Abstract
We have evaluated the potential of conferring protective immunity to herpes simplex virus type 2 (HSV-2) by selectively inducing an HSV-specific CD8+ cytotoxic T-lymphocyte (CTL) response directed against a single major histocompatibility complex class I-restricted CTL recognition epitope. We generated a recombinant vaccinia virus (rVV-ES-gB498-505) which expresses the H-2Kb-restricted, HSV-1/2-cross-reactive CTL recognition epitope, HSV glycoprotein B residues 498 to 505 (SSIEFARL) (gB498-505), fused to the adenovirus type 5 E3/19K endoplasmic reticulum insertion sequence (ES). Mucosal immunization of C57BL/6 mice with this recombinant vaccinia virus induced both a primary CTL response in the draining lymph nodes and a splenic memory CTL response directed against HSV gB498-505. To determine the ability of the gB498-505-specific memory CTL response to provide protection from HSV infection, immunized mice were challenged with a lethal dose of HSV-2 strain 186 by the intranasal (i.n.) route. Development of the gB498-505-specific CTL response conferred resistance in 60 to 75% of mice challenged with a lethal dose of HSV-2 and significantly reduced the levels of infectious virus in the brains and trigeminal ganglia of challenged mice. Finally, i.n. immunization of C57BL/6 mice with either a recombinant influenza virus or a recombinant vaccinia virus expressing HSV gB498-505 without the ES was also demonstrated to induce an HSV-specific CTL response and provide protection from HSV infection. This finding confirms that the induction of an HSV-specific CTL response directed against a single epitope is sufficient for conferring protective immunity to HSV. Our findings support the role of CD8+ T cells in the control of HSV infection of the central nervous system and suggest the potential importance of eliciting HSV-specific mucosal CD8+ CTL in HSV vaccine design.
Both humoral and cell-mediated components of the immune response are involved in controlling herpes simplex virus (HSV) infection (51, 61). Studies of humans and of mice have implicated a role for both CD8+ (6, 25, 32, 33, 47, 65–67) and CD4+ (27, 37–39, 52, 53) T-lymphocyte subsets in mediating protection against HSV infection. For example, CD8+ T cells have been shown to be important in limiting replication of HSV in the footpad (6) and colonization of the spinal dorsal root ganglia (6, 66). In contrast, other studies using a zosteriform model of infection have primarily indicated a role for CD4+ T cells in the clearance of HSV (37–39). Both CD4+ and CD8+ (56, 72, 74–76) HSV-specific T lymphocytes have been detected in humans seropositive for HSV. However, the contribution of each subset in the control of HSV infection has not been clearly defined. This illustrates the controversy regarding the relative roles of each subset in the resolution of HSV infection.
To address the role of the CD8+ T-cell subset in providing acquired immunity to HSV infection, we examined the protection afforded by HSV-specific, CD8+ cytotoxic T lymphocytes (CTL) directed to a single CTL recognition epitope. In previous studies by others, immunization with single CTL epitopes has been effective in controlling viral pathogens including lymphocytic choriomeningitis virus (14, 54, 62, 73), murine cytomegalovirus (15), influenza virus (55), and Sendai virus (28). Although HSV-encoded CTL recognition epitopes have been identified by their ability to serve as targets for HSV-specific CTL (3, 8, 24, 64), the ability of CTL directed to these individual epitopes to confer protection against HSV infection has not been determined. We have designed two separate vaccination strategies which permit the exclusive induction of a single HSV epitope-specific, CD8+ T-lymphocyte response and have evaluated the ability of this response to confer protective immunity to HSV infection.
Hanke et al. (24) broadly identified an immunodominant, H-2Kb-restricted epitope within HSV glycoprotein B (gB). The minimal amino acid sequence of this epitope, gB498-505 (SSIEFARL), was demonstrated by Bonneau et al. (8), using synthetic peptides and an epitope-specific CTL clone. The amino acid sequence, SSIEFARL, is identical in both HSV type 1 (HSV-1) (gB498-505) and HSV-2 (gB496-503) (11). CTL specific for gB498-505 are readily induced by immunization with synthetic peptide (8), a cell line expressing gB498-505 in the context of simian virus 40 (SV40) T antigen (5), and a recombinant viral vector expressing this epitope in the context of a cellular protein (19). In the present study, two recombinant vaccinia viruses (rVV-ES-gB498-505 and rVV-gB498-505) and a recombinant influenza virus (WSN/NA/gB) were generated to express a single HSV-encoded epitope, HSV-1 gB498-505, and were characterized for the ability to induce a potent, HSV-specific CTL response upon mucosal immunization. To determine the protection afforded by immunization with each of the individual recombinant viruses, we used a lethal model of HSV-2 encephalitis. Our findings suggest that the induction of a CTL response directed against a single HSV-specific CTL recognition epitope is sufficient to confer significant protective immunity to HSV infection.
MATERIALS AND METHODS
Mice.
Five- to six-week-old male C57BL/6 mice were purchased from The Jackson Laboratory, Bar Harbor, Maine. Mice were housed in groups of four in a 12-h light/12-h dark cycle and provided with food and water ad libitum. At least 1 week was allowed for mice to acclimatize to these conditions prior to any experimentation.
Cell lines.
The fibroblast cell lines B6/WT-3 (59) and B6/K-1,4,5 (69, 70) were grown in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 20 mM HEPES buffer, 0.075% (wt/vol) NaHCO3, and 5% (vol/vol) heat-inactivated fetal bovine serum (FBS; HyClone, Logan, Utah). HuTK−143 (60), a human osteosarcoma-derived cell line, and CV-1 (36), an African green monkey kidney cell line, were grown in supplemented DMEM. Vero cells were maintained in medium 199 supplemented with 8% tryptose phosphate broth, 0.225% NaHCO3, 4% heat-inactivated FBS, and 4% heat-inactivated newborn calf serum. T2/Kb cells were generously provided by Peter Cresswell (Yale University, New Haven, Conn.) and maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 25 mM HEPES buffer, 0.225% NaHCO3, 5 × 10−5 M 2-mercaptoethanol, 10% FBS, and 100 μg of G418 per ml. The CTL clone 2D5, which specifically recognizes HSV-1 gB498-505 in association with the H-2Kb class I molecule, has been described previously (8) and was maintained in IMDM supplemented with 25 mM HEPES buffer, 0.225% NaHCO3, 5 × 10−5 M 2-mercaptoethanol, 10% FBS, 0.05 M α-methyl-d-mannoside, and 10% (vol/vol) Rat T-Stim culture supplement (Collaborative Biomedical Research Products, Bedford, Mass.). The antigen-specific proliferation of these cells was induced by the presence of 5 × 105 mitomycin C-treated, HSV-infected B6/WT-3 cells (7). The epitope in HSV-1 identified by the CTL clone 2D5 is identical in sequence to HSV-2 gB496-503 (11). The Y-4 CTL clone recognizes H-2Kb-restricted SV40 T-antigen epitope IV (Tag404-411) and was cultured as described previously (50).
Viruses.
Stocks of HSV-2 186 and HSV-1 KOS1.1 were generated by infection of Vero cells at a multiplicity of infection (MOI) of 0.01, and the virus stock titer was determined by plaque assay on Vero cells.
Recombinant vaccinia viruses (rVVs) were generated so as to express the single major histocompatibility complex (MHC) class I-restricted CTL recognition epitopes HSV gB498-505 (SSIEFARL) (8, 24) and SV40 Tag404-411 (VVYDFLKC) (50) as peptides fused to the adenovirus type 5 E3/19k endoplasmic reticulum insertion sequence (ES), RYMILGLLALAAVCSA (2, 68). These viruses were designated rVV-ES-gB498-505 and rVV-ES-IV, respectively (Table 1). The corresponding rVVs were also generated to express the gB498-505 and SV40 Tag404-411 epitopes without the ES and termed rVV-gB498-505 and rVV-IV, respectively (Table 1). Annealed complementary synthetic oligonucleotide pairs encoding each epitope sequence were ligated into a modified pSC11 plasmid, kindly provided by Bernard Moss (National Institutes of Health, Bethesda, Md.), such that an Ala codon was inserted between the 16-residue ES and the N terminus of the CTL epitope. The ES was preceded by a start codon, and the CTL epitope sequence was followed by two stop codons to ensure translation termination. The protocol for generation of rVVs was adapted from a protocol described elsewhere (17, 20). Briefly, CV-1 cells were infected with wild-type vaccinia virus strain WR for 2 h in 1 ml of phosphate-buffered saline supplemented with 0.1% (wt/vol) bovine serum albumin (PBS-BSA). Following the infection, the viral inoculum was aspirated, and calcium-phosphate-DNA precipitate (CellPhect Transfection kit; Pharmacia, Uppsala, Sweden) containing the pSC11-derived DNA encoding a CTL epitope was added. Infected cells were incubated for 2 to 3 days until cytopathic effects were observed, at which time cells were harvested and lysed by three rounds of freeze-thawing and sonication. HuTK−143 cells were infected with cell lysates and overlaid with plaquing medium (1:1 mixture of 1.8% Noble agar and 2× Eagle’s medium supplemented with 10% FBS and 25 μg of 5-bromodeoxyuridine per ml) to select for TK− viruses. Plaques were screened for β-galactosidase expression by adding a 2-ml overlay of plaquing medium containing 0.025% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and three rounds of plaque purification were performed. Virus stocks were generated by infection of HuTK−143 cells.
TABLE 1.
Recombinant viruses used in this study
| Recombinant virus | H-2Kb-restricted CTL epitope expressed | CTL clone |
|---|---|---|
| rVV-SC | None | None |
| rVV-gB498-505 | HSV-1 gB498-505 (SSIEFARL) | 2D5 |
| rVV-ES-gB498-505 | HSV-1 gB498-505 | 2D5 |
| rVV-IV | SV40 Tag404-411 (VVYDFLKC) | Y-4 |
| rVV-ES-IV | SV40 Tag404-411 | Y-4 |
| WSN/NA/gB | HSV-1 gB498-505 | 2D5 |
Recombinant influenza virus WSN/NA/gB, which expresses HSV gB498-505 (SSIEFARL) in the neuraminidase (NA) stalk, was generated as previously described (14) (Table 1), via a reverse genetics procedure (18) using plasmid pT3WSN(NA15) that involved insertion of nucleotides into the NA gene by oligonucleotide-directed mutagenesis (31). Transfectant viruses were plaque purified five times in Madin-Darby bovine kidney (MDBK) cells, and direct sequence analysis of purified viral RNA was performed to verify insertion of the gB498-505 sequence. Wild-type influenza virus strain A/WSN/33 (H1N1) (WSN) was provided by Jack Bennink (National Institutes of Health).
Synthetic peptides.
Synthetic peptides encoding the gB498-505 amino acid sequence (SSIEFARL [8]) and SV40 Tag(404-411) (LT404-411; VVYDFLKC [50]) were generated by using an automated peptide synthesizer (MilliGen PepSynthesizer 9050) at the Pennsylvania State University College of Medicine Macromolecular Core Facility. The sequence and purity of the peptides were assessed by high-pressure liquid chromatography tracing (Waters, MilliGen). Peptide working stocks were prepared by dissolving the lyophilized peptides in dimethyl sulfoxide (DMSO) and then diluted with unsupplemented IMDM so that the DMSO concentration was less than 5% (vol/vol).
Induction of primary and memory gB498-505-specific CTL.
Eight- to twelve-week-old C57BL/6 mice were inoculated intranasally (i.n.) with 107 PFU of either rVV-ES-gB498-505 or rVV-ES-IV. For the analysis of the primary CTL response after immunization with rVV-ES-gB498-505, mice were sacrificed on day 8 after immunization, the hilar and submaxillary lymph nodes were removed, and single-cell suspensions were prepared. The primary CTL response to WSN/NA/gB was assayed 5 days after i.n. immunization in the mediastinal lymph nodes and spleen. Lymph node cells were cultured in supplemented IMDM as described previously (9). For the induction of a memory CTL (CTLm) response, mice were immunized i.n., and 4 to 6 weeks later splenic lymphocytes were cultured in supplemented IMDM as described previously (9, 26, 34). Briefly, 107 cells were seeded per well in a 12-well plate in 4 ml of supplemented IMDM. HSV-infected, mitomycin C-treated B6/WT-3 cells (5 × 105) were added to each well, and the cultures were incubated at 37°C–5% CO2 for 5 days.
Assay for cell-mediated cytotoxicity.
51Cr release assays were performed as described previously (9, 13). Vaccinia virus-infected target cells were prepared by infecting 106 B6/K-1,4,5 cells suspended in 0.5 ml of PBS-BSA supplemented with an equal amount of incomplete DMEM at an MOI of 10 for 1 h. After a 1-h adsorption period, 2 ml of supplemented DMEM and Na251CrO4 (51Cr) were added to the infected cells, which were then incubated for 3 h at 37°C–5% CO2. Peptide-pulsed target cells were prepared by incubating 51Cr-labeled cells in the presence of 1 μM synthetic peptide (9) for at least 30 min at 37°C–5% CO2, after which target cells were washed free of unbound peptide. HSV-2-infected target cells were prepared by infecting B6/WT-3 cells with HSV-2 at an MOI of 10 for 1 h in 1 ml of PBS–1% fetal calf serum (FCS). The infected cells were then labeled with 51Cr for 5 h. Influenza virus-infected target cells were prepared by infecting 5 × 105 cells in 0.5 ml of PBS-BSA for 1.5 h followed by a 5-h infection in DMEM supplemented with 51Cr. For the 51Cr release assay, 5 × 103 to 1 × 104 labeled target cells were combined in triplicate with effector cells at various ratios in 0.2 ml in 96-well, V-bottom microtiter tissue culture plates (Costar, Cambridge, Mass.). The microtiter plates were then centrifuged at 100 × g for 3 min and incubated at 37°C–5% CO2 for 4 to 5 h. At the end of the incubation period, plates were centrifuged at 200 × g, 100 μl of the cell supernatant was removed from each well, and the level of radioactivity contained in each supernatant was determined in a gamma counter. Percent specific lysis was determined by the formula (E − S)/(M − S) × 100, where E represents the counts per minute released by target cells when treated with effector cells, S equals the counts per minute released in the presence of medium alone, and M represents the maximum amount released in the presence of 2.5% sodium dodecyl sulfate.
Determination of gB498-505-specific CTL frequency by LDA.
Limiting dilution analysis (LDA) was performed as described previously (9). Splenocytes from mice immunized i.n. with rVV-ES-gB-498-505 or rVV-ES-IV 6 weeks earlier were cultured in 0.2 ml of supplemented IMDM in replicates of 18 in 96-well, U-bottom microtiter tissue culture plates. Each well also contained 105 gamma-irradiated (2,000 rads) naive C57BL/6 splenocytes, 0.2 U of interleukin-2 (Amgen, Thousand Oaks, Calif.), 10 μl of Rat T-Stim culture supernatant, 0.1 M α-methyl-d-mannoside, and 2 × 103 HSV-1-infected, mitomycin C-treated B6/WT-3 cells. After incubation for 7 days at 37°C–5% CO2, effector cells from each well were mixed with gB498-505 peptide-pulsed or mock-pulsed B6/K-1,4,5 cells in a 51Cr release assay. A single well was determined to contain at least one CTL precursor (CTLp) if the specific lysis was greater than 10%. The CTLp frequency was calculated by the minimal χ2 method (71).
Lethal HSV-2 i.n. challenge.
Control and immunized mice were challenged i.n. with either 1 × 105 or 5 × 105 PFU of HSV-2 186 in 20 μl of PBS–1% FCS. Symptoms of HSV-2 infection included ataxia and a pronounced arch in the backs of mice. These mice were monitored for lethality for over 28 days, and moribund mice were sacrificed.
Quantitation of infectious HSV-2 in brains and trigeminal ganglia.
Mice challenged with 5 × 105 PFU of HSV-2 were sacrificed after 5 days, and the brains and trigeminal ganglia were removed, placed in 1 ml of supplemented medium 199, and stored at −80°C. Tissues were subsequently thawed and homogenized with 1-ml Ten Broeck glass tissue homogenizers (Wheaton Industries, Millville, N.J.). Homogenates were centrifuged at 1,000 × g for 4 min. and 10-fold serial dilutions were performed in PBS–1% FCS. The dilutions were titrated on Vero cells in 60-mm-diameter tissue culture plates by adsorbing 0.1 ml of tissue homogenate dilution onto the Vero cell monolayers for 1 h. Methylcellulose overlay medium (13) was then added, and the plates were incubated at 37°C–5% CO2 for 4 to 5 days. Monolayers of cells were then fixed with 5% formaldehyde and stained with 0.5% crystal violet, and plaques were counted. Ten PFU per tissue sample was the lower limit of detection for the infectious virus. The Mann-Whitney test was used to compare the levels of protection afforded by immunization with the rVVs.
RESULTS
CTL clone recognition of the rVVs expressing HSV gB498-505 and SV40 Tag404-411.
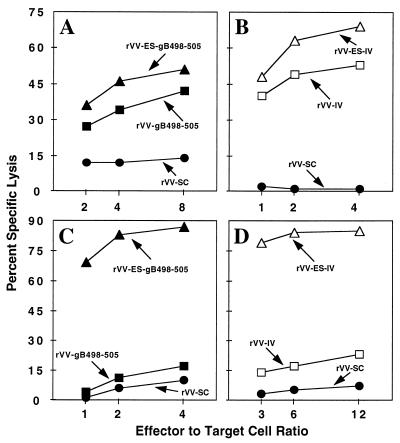
To confirm that the rVVs encoding HSV gB498-505 (SSIEFARL) and SV40 Tag404-411 (VVYDFLKC) fused to the adenovirus type 5 E3/19K glycoprotein ES expressed the respective CTL recognition epitopes, the in vitro expression of rVV-ES-gB498-505, rVV-gB498-505, rVV-ES-IV, and rVV-IV was examined. B6/K-1,4,5 cells were infected with the rVVs and tested in a standard 51Cr release assay for recognition by CTL clones 2D5 (Fig. 1A) and Y-4 (Fig. 1B), which have specific lytic activity for HSV gB498-505 and SV40 Tag404-411, respectively. The 2D5 CTL clone recognized cells infected with rVV-ES-gB498-505 and rVV-gB498-505, and the Y-4 CTL clone recognized cells infected with rVV-ES-IV and rVV-IV, confirming the functional expression of the rVV-encoded minigenes.
FIG. 1.
rVVs express CTL recognition epitopes. B6/K-1,4,5 (A and B) or T2/Kb (C and D) cells were infected with rVV-ES-gB498-505, rVV-gB498-505, rVV-ES-IV, rVV-IV, or rVV-SC for 1 h and incubated in DMEM with 51Cr for an additional 3 h. Virus-infected cells were added to either the gB498-505-specific CTL clone 2D5 (A and C) or the SV40 Tag404-411-specific CTL clone Y-4 (B and D) at the indicated effector-to-target ratios in a standard 51Cr release assay.
To examine the transporter associated with antigen processing (TAP) dependence of the rVVs encoding epitopes fused to the ES sequence, T2 cells expressing H-2Kb molecules (T2/Kb) were used. T2 cells are deficient in MHC class I-restricted antigen presentation due to a deletion in the MHC locus which encodes TAP (1). T2/Kb cells were infected with the rVVs and assayed for recognition by the 2D5 or Y-4 CTL clone. As expected, the ES allowed for the TAP-independent antigen presentation of rVV-ES-gB498-505 (Fig. 1C) and rVV-ES-IV (Figure 1D), thus confirming the functional significance of this sequence.
Intranasal immunization with rVV-ES-gB498-505 induces primary and memory CTL responses.
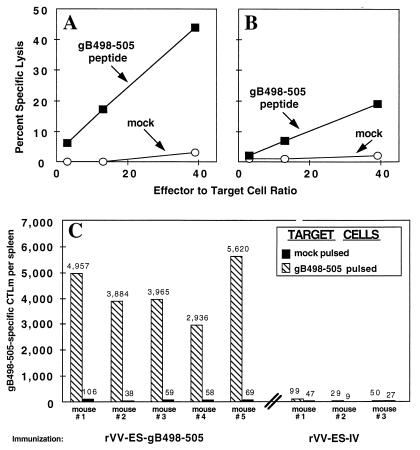
To evaluate the in vivo immunogenicity of rVV-ES-gB498-505, the primary and memory CTL responses induced by i.n. immunization were determined. Eight days after i.n. infection of C57BL/6 mice, we removed the local hilar and submaxillary draining lymph nodes and tested cultured cells in a standard 51Cr release assay for gB498-505-specific lytic activity. Both the hilar (Fig. 2A) and submaxillary (Fig. 2B) lymph nodes contained CTL which were specific for HSV gB498-505, with a higher level of lytic activity detected in the cultures prepared from the hilar lymph nodes. These findings demonstrate that i.n. immunization with rVV-ES-gB498-505 effectively induces a primary CTL response in the local draining lymph nodes.
FIG. 2.
Immunization with rVV-ES-gB498-505 induces primary and memory CTL responses. C57BL/6 mice were immunized i.n. with 107 PFU of rVV-ES gB498-505. Eight days later, the hilar (A) and submaxillary (B) lymph nodes were removed, and single-cell suspensions were formed. The effector lymph node cells were then tested for lytic activity at the indicated effector-to-target ratios against B6/WT-3 cells pulsed with 1 μM gB498-505 synthetic peptide and mock peptide-pulsed B6/WT-3 cells. For analysis of the CTLm response (C), we immunized i.n. five mice with rVV-ES-gB498-505 and three with rVV-ES-IV. Six weeks later, mice were sacrificed and their splenocytes were incubated for 7 days under LDA conditions as described in Materials and Methods. The graded splenocyte cultures were then tested for lytic activity against 51Cr-labeled B6/K-1,4,5 cells which were either pulsed with gB498-505 synthetic peptide or mock pulsed. Total CTLm per spleen was calculated as (CTLm frequency) × (viable cell yield per spleen).
To further characterize the CTL response following immunization with rVV-ES-gB498-505, the magnitude of the CTLm response was evaluated by bulk culture and LDA analysis. The induction of SV40 T-antigen-specific CTLm was also verified following immunization with rVV-ES-IV, expressing the H-2Kb-restricted SV40 T-antigen CTL recognition epitope Tag404-411 (VVYDFLKC), which served as a control for vaccinia virus infections. Mice were immunized with 107 PFU of rVV-ES-gB498-505 or rVV-ES-IV, and 6 weeks later splenocyte bulk cultures and LDA cultures were established. Analysis of bulk culture splenocytes demonstrated the induction of splenic CTL with specificity for the respective viral epitopes, and only splenocytes from rVV-ES-gB498-505-immunized mice had lytic activity against HSV-infected cells (data not shown).
To assess the frequency of gB498-505-specific CTLm induced by i.n. immunization with rVV-ES-gB498-505, splenocytes from immunized mice were cultured under LDA conditions and tested for lytic activity against mock- and gB498-505 peptide-pulsed B6/K-1,4,5 cells. The results (Fig. 2C) of the LDA demonstrate that immunization with rVV-ES-gB498-505 induces a high frequency of gB498-505-specific CTLm in the spleens of immunized mice (range, 2,936 to 4,957 per mouse spleen). As expected, immunization with the control virus rVV-ES-IV did not induce detectable levels of gB498-505-specific CTL. Immunization with rVV-gB498-505 induced a frequency of gB498-505-specific CTLm comparable to that of rVV-ES-gB498-505 (data not shown). These findings demonstrate that rVV-ES-gB498-505 is a potent vector for efficient and consistent induction of CTL with specificity for the HSV-1/2 CTL recognition epitope gB498-505.
Protection from lethal challenge of HSV-2 is conferred by immunization with rVV-ES-gB498-505.
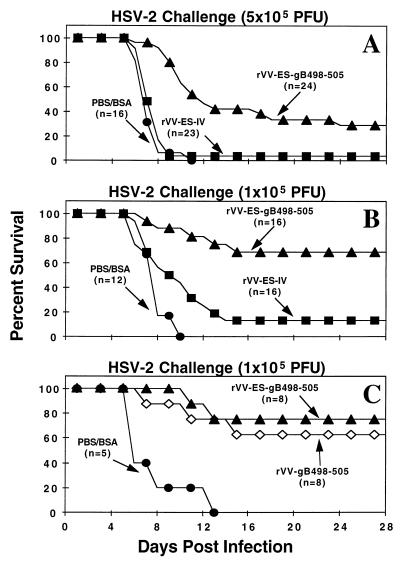
Since immunization with rVV-ES-gB498-505 elicited a significant primary and CTLm response, it was of interest to determine if the presence of CTL directed against a single HSV-1/2 conserved CTL recognition epitope would be sufficient to control HSV infection. To test this hypothesis, we used a model of lethal HSV-2 encephalitis. C57BL/6 mice were immunized i.n. with either PBS-BSA, rVV-ES-IV, or rVV-ES-gB498-505. Four to six weeks later, groups of mice were challenged i.n. with either 5 × 105 or 1 × 105 PFU of HSV-2 186 and were monitored over 28 days for lethality. The results of three separate experiments using the 5 × 105-PFU challenge dose (Fig. 3A) demonstrate that immunization with rVV-ES-gB498-505 conferred a significant delay in the time of death and complete protection for 29% of the challenged mice. All PBS-BSA-immunized mice succumbed to the infection by day 11, while only 1 of 23 mice immunized with rVV-ES-IV survived. However, 7 of 24 rVV-ES-gB498-505-immunized mice survived the challenge with a lethal dose of HSV-2. Since immunization with rVV-ES-gB498-505 conferred only a limited level of protection in mice challenged with 5 × 105 PFU of HSV-2, groups of mice immunized with rVV-ES-gB498-505 were challenged with a fivefold-lower dose (105 PFU) of HSV-2. The results presented in Fig. 3B show that 11 of 16 rVV-ES-gB498-505-immunized mice were protected, while only 2 of 16 mice immunized with rVV-ES-IV survived this lower HSV-2 challenge. Protection was also afforded by immunization with rVV-gB498-505 (Fig. 3C), demonstrating that the observed protection was not dependent on or enhanced by the presence of an ES. These results indicate that immunization with a single CTL recognition epitope of HSV is sufficient to confer protection from lethal HSV-2 infection.
FIG. 3.
Immunization with either rVV-ES-gB498-505 or rVV-gB498-505 protects mice from lethal challenge of HSV-2. Mice were immunized i.n. with either PBS-BSA (A to C), rVV-ES-gB498-505 (A to C), rVV-gB498-505 (C), or rVV-ES-IV (A and B) as described in Materials and Methods. Four to six weeks later, mice were administered i.n. a lethal challenge of 5 × 105 (A) or 105 (B and C) PFU HSV-2 186. Mice were monitored daily for lethality for 28 days. Panels A and B represent data from three and two independent experiments, respectively.
Immunization with rVV-ES-gB498-505 reduces the extent of HSV-2 proliferation in brains and trigeminal ganglia.
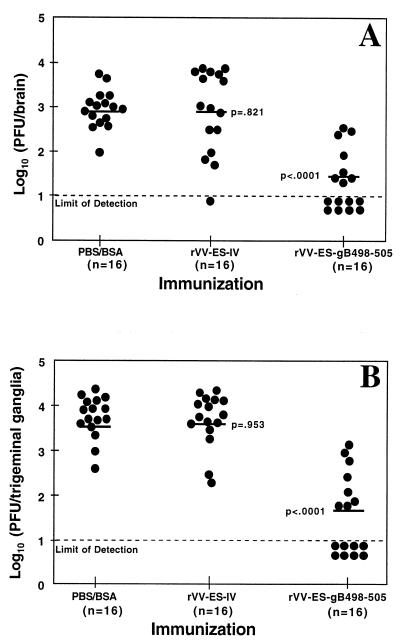
To determine the role of gB498-505-specific CTL in limiting the extent of HSV-2 infection of the central nervous system (CNS), levels of infectious virus were assessed in the brains and trigeminal ganglia of rVV-ES-gB498-505-immunized and control mice after challenge with 5 × 105 PFU of HSV-2. Five days after the lethal challenge, mice were sacrificed and brains and trigeminal ganglia were removed. The level of infectious HSV-2 was determined by plaque assay on Vero cells. The amounts of infectious virus recovered from the brains (Fig. 4A) and trigeminal ganglia (Fig. 4B) of rVV-ES-gB498-505-immunized mice were significantly lower than the levels of HSV-2 in the control PBS-BSA-immunized mice (P < 0.0001). As expected, immunization with rVV-ES-IV did not result in a reduction of infectious virus in the brains (P = 0.821) and trigeminal ganglia (P = 0.953) compared to the PBS-BSA-immunized mice. These results indicate that immunization with rVV-ES-gB498-505 mediated a decrease in the viral colonization of the CNS tissues during the acute stage of HSV-2 infection.
FIG. 4.
Immunization with rVV-ES-gB498-505 reduces the extent of HSV infection. Mice were immunized i.n. with either PBS-BSA or 107 PFU or rVV-ES-gB498-505 or rVV-ES-IV. Four to six weeks later, mice received i.n. a lethal dose of 5 × 105 PFU HSV-2 186. Five days later, the brains (A) and trigeminal ganglia (B) were removed and the level of infectious HSV-2 present in the tissues was determined by plaque analysis. The lower limit of detection (10 PFU/tissue) is indicated by the dashed line. The geometric mean of each immunization group is depicted by the solid horizontal line. The mean level of infectious virus recovered from the rVV-ES-gB498-505-immunized and rVV-ES-IV-immunized mice was compared to that of PBS-BSA-immunized mice, using the nonparametric Mann-Whitney test. Data represent the results of two independent experiments.
A recombinant influenza virus expressing gB498-505 induces protective HSV-specific CTL.
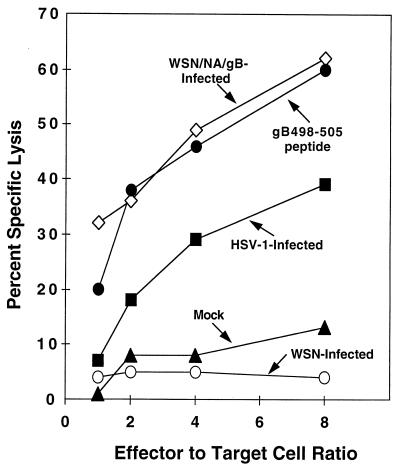
To further confirm the protective ability of gB498-505-specific CTL, we tested the ability of a recombinant influenza virus expressing HSV gB498-505 (SSIEFARL) within the NA stalk (WSN/NA/gB) to provide target cells for CTL clone 2D5 and to elicit HSV-specific CTL. B6/WT-3 cells were infected with either WSN/NA/gB or wild-type influenza virus (WSN) and tested in a standard 51Cr release assay for recognition by the CTL clone 2D5, which has specific lytic activity for HSV gB498-505 (Fig. 5). The 2D5 CTL clone recognized the WSN/NA/gB-infected cells, thus demonstrating that the gB498-505-specific CTL epitope can be functionally processed and presented from the NA stalk. WSN-infected cells were not recognized by the gB498-505-specific CTL, but as expected, CTL clone 2D5 recognized HSV-infected and gB498-505 peptide-pulsed B6/WT-3 cells.
FIG. 5.
WSN/NA/gB expresses the HSV-1 gB498-505 CTL epitope. 51Cr-labeled B6/WT-3 cells were infected with either HSV-1, WSN/NA/gB, or WSN. After a 5-h incubation, virus-infected B6/WT-3 cells pulsed with 1 μM gB498-505 synthetic peptide or mock peptide pulsed were added to the gB498-505-specific CTL clone 2D5 at the indicated effector-to-target ratios in a standard 51Cr release assay.
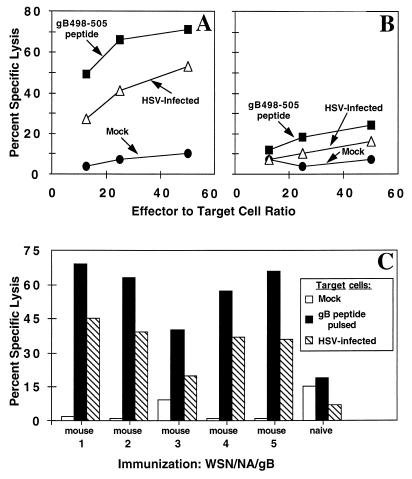
The immunogenicity of WSN/NA/gB was determined by examining primary and memory, gB498-505-specific CTL responses which were induced by i.n. immunization. Five days after i.n. infection of C57BL/6 mice, we removed the mediastinal lymph nodes and spleens and cultured single-cell suspensions with no added stimulator cells for 3 days as described previously (9). The lymphocyte cultures were tested in a standard 51Cr release assay for HSV- and gB498-505-specific lytic activity. HSV-specific CTL activity was readily detected in the mediastinal lymph nodes (Fig. 6A), and a lower level of primary gB498-505-specific lytic activity was observed in the spleens (Fig. 6B) of immunized mice. Splenocytes from mice which had been immunized 4 weeks earlier with WSN/NA/gB were stimulated in vitro for 5 days with HSV-1-infected, mitomycin C-treated B6/WT-3 cells and contained HSV-specific lytic activity (Fig. 6C).
FIG. 6.
Immunization with WSN/NA/gB induces a primary and memory HSV-specific CTL response. For induction of primary CTL, 8 C57BL/6 mice were immunized i.n. with WSN/NA/gB, and 5 days later the mediastinal lymph nodes (A) and spleens (B) were removed. Bulk single-cell suspensions were incubated with no added stimulator cells for 5 days and then tested for lytic activity against HSV-infected 51Cr-labeled B6/WT-3 cells, B6/WT-3 cells pulsed with 1 μM gB498-505 synthetic peptide, and mock peptide-pulsed B6/WT-3 cells. For analysis of a CTLm response (C), five mice were immunized i.n. with WSN/NA/gB. An unimmunized (naive) mouse served as a negative control. Four weeks later, splenocytes from individual mice were cultured with HSV-1-infected, mitomycin C-treated B6/WT-3 cells and tested in a 51Cr release assay for lytic activity against HSV-infected B6/WT-3 cells, B6/WT-3 cells pulsed with 1 μM gB498-505 synthetic peptide, and mock peptide-pulsed B6/WT-3 cells. The results represent an effector-to-target ratio of 30:1.
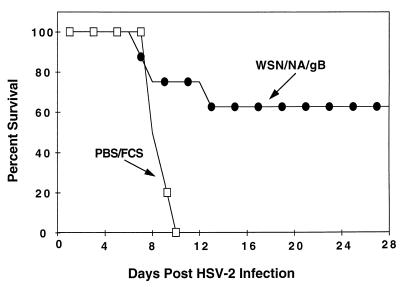
With the demonstration of the effective induction of gB498-505-specific CTL by immunization with WSN/NA/gB, we next assessed the protective ability of the recombinant influenza virus (Fig. 7). Mice were immunized i.n. with either WSN/NA/gB or PBS–1% FCS and four weeks later challenged with 105 PFU of HSV-2 186. All control mice succumbed to the lethal infection, while 63% of the WSN/NA/gB-immunized mice survived the lethal challenge of HSV-2. The epitope-specific protection afforded by this alternate vector clearly confirms the ability of immunization with a single CTL recognition epitope of HSV to confer a significant level of protection from lethal HSV-2 infection.
FIG. 7.
Immunization with WSN/NA/gB confers protection from lethal HSV-2 infection. Mice were immunized i.n. with either PBS–1% FCS (n = 5) or WSN/NA/gB (n = 8) as described in Materials and Methods. Four weeks later, all mice were administered an i.n. challenge of 105 PFU HSV-2 186. Mice were monitored daily for lethality for 28 days.
DISCUSSION
Although we have previously shown that HSV-1 gB498-505-specific CTL can be induced by a variety of methods (5, 8, 19), we had yet to determine the protective nature of CTL directed toward this single, immunodominant epitope against HSV pathogenesis. The data presented here demonstrate that i.n. immunization of C57BL/6 mice with a recombinant vaccinia or influenza virus expressing the single CTL recognition epitope HSV gB498-505 induced both primary and memory CTL responses directed against this epitope. Mice immunized with the rVV showed a reduction in HSV colonization of the CNS and exhibited protection from a lethal challenge of HSV-2. These results indicate that the induction of a single epitope-specific CTL response is sufficient to confer a significant level of protection from lethal HSV-2 infection.
The relative contribution of CD8+ T cells and other components of the adaptive immune response to the control of HSV infection has been controversial. Both T-cell depletion and adoptive transfer studies in mice have supported a role for CD8+ T cells in the control of HSV infections (6, 65–67). However, using a murine zosteriform model of HSV infection, the CD4+ T-cell subset has been demonstrated to be primarily responsible for control of HSV infection (37–39). The apparent discrepancies in the relative importance of each of these subsets may be a function of the particular model of HSV infection used in each of these studies. HSV infection in humans is known to elicit the generation of HSV-specific CD8+ T cells (56, 57, 72), although CD4+ T cells have also been readily detected in individuals seropositive for HSV (74–76). Recently, Posavad et al. demonstrated a correlation between low HSV-specific precursor CD8+ CTL frequencies and severe HSV recurrences in HIV-infected individuals (58). These findings further indicate the importance of CD8+ T cells in the control of HSV infection of humans.
This study used multiple recombinant viral vectors for the induction of HSV-specific CTL with a single specificity. An rVV (rVV-ES-gB498-505) encoding the gB498-505 CTL recognition epitope fused to the adenovirus E3/19K ES (2, 20, 35, 68) was generated. The expression of a gB498-505 CTL epitope peptide fused to this sequence allowed the peptide to be inserted directly into the lumen of the endoplasmic reticulum, thus bypassing the requirement for TAP-dependent peptide transport (Fig. 1C). Our laboratory has previously demonstrated that the fusion of an SV40 T-antigen immunorecessive epitope sequence to the ES can confer increased immunogenicity (20). An rVV expressing gB498-505 without the fused ES and the recombinant influenza virus also served as effective immunogens.
Immunization with rVV-ES-gB498-505 provided protection from i.n. challenge with a lethal dose of HSV-2 (Fig. 3). Although only 29% of immunized mice survived the challenge with 5 × 105 PFU, there was a clear delay in the death of those mice that ultimately succumbed to the lethal HSV-2 infection. However, 69% of rVV-ES-gB498-505-immunized mice survived HSV-2 challenge with a fivefold-lower dose (105 PFU). The lower level of protection seen in mice challenged with the higher dose suggests that there is a critical threshold of infection which the gB498-505-specific CTL can adequately control and that higher levels of viral challenge may overwhelm the capacity of these HSV-specific CTL to control the infection. It is important to note that the observed protection was afforded by a single immunization with rVV-ES-gB498-505. Therefore, it is possible that an increased level of protection is provided by an additional immunization with this or other vectors expressing the gB498-505 epitope. Protection from lethal infection was also observed upon immunization with two other recombinant viral vectors, rVV-gB498-505 (Fig. 3C) and WSN/NA/gB (Fig. 7), confirming that a CTL response directed against the single gB498-505 CTL epitope can confer resistance. The observed protection afforded by immunization with rVV-gB498-505 (Fig. 3C) demonstrated that the gB498-505-specific protection was not dependent on or enhanced by the presence of an ES fused to the gB498-505 epitope. The observed protection was also not dependent on the use of an i.n. route of immunization, since B6 mice immunized intraperitoneally with rVV-ES-gB498-505 were protected from lethal i.n. HSV-2 challenge (data not shown). Overall, these findings demonstrate that the presence of HSV-specific CTLm prior to infection can control a lethal HSV challenge without the additional presence of a preexisting HSV-specific humoral or CD4+ T-cell response. CD4+ T cells have been shown to be required in a primary HSV-specific CTL response but not in a secondary CTL response (26). The CD4+ T-cell help needed for the generation of CTL in B6 mice immunized with the recombinant viruses expressing the gB498-505 epitope may be provided by vector-specific CD4+ T cells.
Our results suggest the possibility that the epitope-specific CTL act in the CNS to control viral infection. However, the possibility also exists that gB498-505-specific CTL limit HSV-2 replication at the initial site of infection, the nasal mucosa, and thus prevent the infection of innervating neurons. Previous studies in mice have demonstrated the sequential course of i.n. HSV infection initiating in the nasal mucosa followed by the dissemination of the infection to the brain and trigeminal ganglia (4, 16). Immunization with rVV-ES-gB498-505 significantly reduced the extent of viral colonization of the CNS tissues (Fig. 4). The mechanisms underlying this protection may include direct CTL-mediated lysis of HSV-2-infected cells or the production of cytokines such as gamma interferon which directly or indirectly serve to limit the establishment and/or spread of the viral infection (46, 67).
Since both HSV-1 and HSV-2 are able to establish infections at mucosal surfaces, the use of a mucosal route of immunization may be most effective in the development of an effective human vaccine. Previous studies have demonstrated that anti-HSV immunity may be induced by mucosal immunization with vectors which express HSV proteins (22, 30, 43, 48). For example, immunization with a recombinant adenovirus expressing full-length HSV gB has been demonstrated to confer protection from HSV infection. Moreover, this protection correlated with the induction of an HSV-specific neutralizing antibody and CTL response (21). Other approaches used for the induction of protective HSV-specific immune responses include immunization with attenuated HSV or replication-defective HSV mutants (9, 44, 45, 49). In addition, vectors expressing HSV-encoded proteins (10, 12, 21, 23, 29, 37, 40–42, 63) have been shown to be effective in mediating protection against HSV infection.
In summary, our results suggest that the induction of HSV-specific CD8+ T cells provides an alternative approach for the generation of acquired immunity to HSV. This study not only confirms the significance of CD8+ CTL in the control of HSV infection but also illustrates the potential importance of designing mucosal HSV vaccines which target specific CTL recognition epitopes encoded within HSV.
ACKNOWLEDGMENTS
This study was supported by research grants AI34070 to S.S.T. and AI29599 to Y.K. from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md. M.A.B. was supported by training grant 5T32CA60395 from the National Cancer Institute.
REFERENCES
- 1.Anderson K, Alexander J, Wei J, Cresswell P. Intracellular transport of class I molecules in antigen processing mutant cell lines. J Immunol. 1993;151:3407–3419. [PubMed] [Google Scholar]
- 2.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweernik H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks T A, Nair S, Rouse B T. Recognition by and in vitro induction of cytotoxic T lymphocytes against predicted epitopes of the immediate-early protein ICP27 of herpes simplex virus. J Virol. 1993;67:613–616. doi: 10.1128/jvi.67.1.613-616.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerman R H, Peters A C B, Bloem B R, Raap A K, van der Ploeg M. Spread of herpes simplex virus to the cerebrospinal fluid and the meninges in experimental mouse encephalitis. Acta Neuropathol. 1992;83:300–307. doi: 10.1007/BF00296793. [DOI] [PubMed] [Google Scholar]
- 5.Bonneau R H, Fu T-M, Tevethia S S. In vivo priming and activation of memory cytotoxic T-lymphocytes (CTL) by a chimeric simian virus 40 T antigen expressing an eight amino acid residue herpes simplex virus gB CTL epitope. Virology. 1993;197:782–787. doi: 10.1006/viro.1993.1657. [DOI] [PubMed] [Google Scholar]
- 6.Bonneau R H, Jennings S R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989;63:1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonneau R H, Jennings S R. Herpes simplex virus-specific cytolytic T lymphocytes restricted to a normally low responder H-2 allele are protective in vivo. Virology. 1990;174:599–604. doi: 10.1016/0042-6822(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 8.Bonneau R H, Salvucci L A, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1- and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 9.Brehm M A, Bonneau R H, Knipe D M, Tevethia S S. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke R L, Goldbeck C, Ng P, Stanberry L, Ott G, Nest G V. The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J Infect Dis. 1994;170:1110–1119. doi: 10.1093/infdis/170.5.1110. [DOI] [PubMed] [Google Scholar]
- 11.Bzik D, Debroy C, Fox B A, Pederson N E, Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986;155:322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- 12.Cantin E M, Eberle R, Baldick J L, Moss B, Willey D E, Notkins A L, Openshaw H. Expression of herpes simplex virus type 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against a lethal herpes simplex 1 infection. Proc Natl Acad Sci USA. 1987;84:5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter V C, Schaffer P A, Tevethia S S. The involvement of herpes simplex virus type-1 glycoproteins in cell mediated immunity. J Immunol. 1981;126:1655–1660. [PubMed] [Google Scholar]
- 14.Castrucci M R, Hou S, Doherty P C, Kawaoka Y. Protection against lethal lymphocytic choriomeningitis virus (LCMV) infection by immunization of mice with an influenza virus containing an LCMV epitope recognized by cytotoxic T lymphocytes. J Virol. 1994;68:3486–3490. doi: 10.1128/jvi.68.6.3486-3490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Val M, Schlicht H-J, Volkmer H, Messerele M, Reddehase M J, Kozinowski U H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991;65:3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond C W E, Eglin R P, Esiri M M. Herpes simplex virus encephalitis in a mouse model: PCR evidence for CNS latency following acute infection. J Neurol Sci. 1994;127:159–163. doi: 10.1016/0022-510x(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 17.Earl P L, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 16.17.1–16.17.16. [Google Scholar]
- 18.Enami M, Luytjes W, Krystal M, Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci USA. 1990;87:3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu T-M, Bonneau R H, Epler M, Tevethia M J, Alam S, Verner K, Tevethia S S. Induction and persistence of a cytotoxic T lymphocyte (CTL) response against a herpes simplex virus-specific CTL epitope expressed in a cellular protein. Virology. 1996;222:269–274. doi: 10.1006/viro.1996.0419. [DOI] [PubMed] [Google Scholar]
- 20.Fu T M, Mylin L M, Schell T D, Bacik I, Russ G, Yewdell J W, Bennink J R, Tevethia S S. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1997;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallichan W S, Johnson D C, Graham F L, Rosenthal K L. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J Infect Dis. 1993;168:622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- 22.Gallichan W S, Rosenthal K L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal immunization but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiasi H, Kaiwar R, Nesburn A B, Slanina S, Wechsler S L. Baculovirus-expressed glycoprotein E (gE) of herpes simplex virus type-1 (HSV-1) protects mice against lethal intraperitoneal and lethal ocular HSV-1 challenge. Virology. 1992;188:469–476. doi: 10.1016/0042-6822(92)90500-o. [DOI] [PubMed] [Google Scholar]
- 24.Hanke T, Graham F L, Rosenthal K L, Johnson D C. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igietseme J U, Calzada P J, Gonzalez A R, Streilein J W, Atherton S S. Protection of mice from herpes simplex virus retinitis by in vitro-activated immune cells. J Virol. 1989;63:4808–4813. doi: 10.1128/jvi.63.11.4808-4813.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings S R, Bonneau R H, Smith P M, Wolcott R M, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor A K, Nash A A, Wildy P, Phelan J, McLean C S, Field H J. Pathogenesis of herpes simplex virus in congenitally athymic mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982;60:225–233. doi: 10.1099/0022-1317-60-2-225. [DOI] [PubMed] [Google Scholar]
- 28.Kast W M, Roux L, Curren J, Blom H J J, Voordouw A C, Meloen R H, Kolakofsky D, Melief C J M. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna S, Blacklaws B A, Overton H A, Bishop D H L, Nash A A. Expression of glycoprotein D of herpes simplex virus type 1 in a recombinant baculovirus: protective responses and T cell recognition of recombinant-infected cell extracts. J Gen Virol. 1989;70:1805–1814. doi: 10.1099/0022-1317-70-7-1805. [DOI] [PubMed] [Google Scholar]
- 30.Kuklin N, Dahesia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen H S, Feng M, Horohov D W, Moore R N, Rouse B T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984;50:56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen H S, Russell R G, Rouse B T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983;41:197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawman M B, Rouse B T, Courtney R J, Walker R D. Cell-mediated immunity against herpes simplex virus: induction of cytotoxic T lymphocytes. Infect Immun. 1980;27:133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson C M, Bennink J R, Restifo N P, Yewdell J W, Murphy B R. Primary pulmonary cytotoxic T lymphocytes induced by immunization with a vaccinia virus recombinant expressing influenza A virus nucleoprotein peptide do not protect mice against challenge. J Virol. 1994;68:3505–3511. doi: 10.1128/jvi.68.6.3505-3511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackett M, Smith G L, Moss B. General method for production and selection of infectious vaccinia virus recombinants. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manickan E, Francotte M, Kuklin N, Dewerchin M, Molitor C, Gheysen D, Slaoui M, Rouse B T. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ cells. J Virol. 1995;69:4711–4716. doi: 10.1128/jvi.69.8.4711-4716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manickan E, Rouse B. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse models. J Virol. 1995;69:8178–8179. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manickan E, Rouse R J D, Yu Z, Wire S, Rouse B T. Genetic immunization against herpes simplex virus. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 40.Martin S, Rouse B T. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J Immunol. 1987;138:3431–3437. [PubMed] [Google Scholar]
- 41.McClements W L, Armstrong M E, Keys R D, Liu M A. Immunization with DNA vaccines encoding glycoprotein D or glycoprotein B, alone or in combination, induces protective immunity in animal models of herpes simplex virus-2 disease. Proc Natl Acad Sci USA. 1996;93:11414–11420. doi: 10.1073/pnas.93.21.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott M, Graham F L, Hanke T, Johnson D C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989;169:244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 43.McDermott M R, Goldsmith G H, Rosenthal K L, Brais L S. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989;159:460–466. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- 44.McLean C S, Erturk M, Jennings R, Challanain D N, Minson A C, Duncan I, Boursnell M E G, Inglis S C. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994;170:1100–1109. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- 45.Meignier B. Genetically engineered attenuated herpes simplex viruses. Rev Infect Dis. 1991;13:s895–s897. doi: 10.1093/clind/13.supplement_11.s895. [DOI] [PubMed] [Google Scholar]
- 46.Milligan G N, Bernstein D I. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell B M, Stevens J G. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J Immunol. 1996;156:246–255. [PubMed] [Google Scholar]
- 48.Morrison L A, Costa X J D, Knipe D M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 49.Morrison L A, Knipe D M. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mylin L M, Deckhut A M, Bonneau R H, Kierstead T D, Tevethia M J, Simmons D T, Tevethia S S. Cytotoxic T lymphocyte escape variants, induced mutations and synthetic peptides define a dominant H-2Kb-restricted determinant in simian virus 40 tumor antigen. Virology. 1995;208:159–172. doi: 10.1006/viro.1995.1139. [DOI] [PubMed] [Google Scholar]
- 51.Nash A A, Cambouropoulos P. The immune response to herpes simplex virus. Semin Virol. 1993;4:181–186. [Google Scholar]
- 52.Nash A A, Field H J, Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980;48:351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- 53.Nash A A, Quartey-Papafio R, Wildy P. Cell-mediated immunity in herpes simplex virus infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980;49:309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- 54.Oldstone M B A, Tishon A, Geckeler R, Lewicki H, Whitton J L. A common antiviral cytotoxic T-lymphocyte epitope for diverse major histocompatibility complex haplotypes: implications for vaccination. Proc Natl Acad Sci USA. 1992;89:2752–2755. doi: 10.1073/pnas.89.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oukka M, Manuguerra J C, Livaditis N, Tourdot S, Riche N, Vergnon I, Cordopatis P, Kosmatopoulos K. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J Immunol. 1996;157:3039–3045. [PubMed] [Google Scholar]
- 56.Posavad C M, Koelle D M, Corey L. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Posavad C M, Koelle D M, Corey L. Tipping the scale of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 58.Posavad C M, Koelle D M, Shaughnessy M F, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pretell J, Greenfield R S, Tevethia S S. Biology of simian virus 40 (SV40) transplantation rejection antigen (TrAg). V. In vitro demonstration of SV40 TrAg in SV40-infected permissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979;97:32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- 60.Rhim J S, Cho H Y, Huebner R J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975;15:23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- 61.Rinaldo C R, Torpey D J. Cell-mediated immunity and immunosuppression in herpes simplex virus infection. Immunodeficiency. 1993;5:33–90. [PubMed] [Google Scholar]
- 62.Rodriguez F, An L L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller J T, Kincaid C, Campbell I L, Whitton J L. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooney J F, Wohlenberg C, Cremer K J, Notkins A L. Immunized mice challenged with herpes simplex virus by the intranasal route show protection against latent infection. J Infect Dis. 1989;159:974–976. doi: 10.1093/infdis/159.5.974. [DOI] [PubMed] [Google Scholar]
- 64.Salvucci L A, Bonneau R H, Tevethia S S. Polymorphism within the herpes simplex virus type 1 (HSV-1) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV-1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–1131. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sethi K K, Omata Y, Schneweis K E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983;64:443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- 66.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 68.Snyder H L, Yewdell J W, Bennink J R. Trimming of antigenic peptides in an early secretory compartment. J Exp Med. 1994;180:2389–2394. doi: 10.1084/jem.180.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka Y, Tevethia S S. In vitro selection of SV40 T antigen epitope loss variants by site specific cytotoxic T lymphocyte clone. J Immunol. 1988;140:4348–4354. [PubMed] [Google Scholar]
- 70.Tanaka Y, Tevethia S S. Loss of immunorecessive cytotoxic T lymphocyte determinant V on SV40 T antigen following cocultivation with site-specific cytotoxic T lymphocyte clone Y-5. Intervirology. 1990;31:197–202. doi: 10.1159/000150154. [DOI] [PubMed] [Google Scholar]
- 71.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1620. [PubMed] [Google Scholar]
- 72.Tigges M A, Koelle D, Hartog K, Sekulovich R E, Corey L, Burke R L. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J Virol. 1992;66:1622–1634. doi: 10.1128/jvi.66.3.1622-1634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitton L J, Sheng N, Oldstone M B A, McKee T A. A “string of beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yasukawa M, Inatsuki A, Kobayashi Y. Differential in vitro activation of CD4+ CD8− and CD8+ CD4− herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989;143:2051–2057. [PubMed] [Google Scholar]
- 75.Yasukawa M, Zarling J M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984;133:2736–2742. [PubMed] [Google Scholar]
- 76.Zarling J M, Moran P A, Brewer L, Ashley R, Corey L. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J Virol. 1988;62:4481–4485. doi: 10.1128/jvi.62.12.4481-4485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]