Abstract
Background
Fat embolism syndrome (FES) is a rare life-threatening complication, which commonly affects the lung. Currently, the most widely accepted criteria for the diagnosis of FES are the Gurd and Wilson Criteria established nearly 40 years ago, but without pulmonary images involved. Our study aims to analyse the pulmonary computed tomography (CT) findings seen in FES.
Case presentation
This report enrolled four cases of FES with lung involvement. The mainly symptoms and signs included dyspnea, disturbance of consciousness, anemia, thrombocytopenia and, most notably, ground-glass opacities, septal thickening, ill-defined centrilobular nodules, and patchy consolidation were demonstrated on bilateral lungs. Combining the clinical manifestations and laboratory tests, the diagnosis of FES was confirmed. With the treatment of steroids, anti-coagulation and supportive treatment, the four patients' symptoms were relieved, abnormalities in chest CT were absorbed significantly and the patients were finally discharged.
Conclusions
There are several common manifestations of FES in pulmonary CT images, and the lung parenchymal features give more information for the diagnosis of FES than the pulmonary vessel findings. Given the absence of a gold standard diagnostic test for FES, further investigation to explore new diagnostic criteria of FES involving pulmonary radiological features is needed in the future.
KEYWORDS: Fat embolism syndrome, pulmonary imaging, chest computed tomography, case report
Background
Fat embolism syndrome (FES) refers to the clinical syndrome where certain triggers stimulate the release of fat into the systemic circulation, causing mechanical obstruction and biochemical injury to the organs and resulting in pulmonary and systemic symptoms.1 Pulmonary fat embolism (PFE) is the presence of fat globules in the pulmonary circulation. Although the fat emboli can reach the vessels of virtually any organs, the organs mainly affected are lungs, brain and skin, which corresponds to the classic triad of FES: dyspnea, neurological abnormalities and petechial rash.2 Common causes of FES include bone fractures,3, 4 orthopedic procedures,5, 6, 7 blunt force trauma,3, 8, 9 liposuction and fat grafting,10, 11 certain diseases (such as sickle cell disease12 and Duchenne muscular dystrophy13, 14), parvovirus infection and others.
FES was a moderately rare life-threatening complication with an incidence ranging from 0.004% in a national hospital charge survey of the United States3 to 8.75% in fracture patients.15 However, in some reported case series, the mortality reached nearly 20%.16 Timely diagnosis and treatment of suspected patients would change their clinical outcomes significantly. Clinically, owning to the unspecific symptoms, relatively low prevalence,3 lack of benchmark tests and standardised diagnostic criteria, and the overlapping symptoms between FES and traumatic injuries, the diagnosis of FES is challenging.2
FES was first described by Zenker in 1862, who observed fat droplets in the lungs of a railway worker who had suffered severe crush injury, and subsequently fat embolism was recognised in trauma patients.17 Gurd first described the diagnostic criteria of FES in 197018 and this was refined by Gurd and Wilson in 1974.19 At least one major criterion and four minor criteria were needed for the diagnosis of FES. The major criteria included respiratory insufficiency, cerebral involvement and petechial rash, and the minor criteria were defined as pyrexia, tachycardia, retinal changes, jaundice and renal changes, anemia, thrombocytopenia, elevated erythrocyte sedimentation rate and fat macroglobulinemia. However, the Gurd and Wilson criteria were based on small series and not validated prospectively, and they did not include pulmonary radiological features. Some recent studies have demonstrated that characteristic imaging findings on chest computed tomography (CT) could assist with the differential diagnosis of FES.20, 21, 22, 23, 24 This study aims to analyse the pulmonary imaging findings and clinical features of four patients with FES in our hospital and other cases diagnosed as FES from the literature with the aim of improving clinicians' understanding of FES.
Methods
Four patients who were diagnosed as FES in our hospital between 2014 and 2020 were enrolled and their clinical information, including clinical manifestations, laboratory findings and pulmonary CT imaging, were collected (Table 1, Table 2). In addition, we performed an extensive literature review of FES cases by searching the MEDLINE database (through PubMed) (Search strategy: Mesh Term' fat embolism syndrome', English language, human species) and EMBASE database (Search strategy: Emtree terms ‘fat embolism syndrome‘/exp, English language, human species) from 1 January 2000 to 31 July 2022. An analysis of the data contained in the case reports was conducted carefully. Patients who met one of the following criteria were included:
-
•
case and case series met the Gurd and Wilson Criteria of FES with pulmonary involvement19
-
•
computed tomographic pulmonary angiography (CTPA) or tissue pathology imply PFE and patients had clinical symptoms.
Table 1.
The characteristic features and laboratory findings
| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age (years) | 39 | 34 | 17 | 41 |
| Gender | Female | Male | Female | Female |
| Type of injuries/surgeries | Liposuction | Liposuction | Right tibia and fibula fractures | Augmentation mammaplasty |
| Time∗ | Peri-operation | Peri-operation | 4 days | 2 hours |
| Dyspnea | P | P | P | P |
| Petechiae rash | P | A | A | A |
| Neurological disorder | P | P | P | P |
| Pyrexia | A | A | A | A |
| Tachycardia | P | A | A | P |
| Retinal changes | A | A | A | A |
| Jaundice | A | A | A | A |
| Renal changes | A | A | A | A |
| Anemia | P | A | P | P |
| Thrombocytopenia | A | A | P | P |
| Elevated ESR | N/A | N/A | P | A |
| Fat macroglobulinemia | N/A | N/A | N/A | N/A |
ESR = erythrocyte sedimentation rate; P = present; A = Absent; N/A = not available.
Indicated the time from injuries/surgeries to symptoms onset.
Table 2.
The clinical parameters and pulmonary CT findings
| Case | ARDS | Assisted ventilation | PO2 (mmHg) | PFE | GGO | Septal thickening | Nodular opacities | Consolidation | Pleural effusion | Resolution time (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A | A | 120 | A | P | P | P | A | A | 6 |
| 2 | P | P | 115.6 (FiO2 0.9) | A | P | P | P | P | A | 5 |
| 3 | A | A | 91 | A | P | P | P | A | A | 26 |
| 4 | A | A | 56.1 | A | P | P | A | P | P | 7 |
ARDS = acute respiratory distress syndrome; PO2 = partial pressure of oxygen; FiO2 = fraction of inspired oxygen; PFE = pulmonary fat embolism; GGO = ground glass opacities; P = present; A = absent.
The exclusion criteria were:
-
•
cases and case series did not include patients' basic information (age, gender etc)
-
•
case and case series did not include CT descriptions of pulmonary parenchyma or pulmonary images after the occurrence of FES.
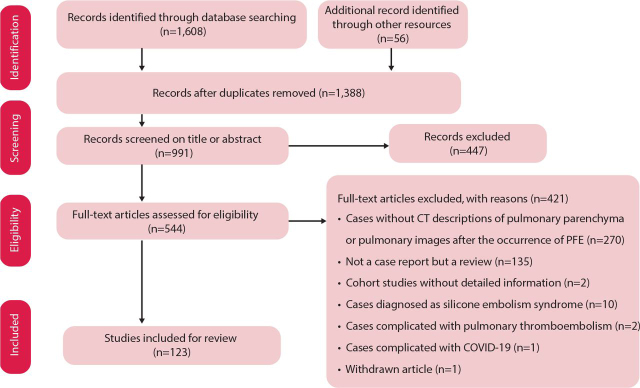
The article screening process is shown in Fig 1.
Fig 1.
Flowchart showing how articles were enrolled.
For all patients, clinical characteristics, including age, gender, type of injuries and surgeries or presence of susceptible diseases conditions, time from injuries or surgeries to symptom onset, clinical symptoms and signs, laboratory findings and pulmonary CT images of lesions at initial presentation were reviewed. Of those, clinical symptoms, signs and laboratory findings mainly involved the parameters mentioned in the Gurd and Wilson criteria.19 Pulmonary CT images showed lesions including ground-glass opacities (GGOs), septal thickening, nodular opacities, consolidation and pleural effusion on CT or high resolution CT,22 and pulmonary fat embolism seen on CTPA.25
For all the 181 patients, the presence of lesions in the bilateral lungs were analysed on thoracic CT images. GGOs were defined as hazy increased lung opacity with preservation of bronchial and vascular margins. Nodular opacities were defined as rounded or irregular opacities, well or poorly defined, measuring within 1 cm in diameter. Septal thickening referred to the thickening of any septa and so render septa visible. Consolidations were defined as homogeneous increases of attenuation in pulmonary parenchyma that obscured the margins of vessels and airway walls.26 Other CT manifestations, like pleural effusion and filling defects of fat attenuation within the pulmonary arteries, were also recorded.
Results
From January 2000 to July 2022, 1,388 related articles were searched for screening. Among them, 123 articles involving 177 FES patients with pulmonary involvement were identified (see supplementary material S1). With the other four FES patients admitted to our hospital, there were 181 cases in total for analysis.
Case presentation
Case 1
A 39-year-old woman presented with dyspnea after liposuction from abdomen and lumbar region and was admitted to our emergency department. She underwent bilateral breast prosthesis implantation 8 years ago. On arrival, she was tachycardic and drowsy, and multiple petechial rashes were seen on the anterior surface of the chest wall. CTPA demonstrated diffuse GGOs. There were no filling defects within pulmonary arteries (Fig 2a and Fig 2b). She was diagnosed with FES and supportive treatments were given. Finally, her symptoms relived and she was discharged.
Fig 2.
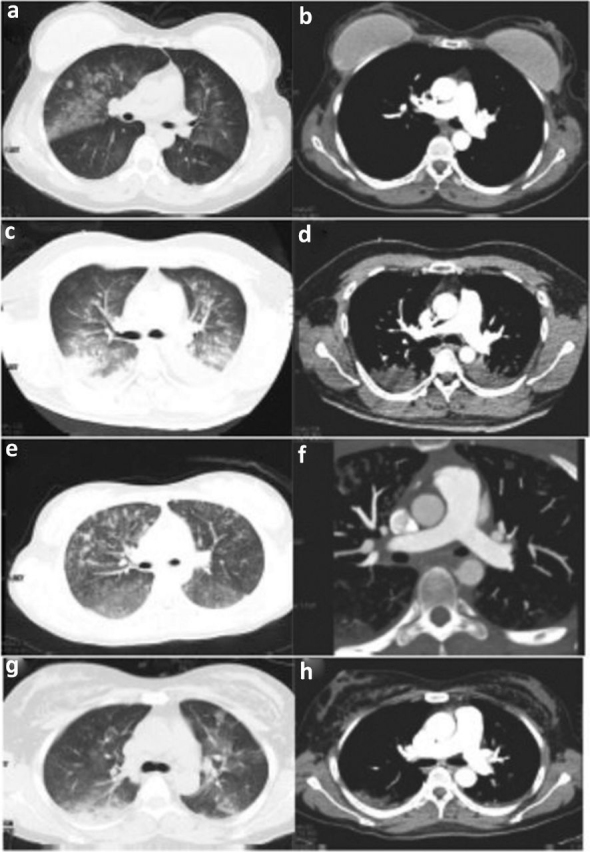
The radiological manifestations of four patients. (a) Pulmonary CT showed diffuse ground-glass opacities (GGOs). (b) Computed tomographic pulmonary angiography (CTPA) revealed no filling defects within pulmonary arteries. (c) Chest CT showed diffuse nodular opacities, bilateral GGOs and consolidation. (d) No filling defects within pulmonary arteries detected by CTPA. (e) Chest CT showed nodular opacities, GGOs and septal thickening. (f) CTPA showed no perfusion defects within pulmonary arteries. (g) Chest CT showed bilateral pulmonary consolidation, GGOs and septal thickening. (h) No filling defects was found by CTPA.
Case 2
A 35-year-old man had undergone liposuction under general anesthesia, with a total aspirate volume of 1.8 liters obtained from the abdomen and lumbar regions. The patient suddenly became unconscious, with dyspnea and cyanosis, and his heart rate and oxygen saturations dropped after he was extubated and transferred to ward for monitoring. Cardiopulmonary resuscitation and vasopressors were administered, and the patient was intubated with invasive ventilation. After the spontaneous rhythm of the heart restored, he was transferred to the intensive care unit of our hospital. The obtained CTPA showed diffuse nodular opacities, bilateral GGOs and consolidation, without filling defects within pulmonary arteries (Fig 2c and Fig 2d). These findings confirmed the diagnosis of FES. After supportive treatment, the patient's pulmonary imaging improved and he was discharged.
Case 3
A young woman aged 17, with her right tibia and fibula fractured in a car accident, received bone casting and traction in a local hospital. 4 days later, she presented with dyspnea and was admitted to our hospital. On examination, her vital signs were stable. The CTPA showed nodular opacities, GGOs and septal thickening, no perfusion defects within pulmonary arteries (Fig 2e and Fig 2f). Blood investigations showed moderate anemia and thrombocytopenia. Due to the combined history and clinical manifestation, the patient was diagnosed with FES. The patient's symptoms relived after 1 week's treatment with steroids and anticoagulation.
Case 4
A 41-year-old woman had an augmentation mammaplasty and liposuction from the abdomen and lumbar region under general anesthesia; the surgery was uneventful. After 2 hours she became drowsy and then lost consciousness, with dyspnea and cyanosis, and oxygen saturation dropped sharply to 16%. Oxygen therapy, epinephrine and dexamethasone were given, and the patient recovered consciousness, but she still felt dyspnea and had cough with blood-tinged sputum and was transferred to our hospital. On arrival, the patient was conscious with vital signs stable. The arterial blood gas (ABG) showed respiratory failure with PO2 56.1 mmHg. Laboratory findings demonstrated anemia and thrombocytopenia. The chest CT showed bilateral pulmonary consolidation, GGOs, and septal thickening, without filling defects within pulmonary arteries in CTPA (Fig Fig 2, Fig 2). The diagnosis of FES was made and steroids and anti-coagulation drugs were given. Finally, the patient fully recovered and was discharged.
Demographic characteristic and causes of fat embolism (Table 3)
Table 3.
Demographic characteristic and causes of FES
| Characteristics | |
| Sex | |
| Male (117/181) | |
| Female (64/181) | |
| Age/yrs (M, IQR) | 29 (22–46) |
| Time from injuries/surgeries to symptom onset in days (median, interquartile range) | 1(0–2) |
| Type of injuries/surgeries/diseases | |
| Femoral fracture | (60/181) |
| Tibial and fibula fractures | (26/181) |
| Multiple bone fractures | (42/181) |
| Vertebral fracture | (3/181) |
| Humerus fracture | (2/181) |
| Total hip arthroplasty | (1/181) |
| Total knee arthroplasty | (3/181) |
| Bilateral sinus floor augmentation | (1/181) |
| Lung transplantation from donor-acquired FES | (4/181) |
| Liposuction and fat grafting | (22/181) |
| Renal angiomyolipoma/malignant tumor | (7/181) |
| Bone marrow necrosis | (6/181) |
| Others (malignant with chemotherapy, hyperinflammation state) | (2/181) |
| Unknown | (2/181) |
Among the enrolled patients, there were 117 males and 64 females, ranging from 11 years to 91 years old, with a median age of 29.0 years. The average time from traumatic injury or surgery procedure to the onset of symptoms was 1 (0–2) days. The most common cause of FES was traumatic bone fracture (133/181), especially the femoral fracture (60/181), followed by multiple bone fractures (42/181), tibial and fibula fractures (26/181), liposuction and fat grafting (22/181), vertebral fractures (3/181), humerus fracture (2/181), hip and knee arthroplasty surgeries (4/181), angiomyolipoma, lung transplantation from donor-acquired FES and bone marrow necrosis caused by hematological system diseases were also reported.
Physical signs and pulmonary imaging
In the 181 patients with corresponding symptoms described, almost all of the included patients presented with dyspnea (161/170), while tachycardia (99/126), neurological disorder (101/137) and acute respiratory failure syndrome (ARDS) (97/149) presented in more than half of the patients. Petechiae was present in 51 out of 105 patients. While in 50 patients retinal signs were mentioned, among them, 20 patients presented with retinal signs and 30 cases without retinal signs. 14 patients presented with renal signs. As with pulmonary imaging, 31 patients had their diagnosis confirmed by CTPA with filling defects in pulmonary arteries. The measurements of the filling defects showed negative mean attenuation values. GGOs were demonstrated in 143 patients, nodular opacities in 125 patients, septal thickening in 122 patients, and consolidation and pleural effusion in 81 and 40 patients, respectively. Among those with follow-up pulmonary CT, the average resolution time was 12.0 (7.0–16.4) days. For those with outcomes recorded, 133 patients survived (133/147).
Discussions and conclusions
Currently, the widely accepted diagnostic criteria for FES are the Gurd and Wilson Criteria established nearly 40 years ago,19 and there is no gold standard test for FES. In 1987, Lindeque et al27 introduced arterial blood analysis into the diagnosis of FES in tibia or femur fracture patients, corresponding to the respiratory insufficiency in Gurd's criteria to some extent. In a prospective randomised study, Schonfeld's criteria were proposed,28 which gave symptoms and fracture sites corresponding weighted scores according to their relative specificity for FES and included chest X-ray; a total score of 5 or more was considered as positive for FES. However, chest X ray with diffuse alveolar infiltrates was hard to differentiate from pulmonary edema, pneumonia and pulmonary hemorrhage. Clinically, chest CT is the first imaging acquired in patients with respiratory distress and can give more information about the lesions of lung parenchyma, airways and vessels than chest X-ray. Therefore, manifestations of pulmonary CT are expected to be potential weighted parameters in the diagnosis of FES.
The common causes of FES in this study were traumatic bone fractures, liposuction and arthroplasty, which is consistent with former research.4, 29 Respiratory symptoms, from hypoxemia to ARDS needing invasive ventilation, dominated the clinical picture in about 90% of the patients23 and were the earliest symptoms in FES patients, which is similar in our analysis.
In our study, chest CT was obtained in all the patients enrolled. GGOs, nodular opacities and septal thickening were the most frequent symptoms. Malagari et al analysed nine patients with mild fat embolism and found that bilateral GGOs (7/9), thickening of the interlobular septa (5/9) were the common pulmonary findings in high-resolution CT, while centrilobular nodular opacities were present in some patients (2/9).4 In some severely ill patients, other manifestations, like lobular consolidation, crazy paving patterns, bronchial wall thickening and pleural effusion also appeared.30 Katrina et al discovered that the presence of pulmonary consolidation and the extent of GGOs correlated with disease severity.22
The exact mechanism that leads to FES is not clear, while mechanical and biochemical theories for what causes the clinical symptoms and pulmonary imaging findings have been widely accepted.31 The mechanical theory suggests that outside mechanical forces, such as traumatic injury, invasive surgery and liposuction, could disrupt fat globules in the bone marrow and fat tissue into ruptured venules which thenenter into pulmonary capillaries or systemic circuit, causing pulmonary and other organs' dysfunction.32 Mechanical obstruction of pulmonary microvasculature by fat emboli would also cause ventilation perfusion mismatch with severity proportional to the burden of fat in the circulation.22 According to the biochemical theory, fat emboli trapped in the pulmonary capillaries release free fatty acids and glycerol, which are toxic to the lung and trigger a cascade of inflammation, resulting in localised endothelial injury, permeability edema and hemorrhage. Both the mechanical and biochemical injuries lead to the clinical manifestations of FES, ranging from hypoxemia, tachypnea and dyspnea to ARDS,33 consistent with the pulmonary imaging findings. As ground-glass opacities presumably mirror the alveolar edema and hemorrhage,33 septal thickening may reflect congestion and edema in the interstitium, and centrilobular nodules are believed to reflect the initial vasculogenic insult of fat emboli. However, the mechanical obstruction seems to play a less important role in the pulmonary injury than the inflammatory cascade trigger.22 This, to some extent, could explain the rarity of visualisation of macroscopic fat emboli within pulmonary vessels in the CTPA.24 As in our study, there were only 31 patients out of 122 patients with computed tomographic angiograms available that presented with fat emboli in the pulmonary vessels.
In practice, FES needs to be differentially diagnosed with pulmonary thromboembolism (PTE) in patients presenting with dyspnea and traumatic history.23 Compared with PTE, FES has no predictable risk factors like deep venous thrombosis, and usually occurs in settings related to disruption of the fatty tissue. There may be an interval of 12–48 hours between the initiation of trauma or surgeries and the onset of clinical and radiologic findings. The relatively specific symptom of petechiae, which presents within 24–36 h of injury or insult, only occurred in 20–50% of patients and resolved quickly.34 Furthermore, the differential diagnosis mainly relies on the attenuation of intraluminal filling defects within pulmonary arteries in CTPA: PTE presents with soft issue attenuation while FES presents with fat attenuation; the emboli thrombus is extremely uncommon.35
During the global pandemic of COVID-19, it is also important and necessary to consider the differential diagnosis of FES and COVID-19, as they have similar symptoms and radiological findings; however, the SARS-CoV-2 RNA test, recent long bone fractures and traumatic history may provide vital clues.36, 37
Our study has several limitations. First, the major limitation is the small sample size, with only four patients included, which limits the further exploring of the new diagnostic criteria that including image findings and the comparison of radiological findings of different etiologies. Second, the pulmonary lobes and lung fields involved were not recorded, which may affect the entire disease evaluation and limit the accurate description of pulmonary images. This study was a retrospective case series and literature review, and full detailed descriptions or CT images could not be acquired in all cases. Last but not least, patients in our study did not receive the same treatment, though there was no standard therapy that would have influenced the resolution time of the radiological lesions.
In conclusion, the occurrence of FES is mostly related to traumatic fracture, arthroplasty, fracture fixation, liposuction and fat transplantation. The common manifestations of FES in pulmonary CT images were GGOs, nodular opacities and septal thickening, and the lung parenchymal features would give more information for the diagnosis of FES than the pulmonary vessel findings. Given the absence of a gold standard diagnostic test for FES, investigation to explore new diagnostic criteria for FES involving pulmonary radiological features is needed in the future.
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee at our institution (Ethical review approval number: 2020-713).
Funding
This study was supported by Sichuan Provincial Health Committee(18PJ398) in data collection and manuscript writing.
Supplementary material
Additional supplementary material may be found in the online version of this article at www.rcpjournals.org:
S1 – Articles included in analysis.
References
- 1.Kosova E, Bergmark B, Piazza G. Fat embolism syndrome. Circulation. 2015;131:317–320. doi: 10.1161/CIRCULATIONAHA.114.010835. [DOI] [PubMed] [Google Scholar]
- 2.Rothberg DL, Makarewich CA. Fat Embolism and Fat Embolism Syndrome. J Am Acad Orthop Surg. 2019;27:e346–e355. doi: 10.5435/JAAOS-D-17-00571. [DOI] [PubMed] [Google Scholar]
- 3.Stein PD, Yaekoub AY, Matta F, et al. Fat embolism syndrome. Am J Med Sci. 2008;336:472–477. doi: 10.1097/MAJ.0b013e318172f5d2. [DOI] [PubMed] [Google Scholar]
- 4.Malagari K, Economopoulos N, Stoupis C, et al. High–resolution CT findings in mild pulmonary fat embolism. Chest. 2003;123:1196–1201. doi: 10.1378/chest.123.4.1196. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio G, Soler I, López-Durán L. Fat embolism syndrome after nailing an isolated open tibial fracture in a stable patient: a case report. BMC Res Notes. 2014;7:237. doi: 10.1186/1756-0500-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bing R, Yiannikas J. Exertional fat embolism after hip joint replacement: a case report. J Med Case Rep. 2014;8:426. doi: 10.1186/1752-1947-8-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustanoja S, Sundararajan S, Strbian D. Unconscious patient after elective bilateral total knee arthroplasty. Stroke. 2014;45:e38–e39. doi: 10.1161/STROKEAHA.113.004011. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Zou D, Qin Z, et al. Nonfracture-associated pulmonary fat embolism after blunt force fatality: case report and review of the literature. Am J Forensic Med Pathol. 2015;36:61–65. doi: 10.1097/PAF.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 9.Cvetković D, Živković V, Nikolić S. An unusual case of pulmonary fat embolism following blunt trauma. Forensic Sci Med Pathol. 2019;15:292–295. doi: 10.1007/s12024-018-0053-0. [DOI] [PubMed] [Google Scholar]
- 10.Byeon SW, Ban TH, Rhee CK. A case of acute fulminant fat embolism syndrome after liposuction surgery. Tuberc Respir Dis (Seoul) 2015;78:423–427. doi: 10.4046/trd.2015.78.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali A, Theobald G, Arshad MA. Fat attacks!: a case of fat embolisation syndrome postliposuction. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220789. bcr2017220789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff DM, Owen E, Bendon R, et al. Distinctive acellular lipid emboli in hemoglobin SC disease following bone marrow infarction with parvovirus infection. Case Rep Hematol. 2015;2015:328065. doi: 10.1155/2015/328065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy LD, Yabrodi M, Lutfi R. Fat embolism syndrome in duchenne muscular dystrophy patients: early recognition and aggressive therapy. Case Rep Crit Care. 2018;2018:3686470. doi: 10.1155/2018/3686470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundeen KM, Bhoopal JR, Simegn MA, et al. Acute hypoxemia and coma in a patient with hemoglobin SC disease. Chest. 2019;155:e21–e23. doi: 10.1016/j.chest.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Chan K, Tham K, Chiu H, et al. Post-traumatic fat embolism–its clinical and subclinical presentations. J Trauma. 1984;24:45–49. [PubMed] [Google Scholar]
- 16.Mudd K, Hunt A, Matherly R, et al. Analysis of pulmonary fat embolism in blunt force fatalities. J Trauma. 2000;48:711–715. doi: 10.1097/00005373-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37:5–8. doi: 10.1007/s00595-006-3307-5. [DOI] [PubMed] [Google Scholar]
- 18.Gurd A. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970;52:732–737. [PubMed] [Google Scholar]
- 19.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Series B. 1974;56:408–416. [PubMed] [Google Scholar]
- 20.Alfudhili K. Pearls in pulmonary computed tomography findings in patients with fat embolism syndrome. Can Assoc Radiol J. 2018;69:479–488. doi: 10.1016/j.carj.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 21.McCabe BE, Veselis CA, Goykhman I, et al. Beyond pulmonary embolism; nonthrombotic pulmonary embolism as diagnostic challenges. Curr Probl Diagn Radiol. 2019;48:387–392. doi: 10.1067/j.cpradiol.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Newbigin K, Souza CA, Armstrong M, et al. Fat embolism syndrome: Do the CT findings correlate with clinical course and severity of symptoms? A clinical-radiological study. Eur J Radiol. 2016;85:422–427. doi: 10.1016/j.ejrad.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Newbigin K, Souza CA, Torres C, et al. Fat embolism syndrome: State-of-the-art review focused on pulmonary imaging findings. Respir Med. 2016;113:93–100. doi: 10.1016/j.rmed.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi S, Kimmel LA, Kirk A, et al. Radiological features of pulmonary fat embolism in trauma patients: a case series. Emerg Radiol. 2022;29:41–47. doi: 10.1007/s10140-021-01969-4. [DOI] [PubMed] [Google Scholar]
- 25.Peña W, Cárdenas-Camarena L, Bayter-Marin JE, et al. Macro fat embolism after gluteal augmentation with fat: first survival case report. Aesthet Surg J. 2019;39:NP380–NP383. doi: 10.1093/asj/sjz151. [DOI] [PubMed] [Google Scholar]
- 26.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 27.Lindeque B, Schoeman H, Dommisse G, et al. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br. 1987;69:128–131. doi: 10.1302/0301-620X.69B1.3818718. [DOI] [PubMed] [Google Scholar]
- 28.Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. a prospective study in high-risk patients. Ann Intern Med. 1983;99:438–443. doi: 10.7326/0003-4819-99-4-438. [DOI] [PubMed] [Google Scholar]
- 29.Costa A, Mendes D, Toufen C, et al. Adult respiratory distress syndrome due to fat embolism in the postoperative period following liposuction and fat grafting. J Bras Pneumol. 2008;34:622–625. doi: 10.1590/s1806-37132008000800013. [DOI] [PubMed] [Google Scholar]
- 30.Piolanti M, Dalpiaz G, Scaglione M, et al. Fat embolism syndrome: lung computed tomography findings in 18 patients. J Comput Assist Tomogr. 2016;40:335–342. doi: 10.1097/RCT.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 31.Rahman SA, Villiani A, Chanda A. In: Intensive Care. Shaikh N, editor. 2017. Fat embolism syndrome.www.intechopen.com/chapters/56283 [Google Scholar]
- 32.Fukumoto LE, Fukumoto KD. Fat Embolism Syndrome. Nurs Clin North Am. 2018;53:335–347. doi: 10.1016/j.cnur.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hulman G. The pathogenesis of fat embolism. J Pathol. 1995;176:3–9. doi: 10.1002/path.1711760103. [DOI] [PubMed] [Google Scholar]
- 34.Montagnana M, Cervellin G, Franchini M, et al. Pathophysiology, clinics and diagnostics of non-thrombotic pulmonary embolism. J Thromb Thrombolysis. 2011;31:436–444. doi: 10.1007/s11239-010-0519-8. [DOI] [PubMed] [Google Scholar]
- 35.Nucifora G, Hysko F, Vit A, et al. Pulmonary fat embolism: common and unusual computed tomography findings. J Comput Assist Tomogr. 2007;31:806–807. doi: 10.1097/rct.0b013e318032566e. [DOI] [PubMed] [Google Scholar]
- 36.Seneviratna A. Fat embolism syndrome or covid-19 pneumonia: A diagnostic dilemma. Sri Lankan J Anaesthesiol. 2021;29:109–113. [Google Scholar]
- 37.Hochhegger B, Zanon M, Altmayer S, et al. COVID-19 mimics on chest CT: a pictorial review and radiologic guide. Br J Radiol. 2021;94:20200703. doi: 10.1259/bjr.20200703. [DOI] [PMC free article] [PubMed] [Google Scholar]