Abstract
There is no evidence evaluating the IL10 epigenetic upregulation among mestizo children in a high‐altitude Andean city in Latin America.
Objective
To identify polymorphisms and methylation profiles in the IL10 gene associated with asthma in children aged 5 to 11.
Methods
A case–control study was conducted with asthmatic and non‐asthmatic children aged 5 to 11 years in Cuenca‐Ecuador. Data on allergic diseases and risk factors were collected through a questionnaire for parents. Atopy was measured by skin prick test (SPT) to relevant aeroallergens. Three IL10 single nucleotide polymorphisms were evaluated in all participants, and methylation analysis was performed in 54 participants. Association between risk factors, allergic diseases and genetic factors were estimated using multivariate logistic regression.
Results
The results of polymorphisms showed no differences between cases and controls when comparing the SNPs rs3024495, rs3024496, rs1800896 allelic and genotypic frequencies. In the methylation analysis, no differences in the IL10 methylation profile were found between cases and controls; however, the multivariate analysis showed an association between the mother's smoking habits and the IL10 methylation profile.
Conclusion
Smoking habit could be essential as an environmental exposure factor in regulating gene expression in children with asthma.
Keywords: asthma, children, IL10, methylation, polymorphisms
In our study conducted in Cuenca‐Ecuador, we explored IL10 gene methylation and its association with asthma among children aged 5–11. While no significant differences in IL10 methylation profiles were observed between asthmatic and non‐asthmatic children, a notable link was found between mothers' smoking habits and alterations in the IL10 methylation pattern. This suggests that maternal smoking could play a pivotal role in modulating gene expression related to asthma in children.
1. INTRODUCTION
Asthma is a chronic respiratory disease that causes variable airflow obstruction, airway hyperresponsiveness and inflammation (Marques et al., 2017; Teixeira et al., 2017). Asthma affects more than 334.000 children worldwide (Forno et al., 2015; Nutten, 2015; Weinmayr et al., 2008) and is the result of complex interactions between genetic susceptibility and environmental exposures (Fiuza et al., 2017; Langie et al., 2016; Miller & Ortega, 2013; Perry et al., 2018).
Most asthma cases begin in childhood with local airway sensitization caused by seasonal viruses and common allergens, such as cockroaches, animal dander, dust mites and pollen (Obando‐Pacheco & Justicia‐Grande, 2018; Teixeira et al., 2017). Nicotine, carcinogens and other tobacco substances are well‐known allergens reported in several epidemiological studies and exposure to these toxicants through fetal and early life could increase the risk of childhood wheezing and asthma (Chilmonczyk et al., 1993; Neuman et al., 2012; Strachan & Cook, 1998). According to the National Health and Nutrition Survey, smoking prevalence in childbearing‐aged Ecuadorian women is around 15.0% (Freire et al., 2015). A lower prevalence was seen in African (2.3%), American (12.9%), eastern Mediterranean (2.3%) and Asian women (2.3%); however, a higher prevalence was observed in European women (21%) (World Health Organization and Others, 2018).
Sensitization occurs through the expansion of Th2 lymphocytes that secrete Th2‐type interleukins (IL‐4, IL‐5, IL‐9, and IL‐13), which are significant in allergic sensitization (Holgate, 2012; Teixeira et al., 2017). In asthma, sensitization of the lower airway causes an incomplete formation of tight junction proteins, which affects the physical barrier function, facilitating the entry of inhaled allergens into the tissue (Holgate, 2012).
The interleukin‐10 (IL10, OMIM accession number: 124092) gene is located on chromosome 1q31‐q32 and encodes for the IL‐10 protein, which significantly modulates allergic pathways (Holgate, 2012; Lyon et al., 2004). Treg cells secreting IL‐10 inhibit Th1, Th2 and Th17 cells, suppress allergen‐specific immunoglobulin E (IgE) and inhibit mast cells, basophils and eosinophils (Holgate, 2012). IL‐10 regulatory effects have been associated with immunological tolerance and protection against asthma through balancing Th1 and Th2 immunity and reducing tissue damage in chronic diseases (Grant et al., 2011; Holgate, 2012; Lyon et al., 2004).
Genetic polymorphisms in IL10 have been associated with increased risk of asthma/wheezing, IgE levels and SPT responses to cockroaches (Figueiredo et al., 2012, 2013; Hunninghake et al., 2008; Lyon et al., 2004). In that sense, the coding variant rs3024496 has been associated with decreased production of IL‐10 in Brazilian children (Figueiredo et al., 2013) and with an increased risk of asthma exacerbations in children previously exposed to mite allergen in Costa Rica (Hunninghake et al., 2008). Additionally, the SNP rs1800896 (or –1082A/G) has been associated with high total serum (IgE) levels, a higher risk for atopic conditions in different populations, and a lower risk of asthma in European and African populations (Ober & Hoffjan, 2006).
Although genetic predisposition is a significant risk factor for developing respiratory diseases, epigenetic modulation, such as DNA methylation, in regulatory sequences of a gene, is one of the mechanisms related to airway diseases (Langie et al., 2016). There has been a growing interest in DNA methylation markers concerning asthma (Ho et al., 2012; Langie et al., 2016, 2018). Evidence from Europe has shown an association between GLI2 promoter region hypermethylation and asthma (Langie et al., 2016, 2018; Xu et al., 2018). However, there is no evidence regarding the methylation status of the IL10 promoter region and its relationship with asthma.
We aim to (i) describe the frequencies of IL10 polymorphisms (rs3024495, rs3024496, rs1800896) among asthmatic and non‐asthmatic children in an Andean city of Ecuador, (ii) compare the IL10 polymorphisms frequencies with other populations, (iii) compare DNA methylation in IL10 promoter region between asthmatic and non‐asthmatic children, and (iv) identify associations between methylation in IL10 promoter with history and family background of allergic diseases and environmental risk factors. The results could provide evidence regarding the role of genetic susceptibility and environmental exposure on the IL10 upregulation in asthmatic and non‐asthmatic children in Latin American populations.
2. METHODS
2.1. Ethical compliance
The Universidad San Francisco de Quito, Ecuador Ethics Committee approved the study protocol (2018‐235E). In an informative meeting, all the procedures were explained to the parents/guardians. Informed consent from the parents and assent from the children older than 10 years were signed. The measurements were taken at the Department of Bioscience, Universidad de Cuenca, in a venue dedicated exclusively to the research.
2.2. Participants' identification and recruitment
We conducted a case‐control study among asthmatic (cases) and non‐asthmatic (controls) children aged 5–11 living in Cuenca, Ecuador, from July 2019 until January 2020. Cuenca is located in the Ecuadorian highlands at 2.550 meters above sea level. Around 90% of the city's population are mestizos (i.e., mixed Spanish‐Indigenous ethnicity) ( Delgado, 2013; Nacional de Estadísticas y Censos INEC, 2010).
Cases were identified from a previous cross‐sectional study undertaken in 2018 (Ochoa‐Avilés, Morillo, Ochoa, et al., 2020) and the medical records of two Pediatric Pulmonologists. At first, 100 potential asthmatic cases were identified; cases were confirmed by a parental report of wheezing in the last 12 months, plus at least one of the following: (i) asthma diagnosis ever, (ii) wheezing during/after physical exercise in the last 12 months, and (iii) sleep interruption due to wheeze in the last 12 months (Pires et al., 2018). Later, 100 potential controls with no parental report of asthma or wheezing were identified in the cross‐sectional study dataset and the medical records; controls were matched (1:1) by age and sex with each case.
2.3. History of allergic diseases, family background of allergic diseases, and environmental risk factors
The ISAAC phase II questionnaire adapted to local conditions (Cooper et al., 2009; Ochoa‐Avilés, Morillo, Rodriguez, et al., 2020; Rodriguez et al., 2017) was applied to collect data on the family background of allergic diseases, risk factors and clinical history data (Ochoa‐Avilés, Morillo, Ochoa, et al., 2020). Data were collected using the KoBoToolBox tool (https://www.kobotoolbox.org/) software. History of allergic diseases comprised of rhinitis and eczema. Rhinitis was defined with a report of sneezing not associated with cold or nasal congestion in the last 12 months. Reports of itchy rash in the previous months, involving folds of the elbows, behind the knees, in front of the ankles, buttocks, or around the neck, ears or eyes, were considered to define eczema (Ochoa‐Avilés, Morillo, Rodriguez, et al., 2020). In addition, data on the following environmental risk factors were collected: mother smoking habits (ever), family smoking exposure (ever), presence of dog(s) in(out)side the house, presence of cat(s) in(out)side the house, contact with farm animals. Parental history of allergic diseases comprises the maternal and/or parental history of asthma, rhinitis and eczema.
2.4. Skin prick testing (SPT)
SPT was performed using the following: saline solution as a negative control, histamine as a positive control, grass mix (Dactylis glomerata, Festuca pratensis, Phoa pratensis, Phelum pratense, and Lolium perenne), tree mix (ash and salix), weed mix (Plantago, Chenopodium, Artermisa, Ambrosia, and Parietaria), fungi (Alternaria, Penicillium, and Cladosporum), dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), tropical mite (Blomia tropicalis), dog and cat dander, feather mix (chicken, duck, and goose), cockroach (Periplaneta americana) and latex. Allergens were pricked on the forearm, and reaction sizes were evaluated after 15 min. Atopy was defined if the mean wheal size was at least 3 mm greater than the negative saline control for any allergen tested (Bousquet et al., 2012).
2.5. Pulmonary function
Trained doctors performed a spirometric evaluation of cases and controls using a previously calibrated Easy One Connect (Medical Technologies, Zurich, Switzerland) portable spirometer. Loeb et al.'s criteria were applied to define the procedure's validity (Loeb et al., 2008). The Forced Expiratory Volume in 1 s (FEV1) was estimated.
2.6. DNA extraction and quantification
Approximately 2 mL of saliva sample was collected from each patient using a kit (OG‐575 Oragene, Assisted Collection kit) designed explicitly for this purpose (Mullegama et al., 2019). According to the manufacturer's instructions, the gDNA was extracted from each sample using the Oragene prepIT‐L2P kit (Genotek Inc., Canada). The concentration of gDNA was quantified with the Quant‐iT dsDNA Broad‐Range Assay kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) on a Qubit 2.0 fluorometer.
2.7. Genotyping
Genotyping was carried out using TaqMan probe‐based 5′‐nuclease assays technology (Applied Biosystems, Foster City, CA, USA) on Applied Biosystem's QuantStudio 12 K equipment. After applying the tests, only single nucleotide polymorphisms (SNPs) with a genotyping rate “call rates” of at least 93% and Hardy–Weinberg equilibrium (HWE > 0.05) and minor allele frequency (MAF > 0.05) were considered for analysis (Table 1), using the PLINK (version 1.9) software. Non‐template negative and genotyping‐positive controls were included in each genotyping plate.
TABLE 1.
Characteristics of the SNPs evaluated.
| SNP | A1 | A2 | MAF | HWE |
|---|---|---|---|---|
| rs3024496 | G | A | 0.24 | 0.78 |
| rs1800896 | C | T | 0.05 | 1.00 |
| rs3024495 | T | C | 0.25 | 0.09 |
Abbreviations: A1, polymorphic allele; A2, wild allele; HWE, Hardy–Weinberg equilibrium; MAF, minor allele frequency; SNP, single‐nucleotide polymorphism.
2.8. Methylation analysis
For a subset of children (n = 27 cases, n = 27 controls). According to the manufacturer's protocol, methylation in the IL10 promoter region was undertaken by real‐time PCR in a QuantStudio 12 K using the OneStep qMethyl™ Kit. About 4 ng of DNA from each sample was accommodated per duplicate in a “reference” and “test” reaction well. DNA in the test reaction was digested with Methylation Sensitive Restriction Enzymes (MSREs), while DNA in the reference reaction was not. Quantification was performed by the comparative threshold cycle method (Hellemans et al., 2007). The PCR for the IL10 promoter region was carried out in a reaction volume of 20ul using the Green‐fluorescent Nucleic Acid Stains (SYTO) technology and 500 nM of the IL10 primer (Thermo Scientific, Invitrogen) 5′TGAAACATGTCAATC3′ (forward) and 5′CCTCAGCTGTCAT3′ (reverse). The PCR thermal profile was 37°C for 2 h for MRSEs digestion, 95°C for 10 min for initial denaturation, 40 cycles of 95°C for 30 s for denaturing, 54°C for 30 s for annealing, and 72°C for 30 s for extension, 42°C for 7 min for final extension, and 4°C for 5 min as a hold step. At the end of each PCR run, the system automatically analyzed the data, and amplification plots were generated for each DNA sample. All experiments were performed in duplicate. The percentage of DNA methylation in the IL10 promoter region was estimated for each participant.
2.9. Statistical analysis
Statistical analyses were conducted using Stata software (Statistical Software Release 12. Stata Corp LLC, College Station, TX). Continuous variables were presented as means with standard deviations; categorical variables were presented as percentages. The commands “gladder” and “ladder” were used in Stata to evaluate the variables' distribution: age, the percentage of DNA methylation in the IL10 promoter region and FEV1. All the variables that did not follow a normal distribution were normalized depending on the best transformation obtained from the ladder command (sqrt, log). Two‐sample t‐test and Chi‐square test were applied to analyze differences in continuous and categorical variables between cases and controls.
The mean percentage of DNA methylation in the IL10 promoter region with its 95% confidence intervals are displayed as error bars. Associations between DNA methylation in the IL10 promoter region (dependent variable) with asthma, pulmonary function, history and family background of allergic diseases and environmental risk factors were analyzed using a multiple linear regression model (predictors). Potential predictors were included in an adjusted model when significantly associated with the dependent variable at a significance level of 10%. The strength of the associations was estimated using the Beta coefficient (β), with 95% confidence intervals (95% CI). All tests were performed with a significance level of 5%.
2.10. In silico analysis
Pairwise Linkage Disequilibrium (LD) was created using Haploview 4.2. IL10 SNPs frequencies in European, African, Asian and Latin American populations were identified using the NCBI platform (https://www.ncbi.nlm.nih.gov/gene) and contrasted with frequencies found in our population.
3. RESULTS
3.1. Study population
Fifty‐one cases and 49 controls agreed to participate in the study. Table 2 summarizes the characteristics of the study participants. Age and gender distribution were equitable between cases and controls. The FEV1 was significantly lower among the cases than controls (88% vs. 95%, p = 0.004). More cases had atopy than the controls (67% vs. 43%, p = 0.01), mainly because of a higher sensitization to mixed pollen (Plantago, Chenopodium, Artermisa, Ambrosia and Parietaria) (80% vs. 20%, p = 0.01).
TABLE 2.
Sociodemographic and clinical characteristics of the study population.
| Cases N (%) | Controls N (%) | p‐value | |
|---|---|---|---|
| Sex | |||
| Male | 23 (45.1) | 27 (55.1) | 0.32 |
| Female | 28 (55.0) | 22 (45.0) | |
| Age | |||
| 3–6 | 36 (70.6) | 32 (65.3) | 0.67 |
| 7–11 | 15 (29.4) | 17 (34.7) | |
| FEV1 (%) (n = 43) | 88.2 (7.3) a | 94.8 (6.6) a | 0.004 |
| Allergic diseases | |||
| Atopy (n = 48) | |||
| Yes | 34 (66.7) | 20 (42.6) | 0.01 |
| No | 17 (33.3) | 27 (57.5) | |
| Rhinitis (n = 49) | |||
| Yes | 34 (66.7) | 28 (58.3) | 0.41 |
| No | 17 (33.3) | 20 (41.7) | |
| Eczema | |||
| Yes | 21 (41.2) | 18 (36.7) | 0.40 |
| No | 30 (58.8) | 31 (63.3) | |
| Environmental risk factors | |||
| Mites (n = 97) b | |||
| Yes | 26 (57.8) | 19 (42.2) | 0.25 |
| No | 24 (46.2) | 28 (57.8) | |
| Dermatophagoides farinae (n = 97) | |||
| Yes | 24 (57.1) | 18 (42.9) | 0.34 |
| No | 26 (47.3) | 29 (52.7) | |
| Dermatophagoides pteronyssius (n = 97) | |||
| Yes | 24 (58.5) | 17 (41.5) | 0.24 |
| No | 26 (46.4) | 30 (53.6) | |
| Blomia tropicalis (n = 97) | |||
| Yes | 10 (58.8) | 7 (41.2) | 0.51 |
| No | 40 (50.0) | 40 (50.0) | |
| Pollen (n = 97) c | |||
| Yes | 12 (80.0) | 3 (20.0) | 0.01 |
| No | 38 (46.3) | 44 (53.6) | |
| Cockroach | |||
| Yes | 2 (66.7) | 1 (33.3) | 0.60 |
| No | 48 (51.1) | 46 (48.9) | |
| Parental smoking habits and history of allergic diseases | |||
| Mother smoking | |||
| Yes | 3 (5.8) | 3 (6.1) | 0.64 |
| No | 48 (94.1) | 46 (93.4) | |
| Family smoking habits | |||
| Yes | 7 (13.7) | 9 (18.4) | 0.53 |
| No | 44 (86.3) | 40 (81.6) | |
| Paternal asthma (n = 81) | |||
| Yes | 8 (16.3) | 7 (21.8) | 0.57 |
| No | 41 (83.7) | 25 (78.1) | |
| Parental rhinitis (n = 81) | |||
| Yes | 35 (71.4) | 20 (62.5) | 0.47 |
| No | 14 (28.6) | 12 (37.35) | |
| Parental eczema (n = 78) | |||
| Yes | 15 (30.6) | 10 (34.5) | 0.80 |
| No | 34 (69.4) | 19 (65.5) | |
Note: Bold, p < 0.05.
Abbreviation: FEV1, forced expiratory volume in 1 s.
Average and standard deviation (SD) are shown.
Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blomia tropicalis.
Plantago, Chenopodium, Artermisa, Ambrosia, and Parietaria.
3.2. IL10 polymorphisms
The genotype distribution and allele frequency were similar between cases and controls (Table 3). We found no differences in genotype and allele frequencies for the three SNPs evaluated in this study between cases and controls.
TABLE 3.
IL10 SNPs allele frequencies for different populations.
| Region | Population | rs1800896 | rs3024495 | rs3024496 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Ref allele | Alt allele | N | Ref allele | Alt allele | N | Ref allele | Alt allele | ||
| Global | ‐ | 301,062 | T = 0.546 | C = 0.453 | 33,692 | C = 0.909 | A = 0.000, T = 0.090 | 165,868 | A = 0.548 | G = 0.451 |
| Europe | European | 266,178 | T = 0.527 | C = 0.473 | 24,122 | C = 0.909 | A = 0.000, T = 0.090 | 142,092 | A = 0.548 | G = 0.451 |
| Africa | African | 8352 | T = 0.668 | C = 0.331 | 6720 | C = 0.979 | A = 0.000, T = 0.020 | 9296 | A = 0.599 | G = 0.401 |
| Asia | Asian | 3938 | T = 0.937 | C = 0.063 | 128 | C = 1.000 | A = 0.000, T = 0.000 | 674 | A = 0.961 | G = 0.039 |
| Latin America | Latin American 1 (afro‐Caribbean) | 1130 | T = 0.643 | C = 0.356 | 140 | C = 0.993 | A = 0.000, T = 0.007 | 752 | A = 0.625 | G = 0.375 |
| Latin American 2 (European and Native American) | 7210 | T = 0.716 | C = 0.283 | 600 | C = 0.992 | A = 0.000, T = 0.008 | 6330 | A = 0.719 | G = 0.2806 | |
| Study population | 100 | T = 0.745 | C = 0.255 | 100 | C = 0.95 | A = 0.000, T = 0.05 | 100 | A = 0.755 | G = 0.245 | |
Abbreviations: Alt allele, alternative allele; N, Number of populations; Ref allele, reference allele.
Genotype distribution and allele frequency were similar between cases and controls (Table 4). We found no differences between cases and controls in both genotype and allele frequencies for the three single nucleotide polymorphisms (SNPs) evaluated in this study.
TABLE 4.
Genotype and allele frequencies for IL10 SNPs.
| Allelic or genotyping profile | Control n (%) | Cases n (%) | p‐value |
|---|---|---|---|
| rs3024496—posición A > G | |||
| AA | 29 (56.9) | 27 (55.1) | 0.3565 |
| GA | 18 (35.3) | 21 (42.9) | |
| GG | 4 (7.8) | 1 (2.0) | |
| A | 0.75 | 0.77 | |
| G | 0.25 | 0.23 | |
| rs1800896—posición T > C | |||
| TT | 29 (56.9) | 26 (53.1) | 0.6001 |
| TC | 18 (35.3) | 21 (42.9) | |
| CC | 4 (7.8) | 2 (4.1) | |
| T | 0.75 | 0.74 | |
| C | 0.25 | 0.26 | |
| rs3024495—posición C > T | |||
| CC | 47 (92.2) | 45 (91.8) | 0.9983 |
| CT | 3 (5.9) | 3 (6.1) | |
| TT | 1 (2.0) | 1 (2.0) | |
| C | 0.95 | 0.95 | |
| T | 0.05 | 0.05 | |
3.3. Linkage disequilibrium
Figure 1 shows the linkage disequilibrium (LD) analysis among the studied IL10 gene SNPs. There is a high degree of linkage disequilibrium between the SNPs rs3024496 and rs1800896.
FIGURE 1.
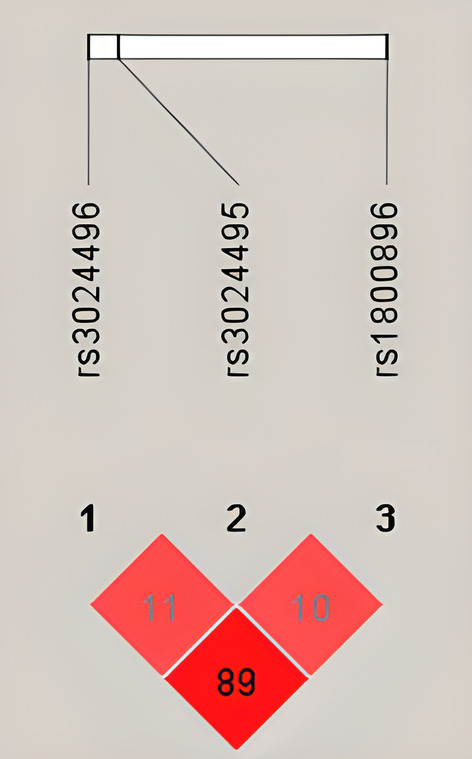
LD plot of the IL10 SNPs analyzed in asthmatic and control patients. The top horizontal bar illustrates thelocation of SNPs on a physical scale. The color of the squares illustrates the strength of pairwise r 2 values on a scale where black indicates perfect LD (r 2 = 1), shades of gray indicate imperfect LD (0 < r 2 < 1) and white indicates perfect equilibrium (r 2 = 0). The r 2 LD value is also indicated within each square.
The top horizontal bar illustrates the location of SNPs on a physical scale. The colour of the squares illustrates the strength of pairwise r 2 values on a scale where black indicates perfect LD (r 2 = 1), shades of grey indicate imperfect LD (0 < r 2 < 1) and white indicates perfect equilibrium (r 2 = 0). The r 2 LD value is also indicated within each square.
3.4. DNA methylation in IL10 promoter region
Figure 2 shows the mean percentage of DNA methylation in the IL10 promoter region with 95% confidence intervals for cases and controls. No differences were found when comparing the percentage of IL10 methylation between cases and controls (47.6 ± 23.1 vs. 41.2 ± 19.0).
FIGURE 2.
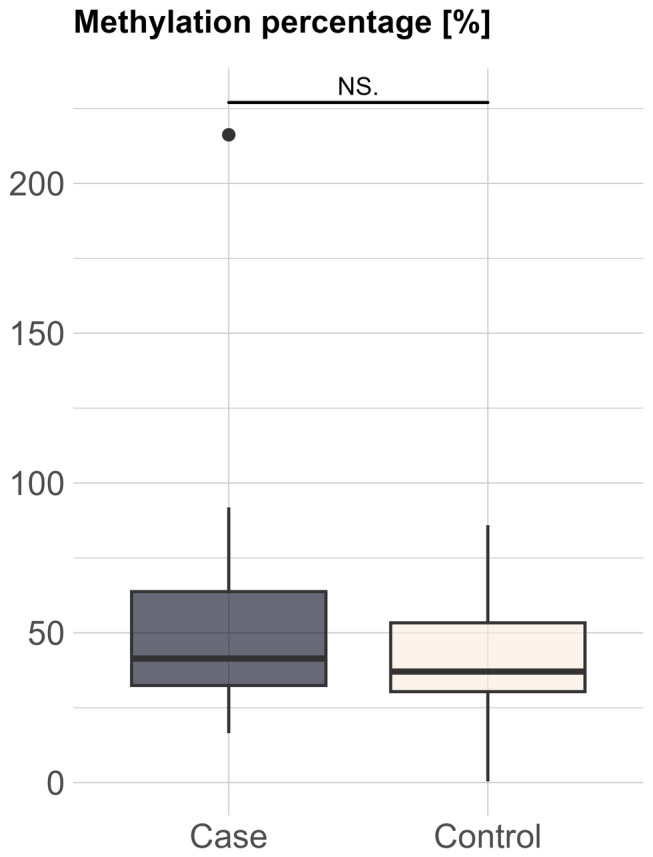
Distribution of cases and controls with respect to methylation percentage.
The associations between the mean percentage of DNA methylation in the IL10 promoter region with asthma, FEV1, history and family background of allergic diseases and environmental risk factors are shown in Table 5. There was no association between DNA methylation in the IL10 promoter region with asthma or FEV1. DNA methylation in the IL10 promoter region was positively associated with maternal smoking habits (ever) (β = 33.06, 95% CI 10.04; 56.07, p < 0.01).
TABLE 5.
Associations between the mean percentage of DNA methylation in IL10 promoter region with asthma, FEV1, history and family background of allergic diseases and environmental risk factors.
| Predictors | IL10 methylation | |
|---|---|---|
| Bivariate β (95% CI) p‐value | Multivariate β (95% CI) p‐value | |
| Asthma and pulmonary function | ||
| Control/case | 6.40 (−5.17–17.97) 0.27 | |
| FEV1 (%) | −0.58 (−1.42–0.26) 0.17 | |
| History of allergic diseases (N/Y) | ||
| Asthma | 2.90 (−8.78–14.58) 0.62 | |
| Rhinitis | −2.22 (−14.46–10.01) 0.72 | |
| Eczema | −2.63 (−14–61‐9.35) 0.66 | |
| Environmental factors (N/Y) | ||
| Mother smoking habits | 28.8 (11.98–45.61) 0.001 | 36.54 (13.45–59.6) <0.01 |
| Family smoking habits | 12.0 (−2.14–26.14) 0.095 | −9.50 (−27.40–8.39) 0.30 |
| Dog inside house | 2.10 (−10.14–14.34) 0.73 | |
| Cat inside house | 7.05 (−9.45–23.54) 0.4 | |
| Dog outside house | −2.20 (−14.78–10.39) 0.73 | |
| Cat outside house | 10.63 (−1.61–22.88) 0.087 | 7.05 (−4.04–18.14) 0.21 |
| Contact with farm animals | −5.42 (−18.40–7.55) 0.41 | |
| Parental history of allergic disorders (N/Y) | ||
| Parental asthma | 3.21 (−13.38–19.80) 0.69 | |
| Parental rhinitis | −7.78 (−20.15–4.58) 0.21 | |
| Parental eczema | −0.51 (−13.25–12.23) 0.94 | |
Abbreviation: FEV1, forced expiratory volume in 1 s.
4. DISCUSSION
To our knowledge, this is the first study to determine SNPs and epigenetic profile of the IL10 gene among asthmatic and non‐asthmatic mestizo children from a high‐altitude setting in Latin America. According to our data, DNA methylation in the IL10 promoter region did not differ between asthmatics and healthy controls. Our results align with those reported in a prospective study with 211 children from Taiwan. Wu et al. (2019) reported that IL10_P325 methylation was not significantly associated with a higher risk of asthma development. To the best of these authors' knowledge, there is little evidence regarding epigenetic IL10 upregulation. A recent study performed by Sharma et al. (2022) reported differential methylation patterns of the FOXP3 transcription factor in asthmatic subjects compared with non‐asthmatic controls. This warrants special attention due to the FOXP3 regulatory role leading to Treg's generation, maintenance and IL10 production (Sharma et al., 2022). The evidence from epigenome‐wide analyses focused on genes involved in the activating of eosinophils and cytotoxic T cells has demonstrated that reduced whole‐blood DNA methylation at different CpG sites was strongly associated with childhood asthma (Reese et al., 2018; Xu et al., 2018). Such studies have shown that distinct DNA methylation patterns are associated with asthma and disease severity (Perry et al., 2018; Sharma et al., 2022; Yang et al., 2021). Although our data show no differences between cases and control DNA methylation in the IL10 promoter region, our results could be the basis for future epigenetic analyses.
DNA methylation in the IL10 promoter region was significantly higher among children whose mothers smoked. Previous reports have indicated that parental tobacco exposure is associated with higher methylation levels of IL10 in children (Wu et al., 2019); additionally, some studies suggest that prenatal exposure to tobacco‐related pollutants can program epigenetic modifications in the IL10 gene, which, in turn, can influence the expression of cytokines, modulating the maturing immune system in early life (Latzin et al., 2011; Sharma et al., 2022). Different immune signalling pathways can be altered by tobacco smoke. Evidence suggests that methylation in a series of genes involved in lymphocyte differentiation, immune regulation, redox reaction and regulatory pathways, including IL10, are influenced by parental tobacco exposure (Hedrich et al., 2010; Natkunam et al., 2007; Sharma et al., 2022; Wu et al., 2014, 2019). Most evidence indicates that the genes involved will likely have high DNA methylation levels (Sharma et al., 2022; Wu et al., 2019). Additionally, an abnormal rate of methylation in the IL10 gene has been associated with several chronic inflammatory diseases, including asthma, lupus erythematosus, rheumatoid arthritis, vitiligo and gastric cancer (Li‐Hong et al., 2007; Lin et al., 2012; Sharma et al., 2022; Tang et al., 2021; Wu et al., 2019; Zhao, Gao, et al., 2010; Zhao, Tang, et al., 2010). Although the methylation rate in the IL10 gene is not associated with asthma in this population, its relationship with high‐prevalence chronic diseases (mentioned earlier) must be considered for future studies and possible strategies to prevent cigarette use.
Tobacco contains more than 4000 substances, 250 known to be toxic (Moritsugu, 2007). Nicotine, a substance present in cigarettes, is highly addictive and prevents many pregnant women who wish to quit smoking from doing so (Zacharasiewicz, 2016). Nicotine concentration is significantly increased in pregnant women (Strachan & Cook, 1998), and the baby is also exposed since the substance can enter through mother–fetus circulation via the placenta (Jauniaux et al., 1999). Part of the nicotine returns to the maternal blood circulation and is subsequently eliminated, but some nicotine accumulates in the amniotic fluid (Jauniaux & Burton, 1992). Rehan et al. (2013) documented the effects of nicotine in a rat model. They demonstrated that an asthma‐like phenotype could be inherited up to two generations after the initial intrauterine exposure.
The IL10 SNPs allele frequencies analyzed did not differ between asthmatics and healthy controls. The SNPs frequencies found in our study are similar to those reported in other populations. The rs1800896 and rs3024496 SNPs frequencies are similar to those reported in European populations, and rs3024495 SNP frequency is comparable to that found in Latin American populations (European and Native American descent). Like other Latin American communities, the Ecuadorian population is multi‐ethnic, with a complex demographic history (Montinaro et al., 2015; Moreno‐Estrada et al., 2013, 2014). Migration and admixture events occurred in the country during pre‐ and post‐Columbian times, including Native Amerindian settlements, European colonization and the import of African slaves (Homburger et al., 2015). The continuous admixture among individuals from different ethnicities has shaped the diversity in the modern Ecuadorian population, contributing to the population's genetic heterogeneity.
Previous studies reported that rs3024496 and rs1800896 SNPs could be associated with decreased production of IL‐10 and increased IgE levels, respectively, triggering the development of inflammatory diseases, including asthma (Figueiredo et al., 2012; Ober & Hoffjan, 2006). We were unable to find such an association in the studied population, perhaps due to a lack of statistical power. Further research is needed to elucidate if other reported IL10 SNPs could be associated with the upregulation of the IL10 gene in Ecuadorian children.
The study has certain limitations that should be considered when interpreting these results. First, the sample size might not represent the Ecuadorian mestizo children population. Second, even if plenty of SNPs are reported in the IL10 gene, we only focused on three of them. Evidence suggests these SNPs could be associated with the IL10 gene regulation in Latino children. However, future studies should focus on evaluating other SNPs and other genes in the mestizo population.
5. CONCLUSIONS
The present study evaluated IL10 genetic and epigenetic upregulation among asthmatic and non‐asthmatic Ecuadorian children. The polymorphism analysis data showed no differences between cases and controls when comparing the frequencies of the rs3024495, rs3024496 and rs1800896 SNPs. Regarding the IL10 methylation profile, although no differences were found between cases and controls, multivariate analysis demonstrated an association of maternal smoking habits with IL10 gene promoter region methylation, possibly indicating a gene–environment interaction. The frequencies of rs1800896 and rs3024496 SNPs were similar to those reported in European populations, and the frequency of rs3024495 SNP is comparable to those found in Latin America (European descendants and Native Americans). Although the study population's small size might not be representative of Ecuadorian mestizo children, our results can be used as a baseline for future research.
AUTHOR CONTRIBUTIONS
Conceptualization, Vivian Alejandra Neira, Angélica Ochoa‐Avilés, Claudia Rodas and Cristina Ochoa Avilés; methodology, Samuel Escandón, Talita Dos Santos‐Jesus, Milca de J. Silva, Valderiene Leão, Marco Salinas, Yosselin Vicuña, Lucy Baldeón, María José Molina‐Cando, Diana Morillo. Marcos Machuca; software, Samuel Escandón, Angélica Ochoa‐Avilés, Diana Morillo; validation, Angélica Ochoa‐Avilés, Lucy Baldeón, Vivian Alejandra Neira, Camila Figueiredo; formal analysis, Samuel Escandón, Cristina Ochoa Avilés; investigation, Cristina Ochoa Avilés; resources, Vivian Alejandra Neira, Camila Figueiredo; data curation, Samuel Escandón, Cristina Ochoa Avilés; writing—original draft preparation, Cristina Ochoa Avilés, Roque Rivas‐Párraga; writing—review and editing, All authors; visualization, Cristina Ochoa Avilés, María José Molina‐Cando; supervision, Angélica Ochoa Avilés; project administration, Vivian Alejandra Neira, Camila Figueiredo; funding acquisition, Vivian Alejandra Neira. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This research was funded by Universidad del Azuay (UDA), grant number 2019‐0056, as well as by Universidad de Cuenca.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Universidad San Francisco de Quito, Quito, Ecuador (approval 2018‐235E, 21‐05‐2019) for studies involving humans.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
ACKNOWLEDGMENTS
We are grateful for the participation and support of children, and parents in the study. Additionally, we acknowledge the contribution of the Vice‐Rectorate of Research at the University of Azuay and the Department of Biosciences at the University of Cuenca.
Ochoa‐Avilés, C. , Ochoa‐Avilés, A. , Rivas‐Párraga, R. , Escandón, S. , Santos‐Jesus, T. D. , Silva, M. d. J. , Leão, V. , Salinas, M. , Vicuña, Y. , Baldeón, L. , Molina‐Cando, M. J. , Morillo, D. , Machuca, M. , Rodas, C. , Figueiredo, C. , & Neira, V. A. (2024). Mother's smoking habits affects IL10 methylation but not asthma in Ecuadorian children. Molecular Genetics & Genomic Medicine, 12, e2438. 10.1002/mgg3.2438
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- Bousquet, J. , Heinzerling, L. , Bachert, C. , Papadopoulos, N. G. , Bousquet, P. J. , Burney, P. G. , Canonica, G. W. , Carlsen, K. H. , Cox, L. , Haahtela, T. , Lodrup Carlsen, K. C. , Price, D. , Samolinski, B. , Simons, F. E. , Wickman, M. , Annesi‐Maesano, I. , Baena‐Cagnani, C. E. , Bergmann, K. C. , Bindslev‐Jensen, C. , … Allergic Rhinitis and its Impact on Asthma . (2012). Practical guide to skin prick tests in allergy to aeroallergens. Allergy, 67(1), 18–24. 10.1111/j.1398-9995.2011.02728.x [DOI] [PubMed] [Google Scholar]
- Chilmonczyk, B. A. , Salmun, L. M. , Megathlin, K. N. , Neveux, L. M. , Palomaki, G. E. , Knight, G. J. , Pulkkinen, A. J. , & Haddow, J. E. (1993). Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. The New England Journal of Medicine, 328(23), 1665–1669. 10.1056/NEJM199306103282303 [DOI] [PubMed] [Google Scholar]
- Cooper, P. J. , Rodrigues, L. C. , Cruz, A. A. , & Barreto, M. L. (2009). Asthma in Latin America: A public heath challenge and research opportunity. Allergy, 64(1), 5–17. 10.1111/j.1398-9995.2008.01902.x [DOI] [PubMed] [Google Scholar]
- Delgado, O. (2013). El plan de desarrollo y ordenamiento territorial del cantón cuenca, Azuay. https://dspace.ups.edu.ec/bitstream/123456789/11170/1/El%20plan%20de%20desarrollo%20y%20ordenamiento%20territorial%20del%20canton%20Cuenca%20Azuay.pdf
- Figueiredo, C. A. , Barreto, M. L. , Alcantara‐Neves, N. M. , Cooper, P. J. , Rodrigues, L. C. , Cruz, A. A. , Pontes‐de‐Carvalho, L. C. , Vergara, C. , Rafaels, N. , Gao, L. , Foster, C. , Campbell, M. , Mathias, R. A. , & Barnes, K. C. (2012). Co‐associations between IL10 genetic variants, IL 10 production and helminth infection in a tropical population of Brazil with high prevalence of asthma. The Journal of Allergy and Clinical Immunology, 129(2), AB62. https://www.jacionline.org/article/S0091‐6749(11)02689‐3/abstract [Google Scholar]
- Figueiredo, C. A. , Barreto, M. L. , Alcantara‐Neves, N. M. , Rodrigues, L. C. , Cooper, P. J. , Cruz, A. A. , Pontes‐de‐Carvalho, L. C. , Lemaire, D. C. , dos Santos Costa, R. , Amorim, L. D. , Vergara, C. , Rafaels, N. , Gao, L. , Foster, C. , Campbell, M. , Mathias, R. A. , & Barnes, K. C. (2013). Coassociations between IL10 polymorphisms, IL‐10 production, helminth infection, and asthma/wheeze in an urban tropical population in Brazil. The Journal of Allergy and Clinical Immunology, 131(6), 1683–1690. 10.1016/j.jaci.2012.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza, B. S. D. , Milca de, J. , Silva, N. M. , Alcântara‐Neves, M. L. , Barreto, R. D. , Costa, S. , & Figueiredo, C. A. (2017). Polymorphisms in DENND1B gene are associated with asthma and atopy phenotypes in Brazilian children. Molecular Immunology, 90, 33–41. 10.1016/j.molimm.2017.06.030 [DOI] [PubMed] [Google Scholar]
- Forno, E. , Gogna, M. , Cepeda, A. , Yañez, A. , Solé, D. , Cooper, P. , Avila, L. , Soto‐Quiros, M. , Castro‐Rodriguez, J. A. , & Celedón, J. C. (2015). Asthma in Latin America. Thorax, 70(9), 898–905. 10.1136/thoraxjnl-2015-207199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire, W. , Ramírez‐Luzuriaga, M. , & Belmont, P. (2015). Tomo I: Encuesta Nacional de Salud y nutrición de la población ecuatoriana de cero a 59 años, ENSANUT‐ECU 2012. Revista Latinoamericana de Políticas Y Acción Pública, 2(1), 117. https://repositorio.flacsoandes.edu.ec/bitstream/10469/7065/2/RFLACSO‐MP2(1).pdf#page=114 [Google Scholar]
- Grant, A. V. , Araujo, M. I. , Ponte, E. V. , Oliveira, R. R. , Cruz, A. A. , Barnes, K. C. , & Beaty, T. H. (2011). Polymorphisms in IL10 are associated with Total immunoglobulin E levels and Schistosoma Mansoni infection intensity in a Brazilian population. Genes and Immunity, 12(1), 46–50. 10.1038/gene.2010.50 [DOI] [PubMed] [Google Scholar]
- Hedrich, C. M. , Ramakrishnan, A. , Dabitao, D. , Wang, F. , Ranatunga, D. , & Bream, J. H. (2010). Dynamic DNA methylation patterns across the mouse and human IL10 genes during CD4+ T cell activation; influence of IL‐27. Molecular Immunology, 48(1–3), 73–81. 10.1016/j.molimm.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans, J. , Mortier, G. , De Paepe, A. , Speleman, F. , & Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real‐time quantitative PCR data. Genome Biology, 8(2), R19. 10.1186/gb-2007-8-2-r19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S.‐M. , Johnson, A. , Tarapore, P. , Janakiram, V. , Zhang, X. , & Leung, Y.‐K. (2012). Environmental epigenetics and its implication on disease risk and health outcomes. ILAR Journal/National Research Council, Institute of Laboratory Animal Resources, 53(3–4), 289–305. 10.1093/ilar.53.3-4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate, S. T. (2012). Innate and adaptive immune responses in asthma. Nature Medicine, 18(5), 673–683. 10.1038/nm.2731 [DOI] [PubMed] [Google Scholar]
- Homburger, J. R. , Moreno‐Estrada, A. , Gignoux, C. R. , Nelson, D. , Sanchez, E. , Ortiz‐Tello, P. , Pons‐Estel, B. A. , Acevedo‐Vasquez, E. , Miranda, P. , Langefeld, C. D. , Gravel, S. , Alarcón‐Riquelme, M. E. , & Bustamante, C. D. (2015). Genomic insights into the ancestry and demographic history of South America. PLoS Genetics, 11(12), e1005602. 10.1371/journal.pgen.1005602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake, G. M. , Soto‐Quirós, M. E. , Lasky‐Su, J. , Avila, L. , Ly, N. P. , Liang, C. , Klanderman, B. J. , Raby, B. A. , Gold, D. R. , Weiss, S. T. , & Celedón, J. C. (2008). Dust mite exposure modifies the effect of functional IL10 polymorphisms on allergy and asthma exacerbations. The Journal of Allergy and Clinical Immunology, 122(1), 93–98. 10.1016/j.jaci.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Estadísticas y Censos INEC . 2010. Resultados Del Censo 2010 de Población Y Vivienda En El Ecuador. INEC. [Google Scholar]
- Jauniaux, E. , & Burton, G. J. (1992). The effect of smoking in pregnancy on early placental morphology. Obstetrics and Gynecology, 79(5), 645–648. https://www.ncbi.nlm.nih.gov/pubmed/ [PubMed] [Google Scholar]
- Jauniaux, E. , Gulbis, B. , Acharya, G. , Thiry, P. , & Rodeck, C. (1999). Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstetrics and Gynecology, 93(1), 25–29. 10.1016/s0029-7844(98)00318-4 [DOI] [PubMed] [Google Scholar]
- Langie, S. A. S. , Moisse, M. , Szic, K. S. V. , Van Der Plas, E. , Koppen, G. , De Prins, S. , Louwies, T. , Nelen, V. , Van Camp, G. , Lambrechts, D. , Schoeters, G. , Vanden Berghe, W. , & De Boever, P. (2018). GLI2 promoter hypermethylation in saliva of children with a respiratory allergy. Clinical Epigenetics, 10, 50. 10.1186/s13148-018-0484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langie, S. A. S. , Szarc vel Szic, K. , Declerck, K. , Traen, S. , Koppen, G. , Van Camp, G. , Schoeters, G. , Vanden Berghe, W. , & De Boever, P. (2016). Whole‐genome saliva and blood DNA methylation profiling in individuals with a respiratory allergy. PLoS One, 11(3), e0151109. 10.1371/journal.pone.0151109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzin, P. , Frey, U. , Armann, J. , Kieninger, E. , Fuchs, O. , Röösli, M. , & Schaub, B. (2011). Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One, 6(8), e23130. 10.1371/journal.pone.0023130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li‐Hong, F. , Bin, C. , Yan‐Feng, Z. , Shu‐Jin, L. , Chun‐Ling, M. , Zhi‐Yu, N. , Guo‐Zhong, Z. , Min, Z. , & Yu‐Xia, Y. (2007). Methylation status of the IL‐10 gene promoter in the peripheral blood mononuclear cells of rheumatoid arthritis patients. Yi Chuan = Hereditas/Zhongguo Yi Chuan Xue Hui Bian Ji, 29(11), 1357–1361. 10.1360/yc-007-1357 [DOI] [PubMed] [Google Scholar]
- Lin, S.‐Y. , Hsieh, S.‐C. , Lin, Y.‐C. , Lee, C.‐N. , Tsai, M.‐H. , Lai, L.‐C. , Chuang, E. Y. , Chen, P. C. , Hung, C. C. , Chen, L. Y. , Hsieh, W. S. , Niu, D. M. , Su, Y. N. , & Ho, H. N. (2012). A whole genome methylation analysis of systemic lupus erythematosus: Hypomethylation of the IL10 and IL1R2 promoters is associated with disease activity. Genes and Immunity, 13(3), 214–220. 10.1038/gene.2011.74 [DOI] [PubMed] [Google Scholar]
- Loeb, J. S. , Blower, W. C. , Feldstein, J. F. , Koch, B. A. , Munlin, A. L. , & Hardie, W. D. (2008). Acceptability and repeatability of spirometry in children using updated ATS/ERS criteria. Pediatric Pulmonology, 43(10), 1020–1024. 10.1002/ppul.20908 [DOI] [PubMed] [Google Scholar]
- Lyon, H. , Lange, C. , Lake, S. , Silverman, E. K. , Randolph, A. G. , Kwiatkowski, D. , Raby, B. A. , Lazarus, R. , Weiland, K. M. , Laird, N. , & Weiss, S. T. (2004). IL10 gene polymorphisms are associated with asthma phenotypes in children. Genetic Epidemiology, 26(2), 155–165. 10.1002/gepi.10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, C. R. , Costa, G. N. O. , & da Silva, T. M. (2017). Suggestive association between variants in IL1RAPL and asthma symptoms in Latin American children. European Journal of Human Genetics https://www.nature.com/articles/ejhg2016197, 25, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. M. , & Ortega, V. E. (2013). Pharmacogenetics and the development of personalized approaches for combination therapy in asthma. Current Allergy and Asthma Reports, 13(5), 443–452. 10.1007/s11882-013-0372-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montinaro, F. , Busby, G. B. J. , Pascali, V. L. , Myers, S. , Hellenthal, G. , & Capelli, C. (2015). Unravelling the hidden ancestry of American admixed populations. Nature Communications, 6, 6596. 10.1038/ncomms7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Estrada, A. , Gignoux, C. R. , Fernández‐López, J. C. , Zakharia, F. , Sikora, M. , Contreras, A. V. , Acuña‐Alonzo, V. , Sandoval, K. , Eng, C. , Romero‐Hidalgo, S. , Ortiz‐Tello, P. , Robles, V. , Kenny, E. E. , Nuño‐Arana, I. , Barquera‐Lozano, R. , Macín‐Pérez, G. , Granados‐Arriola, J. , Huntsman, S. , Galanter, J. M. , … Bustamante, C. D. (2014). Human genetics. The genetics of Mexico recapitulates native American substructure and affects biomedical traits. Science, 344(6189), 1280–1285. 10.1126/science.1251688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Estrada, A. , Gravel, S. , Zakharia, F. , McCauley, J. L. , Byrnes, J. K. , Gignoux, C. R. , Ortiz‐Tello, P. A. , Martínez, R. J. , Hedges, D. J. , Morris, R. W. , Eng, C. , Sandoval, K. , Acevedo‐Acevedo, S. , Norman, P. J. , Layrisse, Z. , Parham, P. , Martínez‐Cruzado, J. C. , Burchard, E. G. , Cuccaro, M. L. , … Bustamante, C. D. (2013). Reconstructing the population genetic history of the Caribbean. PLoS Genetics, 9(11), e1003925. 10.1371/journal.pgen.1003925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritsugu, K. P. (2007). The 2006 report of the surgeon general: The health consequences of involuntary exposure to tobacco smoke. American Journal of Preventive Medicine, 32(6), 542–543. 10.1016/j.amepre.2007.02.026 [DOI] [PubMed] [Google Scholar]
- Mullegama, S. V. , Alberti, M. O. , Cora, A. , Li, Y. , Toy, T. , Tomasian, V. , & Xian, R. R. (2019). Nucleic acid extraction from human biological samples. Methods in Molecular Biology, 1897, 359–383. 10.1007/978-1-4939-8935-5_30 [DOI] [PubMed] [Google Scholar]
- Natkunam, Y. , Zhao, S. , Mason, D. Y. , Chen, J. , Taidi, B. , Jones, M. , Hammer, A. S. , Dutoit, S. H. , Lossos, I. S. , & Levy, R. (2007). The oncoprotein LMO2 is expressed in normal germinal‐center B cells and in human B‐cell lymphomas. Blood, 109(4), 1636–1642. 10.1182/blood-2006-08-039024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman, Å. , Hohmann, C. , Orsini, N. , Pershagen, G. , Eller, E. , Kjaer, H. F. , Gehring, U. , Granell, R. , Henderson, J. , Heinrich, J. , Lau, S. , Nieuwenhuijsen, M. , Sunyer, J. , Tischer, C. , Torrent, M. , Wahn, U. , Wijga, A. H. , Wickman, M. , Keil, T. , … as part of the ENRIECO Consortium . (2012). Maternal smoking in pregnancy and asthma in preschool children: A pooled analysis of eight birth cohorts. American Journal of Respiratory and Critical Care Medicine, 186(10), 1037–1043. 10.1164/rccm.201203-0501OC [DOI] [PubMed] [Google Scholar]
- Nutten, S. (2015). Atopic dermatitis: Global epidemiology and risk factors. Annals of Nutrition & Metabolism, 66(Suppl 1), 8–16. 10.1159/000370220 [DOI] [PubMed] [Google Scholar]
- Obando‐Pacheco, P. , & Justicia‐Grande, A. J. (2018). Respiratory syncytial virus seasonality: A global overview. The Journal of Infectious Diseases https://academic.oup.com/jid/article‐abstract/217/9/1356/4829950, 217, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Ober, C. , & Hoffjan, S. (2006). Asthma genetics 2006: The long and winding road to gene discovery. Genes and Immunity, 7(2), 95–100. 10.1038/sj.gene.6364284 [DOI] [PubMed] [Google Scholar]
- Ochoa‐Avilés, C. , Morillo, D. , Ochoa, A. , Rodriguez, A. , Cooper, P. J. , Andrade, S. , Molina, M. , Parra, M. , Parra‐Ullauri, A. , Mejía, D. , Neira, A. , Rodas‐Espinoza, C. , & Ochoa‐Avilés, A. (2020). Prevalence and risk factors for asthma, rhinitis, eczema and atopy among preschool children in an andean city. PloS one, 15(7), e0234633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa‐Avilés, C. , Morillo, D. , Rodriguez, A. , Cooper, P. J. , Andrade, S. , Molina, M. , Parra, M. , Parra‐Ullauri, A. , Mejía, D. , Neira, A. , Rodas‐Espinoza, C. , & Ochoa‐Avilés, A. (2020). Correction: Prevalence and risk factors for asthma, rhinitis, eczema, and atopy among preschool children in an Andean City. PLoS One, 15(7), e0236843. 10.1371/journal.pone.0236843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, M. M. , Lavender, P. , Kuo, C.‐H. S. , Galea, F. , Michaeloudes, C. , Flanagan, J. M. , Chung, K. F. , & Adcock, I. M. (2018). DNA methylation modules in airway smooth muscle are associated with asthma severity. The European Respiratory Journal, 51(4), 1701068. 10.1183/13993003.01068-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, A. O. , Queiroz, G. A. , de Jesus Silva, M. , da Silva, R. R. , da Silva, H. B. F. , Carneiro, N. V. Q. , Fonseca, H. F. , de Santana, M. B. R. , Nascimento, R. S. , Alcântara‐Neves, N. M. , Costa, G. N. O. , Costa, R. D. S. , Barreto, M. L. , & Figueiredo, C. A. (2018). Polymorphisms in the DAD1 and OXA1L genes are associated with asthma and atopy in a south American population. Molecular Immunology, 101, 294–302. 10.1016/j.molimm.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Reese, S. E. , Cheng‐Jian, X. , den Dekker, H. T. , Lee, M. K. , Sikdar, S. , Ruiz‐Arenas, C. , Merid, S. K. , Rezwan, F. I. , Page, C. M. , Ullemar, V. , Melton, P. E. , Oh, S. S. , Yang, I. V. , Burrows, K. , Söderhäll, C. , Jima, D. D. , Gao, L. , Arathimos, R. , Küpers, L. K. , & Wielscher, M. (2018). Epigenome‐wide meta‐analysis of DNA methylation and childhood asthma. The Journal of Allergy and Clinical Immunology, 143, 2062–2074. 10.1016/j.jaci.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan, V. K. , Liu, J. , Sakurai, R. , & Torday, J. S. (2013). Perinatal nicotine‐induced transgenerational asthma. American Journal of Physiology Lung Cellular and Molecular Physiology, 305(L507), L501–L507. 10.1152/ajplung.00078.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A. , Vaca, M. G. , Chico, M. E. , Rodrigues, L. C. , Barreto, M. L. , & Cooper, P. J. (2017). Rural to urban migration is associated with increased prevalence of childhood wheeze in a Latin‐American City. BMJ Open Respiratory Research, 4(1), e000205. 10.1136/bmjresp-2017-000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Yang, I. V. , & Schwartz, D. A. (2022). Epigenetic regulation of immune function in asthma. The Journal of Allergy and Clinical Immunology, 150(2), 259–265. 10.1016/j.jaci.2022.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan, D. P. , & Cook, D. G. (1998). Health effects of passive smoking. 6. Parental smoking and childhood asthma: Longitudinal and case‐control studies. Thorax, 53(3), 204–212. 10.1136/thx.53.3.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. , Pan, R. , Lele, X. , Ma, Q. , Ying, X. , Zhao, J. , Zhao, H. , et al. (2021). IL10 hypomethylation is associated with the risk of gastric cancer. Oncology Letters, 21(4), 241. 10.3892/ol.2021.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, H. M. P. , Alcantara‐Neves, N. M. , Barreto, M. , Figueiredo, C. A. , & Costa, R. S. (2017). Adenylyl cyclase type 9 gene polymorphisms are associated with asthma and allergy in Brazilian children. Molecular Immunology, 82, 137–145. 10.1016/j.molimm.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Weinmayr, G. , Forastiere, F. , Weiland, S. K. , Rzehak, P. , Abramidze, T. , Annesi‐Maesano, I. , Björkstén, B. , et al. (2008). International variation in prevalence of rhinitis and its relationship with sensitisation to perennial and seasonal allergens. The European Respiratory Journal, 32(5), 1250–1261. 10.1183/09031936.00157807 [DOI] [PubMed] [Google Scholar]
- World Health Organization and Others . (2018). WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025 . https://apps.who.int/iris/bitstream/handle/10665/272694/9789241514170‐eng.pdf
- Wu, C.‐C. , Chia‐Yu, O. , Chang, J.‐C. , Hsu, T.‐Y. , Kuo, H.‐C. , Liu, C.‐A. , Wang, C.‐L. , et al. (2014). Gender‐dependent effect of GSTM1 genotype on childhood asthma associated with prenatal tobacco smoke exposure. BioMed Research International, 2014, 769452. 10.1155/2014/769452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.‐C. , Hsu, T.‐Y. , Chang, J.‐C. , Chia‐Yu, O. , Kuo, H.‐C. , Liu, C.‐A. , Wang, C.‐L. , Chuang, H. , Chen, C.‐P. , & Yang, K. D. (2019). Paternal tobacco smoke correlated to offspring asthma and prenatal epigenetic programming. Frontiers in Genetics, 10, 471. 10.3389/fgene.2019.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C.‐J. , Söderhäll, C. , Bustamante, M. , Baïz, N. , Gruzieva, O. , Gehring, U. , Mason, D. , Chatzi, L. , Basterrechea, M. , Llop, S. , Torrent, M. , Forastiere, F. , Fantini, M. P. , Carlsen, K. C. L. , Haahtela, T. , Morin, A. , Kerkhof, M. , Merid, S. K. , van Rijkom, B. , … Koppelman, G. H. (2018). DNA methylation in childhood asthma: An epigenome‐wide meta‐analysis. The Lancet. Respiratory Medicine, 6(5), 379–388. 10.1016/S2213-2600(18)30052-3 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Yuan, L. , Yang, M. , Xizi, D. , Qin, L. , Wang, L. , Zhou, K. , et al. (2021). Aberrant methylation of aging‐related genes in asthma. Frontiers in Molecular Biosciences, 8, 655285. 10.3389/fmolb.2021.655285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharasiewicz, A. (2016). Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Research, 2(3), 02016. 10.1183/23120541.00042-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M. , Gao, F. , Wu, X. , Tang, J. , & Lu, Q. (2010). Abnormal DNA methylation in peripheral blood mononuclear cells from patients with vitiligo. The British Journal of Dermatology, 163(4), 736–742. 10.1111/j.1365-2133.2010.09919.x [DOI] [PubMed] [Google Scholar]
- Zhao, M. , Tang, J. , Gao, F. , Xiaoyan, W. , Liang, Y. , Yin, H. , & Qianjin, L. (2010). Hypomethylation of IL10 and IL13 promoters in CD4+ T cells of patients with systemic lupus erythematosus. Journal of Biomedicine & Biotechnology, 2010, 931018. 10.1155/2010/931018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


