Abstract
Introduction.
Oscillatory patterns in arterial pressure and blood flow (at ~0.1 Hz) may protect tissue oxygenation during conditions of reduced cerebral perfusion and/or hypoxia. We hypothesized that inducing oscillations in arterial pressure and cerebral blood flow at 0.1 Hz would protect cerebral blood flow and cerebral tissue oxygen saturation during exposure to a combination of simulated hemorrhage and sustained hypobaric hypoxia.
Methods.
Eight healthy human subjects (4 male, 4 female; 30.1 ± 7.6 year) participated in two experiments at high altitude (White Mountain, California, USA; altitude, 3800 m) following rapid ascent and 5–7 d of acclimatization: (1) static lower body negative pressure (LBNP, control condition) was used to induce central hypovolemia by reducing chamber pressure to −60 mmHg for 10 min (0 Hz), and; (2) oscillatory LBNP where chamber pressure was reduced to −60 mmHg, then oscillated every 5 s between −30 mmHg and −90 mmHg for 10 min (0.1 Hz). Measurements included arterial pressure, internal carotid artery (ICA) blood flow, middle cerebral artery velocity (MCAv), and cerebral tissue oxygen saturation (ScO2).
Results.
Forced 0.1 Hz oscillations in mean arterial pressure and mean MCAv were accompanied by a protection of ScO2 (0.1 Hz: −0.67% ± 1.0%; 0 Hz: −4.07% ± 2.0%; P = 0.01). However, the 0.1 Hz profile did not protect against reductions in ICA blood flow (0.1 Hz: −32.5% ± 4.5%; 0 Hz: −19.9% ± 8.9%; P = 0.24) or mean MCAv (0.1 Hz: −18.5% ± 3.4%; 0 Hz: −15.3% ± 5.4%; P = 0.16).
Conclusions.
Induced oscillatory arterial pressure and cerebral blood flow led to protection of ScO2 during combined simulated hemorrhage and sustained hypoxia. This protection was not associated with the preservation of cerebral blood flow suggesting preservation of ScO2 may be due to mechanisms occurring within the microvasculature.
Keywords: cerebral blood flow, hemodynamic oscillations, hypoxia
Introduction
Adjustment of cerebral blood flow is a key mechanism for maintaining adequate oxygen delivery during challenges to cerebral oxygen availability. In addition to increases in absolute blood flow, the pattern of blood flow delivered to the brain may also influence the maintenance of oxygen within the brain tissues. Evidence for the role of oscillations in the maintenance of tissue oxygenation has slowly accumulated within the last 30 years (Tsai and Intaglietta 1993, Goldman and Popel 2001, Anderson et al 2019).
Tsai and Intaglietta were the first to postulate, and then mathematically simulate, the role of vasomotion and oscillatory blood flow on the preservation of tissue oxygen delivery (Intaglietta 1990). Their early models demonstrated a protection of tissue oxygenation when vasomotion was active (Tsai and Intaglietta 1993). This phenomenon was later confirmed through a series of mathematical simulations of oxygen transport through capillaries by Goldman and Popel (2001). Using a model designed from the capillary network within a hamster cheek-pouch retractor muscle, vasomotion (and the resulting oscillatory blood flow) was simulated between 1.5 and 12 cycles per minute (0.025–0.2 Hz) in hypoxic tissues with and without myoglobin. Oscillatory blood flow was effective in improving tissue oxygen delivery when oscillations in blood flow were between 1.5 and 6 cycles per minute (0.025–0.1 Hz), with the most prominent effect observed when tissues lacked myoglobin, so had no internal oxygen stores (Goldman and Popel 2001). In a rat model of reduced muscle tissue perfusion via tourniquet aided constriction of the left femoral artery, the effects of spontaneously occurring vasomotion of about 2 cycles per minute (~0.03 Hz) were compared to when vasomotion was inhibited by calcium-channel blockade (Rücker et al 2000). When present, vasomotion preserved functional capillary density in the surrounding tissues, demonstrating a protection of perfusion to the tissue even when blood flow was reduced (Rücker et al 2000). In human models, oscillations in arterial pressure and cerebral blood flow around 0.1 Hz have been implicated in increased tolerance to central hypovolemia (Rickards et al 2011, Lucas et al 2013, Anderson et al 2019, Hockin and Claydon 2020). During experimentally induced central hypovolemia (via application of lower body negative pressure, LBNP), preservation of brain tissue oxygenation is challenged by a reduction in cerebral blood flow (i.e. oxygen delivery) (Kay and Rickards 2016, Kaur et al 2018). We previously reported the protection of cerebral tissue oxygen saturation by forcing 0.1 Hz oscillations in arterial pressure and cerebral blood flow with LBNP under normoxic conditions (Anderson et al 2019). However, oxygenation of the tissues can also be challenged under hypoxic conditions, including clinical conditions where gas exchange is impaired, and with exposure to high altitude.
Hypoxia stimulates cyclical vasomotion and may be an important compensatory response to the low oxygen conditions (Intaglietta 1990, Bertuglia et al 1991). High altitude exposure imposes a chronic and systemic hypoxic challenge to the body, with reductions in the atmospheric partial pressure of oxygen and subsequent decreases in arterial oxygen saturation, oxygen content, and delivery. Salvi et al reported an increase in vasomotion in the skin microvasculature of the human forearm centered around 0.1 Hz (measured by laser Doppler flux) during high altitude ascent up to 5050 m (Salvi et al 2018). However, the role of 0.1 Hz oscillatory arterial pressure and blood flow on brain tissue oxygenation under the combined stressors of hypoxia and hypovolemia has not been explored. Understanding these responses may assist in the development of potential therapies for clinical use. Generating oscillations in blood flow could be an effective treatment for clinical conditions such as hemorrhagic shock, stroke, and sepsis—all of which challenge the delivery of oxygen to metabolically active tissues due to reduced blood flow and/or reduced arterial oxygen content.
In this study, we examined the effects of inducing 0.1 Hz oscillations in arterial pressure and cerebral blood flow on cerebral tissue oxygenation during the combined stressors of central hypovolemia and sustained hypobaric hypoxia. We tested the hypothesis that inducing oscillations in arterial pressure and cerebral blood flow at 0.1 Hz would protect cerebral blood flow and cerebral tissue oxygen saturation during exposure to a combination of simulated hemorrhage and sustained hypobaric hypoxia via ascent and partial acclimatization to high altitude.
Methods
Ethical approval
This study was conducted in accordance with the Canadian Government Tri-Council Policy on research with human participants, consistent with the Declaration of Helsinki, except for registration in a database. Ethical approval was received from the Mount Royal University Human Research Ethics Board (Protocol 101879), the University of Calgary Conjoint Health Research Ethics Board (Protocol REB18–0374), and the University of North Texas Health Science Center (Protocol 2019–110). This study was a part of a large high altitude research expedition to the Barcoft Laboratory on White Mountain in the Sierra Nevada Mountains, CA, USA in August 2019. However, the specific questions and participant recruitment for this study were determined a priori.
Participants
Young healthy, human participants were recruited for two experimental sessions, both conducted at high altitude (details to follow). Participants were briefed on the purpose, design, and risks of the study, and written informed consent was obtained. Prior to experimentation, participant height, weight, age, and sex were recorded. Female participants completed a urine pregnancy test to ensure they were not pregnant prior to each experimental session. It was not possible to control for menstrual cycle phase due to the nature of this expedition study. All participants abstained from alcohol, exercise, caffeine, dietary supplements, and medications for at least 12 h before experimentation.
Instrumentation
Upon arrival for each experiment, participants laid supine in a LBNP chamber with their iliac crest in line with the chamber opening. They were sealed into the chamber around the waist with heavy-duty plastic, and a neoprene band. Participants were then instrumented for continuous ECG recordings in a lead II configuration (shielded leads, cable and amplifier; AD Instruments, Bella Vista, NSW, Australia), and continuous arterial pressure was monitored and recorded using finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). Aortic diameter was measured (average of 2 measurements) using ultrasound (Terason uSmart 3300, Teratech, Burlington, MA, USA) with a 5 MHz curvilinear probe (5C2), which was subsequently used to correct the Modelflow® stroke volume measurements obtained from the Finometer prior to data collection.
Respiratory gases were monitored using an oral nasal cannula connected to a gas analyzer (ML206 Gas Analyzer; AD Instruments) and used to calculate respiratory rate, pressure of end-tidal CO2 (PETCO2), and O2 (PETO2) corrected for BTPS using atmospheric pressure. Arterial oxygen saturation (SpO2) was continuously monitored via pulse oximetry (Nonin 7500 FO, Nonin Medical Inc., MN, USA).
Oxy-hemoglobin and deoxy-hemoglobin concentrations were measured via near infrared spectroscopy (OxiplexTS; ISS, Champaign-Urbana, IL, USA) in cerebral tissue over the right frontal lobe, and over the flexor carpi ulnaris muscle of the forearm (located by flexion of the fingers). Cerebral and muscle total hemoglobin concentration (THC; oxy-hemoglobin + deoxy-hemoglobin), cerebral tissue oxygen saturation (ScO2; (oxy-hemoglobin/THC)*100), and muscle tissue oxygen saturation (SmO2) were then calculated.
Middle cerebral artery velocity (MCAv) was measured using transcranial Doppler ultrasound and a 2 MHz probe through a temporal window (ST3; Spencer Technologies, Seattle, WA, USA). Duplex Doppler ultrasound (Terason uSmart 3300, Teratech, Burlington, MA, USA) was used to measure internal carotid artery (ICA) diameter and velocity with a 15 MHz linear array probe (15L4 Smart Mark™). Two trained sonographers performed the measurements ensuring that repeated measures on each participant were performed by the same sonographer. A consistent ICA image was ensured between experiments by noting the position in reference to the carotid bifurcation, and the blood velocity profile at rest (Thomas et al 2015). Video recordings of the ultrasound measures were captured using screen recording software (Camtasia, Techsmith Corp, MI, USA) and stored as AVI files for later analysis (see ‘Data Analysis’ section for details).
Hemoglobin (Hb) concentration was measured in participants the morning of each experimental day using finger capillary blood sampling and a hemoglobinometer (Hemocue Hemoglobin System, Hb201 + with microcuvettes; Ängelholm, Sweden).
Experimental design
The combined stressors of hypobaric hypoxia (high altitude exposure) and central hypovolemia (via LBNP) were used in order to assess the effects of oscillatory blood flow when both cerebral blood flow and cerebral tissue oxygen saturation are reduced.
High altitude
Prior to exposure to altitude, all participants lived at or below an altitude of 1045 m and had not traveled to high altitude within the 6 months before the study. All participants flew from Calgary, Canada (1045 m) to Las Vegas, NV, USA (610 m). Less than 12 h after arrival in Las Vegas, participants rapidly ascended to 3800 m (Barcroft Laboratory, White Mountain, California) within 6 h. Participants acclimatized to this high altitude environment for 4–5 d, and were subsequently tested on days 5, 6 and 7 of altitude exposure for this study (figure 1).
Figure 1.
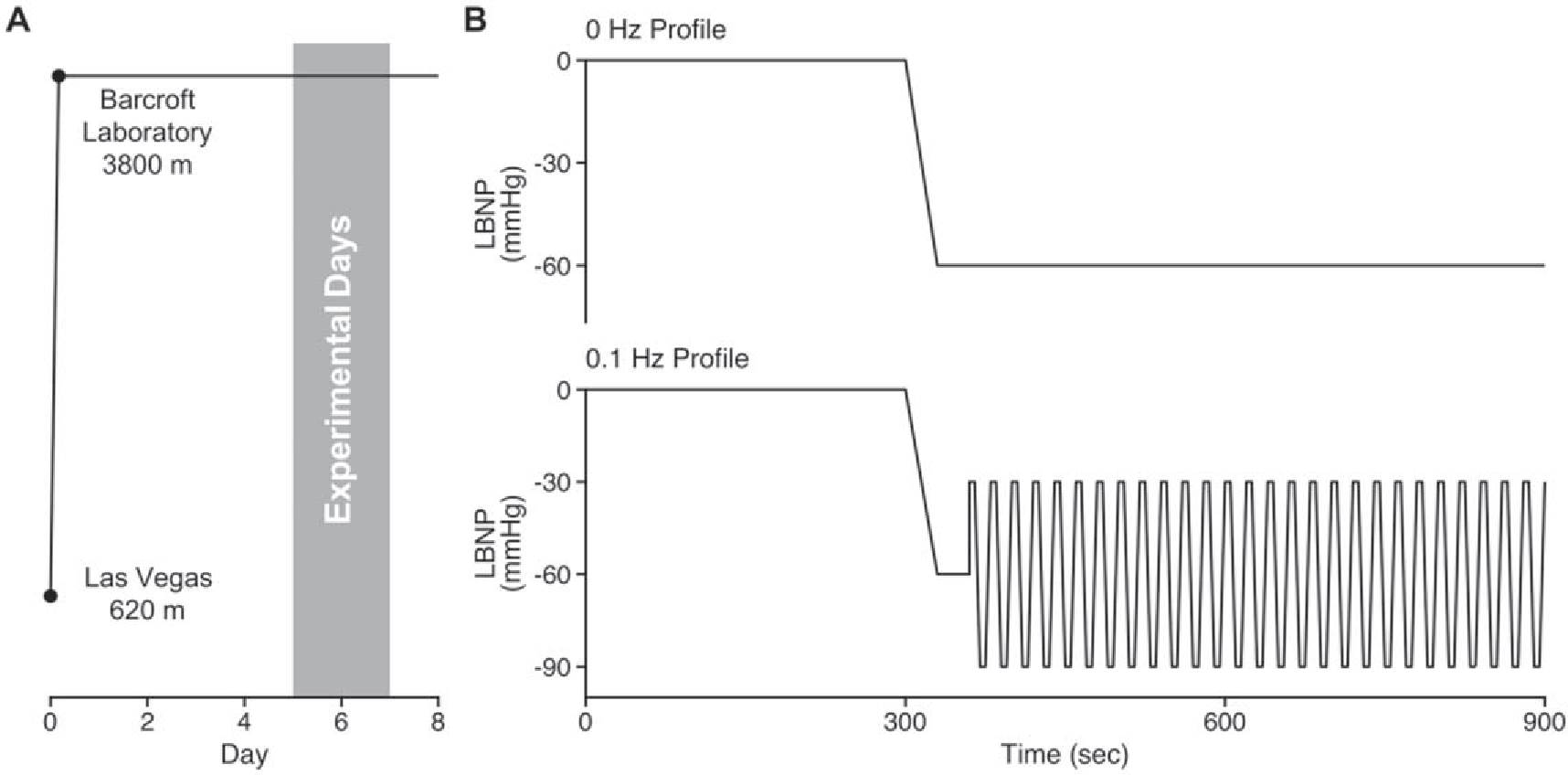
The ascent profile with an ascent of about 4 h on day 0 followed by the timeline of experimentation (A). The representative tracings of the 0 and 0.1 Hz lower body negative pressure (LBNP) profiles (B).
Lower body negative pressure
After instrumentation, a 5 min baseline was recorded, and one of two 10 min LBNP protocols was initiated: (1) a non-oscillatory (0 Hz) condition where chamber pressure was lowered to −60 mmHg over 30 s and maintained at this pressure for 9.5 min, or (2) an oscillatory (0.1 Hz) condition where chamber pressure was lowered to −60 mmHg over 30 s, held at −60 mmHg for 30 s, and then oscillated at 0.1 Hz between −30 mmHg and −90 mmHg for 9 min with 5 s at each pressure (figure 1). The protocol was terminated if the participant’s systolic arterial pressure fell below 80 mmHg, or at the participant’s request due to symptoms of presyncope such as nausea, dizziness, lightheadedness, and loss of color vision. For the 0 Hz condition, if participants completed the 10 min stage of LBNP at −60 mmHg, the chamber was reduced to −70, −80, −90, and −100 mmHg for 5 min each until the onset of presyncope. The data collected for these additional steps are the focus of a separate manuscript and will not be reported here. A 10 min recovery period followed the completion of each LBNP protocol. The order of LBNP profiles was randomized and counterbalanced between experimental sessions. At least 24 h separated the two sessions.
Data analysis
ECG, arterial pressure, stroke volume, MCAv, ScO2, SmO2, SpO2, PETCO2 and PETO2 waveform data were continuously recorded at 1000 Hz (PowerLab/LabChart; AD Instruments) and stored for subsequent analysis. Specialized software (WinCPRS; Absolute Aliens, Turku, Finland) was used for beat-to-beat analysis of hemodynamic data in the time and frequency domains. The final 3 min of the baseline period was used for averaging of time domain measures, while the entire 5 min baseline period was used for frequency domain measures. During LBNP, time domain measures were averaged over the 1 min prior to LBNP release, while frequency domain measures were averaged over the 5 min prior to LBNP release.
R-waves were detected from the ECG recording and used for the calculation of heart rate, and for dividing all other waveform data into cardiac cycles for subsequent analysis. Beat-to-beat systolic and diastolic arterial pressures and cerebral blood velocities were then identified, and mean arterial pressure (MAP) and mean MCAv were calculated using the area under the curve. Cardiac output was calculated by multiplying heart rate by stroke volume, and systemic vascular resistance was calculated by dividing MAP by cardiac output.
Arterial oxygen content was calculated with the following equation:
where was used as a surrogate for PaO2. Simultaneous diameter and blood velocity measurements in the ICA were obtained using specialized wall tracking software, as previously reported (Woodman et al 2001). A minimum of 10 consecutive cardiac cycles were used to average ICA blood velocity and diameter during the last 5 min of each LBNP protocol, as per published guidelines (Thomas et al 2015). The anatomical location of the diameter measurements was matched between experimental sessions during analysis. These data were then used to calculate ICA blood flow as: . Delivery of oxygen () through the ICA was calculated as:
The MCA conductance index and ICA conductance were calculated by dividing mean MCAv or ICA flow by MAP.
The oscillatory characteristics of MAP and MCAv were determined using the fast Fourier-transformation as previously described from our laboratory (Rickards et al 2015). Briefly, data were made equidistant by interpolating linearly and resampling at 5 Hz. Data were then passed through a low-pass impulse response filter using a cut-off frequency of 0.5 Hz. Fast Fourier-transformation was performed using a Hanning window and the Welch Method to compute power spectra. The frequency range used for low frequency (LF) power spectral analysis was 0.07–0.15 Hz. Spectral power is expressed as the integrated area within this range. Spectral power was also calculated as a point measurement at 0.1 Hz.
For each subject and each condition, the percent change from baseline was calculated for any given variable by taking the difference between baseline and LBNP values divided by the baseline value and multiplied by 100.
Statistical analysis
Absolute data were analyzed using a linear mixed model for repeated measures with time point (baseline or hypovolemia) and LBNP condition (0 or 0.1 Hz) as factors in the model followed by Tukey post-hoc tests (JMP, SAS Institute, Cary, NC). The percent change between baseline and LBNP was calculated for the two protocols and compared using paired t-tests after testing for normality with the Shapiro-Wilk test to ensure normal distribution of data. Paired t-tests were also used to compare baseline hemoglobin concentration, chamber pressures, and LBNP experimental time between the two protocols. All data are presented as mean ± standard deviation unless otherwise stated. Exact P-values are reported for all comparisons.
Results
Participants
Nine participants were initially recruited in the study, but one participant was unable to complete the experiments due to mild high altitude illness. Eight individuals (four males, four females) participated in both LBNP conditions (table 1). All participants completed the experiments on days 5–7 of high altitude exposure, with at least 24 h between experiments.
Table 1.
Participant demographics.
| Total group (N = 8) | Range | Males (N = 4) | Females (N = 4) | |
|---|---|---|---|---|
|
| ||||
| Age (year) | 30.1 ± 7.6 | 22–42 | 26.0 ± 4.5 | 34.3 ± 8.3 |
| Height (cm) | 173.0 ± 13.8 | 150.0–188.0 | 184.8 ± 2.4 | 161.3 ± 8.3 |
| Weight (kg) | 72.4 ± 14.5 | 52.2–99.0 | 83.0 ± 11.6 | 61.9 ± 7.5 |
| BMI (kgm−2) | 24.2 ± 4.2 | 18.1–31.1 | 24.3 ± 3.6 | 24.2 ± 5.4 |
| Aortic diameter (mm) | 21.8 ± 0.8 | 19.2–25.5 | 23.8 ± 1.1 | 19.7 ± 0.5 |
Note. Data are mean ± standard deviation.
LBNP results
Average LBNP chamber pressure was slightly lower during the 0.1 Hz protocol by about 6 mmHg (0 Hz, −59.9 ± 0.4 versus 0.1 Hz, 66.6 ± 2.7 mmHg; P <0.01). Six of the eight participants completed the entire 0 Hz profile without becoming presyncopal, and seven participants completed the 0.1 Hz profile, with no difference in completion times (0 Hz, 880.5 ± 40.5 s versus 0.1 Hz, 894.4 ± 15.8 s; P = 0.18). Data from all eight participants in both trials are included in the analysis. A representative figure of physiological signals acquired is shown in figure 2.
Figure 2.
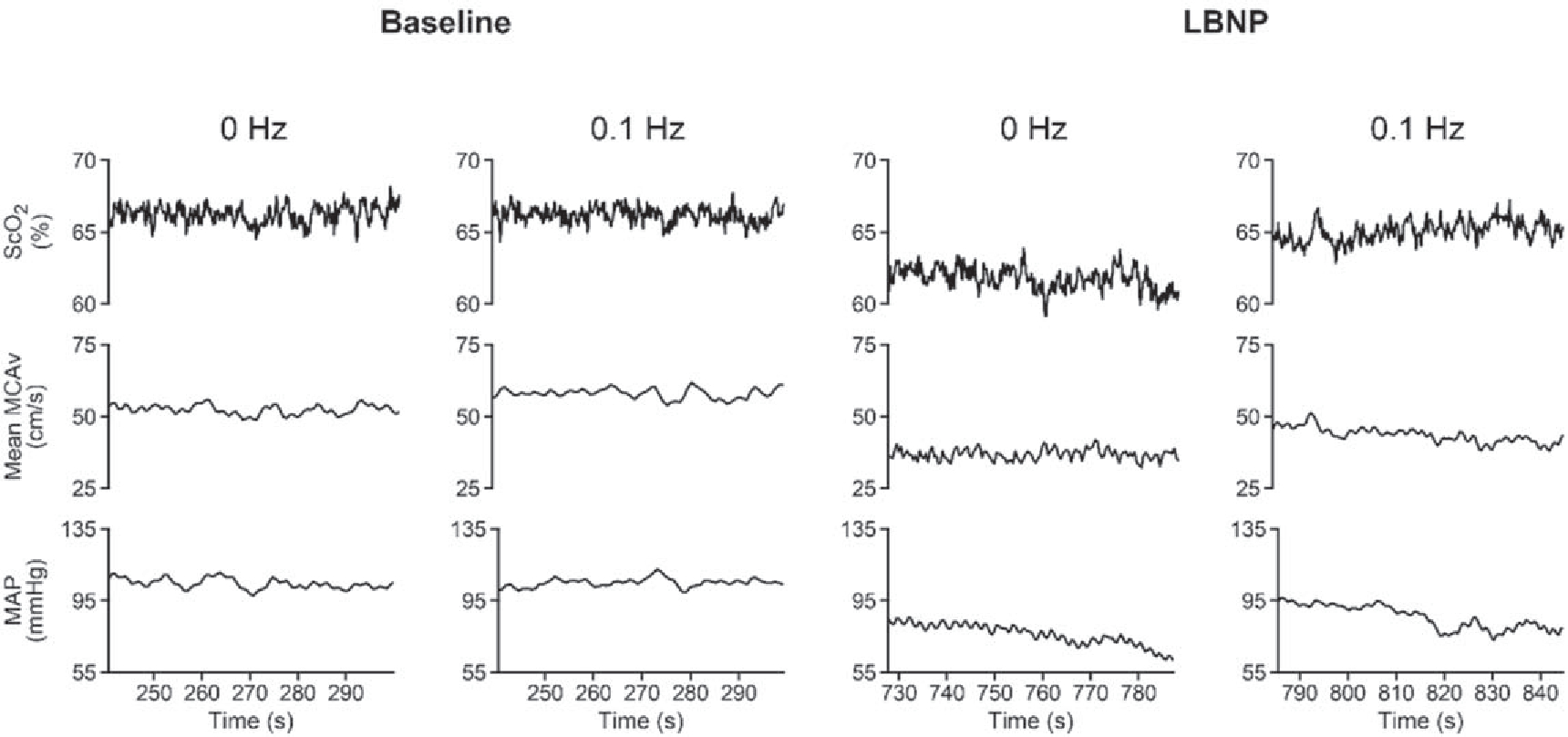
A representative tracing of physiological signals from one subject at baseline, and during the final minute of the 0 and 0.1 Hz lower body negative pressure (LBNP) profiles. ScO2, cerebral oxygen saturation; MCAv, middle cerebral artery velocity; MAP, mean arterial pressure.
Cardiovascular hemodynamic responses
Hemoglobin concentrations measured from the morning of each experiment were not different (P = 0.32; table 2). During the baseline period, prior to application of LBNP, there were no differences in any systemic hemodynamic variables between experimental conditions (‘Cardiovascular’ variables in table 2). When assessing the systemic hemodynamic responses to central hypovolemia with or without 0.1 Hz oscillations, no differences were observed in the responses of stroke volume, heart rate, MAP, or systemic vascular resistance, indicating a similar cardiovascular challenge between profiles (table 2, figure 3). Compared with the 0 Hz condition, PETCO2 was generally higher for the 0.1 Hz condition (LBNP effect, P = 0.04), accompanied by a lower PETO2 (LBNP effect, P = 0.03) and SpO2 (LBNP effect, P = 0.03), despite similar respiration rates (‘Respiratory’ variables in table 2). During LBNP for both conditions, SpO2 increased, although there were no increases in respiration rate (table 2). SmO2 decreased during the 0 Hz condition but this decrease was attenuated during the 0.1 Hz profile (table 2, figure 3).
Table 2.
Time domain physiological responses at baseline and during LBNP.
| Baseline |
LBNP |
P-values |
|||||
|---|---|---|---|---|---|---|---|
| 0 Hz | 0.1 Hz | 0 Hz | 0.1 Hz | Time | LBNP | Interaction | |
|
| |||||||
| Cardiovascular | |||||||
| Heartrate (bpm) | 79.9 ± 11.9 | 79.0 ± 10.2 | 110.6 ± 22.2 * | 106.4 ± 19.8 * | <0.01 | 0.54 | 0.69 |
| Stroke volume (ml) | 82.7 ± 18.6 | 82.8 ± 22.9 | 45.3 ± 16.8 * | 48.2 ± 23.7 * | <0.01 | 0.72 | 0.75 |
| Cardiac output (l min−1) | 6.5 ± 1.2 | 6.5 ± 1.6 | 4.7 ± 1.2 * | 4.7 ± 1.4 * | <0.01 | 0.98 | 0.99 |
| Systolic arterial pressure (mmHg) | 130.8 ± 11.1 | 127.2 ± 12.9 | 108.4 ± 10.5 * | 105.1 ± 7.6 * | <0.01 | 0.29 | 0.97 |
| Diastolic arterial pressure (mmHg) | 75.8 ± 10.3 | 74.8 ± 10.6 | 73.4 ± 8.4 | 72.2 ± 8.1 | 0.16 | 0.53 | 0.94 |
| Mean arterial pressure (mmHg) | 96.8 ± 10.5 | 95.4 ± 11.5 | 86.1 ± 8.2 * | 84.2 ± 6.4 * | <0.01 | 0.45 | 0.92 |
| Systemic vascular resistance (mmHg/(1 min−1)) | 15.5 ± 3.8 | 16.0 ± 6.3 | 19.1 ± 4.9 | 19.6 ± 7.4 | 0.02 | 0.76 | 0.99 |
| Cerebrovascular | |||||||
| Cerebral total hemoglobin (μM) | 47.1 ± 4.6 | 48.2 ± 7.2 | 45.7 ± 4.7 | 46.0 ± 8.2 | 0.24 | 0.44 | 0.97 |
| Cerebral oxygenated hemoglobin (μM) | 31.2 ± 4.5 | 32.2 ± 6.1 | 29.1 ± 4.4 | 30.4 ± 6.8 | 0.16 | 0.32 | 0.67 |
| Cerebral deoxygenated hemoglobin (μM) | 15.9 ± 1.4 | 16.0 ± 2.0 | 16.6 ± 1.5 | 15.6 ± 1.95 | 0.87 | 0.63 | 0.09 |
| Systolic MCAv (cm s −1) | 81.1 ± 11.0 | 89.1 ± 17.2 | 66.1 ± 15.6 * | 62.9 ± 17.1 * | <0.01 | 0.16 | 0.12 |
| Diastolic MCAv (cm s−1) | 42.9 ± 8.7 | 46.9 ± 9.0 | 38.8 ± 10.6 | 35.4 ± 10.7 * | <0.01 | 0.57 | 0.18 |
| MCA conductance index (cm s−1 mmHg−1) | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 * | 0.01 | 0.33 | 0.15 |
| ICA diameter (cm) | 0.57 ± 0.11 | 0.57 ± 0.10 | 0.57 ± 0.12 | 0.56 ± 0.12 * | 0.05 | 0.46 | 0.06 |
| Peak ICA velocity (cm s−1) | 41.1 ± 6.9 | 38.6 ± 6.3 | 31.9 ± 9.6 * | 29.3 ± 8.3 * | <0.01 | 0.55 | 0.98 |
| ICA conductance (ml min −1 mmHg −1) | 3.3 ± 1.2 | 3.2 ± 1.0 | 2.7 ± 0.9 | 2.5 ± 1.1 | 0.01 | 0.96 | 0.75 |
| Peripheral vascular | |||||||
| SmO2 (%) | 75.3 ± 7.2 | 70.6 ± 5.9 | 66.3 ± 8.2 * | 65.8 ± 4.2 | <0.01 | 0.18 | 0.28 |
| Muscle total hemoglobin (μM) | 87.2 ± 22.0 | 83.4 ± 25.3 | 84.2 ± 24.5 | 80.8 ± 25.7 | 0.43 | 0.31 | 0.95 |
| Muscle oxygenated hemoglobin (μM) | 65.2 ± 15.3 | 59.2 ± 20.0 | 54.5 ± 12.3 | 53.1 ± 17.5 | 0.03 | 0.31 | 0.52 |
| Muscle deoxygenated hemoglobin (μM) | 22.0 ± 10.5 | 24.2 ± 7.7 | 29.7 ± 14.9 * | 27.7 ± 9.2 | <0.01 | 0.97 | 0.26 |
| SpO2 (%) | 90.4 ± 2.4 | 88.2 ± 2.3 | 93.9 ± 3.8 * | 92.9 ± 2.9 * | <0.01 | 0.03 | 0.41 |
| Blood hemoglobin concentration(gl−1) | 162.3 ± 14.3 | 157.1 ± 22.1 | — | — | 0.32 (paired t-test) | ||
| Respiratory | |||||||
| PETCO2 (mmHg) | 25.1 ± 3.7 | 27.4 ± 1.7 | 19.4 ± 5.0 | 21.1 ± 3.5 | <0.01 | 0.04 | 0.71 |
| PETO2 (mmHg) | 60.5 ± 4.4 | 57.1 ± 2.9 | 69.7 ± 7.0 * | 67.3 ± 5.8 * | <0.01 | 0.03 | 0.68 |
| Respiration rate (breaths min−1) | 15.3 ± 3.9 | 14.8 ± 4.1 | 15.1 ± 4.7 | 15.6 ± 6.4 | 0.69 | 0.98 | 0.51 |
Note. ScO2, cerebral tissue oxygen saturation; MCAv, middle cerebral artery velocity; ICA, internal carotid artery; SmO2, muscle tissue oxygen saturation; SpO2, arterial oxygen saturation; PETCO2, end-tidal carbon dioxide; PETO2, end-tidal oxygen. All data are presented as mean ± standard deviation. Data analyzed using a linear mixed model for repeated measures, with Tukey post-hoc tests, unless otherwise stated.
0.0001 ⩽ P ⩽ 0.06 versus baseline within condition.
Figure 3.
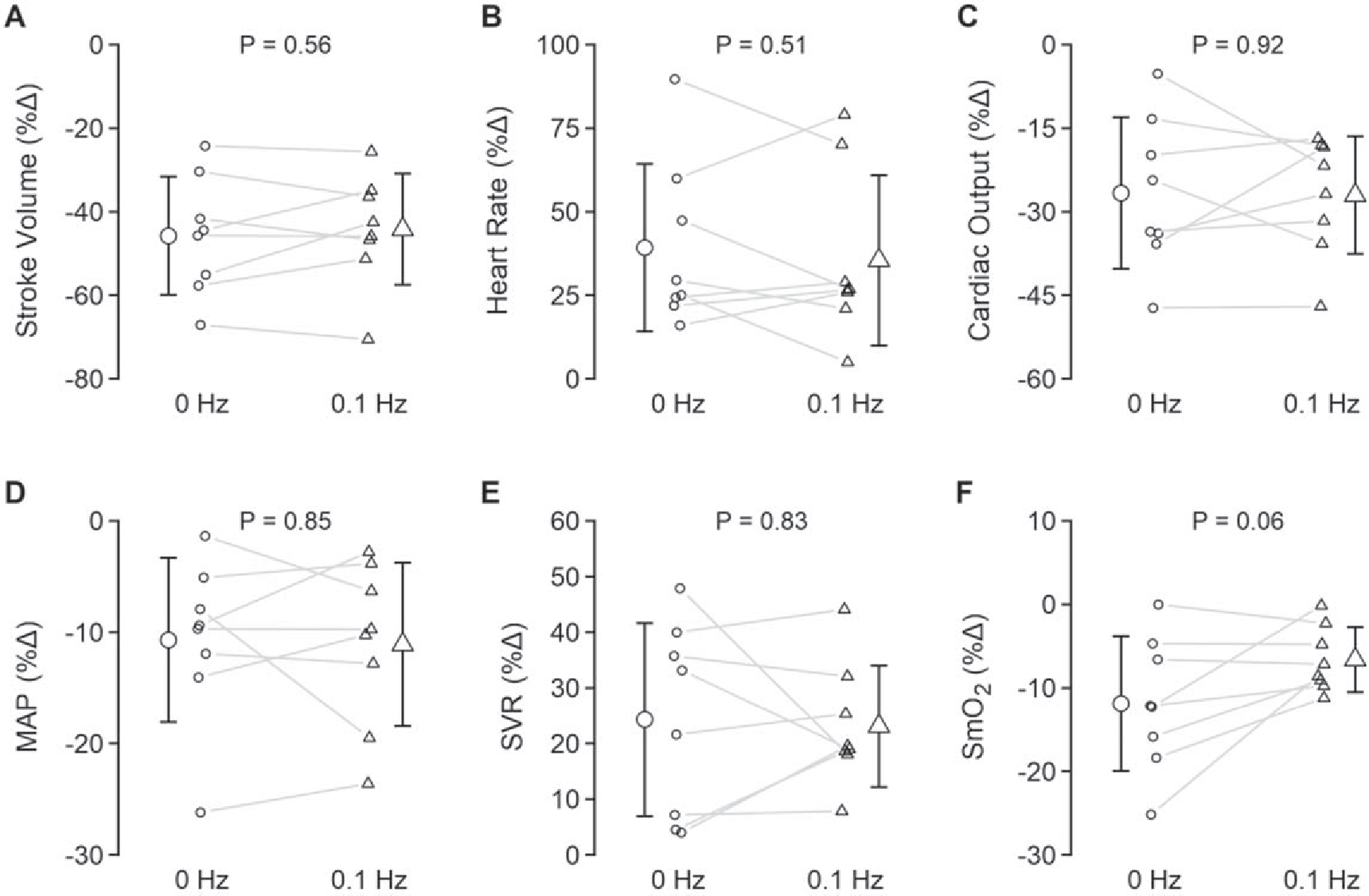
Relative systemic hemodynamic responses during 0 Hz and 0.1 Hz LBNP. MAP, mean arterial pressure; SVR, systemic vascular resistance; SmO2, muscle tissue oxygen saturation. All data are presented as mean ± standard deviation. Data analyzed with paired t-tests. Exact p-values are reported in the figure.
Cerebral hemodynamic responses
Baseline measurements of mean MCAv and ICA blood flow were no different between conditions (P ⩾ 0.38); figures 4(C) and (E)). MCAv recordings were obtained in seven participants during LBNP due to the loss of signal in one participant from headset movement upon onset of LBNP, and ICA blood flow recordings were obtained in five participants during LBNP with two recordings lost due to technical malfunctions and poor recording quality in one participant. ICA velocity decreased with LBNP (time effect, P < 0.01) while a small decrease in diameter was observed only during the 0.1 Hz profile (table 2). MCAv and ICA blood flow decreased in both LBNP conditions, with no differences in responses between 0 and 0.1 Hz LBNP (figures 4(C) and (E)). CaO2 was higher overall for the 0 Hz condition but increased with both LBNP profiles (figure 4(G)). Unilateral DO2 through the ICA decreased by a similar magnitude with both LBNP profiles (figure 4(I)). Despite these similar responses in cerebral blood flow and oxygen delivery between profiles, reductions in ScO2 were attenuated during the 0.1 Hz condition (figures 4(A) and (B)). Importantly, this pattern of preserved ScO2 during the 0.1 Hz condition was consistently observed for every participant.
Figure 4.
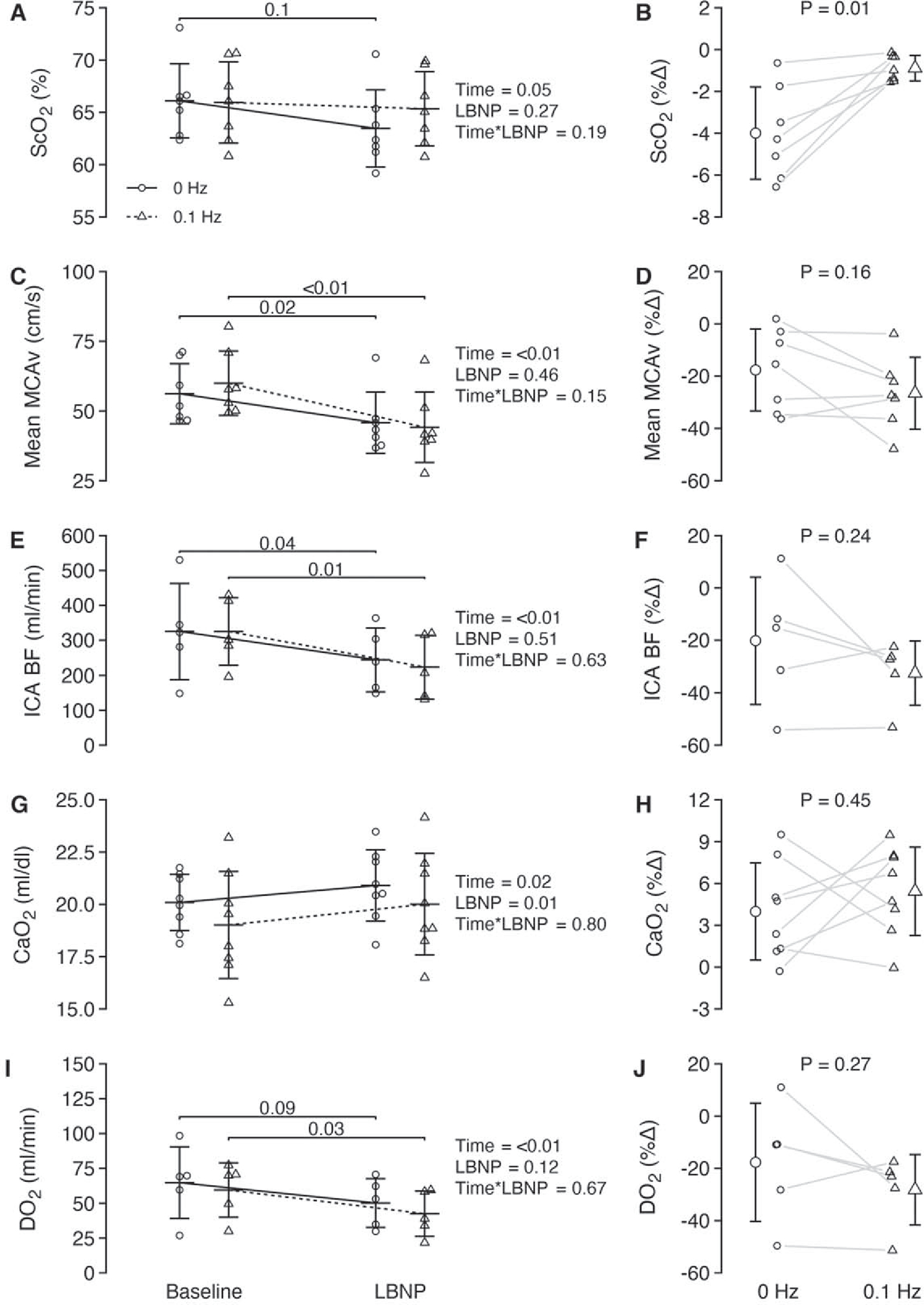
Absolute and relative cerebral tissue oxygenation and cerebral hemodynamic responses during 0 and 0.1 Hz LBNP. ScO2, cerebral tissue oxygen saturation; MCAv, middle cerebral artery velocity; ICA BF, internal carotid artery blood flow; CaO2, arterial oxygen content; DO2, unilateral delivery of oxygen through the internal carotid artery. All data are presented as mean ± standard deviation. Absolute data were analyzed using a linear mixed model for repeated measures followed by Tukey post-hoc tests ((A), (C), (E), (G), (I)). Percent change values in the right column ((B), (D), (F), (H), (J)) were analyzed using paired t-tests. Exact p-values are reported in the figure.
Frequency domain responses
A relative increase in amplitude of oscillations was observed at 0.1 Hz for MAP during the 0.1 Hz condition, consistent with the experimental design (table 3). Oscillations in PETCO2 were also explored as these may have contributed to oscillations in MCAv. Amplitude of PETCO2 oscillations was higher overall for the 0.1 Hz versus 0 Hz condition, with no difference over time (table 3).
Table 3.
Frequency domain physiological responses at baseline and during LBNP.
| Baseline |
LBNP |
P-values |
|||||
|---|---|---|---|---|---|---|---|
| 0Hz | 0.1 Hz | 0 Hz | 0.1 Hz | Time | LBNP | Interaction | |
|
| |||||||
| Power—LF range (0.07-0.15 Hz) | |||||||
| Meanarterial pressure (mmHg2) | 4.9 ± 3.9 | 4.0 ± 1.8 | 11.5 ± 11.0 | 13.3 ± 11.25 * | <0.01 | 0.83 | 0.51 |
| Mean arterial pressure (%Δ) | — | — | 121.9 ± 119.9 | 248.7 ± 219.5 | 0.18 (paired t-test) | ||
| Mean MCAv (cm s−1)2 | 2.2 ± 1.4 | 2.3 ± 1.3 | 2.9 ± 2.6 | 4.9 ± 3.1 *† | 0.02 | 0.02 | 0.02 |
| Mean MCAv (%Δ) | — | — | 44.6 ± 129.0 | 203.1 ± 147.7 | 0.07 (paired t-test) | ||
| PETCO2 (mmHg) | 1.1 ± 0.7 | 2.0 ± 1.1 † | 0.6 ± 0.4 | 1.7 ± 0.7 † | 0.10 | <0.01 | 0.71 |
| Power—0.1 Hz peak | |||||||
| Mean arterial pressure (mmHg2) | 145.5 ± 162.56 | 64.1 ± 52.68 | 160.0 ± 214.2 | 894.3 ± 1074.9 *† | 0.04 | 0.10 | 0.04 |
| Mean arterial pressure (%Δ) | — | — | 20.5 ± 52.9 | 1462.3 ± 1412.5 | 0.02 (paired t-test) | ||
| Mean MCAv (cm s−1)2 | 57.4 ± 54.0 | 38.2 ± 25.4 | 40.5 ± 42.3 | 348.1 ± 313.4 *† | 0.02 | 0.02 | 0.01 |
| Mean MCAv (%Δ) | — | — | 104.8 ± 244.8 | 1486.9 ± 1362.2 | 0.07 (paired t-test) | ||
Note. MCAv, middle cerebral artery velocity; PETCO2, end-tidal carbon dioxide. All data are presented as mean ± standard deviation. Data analyzed using a linear mixed model for repeated measures, with Tukey post-hoc tests, unless otherwise stated.
0.005 ⩽ P ⩽ 0.04 versus baseline within condition.
10.008 ⩽ P ⩽ 0.07 versus 0 Hz at that time point.
Discussion
The aim of this study was to assess the effects of 0.1 Hz hemodynamic oscillations on cerebral tissue oxygenation and cerebral blood flow during the combined stressors of simulated hemorrhage and sustained hypobaric hypoxia with high altitude exposure. The main finding of our study is that oscillations in cerebral blood flow at 0.1 Hz protected against reductions in cerebral tissue oxygenation. Other important findings include: (1) hemodynamic oscillations did not protect against reductions in cerebral blood flow whether indexed by MCAv or ICA flow; and (2) the observed protection in cerebral oxygen saturation under the 0.1 Hz condition was not due to differences in arterial oxygen content or oxygen delivery.
The hemodynamic and tissue oxygenation responses to simulated and actual hemorrhage have been well documented (Manley et al 1999, 2000, Kay and Rickards 2016). As the volume of blood loss increases, both cerebral blood flow and cerebral tissue oxygenation will gradually decrease. Initially, homeostatic mechanisms such as the baroreflex and cerebral autoregulation are engaged in order to maintain arterial pressure and cerebral blood flow, and there is a redistribution of blood volume towards vital organs (Forsyth et al 1970) thus ensuring the delivery of oxygen to cerebral tissues. Eventually, however, these reflex compensatory responses cannot protect cerebral blood flow, leading to reduced oxygen delivery and tissue hypoxia (Manley et al 1999). Increased extraction of oxygen from the blood into the tissues also compensates for this reduced delivery (Powers 1991, Lewis et al 2014), but tissue hypoxia will occur when this compensatory mechanism is exhausted with a persistent reduction in oxygen delivery. Reductions in oxygen delivery via decreases in oxygen content can also occur with hemorrhage due to decreases in arterial oxygen saturation and hemoglobin concentration, which become even more severe with pulmonary disorders (gas exchange limitations), anemia (reduced hemoglobin), or hypoxic conditions (hemoglobin saturation) (Nunn and Freeman 1964).
Exposure to high altitude elicits a number of physiological responses to compensate for the hypoxic environment induced by the lower atmospheric PO2. Initially, ventilation increases to attenuate the reduction in arterial oxygen content and oxygen delivery (Hoiland et al 2018). Heart rate and cardiac output also increase with a chemoreflex-mediated elevation in sympathetic activity, further compensating for reductions in oxygen delivery (Bärtsch and Gibbs 2007). Interestingly, there is an acute reduction in plasma volume upon ascent to high altitude, which increases hematocrit and arterial hemoglobin concentration, and restores arterial oxygen content prior to erythropoietin-induced increases in total red blood cell volume (Siebenmann et al 2017, Schlittler et al 2021). This reduction in plasma volume and increase in hematocrit, however, may be detrimental during a hemorrhagic event at altitude due to increased blood viscosity impeding blood flow. The compensatory cerebrovascular response to hypoxia is a vasodilation and subsequent increase in cerebral blood flow in order to maintain oxygen delivery to the cerebral tissues (Hoiland et al 2015).
In our study, these acute compensatory responses to high altitude exposure and central hypovolemia must be considered. With the reduction in plasma volume and an increased workload placed on the heart to maintain cardiac output and blood flow to the brain with hypoxia, decreased central volume with hemorrhage will further stress the cardiovascular system. In addition, the effects of hypoxia on cerebrovascular regulatory mechanisms are not fully understood. Cerebral autoregulation, for example, may or may not be impaired under hypoxic conditions (Jansen et al 2007, Ainslie et al 2008, Subudhi et al 2010, Smirl et al 2014, Tymko et al 2020), which may further impair physiological compensation to hypovolemia. The additive effects of both hypoxia and hemorrhage on the reduction in cerebral blood flow and cerebral tissue oxygenation highlights the need to explore mechanisms and interventions that may provide benefit to the maintenance of tissue oxygenation during these combined physiological stressors.
Understanding the physiological role of oscillatory blood pressure and blood flow has been elusive. Many studies have sought to understand the mechanisms underlying hemodynamic oscillations at various frequencies, but most have developed mathematical models (Intaglietta 1990, Nilsson and Aalkjaer 2003, Julien 2006, Aalkjaer et al 2011), and only a few studies have sought experimental evidence to explain the physiological benefit of these responses. Early work using computational models suggested that oscillatory blood flow at low frequencies (less than 0.2 Hz) within the microvasculature could protect distal tissue oxygenation during reductions in flow (Tsai and Intaglietta 1993, Goldman and Popel 2001). This idea was further supported by an experimental study in a rat model of reduced perfusion to skeletal muscle via application of a tourniquet around the femoral artery. When endogenously occurring vasomotion was inhibited using calcium-channel blockers during the occlusion, decreases in capillary perfusion in tissues distal to the occlusion were observed compared to when vasomotion (and therefore oscillatory blood flow) was present (Rücker et al 2000).
Data from the current study and a prior study from our laboratory (Anderson et al 2019) also indicate a protection of cerebral tissue oxygenation when oscillatory arterial pressure and cerebral blood flow are induced around 0.1 Hz during conditions of reduced cerebral perfusion and oxygen delivery. Unlike our prior study (Anderson et al 2019), stroke volume was not protected by oscillatory arterial pressure in the current investigation, and cerebral blood flow oscillations were not aided by oscillations in PETCO2, indicating that these variables did not play a role in the protection of ScO2. Similar to our prior study, however, there were no differences between LBNP profiles for other systemic cardiovascular responses such as MAP, cardiac output, or systemic vascular resistance. The magnitude of the response in these variables was also similar to our prior work, except for cardiac output which exhibited a ~10% greater decrease at high altitude for both profiles compared with our previous study at low altitude. This finding highlights the greater physiological stress induced by the combined conditions of central hypovolemia plus hypoxia. We now demonstrate, in two independent studies, that during conditions of reduced cerebral blood flow, induced hemodynamic oscillations at 0.1 Hz protect against reductions in ScO2 despite no protection in cerebral blood flow/velocity. Interestingly, hemodynamic oscillations in skin blood flow at 0.1 Hz measured with laser Doppler flux have also been observed during high altitude ascent and the associated hypobaric hypoxia (Salvi et al 2018). In a separate investigation connected to the current study, we also observed increased 0.1 Hz oscillations in arterial pressure with sustained exposure to high altitude compared with low altitude (unpublished observations). Given these findings, we propose that hemodynamic oscillations are a compensatory mechanism through which tissue oxygenation is protected during states of hypoperfusion and/or hypoxia.
While the precise mechanisms for protection of tissue oxygenation have not been experimentally tested, some hypotheses have been presented. Intaglietta postulated that vasomotion creates a pump-like effect with brief but cyclical increases in both blood velocity and hematocrit down the vascular tree, aiding in the perfusion of more capillaries in hypoperfused tissue (Intaglietta 1990)—a phenomenon that Salvi et al termed the ‘peripheral heart’ (Salvi et al 2018). Hapuarachchi et al suggested that the hemodynamic oscillations in arteriolar beds might have an oxygen sparing effect by preventing the diffusion of oxygen out of the arterioles (a naturally-occurring phenomenon) such that arterial blood arrives at the level of the capillary with a greater concentration of oxygen (Hapuarachchi et al 2010). Hemodynamic oscillations may also alter the distribution of red blood cells within the vessel lumen, where increases in blood velocity converge red blood cells to the center of the lumen and decreases in blood velocity allow for a more uniform distribution (Mchedlishvili and Maeda 2001). This change in red blood cell orientation with oscillatory blood flow may alter oxygen extraction within the microvasculature and help account for the observed differences in tissue oxygenation. Further work is needed to identify and understand the physiological mechanisms underpinning protection of tissue oxygenation with oscillatory blood flow.
Methodological considerations
There are a number of important considerations when interpreting the data from this study. First, experiments were conducted after 5 days at altitude. This exposure to the hypobaric hypoxia of altitude would allow for some compensatory adaptations to start occurring in order to increase arterial oxygen content; indeed, baseline arterial oxygen content was similar to low altitude conditions in our subjects (unpublished observations). It is likely that testing in the acute phase of high altitude exposure may have imposed a greater challenge to maintaining tissue oxygenation under the combined conditions of hypovolemia and hypoxia. Another consideration is the relatively small number of measurements we were able to obtain for ICA flow. While we recognize this limitation, ICA flow responses were similar to the MCA velocity responses (N = 7).
Due to the nature of an expedition study, experiments were scheduled throughout the testing days, without control for circadian variation between participants (time of day was controlled for experiments within each participant), while blood samples for the measurement of hemoglobin concentration were collected in the morning for each individual. Accordingly, hemoglobin concentrations for some subjects were not time-synced with their experiments. However, it is unlikely that hemoglobin concentration varied in a physiologically meaningful way throughout the day of testing.
Another important consideration is the small sample size for some of our measurements. When measurements were successfully recorded, a maximum N of 8 was possible. Due to technical difficulties, we obtained MCA velocity recordings for an N = 7, and N = 5 for ICA blood flow recordings. However, the repeated measures design, and the consistency of some of our key outcome variables (i.e. MCAv and ScO2) with our prior work (Anderson et al 2019), provide added confidence in our conclusions from this small sample.
Lastly, the cerebral tissue oxygen saturation measurement has some important assumptions worth noting. The ScO2 measurement is a mixed sample of oxy- and deoxy-hemoglobin predominantly from the venous blood (approximately 75% venous, 20% arterial, 5% capillary) (Madsen and Secher 1999). As such, ScO2 is affected by both the delivery of oxygen to the tissue and oxygen extraction from the blood. Because indices of cerebral blood flow were no different between experimental conditions, we assume that any changes in ScO2 reflect changes in tissue oxygen extraction. The attenuated decrease in ScO2 for the 0.1 Hz profile thus reflects a decrease in oxygen extraction which may be indicative of reduced metabolic consumption compared to the 0 Hz condition, or may be due to the oxygen sparing effect proposed by Hapuarachchi et al (2010). Further research is needed to address the underlying mechanisms contributing to this effect.
Translational perspective
While recognizing there is much research to be done regarding the therapeutic potential of hemodynamic oscillations, the current findings continue to support potential clinical applications. Inducing oscillations at around 0.1 Hz in either arterial pressure or cerebral blood flow could be used to protect oxygenation of the tissues with reductions in perfusion such as during hemorrhage, sepsis, or during recovery from stroke. This approach could be especially useful in scenarios where access to advanced medical care is limited, such as extended transportation to a hospital, during high altitude/mountaineering expeditions, or for soldiers wounded on the battlefield. We clearly recognize that in such situations, LBNP is not a viable method for inducing hemodynamic oscillations. Other methods of delivery could be used, however, such as pneumatic cuffs that have been utilized by Hockin et al during head-up tilt and LBNP (Hockin et al 2019, Hockin and Claydon 2020). These cuffs could easily be placed around the calf or thigh and rhythmically inflated and deflated at 0.1 Hz. It is plausible that engineering portable technology for therapeutic delivery of hemodynamic oscillations could be a relatively straight-forward task of repurposing this design.
In conclusion, our findings suggests that LF hemodynamic oscillations effectively prevented decreases in cerebral tissue oxygenation under combined central hypovolemia and hypobaric hypoxia, despite no effects on cerebral blood flow or oxygen delivery. Further research is needed to explore the microvascular and tissue responses to hemodynamic oscillations to gain further insight into the mechanisms underpinning these observations. Our current findings support the potential therapeutic use of hemodynamic oscillations in protecting vital organ oxygenation during challenges to blood flow and oxygen delivery.
Acknowledgments
We gratefully acknowledge our research participants for their willingness to contribute to this study, and to all other members of the White Mountain 2019 expedition team for their support. We also sincerely thank the staff at the Barcroft Laboratory for their generous service throughout our expedition.
Funding
Funding for this study was provided, in part, by an American Heart Association Grant-in-Aid (C A R; GRNT 17GRNT33671110), a Natural Sciences and Engineering Research Council of Canada Discovery Grant (TA D; RGPIN-2016–04915), a University of Calgary Research Grant Committee (RJA W), a NSERC Discovery grant (RJ A W), and training fellowships awarded to G K A through a National Institutes of Health-supported Neurobiology of Aging Training Grant (T32 AG020494, Principal Investigator: N Sumien) and an American Heart Association Predoctoral Fellowship (20PRE35210249), and to A J R through a Ruth L Kirchstein NRSA F32 Postdoctoral Fellowship (1F32 HL144082–01A1). N G J is a Parker B Francis Fellowship Recipient.
References
- Aalkjaer C, Boedtkjer D and Matchkov V 2011. Vasomotion—what is currently thought? Acta Physiol. 202 253–69 [DOI] [PubMed] [Google Scholar]
- Ainslie PN et al. 2008. Differential effects of acute hypoxia and high altitude on cerebral blood flow velocity and dynamic cerebral autoregulation: alterations with hyperoxia J. Appl. Physiol. 104 490–8 [DOI] [PubMed] [Google Scholar]
- Anderson GK et al. 2019. Responses of cerebral blood velocity and tissue oxygenation to low-frequency oscillations during simulated haemorrhagic stress in humans Exp. Physiol. 104 1190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bärtsch P and Gibbs JSR 2007. Effect of altitude on the heart and the lungs Circulation 116 2191–202 [DOI] [PubMed] [Google Scholar]
- Bertuglia S et al. 1991. Hypoxia- or hyperoxia-induced changes in arteriolar vasomotion in skeletal muscle microcirculation Am. J. Physiol. Heart. Circ. Physiol. 260 H362–72 [DOI] [PubMed] [Google Scholar]
- Forsyth RP, Hoffbrand BI and Melmon KL 1970. Redistribution of cardiac output during hemorrhage in the unanesthetized Monkey Circ. Res. 27 311–20 [DOI] [PubMed] [Google Scholar]
- Goldman D and Popel AS 2001. A computational study of the effect of vasomotion on oxygen transport from capillary networks J. Theor. Biol. 209 189–99 [DOI] [PubMed] [Google Scholar]
- Hapuarachchi T, Park CS and Payne S 2010. Quantification of the effects of vasomotion on mass transport to tissue from axisymmetric blood vessels J. Theor. Biol. 264 553–9 [DOI] [PubMed] [Google Scholar]
- Hockin BCD et al. 2019. Intermittent calf compression reverses lower limb pooling and improves cardiovascular control during passive orthostasis Auton. Neurosci. 217 102–13 [DOI] [PubMed] [Google Scholar]
- Hockin BCD and Claydon VE 2020. Intermittent calf compression delays the onset of presyncope in young healthy individuals Front. Physiol. 10 1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiland RL et al. 2015. Hypoxemia, oxygen content, and the regulation of cerebral blood flow Am. J. Physiol. Regul. Integr. Comp. Physiol. 310 R398–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiland RL et al. 2018. Ventilatory and cerebrovascular regulation and integration at high-altitude Clin. Auton. Res. 28 423–35 [DOI] [PubMed] [Google Scholar]
- Intaglietta M 1990. Vasomotion and flowmotion: physiological mechanisms and clinical evidence Vascular Med. Rev. vmr–1 101–12 [Google Scholar]
- Jansen GFA et al. 2007. Role of the altitude level on cerebral autoregulation in residents at high altitude J. Appl. Physiol. 103 518–23 [DOI] [PubMed] [Google Scholar]
- Julien C 2006. The enigma of Mayer waves: facts and models Cardiovasc. Res. 70 12–21 [DOI] [PubMed] [Google Scholar]
- Kaur J et al. 2018. Regulation of regional cerebral blood flow during graded reflex-mediated sympathetic activation via lower body negative pressure J. Appl. Physiol. 125 1779–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay VL and Rickards CA 2016. The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia Am. J. Physiol. Regul. Integr. Comp. Physiol. 310 R375–83 [DOI] [PubMed] [Google Scholar]
- Lewis NCS et al. 2014. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance J. Physiol. 592 5203–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJE et al. 2013. Slow breathing as a means to improve orthostatic tolerance: a randomized sham-controlled trial J. Appl. Physiol. 115 202–11 [DOI] [PubMed] [Google Scholar]
- Madsen PL and Secher NH 1999. Near-infrared oximetry of the brain Prog. Neurobiol. 58 541–60 [DOI] [PubMed] [Google Scholar]
- Manley GT et al. 1999. Brain tissue oxygenation during hemorrhagic shock, resuscitation, and alterations in ventilation J. Trauma 46 261–7 [DOI] [PubMed] [Google Scholar]
- Manley GT et al. 2000. Cerebral oxygenation during hemorrhagic shock: perils of hyperventilation and the therapeutic potential of hypoventilation J. Trauma 48 1025–32 [DOI] [PubMed] [Google Scholar]
- Mchedlishvili G and Maeda N 2001. Blood flow structure related to red cell flow: determinant of blood fluidity in narrow microvessels Jpn. J. Physiol. 51 19–30 [DOI] [PubMed] [Google Scholar]
- Nilsson H and Aalkjaer C 2003. Vasomotion: mechanisms and physiological importance Mol. Interv. 3 79–89 [DOI] [PubMed] [Google Scholar]
- Nunn JF and Freeman J 1964. Problems of oxygenation and oxygen transport during haemorrhage Anaesthesia 19 206–16 [DOI] [PubMed] [Google Scholar]
- Powers WJ 1991. Cerebral hemodynamics in ischemic cerebrovascular disease Ann. Neurol. 29 231–40 [DOI] [PubMed] [Google Scholar]
- Rickards CA et al. 2011. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity J. Appl. Physiol. 111 1048–58 [DOI] [PubMed] [Google Scholar]
- Rickards CA et al. 2015. Coupling between arterial pressure, cerebral blood velocity, and cerebral tissue oxygenation with spontaneous and forced oscillations Physiol. Meas. 36 785–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rücker M et al. 2000. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure Am. J. Physiol. Heart. Circ. Physiol. 279 H550–8 [DOI] [PubMed] [Google Scholar]
- Salvi P et al. 2018. Increase in slow-wave vasomotion by hypoxia and ischemia in lowlanders and highlanders J. Appl. Physiol. 125 780–9 [DOI] [PubMed] [Google Scholar]
- Schlittler M et al. 2021. Regulation of plasma volume in male lowlanders during 4 days of exposure to hypobaric hypoxia equivalent to 3500 m altitude J. Physiol. 599 1083–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenmann C, Robach P and Lundby C 2017. Regulation of blood volume in lowlanders exposed to high altitude J. Appl. Physiol. 123 957–66 [DOI] [PubMed] [Google Scholar]
- Smirl JD et al. 2014. Cerebral pressure–flow relationship in lowlanders and natives at high altitude J. Cereb. Blood. Flow. Metab. 34 248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi AW, Panerai RB and Roach RC 2010. Effects of hypobaric hypoxia on cerebral autoregulation Stroke 41 641–6 [DOI] [PubMed] [Google Scholar]
- Thomas KN et al. 2015. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow Am. J. Physiol. Regul. Integr. Comp. Physiol. 309 R707–20 [DOI] [PubMed] [Google Scholar]
- Tsai AG and Intaglietta M 1993. Evidence of flowmotion induced changes in local tissue oxygenation Int. J. Microcirc., Clin. Exp. 12 75–88 [PubMed] [Google Scholar]
- Tymko MM et al. 2020. UBC-Nepal expedition: dynamic cerebral autoregulation is attenuated in lowlanders upon ascent to 5050 m Eur. J. Appl. Physiol. 120 675–86 [DOI] [PubMed] [Google Scholar]
- Woodman RJ et al. 2001. Improved analysis of brachial artery ultrasound using a novel edge-detection software system J. Appl. Physiol. 91 929–37 [DOI] [PubMed] [Google Scholar]


