Abstract
This narrative review provides an overview of the posterior circulation and the clinical features of common posterior circulation stroke (PCS) syndromes in the posterior arterial territories and how to distinguish them from mimics. We outline the hyperacute management of patients with suspected PCS with emphasis on how to identify those who are likely to benefit from intervention based on imaging findings. Finally, we review advances in treatment options, including developments in endovascular thrombectomy (EVT) and intravenous thrombolysis (IVT), and the principles of medical management and indications for neurosurgery. Observational and randomised clinical trial data have been equivocal regarding EVT in PCS, but more recent studies strongly support its efficacy. There have been concomitant advances in imaging of posterior stroke to guide optimal patient selection for thrombectomy. Recent evidence suggests that clinicians should have a heightened suspicion of posterior circulation events with the resultant implementation of timely, evidence-based management.
KEYWORDS: stroke, posterior circulation, posterior stroke, basilar artery occlusion, endovascular thrombectomy, thrombolysis
Introduction
Posterior circulation stroke (PCS) refers to a neurological deficit resulting from impaired perfusion of the brainstem, cerebellum, thalamus and/or occipitoparietal lobe. This is a clinical manifestation of occlusion causing ischaemia of, or haemorrhage from, the vertebrobasilar system. Although relatively less common compared with anterior circulation stroke (ACS), PCS nonetheless accounts for ∼20% of all strokes, with 70,000–100,000 people presenting with PCS in the USA annually.1 The morbidity and mortality associated with PCS are variable: stroke registries have reported mortality rates as low as 3.6% at 30 days2 overall, but some syndromes (notably basilar artery occlusion; BAO), carry an abysmal prognosis, with mortality >80% in some datasets.3 Specifically, occlusions of the proximal and/or middle portions of the basilar artery have been associated with severe deficits and higher mortality compared with the occlusion of the distal one-third of the basilar artery.4
Anatomy and pathophysiology
Fig 1 provides a brief anatomical overview of the posterior circulation. Vertebral arteries are derived from the subclavian arteries bilaterally and supply the posterior inferior cerebellar arteries (PICAs) and the midline anterior spinal artery (ASA) during their ascent. The vertebral arteries fuse at the pontomedullary junction, creating the basilar artery. The basilar gives rise first to the anterior inferior cerebellar arteries (AICAs), small pontine branches and finally the superior cerebellar arteries (SCAs), before terminating as the bilateral posterior cerebral arteries (PCAs). There is considerable interindividual variation: one vertebral artery is frequently dominant, contributing to the majority of basilar supply, and the hypoplastic side can even terminate at the ipsilateral PICAs in ∼2% of patients. Although the majority remain asymptomatic, it has been suggested that the altered haemodynamics potentially increase the likelihood of vertebrobasilar vascular events. Furthermore, ∼20% of patients have an incomplete circle of Willis, meaning that at least one PCA is supplied via the anterior circulation, namely the carotids, the so-called fetal origin of PCA. This does not appear to increase the stroke risk per se, although it can result in carotid occlusion presenting as a PCA syndrome (see below).5 There are also structural differences between the anterior and posterior circulation vessels, including thinner arterial walls and more concentric intimal thickening in the latter.6
Fig 1.
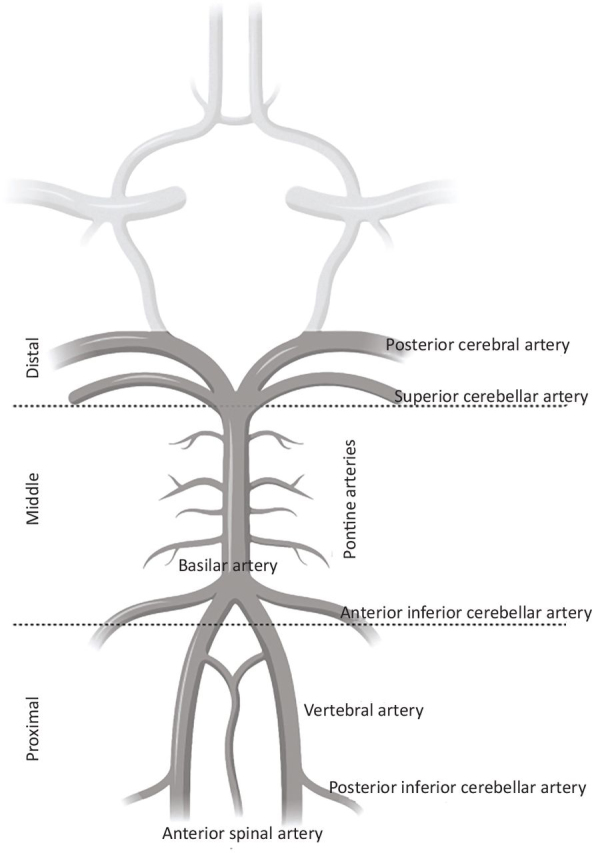
Anatomy of the posterior circulation. Created with BioRender (BioRender.com).
There are differences between PCS and ACS regarding pathology, aetiology, outcome and, eventually, preferred therapeutical approaches.7 PCS aetiology is primarily embolic (40%), with a cardiac source in 24% of cases in the New England registry.2 Large-artery occlusive disease with haemodynamic deficits secondary to intracranial atherosclerotic disease (ICAD) accounted for 32% and branch arteries for 14%.2 Basilar occlusion in isolation is mainly attributed to ICAD (35%) and embolism (36%). Vertebrobasilar dissection, where a tear in the tunica intima allows blood flow to create a false lumen between the intima and media, accounts for 5% of BAO overall; importantly, dissection is relatively more common in younger patients with stroke.8 Although trauma is an important cause of dissection, most cases are spontaneous; a small minority have an underlying cause, such as connective tissue disease. In the Basilar Artery International Cooperation Study (BASICS) registry, the stroke mechanism was not identified in 22% of basilar occlusions.9
The brainstem is vital for physiological homeostasis, accounting for the very high mortality rates in BAO. It has subsequently been observed that there are myriad clinicoradiological subgroups that could have excellent medium- and long-term outcomes despite severe deficits at presentation.10 This might reflect intrinsic regional resistance, because in vivo models consistently suggest that the forebrain is significantly more vulnerable to necrosis compared with the brainstem. Clinical data are relatively more scarce and focus primarily on states of global hypoperfusion, such as cardiac arrest; nonetheless, there are pathological and radiological studies to support regional resistance to prolonged ischaemia in the brainstem, which could relate to relatively lower metabolic demand compared with the cortex.11, 12 The mechanisms for this remain elusive, although it has recently been suggested that Na+/K+ ATPase isoforms are key mediators.13
Clinical presentation
PCS is difficult to diagnose owing to the often stuttering, progressive and/or non-lateralising nature of the symptoms given the blood supply to the brainstem (midbrain, pons and medulla), cerebellum and occipital cortex.14 In one cohort, over 60% of patients had progressive features, and 13% had prodromal symptoms before their stroke presentation.15 Timely diagnosis relies upon a careful history and a high clinical index of suspicion (speed of onset, age and vascular risk factors). Acute onset of 'crossed’ deficits, that is, cranial nerve signs on one side with a deficiency in sensory or motor function in the limbs on the opposite side of the body, as seen in lateral medullary syndrome, is likely to have a posterior circulation aetiology. The most common symptoms patients with PCS experience are listed in Table 1.
Table 1.
Most common posterior circulation symptoms66
| Symptoms | Percentage of patients |
|---|---|
| Dizziness | 47 |
| Unilateral limb weakness | 41 |
| Dysarthria | 31 |
| Headache | 28 |
| Nausea or vomiting | 27 |
'Dizziness’ is often a troublesome symptom to localise. In the context of acute stroke, vertigo is a feeling of true movement relative to the environment. At the bedside, the head impulse test (normal), the pattern of nystagmus (fast-phase changes direction), and the test for skew (skew deviation), collectively termed the HINTS test, has a greater than 90% sensitivity for distinguishing a central from a peripheral cause for vertigo and, in one cross-sectional study, a normal head impulse test with direction-changing nystagmus or skew deviation demonstrated 100% sensitivity and 96% specificity for PCS.16 Table 2 describes the clinical features that localise vascular lesions of the posterior circulation.
Table 2.
Posterior stroke presentations according to vascular territory
| Vascular territory | Anatomical area | Syndrome/clinical features |
|---|---|---|
Branches of basilar artery:
|
Brainstem from medulla upward | Complete basilar syndrome
|
| Top of basilar artery | Bilateral midbrain and thalamic infarction with damage to reticular activating system; also medial and temporal lobe infarction | Top of basilar syndrome
|
| Paramedian perforating vessel | Bilateral infarction of ventral pons (midbrain and supratentorial syndromes are disconnected from nervous system below) | Locked-in syndrome Complete tetraplegia with preserved level of consciousness |
| Superior cerebellar artery (SCA) |
|
|
| Anterior inferior cerebellar artery (AICA) |
|
|
| Posterior inferior cerebellar artery (PICA) |
|
|
Posterior cerebral artery (PCA) (terminal branch of basilar artery)
|
|
|
Assessment and diagnosis
As with any stroke presentation, clinical examination and imaging are crucial for accurate diagnosis and selection of management options. Neurological examination in acute presentations is often difficult and with considerable interobserver variation in findings. Not only is diagnosis more challenging, but so too is estimation of severity. First, the symptomatology and time course of PCS are more variable, difficult to localise and frequently non-specific (Table 1). An estimated 39.4% of PCS cases can be facial drooping, arm weakness, speech difficulties and time (FAST) negative.17 Second, even if the stroke is recognised, there is evidence that the National Institutes of Health Stroke Scale (NIHSS) underestimates severity and is prognostically too optimistic in PCS,18 perhaps reflecting the relatively greater contribution of forebrain functions to the composition of the score. Alternative scores that take greater account of PCS features, including dysphagia and diplopia, have been proposed, such as the Israeli Vertebrobasilar Stroke Scale,19 but their use in everyday clinical settings and research trials remains limited. In basilar occlusions, neurological scores will commonly underestimate prognosis because of the progressive nature of these syndromes in the absence of intervention, in contrast to anterior circulation occlusions.14
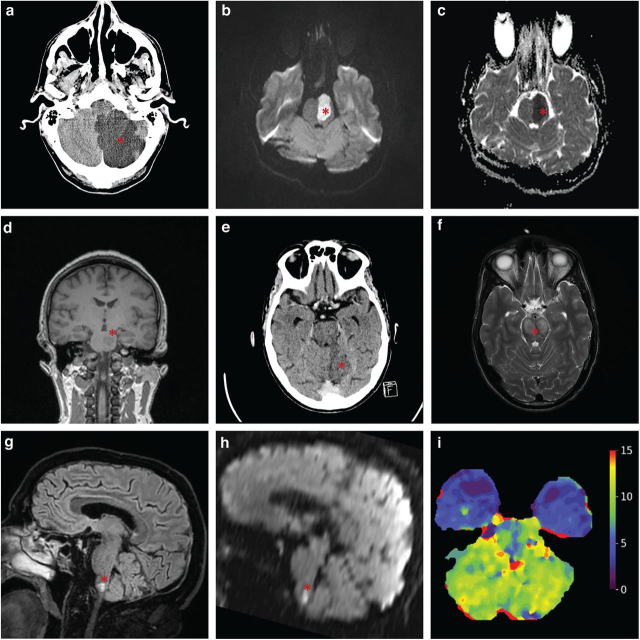
Imaging is central to diagnosis and management decisions in PCS, with various modalities available (Fig 2). Non-contrast computed tomography (NCCT) remains the mainstay of stroke imaging; however, its use to detect posterior fossa ischaemia is complicated by multiple factors. First, the relatively thick skull base degrades NCCT quality because of beam hardening and photon starvation artefacts. Consequently, its sensitivity for early ischaemic changes in PCS has been estimated to be ∼40%, with some patients remaining NCCT negative even at >24 h after onset20; by comparison, the sensitivity in ACS is ∼75%. Second, whereas identification of thrombus on NCCT (a so-called 'hyperdense basilar sign’) has good specificity for large vessel occlusion (LVO) and can even be helpful in prognostication, its sensitivity remains relatively poor at ∼70–75% and is highly dependent on the awareness of the reader to the clinical probability of LVO.21 Consequently, a normal NCCT does not reliably exclude PCS.
Fig 2.
Example infarcts in the posterior circulation (asterisks). (a) Axial non-contrast CT image of a left posterior cerebellar artery infarct with mass effect. (b) Axial MRI DWI image of a large acute left-sided pontine infarct. (c) Axial MRI ADC image of a large acute left-sided pontine infarct. (d) Coronal MRI T1 image of an acute left-sided pontine infarct. (e) Axial non-contrast CT image showing an infarct on the medial aspect of the left cerebellum representing a superior cerebellar artery infarct. (f) Axial T2 MRI image of a bilateral caudal midbrain infarct. (g) Sagittal MRI T2 image with medullary infarct. (h) Sagittal MRI DWI image with restricted diffusion in medullary infarct. (i) Axial CT perfusion image showing time to maximum (Tmax, false colour scale in seconds), with bilateral cerebellar hypoperfusion in basilar artery occlusion. ADC = apparent diffusion coefficient; CT = computed tomography; DWI = diffusion-weighted image; MRI = magnetic resonance imaging.
Similar to current ACS imaging protocols, contrast CT angiography (CTA) is a crucial adjunct in PCS diagnosis. It is widely accessible, easily combined with NCCT acquisition and relatively straightforward to interpret. CTA has excellent sensitivity and specificity (both >98%) compared with the gold standard on digital subtraction angiography (DSA) for LVO detection,22 which is crucial for accurately selecting patients for endovascular thrombectomy (EVT). Moreover, prognostically valuable metrics can be derived from CTA, including thrombus burden, collateral status (as incorporated in the Basilar Artery on Computed Tomography Angiography (BATMAN) score),23 distal versus proximal occlusion site24 and hypoattenuation on CTA source images, although their real-life use remains limited.
CT perfusion (CTP) is another contrast-based technique of significant interest in hyperacute stroke. It quantifies mean transit time (MTT), cerebral blood flow (CBF) and volume (CBV), and differential changes in these hypothetically allow for identification of salvageable versus non-salvageable brain: infarct core should exhibit prolonged MTT with reduced CBF and CBV, whereas penumbra should have a more moderate reduction in CBF and a normal CBV. Adjunct CTP improves sensitivity for PCS over NCCT and CTA alone,25 most notably for cerebellar strokes,26 and could allow for more accurate prognostication.27
Magnetic resonance imaging (MRI) is the most sensitive imaging modality for detecting ischaemia. Diffusion-weighted imaging (DWI) is beneficial because of its high sensitivity for acutely ischaemic tissue, and DWI lesion burden is an independent outcome predictor in PCS.28 Importantly, the odds of a false-negative DWI in PCS are fivefold higher than in ACS.29 Sensitivity can be improved by adding perfusion-weighted imaging (PWI) sequences.30 MR angiography (MRA) can be performed as part of the same scan to characterise the vasculature. However, there is a dearth of prospective evidence to suggest that MRI offers added benefits beyond CT-based imaging in patient selection for reperfusion therapy. This is compounded by its higher cost, longer scan duration, limited availability (particularly out of hours), and possible contraindications (eg metal implants or pacemakers) that might not be identified in an emergency setting.
Treatment
Reperfusion therapy using either intravenous thrombolysis (IVT) alone or in combination with EVT is the mainstay of treatment for both ACS and PCS. There has been a revolution in the use of EVT, driven by the advent of second-generation devices ('stent-retrievers’) and direct aspiration. The evidence basis behind ACS thrombectomy and technical considerations have been reviewed extensively elsewhere31; this review focuses on its application to PCS.
Thrombolysis
IVT with alteplase, recombinant tissue plasminogen activator, used within 4.5 h post stroke is the recommended standard treatment for acute ischaemic stroke, including PCS.32 However, treatment with IVT alone is associated with low recanalisation rates, particularly for BA occlusion.33 There is recent evidence that tenecteplase might be associated with a higher likelihood of reperfusion, although this has not yet been confirmed in randomised controlled trials (RCTs).34
Intra-arterial thrombolysis remains an option and, in the anterior circulation, has been associated with improved clinical outcomes with an increased risk of symptomatic intracranial haemorrhage (sICH).35 The evidence base in PCS is limited, and meta-analyses (MAs) have suggested uncertain benefits, particularly compared with MT.36
Mechanical thrombectomy
Whereas the clinical effectiveness of EVT for treating anterior circulation stroke is well established within 6 h post stroke and up to 24 h post stroke for selected patients, many centres have extended EVT to LVO in the posterior circulation, most notably BAO. To date, there have been four major RCTs examining thrombectomy in BAO. The Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST) trial, a multicenter RCT for BA occlusion within 8 h post stroke, was confounded by an excessive crossover from the medical management group to the EVT group, which led to the non-superiority of EVT over medical treatment in the intention-to-treat analysis,37 with subsequent premature termination of the trial. Importantly, both the as-treated and per-protocol analyses showed an increase in patients achieving a modified Rankin scale (mRS) 0–3, indicating that more patients were left with at most moderate disability following EVT. The BASICS RCT (distinct from the BASICS registry described below) did not demonstrate significant differences between EVT and medical management, including in as-treated analyses, and also had issues with crossover and eligible patients being treated outside the trial, mostly with EVT.38 By contrast, more recent RCTs have provided stronger evidence for the benefit of EVT in BAO. In the Endovascular Treatment for Acute Basilar-Artery Occlusion (ATTENTION) trial, EVT was associated with improved rates of mRS 0–3 and reduced mortality at 90 days, in patients presenting within 12 h post stroke onset.39 Similarly, the Basilar Artery Occlusion Chinese Endovascular (BAOCHE) RCT showed that, in 217 patients, long-term rates of mRS 0–3 were superior with EVT compared with medical management in the extended window of 6–24 h after stroke onset.40 Although both ATTENTION and BAOCHE were conducted in Chinese populations, with certain epidemiological differences (such as higher rates of intracranial arterial stenosis), it is nonetheless likely that these results will be generalisable to more heterogeneous populations. Indeed, MAs of the four trials also strongly support higher rates of mRS 0–3, mRS 0–2 (ie functional independence) and lower mortality with EVT following BAO.41
There have also been varied findings in registry studies. The BASICS registry did not demonstrate any significant difference in the efficacy and prognosis between endovascular treatment (comprising MT, stenting or a combination thereof, with or without IVT) compared with IVT alone in the 30-day clinical outcome.9 Notably, the BASICS registry largely pre-dated the development of new-generation EVT devices, which have been shown to have higher recanalisation rates and improved functional outcomes.42 EVT was supported by the BASILAR non-randomised cohort study, which included 829 patients and found a highly significant improvement in 90-day outcomes of BAO treated with MT, which was maintained at 1 year.43, 44 The largest and most recent registry, ATTENTION, also demonstrated improved functional outcomes and reduced mortality,45 similarly to its RCT counterpart. Other smaller recent prospective and retrospective multicentre46, 47, 48 studies have also shown successful reperfusion and a favourable outcome following treatment of BA occlusion with EVT. It is also important to note that observational data are inherently more prone to bias compared with RCTs, most notably selection bias. Nonetheless, it is reassuring that the most up-to-date observational data are in agreement with the most recent RCT data.
Several systematic reviews (SRs) and MAs of observational studies on EVT have shown successful recanalisation rates with favourable outcomes and reduced mortality. One review of 102 observational studies found no significant difference in favourable outcome odds; however, mortality was significantly reduced (43% with IVT vs. 31% with EVT).49 Another review involving 672 patients from 17 studies showed an excellent recanalisation rate with EVT compared to IVT (88% vs. 60%), leading to improved rates of good clinical outcome (43 versus 31%) and reduced mortality (26% versus 41%,) with EVT versus IVT.50 Unfortunately, MAs have been limited by the small sample sizes and heterogeneity of observational studies and the fact that many preceded the era of modern thrombectomy methods.
Although uncommon, EVT can result in vessel injuries (perforation, dissection or pseudoaneurysm; at the puncture site or intracranially), vasospasm, sICH and infarction in a previously unaffected territory, among other complications.31, 42 MAs have not identified differences in the overall safety profile between stent retrievers and direct aspiration,49 and sICH risk did not appear to differ between EVT and medical management (5% versus 7%),50 similar to data from ACS. However, these analyses preceded ATTENTION and BAOCHE, both of which suggested higher rates of sICH in the EVT group, despite an overall beneficial effect.39, 40 This effect is further borne out in MAs showing higher rates of sICH with EVT.41
Despite these recent developments, solidifying the evidence base for EVT in BAO, many questions remain about optimal patient selection for this procedure to identify those patients with the capacity to benefit from intervention. One key question is the use of bridging IVT before EVT. This has been extensively studied in the anterior circulation: although the results remain somewhat equivocal, current recommendations from the European Stroke Organisation are to offer patients both IVT and EVT if eligible.51 There are fewer data regarding this in BAO, because the RCTs comparing bridging to direct EVT were mostly restricted to anterior circulation LVO. Even in trials that recruited patients with BAO, the sample sizes were very limited; for example, of 295 patients included in DIRECT-SAFE, only 19 had basilar occlusion.52 Both ATTENTION and BAOCHE had very low rates of IVT use because of the extended time windows, limiting the utility of subgroup analyses. Observational studies have supported bridging IVT,53, 54 but BAO-specific RCT data are required for evidence-based guidelines.
Antiplatelet therapy
Two large clinical trials tested the safety and efficacy of administering aspirin doses between 160 and 300 mg to patients with AIS, and their results were recently confirmed by an extensive Cochrane review of aspirin trials.55 Aspirin administration is indicated in patients with AIS within 24 to 48 h after symptom onset. For patients who have received IVT, the aspirin administration should be delayed for 24 h, except in the case of concomitant conditions for which aspirin treatment given in the absence of IVT is known to provide a benefit or withholding such treatment is known to cause harm. In patients with contraindications to aspirin, the administration of alternative antiplatelet agents (eg clopidogrel) should be considered.32 Furthermore, in patients presenting with minor non-cardioembolic ischaemic stroke (NIHSS score ≤3) who did not receive IVT, treatment with dual antiplatelet therapy (aspirin and clopidogrel) started within 24 h after symptom onset and continued for 21 days is effective in reducing recurrent ischaemic stroke for a period of up to 90 days from symptom onset.56 This must be balanced with an increased risk of haemorrhagic complications and does not apply to patients with known underlying cardioembolic causes.57
Monitoring
Patients with confirmed PCS require rapid transfer to a specialist stroke team and, depending on the level of consciousness, intensive care referral might be indicated. Careful assessment of neurological parameters, including NIHSS, should be performed repeatedly and with increased frequency in patients with unstable clinical presentation and evolving deficits.32 This is also useful for prognostication, because NIHSS improvement following EVT predicts long-term functional outcomes.58
Support of respiratory functions and cardiac monitoring
Patients should be maintained at an oxygen saturation of >94% as per American Heart Association (AHA) guidelines.32 Impaired respiratory function resulting from a decreased level of consciousness (with risk of aspiration) or direct stroke involvement of central respiratory centres is a common challenge in BAO, and a low threshold for airway support and ventilatory assistance is recommended. If the suspected cause of stroke is cardiogenic, close cardiac monitoring and consideration for anticoagulation are recommended if a cardiogenic cause is identified.
Physiological parameters
Patients with hyperthermia (>38°C) should be treated with antipyretic medication, and investigated for infective or other treatable causes of fever. This is supported by a large retrospective cohort study in which temperatures of <37°C and >39°C were associated with an increased in-hospital mortality.59 Targeted temperature management to avoid hyperthermia features in numerous guidelines, although the evidence base remains relatively weak.60 The role of hypothermia, although supported by preclinical studies, remains unclear in PCS.61 Hyperglycaemic (blood glucose 180 mg/dL, 10 mmol/L) and hypoglycaemic (<60 mg/dL; 3.3 mmol/L) blood glucose levels should be normalised and uncontrolled diabetes mellitus treated accordingly.
Surgical management
Ischaemic stroke can be complicated by haemorrhagic transformation or cytotoxic oedema, with subsequent mass effect and intracranial pressure rise resulting from the stroke itself or a complication of treatment with EVT or IA/IV thrombolysis.61 The therapeutic approach to addressing this is neurosurgical, either through external ventricular drainage or decompressive suboccipital craniectomy, which is indicated in patients presenting with radiological or clinical signs of mass effect, brain stem compression ascending trans-tentorial herniation or if ventriculostomy fails to improve neurological function.
Secondary prevention
The paucity of primary literature on secondary prevention following PCS obliges the use of recommendations on anticoagulation, blood pressure and lipid control that are mostly based on anterior circulation stroke, although inter-regional differences might be negligible.
A combination of aspirin and clopidogrel is more effective compared with monotherapy with either agent for high-risk transient ischaemic attack (TIA) and minor stroke during the first few weeks after stroke onset.62 Long-term treatment is 75 mg once daily of either clopidogrel or aspirin. In patients with concomitant atrial fibrillation, the mainstay of treatment is with novel oral anticoagulants (NOACs) or warfarin. Increased blood pressure is a risk factor for stroke; however, in the context of significant stenosis in the vertebrobasilar circulation, caution should be exercised when considering aggressive blood pressure control. Evidence is mainly anecdotal; however, those with anatomical variants, such as limited or absent posterior communicating arteries, especially in the context of bilateral vertebral artery stenosis or severe stenosis of the dominant vertebral artery, or severe basilar stenosis, might be at increased risk of infarction in the context of relative hypotension. Carotid intervention is evidence based in ACS and should only be considered in PCS where the ischaemic territory is supplied through a fetal PCA. Although the association between raised plasma cholesterol and ischaemic stroke is less clear than that between cholesterol and coronary artery disease, high-dose statins still reduce overall stroke incidence (with a possible slight increase in haemorrhagic events) and are usually included in standard secondary prevention.32
Vertebral artery stenosis is responsible for ∼20% of PCS that could technically be stented. The Vertebral artery Ischaemia Stenting Trial (VIST) trial compared vertebral stenting/angioplasty and best medical therapy with no difference between groups.63 The management of vertebral artery dissection, a relatively common cause of PCS in younger patients, is controversial. The Cervical Artery Dissection In Stroke Study (CADISS) trial reported no difference between antiplatelet and anticoagulation but was limited by low stroke incidence.64 Stenting can be considered on a case-by-case basis, including for both stenosis and vertebral dissection refractory to medical management, but there is limited evidence to support this.65
Conclusion
Posterior circulation poses a significant clinical challenge because of its variable and non-specific symptoms and the potentially grave consequences of a delayed diagnosis. Broadening access to high-quality imaging has significantly improved our ability to diagnose PCS and identify patients who might benefit from treatment. The rise of interventional approaches and EVT has provided new hope for improving outcomes in PCS secondary to BAO, supported by recent RCTs and observational data. However, further work is required in certain aspects, such as the use of adjunct IVT with EVT.
Summary
-
•
Posterior circulation stroke (PCS) has significant morbidity and mortality but remains diagnostically challenging because of the often non-specific presentations.
-
•
There has been significant progress in using advanced imaging for PCS, which has enabled increased patient selection for endovascular thrombectomy (EVT).
-
•
Until recently, the evidence base for EVT in large vessel occlusion in the posterior circulation was weaker than in the anterior circulation. However, over the past 12 months, convincing observational and randomised trial evidence has emerged to support the efficacy of EVT in basilar artery occlusion.
-
•
For most patients with PCS, medical management remains the treatment mainstay.
Funding
AMB is funded by the NIHR Oxford Biomedical Research Centre.
Conflicts of interest
AMB is a senior medical science advisor and co-founder of Brainomix, a company that develops electronic ASPECTS (e-ASPECTS). GH is chief medical and innovation officer at Brainomix. The other authors do not declare any conflicts of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Caplan L, Chung CS, Wityk R, et al. New England medical center posterior circulation stroke registry: I. Methods, data base, distribution of brain lesions, stroke mechanisms, and outcomes. J Clin Neurol. 2005;1:14–30. doi: 10.3988/jcn.2005.1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacke W, Zeumer H, Ferbert A, et al. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19:1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 4.Brandt T, von Kummer R, Muller-Kuppers M, et al. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 5.de Monye C, Dippel DW, Siepman TA, et al. Is a fetal origin of the posterior cerebral artery a risk factor for TIA or ischemic stroke? A study with 16-multidetector-row CT angiography. J Neurol. 2008;255:239–245. doi: 10.1007/s00415-008-0699-8. [DOI] [PubMed] [Google Scholar]
- 6.Roth W, Morgello S, Goldman J, et al. Histopathological differences between the anterior and posterior brain arteries as a function of aging. Stroke. 2017;48:638–644. doi: 10.1161/STROKEAHA.116.015630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frid P, Drake M, Giese AK, et al. Detailed phenotyping of posterior vs. anterior circulation ischemic stroke: a multi-center MRI study. J Neurol. 2020;267:649–658. doi: 10.1007/s00415-019-09613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji R, Schwamm LH, Pervez MA, et al. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70:51–57. doi: 10.1001/jamaneurol.2013.575. [DOI] [PubMed] [Google Scholar]
- 9.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8:724–730. doi: 10.1016/S1474-4422(09)70173-5. [DOI] [PubMed] [Google Scholar]
- 10.Kumral E, Bayulkem G, Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. J Neurol. 2002;249:1659–1670. doi: 10.1007/s00415-002-0879-x. [DOI] [PubMed] [Google Scholar]
- 11.Luigetti M, Goldsberry GT, Cianfoni A. Brain MRI in global hypoxia-ischemia: a map of selective vulnerability. Acta Neurol Belg. 2012;112:105–107. doi: 10.1007/s13760-012-0007-3. [DOI] [PubMed] [Google Scholar]
- 12.Wytrzes LM, Chatrian GE, Shaw CM, et al. Acute failure of forebrain with sparing of brain-stem function. Electroencephalographic, multimodality evoked potential, and pathologic findings. Arch Neurol. 1989;46:93–97. doi: 10.1001/archneur.1989.00520370095028. [DOI] [PubMed] [Google Scholar]
- 13.Brisson CD, Hsieh YT, Kim D, et al. Brainstem neurons survive the identical ischemic stress that kills higher neurons: insight to the persistent vegetative state. PLoS ONE. 2014;9:e96585. doi: 10.1371/journal.pone.0096585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JD, Rindler RS, Carr C, et al. Delay in diagnosis of basilar artery stroke. Neurocrit Care. 2016;24:172–179. doi: 10.1007/s12028-015-0211-0. [DOI] [PubMed] [Google Scholar]
- 15.Ferbert A, Bruckmann H, Drummen R. Clinical features of proven basilar artery occlusion. Stroke. 1990;21:1135–1142. doi: 10.1161/01.str.21.8.1135. [DOI] [PubMed] [Google Scholar]
- 16.Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulli G, Markus HS. The use of FAST and ABCD2 scores in posterior circulation, compared with anterior circulation, stroke and transient ischemic attack. J Neurol Neurosurg Psychiatry. 2012;83:228–229. doi: 10.1136/jnnp.2010.222091. [DOI] [PubMed] [Google Scholar]
- 18.Kim JT, Park MS, Choi KH, et al. clinical outcomes of posterior versus anterior circulation infarction with low National Institutes of Health Stroke Scale scores. Stroke. 2017;48:55–62. doi: 10.1161/STROKEAHA.116.013432. [DOI] [PubMed] [Google Scholar]
- 19.Gur AY, Lampl Y, Gross B, et al. A new scale for assessing patients with vertebrobasilar stroke-the Israeli Vertebrobasilar Stroke Scale (IVBSS): inter-rater reliability and concurrent validity. Clin Neurol Neurosurg. 2007;109:317–322. doi: 10.1016/j.clineuro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Hwang DY, Silva GS, Furie KL, et al. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. 2012;42:559–565. doi: 10.1016/j.jemermed.2011.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldmakher GV, Camargo EC, Furie KL, et al. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke. 2009;40:134–139. doi: 10.1161/STROKEAHA.108.516690. [DOI] [PubMed] [Google Scholar]
- 22.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 2001;25:520–528. doi: 10.1097/00004728-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Alemseged F, Shah DG, Diomedi M, et al. The Basilar Artery on Computed Tomography Angiography Prognostic Score for basilar artery occlusion. Stroke. 2017;48:631–637. doi: 10.1161/STROKEAHA.116.015492. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer AM, Simpson E, Bradley MD, et al. Computed tomography angiography in hyperacute ischemic stroke: prognostic implications and role in decision-making. Stroke. 2013;44:1480–1488. doi: 10.1161/STROKEAHA.111.679522. [DOI] [PubMed] [Google Scholar]
- 25.van der Hoeven EJ, Dankbaar JW, Algra A, et al. Additional diagnostic value of computed tomography perfusion for detection of acute ischemic stroke in the posterior circulation. Stroke. 2015;46:1113–1115. doi: 10.1161/STROKEAHA.115.008718. [DOI] [PubMed] [Google Scholar]
- 26.Sporns P, Schmidt R, Minnerup J, et al. Computed tomography perfusion improves diagnostic accuracy in acute posterior circulation stroke. Cerebrovasc Dis. 2016;41:242–247. doi: 10.1159/000443618. [DOI] [PubMed] [Google Scholar]
- 27.Pallesen LP, Gerber J, Dzialowski I, et al. Diagnostic and prognostic impact of pc-ASPECTS applied to perfusion CT in the Basilar Artery International Cooperation Study. J Neuroimaging. 2015;25:384–389. doi: 10.1111/jon.12130. [DOI] [PubMed] [Google Scholar]
- 28.Nagel S, Herweh C, Kohrmann M, et al. MRI in patients with acute basilar artery occlusion - DWI lesion scoring is an independent predictor of outcome. Int J Stroke. 2012;7:282–288. doi: 10.1111/j.1747-4949.2011.00705.x. [DOI] [PubMed] [Google Scholar]
- 29.Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI-negative acute ischemic stroke: a meta-analysis. Neurology. 2017;89:256–262. doi: 10.1212/WNL.0000000000004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonsen CZ, Madsen MH, Schmitz ML, et al. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5% Stroke. 2015;46:98–101. doi: 10.1161/STROKEAHA.114.007107. [DOI] [PubMed] [Google Scholar]
- 31.Ganesh A, Goyal M. Thrombectomy for acute ischemic stroke: recent insights and future directions. Curr Neurol Neurosci Rep. 2018;18:59. doi: 10.1007/s11910-018-0869-8. [DOI] [PubMed] [Google Scholar]
- 32.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 33.Kumar G, Shahripour RB, Alexandrov AV. Recanalization of acute basilar artery occlusion improves outcomes: a meta-analysis. J Neurointerv Surg. 2015;7:868–874. doi: 10.1136/neurintsurg-2014-011418. [DOI] [PubMed] [Google Scholar]
- 34.Alemseged F, Campbell BCV. Tenecteplase thrombolysis in posterior circulation stroke. Front Neurol. 2021;12:678887. doi: 10.3389/fneur.2021.678887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 36.Wyszomirski A, Szczyrba S, Tomaka D, et al. Treatment of acute basilar artery occlusion: systematic review and meta-analysis. Neurol Neurochir Pol. 2017;51:486–496. doi: 10.1016/j.pjnns.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 38.Langezaal LCM, van der Hoeven E, Mont'Alverne FJA, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297. [DOI] [PubMed] [Google Scholar]
- 39.Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387:1373–1384. doi: 10.1056/NEJMoa2207576. [DOI] [PubMed] [Google Scholar]
- 40.Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387:1361–1372. doi: 10.1056/NEJMoa2206317. [DOI] [PubMed] [Google Scholar]
- 41.Adusumilli G, Kobeissi H, Ghozy S, et al. Endovascular thrombectomy after acute ischemic stroke of the basilar artery: a meta-analysis of four randomized controlled trials. J Neurointerv Surg. 2022 doi: 10.1136/jnis-2022-019776. Published online 8 December 2022. doi:10.1136/jnis-2022-019776. [DOI] [PubMed] [Google Scholar]
- 42.Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke. 2018;13:348–361. doi: 10.1177/1747493017743051. [DOI] [PubMed] [Google Scholar]
- 43.Writing Group for the BG, Zi W, Qiu Z, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. 2020;77:561–573. doi: 10.1001/jamaneurol.2020.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F, Sang H, Song J, et al. One-year outcome after endovascular treatment for acute basilar artery occlusion. Stroke. 2021;53:e9–13. doi: 10.1161/STROKEAHA.120.033658. [DOI] [PubMed] [Google Scholar]
- 45.Tao C, Qureshi AI, Yin Y, et al. Endovascular treatment versus best medical management in acute basilar artery occlusion strokes: results from the ATTENTION multicenter registry. Circulation. 2022;146:6–17. doi: 10.1161/CIRCULATIONAHA.121.058544. [DOI] [PubMed] [Google Scholar]
- 46.Gory B, Mazighi M, Blanc R, et al. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever) J Neurosurg. 2018;129:1482–1491. doi: 10.3171/2017.7.JNS171043. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko J, Ota T, Unemoto K, et al. Endovascular treatment of acute basilar artery occlusion: Outcomes, influencing factors and imaging characteristics from the Tama-REgistry of acute thrombectomy (TREAT) study. J Clin Neurosci. 2021;86:184–189. doi: 10.1016/j.jocn.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 48.Pirson FAV, Boodt N, Brouwer J, et al. Endovascular treatment for posterior circulation stroke in routine clinical practice: results of the multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands Registry. Stroke. 2022;53:758–768. doi: 10.1161/STROKEAHA.121.034786. [DOI] [PubMed] [Google Scholar]
- 49.Sheng K, Tong M. Therapy for acute basilar artery occlusion: a systematic review and meta-analysis. F1000Res. 2019;8:165. doi: 10.12688/f1000research.18042.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu L, Salehi Ravesh M, Jansen O, et al. Stent retriever thrombectomy potentially increases the recanalization rate, improves clinical outcome, and decreases mortality in acute basilar occlusion: a systematic review and meta-analysis. Cerebrovasc Dis Extra. 2019;9:46–56. doi: 10.1159/000499665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turc G, Tsivgoulis G, Audebert HJ, et al. European Stroke Organisation - European Society for Minimally Invasive Neurological Therapy expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischaemic stroke and anterior circulation large vessel occlusion. Eur Stroke J. 2022;7:1–26. doi: 10.1177/23969873221076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell PJ, Yan B, Churilov L, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. 2022;400:116–125. doi: 10.1016/S0140-6736(22)00564-5. [DOI] [PubMed] [Google Scholar]
- 53.Nie X, Wang D, Pu Y, et al. Endovascular treatment with or without intravenous alteplase for acute ischaemic stroke due to basilar artery occlusion. Stroke Vasc Neurol. 2022;7:190–199. doi: 10.1136/svn-2021-001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nappini S, Arba F, Pracucci G, et al. Bridging versus direct endovascular therapy in basilar artery occlusion. J Neurol Neurosurg Psychiatry. 2021;92:956–962. doi: 10.1136/jnnp-2020-325328. [DOI] [PubMed] [Google Scholar]
- 55.Sandercock PA, Counsell C, Tseng MC, et al. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;2014:CD000029. doi: 10.1002/14651858.CD000029.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong J, Wang F, Sundararajan S. Use of dual antiplatelet therapy following ischemic stroke. Stroke. 2020;51:e78–e80. doi: 10.1161/STROKEAHA.119.028400. [DOI] [PubMed] [Google Scholar]
- 58.Guenego A, Bourcier R, Guillen M, et al. Neurological improvement predicts clinical outcome after acute basilar artery stroke thrombectomy. Eur J Neurol. 2021;28:117–123. doi: 10.1111/ene.14487. [DOI] [PubMed] [Google Scholar]
- 59.Saxena M, Young P, Pilcher D, et al. Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med. 2015;41:823–832. doi: 10.1007/s00134-015-3676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews PJD, Verma V, Healy M, et al. Targeted temperature management in patients with intracerebral haemorrhage, subarachnoid haemorrhage, or acute ischaemic stroke: consensus recommendations. Br J Anaesth. 2018;121:768–775. doi: 10.1016/j.bja.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Jeon SB, Koh Y, Choi HA, et al. Critical care for patients with massive ischemic stroke. J Stroke. 2014;16:146–160. doi: 10.5853/jos.2014.16.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217–e223. doi: 10.1161/STROKEAHA.120.033033. [DOI] [PubMed] [Google Scholar]
- 63.Markus HS, Larsson SC, Dennis J, et al. Vertebral artery stenting to prevent recurrent stroke in symptomatic vertebral artery stenosis: the VIST RCT. Health Technol Assess. 2019;23(41):1–30. doi: 10.3310/hta23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trialLancet Neurol. 2015;14:361–367. doi: 10.1016/S1474-4422(15)70018-9. [DOI] [PubMed] [Google Scholar]
- 65.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Stroke. 2011;42:e420–e463. doi: 10.1161/STR.0b013e3182112d08. [DOI] [PubMed] [Google Scholar]
- 66.Searls DE, Pazdera L, Korbel E, et al. Symptoms and signs of posterior circulation ischemia in the new England medical center posterior circulation registry. Arch Neurol. 2012;69:346–351. doi: 10.1001/archneurol.2011.2083. [DOI] [PubMed] [Google Scholar]