Abstract
From the approval of COVID-19 mRNA vaccines to the 2023 Nobel Prize awarded for nucleoside base modifications, RNA therapeutics have entered the spotlight and are transforming drug development. While the term “RNA therapeutics” has been used in various contexts, this review focuses on treatments that utilize RNA as a component or target RNA for therapeutic effects. We summarize the latest advances in RNA-targeting tools and RNA-based technologies, including but not limited to mRNA, antisense oligos, siRNAs, small molecules and RNA editors. We focus on the mechanisms of current FDA-approved therapeutics but also provide a discussion on the upcoming workforces. The clinical utility of RNA-based therapeutics is enabled not only by the advances in RNA technologies but in conjunction with the significant improvements in chemical modifications and delivery platforms, which are also briefly discussed in the review. We summarize the latest RNA therapeutics based on their mechanisms and therapeutic effects, which include expressing proteins for vaccination and protein replacement therapies, degrading deleterious RNA, modulating transcription and translation efficiency, targeting noncoding RNAs, binding and modulating protein activity and editing RNA sequences and modifications. This review emphasizes the concept of an RNA therapeutic toolbox, pinpointing the readers to all the tools available for their desired research and clinical goals. As the field advances, the catalog of RNA therapeutic tools continues to grow, further allowing researchers to combine appropriate RNA technologies with suitable chemical modifications and delivery platforms to develop therapeutics tailored to their specific clinical challenges.
Keywords: RNA therapeutics, delivery of nucleic acids, chemical modifications
Introduction
Looking back at history, RNA therapeutics has been rapidly advancing since the initial key discoveries, for example, the discovery of mRNA in the 1960s [1]. In the following decades, additional milestones were made on mRNA, including the discovery of the 5′-cap, the first delivery to cells with liposomes, the development of in vitro mRNA synthesis platforms as well as the first cationic lipid-mediated mRNA delivery [2], [3], [4], [5], [6]. In the late 1990s, RNA interference (RNAi) was discovered and the first antisense oligo (ASO) drug was approved for cytomegalovirus (CMV) retinitis [7, 8]. During the 2000s, the importance of pseudouridine modification on mRNA was recognized and the first human trial of an mRNA vaccine against melanoma was initiated [9, 10]. In 2018, the first short interfering RNA (siRNA) drug patisiran was approved, showcasing the rapid therapeutic development in a short span of 20 years given that siRNA was initially discovered in 1998 [11]. In 2020, two COVID-19 mRNA vaccines were approved for emergency use and administered to millions of individuals worldwide, marking a historical milestone as the first widely-used RNA therapeutics [12, 13].
The development of RNA technologies stems from the basic understanding of how cellular RNA functions, for example, how endogenous mRNA is translated into proteins by cellular machinery and how siRNA and microRNA (miRNA) mediate gene silencing. In addition, understanding how endogenous RNAs are dysregulated in disease context can reveal novel therapeutic targets. This review will first introduce the types and functions of naturally occurring RNAs in cells and how they can be leveraged for therapeutic development. Following that, different types of RNA therapeutic agents will be summarized along with short briefings on the advances in medicinal chemistry and delivery strategies, although the latter is not the main focus of this review. Together, the RNA therapeutics toolbox is assembled and continues to expand for diverse cellular applications.
Types of naturally occurring RNAs
The repository of naturally occurring RNA in biological systems is highly versatile and covers a wide range of cellular functions. In general, cellular RNA can be grouped into two categories based on their coding potential: (1) messenger RNA (mRNA) that acts as templates for protein translation and (2) noncoding RNA (ncRNA) that does not associate with ribosomes or encode for proteins. While it is estimated that over 75 % of the human genome can be transcribed, only around 1 % of it encodes proteins and the majority are transcribed into ncRNA [14, 15]. ncRNA can be broadly categorized into three classes: (1) infrastructural or “housekeeping” RNA like ribosomal RNA (rRNA), transfer RNA (tRNA), spliceosomal RNA and small nucleolar RNA (snoRNA), (2) small regulatory RNA like siRNA, miRNA and small PIWI-interacting RNA (piRNA), and lastly (3) long noncoding RNAs (lncRNAs) that are typically longer than 200 nucleotides [16].
Coding RNA – mRNA
The biological significance of mRNA has been long recognized since the first discovery that they serve as templates for protein translation. Pre-mRNA is typically transcribed by RNA polymerase II [17]. The maturation of pre-mRNA can occur co- or post-transcriptionally, which typically includes intron excision, exon ligations, 7-methylguanosine (m7G) capping to the 5′ end, and formation of a 3′ end by cleavage and addition of a poly(A) tail [18]. Transcriptome-wide analyses revealed that more than 90 % of genes can undergo alternative splicing events and incorporate different combinations of exons or intron retention, leading to different coding sequences and downstream protein sequences [19], [20], [21]. Most mRNA undergoes the canonical translation initiation in the cytoplasm, which involves the binding of the ribosomal preinitiation complex to the 5′ cap [22]. The transcription, processing, and translation of mRNA are tightly regulated by multiple quality control mechanisms, which may be dysregulated in diseases, leading to mRNA abnormality that can occur at any stage of mRNA biogenesis.
Noncoding RNA – infrastructural RNA
Infrastructural RNAs like rRNA, tRNA, and spliceosomal RNA form machinery crucial for protein synthesis and life. rRNA represents the most abundant class of RNA, accounting for 80–90 % of total RNA by mass inside the cell [23, 24]. It is produced from ribosomal DNA clusters organized in the nucleolus and is transcribed by RNA polymerase I. tRNA makes up around 10–15 % of total RNA by mass and is transcribed by RNA polymerase III (Pol III) [24, 25]. It is an adapter molecule that links a specific codon in the mRNA with its corresponding amino acid. tRNA displays a highly conserved “cloverleaf” secondary structure and represents the most extensively modified RNA in cells, with about 10–20 % of residues being modified [26], [27], [28]. Other infrastructural “housekeeping” RNAs include spliceosomal small nuclear RNAs (snRNAs), which are about 150 nucleotides long on average. They are assembled with proteins to form the spliceosomes and allow for catalysis in the splicing reaction [29]. snoRNAs are widely present in the nucleoli of eukaryotic cells and are reported to play a role in rRNA, tRNA, and mRNA modifications [30].
Small noncoding RNA
miRNA and siRNA
miRNA and siRNA are around 20–35 nucleotides long and represent one of the most studied ncRNA classes due to their critical regulations on gene expression, mostly gene silencing. miRNAs are endogenous and expressed from an organism’s genome, whereas siRNAs are typically thought to be primarily exogenous in origin, derived from viruses, transposons, or transgene triggers [31]. miRNAs are processed from stem-loop precursors with incomplete double-stranded regions, while siRNAs are excised from long, fully double-stranded RNAs [32]. Despite some differences, they both associate with Argonaute proteins, especially Argonaute 2 (AGO2), and form core components of the RNA-induced silencing complex (RISC) [31], [32], [33]. Once associated with RISC, the sense strand (or passenger strand) is cleaved by AGO2 while the antisense strand (or guide strand) remains in the complex to guide RNA target selection [34, 35]. The regulatory effect following target binding depends on the degree of sequence complementarity. siRNA typically directs RISC to perfectly complementary targets and induces target degradation [36]. miRNA often binds to the 3′ untranslated region (UTR) of mRNA with some mismatches and bulges. miRNA nucleotides 2–8 represent the seed region, which is the key region for target base pairing and recognition [31]. In general, a perfect complementarity between miRNA and target would induce target cleavage, whereas the presence of mismatches would promote repression of mRNA translation. Given their critical gene silencing functions, the potential of utilizing endogenous or engineered miRNA and siRNA to modify gene expression is quickly recognized and they are heavily investigated as tools for RNA therapeutics. The landscape of miRNA and siRNA is frequently altered during disease progression, marking them as potential therapeutic targets.
piRNA
piRNA is an animal-specific class of small ncRNA with a length of 21–35 nucleotides that primarily silence transposable elements, especially in the germline, regulate gene expression, and fight against viral infection [37]. Over 90 % of the primary piRNAs are single-stranded and are transcribed by Pol II from piRNA-encoding genes located on specific genomic loci called “Piwi clusters” [38]. Modified and processed piRNAs are loaded into the PIWI-clade Argonautes (PIWI proteins), which then guide the complex to the nascent target transcript and induce target degradation or heterochromatin formation at the gene locus, thereby suppressing the target gene expression [39, 40].
Long noncoding RNA (lncRNA)
lncRNAs are typically longer than 200 nucleotides to differentiate from small ncRNAs like miRNA and tRNA. They are mainly transcribed by RNA pol II and can be spliced and polyadenylated similar to mRNA transcripts, although not required [16], display tissue-specific expression patterns and are dynamically regulated during development and disease progression. lncRNA has been suspected to be transcriptional noise due to their generally low expressions, low levels of sequence conservation compared to protein-coding genes, and low visibility in genetic screens. However, a couple of lncRNAs have been reported with conserved functions, such as H19 and XIST, essential for developmental processes [41], [42], [43]. Characterized examples of lncRNA have demonstrated that their functions include but are not limited to regulating genome organization, the assembly of membraneless organelles, and mRNA stability and translation, through general mechanisms of RNA-RNA, RNA-DNA, and RNA-protein interactions [16, 44], [45], [46]. lncRNA has been gaining interest as therapeutic targets given their gene expression regulations, functional versatility and dysregulation in diseases.
Circular RNA (circRNA)
circRNA is a class of lncRNA with a covalently enclosed loop structure, which confers higher stability than linear RNA and intrinsic resistance to exonuclease degradation [47], [48], [49]. They are produced by a non-canonical splicing event called backsplicing, in which a downstream splice site is linked to an upstream splice site [50]. High-throughput total RNA-seq has revealed that thousands of circRNAs exist in eukaryotic cells with tissue-specific expression patterns, including in fungi, plants, worms, fish, insects, and mammals [51], [52], [53], [54], [55]. circRNA can exert diverse regulations in biological processes through mechanisms of RNA-RNA, RNA-protein, and RNA-DNA interactions, similar to other lncRNAs. Although the majority of circRNAs are noncoding, a small fraction of them may encode for peptides through the presence of internal ribosomal entry site (IRES) and, in some cases, N6-methyladenosine (m6A) dependent protein translation [56], [57], [58], [59].
Enhancer RNA (eRNA)
eRNAs are transcribed from gene enhancers, which are cis-acting regulatory DNA elements that work in tandem with a given promoter to regulate the transcription of the downstream genes [60], [61], [62], [63]. Global profiling and annotation estimated 40,000–65,000 eRNAs expressed in human cells [64], [65], [66], [67]. Evidence suggests that eRNA can be functional and contribute to gene control by altering the chromatin environment and by interacting with transcriptional regulators [61].
RNA therapeutics toolbox
RNA therapeutics offer several advantages over other types of therapeutics, such as small molecules or antibodies. Small molecules are traditionally developed to bind structured regions of a protein, which may not be applicable to all proteins due to the presence of intrinsically “disordered” domains that lack definitive structure. In fact, it is estimated that only about 3,000 out of the 20,000 human proteins are “druggable” and approved drugs target only about 700 proteins so far [68, 69]. Antibodies are effective against cell-surface or circulating proteins but cannot steadily access cytoplasmic targets. In addition, many ncRNAs have been reported to be significantly altered in diseases and can drive disease progression. The existence of “undruggable” proteins and ncRNA targets emphasizes the need for alternative avenues and targeting RNA shows great promise for its diverse potential. In comparison to small molecules and antibodies, RNA-based therapeutic agents like ASO, siRNA and mRNA can be manufactured quickly at a large scale. The incredible speed of production is demonstrated by the COVID-19 mRNA vaccines, which were developed a few months after the start of the pandemic. Another advantage of RNA-based therapeutics is that they represent a streamlined platform and can be quickly modified to target different RNAs or express different proteins. In addition, RNA tools are highly engineerable and can be designed to mediate different effects tailoring to various purposes. For example, ASOs can be engineered to induce target RNA cleavage as well as splicing modulation. In the following sections, RNA-targeting tools and RNA-based therapeutics will be discussed in detail based on their mechanisms of action and therapeutic effects, covering both FDA-approved therapeutics as well as upcoming technologies with clinical potentials.
Templates for protein expression
The feasibility of using in vitro transcribed (IVT) mRNA as templates for protein production was first demonstrated in 1990 by directly injecting naked mRNA encoding for chloramphenicol acetyl-transferase, luciferase and β-galactosidase in the mouse skeletal muscle [70]. Since the initial success, IVT mRNA quickly gained interest for various applications, especially for protein replacement for diseases caused by endogenous protein dysregulation and for expressing antigens for cancer and infectious disease vaccines. IVT mRNA typically mimics the endogenous mRNA with 5 sections: 5′ cap, 5′ UTR, an open reading frame that encodes the protein of interest, 3′ UTR and poly(A) tail. With advances in RNA synthesis and delivery technology, it becomes clear that mRNA has the potential to be engineered to encode for virtually any peptides or proteins in a time-efficient fashion.
Antigen expression for vaccination against infectious disease and cancer
In 2020, the Food and Drug Administration (FDA) approved the very first mRNA vaccines delivered by lipid nanoparticle (LNP) for providing immunity against the SARS-CoV-2 virus that initiated the global COVID-19 pandemic, BNT162b2 mRNA produced by Pfizer and BioNTech, and mRNA-1273 produced by Moderna (Table 1) (Figure 1) [12, 13]. Both mRNAs encode the SARS-CoV-2 spike protein, which mediates receptor binding and fusion of the viral and cellular membranes, and mutations are introduced to the coding sequence to stabilize the protein in the prefusion conformation [12, 13, 71]. mRNA vaccines have several advantages over traditional vaccines using live and attenuated pathogens, protein subunits or DNA: (1) mRNA does not integrate with the host DNA and is non-infectious, (2) the production of mRNA can be rapid, scalable and cost-effective and (3) mRNA can be engineered to encode for multiple antigens or variants of antigens to strengthen immune response [72, 73]. Since 2020, the formulations for the COVID-19 vaccines were quickly updated to protect against variants of the virus such as BA.4/5 and XBB, highlighting the feasibility of mRNA vaccines to be modified to closely follow virus evolution [74, 75]. To note, the versatility of mRNA vaccines was further demonstrated through the release of bivalent COVID-19 vaccines containing mRNAs encoding ancestral SARS-CoV-2 and variant spike proteins [76, 77]. Following the success of COVID-19 mRNA vaccines, mRNA vaccines against other infectious viruses, including ZIKA, cytomegalovirus (CMV), influenza, human metapneumovirus (hMPV), respiratory syncytial virus (RSV), Epstein-Barr, chikungunya, Nipah and rabies, are undergoing clinical trials.
Table 1:
Current FDA-approved RNA therapeutics.
| Therapeutics | Approval year | Disease (cellular target) | Type | Mechanism of action | Key modifications | Delivery strategy |
|---|---|---|---|---|---|---|
| Formivirsen | 1998 | CMV retinitis | ASO | RNA degradation | PS | Intravitreal |
| Pegaptanib | 2004 | Macular degeneration (VEGF) | Aptamer | VEGF antagonist | 2′-OMe, 2′-F | Intravitreal, PEG conjugated |
| Mipomersen | 2013 | Familial hypercholesterolemia (ApoB) | ASO | RNA degradation | 2′-MOE, PS | Subcutaneous |
| Nusinersen | 2016 | Spinal muscular atrophy (SMN2) | ASO | Splice switching | 2′-MOE, PS | Intrathecal |
| Eteplirsen | 2016 | DMD (dystrophin exon 51) | ASO | Splice switching | PMO | Intravenous infusion |
| Patisiran | 2018 | hATTR amyloidosis (TTR) | siRNA | RNA degradation | 2′-OMe, dT | Intravenous, LNP packaged |
| Inotersen | 2018 | hATTR amyloidosis (TTR) | ASO | RNA degradation | 2′-MOE | Subcutaneous |
| Milasen | 2018 | Ceroid lipofuscinosis 7 (MFSD8) | ASO | Splice switching | PS, 2′-OMe | Intrathecal |
| Givosiran | 2019 | Acute hepatic porphyria (aminolevulinate synthase) | siRNA | RNA degradation | 2′-OMe, 2′-deoxy-2′-F, PS | Subcutaneous, GalNAc conjugated |
| Golodirsen | 2019 | DMD (dystrophin exon 53) | ASO | Splice switching | PMO | Intravenous infusion |
| Lumasiran | 2020 | Primary hyperoxaluria type 1 (HAO1) | siRNA | RNA degradation | 2′-OMe, 2′-deoxy-2′-F, PS | Subcutaneous, GalNAc conjugated |
| Inclisiran | 2020 | Hypercholesterolemia (PCSK9) | siRNA | RNA degradation | 2′-OMe, 2′-deoxy-2′-F, PS | Subcutaneous, GalNAc conjugated |
| Viltolarsen | 2020 | DMD (dystrophin exon 53) | ASO | Splice switching | PMO | Intravenous infusion |
| Risdiplam | 2020 | Spinal muscular atrophy (SMN2) | Small molecule | Splice switching | NA | Oral |
| BNT162b2 | 2020 | COVID | mRNA | Antigen expression | m1Ψ | Intramuscular, LNP packaged |
| mRNA-1273 | 2020 | COVID | mRNA | Antigen expression | m1Ψ | Intramuscular, LNP packaged |
| Casimersen | 2021 | DMD (dystrophin exon 45) | ASO | Splice switching | PMO | Intravenous infusion |
| Vutrisiran | 2022 | hATTR amyloidosis (TTR) | siRNA | RNA degradation | 2′-OMe, 2′-deoxy-2′-F, PS | Subcutaneous, GalNAc conjugated |
| Avacincaptad pegol | 2023 | Geographic atrophy (C5) | Aptamer | C5 antagonist | 2′-F, 2′-OMe | Intravitreal, PEG conjugated |
| Tofersen | 2023 | Amyotrophic lateral sclerosis (SOD1) | ASO | RNA degradation | 2′-MOE, PS | Intrathecal |
| Nedosiran | 2023 | Primary hyperoxaluria type 1 (LDH) | siRNA | RNA degradation | 2′-Deoxy-2′-F, 2′-OMe, PS | Subcutaneous, GalNAc conjugated |
| Eplontersen | 2023 | hATTR amyloidosis (TTR) | ASO | RNA degradation | 2′-MOE, PS | Subcutaneous, GalNAc conjugated |
Note: CMV, cytomegalovirus; PEG, polyethylene glycol; VEGF, vascular endothelial growth factor; 2′-OMe, 2′-O-methyl; 2′-F, 2′-fluoro; 2′-MOE, 2′-methoxyethyl; PS, phosphorothioate; hATTR, hereditary transthyretin-mediated; LNP, lipid nanoparticle; PMO, phosphorodiamidate morpholino oligonucleotide; dT, 2′-deoxythymidine; DMD, Duchenne muscular dystrophy; m1Ψ, 1-methyl-3′-pseudouridylyl; LDH, lactate dehydrogenase; GalNAc, N-acetylgalactosamine; ApoB, apolipoprotein B; SMN2, survival motor neuron 2; MFSD8, major facilitator superfamily domain containing 8; HAO1, hydroxyacid oxidase 1; PCSK9, proprotein convertase subtilisin/kexin type 9; LDH, lactate dehydrogenase; SOD1, superoxide dismutase 1; NA, not applicable.
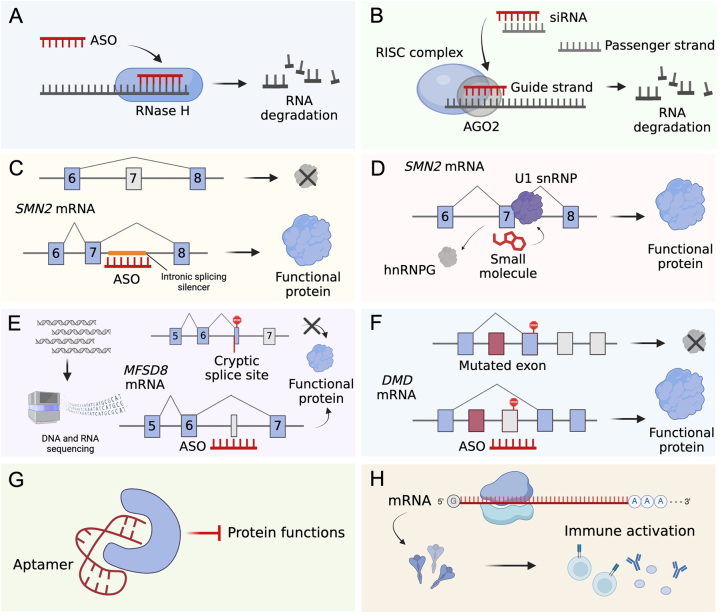
Figure 1:
Mechanisms of action of FDA-approved RNA therapeutics. (A) ASO hybridizes to target RNA through sequence complementarity and recruits RNase H to mediate target degradation and sequential protein downregulation. Current FDA-approved degradation-inducing ASOs include formivirsen, mipomersen, inotersen, tofersen and eplontersen. (B) siRNA therapeutics are delivered as double-stranded molecules. The guide strand is loaded into the RISC complex and guides the complex to the RNA target for degradation. Current FDA-approved degradation-inducing siRNAs include patisiran, givosiran, lumasiran, inclisiran, vutrisiran and nedosiran. (C) Splice-switching ASO nusinersen binds to an intronic splicing silencer located in the intron of SMN2 pre-mRNA and promotes exon 7 inclusion, which leads to functional protein production [230]. (D) Small molecule risdiplam binds to exon 7 of SMN2 pre-mRNA and mediates exon inclusion. The exact mechanism remains to be elucidated but it is proposed that risdiplam promotes the recruitment of U1 snRNP while displacing hnRNPG [231]. (E) Splice-switching ASO can be personalized based on a patient’s specific mutational profiles, seen in the case of milasen. DNA and RNA sequencing of a patient revealed a cryptic splice site in the intron following exon 6 of MFSD8 mRNA, leading to truncated transcript production. Milasen inhibits the splicing to the cryptic splice site and promotes functional exon 6-exon 7 splicing [121]. (F) Splice-switching ASO can bind to an exon of pre-mRNA and leads to the exclusion of that exon in the final mRNA, seen in the cases of eteplirsen, golodirsen, viltolarsen and casimersen. This promotes the generation of functional DMD proteins by skipping a mutated exon with premature stop codon or exon that induces frameshifts [119, 120, 122, 232]. (G) Aptamer folds into defined structures and suppresses protein functions, seen in the cases of pegaptanib and avacincaptad pegol. (H) In vitro transcribed mRNA encodes antigens and leads to downstream immune activation, seen in the cases of COVID-19 vaccines.
In addition to infectious diseases, mRNA vaccines are being developed against cancer with the goal of activating endogenous immune response to eradicate cancer cells. The tumor antigen selection is a key step to effective vaccine design. Tumor antigens can be categorized into tumor-associated self antigens (TAAs) which are overexpressed in tumor cells but can also be present in normal tissues, and tumor- specific antigens (TSAs) which are neoantigens derived from mutations in tumor cells [78], [79], [80]. The delivery strategy for mRNA cancer vaccines can be cellular-based, such as through engineered dendritic cells, or through synthetic vectors such as LNP [78]. For example, dendritic cells transfected with mRNA encoding for antigens WT1, PRAME, and CMVpp65 are undergoing clinical trials for eliminating residual acute myeloid leukemia cells post-remission, and treatments showed good toleration by the patients (NCT02405338) [81, 82]. mRNA-4157 is an example of an mRNA cancer vaccine delivered by LNP for treating high-risk melanoma patients who have undergone surgery in combination with the anti-PD-1 drug pembrolizumab (NCT03897881). It is personalized to encode up to 34 different patient-specific neoantigens, demonstrating the feasibility of utilizing mRNA for personalized medicine, which may be technically challenging for traditional therapies [83]. In addition to expressing antigens, other immune activation strategies include delivering mRNA encoding inflammatory cytokines as well as antibodies [84], [85], [86].
Protein replacement therapies
The main goal of protein replacement therapy is to substitute or replenish specific protein deficiencies that result either from the protein being absent or non-functional due to mutations in affected patients. Gene or protein supplementation could be achieved in different ways including delivering DNA, editing the defective DNA sequence, delivering mRNA as well as directly delivering proteins. mRNA arises as an attractive candidate over proteins given the short half-life of administered proteins and the ineligibility to substitute intracellular proteins such as transcription factors [87]. Compared to DNA delivery or gene editing, mRNA is generally considered safer due to its non-integrative nature and does not induce changes to the genome. mRNA-based replacement therapy has been extensively explored in various fields, including hematologic diseases, metabolic diseases, cancer, cardiac diseases, lung diseases, orthopedic diseases, and many more [88], [89], [90], [91], [92]. For example, AZD8601 is an mRNA encoding vascular endothelial growth factor A (VEGF-A) formulated in biocompatible citrate-buffered saline and is undergoing clinical trials to improve angiogenesis in patients undergoing coronary artery bypass grafting (NCT03370887) [93, 94].
Templates for CRISPR-Cas9 effector
CRISPR/Cas9-mediated genome editing has become a heavily-investigated approach to treat genetic diseases as it can correct DNA sequences and provide long-lasting therapeutic benefits after a single treatment. Since genome editing occurs at the DNA level, it is out of the scope of this review. However, it is worth noting that delivering mRNA encoding Cas9 effector has been extensively studied as an alternative to delivering Cas9 protein or DNA plasmids [95], [96], [97], [98]. For example, NTLA-2001 is an in vivo gene editing agent composed of LNP encapsulating Cas9 mRNA and sgRNA targeting transthyretin (TTR) for the treatment of transthyretin amyloidosis [99]. It is undergoing phase I clinical trials, and TTR protein reduction was observed in patients (NCT04601051). Very recently, in November and December of 2023, the first CRISPR-based gene editing was approved for treating sickle cell disease in the UK and USA, marking a milestone [100]. As the technology continues to be approved and expanded, delivering Cas9 mRNA and guide RNA would be promising alternatives to the current Cas9 protein delivery approach.
Upcoming templates for protein expression
Prolonged RNA half-life and protein translation are often desired to increase vaccine immunogenicity and the duration of therapeutic effect while lowering the RNA amount required per dose. Several modified forms of translation templates are under investigation, including self-amplifying RNA (saRNA), circRNA and trans-amplifying RNA (taRNA). saRNA originates from alphavirus structures and is engineered by replacing the gene sequence coding for virus structural proteins with the gene of interest [101], [102], [103]. RNA from alphaviruses contains sequences coding for nonstructural proteins (nsP1-4) that function as replicases through RNA-dependent RNA synthesis [101], [102], [103]. Using the COVID-19 vaccine as an example, saRNA encoding the spike protein showed elicited a high immune response and may induce a higher neutralizing antibody titer than traditional mRNA [104], [105], [106]. However, one limitation is that saRNA is generally longer than traditional mRNA, which challenges its production and delivery. To overcome this challenge, trans-amplifying RNA (taRNA) has been developed, which involves a second mRNA encoding the alphaviral replicase. Preclinical studies of taRNA vaccines encoding virus antigens showed high immunogenicity and good antibody titer, demonstrating potential clinical utility [107], [108], [109]. circRNA as protein templates is also gaining interest due to their longer half-life than linear mRNA. Despite the lack of a 5′ cap, circRNA can be engineered to efficiently express proteins through IRES-dependent translation mechanisms [110], [111], [112]. In preclinical models, circRNA vaccines can be effectively translated into antigens and can elicit immune responses more potent and long-lasting than linear mRNA [113, 114]. These newer forms of translation templates hold great promise for bringing prolonged therapeutic effects for antigen expression and protein replacements.
Splicing regulators
RNA mis-splicing underlies numerous human diseases and may result from mutations or dysregulation in DNA sequences, RNA cis-regulatory elements, and spliceosomal factors [115]. ASOs are single-stranded synthetic nucleic acids (typically DNA) that are 8–50 nucleotides long and are designed to selectively base-pair to RNA of interest [116, 117]. For splicing modulation, ASOs (also referred to as splice-switching oligonucleotides [SSOs]) can be designed to promote both exon exclusion and inclusion by binding to intron-exon junctions or sequences involved in splicing (Figure 1) [116, 117]. Several ASOs are approved by the FDA to modulate RNA splicing in diseases like spinal muscular atrophy (SMA), Duchenne muscular dystrophy (DMD), and Batten disease (Table 1) [118], [119], [120], [121], [122]. For example, nusinersen is the first splicing-correcting ASO approved for SMA treatment, which is a neuromuscular disorder caused by loss of survival motor neuron (SMN) proteins. It promotes exon 7 inclusion of SMN2 mRNA and increases the expression of SMN protein by binding to the intronic splicing inhibitor in intron 7 (Figure 1) [118]. Recent studies demonstrated that ASOs can be personalized and designed to specifically correct splicing based on the patient’s unique mutational profile, exemplified by Milasen, highlighting its potential in precision medicine [121].
Given that RNAs can adopt stable secondary and tertiary structures, small molecules targeting RNA have been investigated and they offer several advantages over RNA-based therapeutics including well-established delivery systems. The development of RNA-targeting small molecules has been challenging since only a few RNA structures have been solved and experimentally validated [123]. In recent years, several high throughput screening methods have been developed and identified thousands of compounds that can bind RNA [124]. Machine learning approaches are also being implemented to increase the efficiency of small molecule designs by predicting RNA structures as well as the binding affinity to RNAs [125, 126]. Risdiplam is the first FDA-approved small molecule that modifies SMN2 mRNA splicing for the treatment of SMA, similar to nusinersen’s mode of action as discussed above (Table 1) [127]. Some advantages of risdiplam over ASOs are the effective brain penetration and oral bioavailability. With advances in chemical synthesis, screening methods, and RNA structure availability, increasing small molecule candidates are being reported in preclinical studies for various disease models [123].
RNA degraders
Many human diseases are driven by the overexpression and the accumulation of unwanted proteins, such as oncogenes in cancer. Small molecules targeting proteins have been the mainstay of drug development; however, many proteins remain untargetable due to the lack of definitive structures. Degrading RNA is a promising alternative and can be applied to virtually any target in theory. ASOs and siRNAs are the main workforces currently approved by the FDA to degrade and downregulate RNA (Table 1) (Figure 1). The binding of ASOs to RNA forms an RNA-DNA hybrid that becomes a substrate for RNase H, especially RNase H1, which leads to RNA degradation [128, 129]. RNase H-recruiting ASOs can possess different chemical modifications compared to the splicing modulating ASOs, which will be discussed in detail in the chemical modifications section [117, 130, 131]. An example of an FDA-approved RNase H-recruiting ASO is mipomersen, which binds to mRNA encoding human apolipoprotein B (ApoB), leading to its degradation and thus a decrease in ApoB protein production (Table 1). The treatment preferentially reduces small low-density lipoprotein particle number in patients with hypercholesterolemia [132].
siRNA-mediated RNAi is another powerful tool to downregulate RNA and many are approved by the FDA. In contrast to single-stranded ASOs, siRNAs are double-stranded and trigger AGO2 and RISC-dependent RNA degradation. The selection of antisense or guide strand is essential to therapeutic efficacy and how to chemically engineer siRNAs to maximize the specific loading has been a subject of significant scientific inquiry, which will be discussed in later sections [131, 133]. The first siRNA therapeutics, patisiran, delivered by LNP, was approved by the FDA in 2018 to treat hereditary transthyretin-mediated (hATTR) amyloidosis by targeting the 3′ UTR of mRNA encoding both wild type and mutant transthyretin (TTR) [11, 134]. Other FDA-approved siRNAs, such as givosiran and inclisiran, are conjugated to N-acetylgalactosamine (GalNAc), which is a sugar molecule that can bind to the asialoglycoprotein receptor abundantly expressed on liver cells for liver-specific delivery [135, 136]. Due to the delivery limitations (discussed in sections below), the current FDA-approved ASOs and siRNAs are targeting liver RNAs for liver-related diseases. However, numerous ASOs and siRNAs are under clinical trials for other diseases such as lymphoma, prostate cancer, pancreatic cancer, and many other cancers [137, 138]. As an example, siG12D-LODER is a biodegradable implant that releases siRNA against KRAS (G12D) for treating locally advanced pancreatic cancer in combination with chemotherapy [139]. KRAS is an example of oncogene with limited small molecule inhibitors, which highlights the need for RNAi therapeutics and its versatility to target the “undruggable” proteins.
Upcoming RNA degraders
Similar to the mechanisms of siRNA, short hairpin RNAs (shRNAs) can also be loaded into the RISC complex and undergo RNAi after being processed by Dicer. To further improve gene knockdown efficacy, bifunctional shRNAs have been developed that both degrade mRNA transcripts as well as inhibit mRNA translation in preclinical models [140]. Bifunctional shRNAs contain two stem-loop structures and get processed into siRNA-like (fully complementary to target) and miRNA-like (contains mismatches) effectors, which promote cleavage and translation inhibition, respectively [140]. pbi-shRNA™ EWS/FLI1 Type 1 LPX is an example of bifunctional shRNAs in clinical development for treating patients with advanced Ewing’s sarcoma by targeting EWS-FLI1 fusion protein (NCT02736565) [141].
In addition to ASOs and RNAi-based degraders, CRISPR-Cas13 systems have emerged as engineerable tools to target RNA for cleavage as well as for other purposes such as RNA editing. Several Cas13 variants have been reported, such as Cas13a, Cas13b, Cas13c, Cas13d, and Cas13X, which all involve an effector enzyme that cleaves RNA and a single-guide RNA (sgRNA) that guides the system to RNA of interest [142], [143], [144]. In cell line models, Cas13 systems have been reported with fewer off-targets compared to ASOs or RNAi, which could be advantageous [145]. Recent studies have demonstrated that Cas13 protein effector and sgRNA can be delivered in mice using adeno-associated viruses (AAV) as therapeutics to treat RNA viral infections and silence gene expressions in numerous disease models, indicating potential clinical utility [146], [147], [148], [149].
Transcription and translation regulators
In some cases, modulating the transcription and translation efficiency of mRNA is desired without altering the copies of mRNA molecules itself. RNA oligonucleotides can be engineered to activate gene transcription and modulate translation efficiency, distinctive from the actions of the RNA degraders mentioned above. Small activating RNAs (also known as saRNAs, a separate identity from self-amplifying RNAs mentioned in previous sections) are about 20-nucleotide long double-stranded RNA that can target regions in the gene promoter and mediates RNA activation pathway (RNAa) to increase gene transcription through an AGO2-dependent mechanism [150], [151], [152], [153]. Although no small activating RNAs have been approved by the FDA currently, many candidates are undergoing preclinical studies and early phases of clinical trials. For example, MTL-CEBPA is the first small activating RNA in clinical trials to upregulate the expression of CEBPA, a downregulated tumor suppressor, in patients with advanced liver cancer [154, 155].
RNA therapeutics can also be engineered to modulate translation efficiency to upregulate or downregulate protein expression. In preclinical studies, ASOs can inhibit protein translation through steric effects [156, 157]. On the other hand, ASOs can also increase protein production by blocking translation from undesired open reading frames and thus promoting production from the desired open reading frames [158], [159], [160]. Small molecules have also been identified in preclinical studies to modulate mRNA translation efficiency. For instance, synucleozid is a small molecule that binds to an iron-responsive element in the mRNA and inhibits the translation of α-synuclein that links to Parkinson’s disease [161]. As the α-synuclein protein lacks a defined structure, this again highlights that targeting mRNA is a promising approach to address these “undruggable” proteins.
In recent years, synthetic tRNAs have gained interest for modulating translation. Nonsense mutations or DNA mutations that change a sense codon to a premature stop codon account for 11 % of human pathogenic mutations. Suppressor tRNAs have been engineered to recognize stop codons, leading to continued protein translation from transcripts with premature stop codons in cell models and preclinical animal models [162], [163], [164]. In theory, one suppressor tRNA could be applied to multiple diseases caused by nonsense mutations, signifying a wide range of potential applications.
RNA aptamers
RNA aptamers are single-stranded oligonucleotides (can also be DNA for DNA aptamers) with well-defined three dimensional structures that can directly bind to specific target molecules, such as proteins and biomolecules (Figure 1) [165, 166]. Given their abilities to bind targets with high affinity and specificity, RNA aptamers are also referred to as nucleic acid antibodies. In comparison with traditional antibodies, RNA aptamers are advantageous in several aspects, including longer shelf life and ease of synthesis and modifications [167]. Pegaptanib is the first RNA aptamer approved by the FDA in 2004 for treating age-related macular degeneration (AMD) by blocking vascular endothelial growth factor (VEGF) binding to its receptor [168]. Recently in 2023, a second RNA aptamer, avacincaptad pegol, was approved by the FDA to inhibit complement C5 for treating geographic atrophy, an advanced form of AMD [169]. RNA aptamers are generated using systematic evolution of ligands by exponential enrichment (SELEX) to optimize for affinity and specificity [170]. In addition to the direct therapeutic effect, RNA aptamers can also serve as guides for site-specific drug delivery by directly conjugating to the drug molecules or by assembling onto the surface of nanocarriers [171].
Noncoding RNA mimics and inhibitors
miRNAs are key mediators of RNAi in cells and have the ability to regulate complex transcriptional networks. While there are no miRNA-based therapeutics approved by FDA currently, many mimics and inhibitors are in clinical development. miRNA mimics exploit the main advantage of endogenous miRNAs being able to target multiple mRNAs at once. TargomiR is an example of a miR-16 mimic in clinical development for treating patients with recurrent malignant pleural mesothelioma and non-small cell lung cancer (NCT02369198) [172]. To inhibit miRNA activity, single-stranded modified antisense nucleotides anti-miRs have been developed and numerous are undergoing clinical trials. RG-012 is an example of a complementary molecule that inhibits miR-21 from binding to other targets and was investigated for treating Alport syndrome (NCT03373786). Despite great therapeutic benefits in preclinical models, the development of RG-012 and many other miRNA-based therapeutics has been halted in clinics due to a lack of efficacy or adverse health effects, which will be discussed in later sections [173].
Several other noncoding RNA-based tools have been explored in the laboratory but have not yet been applied in the clinic. In addition to anti-miRs that specifically inhibit one miRNA, miRNA sponges have been engineered to prevent one or more miRNAs binding to other cellular targets, thus inhibiting miRNA activity [174, 175]. miRNA sponges are synthetic linear or circular transcripts with tandem binding sites to one or more miRNAs of interest and compete with other cellular mRNAs for miRNA binding [175]. In particular, circRNAs have the potential to be potent sponges, as demonstrated by ciRS-7, a natural circle containing more than 70 binding sites for miR-7 [176, 177]. lncRNAs and circRNAs have been reported to be extensively dysregulated in diseases compared to normal tissues and can drive pathogenesis. Although no therapeutics are currently in clinical trials to target or mimic lncRNAs, they can be novel targets given further understanding of their functions. lncRNAs have been explored as biomarkers and several are evaluated in clinical trials, for example, NCT03830619 for lung cancer and NCT04175691 for acute ischemic stroke [178, 179].
RNA base editors
RNA editing has emerged as a powerful tool to correct disease-causing mutations and to tackle situations where transient effect is desirable as opposed to permanent genome editing, such as acute pain, viral infection and inflammation. Although no editors have entered the clinical space, preclinical studies have demonstrated efficacy for various disease models mostly utilizing the endogenous effector enzyme adenosine deaminases (ADARs). ADAR represents a family of enzymes that deaminates double-stranded RNA and edits adenosine (A) to inosine (I), which is then functionally recognized as guanosine (G) by cellular machinery [180]. Notably, G-to-A mutations were estimated to account for 28 % of disease-causing mutations and, therefore, can be potentially corrected by ADAR-mediated RNA editing [181]. In addition to ADARs, APOBEC enzymes are another endogenous family of editors that mediate cytosine (C)-to-uracil (U) editing [182]. To recruit endogenous RNA editors to target RNA, a guide complementary to the target RNA must be delivered to the cells and its design is critical for target specificity and efficacy. The guide can be delivered as ASOs, gRNAs or DNA templates that are transcribed into gRNAs upon entry of the cell. In October 2023, Wave Life Sciences submitted the first RNA editing clinical trial application for WVE-006, which is a GalNAc-conjugated oligonucleotide that recruits endogenous ADAR to correct SERPINA1 mutations for treating alpha-1 antitrypsin deficiency (AATD). RNA editors can also be exogenously delivered to cells which enable engineering of the proteins and the gRNA. Available engineered systems include: (1) covalently linking gRNA to the deamination effector, (2) fusing deaminase to an RNA-binding domain which binds to a corresponding RNA motif on the gRNA and (3) fusing deaminase to a catalytically inactive Cas13 protein [183]. ADAR deaminase domain has also been engineered to mediate C-to-U editing [184]. Besides sequence editing, tools are also available to edit RNA modifications, such as N6-methyladenosine (m6A), which contribute to the regulation of RNA stability, splicing, and translation [185].
Chemical modifications and delivery strategies
One of the biggest challenges to translate RNA therapeutics into the clinic is to effectively deliver the molecules to the target site. Oligonucleotides and mRNAs are typically large (mRNAs are about 300–1,500 kDa, double-stranded siRNAs are about 14 kDa, single-stranded ASOs are 4–10 kDa) and negatively charged, meaning that they do not readily pass through the plasma membrane [186, 187]. To achieve therapeutic effect, RNA therapeutics must resist nuclease degradation in the extracellular space, bypass renal clearance, evade sequestration by plasma proteins, traverse the plasma membrane, escape the endosomes, and avoid re-export by exocytosis [186, 188]. The therapeutics would need to overcome additional barriers such as the blood-brain barrier if the central nervous system is the desired target site.
One key approach to optimize the pharmacodynamics and pharmacokinetics of RNA therapeutics is to chemically modify the DNA and RNA molecules (Figure 2). While this review does not aim to cover the modifications in detail, several are worth mentioning given their incorporation in the FDA-approved therapeutics. The main goal of chemical modification is to increase the nuclease degradation resistance when delivered to the extracellular space while still retaining the affinity for complementary targets and preserving the mode of action. The exchange of phosphodiester (PO) to phosphorothioate (PS), the substitution of ribose 2′-O-position to 2′-O-methoxyethyl (MOE) and the development of phosphorodiamidate morpholino oligonucleotide (PMO) are examples of modifications incorporated into the FDA-approved therapeutics (Table 1) [131, 189]. It is worth noting that some modifications, such as MOE, at certain positions can affect RISC loading in the case of siRNAs and may not be suitable for RNA degradation-inducing therapeutics. Some common modifications adopted for siRNAs include 2′-O-methyl (OMe) and 2′-fluoro (F) nucleotides, which still enable RISC loading and target cleavage (Table 1) [131, 189]. On the other hand, target cleavage is not desired for splicing modulators like splice-switching ASOs. They commonly possess modifications such as MOE, PS, and PMO that confer high stability but do not trigger mRNA degradation [186, 190]. Similarly, aptamers are chemically modified to prevent rapid clearance in vivo. Spiegelmers are examples of modified aptamers composed of L-nucleotide chains as opposed to the natural D-nucleotides, which exhibit exceptional stability in serum [191]. While various chemical modifications are available, the optimal choice depends on the application and the desired mode of action, whether it is to trigger target degradation or act as steric blocks or aptamers. Therapeutic mRNAs face similar challenges, they are prone to nuclease degradation especially given their larger sizes and can lead to unwanted immunogenicity. One key solution is to incorporate pseudouridine (Ψ), which enhances RNA stability and decreases anti-RNA immune response given that it is an abundant modification found on naturally occurring cellular RNAs [192]. This finding was utilized in the COVID mRNA vaccine designs and awarded the Nobel Prize in 2023 given its significance [193], [194], [195]. A recent study indicates that pseudouridine in mRNA can cause +1 ribosomal frameshift in animals injected with BNT162b2 COVID mRNA vaccines, indicating potential off-target effects [196]. Although no adverse outcomes have been reported from mistranslation so far, the effect of chemical modifications requires further examination and optimization.
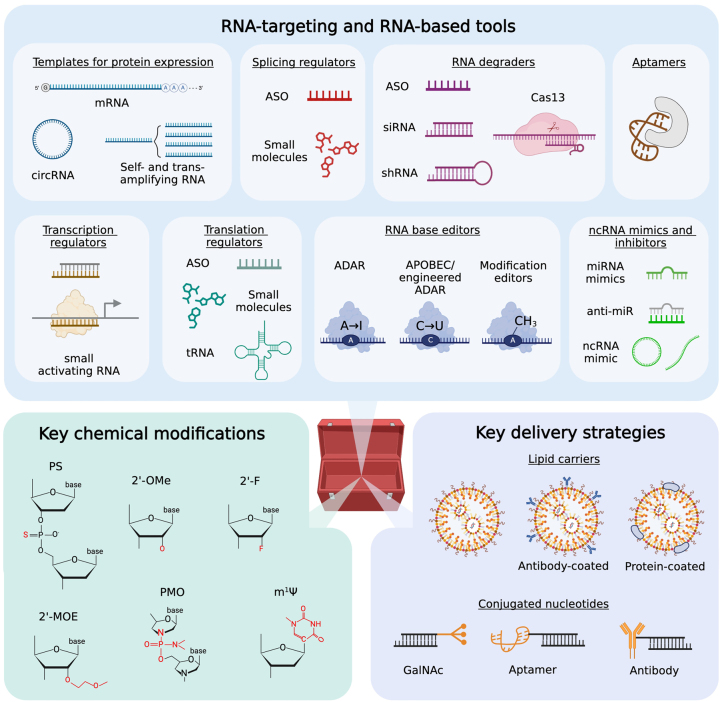
Figure 2:
Overview of the RNA therapeutics toolbox. FDA-approved and upcoming tools are categorized based on their mechanisms of action and therapeutic effects. Examples of key chemical modifications and delivery strategies are also summarized, but these do not represent the full catalog of all existing ones.
The current main delivery strategies for RNA therapeutics include: (1) delivering nucleic acids without carriers, (2) conjugating nucleic acids to biomolecules such as lipids, aptamers, antibodies and sugars, and (3) packaging into cellular carriers like dendritic cells, or synthetic carriers like LNPs and polymeric nanoparticles (Figure 2). The “optimal” delivery platform depends greatly on the type of nucleic acid payload and the target tissue. While small RNA therapeutics such as ASOs and siRNAs can be delivered without carriers, mRNAs require a vehicle for entry into a cell due to their large sizes. The delivery of naked nucleic acids is typically through local injection. For example, splicing switch ASO nusinersen for treating spinal muscular atrophy is delivered through intrathecal injection or direct injection into the fluid surrounding the spinal cord [118]. However, these injections are invasive and methods to deliver the therapeutics across the blood-brain barrier are in need. LNP is a key class of drug delivery system approved by the FDA for liver siRNA (patisiran) delivery and for COVID mRNA vaccine delivery [11], [12], [13]. It is advantageous over other delivery platforms due to its good tissue penetration, weak immunogenicity, and lower toxicity compared to some other polymeric materials [188, 197]. These LNPs contain variations of four basic components: (1) ionizable lipids, (2) cholesterol, (3) helper lipids and (4) poly(ethylene glycol) (PEG)-lipid. One limitation of the current LNP formulations is that they mainly accumulate in the liver. While it is acceptable for treating liver diseases and producing antigens or proteins that are missing in inherited metabolic and hematological disorders, effective LNP delivery to other tissues remains challenging but has shown good improvements in preclinical studies by modifying the formulation and coating LNPs with tissue-targeting macromolecules [188, 198], [199], [200], [201]. Using lung tissue as an example, numerous efforts are spent on developing inhalable or nebulized LNPs to maximize therapeutic concentration in the lungs while limiting systemic exposure [202]. To withstand the shear stress during nebulization, LNP formulation is being optimized by modifying lipid components and composition as well as incorporating additional elements such as DNA hydrogel [203], [204], [205]. In addition to LNPs, other synthetic carriers include polymeric nanoparticles, micelles, dendrimers, and liposomes [188, 197]. Altogether, synthetic carriers are continuing to be optimized for composition as well as synthesis methods [206], [207], [208], [209]. Other RNA therapeutic delivery strategies include directly conjugating nucleic acids to tissue-targeting macromolecules, for example, GalNAc for FDA-approved liver targeting. Asialoglycoprotein receptor that GalNAc binds to is an ideal receptor for active targeting since it is specifically expressed in liver cells and can lead to rapid endocytosis of nucleic acids upon GalNAc binding [188]. Ongoing research has reported that ligands, in the forms of sugars, proteins, aptamers, lipids, and more, can increase delivery to other organs, such as the kidney, lung, and heart [210], [211], [212]. This review offers an overview of some of the common chemical modifications and delivery strategies, however, ongoing research and upcoming technologies extend far beyond the material covered here and will likely transform the clinical application of RNA therapeutics in coming years.
Challenges and future outlooks
While the success and promise of RNA therapeutics are evident, several challenges remain to be tackled. As discussed in the above section, tissue-specific and extrahepatic deliveries remain an obstacle but multiple upcoming strategies have reported good improvement in preclinical studies. Other delivery challenges related to LNP include inefficient cellular uptake and poor endosomal escape to release cargo, which are under extensive investigation and optimization [188, 213].
A concern regarding therapeutics like ASOs and siRNAs is the target specificity and the undesired off-target effects. To improve target specificity and effectively select target sequences, machine learning algorithms have been developed to predict silencing efficacy as well as off-target effects, which can account for siRNA sequence features, RNA target sequence features and the presence of chemical modifications [214], [215], [216]. It is worth noting that machine learning algorithms are also being applied to improve LNP formulations, RNA aptamer, and small molecule designs, and will be versatile tools for various aspects of RNA therapeutic development [217], [218], [219]. Chemical modification is another method to improve siRNA target specificity. Although siRNAs are designed fully complementary to their targets, they can exhibit miRNA-like activities and bind to undefined sites through the miRNA-like seed region. To tackle that, modifications such as 2′-OMe can be introduced in the siRNA seed region and weaken the interaction with undesired transcripts [220]. Off-target effects can also be mediated by loading the passenger strand into RISC, which can be limited by several strategies including introducing different chemical modifications to the passenger and guide strands and trimming or fragmenting the passenger strand [221], [222], [223], [224].
Another challenge faced by RNA therapeutics, or therapeutics in general, is the translation of experimental agents into clinical practice. Many potential RNA therapeutics have demonstrated promising results in preclinical studies, however, failed to translate into the clinics due to severe toxicity and limited efficacy in humans. For example, miR-21 inhibitor RG-012 and miR-34a mimic MRX34 both demonstrated good efficacy in preclinical models but clinical trials were halted due to severe adverse events [173]. The toxicity in some cases can be associated with off-target effects of the oligonucleotides and undesired on-target effects in tissues other than the intended target site due to systemic administration [179]. The dosing of RNA therapeutics is another factor that needs to be taken into consideration, which can impact the off-target rates and affect endogenous RNAi machinery [225, 226]. Another major cause of toxicity is the undesired immunogenicity. As a viral defense mechanism, multiple extra- and intracellular receptors exist to recognize both single-stranded and double-stranded RNA, leading to downstream immune activation. As an example, anti-VEGFR1 siRNA bevasiranib can induce anti-angiogenic effects through direct TLR3 stimulation, resulting in its termination of clinical development (NCT00499590) [227]. As introduced in previous sections, chemical modifications on oligonucleotides can significantly reduce immunogenicity by limiting their interaction with DNA or RNA sensors [228, 229]. Other upcoming strategies include designing smaller RNA therapeutics, given that TLR activation requires a length of at least 21 nucleotides, and combining RNA therapeutics with other therapies to reduce the dosing requirement [179].
The development of RNA therapeutics is an interdisciplinary effort and depends on advances in RNA-targeting and RNA-based technologies, molecular understanding of the mechanism of action, chemical modifications, delivery platforms and immunology. In this review, we summarize the latest tools available for the RNA therapeutic toolbox and highlight the diverse applications ranging from antigen expression for vaccines to mimicking and targeting ncRNA (Figur 2). As the toolbox continues to expand, researchers can assemble the “optimal” therapeutics by selecting the appropriate tools for the desired mode of action and combine with chemical modifications and delivery strategies for a specific tissue of target, tailored for a clinical application. With more than dozens of RNA therapeutics currently undergoing clinical trials, there is no doubt that we will continue to witness growth of the field and application in clinics in the upcoming years, bringing benefits to patients, especially those with dire need for novel therapeutics.
Footnotes
Research ethics: The local Institutional Review Board deemed the study exempt from review.
Informed consent: Not applicable.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Research funding: This work was supported by the Terry Fox New Frontiers Program Project Grant (PPG19-1019 and 23-1124 to H.H.H). H.H.H. holds Tier 1 Canada Research Chair in RNA Medicine. M.T. is supported by the Canadian Institutes of Health Research (CIHR) Doctoral Fellowship for graduate students.
Data availability: Not applicable.
References
- 1.Brenner S, Jacob F, Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190:576–81. doi: 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- 2.Muthukrishnan S, Both GW, Furuichi Y, Shatkin AJ. 5′-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975;255:33–7. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriadis GJ. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature. 1978;274:923–4. doi: 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- 4.Krieg PA, Melton DA. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–70. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989;86:6077–81. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 8.Piascik P. Fomiversen sodium approved to treat CMV retinitis. J Am Pharmaceut Assoc. 1999;39:84–5. doi: 10.1016/s1086-5802(16)30428-4. [DOI] [PubMed] [Google Scholar]
- 9.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 11.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 12.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–47. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole CN. Choreographing mRNA biogenesis. Nat Genet. 2001;29:6–7. doi: 10.1038/ng0901-6. [DOI] [PubMed] [Google Scholar]
- 18.Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet. 2014;15:163–75. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–5. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 20.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–6. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braunschweig U, Barbosa-Morais NL, Pan Q, Nachman EN, Alipanahi B, Gonatopoulos-Pournatzis T, et al. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 2014;24:1774–86. doi: 10.1101/gr.177790.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson RJ, Hellen CUT, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–27. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blobel G, Potter VR. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967;26:279–92. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- 24.Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldron C, Lacroute F. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J Bacteriol. 1975;122:855–65. doi: 10.1128/jb.122.3.855-865.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orellana EA, Siegal E, Gregory RI. tRNA dysregulation and disease. Nat Rev Genet. 2022;23:651–64. doi: 10.1038/s41576-022-00501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat Rev Mol Cell Biol. 2021;22:375–92. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]
- 28.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–62. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matera AG, Terns RM, Terns MP. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol. 2007;8:209–20. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- 30.Huang ZH, Du YP, Wen JT, Lu BF, Zhao Y. snoRNAs: functions and mechanisms in biological processes, and roles in tumor pathophysiology. Cell Death Dis. 2022;8:259. doi: 10.1038/s41420-022-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–29. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 33.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–9. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 34.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–20. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Li S, Qi HH, Chowdhury D, Shi Y, Novina CD. Distinct passenger strand and mRNA cleavage activities of human Argonaute proteins. Nat Struct Mol Biol. 2009;16:1259–66. doi: 10.1038/nsmb.1712. [DOI] [PubMed] [Google Scholar]
- 36.Ryther RCC, Flynt AS, Phillips JA, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene Ther. 2005;12:5–11. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 37.Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2019;20:89–108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Ben S, Xin J, Li S, Zheng R, Wang H, et al. The biogenesis and biological function of PIWI-interacting RNA in cancer. J Hematol Oncol. 2021;14:93. doi: 10.1186/s13045-021-01104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Lin H. Roles of piRNAs in transposon and pseudogene regulation of germline mRNAs and lncRNAs. Genome Biol. 2021;22:27. doi: 10.1186/s13059-020-02221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Pan Y, Fang Y, Zhang J, Xie M, Yang F, et al. The biogenesis and functions of piRNAs in human diseases. Mol Ther Nucleic Acids. 2020;21:108–20. doi: 10.1016/j.omtn.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–5. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 42.Brown CJ, Hendrich BD, Rupert JL, Lafrenière RG, Xing Y, Lawrence J, et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–42. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 43.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 44.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:159. doi: 10.1038/s41580-021-00330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enuka Y, Lauriola M, Feldman ME, Sas-Chen A, Ulitsky I, Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–83. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Xue W, Li X, Zhang J, Chen S, Zhang JL, et al. The biogenesis of nascent circular RNAs. Cell Rep. 2016;15:611–24. doi: 10.1016/j.celrep.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 50.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–7. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, et al. Circular RNA is expressed across the eukaryotic tree of life. PLoS One. 2014;9:e90859. doi: 10.1371/journal.pone.0090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia S, Feng J, Lei L, Hu J, Xia L, Wang J, et al. Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Briefings Bioinf. 2017;18:984–92. doi: 10.1093/bib/bbw081. [DOI] [PubMed] [Google Scholar]
- 56.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST, et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84. doi: 10.1186/s13059-019-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al. Translation of CircRNAs. Mol Cell. 2017;66:9–21.e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626–41. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panigrahi A, O’Malley BW. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 2021;22:108. doi: 10.1186/s13059-021-02322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartorelli V, Lauberth SM. Enhancer RNAs are an important regulatory layer of the epigenome. Nat Struct Mol Biol. 2020;27:521–8. doi: 10.1038/s41594-020-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–61. doi: 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drabløs F, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347:1010–4. doi: 10.1126/science.1259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meers MP, Adelman K, Duronio RJ, Strahl BD, McKay DJ, Matera AG. Transcription start site profiling uncovers divergent transcription and enhancer-associated RNAs in Drosophila melanogaster. BMC Genom. 2018;19:157. doi: 10.1186/s12864-018-4510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen RAJ, Down TA, Stempor P, Chen QB, Egelhofer TA, Hillier LW, et al. The landscape of RNA polymerase II transcription initiation in C. elegans reveals promoter and enhancer architectures. Genome Res. 2013;23:1339–47. doi: 10.1101/gr.153668.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodgers G, Austin C, Anderson J, Pawlyk A, Colvis C, Margolis R, et al. Glimmers in illuminating the druggable genome. Nat Rev Drug Discov. 2018;17:301–2. doi: 10.1038/nrd.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 71.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–55. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gote V, Bolla PK, Kommineni N, Butreddy A, Nukala PK, Palakurthi SS, et al. A comprehensive review of mRNA vaccines. Int J Mol Sci. 2023;24:2700. doi: 10.3390/ijms24032700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817–38. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK, et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2023;11:1089–100. doi: 10.1016/S2213-2600(23)00306-5. [DOI] [PubMed] [Google Scholar]
- 75.Chalkias S, McGhee N, Whatley JL, Essink B, Brosz A, Tomassini JE, et al. Safety and immunogenicity of XBB.1.5-containing mRNA vaccines. bioRxiv. 2023 https://www.medrxiv.org/content/10.1101/2023.08.22.23293434.abstract Available from. [Google Scholar]
- 76.Chalkias S, Eder F, Essink B, Khetan S, Nestorova B, Feng J, et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine against COVID-19: a phase 2/3 trial. Nat Med. 2022;28:2388–97. doi: 10.1038/s41591-022-02031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med. 2022;387:1279–91. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C, Shi Q, Huang X, Koo S, Kong N, Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer. 2023;23:526–43. doi: 10.1038/s41568-023-00586-2. [DOI] [PubMed] [Google Scholar]
- 79.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–60. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 80.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–29. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lichtenegger FS, Schnorfeil FM, Rothe M, Deiser K, Altmann T, Bücklein VL, et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: results of a phase I trial. Clin Transl Immunol. 2020;9:e1117. doi: 10.1002/cti2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fløisand Y, Remberger M, Bigalke I, Josefsen D, Vålerhaugen H, Inderberg EM, et al. WT1 and PRAME RNA-loaded dendritic cell vaccine as maintenance therapy in de novo AML after intensive induction chemotherapy. Leukemia. 2023;37:1842–9. doi: 10.1038/s41375-023-01980-3. [DOI] [PubMed] [Google Scholar]
- 83.Carvalho T. Personalized anti-cancer vaccine combining mRNA and immunotherapy tested in melanoma trial. Nat Med. 2023;29:2379–80. doi: 10.1038/d41591-023-00072-0. [DOI] [PubMed] [Google Scholar]
- 84.Etxeberria I, Bolaños E, Quetglas JI, Gros A, Villanueva A, Palomero J, et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T cells. Cancer Cell. 2019;36:613–29.e7. doi: 10.1016/j.ccell.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Lai I, Swaminathan S, Baylot V, Mosley A, Dhanasekaran R, Gabay M, et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6:125. doi: 10.1186/s40425-018-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schlake T, Thran M, Fiedler K, Heidenreich R, Petsch B, Fotin-Mleczek M. mRNA: a novel avenue to antibody therapy? Mol Ther. 2019;27:773–84. doi: 10.1016/j.ymthe.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vavilis T, Stamoula E, Ainatzoglou A, Sachinidis A, Lamprinou M, Dardalas I, et al. mRNA in the context of protein replacement therapy. Pharmaceutics. 2023;15:166. doi: 10.3390/pharmaceutics15010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magadum A, Kaur K, Zangi L. mRNA-based protein replacement therapy for the heart. Mol Ther. 2019;27:785–93. doi: 10.1016/j.ymthe.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahu I, Haque AKMA, Weidensee B, Weinmann P, Kormann MSD. Recent developments in mRNA-based protein supplementation therapy to target lung diseases. Mol Ther. 2019;27:803–23. doi: 10.1016/j.ymthe.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trepotec Z, Lichtenegger E, Plank C, Aneja MK, Rudolph C. Delivery of mRNA therapeutics for the treatment of hepatic diseases. Mol Ther. 2019;27:794–802. doi: 10.1016/j.ymthe.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berraondo P, Martini PGV, Avila MA, Fontanellas A. Messenger RNA therapy for rare genetic metabolic diseases. Gut. 2019;68:1323–30. doi: 10.1136/gutjnl-2019-318269. [DOI] [PubMed] [Google Scholar]
- 92.Badieyan ZS, Berezhanskyy T, Utzinger M, Aneja MK, Emrich D, Erben R, et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J Contr Release. 2016;239:137–48. doi: 10.1016/j.jconrel.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 93.Anttila V, Saraste A, Knuuti J, Jaakkola P, Hedman M, Svedlund S, et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol Ther-Methods Clin Dev. 2020;18:464–72. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anttila V, Saraste A, Knuuti J, Hedman M, Jaakkola P, Laugwitz KL, et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol Ther. 2023;31:866–74. doi: 10.1016/j.ymthe.2022.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han JP, Kim M, Choi BS, Lee JH, Lee GS, Jeong M, et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci Adv. 2022;8:eabj6901. doi: 10.1126/sciadv.abj6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ling S, Yang S, Hu X, Yin D, Dai Y, Qian X, et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat Biomed Eng. 2021;5:144–56. doi: 10.1038/s41551-020-00656-y. [DOI] [PubMed] [Google Scholar]
- 97.Kenjo E, Hozumi H, Makita Y, Iwabuchi KA, Fujimoto N, Matsumoto S, et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat Commun. 2021;12:7101. doi: 10.1038/s41467-021-26714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci U S A. 2021;118:e2020401118. doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillmore JD, Maitland ML, Lebwohl D. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385:1722–3. doi: 10.1056/NEJMc2114592. [DOI] [PubMed] [Google Scholar]
- 100.Sheridan C. The world’s first CRISPR therapy is approved: who will receive it? Nat Biotechnol. 2024;42:3–4. doi: 10.1038/d41587-023-00016-6. [DOI] [PubMed] [Google Scholar]
- 101.Lundstrom K. Self-amplifying RNA viruses as RNA vaccines. Int J Mol Sci. 2020;21:5130. doi: 10.3390/ijms21145130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28:117–29. doi: 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ballesteros-Briones MC, Silva-Pilipich N, Herrador-Cañete G, Vanrell L, Smerdou C. A new generation of vaccines based on alphavirus self-amplifying RNA. Curr Opin Virol. 2020;44:145–53. doi: 10.1016/j.coviro.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oda Y, Kumagai Y, Kanai M, Iwama Y, Okura I, Minamida T, et al. Booster dose of self-amplifying SARS-CoV-2 RNA vaccine vs. mRNA vaccine: a phase 3 comparison of ARCT-154 with comirnaty. bioRxiv. 2023 https://www.medrxiv.org/content/10.1101/2023.07.13.23292597.abstract Available from. [Google Scholar]
- 105.Voigt EA, Gerhardt A, Hanson D, Jennewein MF, Battisti P, Reed S, et al. A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability. NPJ Vaccines. 2022;7:150. doi: 10.1038/s41541-022-00578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McKay PF, Hu K, Blakney AK, Samnuan K, Brown JC, Penn R, et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmidt C, Hastert FD, Gerbeth J, Beissert T, Sahin U, Perkovic M, et al. A bivalent trans-amplifying RNA vaccine candidate induces potent chikungunya and ross river virus specific immune responses. Vaccines. 2022;10:1374. doi: 10.3390/vaccines10091374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perkovic M, Gawletta S, Hempel T, Brill S, Nett E, Sahin U, et al. A trans-amplifying RNA simplified to essential elements is highly replicative and robustly immunogenic in mice. Mol Ther. 2023;31:2297. doi: 10.1016/j.ymthe.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beissert T, Perkovic M, Vogel A, Erbar S, Walzer KC, Hempel T, et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol Ther. 2020;28:119–28. doi: 10.1016/j.ymthe.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen CK, Cheng R, Demeter J, Chen J, Weingarten-Gabbay S, Jiang L, et al. Structured elements drive extensive circular RNA translation. Mol Cell. 2021;81:4300–18.e13. doi: 10.1016/j.molcel.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen R, Wang SK, Belk JA, Amaya L, Li Z, Cardenas A, et al. Engineering circular RNA for enhanced protein production. Nat Biotechnol. 2023;41:262–72. doi: 10.1038/s41587-022-01393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Unti MJ, Jaffrey SR. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem Biol. 2023;31:163–76. doi: 10.1016/j.chembiol.2023.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amaya L, Grigoryan L, Li Z, Lee A, Wender PA, Pulendran B, et al. Circular RNA vaccine induces potent T cell responses. Proc Natl Acad Sci U S A. 2023;120:e2302191120. doi: 10.1073/pnas.2302191120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qu L, Yi Z, Shen Y, Lin L, Chen F, Xu Y, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. 2022;185:1728–44.e16. doi: 10.1016/j.cell.2022.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scotti MM, Swanson MS. RNA mis-splicing in disease. Nat Rev Genet. 2016;17:19–32. doi: 10.1038/nrg.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bajan S, Hutvagner G. RNA-based therapeutics: from antisense oligonucleotides to miRNAs. Cells. 2020;9:137. doi: 10.3390/cells9010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rinaldi C, Wood MJA. Antisense oligonucleotides: the next Frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 118.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–35. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 119.Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74:637–47. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 120.Scaglioni D, Catapano F, Ellis M, Torelli S, Chambers D, Feng L, et al. The administration of antisense oligonucleotide golodirsen reduces pathological regeneration in patients with Duchenne muscular dystrophy. Acta Neuropathol Commun. 2021;9:7. doi: 10.1186/s40478-020-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381:1644–52. doi: 10.1056/NEJMoa1813279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, Smith EC, et al. Safety, tolerability, and efficacy of viltolarsen in boys with duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 2020;77:982–91. doi: 10.1001/jamaneurol.2020.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Childs-Disney JL, Yang X, Gibaut QMR, Tong Y, Batey RT, Disney MD. Targeting RNA structures with small molecules. Nat Rev Drug Discov. 2022;21:736–62. doi: 10.1038/s41573-022-00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rizvi NF, Santa Maria JP, Nahvi A, Klappenbach J, Klein DJ, Curran PJ, et al. Targeting RNA with small molecules: identification of selective, RNA-binding small molecules occupying drug-like chemical space. SLAS Discovery. 2020;25:384–96. doi: 10.1177/2472555219885373. [DOI] [PubMed] [Google Scholar]
- 125.Xiao H, Yang X, Zhang Y, Zhang Z, Zhang G, Zhang BT. RNA-targeted small-molecule drug discoveries: a machine-learning perspective. RNA Biol. 2023;20:384–97. doi: 10.1080/15476286.2023.2223498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garber K. Drugging RNA. Nat Biotechnol. 2023;41:745–9. doi: 10.1038/s41587-023-01790-z. [DOI] [PubMed] [Google Scholar]
- 127.Ratni H, Ebeling M, Baird J, Bendels S, Bylund J, Chen KS, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA) J Med Chem. 2018;61:6501–17. doi: 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- 128.Wu H, Lima WF, Zhang H, Fan A, Sun H, Crooke ST. Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J Biol Chem. 2004;279:17181–9. doi: 10.1074/jbc.M311683200. [DOI] [PubMed] [Google Scholar]
- 129.Majorek KA, Dunin-Horkawicz S, Steczkiewicz K, Muszewska A, Nowotny M, Ginalski K, et al. The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 2014;42:4160–79. doi: 10.1093/nar/gkt1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deleavey GF, Damha MJ. Designing chemically modified oligonucleotides for targeted gene silencing. Chem Biol. 2012;19:937–54. doi: 10.1016/j.chembiol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 131.Hall J. Future directions for medicinal chemistry in the field of oligonucleotide therapeutics. RNA. 2023;29:423–33. doi: 10.1261/rna.079511.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santos RD, Raal FJ, Donovan JM, Cromwell WC. Mipomersen preferentially reduces small low-density lipoprotein particle number in patients with hypercholesterolemia. J Clin Lipidol. 2015;9:201–9. doi: 10.1016/j.jacl.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 133.Chen PY, Weinmann L, Gaidatzis D, Pei Y, Zavolan M, Tuschl T, et al. Strand-specific 5′-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA. 2008;14:263–74. doi: 10.1261/rna.789808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 135.D’Souza AA, Devarajan PV. Asialoglycoprotein receptor mediated hepatocyte targeting – strategies and applications. J Contr Release. 2015;203:126–39. doi: 10.1016/j.jconrel.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 136.Springer AD, Dowdy SF. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Therapeut. 2018;28:109–18. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zogg H, Singh R, Ro S. Current advances in RNA therapeutics for human diseases. Int J Mol Sci. 2022;23:2736. doi: 10.3390/ijms23052736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim YK. RNA therapy: rich history, various applications and unlimited future prospects. Exp Mol Med. 2022;54:455–65. doi: 10.1038/s12276-022-00757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–70. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rao DD, Maples PB, Senzer N, Kumar P, Wang Z, Pappen BO, et al. Enhanced target gene knockdown by a bifunctional shRNA: a novel approach of RNA interference. Cancer Gene Ther. 2010;17:780–91. doi: 10.1038/cgt.2010.35. [DOI] [PubMed] [Google Scholar]
- 141.Rao DD, Jay C, Wang Z, Luo X, Kumar P, Eysenbach H, et al. Preclinical justification of pbi-shRNA EWS/FLI1 lipoplex (LPX) treatment for Ewing’s sarcoma. Mol Ther. 2016;24:1412–22. doi: 10.1038/mt.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Granados-Riveron JT, Aquino-Jarquin G. CRISPR–Cas13 precision transcriptome engineering in cancer. Cancer Res. 2018;78:4107–13. doi: 10.1158/0008-5472.can-18-0785. [DOI] [PubMed] [Google Scholar]
- 143.Xu C, Zhou Y, Xiao Q, He B, Geng G, Wang Z, et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat Methods. 2021;18:499–506. doi: 10.1038/s41592-021-01124-4. [DOI] [PubMed] [Google Scholar]
- 144.Gupta R, Ghosh A, Chakravarti R, Singh R, Ravichandiran V, Swarnakar S, et al. Cas13d: a new molecular scissor for transcriptome engineering. Front Cell Dev Biol. 2022;10:866800. doi: 10.3389/fcell.2022.866800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, Hsu PD. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173:665–76.e14. doi: 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Powell JE, Lim CKW, Krishnan R, McCallister TX, Saporito-Magriña C, Zeballos MA, et al. Targeted gene silencing in the nervous system with CRISPR-Cas13. Sci Adv. 2022;8:eabk2485. doi: 10.1126/sciadv.abk2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan Z, Yao Y, Li L, Cai L, Zhang H, Zhang S, et al. Treatment of autosomal dominant retinitis pigmentosa caused by RHO-P23H mutation with high-fidelity Cas13X in mice. Mol Ther Nucleic Acids. 2023;33:750–61. doi: 10.1016/j.omtn.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keng CT, Yogarajah T, Lee RCH, Muhammad IBH, Chia BS, Vasandani SR, et al. AAV-CRISPR-Cas13 eliminates human enterovirus and prevents death of infected mice. EBioMedicine. 2023;93:104682. doi: 10.1016/j.ebiom.2023.104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morelli KH, Wu Q, Gosztyla ML, Liu H, Yao M, Zhang C, et al. An RNA-targeting CRISPR–Cas13d system alleviates disease-related phenotypes in Huntington’s disease models. Nat Neurosci. 2022;26:27–38. doi: 10.1038/s41593-022-01207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ghanbarian H, Aghamiri S, Eftekhary M, Wagner N, Wagner KD. Small activating RNAs: towards the development of new therapeutic agents and clinical treatments. Cells. 2021;10:591. doi: 10.3390/cells10030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meng X, Jiang Q, Chang N, Wang X, Liu C, Xiong J, et al. Small activating RNA binds to the genomic target site in a seed-region-dependent manner. Nucleic Acids Res. 2016;44:2274–82. doi: 10.1093/nar/gkw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–42. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yoon S, Rossi JJ. Therapeutic potential of small activating RNAs (saRNAs) in human cancers. Curr Pharmaceut Biotechnol. 2018;19:604–10. doi: 10.2174/1389201019666180528084059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarker D, Plummer R, Meyer T, Sodergren MH, Basu B, Chee CE, et al. MTL-CEBPA, a small activating RNA therapeutic upregulating C/EBP-α, in patients with advanced liver cancer: a first-in-human, multicenter, open-label, phase I trial. Clin Cancer Res. 2020;26:3936–46. doi: 10.1158/1078-0432.CCR-20-0414. [DOI] [PubMed] [Google Scholar]
- 155.Kingwell K. Small activating RNAs lead the charge to turn up gene expression. Nat Rev Drug Discov. 2021;20:573–4. doi: 10.1038/d41573-021-00127-2. [DOI] [PubMed] [Google Scholar]
- 156.Sasaki S, Sun R, Bui HH, Crosby JR, Monia BP, Guo S. Steric inhibition of 5′ UTR regulatory elements results in upregulation of human CFTR. Mol Ther. 2019;27:1749–57. doi: 10.1016/j.ymthe.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vickers TA, Wyatt JR, Burckin T, Bennett CF, Freier SM. Fully modified 2’ MOE oligonucleotides redirect polyadenylation. Nucleic Acids Res. 2001;29:1293–9. doi: 10.1093/nar/29.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liang XH, Shen W, Crooke ST. Specific increase of protein levels by enhancing translation using antisense oligonucleotides targeting upstream open frames. Adv Exp Med Biol. 2017;983:129–46. doi: 10.1007/978-981-10-4310-9_9. [DOI] [PubMed] [Google Scholar]
- 159.Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci U S A. 2009;106:7507–12. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liang XH, Shen W, Sun H, Migawa MT, Vickers TA, Crooke ST. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat Biotechnol. 2016;34:875–80. doi: 10.1038/nbt.3589. [DOI] [PubMed] [Google Scholar]
- 161.Zhang P, Park HJ, Zhang J, Junn E, Andrews RJ, Velagapudi SP, et al. Translation of the intrinsically disordered protein α-synuclein is inhibited by a small molecule targeting its structured mRNA. Proc Natl Acad Sci U S A. 2020;117:1457–67. doi: 10.1073/pnas.1905057117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Albers S, Allen EC, Bharti N, Davyt M, Joshi D, Perez-Garcia CG, et al. Engineered tRNAs suppress nonsense mutations in cells and in vivo. Nature. 2023;618:842–8. doi: 10.1038/s41586-023-06133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang J, Zhang Y, Mendonca CA, Yukselen O, Muneeruddin K, Ren L, et al. AAV-delivered suppressor tRNA overcomes a nonsense mutation in mice. Nature. 2022;604:343–8. doi: 10.1038/s41586-022-04533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dolgin E. tRNA therapeutics burst onto startup scene. Nat Biotechnol. 2022;40:283–6. doi: 10.1038/s41587-022-01252-y. [DOI] [PubMed] [Google Scholar]
- 165.Di Ruscio A, de Franciscis V. Minding the gap: unlocking the therapeutic potential of aptamers and making up for lost time. Mol Ther Nucleic Acids. 2022;29:384–6. doi: 10.1016/j.omtn.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adachi T, Nakamura Y. Aptamers: a review of their chemical properties and modifications for therapeutic application. Molecules. 2019;24:4229. doi: 10.3390/molecules24234229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ni S, Zhuo Z, Pan Y, Yu Y, Li F, Liu J, et al. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces. 2021;13:9500–19. doi: 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
- 168.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–32. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 169.Khanani AM, Patel SS, Staurenghi G, Tadayoni R, Danzig CJ, Eichenbaum DA, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402:1449–58. doi: 10.1016/S0140-6736(23)01583-0. [DOI] [PubMed] [Google Scholar]
- 170.Ellington AD, Szostak JW. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature. 1992;355:850–2. doi: 10.1038/355850a0. [DOI] [PubMed] [Google Scholar]
- 171.Zhou J, Rossi JJ. Cell-type-specific, aptamer-functionalized agents for targeted disease therapy. Mol Ther Nucleic Acids. 2014;3:e169. doi: 10.1038/mtna.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reid G, Kao SC, Pavlakis N, Brahmbhatt H, MacDiarmid J, Clarke S, et al. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics. 2016;8:1079–85. doi: 10.2217/epi-2016-0035. [DOI] [PubMed] [Google Scholar]
- 173.Jones D. Setbacks shadow microRNA therapies in the clinic. Nat Biotechnol. 2018;36:909–10. doi: 10.1038/nbt1018-909. [DOI] [PubMed] [Google Scholar]
- 174.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–50. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 177.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 178.Deng QW, Huang S, Li S, Zhai Q, Zhang Q, Wang ZJ, et al. Inflammatory factors as potential markers of early neurological deterioration in acute ischemic stroke patients receiving endovascular therapy – the AISRNA study. J Inflamm Res. 2021;14:4399–407. doi: 10.2147/JIR.S317147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics – challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mannion N, Arieti F, Gallo A, Keegan LP, O’Connell MA. New insights into the biological role of mammalian ADARs; the RNA editing proteins. Biomolecules. 2015;5:2338–62. doi: 10.3390/biom5042338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Booth BJ, Nourreddine S, Katrekar D, Savva Y, Bose D, Long TJ, et al. RNA editing: expanding the potential of RNA therapeutics. Mol Ther. 2023;31:1533–49. doi: 10.1016/j.ymthe.2023.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pecori R, Di Giorgio S, Paulo Lorenzo J, Nina Papavasiliou F. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat Rev Genet. 2022;23:505–18. doi: 10.1038/s41576-022-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pfeiffer LS, Stafforst T. Precision RNA base editing with engineered and endogenous effectors. Nat Biotechnol. 2023;41:1526–42. doi: 10.1038/s41587-023-01927-0. [DOI] [PubMed] [Google Scholar]
- 184.Abudayyeh OO, Gootenberg JS, Franklin B, Koob J, Kellner MJ, Ladha A, et al. A cytosine deaminase for programmable single-base RNA editing. Science. 2019;365:382–6. doi: 10.1126/science.aax7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lo N, Xu X, Soares F, He HH. The basis and promise of programmable RNA editing and modification. Front Genet. 2022;13:834413. doi: 10.3389/fgene.2022.834413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673–94. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nitika, Wei J, Hui AM. The delivery of mRNA vaccines for therapeutics. Life. 2022;12:1254. doi: 10.3390/life12081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paunovska K, Loughrey D, Dahlman JE. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23:265–80. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Foster DJ, Brown CR, Shaikh S, Trapp C, Schlegel MK, Qian K, et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther. 2018;26:708–17. doi: 10.1016/j.ymthe.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Egli M, Manoharan M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023;51:2529–73. doi: 10.1093/nar/gkad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eulberg D, Klussmann S. Spiegelmers: biostable aptamers. Chembiochem. 2003;4:979–83. doi: 10.1002/cbic.200300663. [DOI] [PubMed] [Google Scholar]
- 192.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim KQ, Burgute BD, Tzeng SC, Jing C, Jungers C, Zhang J, et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 2022;40:111300. doi: 10.1016/j.celrep.2022.111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morais P, Adachi H, Yu YT. The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Front Cell Dev Biol. 2021;9:789427. doi: 10.3389/fcell.2021.789427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nance KD, Meier JL. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent Sci. 2021;7:748–56. doi: 10.1021/acscentsci.1c00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mulroney TE, Pöyry T, Yam-Puc JC, Rust M, Harvey RF, Kalmar L, et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature. 2024;625:189–94. doi: 10.1038/s41586-023-06800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rhym LH, Anderson DG. Nanoscale delivery platforms for RNA therapeutics: challenges and the current state of the art. Med. 2022;3:167–87. doi: 10.1016/j.medj.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 198.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol. 2020;15:313–20. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang H, Leal J, Soto MR, Smyth HDC, Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12:1042. doi: 10.3390/pharmaceutics12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li B, Manan RS, Liang SQ, Gordon A, Jiang A, Varley A, et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat Biotechnol. 2023;41:1410–5. doi: 10.1038/s41587-023-01679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pardridge WM. Brain gene therapy with Trojan horse lipid nanoparticles. Trends Mol Med. 2023;29:343–53. doi: 10.1016/j.molmed.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chang RYK, Chan HK. Lipid nanoparticles for the inhalation of mRNA. Nat Biomed Eng. 2021;5:949–50. doi: 10.1038/s41551-021-00794-x. [DOI] [PubMed] [Google Scholar]
- 203.Wei X, Li Y, Cheng X, Wen Y, Yuan W, Chen R, et al. Increase nebulization stability of lipid nanoparticles by integrating a DNA supramolecular hydrogel. ACS Macro Lett. 2023;12:745–50. doi: 10.1021/acsmacrolett.3c00183. [DOI] [PubMed] [Google Scholar]
- 204.Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5:1059–68. doi: 10.1038/s41551-021-00786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miao H, Huang K, Li Y, Li R, Zhou X, Shi J, et al. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. Int J Pharm. 2023;640:123050. doi: 10.1016/j.ijpharm.2023.123050. [DOI] [PubMed] [Google Scholar]
- 206.Yuan W, Cheng J, Zhu C, Dong G, Zhao X, Meng S, et al. Preparing liposomes through frame guided assembly with high-loading functional nucleic acids. Nanoscale. 2023;15:9946–53. doi: 10.1039/d3nr01412f. [DOI] [PubMed] [Google Scholar]
- 207.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–63. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers. 2019;11:745. doi: 10.3390/polym11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li Y, Yuan W, Tian X, Zhu C, Li X, Chen R, et al. A facile method to prepare non-cationic mRNA lipid-nanoparticles based on frame guided assembly strategy. Nano Today. 2023;52:101991. doi: 10.1016/j.nantod.2023.101991. [DOI] [Google Scholar]
- 210.Biscans A, Coles A, Haraszti R, Echeverria D, Hassler M, Osborn M, et al. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 2019;47:1082–96. doi: 10.1093/nar/gky1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nagata T, Dwyer CA, Yoshida-Tanaka K, Ihara K, Ohyagi M, Kaburagi H, et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood–brain barrier and knock down genes in the rodent CNS. Nat Biotechnol. 2021;39:1529–36. doi: 10.1038/s41587-021-00972-x. [DOI] [PubMed] [Google Scholar]
- 212.Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16:440. doi: 10.1038/nrd.2017.86. [DOI] [PubMed] [Google Scholar]
- 213.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–94. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Monopoli KR, Korkin D, Khvorova A. Asymmetric trichotomous partitioning overcomes dataset limitations in building machine learning models for predicting siRNA efficacy. Mol Ther Nucleic Acids. 2023;33:93–109. doi: 10.1016/j.omtn.2023.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Han Y, He F, Chen Y, Liu Y, Yu H. SiRNA silencing efficacy prediction based on a deep architecture. BMC Genom. 2018;19:669. doi: 10.1186/s12864-018-5028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee M. Machine learning for small interfering RNAs: a concise review of recent developments. Front Genet. 2023;14:1226336. doi: 10.3389/fgene.2023.1226336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gao H, Kan S, Ye Z, Feng Y, Jin L, Zhang X, et al. Development of in silico methodology for siRNA lipid nanoparticle formulations. Chem Eng J. 2022;442:136310. doi: 10.1016/j.cej.2022.136310. [DOI] [Google Scholar]
- 218.Metwally AA, Nayel AA, Hathout RM. In silico prediction of siRNA ionizable-lipid nanoparticles in vivo efficacy: machine learning modeling based on formulation and molecular descriptors. Front Mol Biosci. 2022;9:1042720. doi: 10.3389/fmolb.2022.1042720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bashir A, Yang Q, Wang J, Hoyer S, Chou W, McLean C, et al. Machine learning guided aptamer refinement and discovery. Nat Commun. 2021;12:2366. doi: 10.1038/s41467-021-22555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Seok H, Lee H, Jang ES, Chi SW. Evaluation and control of miRNA-like off-target repression for RNA interference. Cell Mol Life Sci. 2018;75:797–814. doi: 10.1007/s00018-017-2656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Elmén J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33:439–47. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wu SY, Yang X, Gharpure KM, Hatakeyama H, Egli M, McGuire MH, et al. 2′-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat Commun. 2014;5:3459. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chang CI, Yoo JW, Hong SW, Lee SE, Kang HS, Sun X, et al. Asymmetric shorter-duplex siRNA structures trigger efficient gene silencing with reduced nonspecific effects. Mol Ther. 2009;17:725–32. doi: 10.1038/mt.2008.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bramsen JB, Laursen MB, Damgaard CK, Lena SW, Babu BR, Wengel J, et al. Improved silencing properties using small internally segmented interfering RNAs. Nucleic Acids Res. 2007;35:5886–97. doi: 10.1093/nar/gkm548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009;27:549–55. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ui-Tei K, Naito Y, Nishi K, Juni A, Saigo K. Thermodynamic stability and Watson–Crick base pairing in the seed duplex are major determinants of the efficiency of the siRNA-based off-target effect. Nucleic Acids Res. 2008;36:7100–9. doi: 10.1093/nar/gkn902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–7. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Judge AD, Bola G, Lee ACH, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 229.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–7. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 230.Singh RN, Singh NN. Mechanism of splicing regulation of spinal muscular atrophy genes. Adv Neurobiol. 2018;20:31–61. doi: 10.1007/978-3-319-89689-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Singh RN, Ottesen EW, Singh NN. The first orally deliverable small molecule for the treatment of spinal muscular atrophy. Neurosci Insights. 2020;15 doi: 10.1177/2633105520973985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zakeri SE, Pradeep SP, Kasina V, Laddha AP, Manautou JE, Bahal R. Casimersen for the treatment of Duchenne muscular dystrophy. Trends Pharmacol Sci. 2022;43:607–8. doi: 10.1016/j.tips.2022.04.009. [DOI] [PubMed] [Google Scholar]