Abstract
Background
We present the largest study of the frequency and nature of visual complications in a cohort of 350 patients consecutively diagnosed with giant cell arteritis (GCA).
Methods
All individuals were assessed using structured forms and diagnosed using imaging or biopsy. A binary logistic regression model was used to analyse data for predicting visual loss.
Results
Visual symptoms occurred in 101 (28.9%) patients, with visual loss in one or both eyes in 48 (13.7%) patients. Four patients had binocular visual loss. Anterior ischaemic optic neuropathy (N=31), retinal artery obstruction (N=8) and occipital stroke (N=2) were the main causes of visual loss. Of the 47 individuals who had repeat visual acuity testing at 7 days, three individuals had improvement to 6/9 or better. After introducing the fast-track pathway, the frequency of visual loss decreased from 18.7% to 11.5%. Age at diagnosis (odds ratio (OR) 1.12) and headache (OR 0.22) were significant determinants of visual loss in a multivariate model. Jaw claudication trended to significance (OR 1.96, p=0.054).
Conclusions
We recorded a visual loss frequency of 13.7% in the largest cohort of patients with GCA examined from a single centre. Although improvement in vision was rare, a dedicated fast-track pathway reduced visual loss. Headache could result in earlier diagnosis and protect against visual loss.
KEYWORDS: Giant cell arteritis, visual manifestations, arteritic anterior ischaemic optic neuropathy, visual loss, GCA fast track pathway
Introduction
Giant cell arteritis (GCA) is a large-vessel vasculitis that preferentially affects people of Northern European ancestry, peaking in the eighth decade of life.1 It has a predilection for branches of the external carotid and/or subclavian artery.2 However, one of its most feared complications is visual loss, which is presumed to result from direct involvement of the ophthalmic artery, which is usually a branch of the internal carotid artery. The vascular supply to the eye has two main sources: the posterior ciliary arteries and the central retinal artery.3 The posterior ciliary arteries supply the optic nerve head, the choroid up to the equator, the retinal pigmented epithelium and the outer 130 μm of the retina.4 Their involvement can cause anterior ischaemic optic neuropathy (AION). The central retinal artery runs inside the optic nerve and its blockage presents as central retinal artery obstruction (CRAO). In GCA, these two ocular syndromes can cause permanent visual loss. In addition, choroidal infarction and posterior ischaemic optic neuropathy can also cause visual loss. The latter presents with a normal fundus examination and is a diagnosis of exclusion. Patients with GCA and monocular visual loss need prompt treatment to lessen the risk of visual loss in the fellow eye. Posterior circulation strokes because of involvement of the vertebral artery, a branch of the subclavian artery, can result in hemifield loss because of involvement of the occipital cortex.3 Double vision has also been reported commonly in GCA, but it is not clear whether this is because of involvement of the vasculature of the extraocular muscles or the cranial nerves.
Visual loss in GCA has been reported to occur in 12% to 70% of patients.5, 6, 7, 8, 9, 10, 11, 12, 13 This wide variation in data could result, in part, from the specialty reporting those data, being 12–16% in patients from internal medicine,8, 11 14–26% from rheumatology,9, 13 20% from neurology5 and 48–70% from ophthalmology.6, 7, 10, 12
To avert these and further complications in GCA, it is important that GCA as a possible differential diagnosis is considered in the acute setting. Suspected cases should be promptly and appropriately treated and reviewed by a specialist for definitive long-term management.
At our centre, we run an interdisciplinary fast-track service for the diagnosis and management of individuals with suspected GCA. The details of this service are published elsewhere.14 Briefly, referrals are triaged from primary care or internal medicine and managed jointly by ophthalmology and rheumatology. The patient pathway takes every individual with suspected GCA through a validated ultrasonography service.15 We accept that there is no gold standard for the diagnosis of GCA. Our experience and formal validation allow for minimisation of false positive results. A second test is arranged in those individuals in whom the pre-test probability remains high after a negative or equivocal result as per international recommendations to minimise the risk of false negative ultrasound scans.16 The data presented here are likely to be more representative of the entire spectrum of presentations of GCA.
Methods
Setting
A tertiary interdisciplinary vasculitis service in a large predominantly rural county in the UK.
Participants
Data from individuals consecutively diagnosed with GCA diagnosed by ultrasonography, temporal artery biopsy or positron emission tomography (PET) were used in the study. The presence of inflammatory infiltrate in at least one layer of the temporal artery biopsy was accepted as a positive biopsy in the correct clinical context. The presence of a non-compressible halo sign in at least two arteries was accepted as a positive ultrasonography result in the appropriate clinical context. The halo sign was defined as per internationally accepted consensus.17 PET was accepted to be positive when there appeared to be more 18-fluorodeoxyglucose uptake in an artery than in the liver.
Data collection
The NHS in England has commissioned select centres in England for the delivery of specialised care, including that of GCA.18 As part of this programme, our centre is required to maintain a secure database to allow returns to the NHS of nationally benchmarked metrics. Data entry is done prospectively at time of diagnosis and every follow-up. For patients with GCA, the recorded dataset was revised after the publication of European Alliance of Associations for Rheumatology (EULAR) recommendations for a minimum core dataset.19
No ethics approval was required because the data presented here were collected as part of routine care of all the patients directly under our care.
Outcomes of interest
The primary outcome of interest was the frequency of permanent visual loss in GCA. Other outcomes of interest were frequency of visual symptoms, the ocular syndrome leading to permanent visual loss, the visual acuities at baseline and 7 days, the effect of a fast-track pathway on incidence of visual loss, and the value of the recorded symptoms, clinical signs and laboratory markers to predict permanent visual loss.
Statistics
All statistical analyses were performed using SPSS 28 (IBM, Armonk, NY, USA). Continuous variables were tested for normality using the Shapiro–Wilk test. Normally distributed variables were compared across the two groups (with and without visual loss) using the independent samples t-test. Non-parametric variables were compared using the Mann–Whitney U test. The effect of the categorical variables on visual loss was calculated using the Chi-squared test or the Fisher's exact test as appropriate. All variables with p>0.2 were included in a binary logistic regression using the Block Entry method. The categorical variables were denoted using simple contrast, with the absence of the variable being the reference.
Results
At our centre, 350 patients were diagnosed with GCA between 2012 and 2021. The mean (standard deviation) age was 74.6 (7.8) and 236 (67.4%) were female. The diagnosis was established in 243 patients using ultrasonography, in 80 by temporal artery biopsy and in 27 by PET.
The mean haemoglobin was 121.1 (14.4) g/dL. The median (interquartile range (IQR)) erythrocyte sedimentation rate (ESR) was 63 (44) mm/h. The median (IQR) C-reactive protein was 65 (77) mg/dL. In those with visual loss, the mean CRP was 67.5 mg/dL and the mean ESR was 68 mm/h. In those without visual loss, the mean CRP was 62 mg/dL and mean ESR was 62 mm/h. Table 1 details further statistical analyses.
Table 1.
Univariate analysis testing significance of variables to predict visual loss in patients with giant cell arteritisa
| Variable | With visual loss | Without visual loss | Test used | p-value |
|---|---|---|---|---|
| Age (mean ± SD) | 80.1±6.3 | 73.7±7.6 | t-test | <0.001 |
| Gender (females) | 30/48 | 206/302 | Chi-square | 0.43 |
| Haemoglobin in g/L (mean ± SD) | 119.0±15.3 (for n=46) | 121.4±14.2 | t-test | 0.30 |
| C-reactive protein in mg/L (median (IQR)) | 67.5 (75) | 65 (78) (for n=289) | Mann Whitney U | 0.89 |
| Erythrocyte sedimentation rate in mm/h (median (IQR)) | 68 (43) (for n=47) | 62 (42) (for n=281) | Mann Whitney U | 0.60 |
| Smoking status | ||||
| Never smoked | 10/16 | 59/134 | Fisher's exact | 0.408 |
| Ex-smokers | 4/16 | 52/134 | ||
| Current smoker | 2/16 | 23/134 | ||
| Scalp tenderness | 7/48 | 65/302 | Chi-square | 0.27 |
| Headache | 24/48 | 229/302 | Chi-square | <0.001 |
| Jaw claudication | 25/48 | 105/302 | Chi-square | 0.02 |
| Shoulder girdle pain | 5/48 | 63/302 | Chi-square | 0.09 |
| Temporal artery abnormality | 12/48 | 85/302 | Chi-square | 0.65 |
| Fever >38°C | 1/48 | 12/302 | Fisher's exact | 1.00 |
| Weight loss >2 kg | 11/48 | 73/302 | Chi-square | 0.85 |
| Drenching night sweats | 4/48 | 58/302 | Chi-square | 0.07 |
| Loss of appetite | 9/48 | 58/302 | Chi-square | 0.94 |
Unless stated, the numbers are for 48 individuals with visual loss and 302 individuals without visual loss.
Visual symptoms and signs
Of the patients, 101 (28.9%) had visual symptoms: 27 had diplopia and 80 had blurring or loss of vision. Visual acuity had been recorded for 89 of the individuals with visual symptoms. The visual acuities at baseline are shown in Table 2. Of the 80 who complained of blurred vision, 48 (13.7% of cohort) individuals had objective permanent visual loss. The causes of visual loss are detailed in Table 3. Of those with visual loss, 12.5% (6/48) had no other cranial or systemic symptoms associated with GCA.
Table 2.
Visual acuity in 89 of 101 individuals with giant cell arteritis and visual symptoms
| Visual acuity scale | Right eye (n) | Left eye (n) |
|---|---|---|
| Better than 6/6 | 6 | 3 |
| 6/6 to 6/7.5 | 16 | 21 |
| 6/7.5 to 6/9 | 34 | 21 |
| 6/9 to 6/12 | 7 | 14 |
| 6/12 to 6/15 | 0 | 0 |
| 6/15 to 6/18 | 4 | 4 |
| 6/18 to 6/24 | 3 | 2 |
| 6/24 to 6/36 | 2 | 3 |
| 6/36 to 6/48 | 0 | 0 |
| 6/48 to 6/60 | 2 | 2 |
| Worse than 6/60 | 15 | 19 |
Table 3.
Causes of visual loss in 48 individuals with giant cell arteritis
| Ocular presentation | No. of patients (n=48) |
|---|---|
| Anterior ischaemic optic neuropathy | 31 (3 of which were binocular) |
| Retinal artery obstruction | 8 (1 of which was binocular) |
| Posterior circulation stroke | 2 (homonymous field loss) |
| Undetermined cause | 7 (all monocular) |
Comparing visual acuity at Day 0 and Day 7
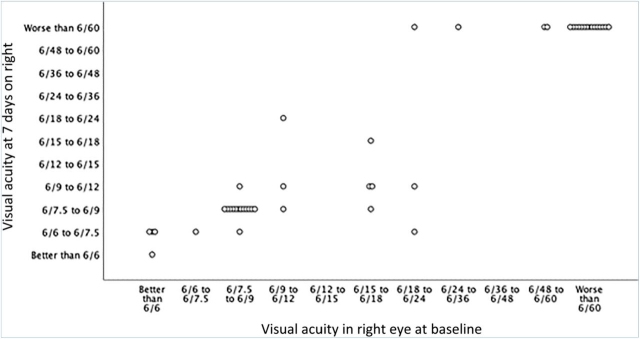
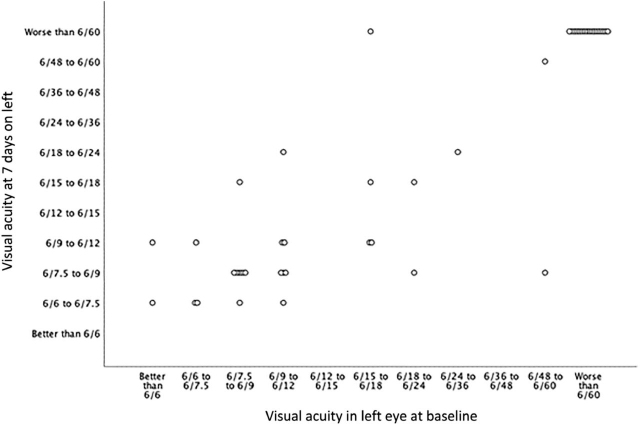
Repeat visual acuities were performed at Day 7 in 47 of the 48 individuals who had visual loss. A comparison of the repeat examination is presented in Figs Fig 1, Fig 2. Of those 47 individuals, 21 had no difference in the visual acuity at Day 0 and 7, whereas 10 had worsening of one eye with no change in the other, two had worsening in both eyes, nine had improvement in one eye without any change in the other, one had improvement in one eye and worsening in the other, and four had improved visual acuity in both eyes.
Fig 1.
Visual acuity at Day 0 and Day 7 in the right eye in 47 individuals with giant cell arteritis and permanent visual loss.
Fig 2.
Visual acuity at Day 0 and Day 7 in the left eye in 47 individuals with giant cell arteritis and permanent visual loss.
Effect of fast-track clinic on incidence of visual loss
Between 2012 and 2016, 107 individuals were diagnosed with GCA in our centre. Of those, 20 (18.7%) had visual loss at diagnosis. From 2017 onward, the fast-track pathway picked up 243 new diagnoses, of which 28 (11.5%) had visual loss at diagnosis.
Identifying predictors of visual loss
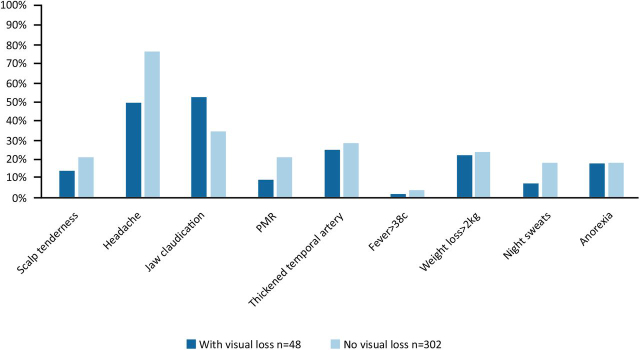
Fig 3 shows the incidence of systemic symptoms in our patients with GCA with and without visual loss. Table 1 shows all the factors that were tested separately for their status in predicting visual loss in our cohort. The 48 individuals with visual loss were statistically significantly older by 6.4 years. Jaw claudication appeared to be positively related to visual loss and headache appeared to be negatively related. Six individuals with visual loss did not have any systemic symptoms.
Fig 3.
Systemic symptoms in patients with giant cell arteritis with and without visual loss. PMR = polymyalgia rheumatica.
Logistic regression
A model was created using age, headache, jaw claudication, shoulder girdle pain and drenching night sweats as inputs. The Nagelkerke R2 was 0.26. Classification improved from 86.3 to 89.4 with the entry of those variables. The odds ratios (ORs) are shown in Table 4.
Table 4.
Odds ratios for visual loss of factors included in multivariate logistic regression
| Variable | Adjusted odds ratio (95% confidence interval) for visual loss | p-value |
|---|---|---|
| Age at diagnosis (for each year increase in age) | 1.12 (1.06–1.17) | <0.001 |
| Headache | 0.22 (0.11–0.46) | <0.001 |
| Jaw claudication | 1.96 (0.99–3.90) | 0.05 |
| Shoulder girdle pain | 0.38 (0.13–1.10) | 0.08 |
| Drenching night sweats | 0.47 (0.15–1.46) | 0.19 |
Discussion
We present the largest study of the incidence and nature of visual complications in a cohort of 350 individuals consecutively objectively diagnosed with GCA either by imaging or biopsy. Yates et al published a larger cohort, but these patients were not consecutively diagnosed and neither were they all diagnosed based on objective tests.20 Our work has several strengths. It represents all individuals diagnosed with GCA within a 10-year period in our catchment area without any case selection bias. Our hospital serves a population of 900,000 using a common pathway agreed between all internal medicine specialities as well as ophthalmology. Thus, we have ensured that the full spectrum of GCA is captured. We have structured assessment clerking sheets that are used for the assessment of individuals with suspected GCA, thus having very few missing data. All the ultrasonography examinations are carried out by one experienced sonographer (CBM), who has participated in local and international validation exercises for this technique.15, 17, 21, 22 In a minority of patients, PET computed tomography (CT) was used to confirm the diagnosis of GCA in those with predominantly extracranial symptoms when a more systemic large vessel vasculitis was suspected.
We also recognise that our work has some limitations. The incidence of visual loss because of GCA is dependent on social factors as well as biological factors. Biological factors, such as signs, symptoms and blood test results, are generalisable, but social factors, such as the distance from a hospital, access to public transport or access to primary care are not. Our data were from a predominantly rural county in the UK without any motorways (controlled-access vehicular highways). This could result in reduced and/or delayed access to healthcare, causing an altered incidence of visual manifestations.
Visual symptoms, including diplopia and blurred or lost vision, occurred in 29% of our GCA cohort. The aetiology of diplopia is unknown. The potential anatomical sites for the lesion could be the vasculature of the midbrain, the vasa nervosa of the cranial nerves or the vasculature of the extraocular muscles. There has been some evidence suggesting that it is the nerve that is affected.23 However, GCA is a disease predominantly affecting large vessels. In the context of the eye, it also affects medium-sized vessels (the central retinal artery and the posterior ciliary arteries are classified as medium-sized vessels as per the Chapel Hill definitions). The recovery of diplopia after the start of glucocorticoid therapy is relatively rapid and unlike the prolonged recovery typical of a nerve injury. This suggests that diplopia is related to muscle ischaemia.
In our cohort, 48 (13.7%) patients were reported to have suffered permanent visual loss. This is similar to the visual involvement reported by other interdisciplinary services. Gonzalez-Gay et al reported the incidence of visual loss in Lugo, Spain to be 12.5%.24 Similarly, when Font et al studied all patients diagnosed with GCA in an internal medicine setting, they reported a incidence of visual loss of 15.8%.25 Rheumatology-only units have reported incidences of visual loss as low as 5%,26 whereas neuro-ophthalmology units have reported visual loss incidence to be 50%.27 Our interdisciplinary work shows that the true incidence of visual loss is more likely to be in the region of 12–15%, by virtue of reporting from a larger cohort. AION and CRAO are the commonest ocular syndromes that cause blindness.27 In our study, we reported that AION was approximately three times as common as CRAO. This is similar to the finding by Baalbaki et al from a cohort of 100 cases from a vasculitis clinic.28 We identified homonymous field loss in two cases, perhaps as a result of posterior circulation compromise because of vertebrobasilar insufficiency (Table 3). Seven individuals with decreased visual acuity had a normal fundoscopic appearance. Putative causes included posterior ischaemic optic neuropathy or choroidal infarction resulting from involvement of the posterior ciliary.29, 30, 31
In our study, there was a 12% rise in the odds of developing visual loss with each advancing year (Table 4). Liozon et al found an increase of a similar magnitude of 6% in the odds for visual loss with every advancing year.32 Other researchers have also found that the age of those with visual loss in their cohorts was older than those who did not have visual loss.28, 33, 34 We suggest that this might not only be because of biologically older arteries, but might also include social factors, including the likelihood of greater dependence on accessing healthcare. Thus, further work is needed to investigate the relationship between socioeconomic conditions and visual loss. We reported that the presence of a headache was associated with a reduction in the odds of developing visual loss. This surprising finding has also been reported by at least four other studies.28, 28, 33, 33 Salvarani et al reported an adjusted OR (95% confidence interval (CI)) of 0.41 (0.07–2.5), which is comparable to our adjusted OR of 0.22 (0.11–0.46).35 This is inexplicable anatomically and we hypothesise that the absence of headache makes individuals less likely to seek appropriate medical attention. Jaw claudication has been traditionally believed to be a risk factor for visual loss in GCA. We reported that there appeared to be a trend for this, but the CI crosses 1 and the p-value was 0.054. To date, this association remains controversial. Two studies from Canada and Italy both found that jaw claudication increased the odds of developing visual loss.32, 35 However, similar to our study, three other studies did not find this to be a statistically significant finding.28, 33, 34 Anatomically, it make sense for jaw claudication to be associated with visual involvement. The maxillary artery is responsible for the vascular nourishment of the muscles of mastication. Its terminal branch is the infraorbital artery, which supplies some of the extraocular muscles. However, in a small number of individuals, the ophthalmic artery has been known to arise from the middle meningeal artery, which is itself a branch of the maxillary artery.36 Thus, this relationship might rely on anatomical variations leading to a trend toward association rather than an unequivocal relationship.
The development of fast-track pathways leading to rapid diagnosis using ultrasonography appears to be cost-effective14, 37 and to lead to a reduction in visual loss.34, 37 Diamantopoulos et al reported a reduced frequency of visual loss from 6/32 in a conventional pathway to 1/43 in a fast-track pathway.37 Patil et al revealed that the frequency of visual loss decreased from 17/46 to 6/67 in a similar comparison.34 In the current study, we found a decrease in the incidence of visual loss from 20/107 to 28/243. Combining the results of these three studies results in a frequency of visual loss of 43/185 (23%) using conventional pathways and of 35/353 (10%) using fast-track pathways. We accept that this is a crude analysis because the three conventional pathways might have been vastly different. However, that would also make this figure more representative of the frequency of visual loss if rapid diagnostics are not available. By the same token, fast-track pathways halve the risk of visual loss, and their development and proliferation should be encouraged. Nevertheless, qualitative research is required to provide further insights into the 10% of patients for whom sight loss remains an issue despite rapid diagnostics, and whose condition is likely to be the result of both social and primary care factors.
Of our cohort, 47/48 individuals who had visual loss had repeat visual acuity testing after 7 days of glucocorticoid treatment. Of those, 13 individuals showed an improvement in visual acuity. In seven, the worse eye had a visual acuity of 6/60 or worse; three had a visual acuity of worse than 6/12 in at least one eye (which is the legal requirement to be able to drive in the UK). Three individuals had improvement of vision to 6/9 or better. None had been treated with intravenous methylprednisolone. The role of intravenous methylprednisolone to improve vision is contentious and international recommendations admit to the quality of evidence being low and only recommend that its use be considered.16 In our centre, we use a lean body mass-based regimen of oral prednisolone from diagnosis.38
In conclusion, we present the frequency of visual manifestations of GCA in the largest cohort of patients objectively diagnosed with GCA. Of our cohort, 28.9% had visual symptoms and 13.7% suffered visual loss. The main predictors of visual loss were increasing age and absence of headache. The predictive value of jaw claudication remains equivocal. The frequency of visual loss was reduced to 11.5% with the introduction of the fast-track pathway, although more work needs to be done to understand the causes of the visual loss, which could include social factors.
Summary box
What is known:
The frequency of visual loss in GCA has been variably reported in the literature to be between 12% and 70%, likely dependent on the speciality reporting the data. Although the range of possible ocular manifestations of GCA is well known, this was the largest single centre study to look at this in depth.
What is the question:
What was the frequency of visual loss in a cohort of 350 patients diagnosed with GCA over a 10-year period? At our centre, patients are managed through an interdisciplinary pathway; therefore, our results reflect the true incidence of visual loss.
What was found:
-
•
Out of 350 patients with GCA, vision loss occurred in 13.7% of 350 patients with GCA and rarely recovered
-
•
Visual loss was statistically more likely with age and less likely with headache
-
•
After introduction of the fast-track pathway, the frequency of visual loss decreased from 18.7% to 11.5%.
Implications:
Creation of fast-track pathways should be encouraged, given that a decrease in visual loss was recorded in patients following the introduction of such a pathway at our centre.
Acknowledgments
Some of the data presented here were presented at the following conferences: 20th International Vasculitis + ANCA workshop (3–6 April 2022); British Society for Rheumatology Annual Conference (April 2022); Royal College of Ophthalmology Annual Conference (May 2022); and EULAR (June 2022). No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
References
- 1.Brekke LK, Diamantopoulos AP, Fevang BT, et al. Incidence of giant cell arteritis in Western Norway 1972-2012: a retrospective cohort study. Arthritis Res Ther. 2017;19:278. doi: 10.1186/s13075-017-1479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 3.De Smit E, O'Sullivan E, Mackey DA, et al. Giant cell arteritis: ophthalmic manifestations of a systemic disease. Graefes Arch Clin Exp Ophthalmol. 2016;254:2291–2306. doi: 10.1007/s00417-016-3434-7. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS. Posterior ciliary arterial occlusive disorders. Trans Ophthalmol Soc UK (1962) 1971;91:291–303. [PubMed] [Google Scholar]
- 5.Sorensen S, Lorenzen I. Giant-cell arteritis, temporal arteritis and polymyalgia rheumatica. A retrospective study of 63 patients. Acta Med Scand. 1977;201:207–213. doi: 10.1111/j.0954-6820.1977.tb15683.x. [DOI] [PubMed] [Google Scholar]
- 6.Jonasson F, Cullen JF, Elton RA. Temporal arteritis. A 14-year epidemiological, clinical and prognostic study. Scott Med J. 1979;24:111–117. doi: 10.1177/003693307902400203. [DOI] [PubMed] [Google Scholar]
- 7.Graham E, Holland A, Avery A, et al. Prognosis in giant-cell arteritis. Br Med J (Clin Res Ed) 1981;282:269–271. doi: 10.1136/bmj.282.6260.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengtsson BA, Malmvall BE. The epidemiology of giant cell arteritis including temporal arteritis and polymyalgia rheumatica. Incidences of different clinical presentations and eye complications. Arthritis Rheum. 1981;24:899–904. doi: 10.1002/art.1780240706. [DOI] [PubMed] [Google Scholar]
- 9.Aiello PD, Trautmann JC, McPhee TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100:550–555. doi: 10.1016/s0161-6420(93)31608-8. [DOI] [PubMed] [Google Scholar]
- 10.Glutz von Blotzheim S, Borruat FX. Neuro-ophthalmic complications of biopsy-proven giant cell arteritis. Eur J Ophthalmol. 1997;7:375–382. doi: 10.1177/112067219700700412. [DOI] [PubMed] [Google Scholar]
- 11.Cid MC, Font C, Oristrell J, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 1998;41:26–32. doi: 10.1002/1529-0131(199801)41:1<26::AID-ART4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore) 2000;79:283–292. doi: 10.1097/00005792-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Mukhtyar C, Ducker G, Fordham S, et al. Improving the quality of care for people with giant cell arteritis. Clin Med (Lond) 2021;21:e371–e374. doi: 10.7861/clinmed.2021-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukhtyar C, Myers H, Scott DGI, et al. Validating a diagnostic GCA ultrasonography service against temporal artery biopsy and long-term clinical outcomes. Clin Rheumatol. 2020;39:1325–1329. doi: 10.1007/s10067-019-04772-2. [DOI] [PubMed] [Google Scholar]
- 16.Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. doi: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 17.Chrysidis S, Duftner C, Dejaco C, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open. 2018;4:e000598. doi: 10.1136/rmdopen-2017-000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhtyar C, Hodgson H. The need to establish standards of care for Giant Cell Arteritis. Rheumatology (Oxford) 2020;59:702–704. doi: 10.1093/rheumatology/kez548. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers L, Askling J, Bijlsma HW, et al. 2018 EULAR recommendations for a core data set to support observational research and clinical care in giant cell arteritis. Ann Rheum Dis. 2019;78:1160–1166. doi: 10.1136/annrheumdis-2018-214755. [DOI] [PubMed] [Google Scholar]
- 20.Yates M, MacGregor AJ, Robson J, et al. The association of vascular risk factors with visual loss in giant cell arteritis. Rheumatology (Oxford) 2017;56:524–528. doi: 10.1093/rheumatology/kew397. [DOI] [PubMed] [Google Scholar]
- 21.Schafer VS, Chrysidis S, Dejaco C, et al. Assessing Vasculitis in Giant Cell Arteritis by Ultrasound: Results of OMERACT Patient-based Reliability Exercises. J Rheumatol. 2018;45:1289–1295. doi: 10.3899/jrheum.171428. [DOI] [PubMed] [Google Scholar]
- 22.Schafer VS, Chrysidis S, Schmidt WA, et al. OMERACT definition and reliability assessment of chronic ultrasound lesions of the axillary artery in giant cell arteritis. Semin Arthritis Rheum. 2021;51:951–956. doi: 10.1016/j.semarthrit.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Mournet S, Sene T, Charbonneau F, et al. High-resolution MRI demonstrates signal abnormalities of the 3rd cranial nerve in giant cell arteritis patients with 3rd cranial nerve impairment. Eur Radiol. 2021;31:4472–4480. doi: 10.1007/s00330-020-07595-x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Gay MA, Miranda-Filloy JA, Lopez-Diaz MJ, et al. Giant cell arteritis in northwestern Spain: a 25-year epidemiologic study. Medicine (Baltimore) 2007;86:61–68. doi: 10.1097/md.0b013e31803d1764. [DOI] [PubMed] [Google Scholar]
- 25.Font C, Cid MC, Coll-Vinent B, et al. Clinical features in patients with permanent visual loss due to biopsy-proven giant cell arteritis. Br J Rheumatol. 1997;36:251–254. doi: 10.1093/rheumatology/36.2.251. [DOI] [PubMed] [Google Scholar]
- 26.Hocevar A, Rotar Z, Jese R, et al. Do early diagnosis and glucocorticoid treatment decrease the risk of permanent visual loss and early relapses in giant cell arteritis: a prospective longitudinal study. Medicine (Baltimore) 2016;95:e3210. doi: 10.1097/MD.0000000000003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998;125:521–526. doi: 10.1016/s0002-9394(99)80193-7. [DOI] [PubMed] [Google Scholar]
- 28.Baalbaki H, Jalaledin D, Lachance C, et al. Characterization of visual manifestations and identification of risk factors for permanent vision loss in patients with giant cell arteritis. Clin Rheumatol. 2021;40:3207–3217. doi: 10.1007/s10067-021-05643-5. [DOI] [PubMed] [Google Scholar]
- 29.Ghanchi FD, Williamson TH, Lim CS, et al. Colour Doppler imaging in giant cell (temporal) arteritis: serial examination and comparison with non-arteritic anterior ischaemic optic neuropathy. Eye (Lond) 1996;10:459–464. doi: 10.1038/eye.1996.101. [DOI] [PubMed] [Google Scholar]
- 30.Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol Scand. 2002;80:355–367. doi: 10.1034/j.1600-0420.2002.800403.x. [DOI] [PubMed] [Google Scholar]
- 31.Issa M, Donaldson L, Jeeva-Patel T, et al. Ischemic ocular manifestations of giant cell arteritis: A Canadian case series. J Neurol Sci. 2022;436:120222. doi: 10.1016/j.jns.2022.120222. [DOI] [PubMed] [Google Scholar]
- 32.Liozon E, Dalmay F, Lalloue F, et al. Risk Factors for Permanent Visual Loss in Biopsy-proven Giant Cell Arteritis: A Study of 339 Patients. J Rheumatol. 2016;43:1393–1399. doi: 10.3899/jrheum.151135. [DOI] [PubMed] [Google Scholar]
- 33.Chen JJ, Leavitt JA, Fang C, et al. Evaluating the incidence of arteritic ischemic optic neuropathy and other causes of vision loss from giant cell arteritis. Ophthalmology. 2016;123:1999–2003. doi: 10.1016/j.ophtha.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patil P, Williams M, Maw WW, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33:S103–S106. [PubMed] [Google Scholar]
- 35.Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum. 2005;53:293–297. doi: 10.1002/art.21075. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Rhoton AL., Jr Middle meningeal origin of the ophthalmic artery. Neurosurgery. 2001;49:401–407. doi: 10.1097/00006123-200108000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Diamantopoulos AP, Haugeberg G, Lindland A, et al. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford) 2016;55:66–70. doi: 10.1093/rheumatology/kev289. [DOI] [PubMed] [Google Scholar]
- 38.Mukhtyar C, Cate H, Graham C, et al. Development of an evidence-based regimen of prednisolone to treat giant cell arteritis - the Norwich regimen. Rheumatol Adv Pract. 2019;3:rkz001. doi: 10.1093/rap/rkz001. [DOI] [PMC free article] [PubMed] [Google Scholar]