Abstract
Admission care bundles have been demonstrated to improve clinical outcomes for patients in several settings. Decompensated cirrhosis care bundles have been developed following previous reports demonstrating poor care for inpatients with alcohol-related liver disease (ARLD). We performed a UK multi-centred retrospective observational study to understand how frequently decompensated cirrhosis admission care bundles were utilised, who they were used for and their impact on outcomes. In this study (1,224 admissions, 104 hospitals), we demonstrated that admission care bundle usage was low across the UK (11.44%). They were more likely to be utilised in patients with ARLD or who were jaundiced, and less likely to be used in patients admitted for gastrointestinal bleeding. The admission care bundle improved the standard of alcohol care and requesting initial investigations. However, there were areas where more than 80% compliance was achieved without the use of a care bundle and areas where less than 50% compliance was achieved with the use of a care bundle. Given the low utilisation of care bundles, we were unable to demonstrate an effect on risk-adjusted mortality. Thus, interdisciplinary work is required to develop tools which are widely used and improve care and outcomes for patients with decompensated cirrhosis.
KEYWORDS: cirrhosis, care bundle, equity, variation
Introduction
Morbidity and mortality from chronic liver disease in the UK has significantly increased over the past 50 years.1 Compounding this have been concerns regarding the standard of inpatient care received by patients with decompensated cirrhosis. The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report into patients admitted to hospital in the UK with alcohol-related liver disease (ARLD) demonstrated that less than 50% of patients received good care.2 As a response, standards of care for the first 24 h of admission of patients with decompensated cirrhosis were established on behalf of the British Society of Gastroenterology (BSG) and the British Association for the Study of the Liver (BASL).3 These standards have subsequently been used to develop care bundles that are integrated within patient notes to provide a step-wise framework to managing patients admitted with decompensated cirrhosis. These admission care bundles have been demonstrated to improve inpatient care but have not been shown to impact mortality to this point.4 This is unlike other care bundles, such as the 'Sepsis Six’ care bundle.5 Thus, there is a need to ensure that admission care bundles for decompensated cirrhosis achieve their intended goal of improved outcomes for this cohort of patients.
In this study, the primary aim was to ascertain how frequently admission care bundles were utilised for patients with decompensated cirrhosis across the UK. Secondary aims included to determine patient cohorts for whom the admission bundle is more likely to be utilised and to understand whether admission care bundle use impacts outcomes for patients with decompensated cirrhosis.
Method
Study design
This was a UK multicentre, retrospective, observational cohort study including patients acutely admitted to UK hospitals with decompensated cirrhosis in November 2019. November 2019 was selected as a month for data collection because of the lack of UK public holidays and junior doctor changeovers and because it pre-dated the Coronavirus 2019 (COVID-19) pandemic. Trainees were invited to participate in this study via the Trainee Collaborative for Research and Audit in Hepatology UK (ToRcH-UK) network.6 Sites were invited by email through society mailing lists (BASL, BSG, Scottish Society of Gastroenterology and Welsh Association for Gastroenterology and Endoscopy) and through Twitter via the @uk_torch account. The study was registered at all participating sites as an audit through local audit departments (host site: King's College Hospital; audit reference number LIV16062021). Patient hospital admissions were identified via coding utilised by the NHS England Cirrhosis Quality Dashboard before exclusion criteria were then applied.7 Admissions were excluded if they were for patients who were: less than 18 years old; not admitted as an emergency; not felt to have chronic liver disease (histologically, radiologically or clinically) or compensated chronic liver disease; admitted for alternate non-liver pathology; included in clinical trials during that admission; transferred from an alternate hospital; previous liver transplant; known to have active non-liver cancer or metastatic liver cancer; or pregnant (supplementary material S2). Reporting of the analysis of this study complies with STROBE guidelines for the reporting of cohort studies.8 Pseudonymised data were transferred in a standardised password-protected Microsoft® Excel® data collection spread sheets via encrypted NHS email addresses.
Data collection
Each registered site was issued with guidance to assist with data collection (supplementary material S3). Data were acquired for each hospital, including regarding whether it was designated as a specialist hepatology training centre (as determined by the BSG9). Hospitals were grouped by NHS region in England,10 with hospitals in Northern Ireland, Scotland and Wales grouped into Health and Social Care Northern Ireland (HSCNI), NHS Scotland and NHS Wales, respectively.
Patient notes were interrogated for: demographic, clinical and laboratory data at admission; documented use of an admission decompensated cirrhosis care bundle; whether component standards set out within the BSG/BASL admission care bundle were met; and clinical outcome data following the first 24 h post-admission. Aetiology of liver disease was recorded as per documentation within the clinical notes. Dual aetiology of liver disease was permitted and patients were categorised within each cohort (eg non-alcoholic fatty liver disease (NAFLD) and concomitant ARLD). The primary presenting complaint leading to hospital presentation was recorded (eg if a patient presented with a large volume variceal bleed, was found to be jaundiced, had low-grade hepatic encephalopathy (HE), and an acute kidney injury (AKI), the presenting complaint was recorded as variceal bleed). Laboratory and clinical data were recorded for calculation of liver-related prognostic scores including: Child-Pugh score,11, 12 UK End-stage Liver Disease score13 and Model for End-stage Liver Disease (MELD) score.14 Time and date of patient admission were recorded, which allowed admissions to be classified as in hours (Monday–Friday between 09.00 h and 17.00 h) or out of hours (OOH). Individual standards set out within the BSG/BASL admission care bundle for patients with decompensated cirrhosis were evaluated as met/not met across all seven domains (investigations, alcohol, infections, AKI and/or hyponatraemia, gastrointestinal bleeding, encephalopathy, or other). If a particular domain was not relevant to a patient admission, it was not completed. Clinical outcome data included patient care location (including critical care admission), and whether they survived until hospital discharge.
Data analysis
Comparisons were made between patient admissions for which an admission care bundle was used and those where they were not. If a patient admission resulted in a transfer to another centre, it was excluded from the in-hospital mortality analysis because of a lack of information regarding survival/mortality from that encounter. Continuous demographic, clinical and laboratory variables were analysed for normality using the D'Agostino and Pearson tests. All data were non-normally distributed and analysed using Mann-Whitney U tests (two groups) or Kruskal–Wallis test (three or more groups) with results reported as median (interquartile range (IQR)). Categorical data were analysed by Fisher's exact tests (two groups) or Chi-square test (three or more groups) and results reported as number (%).
We adjusted the admission care bundle mortality for patient-specific variables associated with poor prognosis in decompensated cirrhosis. Multiple logistic regression was used to adjust for patient age, MELD score and critical care admission. Variables within each model were recorded as an odds ratio (OR) and p-value. Model performance was recorded as area under the curve (AUC) (95% confidence interval (CI)). Goodness-of-fit was recorded by pseudo-r2, Hosmer-Lemeshow (HL) statistic and log likelihood ratio statistic. Complete case analysis was used excluding individuals with missing data. Correction for multiple comparisons was performed using the Benjamini–Hochberg procedure with a false discovery rate (FDR) set at 0.05.15 All univariable and multivariable analyses were performed using Prism V9.2.0 (GraphPad, San Diego, CA, USA).
Results
Completed datasets were received from 104 hospitals across the UK. A total of 11,045 patient admissions were identified from coding. After application of the exclusion criteria (supplementary material S2), 1,224 patient admissions from 1,168 patients were included in the final analysis. The number of sites participating varied regionally, but there were no significant differences in median regional admission number (supplementary material S4).
An admission cirrhosis care bundle was used in 11.44% (140/1,244) of admissions. There was significant regional variation in bundle utilisation, with the highest utilisation in NHS North East and Yorkshire (26.88% of admissions) followed by NHS South West (24.47% of admissions), and lowest in NHS Wales (0% of admissions) followed by NHS Midlands (2.65% of admissions) (Fig 1). Baseline characteristics of admissions where an admission care bundle was used compared with those where one was not are shown in Table 1. Care bundles were significantly more likely to be used in admissions for patients with ARLD, presenting primarily with jaundice, and with worse prognostic scores (MELD, UKELD and Child-Pugh scores). Admissions for patients presenting primarily with gastrointestinal bleeding were significantly less likely to have an admission care bundle used. However, whether the patient was admitted to a specialist hepatology centre, was admitted out of hours, had a known diagnosis of liver disease or previous decompensation episode or continued to regularly use alcohol did not impact whether an admission care bundle was utilised (Table 1).
Fig 1.
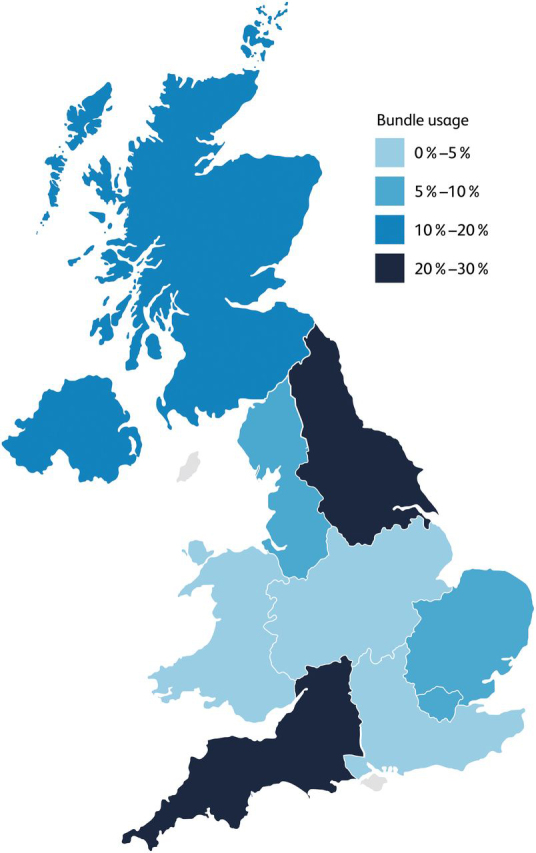
Admission care bundle utilisation across the UK. Percentage of regional admissions for whom an admission care bundle was utilized, compared with Chi-square test (p<0.0001). See supplementary material S5 for more information.
Table 1.
Characteristics of patient admissions for whom an admission care bundle was or was not utiliseda
| Characteristic | N | Admission care bundle utilised | N | Admission care bundle not utilised | p value |
|---|---|---|---|---|---|
| Age | 140 | 56.00 (46.25–64.75) | 1,084 | 58.00 (48.00–68.00) | 0.04 |
| Male sex | 140 | 96 (68.57%) | 1,084 | 658 (60.70%) | 0.08 |
| Admitted out of hours | 138 | 92 (66.67%) | 1,070 | 671 (62.71%) | 0.40 |
| Specialist hepatology centre admission | 140 | 68 (48.57%) | 1,084 | 537 (49.54%) | 0.86 |
| Alcohol included in aetiology (can be a co-factor) | 140 | 124 (88.57%) | 1,084 | 791 (72.97%) | <0.0001∗ |
| NAFLD included in aetiology (can be a co-factor) | 140 | 14 (10.00%) | 1,084 | 162 (14.94%) | 0.13 |
| Current alcohol use | 121 | 78 (64.46%) | 935 | 446 (54.79%) | 0.05 |
| Previously known liver disease | 140 | 118 (84.29%) | 1,084 | 923 (85.15%) | 0.80 |
| Previous known decompensation | 140 | 92 (65.71%) | 1,084 | 730 (67.34%) | 0.70 |
| Known hepatocellular carcinoma | 140 | 9 (6.43%) | 1,084 | 55 (5.07%) | 0.54 |
| Primary reason for admission | |||||
| Acute kidney injury | 140 | 5 (3.57%) | 1,084 | 39 (3.60%) | >0.9999 |
| Ascites | 140 | 50 (35.71%) | 1,084 | 358 (33.03%) | 0.57 |
| Encephalopathy | 140 | 26 (18.57%) | 1,084 | 185 (17.07%) | 0.64 |
| Gastrointestinal bleeding | 140 | 10 (7.14%) | 1,084 | 170 (15.68%) | 0.005∗ |
| Jaundice | 140 | 33 (23.57%) | 1,084 | 152 (14.02%) | 0.005∗ |
| Sepsis | 140 | 8 (5.71%) | 1,084 | 71 (6.55%) | 0.86 |
| Other | 140 | 8 (5.71%) | 1,084 | 109 (10.06%) | 0.13 |
| Prognostic scores | |||||
| MELD score | 135 | 19.00 (14.00–23.00) | 967 | 16.00 (12.00–21.00) | 0.001∗ |
| UKELD score | 135 | 59.00 (55.00–64.00) | 967 | 56.00 (52.00–61.00) | 0.0003∗ |
| Child-Pugh Score | 128 | 9.00 (8.00–11.00) | 943 | 9.00 (8.00–10.00) | 0.001∗ |
Results from Mann–Whitney U tests presented as median (IQR). Results of Fisher's Exact tests presented as number (%). Asterisks denote significance.
NAFLD = non-alcoholic fatty liver disease; MELD = Model for End-stage Liver Disease; UKELD = UK End-stage Liver Disease score.
Overall compliance with standards of care varied across the different domains in this cohort. The standards of care with the highest overall compliance were documentation of NEWS score (92.89%) and antibiotics prescription as per Trust guidelines (91.30%). However, all domains had standards of care where overall performance could be improved (Table 2). Admission care bundle use led to several significant improvements in standard of care, including around documentation of National Early Warning Scores (NEWS), completion of initial blood tests, septic screens and ascitic taps, ultrasounds being requested, alcohol use being recorded, appropriate Pabrinex® prescription, Clinical Institute Withdrawal assessment use and a mean arterial pressure (MAP) target being set in patients with AKI (Table 2). However, although there were significant differences in these domains between admissions managed with a care bundle and those for whom one was not utilised, only NEWS documentation (97.86%) and appropriate Pabrinex prescription (94.85%) achieved compliance of >90% with septic screens (25.00%) and MAP targets in AKI (28.57%) having a compliance of <50% in admissions for whom a care bundle was utilised. Given the low number of admissions for whom a care bundle was utilised, some comparisons were underpowered to appropriately assess the impact of completion of a care bundle on specific domains (Table 2).
Table 2.
Admission care bundle use impact on standards of care within the first 24 h after presentationa
| Variable | n | Overall | n | Admission care bundle utilised | n | Admission care bundle not utilised | p value |
|---|---|---|---|---|---|---|---|
| Investigations | |||||||
| National Early Warning Score documentation | 1,224 | 1,137 (92.89%) | 140 | 137 (97.86%) | 1,084 | 1,000 (92.25%) | 0.01∗ |
| Complete blood tests | 1,214 | 506 (41.68%) | 139 | 94 (67.63%) | 1,075 | 412 (38.33%) | <0.0001∗ |
| Septic screen (excluding ascitic tap) | 1,216 | 168 (13.82%) | 140 | 35 (25.00%) | 1,076 | 133 (12.36%) | 0.0001∗ |
| Ultrasound scan of abdomen request | 1,218 | 609 (50.00%) | 140 | 90 (64.29%) | 1,078 | 519 (38.14%) | 0.0004∗ |
| Ascitic tap | 738 | 397 (53.79%) | 100 | 67 (67.00%) | 638 | 330 (51.72%) | 0.005∗ |
| Alcohol | |||||||
| Daily alcohol intake recorded | 1,224 | 932 (76.14%) | 140 | 121 (86.43%) | 1,084 | 811 (74.82%) | 0.002∗ |
| Pabrinex prescribed (if >8 units for men, >6 units for women) | 691 | 577 (83.50%) | 97 | 92 (94.85%) | 594 | 485 (81.65%) | 0.0006∗ |
| Clinical Institute Withdrawal Assessment score if withdrawing | 585 | 387 (66.15%) | 85 | 72 (84.71%) | 500 | 315 (63.00%) | <0.0001∗ |
| Infections | |||||||
| Spontaneous bacterial peritonitis suspected | 414 | 100 (24.15%) | 55 | 18 (32.73%) | 359 | 82 (22.84%) | 0.13 |
| Antibiotics as per Trust protocol | 322 | 294 (91.30%) | 52 | 45 (86.54%) | 270 | 249 (92.22%) | 0.18 |
| If spontaneous bacterial peritonitis: human albumin solution 1.5 g/kg | 77 | 38 (49.35%) | 17 | 19 (58.82%) | 60 | 19 (31.67%) | 0.05 |
| Acute kidney injury/hyponatraemia | |||||||
| Diuretics and nephrotoxics stopped | 286 | 233 (81.47%) | 49 | 44 (89.80%) | 237 | 189 (79.75%) | 0.11 |
| Fluid resuscitation | 323 | 250 (77.40%) | 55 | 40 (72.73%) | 268 | 210 (78.36%) | 0.38 |
| Fluid balance chart and daily weights | 353 | 211 (59.77%) | 59 | 39 (66.10%) | 294 | 172 (58.50%) | 0.31 |
| MAP aim >80 mmHg | 328 | 43 (13.11%) | 56 | 16 (28.57%) | 272 | 27 (9.93%) | 0.0007∗ |
| Gastrointestinal bleeding | |||||||
| Fluid resuscitated (MAP >65 mmHg) | 230 | 175 (76.09%) | 21 | 16 (76.19%) | 209 | 159 (76.08%) | >0.9999 |
| Terlipressin prescribed | 237 | 171 (72.15%) | 23 | 17 (73.91%) | 214 | 154 (71.96%) | >0.9999 |
| Antibiotics prescribed | 242 | 177 (73.14%) | 24 | 8 (75.00%) | 218 | 169 (77.52%) | 0.80 |
| Red blood cells given if haemoglobin <70 g/L | 144 | 80 (55.56%) | 13 | 7 (53.85%) | 131 | 73 (55.73%) | >0.9999 |
| Fresh frozen plasma given if INR >2.0 | 182 | 38 (20.88%) | 17 | 3 (17.65%) | 165 | 35 (21.21%) | >0.9999 |
| Platelets if <50×109/L | 59 | 22 (37.29%) | 6 | 2 (33.33%) | 53 | 20 (37.74%) | >0.9999 |
| Endoscopy in <12 h | 264 | 88 (33.33%) | 28 | 4 (14.29%) | 236 | 84 (35.59%) | 0.03 |
| Encephalopathy | |||||||
| Precipitant identified | 360 | 237 (65.83%) | 61 | 41 (67.21%) | 299 | 196 (65.55%) | 0.88 |
| Lactulose/enema prescribed | 378 | 331 (87.57%) | 66 | 61 (92.42%) | 312 | 270 (86.54%) | 0.23 |
| CT scan of head requested | 356 | 163 (45.79%) | 64 | 28 (43.75%) | 292 | 135 (46.23%) | 0.78 |
| Other | |||||||
| VTE | 1,209 | 817 (67.58%) | 139 | 104 (74.82%) | 1,070 | 713 (66.64%) | 0.05 |
| GI/Hep review <24 h | 1,111 | 748 (67.33%) | 127 | 94 (74.02%) | 984 | 654 (66.46%) | 0.11 |
Results of Fisher's exact tests comparing care within the first 24 h following presenting to hospital in admissions where a care bundle was utilised and those where it was not. All results described as n (%). Asterisks denote significance.
CT = computed tomography; GI/Hep = gastrointestinal/hepatology; INR = international normalised ratio; MAP = mean arterial pressure; VTE = venous thromboembolism.
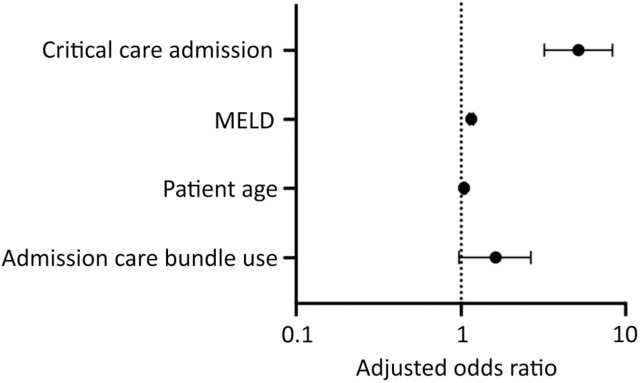
Admission care bundle use was not associated with a significantly increased likelihood of critical care admissions on univariable analysis (13.67% vs 10.18%, p=0.24) (supplementary material S6). However, admission care bundle usage was found to be associated with admission mortality (23.02% vs 14.61%, p=0.01) (supplementary material S6). Following adjustment for variables associated with poor prognosis (patient age, MELD score and critical care admissions), this association was not significant (adjusted OR 1.62 (95% CI 0.97–2.65)) (Fig 2).
Fig 2.
Impact of admission care bundles on admission mortality after adjustment for patient age, MELD score and critical care admission. Odds ratio plot demonstrating admission care bundle use association with admission mortality when adjusted for model variables (n=1,080, area under the curve (AUC) 0.78 (0.74–0.82), pseudo r2=0.22, HL Statistics 9.58 (p=0.30), log-likelihood ratio statistic 195.70 (p<0.0001∗)) (see supplementary material S7). MELD = Model for End-stage Liver Disease.
Discussion
To our knowledge, we have performed the largest, regionally representative UK study of admission care bundle use for patients with decompensated cirrhosis. We found low utilisation across the UK with significant regional variation in admission care bundle use. The low utilisation of the admission care bundle makes meaningful assessment of its impact on decompensated cirrhosis patient clinical outcomes not currently possible. However, we highlight areas where gold standard care is met irrespective of bundle use and demonstrate areas where the admission care bundle improves care. These data will help guide future tools focused on improving outcomes for this cohort of patients.
The care bundle was developed to address concerning deficits in care received by patients with decompensated cirrhosis within the first 24 h of admission.3 We confirm previous findings that admission care bundles do significantly improve standards of care, particularly in regards to alcohol care and initial investigations.4 However, documentation of NEWS score was 92.25% in admissions for whom a care bundle was not used and, despite being a significant improvement, septic screens were performed in only 25.00% of patients for whom a care bundle was used. This would suggest that some standards are generally met irrespective of admission care bundle usage and that there are areas where healthcare professionals (HCPs) require further support and training to achieve gold standard care in combination with care bundle utilisation. Compounding this is the evolution of the evidence base over time. Since the development of the first admission care bundle, evidence has evolved regarding blood product use in cirrhosis, with recent American and European guidance advising against routine use of fresh frozen plasma in patients with prolonged international normalised ratio (INR) results.16, 17 Our findings demonstrate a need to develop evidence-based tools to support HCPs managing patients admitted with decompensated cirrhosis, with a focus on areas of care where performance is currently globally poor. This will need to be accompanied by an upskilling of HCPs who are involved in the management of these patients.
Utilisation of admission care bundles is poor across the UK, with the two regions with the highest utilisation being the ones where admission care bundles were first developed.4 This is despite 69% of Trusts stating in a recent NCEPOD survey that they utilised the BSG/BASL admission care bundle.18 It is unclear why there is such a disparity between Trust-reported performance and 'real-world’ performance in the usage of admission care bundles for patients with decompensated cirrhosis. It is vital to understand the barriers to the uptake in the bundle. From our data, we hypothesise that this may relate to: challenges among HCPs in the recognition of liver disease, a focus on the management of gastrointestinal bleeding and a lack of engagement of key stakeholders providing acute care.
We demonstrate that admissions for patients with ARLD or who were jaundiced were more likely to result in admission care bundle use. This suggests that there are challenges in identifying patients with decompensated cirrhosis who do not present in this manner. The global prevalence of NAFLD has continued to rise in recent years and now stands at >30%.19 However, despite this increasing prevalence, a previous global survey demonstrated significant knowledge gaps in physicians who typically engage with these patients.20 Similarly, it is acknowledged that presentations of decompensation, such as HE, can be challenging to identify within the acute setting.21 The lack of utilisation across the UK may, in part, relate to a lack of recognition of certain patients with decompensated cirrhosis. Serial targeted educational sessions have been previously shown to increase adherence to the decompensated admission care bundle to 90%.4 This likely represents the value in educating HCPs managing patients at the time of their admission to not only utilise care bundles, but also recognise and identify patients who may be presenting with decompensated cirrhosis.
We also demonstrate the patients who presented primarily with gastrointestinal bleeding were significantly less likely to have an admission care bundle utilised. There has been significant focus on improving outcomes for patients with gastrointestinal bleeds in the UK because previous studies demonstrated suboptimal care and outcomes.22, 23 Although time to endoscopy has not been demonstrated to impact outcomes for patients with upper gastrointestinal bleeds,24 there remains significant focus on determining the need for endoscopy early in this cohort, which features within recent multi-societal guidance.25 This guidance has led to the development of an upper gastrointestinal bleed care bundle, which does not include guidance regarding the management of other decompensated cirrhosis complications (eg HE).25 This may unintentionally detract from the management of decompensated cirrhosis. It is important that both gastrointestinal bleeding and decompensated cirrhosis are managed optimally and that tools developed to improve patient care in these domains should reference each other.
It is important to recognise that, although the admission care bundle for decompensated cirrhosis was created to improve care at the time of admission, the standards were set by gastroenterologists and hepatologists of the two major specialty societies (BSG and BASL) without the inclusion of other care providers of this cohort on admission, including emergency medicine and acute medicine HCPs.3 There is the potential that this leads to certain standards included within the care bundle not being feasible within the first 24 h of care because of stresses currently faced in providing acute care in the NHS. This may be reflected in poor compliance with certain standards, even where an admission care bundle was used. Given the evolution of the evidence-base combined with the changes in pressures of acute care provision since the initial standards were set, this may be the opportunity for interdisciplinary consensus on care provision, which could lead to the development of a new tool to improve patient care and clinical outcomes.
There are several limitations of this study, including the retrospective design. Given the 'real-world’ nature of this study, clinical diagnoses were taken from the records because validated diagnostic criteria could not be applied. The use of a single month may highlight anomalies in care provision, which are not consistent over a longer period as well as seasonal variations of certain illnesses. Although data were submitted from all NHS regions, some regions had a lower proportion of hospitals completing data collection, which may impact regional performance within the study. Given that trainees were invited to participate in this study, this may have led to selection bias and may explain why specialist hepatology centres were over-represented within the final data set. Future studies should be prospectively designed utilising proformas with validated diagnostic criteria and should include representative samples from all NHS regions across a broad time-frame. Admissions were selected by coding; therefore, incorrectly coded admissions would not have been detected. However, we utilised established coding from NHS England7 and applied strict exclusion criteria to ensure the quality of admissions included. We demonstrated that the codes utilised within the NHS England Cirrhosis Dashboard lack specificity for admissions with decompensated cirrhosis, but we do not have a breakdown of why admissions were excluded. There is significant variation in performance of conventional coding sets to detect cirrhosis.26 The NHS England Cirrhosis Dashboard uses a code set that is broader than that typically utilised in clinical studies, which may increase sensitivity for detecting patients with cirrhosis but impact specificity.7 Further work is required to evaluate and optimise these codes to ensure the accuracy of data recorded and to ensure that they are applicable across the UK. However, the strengths of the study should also be acknowledged. This is a large-scale multicentred study with data submitted from all NHS regions, with multiple data points encompassing patient care across each admission with appropriate comparisons to assess the impact of admission care bundles on care provision.
In conclusion, we demonstrate that there is low utilisation of the admission care bundle across the UK. It is likely that there are multiple barriers to use, including recognition of disease aetiologies and presenting symptoms of patients with decompensated cirrhosis. To improve care for this cohort, we would recommend an interdisciplinary consensus of care standards for the first 24 h, which could be developed into a user-friendly tool that improves care and clinical outcomes for patients with decompensated cirrhosis.
Summary box
What is known?
-
•
There have been previous concerns raised regarding the standard of care for inpatients with decompensated cirrhosis in the UK.
-
•
Standards for the first 24 h of care for patients with decompensated cirrhosis have been established, which have been developed into admission care bundles.
What is the question?
-
•
How frequently are admission care bundles utilised for patients with decompensated cirrhosis in the UK?
-
•
Which patients are more likely to have an admission care bundle utilised for their admission and what is the impact of admission care bundles on the clinical outcomes for patients with decompensated cirrhosis?
What was found?
-
•
Utilisation of the bundle was globally poor across the UK.
-
•
Patients who had alcohol-related liver disease or were jaundiced were more likely to have an admission care bundle utilised.
-
•
Admission care bundles improved alcohol and acute kidney injury care and requests of initial investigations, although there were areas where there was ‘good’ performance in the non-admission care bundle cohort and ‘poor’ performance in the admission care bundle cohort.
-
•
Meaningful assessment of impact on clinical outcomes was not possible because of the low utilisation rate.
What are the implications for practice?
-
•
Education is required for healthcare professionals to help improve identification and management of patients with decompensated cirrhosis.
-
•
Interdisciplinary work is required to update standards and develop a tool that improves care and outcomes for patient with decompensated chronic liver disease and that will be utilised widely across the UK.
Funding
We are grateful to Guts-UK for funding this work. The funder did not have any influence on the study or the manuscript.
Acknowledgements
We are grateful for the support and endorsement from the British Society of Gastroenterology, British Association for the Study of the Liver, Scottish Society of Gastroenterology and the Welsh Association for Gastroenterology and Endoscopy. We are also grateful for the funding support provided by Guts-UK.
Supplemental information
Additional supplementary material may be found in the online version of this article at www.rcpjournals.org/content/clinmedicine
S1 – List of collaborators
S2 – Inclusion and exclusion criteria
S3 – Supplementary methods
S4 – Regional admission numbers
S5 – Comparison of admission care bundle use across regions
S6 – Clinical outcomes in admissions where an admission care bundle was utilised and where one was not
S7 – Admission care bundle use association with admission mortality when adjusted for patient age, MELD score and critical care admissions
Supplementary Material
References
- 1.Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 2.NCEPOD . NCEPOD; London: 2013. “Measuring the Units” - A review of patients who died with alcohol related liver disease. National Confidential Enquiry into Patient Outcome and Death (UK) 2013. [Google Scholar]
- 3.McPherson S, Dyson J, Austin A, et al. Response to the NCEPOD report: development of a care bundle for patients admitted with decompensated cirrhosis—the first 24 h. Frontline Gastroenterol. 2016;7:16–23. doi: 10.1136/flgastro-2014-100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyson JK, Rajasekhar P, Wetten A, et al. Implementation of a ‘care bundle’ improves the management of patients admitted to hospital with decompensated cirrhosis. Aliment Pharmacol Ther. 2016;44:1030–1038. doi: 10.1111/apt.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28:507–512. doi: 10.1136/emj.2010.095067. [DOI] [PubMed] [Google Scholar]
- 6.Trainee Collaborative for Research and Audit in Hepatology UK ToRcH-UK: shining a light on liver disease in the UK. Frontline Gastroenterol. 2022;13:266–268. doi: 10.1136/flgastro-2021-101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.7 NHS England,. Hepatobiliary and pancreas - cirrhosis of the liver (adults) quality dashboard 2021/2022. www.england.nhs.uk/wp-content/uploads/2021/05/HPB-Cirrhosis-Metric-Definitions-202122.pdf [Accessed 5 May 2022].
- 8.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.9 British Society of Gastroenterology,. UK Level 2 (and 3) hepatology training centres, 2021. www.bsg.org.uk/wp-content/uploads/2021/03/Enc.-J-UK-level-2-hepatology-training-centres-2021-v2.pdf [Accessed 5 May 2022].
- 10.10 NHS England,. Regional teams, 2022. www.england.nhs.uk/about/regional-area-teams/ [Accessed 5 May 2022].
- 11.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 13.Barber K, Madden S, Allen J, et al. Elective liver transplant list mortality: development of a United Kingdom end-stage liver disease score. Transplantation. 2011;92:469–476. doi: 10.1097/TP.0b013e318225db4d. [DOI] [PubMed] [Google Scholar]
- 14.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Royal Statistical Soc. 1995;57:289–300. [Google Scholar]
- 16.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. 2022;76:1151–1184. doi: 10.1016/j.jhep.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366–413. doi: 10.1002/hep.31646. [DOI] [PubMed] [Google Scholar]
- 18.18 NCEPOD,. “Remeasuring the units” – an update on the organisation of alcohol-related liver disease services. National Confidential Enquiry into Patient Outcome and Death (UK,) 2022. www.ncepod.org.uk/pdf/current/Remeasuring%20the%20Units_full%20report.pdf [Accessed 2 December 2023].
- 19.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Ong JP, Takahashi H, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022;20:e1456–e1468. doi: 10.1016/j.cgh.2021.06.048. [DOI] [PubMed] [Google Scholar]
- 21.Shawcross DL, Dunk AA, Jalan R, et al. New Insights Steering Committee. How to diagnose and manage hepatic encephalopathy: a consensus statement on roles and responsibilities beyond the liver specialist. Eur J Gastroenterol Hepatol. 2016;28:146–152. doi: 10.1097/MEG.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hearnshaw SA, Logan RF, Lowe D, et al. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59:1022–1029. doi: 10.1136/gut.2008.174599. [DOI] [PubMed] [Google Scholar]
- 23.Rockall TA, Logan RF, Devlin HB, et al. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National audit of acute upper gastrointestinal haemorrhage. BMJ. 1995;311:222–226. doi: 10.1136/bmj.311.6999.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau JYW, Yu Y, Tang RSY, et al. Timing of Endoscopy for Acute Upper Gastrointestinal Bleeding. N Engl J Med. 2020;382:1299–1308. doi: 10.1056/NEJMoa1912484. [DOI] [PubMed] [Google Scholar]
- 25.Siau K, Hearnshaw S, Stanley AJ, et al. British Society of Gastroenterology (BSG)-led multisociety consensus care bundle for the early clinical management of acute upper gastrointestinal bleeding. Frontline Gastroenterol. 2020;11:311–323. doi: 10.1136/flgastro-2019-101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer JE, Gonzalez JJ, Min T, et al. Systematic review: development of a consensus code set to identify cirrhosis in electronic health records. Aliment Pharmacol Ther. 2022;55:645–657. doi: 10.1111/apt.16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.