Abstract
Background
Open Abdomen (OA) cases represent a significant surgical and resource challenge. AbClo is a novel non-invasive abdominal fascial closure device that engages lateral components of the abdominal wall muscles to support gradual approximation of the fascia and reduce the fascial gap. The study objective was to assess the economic implications of AbClo compared to negative pressure wound therapy (NPWT) alone on OA management.
Methods
We conducted a cost-minimization analysis using a decision tree comparing the use of the AbClo device to NPWT alone among patients with midline laparotomy for trauma or acute abdominal surgery who were ineligible for primary fascial closure. The time horizon was limited to the length of the inpatient hospital stay, and costs were considered from the perspective of the US Medicare payer. Clinical effectiveness data for AbClo was obtained from a randomized clinical trial. Cost data was obtained from the published literature. Probabilistic and deterministic sensitivity analyses were performed. The primary outcome was incremental cost.
Results
The mean cumulative costs per patient were $76 582 for those treated with NPWT alone and $70,582 for those in the group treated with the AbClo device. Compared to NPWT alone, AbClo was associated with lower incremental costs of −$6012 (95% CI −$19 449 to +$1996). The probability that AbClo was cost-savings compared to NPWT alone was 94%.
Conclusions
The use of AbClo is an economically attractive strategy for management of OA in in patients with midline laparotomy for trauma or acute abdominal surgery who were ineligible for primary fascial closure.
Keywords: acute care surgery, general surgery, the business of surgery, health economics, cost-benefit
Introduction
The open abdomen (OA) strategy is commonly employed in surgical situations where primary abdominal closure is not feasible or may lead to complications, such as in severe abdominal trauma following damage control surgery, abdominal sepsis, or abdominal compartment syndrome. 1 Open abdomen surgical cases represent a significant surgical and resource challenge. In the United States, it is estimated that there are more than 2 million open abdomen surgery cases per year. 2 The economic burden associated with OA management is associated with prolonged lengths of hospital stay, and the use of current in-hospital management strategies to achieve eventual primary closure which have variable and often suboptimal efficacy.3-5
Currently, vacuum assisted techniques (VAT) or negative pressure wound therapy (NPWT) are the most common for temporary abdominal closure (TAC) and coverage with the goals of preventing further bowel injury, managing fluid losses from the abdominal cavity, and ultimately facilitating primary closure. While VAT/NPWT are useful in the management of abdominal fluids and secretions, these techniques provide minimal support to prevent lateral retraction of the abdominal wall fascial edges and rectus muscles. Additional management strategies for OA include the application of devices that are surgically fixed to the abdominal wall and provide mechanical support to prevent the lateral retraction of fascial edges. However, these devices are stitched through the abdominal wall, and may cause further damage to the healing abdomen. Furthermore, these devices are resource intensive and costly to the health care system due to the necessity of frequent trips to the operating room for application and readjustment. 6
Most recently, a non-invasive approach has been developed to prevent the lateral retraction of fascial edges and provide mechanical support. AbClo is a novel non-invasive abdominal fascial closure device that engages lateral components of the abdominal wall muscles to and support gradual approximation of the fascia and reduce the fascial gap. AbClo uses principles and mechanics of hoop stress whereby it provides a consistent, uniform and distributed appositional force to prevent retraction of the abdominal muscles. AbClo consists of 2 rectus muscle splints, a circumferential dynamic retainer (CDR) and a tension gauge. The RMS stabilize the abdominal muscles in place; the CDR provides circumferential appositional support to overcome the hoop stress and the tensioner gauge ensures that the correct tension is being applied to the muscles and fascia to achieve gradual re-approximation of abdominal fascia. In a randomized study of 38 patients, the combined application of AbClo and VAT to patients with an OA resulted in an 85% primary facial closure rate compared to only 56% when VAT alone was applied. 7 In addition to the increased clinical efficacy associated with the AbClo device, it also has distinct advantages when considering health resource use including point-of-care application. That is, given its non-invasive design, it can be applied and adjusted at the bedside within the intensive care unit without the need for use of the operative room.
While the clinical outcomes and techniques for open abdomen management have evolved, the economic implications of the newly available management strategies and potentially more efficacious technologies warrant careful consideration. Healthcare systems worldwide have experienced growing pressure to optimize resource allocation and contain healthcare costs. In this context, we report the economic analysis of the AbClo device from the perspective of the US Medicare health payor.
Methods
The study protocol and report were prepared in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. 8 Formal ethics board review was not required for model-based studies using data from the published literature.
Model Design
This study was a cost-minimization analysis to compare the use of the AbClo device + negative pressure therapy to negative pressure therapy alone among patients with midline laparotomy for trauma or acute abdominal surgery who were ineligible for primary fascial closure. The economic analysis adopted the perspective of the US Medicare payor. The time horizon was limited to the length of the inpatient hospital stay based on available empiric data assessing the efficacy of the AbClo device. 7 Due to the uncertainty of the impact on health-related quality of life, mortality and long term medical resource use (such as rehospitalizations or emergency department visits), a longer term cost-effectiveness and/or cost-utility analysis was not undertaken.
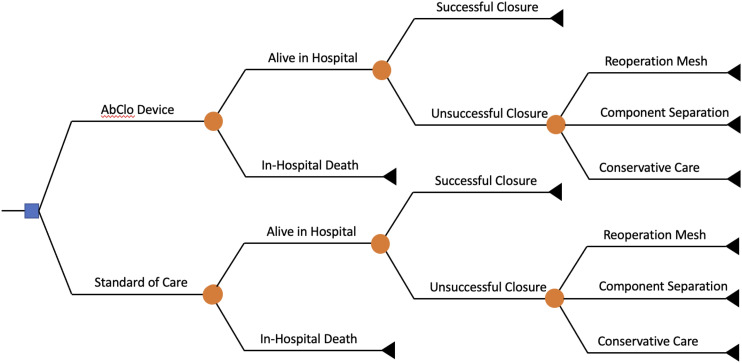
The base case economic model used a simple decision tree, which was reviewed for face validity by clinical experts in the trauma and general surgery fields (Figure 1). The decision tree is briefly described as follows. Following management with the AbClo device or standard of care, patients underwent reoperation to close the laparotomy incision. These operations could result in a successful primary fascial closure, or failure closure. Those with failure fascial closure could be further managed with a second reoperation to under component separation with or without mesh. The model assumed that the days spent in an intensive care unit was different based on successful vs failed primary closure, which is consistent with prior prospective cohort studies on patients with OA following damage control laparotomies for trauma. 9 The primary model outcome was direct healthcare costs. Modelling was performed in TreeAge Pro Healthcare 2022 (Williamstown, MA).
Figure 1.
Decision tree.
Model Inputs
The clinical effectiveness of the AbClo device was studied in a randomized, single-center, open-label trial of 38 patients (age > 16 years), who were mechanically ventilated and had their abdominal wall fascia and skin left open at conclusion of a midline laparotomy for trauma or acute care surgery. 7 Patients were randomized to (a) the AbClo device + negative pressure or (b) negative pressure alone. The study found a high proportion of patients with successful primary closure of the abdomen when randomized to the AbClo device (85%) compared to standard of care (55.6%; P = .046). In this study, AbClo Device patients were more likely to experience primary fascial closure compared to those treated with standard of care (RR 1.53, 95% CI 0.99 to 2.80). 7
Cost Inputs
Hospitalization costs were assigned using Diagnosis-Related Group (DRG)-based 2021 US Medicare reimbursement rates. 10 The DRG code 963 (“Other multiple significant trauma with major complication or comorbidity”) was applied to calculate average cost per inpatient day, which was estimated by dividing the 2021 Medicare reimbursement by the median length of stay (LOS) published by the Centers for Medicare and Medicaid Services. Then, these estimates of daily reimbursement were multiplied by length of stay to calculate costs of each hospitalization. Hospital length of stay depended on success of primary closure; those with unsuccessful primary closure remained in hospital for an average of 13.9 days longer. 9
The model also accounted for the additional the length of ICU stay for those with a failed primary closure, necessitating additional intervention such as dressing changes, and possible repair of failed closure by component separation with or without mesh. 9 We included costs associated with dressing changes performed every 2 to 3 days.11,12 These dressing changes were performed in the operating room and each procedure lasted on average 98 minutes. 11 Operating room costs were estimated from the average per minute cost ($45.26 per minute) derived from California hospitals. 13 Costs for reoperation for failed primary closure were obtained from a prior costing analysis at the University of Kentucky Medical Center that used detailed cost accounting systems. 14 Costs for inpatients requiring ventral hernia repairs were calculated for those requiring component separation without mesh, repair with synthetic mesh, and repair with biologic mesh. Based on discussion with clinical experts, the model assumed a 3:1 use of synthetic vs biologic mesh.
This analysis did not include the cost of initial operative management for an open abdomen since these costs were assumed similar regardless of AbClo or standard of care strategy. The additive costs would be due to the AbClo device itself (estimated $5600 USD).
Costs were reported in 2021 USD from the perspective of the US health care system, and adjusted for inflation using the US Medical Care Consumer Price Index, where appropriate. 15 Discounting was not applied due to the short time horizon of the decision tree.
Sensitivity Analyses
Both deterministic and probabilistic sensitivity analyses were performed to estimate the influence that the range of input values had on incremental costs. The one-way sensitivity analyses varied one input parameter at a time using the lower and upper 95% interval bounds and recorded the change in the incremental cost. Variables, for which confidence intervals were not provided were modelled with wide distributions (±25%). For the probabilistic sensitivity analysis, distributional assumptions of the input parameters were made (see Table 1). Distributions were estimated with the means and standard deviations from source documentation. A Monte Carlo simulation with 10 000 iterations was used to propagate the uncertainty in individual model parameters to produce a distribution of expected costs.
Table 1.
Model Inputs.
| Base Case | Range | Distribution | Source | |
|---|---|---|---|---|
| Clinical inputs | ||||
| Probability of primary closure with SoC | .556 | ±25% | Beta | 7 |
| Risk ratio of closure (AbClo vs SoC) | 1.53 | .99–2.80 | Triangle | 7 |
| Risk of inpatient mortality | .21 | ±25% | n/a | 18 |
| ICU days (if successful closure) | 14.4 | 13.1–15.7 | Triangle | 9 |
| ICU days (if failed closure) | 22.0 | 19.0–25.0 | Triangle | 9 |
| Hospital length of stay (if successful closure) | 23.3 | 21.6–25.0 | Triangle | 9 |
| Hospital length of stay (if failed closure) | 37.2 | 32.2–42.2 | Triangle | 9 |
| Cost inputs (2021 USD) | ||||
| Daily cost of hospitalization (DRG 963) | 2112 | ±25% | Gamma | 10 |
| Operative room cost per dressing change | 4436 | ±25% | Gamma | 11,13 |
| Cost of operation to manage failed closure using synthetic mesh | 9995 | 7743–12,563 | Gamma | 14 |
| Cost of operation to manage failed closure using biologic mesh | 22,347 | 19,845–28,444 | Gamma | 14 |
| Cost of operation to manage failed closure using component separation | 7137 | 4767–12,379 | Gamma | 14 |
| Cost of AbClo device | 5600 | 3000–8000 | Gamma | PC |
Results
Base Case Results
The mean cumulative costs per patient were $70 570 for those in the standard of care group (i.e., those treated with negative pressure alone) and $76 582 for those in the group treated with the AbClo device. Compared to standard of care, AbClo was associated with lower incremental costs of -$6012 (95% CI −$19 449 to +$1996). That is, AbClo was cost-savings compared to standard of care in 94% of simulations (Table 2).
Table 2.
Base Case Results.
| Strategy | Total Costs (2021 USD) | Cost Difference |
|---|---|---|
| Standard of care | 76,582 (56,102–107,550) | Reference |
| AbClo device | 70,582 (51,777–95,592) | −6012 (−19,449 to 1996) |
Sensitivity Analyses
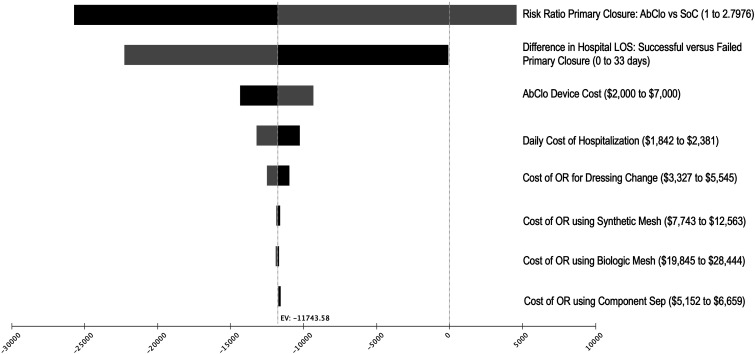
For one-way sensitivity analyses, we varied each model input to assess the effect on incremental costs (Figure 2). The input with greatest variation on the model outcome was the clinical effectiveness of the AbClo Device on primary closure rates. The clinical effectiveness of AbClo is obtained from a single-center randomized control trial, which showed a 53% increased likelihood of primary closure compared to standard of care (risk ratio 1.53, 95% CI 0.99 to 2.80). As the confidence interval crosses the null of 1.0, cost-savings is no longer estimated. For AbClo to be cost savings compared to standard of care, AbClo would need to demonstrate an 14% or greater increased likelihood of primary closure in future confirmatory studies.
Figure 2.
Tornado diagram of 1-way sensitivity analyses.
Other model inputs that influenced the model outcome included the cost of the AbClo device, the difference in hospital length of stay for individuals with unsuccessful primary closure vs successful closure, daily cost of hospitalization, and the cost of an operating room for dressing changes upon failed primary closure. However, cost-savings was estimated in all one-way sensitivity analyses across the plausible range for each of these model inputs.
To assess the maximum reimbursement for AbClo where the device would still be considered cost-savings, we conducted a post-hoc threshold analysis. Any AbClo reimbursement cost below $11 612 would remain cost savings compared to standard of care. However, this threshold analysis is accompanied by several important caveats. First, this threshold assumes that AbClo is associated with a 53% increase in primary closure rates. This effect size needs to be confirmed in larger randomized trials. Second, this economic model assumes that individuals with an unsuccessful primary closure will require longer hospital lengths of stay while awaiting management of failed closure, such as component separation with or without mesh. In this model, cost savings was driven by a reduced mean length of hospital stay in the AbClo group due to higher rates of successful primary closure. This is assumption is consistent with outcomes reported from prospective observational cohort studies of patients OA in the setting of trauma. 9 Furthermore, AbClo was associated with cost savings when varying hospital length of stay across a plausible range in the sensitivity analyses.
Discussion
The main finding of our analysis indicates that use of the AbClo device among patients with midline laparotomy for trauma or acute abdominal surgery who were ineligible for primary fascial closure is economically attractive through provision of cost-savings to the US Medicare payor compared to current standard of care using negative pressure wound therapy alone. Assuming a single hospital system was responsible for treating 100 patients with OA per year, the budget impact of the AbClo device would estimate an annual cost savings of approximately $60 000.
The estimates of cost-savings remained consistent across the majority of sensitivity analyses, where the model inputs were varied over their reported ranges. However, the input with greatest variation on the model was the clinical effectiveness of the AbClo device on primary closure rates. That is, cost savings of the AbClo device is driven by the 53% increased likelihood of primary closure compared to standard of care (risk ratio 1.53, 95% CI 0.99 to 2.80). When the AbClo clinical effectiveness approaches the lower confidence interval (i.e., crosses the null of 1.0), AbClo is no longer cost savings compared to standard of care. A larger clinical trial is currently ongoing to corroborate the effect size of AbClo on improving primary fascial closure rates and improve the precision of the confidence interval (clinicaltrials.gov; NCT03815370).
As previously described, the clinical applications of the AbClo device focus on temporal closure to prevent further bowel injury, to manage fluid losses from the abdominal cavity and to facilitate primary closure. Early closure is dependent of several technical factors and the patient’s underlying clinical condition; however, improved closure rates have been reported using a combined approach of vacuum-assisted devices with methods that provide gradual midline dynamic tension of the abdominal wall fascia.6,16,17 A key advantage to the AbClo device is the provision of abdominal wall support using non-invasive applications. Prior devices require fixation by stitching through the skin, subcutaneous tissue, muscle, and fascial layers. Furthermore, another important limitation is that initial placement and subsequent adjustments require frequent trips to the operating room, which delays timely management and consumes valuable operating room resources. In contrast, the AbClo device is non-invasive and can be applied, adjusted, and removed at point-of-care at the ICU bedside. In addition to the clinical advantages offered by the AbClo device, the current study demonstrates an economic benefit to its use. The cost-savings is likely achieved by improved closure rates, and subsequent reduced ICU lengths of stays, as well as a reduction operating room costs given that AbClo can be managed at the bedside.
Limitations
The economic model has several caveats. First, the clinical effectiveness data is based on a single study of 38 patients. The effectiveness of the AbClo device will need to be externally validated in additional studies with a larger cohort that includes the range of comorbidities observed in clinical practice. The value proposition of the device may be influenced by patients with increased comorbidity burden, which is associated with increased post-operative complications and may influence the clinical effectiveness (and thus cost-effectiveness) of the AbClo device. Second, the availability of cost data is limited in the current literature. This study calculated costs using a top-down approach based on DRGs to estimate the daily inpatient hospital costs (accounting for patient complexity associated with the trauma admissions, DRG 963) then multiplied by lengths of stay associated with successful vs failed primary closure following laparotomy. Third, this economic study was specified as a cost-minimization analysis and did not estimate differences in clinical outcomes such as health-related quality of life, time to hospital discharge or mortality. The ability to conduct a cost-consequence or cost-utility analysis is limited by the availability of long-term data related to the AbClo device. However, this data will be available in the upcoming years upon completion of a multi-center randomized control trial comparing the AbClo device to usual care.
Conclusions
In this economic model, the use of the AbClo device is cost-savings compared to standard of care (i.e., negative pressure alone) in patients with midline laparotomy for trauma or acute abdominal surgery who were ineligible for primary fascial closure. The AbClo device provides $6012 of cost savings per patient with a 94% probability of cost-savings compared to standard of care.
Supplemental Material
Supplemental Material for Economic Analysis of AbClo, a Novel Abdominal Fascia Closure Device, for Patients With an Open Abdomen Following Trauma or Acute Abdominal Surgery by Derek S. Chew and Taranvir Dayal in Surgical Innovation
Author Contributions: DC was responsible for study design, analysis, interpretation, writing and critical revision. TD was responsible for writing and critical revision.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TD is an employee of InventoRR MD Inc. DC reports consulting fees through the Integrated Management Platform to Accelerate Clinical Trials (IMPACT) at the University of Calgary.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support of this study was provided by InventoRR MD Inc.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Derek S. Chew https://orcid.org/0000-0003-3539-7485
References
- 1.Chabot E, Nirula R. Open abdomen critical care management principles: resuscitation, fluid balance, nutrition, and ventilator management. Trauma Surg Acute Care Open. 2017;2(1):e000063. doi: 10.1136/tsaco-2016-000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carney MJ, Weissler JM, Fox JP, Tecce MG, Hsu JY, Fischer JP. Trends in open abdominal surgery in the United States-Observations from 9,950,759 discharges using the 2009-2013 National Inpatient Sample (NIS) datasets. Am J Surg. 2017;214(2):287-292. doi: 10.1016/j.amjsurg.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Betancourt AS, Milagros GC, Sibaja P, Fernandez L, Norwood S. Cost evaluation of temporary abdominal closure methods in abdominal sepsis patients successfully treated with an open abdomen. Should we take temporary abdominal closure methods at face value? Health economic evaluation. Ann Med Surg (Lond). 2020;56:11-16. doi: 10.1016/j.amsu.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez LG. Management of the open abdomen: clinical recommendations for the trauma/acute care surgeon and general surgeon. Int Wound J. 2016;13(Suppl 3):25-34. doi: 10.1111/iwj.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Ye J, Song W, Chen J, Yuan Y, Ren J. Comparison of outcomes between early fascial closure and delayed abdominal closure in patients with open abdomen: a systematic review and meta-analysis. Gastroenterol Res Pract. 2014;2014:784056. doi: 10.1155/2014/784056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu P, Uhlich R, Gleason F, Kerby J, Bosarge P. Impact of initial temporary abdominal closure in damage control surgery: a retrospective analysis. World J Emerg Surg. 2018;13:43. doi: 10.1186/s13017-018-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezende-Neto JB, Camilotti BG. New non-invasive device to promote primary closure of the fascia and prevent loss of domain in the open abdomen: a pilot study. Trauma Surg Acute Care Open. 2020;5(1):e000523. doi: 10.1136/tsaco-2020-000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 9.Dubose JJ, Scalea TM, Holcomb JB, et al. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2013;74(1):113-120; discussion 1120-2. doi: 10.1097/TA.0b013e31827891ce. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid . Acute Inpatient Prospective Payment System. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS. Accessed March 2020. [Google Scholar]
- 11.Seternes A, Fasting S, Klepstad P, et al. Bedside dressing changes for open abdomen in the intensive care unit is safe and time and staff efficient. Crit Care. 2016;20(1):164. doi: 10.1186/s13054-016-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasilainen S, Mentula P, Salminen P, et al. Superior primary fascial closure rate and lower mortality after open abdomen using negative pressure wound therapy with continuous fascial traction. J Trauma Acute Care Surg. 2020;89(6):1136-1142. doi: 10.1097/TA.0000000000002889. [DOI] [PubMed] [Google Scholar]
- 13.Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153(4):e176233. doi: 10.1001/jamasurg.2017.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds D, Davenport DL, Korosec RL, Roth JS. Financial implications of ventral hernia repair: a hospital cost analysis. J Gastrointest Surg. 2013;17(1):159-166; discussion p 166-7. doi: 10.1007/s11605-012-1999-y. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Labor: Bureau of Labor Statistics . Consumer Price Index- All Urban Consumers; 2020. https://www.bls.gov/cpi/. [Google Scholar]
- 16.Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192(2):238-242. doi: 10.1016/j.amjsurg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Fantus RJ, Mellett MM, Kirby JP. Use of controlled fascial tension and an adhesion preventing barrier to achieve delayed primary fascial closure in patients managed with an open abdomen. Am J Surg. 2006;192(2):243-247. doi: 10.1016/j.amjsurg.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Harvin JA, Maxim T, Inaba K, et al. Mortality after emergent trauma laparotomy: a multicenter, retrospective study. J Trauma Acute Care Surg. 2017;83(3):464-468. doi: 10.1097/TA.0000000000001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Economic Analysis of AbClo, a Novel Abdominal Fascia Closure Device, for Patients With an Open Abdomen Following Trauma or Acute Abdominal Surgery by Derek S. Chew and Taranvir Dayal in Surgical Innovation