Abstract
Background
Spinal metastases are a significant complication of advanced cancer. In this study, we assess temporal trends in the incidence and timing of spinal metastases and examine underlying patient demographics and primary cancer associations.
Methods
In this population-based retrospective cohort study, health data from 2007 to 2019 in Ontario, Canada were analyzed (n = 37, 375 patients identified with spine metastases). Primary outcomes were annual incidence of spinal metastasis, and time to metastasis after primary diagnosis.
Results
The age-standardized incidence of spinal metastases increased from 229 to 302 cases per million over the 13-year study period. The average annual percent change (AAPC) in incidence was 2.2% (95% CI: 1.4% to 3.0%) with patients aged ≥85 years demonstrating the largest increase (AAPC 5.2%; 95% CI: 2.3% to 8.3%). Lung cancer had the greatest annual incidence, while prostate cancer had the greatest increase in annual incidence (AAPC 6.5; 95% CI: 4.1% to 9.0%). Lung cancer patients were found to have the highest risk of spine metastasis with 10.3% (95% CI: 10.1% to 10.5%) of patients being diagnosed at 10 years. Gastrointestinal cancer patients were found to have the lowest risk of spine metastasis with 1.0% (95% CI: 0.9% to 1.0%) of patients being diagnosed at 10 years.
Conclusions
The incidence of spinal metastases has increased in recent years, particularly among older patients. The incidence and timing vary substantially among different primary cancer types. These findings contribute to the understanding of disease trends and emphasize a growing population of patients who require subspecialty care.
Keywords: cohort studies, epidemiology, incidence, Ontario, spinal metastasis
Key Points.
The incidence of spinal metastasis increased from 229 to 302 cases per million.
Incidence and timing of spinal metastasis varied by age and primary cancer type.
Lung cancer patients have the highest risk of spinal metastasis after diagnosis.
Importance of the Study.
Our results point to a growing population of patients requiring treatment for spinal metastases. The demographics of this population are changing, and we can expect a larger proportion of the patient pool to be of older age and with metastatic prostate cancer. Furthermore, we demonstrate variation in timing of metastases based on primary cancer type. Lung cancer patients have the greatest risk of metastasis after diagnosis, and gastrointestinal cancer patients have the lowest. These findings have health policy implications and can assist in patient counseling and surveillance at the time of cancer diagnosis.
Spinal metastases are a consequential complication of advanced cancer. Patients with metastatic tumors to the spine often suffer from debilitating pain and neurologic deficits due to pathologic fractures or tumor-related compression of the spinal cord or spinal nerves.1 This complication is also costly to the healthcare system, with direct healthcare cost estimates being upwards of $554 323 USD per patient for the first 60 days of treatment.2 Appropriate understanding of epidemiology is paramount for counseling patients, optimizing health policy, and improving the efficiency of health care delivery.3,4
The cancer population is changing, driven largely by increased life expectancy, improved systemic cancer therapy, and a growing population of patients living with primary tumors.5 The World Health Organization has estimated that 29.4 million new cancer patients will be diagnosed in 2040, a roughly 60% increase.6 The spine is commonly cited to be the most common site of bony metastases.7 The evaluation and treatment of metastatic spine disease, therefore, will be a growing burden. reevaluation of the epidemiology of patients with spinal metastases is therefore needed, to keep our estimates current. Prior estimates of incidence and temporal trends of spine metastases are either outdated or based on single-center data.8–12
The purpose of this study was to evaluate the temporal trends in the incidence and timing of spine metastases over a 13-year period from 2007 to 2019. In addition, age, sex, and cancer subtype-specific estimates of the risk of spinal metastases are evaluated and compared.
Materials and Methods
Study Design
This longitudinal population-based retrospective cohort study was completed in Ontario, where universal health care services are accessible through the Ontario Health Insurance Plan (OHIP). Ontario has the largest population among provinces in Canada, with estimates growing from 12.8 to 14.6 million people during the study.13 This total accounts for nearly half of the Canadian population. The study was approved by the Unity Health Toronto research ethics board (REB 21–149). In addition, this study followed the Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) guidelines.14
Data Source
Data was retrieved from administrative health databases for the years 2007 to 2019. All data were available through the Institute for Clinical Evaluative Sciences (ICES).15 ICES is an independent, nonprofit research institute funded by an annual grant from the Ontario Ministry of Health. As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario.15To define the patient cohort unique ICES patient identifiers were used to link data from the Ontario Cancer Registry (OCR), the OHIP Claims database, and the Cancer Care of Ontario Activity Level Reporting Database for Radiotherapy (ALR). Patient demographics were derived from the Registered Person’s Database (RPDB). A summary of data sources is provided in Supplementary Table 1.
Patients
We identified patients by linking the OCR, OHIP, and ALR with unique individual identifiers. Inclusion criteria included all OHIP-eligible adults over 25 years registered in the OCR and alive at the time of diagnosis of their primary cancer. This age range was chosen to align with age ranges available for reference populations within Statistics Canada. The OCR identifies all new cancer cases diagnosed in persons living within Ontario.16 To identify those who subsequently developed spine metastases we identified cancer patients with either an OHIP fee code for extradural tumor decompression linked with an OHIP neoplastic diagnosis code or a spine body region radiation code within ALR. We excluded patients known to have primary spine, peripheral nerve, intradural, or intramedullary tumors. This method of identification of patients with spinal metastases has been adapted from previous publications.17–19Supplementary Figure 1 outlines cohort creation as per RECORD guidelines. Codes used for patient inclusion and exclusion are listed in Supplementary Table 2.
Subgroups
Patient subgroups were prespecified for evaluation. Age subgroups were defined as 25–44, 45–64, 65–74, 75–84, and ≥85 years. Sex subgroups were male and female. Primary cancer types were grouped as lung, breast, prostate, gastrointestinal, myeloma, urological, lymphoma, hepatobiliary, melanoma, gynecological, thyroid, or miscellaneous. These primary cancer groups were selected based on available International Classification of Diseases Oncology 3 Topography Codes as described in prior studies.17,19 Outcomes were provided for the 6 most common primary cancers within our spine metastases cohort; namely, lung, breast, prostate, gastrointestinal, myeloma, and urological. Diagnostic codes for primary cancer subtypes are provided in Supplementary Table 4.
Outcomes
The primary outcome of annual incidence of spine metastases was estimated by identifying new cases in Ontario from January 1st through December 31st of each year from 2007 to 2019. Crude incidences were estimated using the annual counts with reference to the Ontario population for the given year. Age and sex subgroup incidence were estimated by using age group or sex group-specific Ontario populations. Primary cancer subgroups were referenced to the general Ontario population for the given year, to provide their year-specific contribution to the overall incidence for the year.
Total population, and subgroup estimates by age and sex were based on data available from Statistics Canada.20 Age-standardized incidence was calculated by re-weighting crude incidence to match a 2006 Ontario population distribution. This was done to accurately compare incidence across years independent of differences in age distribution, as recommended by Statistics Canada and the National Cancer Institute Division of Cancer Control & Population Sciences.21,22 For comparison, we estimated the age-standardized overall incidence of new cancer diagnoses for each primary cancer type.
The timing of metastases was assessed as a time-to-event variable by calculating the time between the date of primary cancer diagnosis and the date of the first spine metastases intervention. The earliest date of entry between OHIP and ALR was used as an estimate of the date of spinal metastases (Supplementary Table 2). In cases where the date of entry of these codes was at the same date or prior to the date of primary cancer diagnosis, then a synchronous presentation of cancer and spinal metastases was assumed, with a of zero days.
The incidence rate of spine metastases after cancer diagnosis was calculated for each cancer type using a person-years approach.23 Estimates were calculated by dividing the number of spinal metastases occurring among patients at risk during the study period, divided by the total time at risk for the subgroup. Individual patient time at risk was defined to be the interval between cancer diagnosis and the date of earliest spine metastasis intervention for the patients with spinal metastasis. For cancer patients not found to have a spine metastasis during the study period, the time at risk was the interval between cancer diagnosis and the date of death or final follow-up.
Statistical Analyses
Statistical analyses were performed using R version 4.2.1 with a prespecified significance level of P = .05 for 2-tailed tests. Descriptive statistics were reported as mean, standard deviation (SD), minimum, median, and maximum for continuous variables and counts with proportions for categorical variables. Standard errors and 95% confidence intervals for crude incidence and incidence rates were computed by assuming a Poisson distribution of population rates, as recommended for rare outcomes.24
Temporal trend analysis was done using Join-point regression software available through the National Cancer Institute Division of Cancer Control & Population Sciences.25,26 Join-point software was used to compute an average annual percent change (AAPC), using the grid search method, with model selection done using the Weighted Bayesian Information Criterion, and 95% confidence intervals computed with a parametric method.27 AAPC confidence intervals for the incidence of spinal metastases were compared to the overall incidence of new cancer diagnoses to detect statistically significant differences.
Time to metastasis for the cohort and for primary cancer subgroups were analyzed using a competing risks approach.28 The time of death was recorded as a competing event for cancer patients in OCR not found to have a spine metastasis during the study period. The cumulative incidence function with log-based 95% confidence intervals were estimated using the Nelson-Aelen method.29,30 Point estimates for the cumulative incidence of spinal metastasis at 1, 5, and 10 years were computed with 95% confidence intervals. Time points were chosen to respect the range of event times available within the cohort. Gray’s test was used to compare cumulative incidence curves between primary cancer subgroups.31
Results
Overview
The study cohort consisted of 37 375 patients identified with spinal metastases from 2007 to 2019. The mean age was 64.4 years (12.6 years SD) and 55% were male. The 6 most common primary cancer types within the cohort were lung (27%), breast (16%), prostate (16%), gastrointestinal (8%), myeloma (8%), and urological (7%). A baseline description of the cohort is provided in Table 1.
Table 1.
Baseline Characteristics of Study Cohort of Patients With Age-Standardized Annual Incidence of Spinal Metastases, and Average Annual Percent Change (AAPC) in the Incidence of Spine Metastasis SD Standard Deviation
| Year | No. of Patients | Age, years (SD) | Male (%) | Lung (%) | Breast (%) | Prostate (%) | Gastrointestinal (%) | Myeloma (%) | Urological (%) | Age—Standardized annual incidence of spinal metastases (95% CI)—/Million | AAPC in spinal metastases incidence (95% CI), P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007–2019 | 37 375 | 64.4 (12.6) | 20 564 (55.0) | 10 150 (27.2) | 5958 (15.9) | 5814 (15.6) | 3146 (8.4) | 3061 (8.2) | 2759 (7.4) | — | 2.2 (1.4 to 3.0), P < .01 |
| 2007 | 2033 | 63.7 (12.8) | 1059 (52.1) | 626 (30.8) | 302 (14.9) | 228 (11.2) | 172 (8.5) | 192 (9.4) | 146 (7.2) | 229 (219–239) | |
| 2008 | 2203 | 63.7 (12.6) | 1206 (54.7) | 698 (31.7) | 295 (13.4) | 275 (12.5) | 195 (8.9) | 197 (8.9) | 139 (6.3) | 242 (232–252) | |
| 2009 | 2400 | 64.1 (12.6) | 1300 (54.2) | 692 (28.8) | 361 (15.0) | 320 (13.3) | 226 (9.4) | 203 (8.5) | 185 (7.7) | 257 (247–268) | |
| 2010 | 2450 | 64.3 (12.7) | 1365 (55.7) | 714 (29.1) | 385 (15.7) | 329 (13.4) | 205 (8.4) | 206 (8.4) | 199 (8.1) | 255 (245–265) | |
| 2011 | 2691 | 64.4 (12.9) | 1473 (54.7) | 805 (29.9) | 385 (14.3) | 375 (13.9) | 239 (8.9) | 219 (8.1) | 188 (7.0) | 273 (263–284) | |
| 2012 | 2828 | 64.3 (12.8) | 1548 (54.7) | 782 (27.7) | 443 (15.7) | 416 (14.7) | 249 (8.8) | 261 (9.2) | 187 (6.6) | 282 (272–293) | |
| 2013 | 2946 | 64.2 (12.7) | 1611 (54.7) | 796 (27.0) | 479 (16.3) | 434 (14.7) | 249 (8.5) | 223 (7.6) | 234 (7.9) | 285 (275–296) | |
| 2014 | 2987 | 64.4 (12.9) | 1622 (54.3) | 827 (27.7) | 505 (16.9) | 459 (15.4) | 250 (8.4) | 260 (8.7) | 191 (6.4) | 284 (273–294) | |
| 2015 | 3164 | 64.5 (12.5) | 1722 (54.4) | 825 (26.1) | 519 (16.4) | 522 (16.5) | 268 (8.5) | 257 (8.1) | 208 (6.6) | 293 (283–304) | |
| 2016 | 3380 | 64.8 (12.6) | 1921 (56.8) | 851 (25.2) | 554 (16.4) | 591 (17.5) | 277 (8.2) | 244 (7.2) | 278 (8.2) | 305 (295–316) | |
| 2017 | 3293 | 64.3 (12.6) | 1829 (55.5) | 800 (24.3) | 560 (17.0) | 561 (17.0) | 262 (8.0) | 247 (7.5) | 270 (8.2) | 291 (281–301) | |
| 2018 | 3412 | 64.8 (12.4) | 1912 (56.0) | 835 (24.5) | 556 (16.3) | 611 (17.9) | 280 (8.2) | 272 (8.0) | 250 (7.3) | 294 (284–304) | |
| 2019 | 3588 | 65.0 (12.3) | 1996 (55.6) | 899 (25.1) | 614 (17.1) | 693 (19.3) | 274 (7.6) | 280 (7.8) | 284 (7.9) | 302 (292–312) |
Annual Incidence of Spinal Metastases
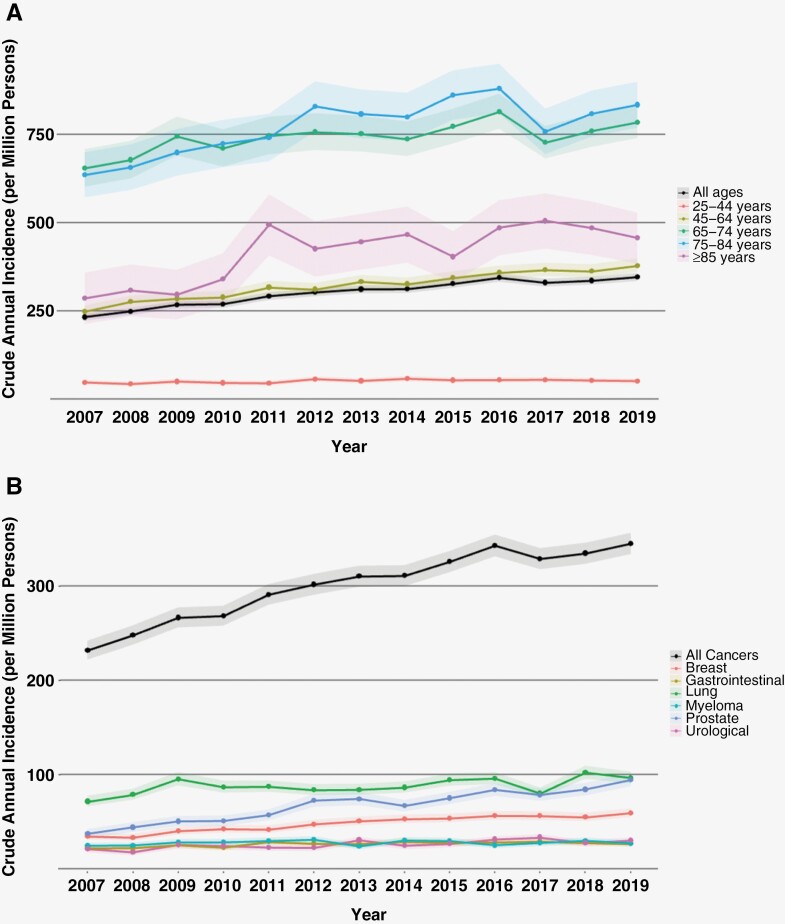
A significant increase in the incidence of spinal metastases was noted across the study years (AAPC 2.2; 95% CI: 1.4 to 3.0). We found no significant difference between males and females in the annual age-standardized incidence of spinal metastasis, or the AAPC across the study period (Table 2). The age groups with the highest annual incidence across the study period were 65–74 years, and 75–84 years (Figure 1A). Temporal trend analysis provided in Table 2 demonstrates that all age groups above 45 years demonstrated a significant increase in the AAPC across the study period. The age group demonstrating the largest growth in annual incidence were patients aged ≥85 years (AAPC 5.2; 95% CI: 2.3 to 8.3).
Table 2.
Number of Cases, Annual Incidence, and Average Annual Percent Change (AAPC) in Incidence of Spine Metastasis by Age Groups and Sex
| Age 25–44 years | Age 45–64 years | Age 65–74 years | Age 75–84 years | Age ≥ 85 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Count | Annual incidence (95% CI) -/Million | AAPC (95% CI), P-value |
Count | Crude annual incidence (95% CI)/Million | AAPC (95% CI) P-value |
Count | Annual incidence (95% CI) -/Million | AAPC (95% CI), P-value |
Count | Annual incidence (95% CI) -/Million | AAPC (%), P-value |
Count | Annual incidence (95% CI) -/Million | AAPC (95% CI) P-value |
| 2007 | 172 | 47 (40–54) | 1.3 (−0.9 to 3.6) P = .25 |
842 | 248 (231–265) | 3.4 (2.4 to 4.3) P < .01 |
581 | 654 (601–707) | 1.0 (0.3 to 1.7) P < .01 |
380 | 635 (571–699) | 2.3 (0.7 to 4.0) P < .01 |
58 | 285 (212–358) | 5.2 (2.3 to 8.3) P < .01 |
| 2008 | 154 | 42 (35–49) | 965 | 276 (258–293) | 620 | 678 (624–731) | 398 | 657 (592–721) | 66 | 307 (233–381) | |||||
| 2009 | 178 | 49 (42–56) | 1024 | 284 (267–302) | 704 | 745 (690–800) | 427 | 699 (632–765) | 67 | 295 (225–366) | |||||
| 2010 | 163 | 45 (38–52) | 1065 | 288 (271–306) | 693 | 711 (658–764) | 448 | 724 (657–791) | 81 | 339 (265–413) | |||||
| 2011 | 159 | 44 (37–51) | 1189 | 316 (298–334) | 755 | 746 (693–799) | 465 | 741 (673–808) | 123 | 494 (406–581) | |||||
| 2012 | 202 | 56 (48–64) | 1176 | 311 (293–328) | 811 | 757 (705–809) | 528 | 829 (759–900) | 111 | 425 (346–504) | |||||
| 2013 | 184 | 51 (43–58) | 1269 | 333 (314–351) | 849 | 752 (701–802) | 523 | 808 (739–877) | 121 | 445 (366–525) | |||||
| 2014 | 207 | 57 (49–65) | 1252 | 326 (308–344) | 869 | 737 (688–786) | 528 | 800 (731–868) | 131 | 466 (386–546) | |||||
| 2015 | 192 | 53 (45–60) | 1329 | 343 (325–362) | 948 | 773 (724–822) | 578 | 861 (791–931) | 117 | 403 (330–476) | |||||
| 2016 | 196 | 53 (46–61) | 1397 | 358 (339–377) | 1038 | 815 (765–864) | 602 | 879 (809–950) | 147 | 485 (407–564) | |||||
| 2017 | 202 | 54 (47–62) | 1436 | 366 (347–385) | 963 | 727 (681–773) | 534 | 758 (694–822) | 158 | 505 (426–583) | |||||
| 2018 | 199 | 52 (45–59) | 1425 | 362 (343–381) | 1043 | 760 (714–806) | 589 | 808 (743–874) | 156 | 484 (408–560) | |||||
| 2019 | 198 | 50 (43–57) | 1490 | 378 (359–397) | 1118 | 785 (739–831) | 631 | 834 (769–899) | 151 | 456 (383–529) | |||||
| Males | Females | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Count | Age-adjusted incidence (95% CI)—/Million | AAPC (95% CI), P-value |
Count | Age—standardized incidence (95% CI) -/Million | AAPC (95% CI), P-value |
|||||||||
| 2007 | 1059 | 258 (242–273) | 2.3 (1.0 to 3.6), P < .01 |
974 | 209 (196–223) | 2.1 (1.3 to 2.9), P < .01 |
|||||||||
| 2008 | 1206 | 285 (269–301) | 997 | 210 (197–223) | |||||||||||
| 2009 | 1300 | 299 (283–315) | 1100 | 226 (213–240) | |||||||||||
| 2010 | 1365 | 303 (287–320) | 1085 | 218 (205–231) | |||||||||||
| 2011 | 1473 | 320 (304–337) | 1218 | 238 (224–251) | |||||||||||
| 2012 | 1548 | 329 (312–345) | 1280 | 246 (232–260) | |||||||||||
| 2013 | 1611 | 330 (314–346) | 1335 | 250 (236–264) | |||||||||||
| 2014 | 1622 | 324 (309–340) | 1365 | 253 (240–267) | |||||||||||
| 2015 | 1722 | 336 (320–352) | 1442 | 261 (247–275) | |||||||||||
| 2016 | 1921 | 364 (347–380) | 1459 | 258 (245–275) | |||||||||||
| 2017 | 1829 | 335 (319–350) | 1464 | 257 (243–270) | |||||||||||
| 2018 | 1912 | 339 (324–355) | 1500 | 258 (245–272) | |||||||||||
| 2019 | 1996 | 344 (329–359) | 1592 | 268 (255–282) | |||||||||||
Figure 1.
Annual trends in incidence of spinal metastases by age group (A) and primary cancer type (B). Dots represent point estimates for a given year, with shaded regions representing 95% confidence intervals.
Lung cancer had the highest crude incidence of spinal metastasis among all primary cancers (Figure 1B). This remained consistent after age standardization to account for differences in age distribution (Table 3). Breast, prostate, and urological cancers demonstrated a significant increase in the incidence of spinal metastasis over the study period. However, prostate cancer demonstrated the greatest growth in incidence (AAPC 6.5; 95% CI: 4.1 to 9.0).
Table 3.
Number of Cases, Age-Standardized Annual Incidence, and Average Annual Percent Change (AAPC) in Incidence of Spine Metastases by Primary Cancer Type
| Lung | Breast | Prostate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Count | Age—standardized incidence (95% CI)/—Million | AAPC (95% CI), P-value |
Count | Age—standardized incidence (95% CI)—/Million | AAPC (95% CI), P-value |
Count | Age—standardized incidence (95% CI)—/Million | AAPC (95% CI), P-value |
| 2007 | 626 | 71 (65–77) | 0.7 (−0.6 to 2.1) P = .25 |
302 | 34 (30–38) | 4.4 (3.0 to 5.8) P < .01 |
228 | 37 (32–42) | 6.5 (4.1 to 9.0) P < .01 |
| 2008 | 698 | 78 (72–84) | 295 | 33 (29–37) | 275 | 44 (38–49) | |||
| 2009 | 692 | 94 (87–101) | 361 | 40 (36–44) | 320 | 50 (44–55) | |||
| 2010 | 714 | 85 (79–91) | 385 | 42 (38–46) | 329 | 50 (45–55) | |||
| 2011 | 805 | 85 (79–91) | 385 | 41 (37–45) | 375 | 56 (50–61) | |||
| 2012 | 782 | 80 (75–86) | 443 | 47 (42–51) | 416 | 71 (65–78) | |||
| 2013 | 796 | 80 (74–85) | 479 | 50 (45–54) | 434 | 72 (66–79) | |||
| 2014 | 827 | 81 (75–87) | 505 | 52 (47–56) | 459 | 63 (57–68) | |||
| 2015 | 825 | 88 (81–94) | 519 | 52 (48–57) | 522 | 70 (64–76) | |||
| 2016 | 851 | 89 (83–95) | 554 | 55 (50–60) | 591 | 77 (71–83) | |||
| 2017 | 800 | 74 (69–79) | 560 | 55 (50–59) | 561 | 73 (67–79) | |||
| 2018 | 835 | 93 (87–100) | 556 | 53 (49–57) | 611 | 77 (71–83) | |||
| 2019 | 899 | 87 (81–93) | 614 | 58 (53–62) | 693 | 85 (78–91) | |||
| Gastrointestinal | Myeloma | Urological | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Count | Age—standardized incidence (95% CI)—/Million | AAPC (95% CI), P-value |
Count | Age—standardized incidence (95% CI)—/Million | AAPC (95% CI), P-value |
Count | Age—standardized incidence (95% CI)—/Million | AAPC (95% CI), P-value |
| 2007 | 172 | 22 (18–25) | 1.8 (−0.2 to 3.8) P = .08 |
192 | 24 (21–28) | −0.3 (−1.8 to 1.3) P = .72 |
146 | 21 (17–24) | 2.9 (0.9 to 4.9) P < .01 |
| 2008 | 195 | 22 (19–25) | 197 | 24 (21–28) | 139 | 17 (14–20) | |||
| 2009 | 226 | 25 (22–28) | 203 | 28 (24–31) | 185 | 25 (21–29) | |||
| 2010 | 205 | 22 (19–25) | 206 | 27 (24–31) | 199 | 24 (20–27) | |||
| 2011 | 239 | 28 (25–32) | 219 | 28 (25–32) | 188 | 22 (19–25) | |||
| 2012 | 249 | 26 (22–29) | 261 | 29 (26–33) | 187 | 21 (18–24) | |||
| 2013 | 249 | 26 (22–29) | 223 | 22 (19–25) | 234 | 29 (25–33) | |||
| 2014 | 250 | 28 (24–31) | 260 | 28 (25–31) | 191 | 23 (20–27) | |||
| 2015 | 268 | 26 (23–29) | 257 | 28 (24–31) | 208 | 25 (22–29) | |||
| 2016 | 277 | 27 (23–30) | 244 | 23 (20–26) | 278 | 29 (25–32) | |||
| 2017 | 262 | 28 (24–31) | 247 | 25 (22–28) | 270 | 31 (27–35) | |||
| 2018 | 280 | 26 (23–29) | 272 | 27 (24–31) | 250 | 25 (22–28) | |||
| 2019 | 274 | 25 (22–28) | 280 | 24 (21–27) | 284 | 28 (25–31) | |||
Incidence of Spinal Metastases Compared to Overall Incidence of Cancer
Trends in the incidence of spinal metastases were compared to trends in the overall incidence of new primary cancer diagnoses (Supplementary Table 5). The AAPC in the incidence of spinal metastases was significantly different than the overall incidence of new cancer diagnoses for lung cancer, breast cancer, prostate cancer, gastrointestinal cancer, and myeloma. We found a significant increase in the incidence of spinal metastases for prostate cancer patients (AAPC 6.5; 95% CI: 4.1 to 9.0) despite a significant decrease in the overall incidence of prostate cancer diagnoses over the study period (AAPC −3.7; 95% CI: −6.1 to −1.2). Similarly, we found a significant increase in the incidence of spinal metastases for breast cancer patients (AAPC 4.4; 95% CI: 3.0 to 5.8) despite no change in the background incidence of breast cancer diagnoses over the study period (AAPC 0.2; 95% CI: −0.6 to 0.9).
Timing of Metastases
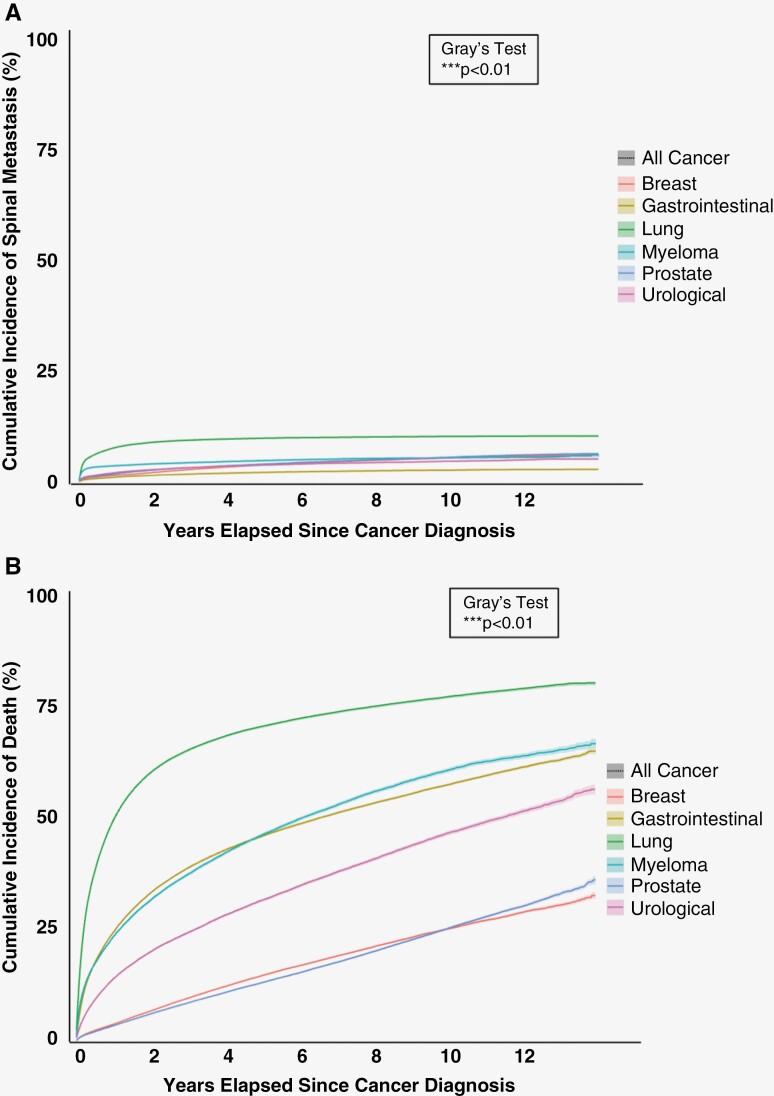
There was a significant difference in the cumulative incidence of spine metastasis across primary cancer types (Figure 2A). Lung cancer patients exhibited the highest risk of spine metastasis at selected time points (Table 4). The cumulative incidence of spine metastasis for lung cancer patients was found to be 7.8% (95% CI: 7.7% to 8.0%) at 1 year, 9.9% (95% CI: 9.7% to 10.1%) at 5 years, and 10.3% (95% CI: 10.1% to 10.5%) at 10 years. Conversely, gastrointestinal cancer patients exhibited the lowest risk of spine metastasis at selected time points. The cumulative incidence of spine metastasis for gastrointestinal cancer patients at 1 year was 1.0% (95% CI: 0.9% to 1.0%), 2.0% (95% CI: 2.0% to 2.1%) at 5 years, and 2.6% (95% CI: 2.5% to 2.7%) at 10 years.
Figure 2.
Cumulative incidence functions of (A) spinal metastasis and (B) the competing risk of death after cancer diagnosis. Shaded regions represent 95% confidence intervals.
Table 4.
Cumulative Incidence Point Estimates, and Person-Time Incidence Rate of Spine Metastasis by Primary Cancer Type
| Years elapsed since primary cancer diagnosis | Cumulative incidence percent (95% CI) | Incidence rate per 1000 person-years (95% CI) |
Risk ratio (95% CI) |
|
|---|---|---|---|---|
| Lung | 1-year | 7.8 (7.7–8.0) | 54.3 (53.2–55.4) | 10.3 (9.7–10.9) |
| 5-year | 9.9 (9.7–10.1) | |||
| 10-year | 10.3 (10.1–10.5) | |||
| Breast | 1-year | 1.5 (1.4–1.5) | 7.1 (6.9–7.3) | 1.4 (1.3–1.4) |
| 5-year | 3.8 (3.7–3.9) | |||
| 10-year | 5.4 (5.2–5.5) | |||
| Prostate | 1-year | 1.8 (1.7–1.9) | 7.3 (7.1–7.5) | 1.4 (1.3–1.5) |
| 5-year | 4.0 (3.9–4.1) | |||
| 10-year | 5.5 (5.4–5.7) | |||
| Gastrointestinal | 1-year | 1.0 (0.9–1.0) | 5.3 (5.1–5.5) | Reference |
| 5-year | 2.0 (2.0–2.1) | |||
| 10-year | 2.6 (2.5–2.7) | |||
| Myeloma | 1-year | 3.6 (3.4–3.7) | 13.4 (12.9–13.9) | 2.5 (2.4–2.7) |
| 5-year | 4.8 (4.6–4.9) | |||
| 10-year | 5.4 (5.2–5.6) | |||
| Urological | 1-year | 2.0 (1.9–2.1) | 8.3 (8.0–8.7) | 1.6 (1.5–1.7) |
| 5-year | 3.7 (3.6–3.9) | |||
| 10-year | 4.6 (4.4–4.8) |
Estimating risk of spine metastasis through the person-time approach found similar relationships across primary cancer types with respect to risk of spine metastasis. Lung cancer had the highest incidence rate of spine metastasis (54.3 cases per 1000 person-years; 95% CI: 53.2 to 55.4), while gastrointestinal cancer had the lowest incidence rate of spine metastasis (5.3 cases per 1000 person-years; 95% CI: 5.1 to 5.5). Overall, the risk ratio of spine metastasis incidence rate between lung cancer and gastrointestinal cancer was 10.3 (95% CI: 9.7 to 10.9).
Discussion
In this population-based study, we examined trends in the incidence and timing of spine metastases from 2007 to 2019. We found the age-standardized incidence in spine metastases increased from 229 cases per million to 302 cases per million over the study period. We noted a 2.2% average annual percent increase in the age-standardized incidence, which remained consistent across male and female patients. The incidence grew fastest among older patients, with those above 85 years demonstrating the largest AAPC of 5.2%. Lung cancer patients had the largest age-standardized incidence per study year. However, prostate cancer patients had the greatest AAPC. Temporal trends in the incidence of spinal metastasis were significantly different than trends in the overall incidence of primary cancers. Lung cancer patients displayed the greatest risk of spinal metastasis after primary diagnosis. These data provide a contemporary benchmark for future health policy.3,32 Furthermore, findings have direct implications for resource allocation, health policy, and care coordination. The Ontario health system will need to prepare for a growing population of older patients with spinal metastasis, for which direct healthcare cost estimates are upwards of $554 323 USD per patient for the first 60 days of treatment.2 Moreover, our data suggest the need for close surveillance of lung cancer patients around the time of cancer diagnosis.
A systematic review conducted by Van Den Brande in 2022 surveyed available literature on the epidemiology of spinal metastases.33 The authors found 15 studies that reported on the epidemiology of spinal metastases from any primary tumor, of which only 6 were conducted within North America. The most recent of these 6 studies represented a 2-year cohort between 2012 and 2014.34 The longest cohort reflected data from 1995 to 2009.35 In our study, we report results on the epidemiology of spinal metastases over a recent 13-year period from 2007 to 2019, which provides a more contemporary longitudinal evaluation.
Three prior studies have described the annual incidence of spinal metastases. Zaikova et al report an annual incidence of 26.0 per 100 000 (260 per Million) between 2007 and 2008 within South-East Norway.36 Conversely, Choi et al. report an annual incidence of 6.68 per 100 000 (66.8 per Million) between 2008 and 2017 in South Korea.37 Similarly, Sohn et al. report an annual incidence between 6.04 and 6.83 per 100 000 (66.8 and 68.3 per Million) between 2009 and 2012 in South Korea.38 In our study, we estimated crude annual incidence as well as age-standardized annual incidence for accurate comparison across years. Between 2007 and 2019 we noted a significantly increasing age-standardized incidence from 22.9 per 100 000 (229 per Million) to 30.2 per 100 000 (302 per Million). We therefore found a comparable annual incidence to South-East Norway, and greater than South Korea.
A study by Hong et al. from 2002 to 2012 in Korea evaluated the time to bony metastasis for various primary solid tumors.39 They noted that lung cancer patients had the shortest mean time to bone metastasis (9.0 ± 15.2 months). Conversely, colorectal cancer patients had the longest mean time to bone metastasis (28.9 ± 25.5 months). The cumulative incidence function for time to metastases estimated in our study demonstrates that lung cancer had the highest risk of metastases whereas gastrointestinal cancers had the lowest. This finding was also corroborated through a person-time approach. We noted an RR of 10.3 (95% CI: 9.7 to 10.9) of spinal metastasis for lung cancer patients relative to gastrointestinal cancer patients.
Few studies have analyzed trends between years in the incidence and timing of spine metastasis. Sohn et al. found an increase in their estimated annual incidence between 2012 to 2014 from 6.04 per 100 000 to 6.83 per 100 000.38 A study by Mak et al. on cases in the United States from 1998 to 2006, reported a significant increase in hospitalization for malignant spinal cord compression at an annual rate of 6%–8%.40 Our study found a significant increase in the annual incidence of spine metastases, with an AAPC of 2.2% between 2007 and 2019. We further compared rates of change among patient subgroups and identified the highest growth rate among patients 85 years and older (AAPC 5.2%) and patients with prostate cancer (AAPC 6.4%). Prostate cancer patients may have demonstrated the largest growth due to advancements in systemic therapies developed during this time. Dendritic cell immunotherapy was developed between 2014 to 2017 and has been shown to improve overall survival in patients with prostate cancer.41
Our study has notable limitations. Misclassification bias may underestimate results, as data was collected retrospectively from administrative health databases. Alternatively, temporal trends may have been overestimated due to ascertainment bias related to more intensive investigations and improved electronic charting over the study period. Furthermore, no studies have validated the use of Ontario administrative health databases for the identification of patients with spinal metastases. However, a recent study of ICES databases including OCR and OHIP validated their use for identifying patients with metastatic gastric cancer, with some algorithms having a positive predictive value of 88.8%, sensitivity of 90.1%, and specificity of 92.5%.42 Finally, our study is limited to patients undergoing treatment for their disease, and therefore underestimates the true annual incidence of spinal metastasis, as palliative patients not receiving either radiotherapy or surgery would not be captured.
Conclusion
The annual incidence of spinal metastases increased between 2007 and 2019. Older patients were increasingly affected. Differences between cancer patients were apparent in both the incidence and timing of spinal metastasis. Our findings provide insights into the evolving landscape of spinal metastatic disease within the context of the Canadian healthcare system, and provide a benchmark for future health policy. We emphasize the need to prepare for a growing elderly population of patients needing multidisciplinary subspecialty care.
Supplementary Material
Acknowledgment
This study contracted ICES Data & Analytic Services (DAS) and used de-identified data from the ICES Data Repository, which is managed by ICES with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research, and the Government of Ontario. This study was supported through provision of data by ICES and Cancer Care Ontario (CCO) and through funding support to ICES from an annual grant by the Ministry of Health (MOH) and the Ontario Institute for Cancer Research (OICR). The opinions, results, and conclusions reported in this paper are those of the authors. No endorsement by ICES or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and information provided by Ontario Health (OH). The opinions, results, views, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of OH. No endorsement by OH is intended or should be inferred. We thank Refik Saskin and Eliane Kim for their technical and methodological support in using ICES data.
Contributor Information
Husain Shakil, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Armaan K Malhotra, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Jetan H Badhiwala, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada.
Vishwathsen Karthikeyan, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada.
Ahmad Essa, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Yingshi He, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Michael G Fehlings, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Krembil Research Institute, Toronto Western Hospital, Toronto, Ontario, Canada.
Arjun Sahgal, Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Nicolas Dea, Neurosurgical and Orthopedic Spine Program, Vancouver General Hospital, University of British Columbia, Vancouver, British Columbia, Canada.
Alex Kiss, Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; ICES, Toronto, Ontario, Canada.
Christopher D Witiw, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Donald A Redelmeier, Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; ICES, Toronto, Ontario, Canada; Department of Medicine, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Jefferson R Wilson, Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario, Canada.
Funding
This research received support from the Canadian Institutes of Health Research, the Unity Health Labatt Chari in Neurosurgery, and the University of Toronto Temerty Faculty of Medicine. The study was conducted using data sets stored at the ICES, which is funded through an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Conflict of interest statement
No authors reported conflicts of interest related to this study. Outside of this study, AS has been a consultant for Varian, Elekta (Gamma Knife Icon), BrainLAB, Merck, Abbvie, and Roche; Vice President of the International Stereotactic Radiosurgery Society (ISRS); Co-Chair of the AO Spine Knowledge Forum Tumor; received honorarium for past educational seminars for AstraZeneca, Elekta AB, Varian, BrainLAB, Accuray, Seagen Inc.; research grant with Elekta AB, Varian, Seagen Inc., BrainLAB; and travel accommodations/expenses with Elekta, Varian, and BrainLAB. AS also belongs to the Elekta MR Linac Research Consortium and is a Clinical Steering Committee Member, and chairs the Elekta Oligometastases Group and the Elekta
Gamma Knife Icon Group outside of this study. ND reported personal feeds from Stryker, Medtronic, Cerapedics, and Baxter outside the submitted work. ND is a stockholder of Medtronic and received fellowship support from Medtronic, AOSpine, and JJ/Synthes outside the submitted work. CDW reported grants from Cerapedics and personal fees from Stryker outside the submitted work. DAR reported research support from a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the PSI Foundation of Ontario, and the Kimel-Schatzky Traumatic Brain Injury Research Fund outside the submitted work. JRW reported personal fees from Stryker Canada outside the submitted work.
Authorship statement
H.S. Study design, data analysis, manuscript preparation, and revisions. A.K.M. Data analysis and manuscript revision. J.H.B. Manuscript preparation and revision. V.K. Manuscript preparation and revision. A.E. Manuscript revision. Y.H. Manuscript preparation and revision. M.G.F. Manuscript preparation and revision. A.S. Manuscript preparation and revision. N.D. Manuscript preparation and revision. A.K. Study design, data analysis, manuscript preparation, and revision. C.D.W. Study design, data analysis, manuscript preparation, and revision. D.A.R. Study design, data analysis, manuscript preparation, and revision. J.R.W. Study design, data analysis, manuscript preparation, and revision.
Data availability
The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
- 1. Nater A, Sahgal A, Fehlings M.. Chapter 16 - Management - spinal metastases. Handb Clin Neurol. 2018;149:239–255. [DOI] [PubMed] [Google Scholar]
- 2. Fehlings MG, Nater A, Holmer H.. Cost-effectiveness of surgery in the management of metastatic epidural spinal cord compression: A systematic review. Spine (Phila Pa 1976). 2014;39(22):S99–S105. [DOI] [PubMed] [Google Scholar]
- 3. Nater A, Chuang J, Liu K, et al. A personalized medicine approach for the management of spinal metastases with cord compression: Development of a novel clinical prediction model for postoperative survival and quality of life. World Neurosurg. 2020;140:654–663.e13. [DOI] [PubMed] [Google Scholar]
- 4. Nater A, Tetreault LA, Kopjar B, et al. Predictive factors of survival in a surgical series of metastatic epidural spinal cord compression and complete external validation of 8 multivariate models of survival in a prospective North American multicenter study. Cancer. 2018;124(17):3536–3550. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all - PAHO/WHO | Pan American Health Organization. Accessed May 15, 2023. https://www.paho.org/en/node/69004 [Google Scholar]
- 7. Coleman RE, Roodman, Smith, Body, Suva, Vessella. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 8. Jacobs WB, Perrin RG.. Evaluation and treatment of spinal metastases: An overview. Neurosurg Focus. 2001;11(6):1–11. [DOI] [PubMed] [Google Scholar]
- 9. Loblaw DA, Laperriere NJ, Mackillop WJ.. A population-based study of malignant spinal cord compression in ontario. Clin Oncol. 2003;15(4):211–217. [DOI] [PubMed] [Google Scholar]
- 10. Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wewel JT, O’toole JE.. Epidemiology of spinal cord and column tumors. Neurooncol. Pract.. 2020;7(suppl 1):ii5–ii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Statistics Canada. Population estimates, quarterly. Accessed May 16, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901
- 14. Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10): e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schull MJ, Azimaee M, Marra M, et al. ICES: Data, discovery, better health. Int J Popul Data Sci. 2020;4(2):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robles SC, Marrett LD, Aileen Clarke E, Risch HA.. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495–501. [DOI] [PubMed] [Google Scholar]
- 17. Finkelstein JA, Zaveri G, Wai E, et al. A population-based study of surgery for spinal metastases. Journal of Bone and Joint Surgery - Series B. 2003;85(7):1045–1050. [DOI] [PubMed] [Google Scholar]
- 18. Brastianos HC, Nguyen P, Sahgal A, et al. Association of innovations in radiotherapy and systemic treatments with clinical outcomes in patients with melanoma brain metastasis from 2007 to 2016. JAMA Netw Open. 2020;3(7):e208204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhanot K, Widdifield J, Huang A, et al. Survival after surgery for spinal metastases: A population-based study. Can J Surg. 2022;65(4):E512–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Statistics Canada. Population estimates on July 1st, by age and sex. Accessed June 6, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 [Google Scholar]
- 21. Statistics Canada. Age-standardized Rates. Accessed August 25, 2023.https://www.statcan.gc.ca/en/dai/btd/asr [Google Scholar]
- 22. How to Compute Age-Adjusted Rates in Joinpoint — Joinpoint Help System. Accessed August 25, 2023. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/input-file-tab/how-to-compute-age-adjusted-rates-in-joinpoint [Google Scholar]
- 23. Noordzij M, Dekker FW, Zoccali C, Jager KJ.. Measures of disease frequency: Prevalence and incidence. Nephron Clin Pract. 2010;115(1):c17–c20. [DOI] [PubMed] [Google Scholar]
- 24. Hoffman JIE. Chapter 18 - the poisson distribution. Biostatistics for Medical and Biomedical Practitioners. 2015:259–278. [Google Scholar]
- 25. Surveillance Research Program. Joinpoint Regression Program. Accessed July 11, 2023. https://surveillance.cancer.gov/joinpoint/
- 26. Kim HJ, Fay MP, Feuer EJ, Midthune DN.. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 27. Method and Parameters Tab — Joinpoint Help System. Accessed August 25, 2023. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab [Google Scholar]
- 28. Austin PC, Lee DS, Fine JP.. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bewick V, Cheek L, Ball J.. Statistics review 12: Survival analysis. Crit Care. 2004;8(5):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen PK, Geskus RB, De Witte T, Putter H.. Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol. 2012;41(3): 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dignam JJ, Kocherginsky MN.. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26(24):4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fehlings MG, Nater A, Holmer H.. Cost-effectiveness of surgery in the management of metastatic epidural spinal cord compression: a systematic review. Spine (Phila Pa 1976). 2014;39(22 suppl 1):S99–S105. [DOI] [PubMed] [Google Scholar]
- 33. Van den Brande R, Cornips E MJ, Peeters M, et al. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J Bone Oncol. 2022;35:100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price M, Goodwin JC, De la Garza Ramos R, et al. Gender disparities in clinical presentation, treatment, and outcomes in metastatic spine disease. Cancer Epidemiol. 2021;70:101856. [DOI] [PubMed] [Google Scholar]
- 35. Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support Care Cancer. 2013;21(12):3279–3286. [DOI] [PubMed] [Google Scholar]
- 36. Zaikova O, Giercksky KE, Fosså SD, et al. A population-based study of spinal metastatic disease in South-East Norway. Clin Oncol (R Coll Radiol). 2009;21(10):753–759. [DOI] [PubMed] [Google Scholar]
- 37. Choi SH, Koo JW, Choe D, Kang CN.. The incidence and management trends of metastatic spinal tumors in south korea: A nationwide population-based study. Spine (Phila Pa 1976). 2020;45(14):E856–E863. [DOI] [PubMed] [Google Scholar]
- 38. Sohn S, Kim J, Chung CK, et al. Nationwide epidemiology and healthcare utilization of spine tumor patients in the adult Korean population, 2009-2012. Neurooncol. Pract.. 2015;2(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM.. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS One. 2020;15(7): e0234927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824–831. [DOI] [PubMed] [Google Scholar]
- 41. Vogelzang NJ, Beer TM, Gerritsen W, et al. ; VIABLE Investigators. Efficacy and safety of autologous dendritic cell–based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: The viable phase 3 randomized clinical trial. JAMA Oncol. 2022;8(4):546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahar AL, Jeong Y, Zagorski B, Coburn N.. Validating an algorithm to identify metastatic gastric cancer in the absence of routinely collected TNM staging data. BMC Health Serv Res. 2018;18(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.