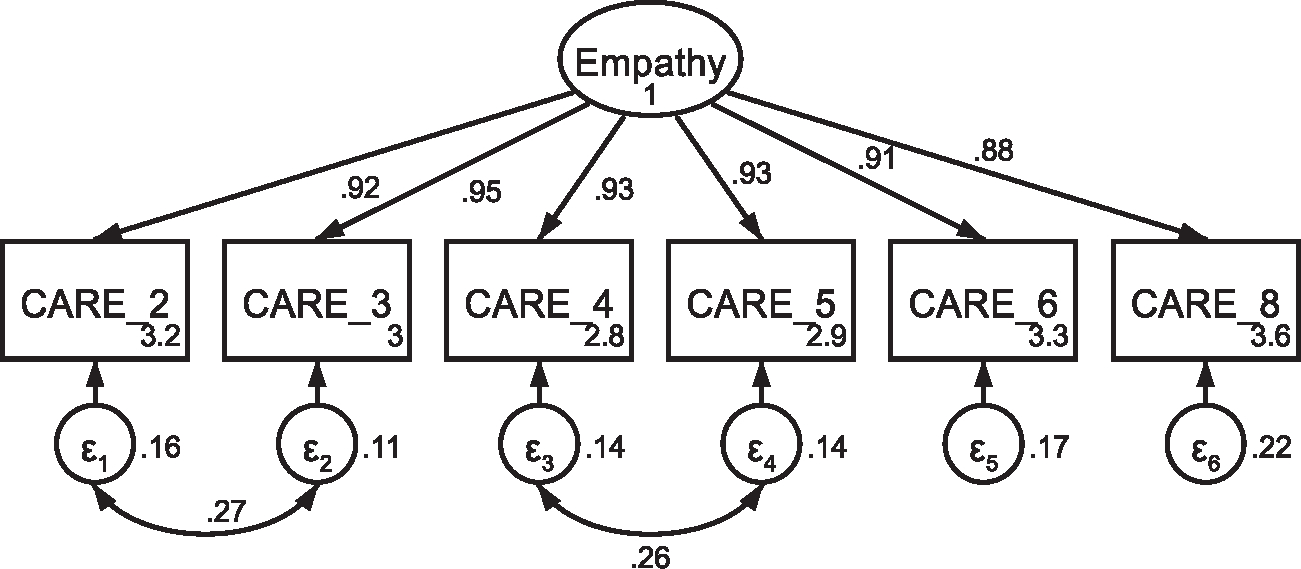
CFA of retained items in the Consultation and Relational Empathy (CARE) questionnaire with covaried error terms Note: Parameter estimates are standardized. Model fit indices were χ2(7) = 14.16, p = .048; RMSEA = .058 (CI .005, .101); CFI = .997; SRMR = .008. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 21.86 Δχ2 improvement of fit for the covariation of items two and three (χ2(8) = 25.54, p = .001; RMSEA = .085 (CI .049, .122); CFI = .993; SRMR = .011). Modification indices next supported a 12.64 Δχ2 improvement of fit for the covariation of items four and five, resulting in the above model that was utilized in subsequent analyses.
A.4. Adjustment to the cancer diagnosis
Table A4.
Means, standard deviations, and items of the modified Mini-Mental Adjustment to Cancer scale.
| Current patients | Former patients | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Item | Item wording | Rotated factor loadings | Retained? | M | SD | M | SD |
|
| |||||||
| MMAC_1 | I am determined to do everything I can to beat this disease. | .203 | N | 4.32 | .93 | 4.22 | .93 |
| MMAC_2 | I am very optimistic. | .644 | Y | 3.98 | 1.11 | 3.88 | .99 |
| MMAC_3 | I feel completely at a loss about what to do. (Reverse coded) | .714 | Y | 4.09 | 1.17 | 4.46 | .78 |
| MMAC_4 | I feel there is nothing I can do to help myself. (Reverse coded) | .746 | Y | 4.13 | 1.19 | 4.50 | .83 |
| MMAC_5 | I suffer great anxiety about having cancer. (Reverse coded) | .781 | Y | 3.04 | 1.33 | 3.64 | 1.22 |
| MMAC_6 | I am apprehensive about my cancer progressing. (Reverse coded) | .641 | Y | 2.63 | 1.20 | 2.97 | 1.12 |
| MMAC_7 | I make a positive effort not to think about my cancer. | .003 | N | 3.60 | 1.22 | 3.15 | 1.21 |
| MMAC_8 | I distract myself when thoughts about my cancer come into my head. | −.045 | N | 3.12 | 1.43 | 2.86 | 1.20 |
| Mean composite score | 3.61 | .70 | 3.71 | .59 | |||
Note. All participants included in factor analysis. M = mean, SD = standard deviation. Y = yes, retained; N = not retained. Items with loadings < .60 were removed from the scale one at a time, with the lowest loading (item 7) removed first. Loadings were reassessed after each item was removed.
Fig. A6.
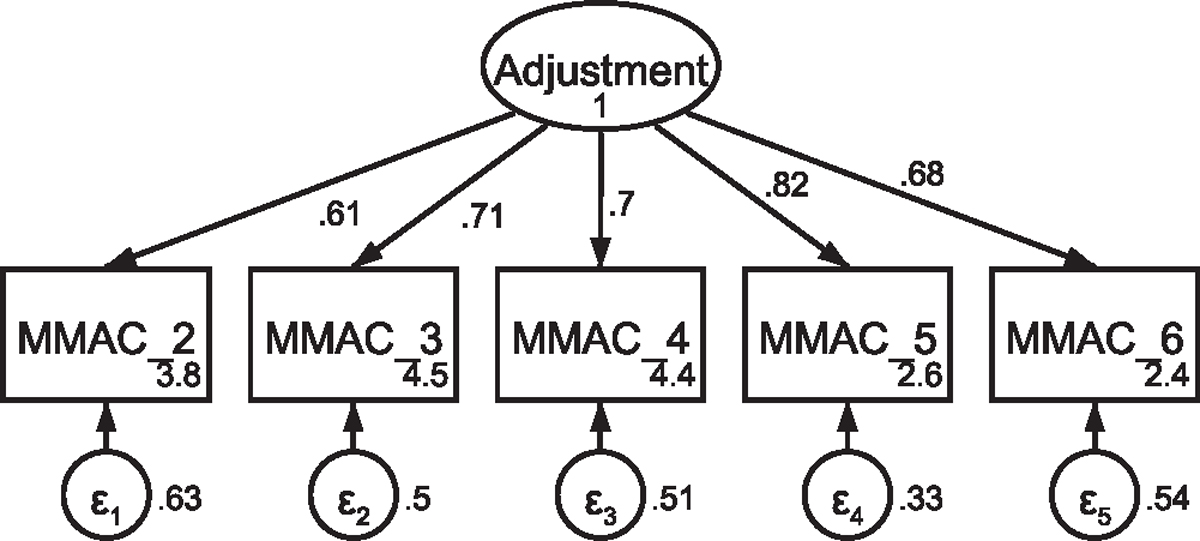
CFA of retained items in the modified Mini-Mental Adjustment to Cancer scale without covaried error terms Note: Parameter estimates are standardized. Model fit indices were χ2(5) = 101.87, p < .001; RMSEA = .251 (CI .210, .295); CFI = .843; SRMR = .074.
Fig. A7.
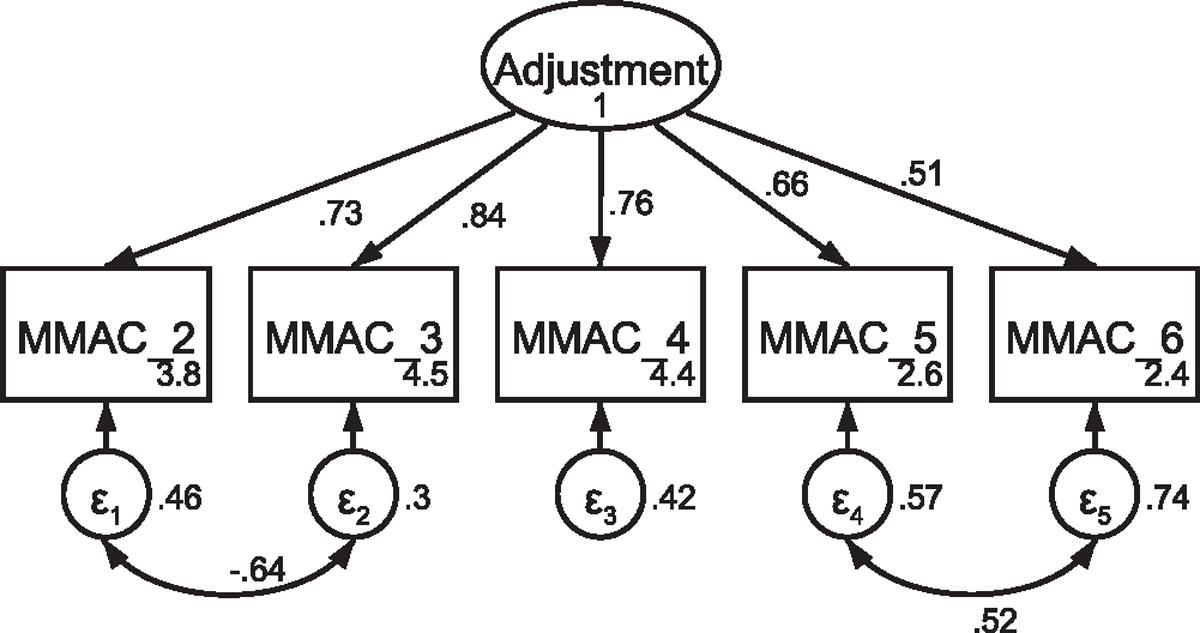
CFA of retained items in the modified Mini-Mental Adjustment to Cancer scale withcovaried error terms Note: Parameter estimates are standardized. Model fit indices were χ2(3) = 5.31, p = .15; RMSEA = .050 (CI <.001, .119); CFI = .996; SRMR = .014. Covariations were added stepwise, with a new model assessed after each modification. Modification indices first supported a 70.21 Δχ2 improvement of fit for the covariation of items five and six (χ2(4) = 34.58, p < .001; RMSEA = .158 (CI .112, .208); CFI = .950; SRMR = .046). Modification indices next supported a 24.68 Δχ2 improvement of fit for the covariation of items two and three, resulting in the above model that was used in subsequent analyses.
A.5. Full structural equation models
Fig. A8.
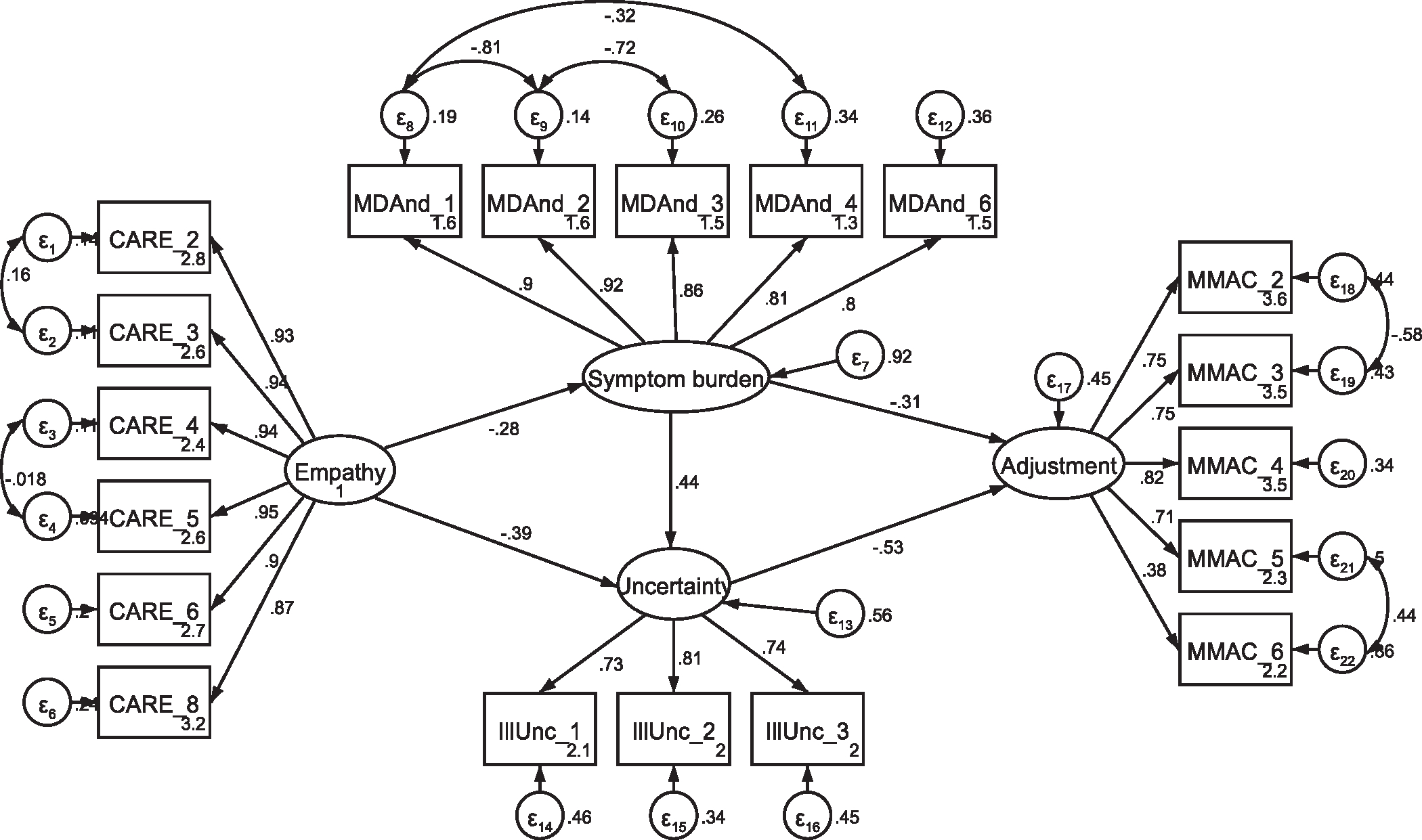
Initial SEM with current patients Note: Parameter estimates are standardized. Model fit indices were χ2(140) = 231.87, p < .001; RMSEA = .074 (CI .057, .091); CFI = .953; SRMR = .068.
Fig. A9.
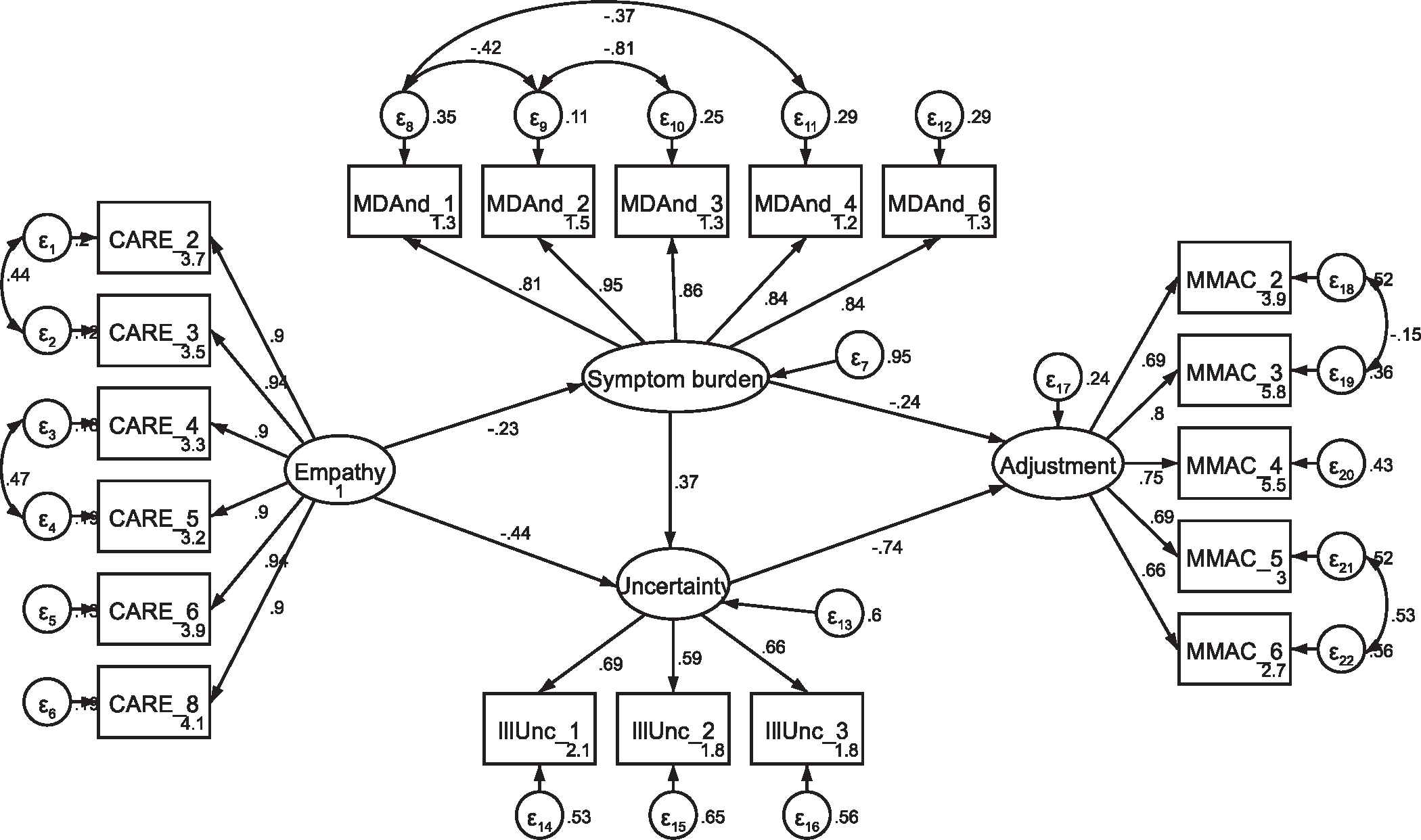
Initial SEM with former patients Note: Parameter estimates are standardized. Model fit indices were χ2(140) = 370.91, p < .001; RMSEA = .094 (CI .083, .106); CFI = .927; SRMR = .070. Modification indices support a 22.94 Δχ2 improvement in model fit with the covariation of error terms between illunc_1 and illunc_2.
Fig. A10.
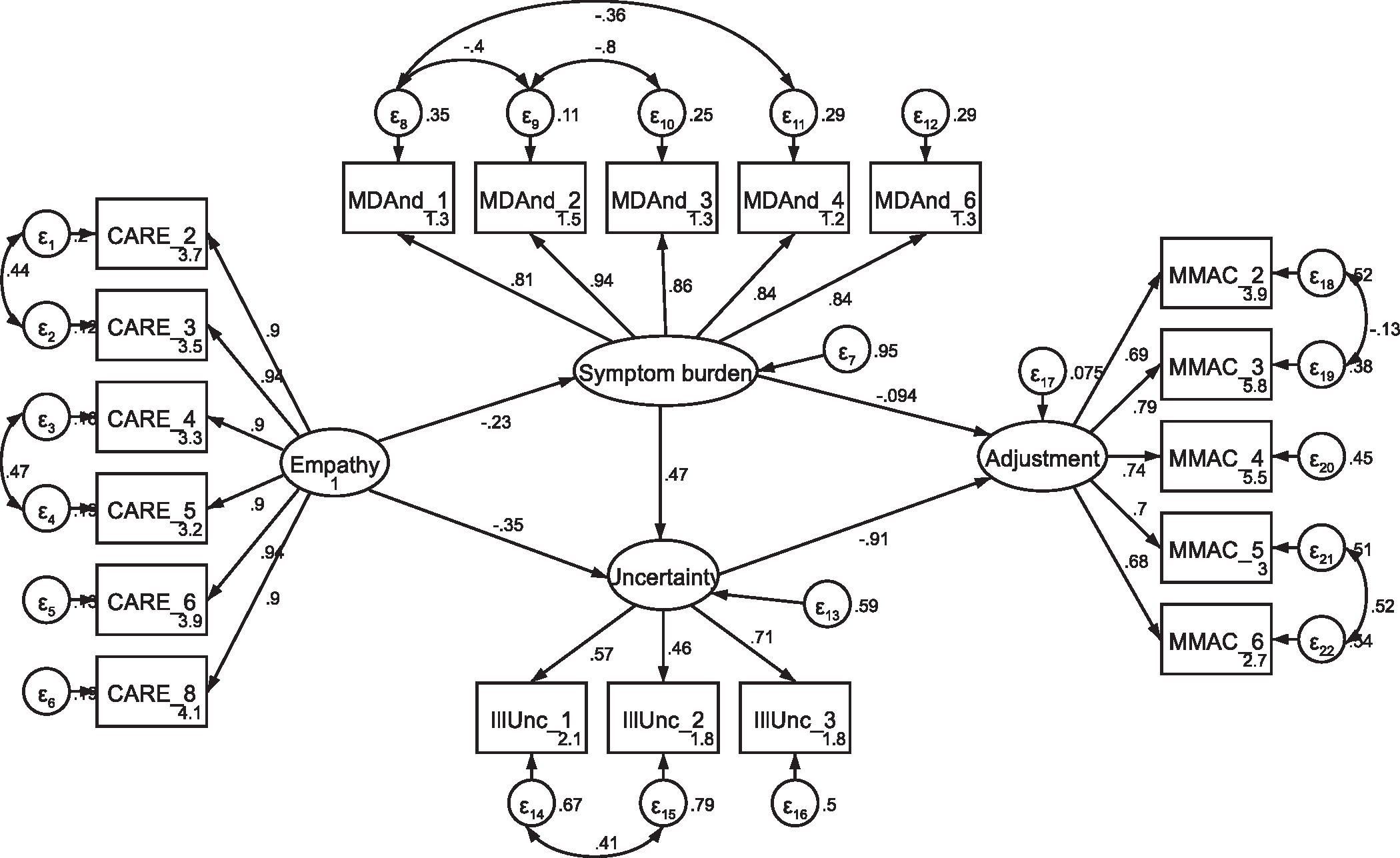
SEM with former patients, including covaried error term between illunc_1 and illunc_2 Note: Parameter estimates are standardized. Model fit indices were χ2(139) = 345.46, p < .001; RMSEA = .089 (CI .078, .101); CFI = .934; SRMR = .071. This model demonstrated a markedly better fit to the data than the model in Fig. A9 (Δχ2 = 25.45, Δdf = 1, p < .001).
Fig. A11.
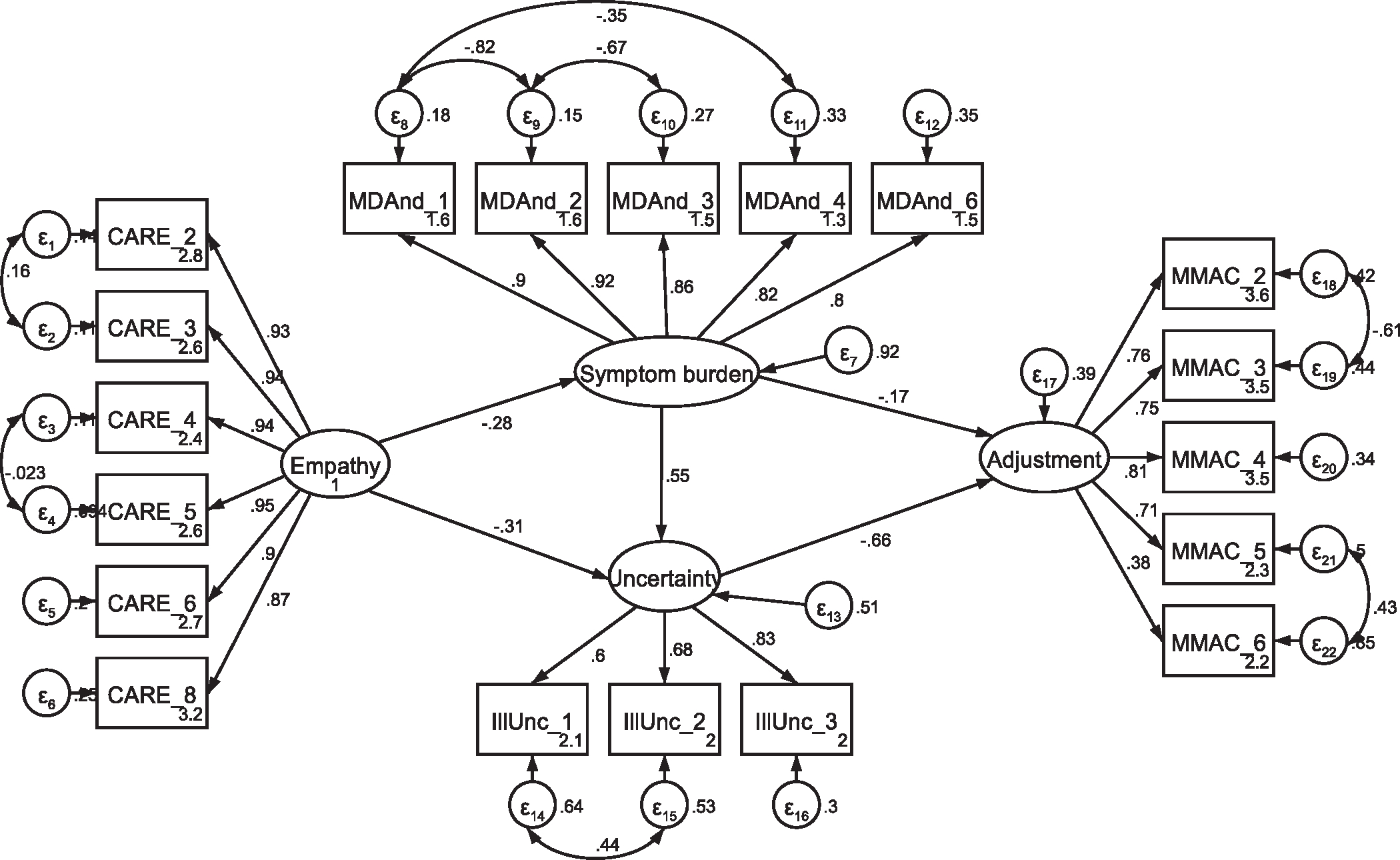
SEM with current patients, including covaried error term between illunc_1 and illunc_2 for direct comparison with the former patient model Note: Parameter estimates are standardized. Model fit indices were χ2(139) = 216.84, p < .001; RMSEA = .068 (CI .050, .085); CFI = .961; SRMR = .067. This model demonstrated a markedly better fit to the data than the model in Fig. A8 (Δχ2 = 15.03, Δdf = 1, p < .001).
Footnotes
Communication Studies.
We use the terms “current” and “former” patients to distinguish between groups at different points in the cancer illness trajectory (rather than “survivors”) though we recognize that all patients are survivors from the time of diagnosis.
Male breast cancer patients were excluded because there were insufficient participants to draw meaningful inferences.
Prior to adding the covaried error term, the moderation effect was also present (Δχ2 = 343.62, Δdf = 145, p < .001).
We would like to thank our anonymous reviewers and editors for encouraging our team to further discuss and transparently present in detail our approach to measurement and analyses.
Declaration of Competing Interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Rutgers University Institutional Review Board (protocol and approval # E17–664).
CRediT authorship contribution statement
Liesl Broadbridge: Conceptualization, Formal analysis, Writing – original draft preparation, visualization, Funding acquisition (CSOC). Kathryn Greene: Conceptualization, Methodology, investigation, Writing – review & editing, supervision, Funding acquisition (GIFR, CSOC). Maria K. Venetis: Conceptualization, Methodology, Investigation, Writing – review & editing. Lauren E. Lee: Conceptualization, Methodology, Investigation. Smita C. Banerjee: Conceptualization, Methodology, Writing – review & editing. Biren Saraiya: Conceptualization, Writing – review & editing. Katie A. Devine: Conceptualization, methodology, Investigation, Writing – review & editing, Funding acquisition (CSOC).
References
- [1].Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology 2007;32:22–35. 10.1016/J.PSYNEUEN.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol 2014;32:3540–6. 10.1200/JCO.2014.56.0086. [DOI] [PubMed] [Google Scholar]
- [3].Mehnert A, Hartung TJ, Friedrich M, Vehling S, Brähler E, Härter M, et al. One in two cancer patients is significantly distressed: Prevalence and indicators of distress. Psychooncology 2018;27:75–82. 10.1002/pon.4464. [DOI] [PubMed] [Google Scholar]
- [4].Nosarti C, Roberts JV, Crayford T, McKenzie K, David AS. Early psychological adjustment in breast cancer patients: A prospective study. J Psychosom Res 2002;53:1123–30. 10.1016/S0022-3999(02)00350-1. [DOI] [PubMed] [Google Scholar]
- [5].Sutton TL, Koprowski MA, Grossblatt-Wait A, Brown S, McCarthy G, Liu B, et al. Psychosocial distress is dynamic across the spectrum of cancer care and requires longitudinal screening for patient-centered care. Support Care Cancer 2022;30:4255–64. 10.1007/s00520-022-06814-z. [DOI] [PubMed] [Google Scholar]
- [6].Lam WWT, Soong I, Yau TK, Wong KY, Tsang J, Yeo W, et al. The evolution of psychological distress trajectories in women diagnosed with advanced breast cancer: a longitudinal study. Psychooncology 2013;22:2831–9. 10.1002/PON.3361. [DOI] [PubMed] [Google Scholar]
- [7].Park J-H, Sik Jung Y, Young Kim J, Jo Y, Hyoung Bae S. Trajectories of health-related quality of life in breast cancer patients. Support Care Cancer 2020:28. 10.1007/s00520-019-05184-3. [DOI] [PubMed] [Google Scholar]
- [8].Rogers CC, Pope S, Whitfield F, Cohn WF, Valdez RS. The lived experience during the peri-diagnostic period of breast cancer: A scoping review. Patient Educ Couns 2022;105:547–85. 10.1016/J.PEC.2021.06.017. [DOI] [PubMed] [Google Scholar]
- [9].Engelhardt EG, Pieterse AH, Han PKJ, van Duijn-Bakker N, Cluitmans F, Maartense E, et al. Disclosing the uncertainty associated with prognostic estimates in breast cancer: Current practices and patients’ perceptions of uncertainty. Med Decis Mak 2017;37:179–92. . [DOI] [PubMed] [Google Scholar]
- [10].Fridfinnsdottir EB. Icelandic women’s identifications of stressors and social support during the diagnostic phase of breast cancer. J Adv Nurs 1997;25:526–31. 10.1046/j.1365-2648.1997.t01-1-1997025526.x. [DOI] [PubMed] [Google Scholar]
- [11].Liu L-N, Li C-Y, Tzuh S, Huang C-S, Chiou A-F. Role of continuing supportive cares in increasing social support and reducing perceived uncertainty among women with newly diagnosed breast cancer in Taiwan. Cancer Nurs 2006;29:273–82. 10.1097/00002820-200607000-00004. [DOI] [PubMed] [Google Scholar]
- [12].Parker PA, Alba F, Fellman B, Urbauer DL, Li Y, Karam JA, et al. Illness uncertainty and quality of life of patients with small renal tumors undergoing watchful waiting: A 2-year prospective study. Eur Urol 2013;63:1122–7. 10.1016/j.eururo.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gill KM, Mishel MH, Belyea M, Germino B, Germino LS, Porter L, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum 2004;31:633–9. 10.1188/04.ONF.633-639. [DOI] [PubMed] [Google Scholar]
- [14].Maheu C, Singh M, Tock WL, Eyrenci A, Galica J, Hébert M, et al. Fear of cancer recurrence, health anxiety, worry, and uncertainty: A scoping review about their conceptualization and measurement within breast cancer survivorship research. Front Psychol 2021:12. 10.3389/fpsyg.2021.644932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhuang H, Wang L, Yu X, Sally |, Chan W-C, Gao Y, et al. Effects of decisional conflict, decision regret and self-stigma on quality of life for breast cancer survivors: A cross-sectional, multisite study in China. J Adv Nurs 2022;78:3261–72. 10.1111/jan.15250. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Y, Kwekkeboom K, Petrini M. Uncertainty, self-efficacy, and self-care behavior in patients with breast cancer undergoing chemotherapy in China. Cancer Nurs 2015;38:E19–26. 10.1097/NCC.0000000000000165. [DOI] [PubMed] [Google Scholar]
- [17].Clayton MF, Mishel MH, Belyea M. Testing a model of symptoms, communication, uncertainty, and well-being, in older breast cancer survivors. Res Nurs Health 2006;29:18–39. 10.1002/nur.20108. [DOI] [PubMed] [Google Scholar]
- [18].Clayton MF, Dudley WN, Musters A. Communication with breast cancer survivors. Health Commun 2008;23:207–21. 10.1080/10410230701808376. [DOI] [PubMed] [Google Scholar]
- [19].Lake PW, Conley CC, Pal T, Sutton SK, Vadaparampil ST. Anxiety and depression among Black breast cancer survivors: Examining the role of patient-provider communication and cultural values. Patient Educ Couns 2022;105:2391–6. 10.1016/j.pec.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bylund CL, Makoul G. Empathic communication and gender in the physician–patient encounter. Patient Educ Couns 2002;48:207–16. 10.1016/S0738-3991(02)00173-8. [DOI] [PubMed] [Google Scholar]
- [21].Halpern J. Empathy and patient-physician conflicts. J Gen Intern Med 2007;22:696–700. 10.1007/s11606-006-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Butow PN, Kazemi JN, Beeney LJ, Griffin A-M, Dunn SM, Tattersall MHN. When the diagnosis is cancer: Patient communication experiences and preferences. Cancer 1996;77:2630–7. . [DOI] [PubMed] [Google Scholar]
- [23].Dean M, Street RL. A 3-stage model of patient-centered communication for addressing cancer patients’ emotional distress. Patient Educ Couns 2014;94:143–8. 10.1016/J.PEC.2013.09.025. [DOI] [PubMed] [Google Scholar]
- [24].Chen Z, He G, Zhao Y, Han C, Xu L, Jian H, et al. Symptom burden and emotional distress in advanced lung cancer: The moderating effects of physicians’ communication skills and patients’ disease understanding. Support Care Cancer 2022;30:9497–505. 10.1007/s00520-022-07323-9. [DOI] [PubMed] [Google Scholar]
- [25].Liu J-E, Mok E, Wong T. Caring in nursing: Investigating the meaning of caring from the perspective of cancer patients in Beijing, China. J Clin Nurs 2006;15:188–96. 10.1111/j.1365-2702.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- [26].Brashers DE, Babrow A, Goldsmith D, Jackson S, Jacobs S, Neidig J. Communication and uncertainty management. J Commun 2001;51:477–97. 10.1111/J.1460-2466.2001.TB02892.X. [DOI] [Google Scholar]
- [27].Gustafson A Reducing patient uncertainty: Implementation of a shared decision- making process enhances treatment quality and provider communication. Clin J Oncol Nurs 2017;21:113–5. 10.1188/17.CJON.113-115. [DOI] [PubMed] [Google Scholar]
- [28].Mishel MH. Uncertainty in illness. Image J Nurs Sch 1988;20:225–32. 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- [29].Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model A Multidiscip J 1999;6:1–55. 10.1080/10705519909540118. [DOI] [Google Scholar]
- [30].Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model 2009;6:1–55. 10.1080/10705519909540118. [DOI] [Google Scholar]
- [31].Herzog W, Boomsma A. Small-sample robust estimators of noncentrality-based and incremental model fit. Struct Equ Model 2009;16:1–27. 10.1080/10705510802561279. [DOI] [Google Scholar]
- [32].McNeish D Should we use F-tests for model fit instead of Chi-Square in overidentified structural equation models? Oran Res. Methods 2018;23:487–510. 10.1177/1094428118809495. [DOI] [Google Scholar]
- [33].Bentler PM, Yuan KH. Structural equation modeling with small samples: Test statistics. Multivar Behav Res 2010;34:181–97. 10.1207/S15327906Mb340203. [DOI] [PubMed] [Google Scholar]
- [34].Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients. Cancer 2000;89:1634–46. . [DOI] [PubMed] [Google Scholar]
- [35].Mishel MH Uncertainty in illness scales manual 1996. [Google Scholar]
- [36].Mercer SW, Maxwell M, Heaney D, Watt GCM. The consultation and relational empathy (CARE) measure: Development and preliminary validation and reliability of an empathy-based consultation process measure. Fam Pr 2004;21:699–705. 10.1093/FAMPRA/CMH621. [DOI] [PubMed] [Google Scholar]
- [37].Watson M, Law MG, Santos MD, Greer S, Baruch J, Bliss J. The mini-mac: Further development of the mental adjustment to cancer scale. J Psychosoc Oncol 1994;12:33–46. 10.1300/J077V12N03_03. [DOI] [Google Scholar]
- [38].Venetis MK Communication-participation behavior during the delivery of breast-cancer care. Rutgers University - Graduate School - New Brunswick, 2010. 10.7282/T3K9377N. [DOI] [Google Scholar]
- [39].Street RL, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 2009;74:295–301. 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- [40].Schiele SE, Emery CF, Jackson JL. The role of illness uncertainty in the relationship between disease knowledge and patient-reported outcomes among adolescents and adults with congenital heart disease. Heart Lung 2019;48:325–30. 10.1016/J.HRTLNG.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guan T, Santacroce SJ, Chen D-G, Song L. Illness uncertainty, coping, and quality of life among patients with prostate cancer. Psychooncology 2020;29:1019–25. 10.1002/pon.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cripe LD, Vater LB, Lilly JA, Larimer A, Hoffmann ML, Frankel RM. Goals of care communication and higher-value care for patients with advanced-stage cancer: A systematic review of the evidence. Patient Educ Couns 2022;105:1138–51. 10.1016/j.pec.2021.08.016. [DOI] [PubMed] [Google Scholar]
- [43].Hilarius DL, Kloeg PHAM, Detmar SB, Muller MJ, Aaronson NK. Level of agreement between patient self-report and observer ratings of health-related quality of life communication in oncology. Patient Educ Couns 2007;65:95–100. 10.1016/J.PEC.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [44].Claramita M, Arininta N, Fathonah Y, Kartika S, Prabandari YS, Pramantara IDP. A partnership-oriented and culturally-sensitive communication style of doctors can impact the health outcomes of patients with chronic illnesses in Indonesia. Patient Educ Couns 2020;103:292–300. 10.1016/j.pec.2019.08.033. [DOI] [PubMed] [Google Scholar]
- [45].Zwingmann J, Baile WF, Schmier JW, Bernhard J, Keller M. Effects of patient-centered communication on anxiety, negative affect, and trust in the physician in delivering a cancer diagnosis: A randomized, experimental study. Cancer 2017;123:3167–75. 10.1002/cncr.30694. [DOI] [PubMed] [Google Scholar]
- [46].Hermida R The problem of allowing correlated errors in structural equation modeling: Concerns and considerations. Comput Methods Soc Sci 2015;3:5–17. [Google Scholar]


