Abstract
Cystic fibrosis (CF) is the most common monogenic autosomal recessive disease in Caucasians caused by pathogenic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (CFTR). Significant small molecule therapeutic advances over the past two decades have been made to target the defective CFTR protein and enhance its function. To address the most prevalent defect of the defective CFTR protein (i.e., F508del mutation) in CF, two biomolecular activities are required, namely, correctors to increase the amount of properly folded F508delCFTR levels at the cell surface and potentiators to allow the effective opening, i.e., function of the F508delCFTR channel. Combined, these activities enhance chloride ion transport yielding improved hydration of the lung surface and subsequent restoration of mucociliary clearance. To enhance clinical benefits to CF patients, a complementary triple combination therapy consisting of two corrector molecules, type 1 (C1) and type 2, with additive mechanisms along with a potentiator are being investigated in the clinic for maximum restoration of mutated CFTR function. We report the identification and in vitro biologic characterization of ABBV-2222/GLPG2222 (4-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic acid),—a novel, potent, and orally bioavailable C1 corrector developed by AbbVie-Galapagos and currently in clinical trials—which exhibits substantial improvements over the existing C1 correctors. This includes improvements in potency and drug-drug interaction (DDI) compared with 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (VX-809, Lumacaftor) and improvements in potency and efficacy compared with 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-[1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide (VX-661, Tezacaftor). ABBV-2222/GLPG2222 exhibits potent in vitro functional activity in primary patient cells harboring F508del/F508del CFTR with an EC50 value <10 nM.
SIGNIFICANCE STATEMENT
To address the most prevalent defect of the defective CFTR protein (i.e., F508del mutation) in cystic fibrosis, AbbVie-Galapagos has developed ABBV-2222/GLPG2222, a novel, potent, and orally bioavailable C1 corrector of this protein. ABBV-2222/GLPG2222, which is currently in clinical trials, exhibits potent in vitro functional activity in primary patient cells harboring F508del/F508del CFTR and substantial improvements over the existing C1 correctors.
Introduction
Cystic fibrosis (CF) is one of the most common lethal autosomal recessive Mendelian disorders [https://www.omim.org/], the gene for which was discovered >25 years ago (Kerem et al., 1989; Rommens et al., 1989). Affecting an estimated 75,000 people worldwide with ∼30,000 living in the United States (Cystic Fibrosis Foundation; https://www.cff.org/), CF is caused by loss-of-function mutations in the cftr gene protein product, cystic fibrosis transmembrane conductance regulator (CFTR) (Riordan et al., 1989), which belongs to the ATP-binding cassette transporter family. CFTR is primarily expressed at the apical membrane of secretory epithelia of the airways, intestine, and hepatobiliary and reproductive tracts, as well as the pancreas and sweat gland secretory coil, where it acts as a cAMP-regulated (Kartner et al., 1991) and ATP-gated anion channel (Hwang and Sheppard, 2009; Hwang et al., 2018). Through its anion channel function, CFTR plays an important role in the transport of salt and water across these secretory epithelia, which is required for effective hydration of epithelial surfaces. Over 2000 CFTR genetic variants have been identified within the general and disease populations, and with systematic efforts 336 disease-causing variants in addition to 35 variants with varying clinical consequence have been distinguished from neutral variants (Cutting, 2015). The most prevalent mutation present on at least one allele in ∼90% of CF patients eliminates three base pairs, deleting phenylalanine 508 in the CFTR protein (Phe508del; F508del). To effectively treat patients carrying the most prevalent CF-causing mutation, there is a need for two biomolecular activities, namely, CFTR correctors that will increase the amount of F508del-CFTR protein at the plasma membrane, and CFTR potentiators that will allow effective functioning (gating) of the F508del-CFTR protein (Cheng et al., 1990; Dalemans et al., 1991; Lukacs et al., 1993; Cai et al., 2011; Lukacs and Verkman, 2012). For CF patients with the homozygous F508del mutation, both preclinical and clinical data indicate that a combination of these biomolecular activities is required for restoring F508del-CFTR function to levels projected to yield a significant clinical benefit. The advent of novel CFTR modulator therapies targeting this underlying CFTR defect have resulted in the development and marketing by Vertex Pharmaceuticals of the potentiators KALYDECO [N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (ivacaftor)] (Van Goor et al., 2009), for patients with 33 CFTR mutations causing gating defects, and ORKAMBI [a dual combination of ivacaftor and 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (lumacaftor), a C1 corrector for patients homozygous with the F508delCFTR mutation (Van Goor et al., 2011). Recently, another dual combination of ivacaftor and 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-[1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide (tezacaftor) has been marketed by Vertex as SYMDEKO (Tezacaftor/Ivacaftor 2018). Both of these dual combination therapies have shown only modest improvements in lung function [percentage of forced expiratory volume in one second (FEV1)] in patients homozygous for F508delCFTR mutations. In addition to being a cytochrome CYP3A4 inducer (www.ema.europa.eu/en/documents/annual-report/2015-annual-report-european-medicines-agency_en.pdf), ORKAMBI has been reported to demonstrate bronchoconstriction in some patients, most likely due to lumacaftor’s off-target effects (Elborn et al., 2016; Hubert et al., 2017; Marigowda et al., 2017). These liabilities were not observed with tezacaftor/ivacaftor in dual combination clinical studies, but still only modest lung function improvements (%FEV1) in addition to the reduced pulmonary exacerbations were observed for individuals that were homozygous with F508delCFTR or heterozygous with a second variant having residual function (Rowe et al., 2017; Taylor-Cousar et al., 2017). The C1 correctors target early folding of the mutant CFTR protein and could be combined with C2-type correctors with complementary mechanisms of action to deliver increased levels of F508del-CFTR protein to the plasma membrane. A triple combination of two correctors and a potentiator is expected to attain greater clinical benefits (Fig. 1). Proof of concept for such triple combination small molecule therapy to yield an efficacious readout was recently demonstrated by Vertex Pharmaceuticals in their phase 1 and 2 clinical trial results (Davies et al., 2018b; Taylor-Cousar et al., 2018) and in subsequent results from their phase 3 clinical trials with one triple combination with VX-659 (3-Pyridinecarboxamide, N-(phenylsulfonyl)-6-(3-(2-(1-(trifluoromethyl)cyclopropyl)ethoxy)-1H-pyrazol-1-yl)-2-((4S)-2,2,4-trimethyl-1-pyrrolidinyl)-, potassium salt) (Davies et al., 2018a) and the other with VX-445 (3-Pyridinecarboxamide, N-((1,3-dimethyl-1H-pyrazol-4-yl)sulfonyl)-6-(3-(3,3,3-trifluoro-2,2-dimethylpropoxy)-1H-pyrazol-1-yl)-2-((4S)-2,2,4-trimethyl-1-pyrrolidinyl)-) (https://investors.vrtx.com/news-releases/news-release-details/correcting-and-replacing-two-phase-3-studies-triple-combination).
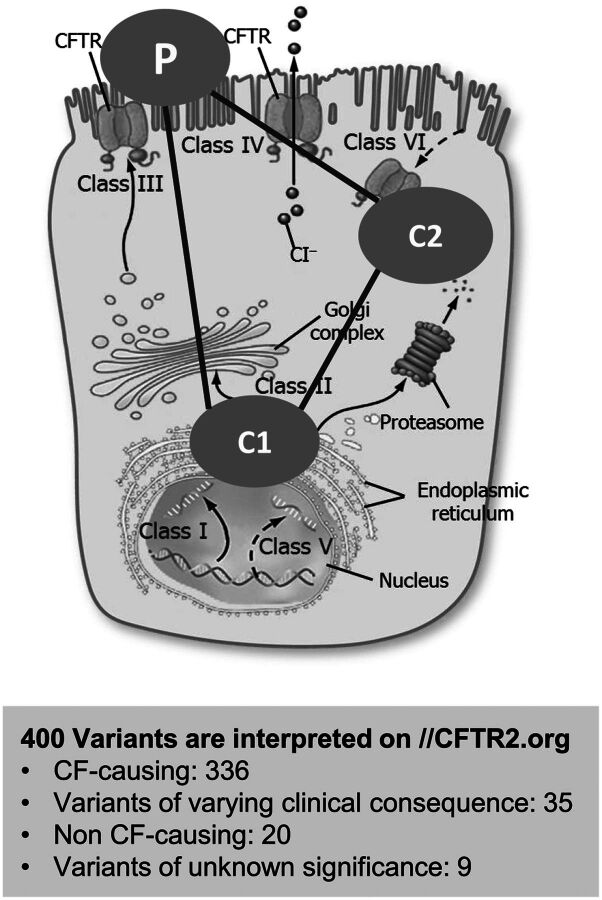
Fig. 1.
CFTR modulators. A total of 336 disease-related variants/mutations have been listed for the CFTR protein at https://www.cftr2.org/mutations_history. A complementary triple combination therapy consisting of two corrector molecules, type 1 (C1) and type 2 (C2) with additive mechanisms along with a potentiator (P) are being developed by several investigators for maximum restoration of mutated CFTR function.
Here, we report the identification and characterization of ABBV-2222/GLPG2222 (hereinafter referred to as ABBV-2222) (Fig. 2), a C1 CFTR corrector with significant improvements in potency and efficacy in vitro compared with the currently available C1 correctors lumacaftor and tezacaftor. C1 compounds like ABBV-2222, likely operate in a similar mechanism as described for lumacaftor and possibly tezacaftor and will be needed for novel triple combination therapies in addition to future therapies based on tezacaftor. ABBV-2222 possesses excellent potency and efficacy compared with other C1 correctors in human bronchial epithelial (HBE) transepithelial current clamp (TECC) functional assays; however, there will still be need for efficacious triple combination CFTR modulators. Subsequent analysis of the drug properties of this molecule, such as the pharmacokinetics and other preclinical characterizations showed it to be a suitable candidate for clinical development, and hence advancement into clinical trials.
Fig. 2.
Chemical structures of CFTR modulators discussed in the paper. Lumacaftor and tezacaftor are C1 class correctors and ivacaftor is a CFTR potentiator, which are drugs marketed by Vertex Pharmaceuticals for treating CF. ABBV-2222 is a C1 class corrector, which is currently in phase II clinical trials; compound 15 is a precursor of ABBV-2222, which is used as an on-plate control in the biologic assays described in the paper; and A-9433 is the inactive enantiomer of ABBV-2222. GLPG1837 is the potentiator of CFTR used in this paper.
Materials and Methods
Cell Surface Expression/Horseradish Peroxidase Assay.
A human lung–derived epithelial cell line [cystic fibrosis bronchial epithelial (CFBE) cells, CFBE41o-] (Ehrhardt et al., 2006) was used to develop a cellular assay for measuring the F508del CFTR cell surface expression (CSE) after correction with test compounds. This was achieved by expressing the F508del CFTR mutation along with horseradish peroxidase (HRP) activity in the fourth exofacial loop. The HRP activity was then measured using luminescence readout from these cells (CFBE41o-F508del CFTR-HRP), which were incubated overnight with the test corrector compounds (Veit et al., 2014). Briefly, for this primary assay, the CFBE41o-F508del CFTR-HRP cells were plated in 384-well plates (Greiner Bio-one) at 4000 cells/well along with 0.5 µg/ml doxycycline to induce the F508del CFTR-HRP expression, and then further incubated at 37°C and 5% CO2 for 72 hours. The test compounds were then added at the required concentrations and further incubated for 18–24 hours at 33°C. The highest concentration tested was 20 µM with an 8–12 point concentration-response curve using 3-fold dilution. Three replicate plates were run to determine one EC50 value. The EC50 values are represented as pEC50 values, defined as the negative log of EC50 values. All plates contained DMSO as the negative control and a single concentration of 2 to 3 µM of 3-[(2R,4R)-4-([[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl]amino)-7-methoxy-3,4-dihydro-2H-chromen-2-yl]benzoic acid (compound 15) (Wang et al., 2018) as the positive control, as well as the on-plate concentration response of the positive control. The plates were washed 5× times with Dulbecco’s PBS postincubation, followed by the addition of luminol (50 µl), as the HRP substrate. This was followed by measuring the HRP activity using luminescence readout on an EnVision Multilabel Plate Reader (Perkin Elmer). The raw counts from the experiment were analyzed using Accelrys Assay Explorer version 3.3.
The percentage of activity measured at each of the eight test concentrations of the test compound was normalized to the on-plate positive control using the following formula:
The maximum percentage of activity achieved for the test compound at any tested concentration is presented along with the pEC50 value calculated using the general sigmoidal curve with the variable Hill slope equation.
Western Blot Analysis of F508delCFTR Band C/B in CFBE Cells.
CFBE cells stably expressing F508delCFTR (clone DG3) were obtained from the laboratory of Dr. Bob Bridges (Rosalind Franklin University of Medical Sciences, North Chicago, IL) and grown in minimal essential medium (MEM) (Gibco, Grand Island, NY), supplemented with 10% FBS (Hyclone), 1% penicillin/streptomycin, and 500 µg/ml G418 (Geneticin) (Gibco) at 37°C in a 5% CO2 humidified incubator. CFBE cells were seeded at 106 cells per well onto six-well dishes overnight and then treated with either DMSO or the indicated compound for 18–24 hours at 37°C in a 5% CO2 humidified incubator. Prior to lysis, cell monolayers were rinsed twice with cold PBS to remove serum and medium. Cell plates were put on ice and lysed with the addition of radioimmunoprecipitation assay buffer (Sigma) containing protease inhibitors (Roche). Lysates were centrifuged at 12,000g for 10 minutes at 4°C to pellet insoluble material and total protein concentration was measured using the bicinchoninic acid (BCA) protein assay (ThermoFisher, Waltham, MA). Equal amounts of protein were electrophoresed on NuPAGE Novex 7% Tris-acetate gels for 2 hours at 150 V. Proteins were then transferred onto polyvinylidene difluoride (PVDF) membranes at 20 V for 1 hour using a Novex Semi-Dry Blotter (Invitrogen). Membranes were incubated overnight at 4°C with primary mouse monoclonal anti-CFTR 596 antibody (1:5000, UNC596; University of North Carolina at Chapel Hill). Secondary antibody [IRDye 800CW goat anti-mouse IgG (1:15,000; LI-COR)] was incubated for 1 hour at room temperature in the dark. Immunoblots were scanned on a LI-COR Odyssey infrared imaging system and quantified using the system software. Mouse monoclonal α-Na/K ATPase antibody (1:5000; Abcam) was used to normalize sample loading.
Western Blot Analysis of F508delCFTR Band C/B in Baby Hamster Kidney Cells Expressing Suppressor Mutations.
Suppressor mutations were used as a tool for a structural-based corrector screen in order to identify correctors that either stabilize nucleotide binding domain (NBD) 1 and/or the NBD1/membrane-spanning domain (MSD) 2 interface of the F508del CFTR protein based on the possible synergism. The two suppressor mutant variants used in these studies were R1S (G550E, R553Q, R555K, and F494N mutations that stabilize the NBD1 domain) and R1070W (which stabilizes the NBD1-MSD2 interface).
Baby hamster kidney (BHK)-21 cells, stably expressing the R1S or R1070W F508del CFTR with three tandem hemagglutinin epitopes (3HA) in the fourth extracellular loop, were obtained from the laboratory of Dr. Gergely Lukacs (McGill University, Montreal, Canada) and maintained as described previously (Sharma et al., 2004; Du et al., 2005). The HA tags preserved the functional and biochemical characteristics of CFTR (Haardt et al., 1999). BHK cells were grown in Dulbecco’s modified Eagle’s medium/F-12 (Gibco), supplemented with 5% FBS (Hyclone) and 500 µM methotrexate (Hospira), at 37°C in a 5% CO2 humidified incubator.
Briefly, the BHK suppressor mutant cells were seeded at 1 × 106 cells per well onto six-well dishes overnight and then treated with either 0.2% DMSO or 1 µM ABBV-2222 for 18–24 hours at 37°C in a 5% CO2 humidified incubator. Just prior to lysis, cell monolayers were rinsed twice with cold PBS to remove serum and medium. Cell plates were put on ice and lysed with the addition of radioimmunoprecipitation assay buffer containing protease inhibitors (cOmplete Protease Inhibitor Cocktail, EDTA-free; Roche). Lysates were centrifuged at 14,000g for 15 minutes at 4°C to pellet insoluble material and total protein concentration was measured using the bicinchoninic acid protein assay (Thermo). Next, 50 µg of protein was electrophoresed on NuPAGE Novex 3%–8% Tris-acetate gels for 2 hours at 150 V. Proteins were then transferred onto polyvinylidene difluoride membranes at 20 V for 1 hour using a Novex Semi-Dry Blotter (Invitrogen). Membranes were incubated with primary antibody overnight at 4°C to detect the HA-tagged CFTR (mouse monoclonal anti-HA antibody (1:5000; Covance Innovative Antibodies). Secondary antibody [IRDye 800CW goat anti-mouse IgG (1:15,000; LI-COR)] was incubated for 1 hour at room temperature in the dark. Immunoblots were scanned on a LI-COR Odyssey infrared imaging system and quantified using the system software. Mouse monoclonal anti-α-1 Na/K ATPase antibody (1:5000; Abcam) was used to normalize sample loading.
Correction of CFTR Protein through Action on Membrane-Spanning Domain 1.
Human embryonic kidney 293 (HEK-293)cells from American Type Culture Collection (Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 1% FBS (Thermo Scientific) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Gibco) at 37°C in an atmosphere of 5% CO2. Cell transfections were performed using Effectene reagent (Qiagen, Valencia, CA). The empty pcDNA3.1(+) vector was used to ensure equal microgram quantities of DNA were used in all transfection reactions.
Transepithelial Current Clamp Assay Using CFBE41o- (Clone DG3) or Primary Human Bronchial Epithelial Cells.
A cell-based assay was developed using the CFBE41o- (clone DG3) of the primary HBE cells and used as a secondary assay to test novel F508del CFTR correctors for their activity on primary HBE cells with the F508del/F508del CFTR mutation.
CFBE-DG3 Cells.
CFBE41o- cells stably expressing F508del CFTR (clone DG3) were obtained from the laboratory of Dr. Bridges (Rosalind Franklin University of Medical Sciences) and cultured in minimal essential medium (Gibco) supplemented with 10% FBS, 1% penicillin/streptomycin, and G418 (Geneticin) selection (0.5 mg/ml) in a humidified 5% CO2/95% O2 incubator at 37°C. For propagation, the CFBE41o- cells were cultured in plastic flasks coated with an extracellular matrix consisting of 10 μg/ml human fibronectin (EMD), 30 μg/ml PureCol collagen (Advanced Biomatrix), and 100 μg/ml bovine serum albumin (Sigma-Aldrich) diluted in minimal essential basal medium. For TECC measurements, CFBE41o- cells were seeded onto collagen-coated 24-well filters at a density of 1.5 × 105 cells/filter and cultured for 5–7 days in the aforementioned growing media to form a tight monolayer.
Human Bronchial Epithelial Cells.
Primary HBE cells from CFTR patients with homozygous F508del/F508del mutation were expanded from 1 to 250 × 106 cells (Neuberger et al., 2011). For this purpose, cells isolated from CF patients with the homozygous mutation, procured from the CF center tissue procurement and cell culture core at the Marsico Lung Institute at the University of North Carolina at Chapel Hill (https://infoporte.unc.edu/cores/buy.php?cid=135) as well as the Cystic Fibrosis Translational Research Center at McGill University (https://mcgill.ca/cftrc/platforms/primary-airway-cell-biobank-pacb/services), were seeded onto 24-well Corning filter plates coated with 3T3 conditioned media and grown at an air-liquid interface for 35 days using Ultroser G supplemented differentiation media. All of the primary human bronchial epithelial cells were collected in accordance with institutional review board (IRB) approval protocols. Apical surface mucus was removed 72 hours before the experiment by incubating the apical surface of the cells for 30 minutes with 3 mM dithiothreitol prepared in Dulbecco’s PBS with Ca2+ and Mg2+. This was followed by aspiration of the mucus from the apical surface along with Dulbecco’s PBS. The apical surface is re-washed with PBS incubated for 30 minutes followed with aspiration. These HBE cells were then incubated with the desired concentrations of the corrector compounds 18–24 hours at 37°C and 5% CO2. The desired concentrations of the corrector compounds were prepared from the 10 mM stocks in differentiation media and were always applied on the basolateral side of the epithelial cells. We also used an assay format, where a fixed concentration of potentiator was added chronically along with the corrector compounds. This chronic treatment with the potentiator helps eliminate any interaction that might happen with CFTR modulators (correctors and potentiators) (Cholon et al., 2014; Veit et al., 2014) and thereby determine the true efficacy of the modulator combinations reflective of clinical relevance.
Transepithelial Current Clamp Assay.
The assay uses a transepithelial current clamp (Vu et al., 2017) instrument that can measure the functionality of the mutated channel by measuring the CFTR equivalent current (IEQ) generated by the polarized primary epithelial cells. We used a TECC-24 with a 24-channel electrode manifold allowing for the simultaneous measurement of the transepithelial voltage (VT) and transepithelial resistance (RT) under current clamp conditions from 24 filters using a 24-well Costar filter plate. The design of the filters in the 24-well filter plates is exactly the same as the design of an individual Transwell filter used in the classic Ussing chamber with a surface area of 0.33 cm2. Each measured VT value is corrected for the electrode offset potential measured using buffer alone in a separate plate, and each measured RT value is then corrected for the combined solution series and empty filter resistances. The corrected VT and RT values were then used to calculate the equivalent current using Ohm’s law (IEQ = VT/RT). The area under the curve for the time period between the forskolin peak IEQ response and the time of bumetanide addition was also calculated using a one-third trapezoid method, in addition to calculating the IEQ value. Unlike Fischer rat thyroid (FRT) cells, where the expression of CFTR is not polarized, expression of CFTR in CFBE cells is restricted to the apical membrane, and in cells overexpressing CFTR the transepithelial conductance (Gt) response largely reflects apical membrane conductance changes. When using CFBE cells in the TECC, Gt was measured using a custom microcontrolled TECC. Cells were simulated with a 30 mV sine wave at a frequency of 1 Hz and the current response was sampled at 8 kHz. The Gt value was calculated using Ohm’s law, where Gt = ΔI/ΔVt (in which ΔI is the delta current response to the 30 mV voltage sine wave, ΔVt). The assay was run in a 24-well format and all 24 wells were measured at the same time point giving a higher throughput for this assay. On the day of measuring the corrector activity on the TECC, the cells were switched into a bicarbonate- and serum-free F-12 Coon’s medium and allowed to equilibrate for 180 minutes for the CFBE-DG3 cells and 30 minutes for the HBE cells in a CO2 free incubator. At the time of measurement, the apical and basolateral sides of the filter were bathed with the F-12 Coon’s modification media (with 20 mM HEPES), pH 7.4 (using 1 M Tris), and the measurements were made at 36.5°C. Current responses before and after the sequential addition of benzamil (apical 6 µM addition; for inhibiting the epithelial sodium channel), forskolin (apical and basolateral 10 µM addition; for activating the CFTR channel), control potentiator, N-(3-carbamoyl-5,5,7,7-tetramethyl-4,7-dihydro-5H-thieno[2,3-c]pyran-2-yl)-1H-pyrazole-5-carboxamide [(GLPG1837), apical and basolateral 1 µM addition; for potentiating the CFTR channel] (Van der Plas et al., 2018), and bumetanide (basolateral 20 µM addition; for inhibiting the Na:2Cl:K cotransporter, an indirect measure of inhibiting the Cl− secretion driven by the CFTR channel) were measured.
All plates contained negative controls (DMSO), which coupled with the control potentiator (GLPG1837) set the null response and positive controls (3 µM of compound 15) (Wang et al., 2018) coupled with the control potentiator that set the 100% response to measure the correction of the mutated CFTR channel. The maximum percentage of activity is reported relative to the positive control value.
The percentage of activity measured at each of the six test concentrations of the test compound was normalized to the on-plate positive control using the following formula:
The IEQ and area under the curve at different test concentrations were fit and an EC50 value was calculated using the general sigmoidal curve with the variable Hill slope equation included in the Prism version 5 software program.
Patch-Clamp Electrophysiological Assay.
To examine the open probability (Po) of F508delCFTR after pretreatment with ABBV-2222, CHO cells transiently transfected with CFTR-cDNA [pcDNA 3.1 Zeo (+) vector; Invitrogen] and GFP encoding pEGFP-C3 (Takara Bio Inc., Shiga, Japan) were incubated with ABBV-2222 overnight before patch-clamp experiments. To avoid repetition, detailed experimental methods, materials, and data analysis can be found in our latest publication (Yeh et al., 2019).
Chemicals and Reagents.
Benzamil and Forskolin were purchased from Sigma-Aldrich. The selective CFTR inhibitor, CFTRInhib-172, was purchased from Tocris Biosciences. The Na-K-2Cl cotransporter inhibitor, bumetanide, was purchased from Sigma-Aldrich. The Vertex CFTR modulators, lumacaftor, tezacaftor, and ivacaftor were purchased from SellekChem. All AbbVie/Galapagos CFTR modulators were synthesized at AbbVie or Galapagos and these test compounds were tested from 10-mM stock solution made with DMSO and stored at room temperature.
CFTR expression plasmids pcDNA3.1(+)-CFTR and pcDNA3.1(+)ΔF508-CFTR have been described elsewhere (Meacham et al., 2001; Younger et al., 2006). CFTR constructs representing CF disease–causing point mutants or truncated biogenic intermediates were made using the QuikChange protocol (Stratagene, Santa Clara, CA). The CFTR antibody used in this study was MM13-4 (N-terminal tail epitope) obtained from Millipore, Darmstadt, Germany.
Statistical Analysis.
The results are expressed as mean ± S.D. or S.E.M. of n observations. Statistical analysis was carried out using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA) for Windows.
Results
Identification of an Active Chemotype and ABBV-2222 Using a Cell Surface Expression Assay
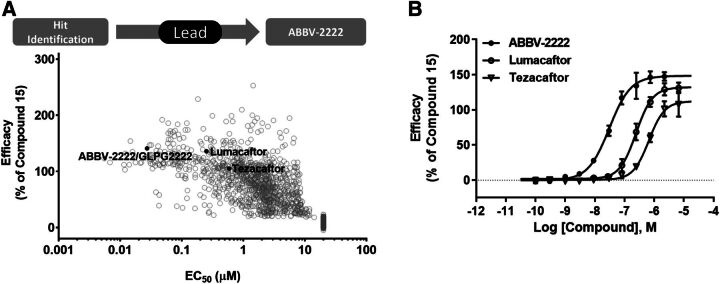
The first-in-class first generation of C1 correctors, lumacaftor and tezacaftor, were developed and marketed by Vertex Pharmaceuticals. We have identified and developed a novel C1 corrector, ABBV-2222, that has a differentiated structure with improved potency and enhanced clinical characteristics (Wang et al., 2018). Figure 2 shows the structure of these correctors and corrector compound 15, a precursor of ABBV-2222 used as the control in our biologic assays, as well as A-9433, which is the inactive enantiomer of ABBV-2222; and the structure of the Vertex marketed CFTR potentiator, KALYDECO (ivacaftor) along with GLPG1837, the potentiator used in our biologic assays described in this paper. Figure 3A shows the medicinal chemistry process that started from the hit identified from a high-throughput primary screen. The virtual libraries were enumerated from selected proprietary acid and amine monomers present in AbbVie’s collection to assemble novel lead compounds through a final amide bond coupling reaction. The final products were then filtered based on optimized chemical and physiochemical properties and actual compounds synthesized for their structure-activity relationship (SAR). After the initial SAR to identify sets of active compounds, an empirical-based SAR effort was used for optimizing compound potency, its clearance, and other drug-like properties. The high-throughput CSE-HRP assay for measuring the F508del CFTR cell surface expression after correction with test compounds was used to identify the chemotype and all of the further primary SAR studies. The medicinal chemistry cycle of compound synthesis and SAR analysis was repeated to refine and finally generate potent lead compounds with good drug-like properties. ABBV-2222 was identified from these sets of lead molecules. A plot representing the potency and efficacy improvement progression through understanding of the SAR of the compounds is also shown in Fig. 3A. The concentration-response curve for membrane expression efficacy of ABBV-2222 relative to lumacaftor and tezacaftor as measured by the CSE-HRP assay is shown in Fig. 3B. ABBV-2222 was approximately 10- and 20-fold more potent than lumacaftor and tezacaftor, respectively. It had an EC50 value of 27 nM and pEC50 value of 7.56 ± 0.16 (n = 19), compared with EC50 = 251 nM and pEC50 = 6.6 ± 0.08 (n = 3) and EC50 = 586 nM and pEC50 = 6.23 ± 0.05 (n = 6) for lumacaftor and tezacaftor, respectively. The relative efficacies from these experiments were 141 ± 15 (n = 19), 136 ± 5 (n = 3), and 105 ± 14 (n = 6) for ABBV-2222, lumacaftor, and tezacaftor, respectively. An efficacy drop-off was observed for ABBV-2222 above 2.22 µM and lumacaftor above 6.67 µM (data not shown in the plot and not used for the EC50 calculations). Compound 15 showed an EC50 value of 230 nM with relative efficacy of 101% in this assay and was subsequently used as an on-plate control for our internal biologic assays.
Fig. 3.
(A) Medicinal chemistry process of Lead generation followed by the identification of ABBV-2222. Detailed SAR data for the chemical series using the CSE-HRP assay showing a distribution of the potency and efficacy of ABBV-2222 relative to other derivatives as well as lumacaftor and tezacaftor. (B) Representative concentration-response of ABBV-2222 in the CSE assay in comparison with lumacaftor and tezacaftor. Data are shown as mean ± S.D. of three replicates from a representative experiment; however, several replicates were run as described below. ABBV-2222 was approximately 10- and 20-fold more potent than lumacaftor and tezacaftor, respectively. It had values of EC50 = 27 nM and pEC50 = 7.56 ± 0.16 (n = 19), compared with EC50 = 251 nM and pEC50 = 6.6 ± 0.08 (n = 3) and EC50 = 586 nM and pEC50 = 6.23 ± 0.05 (n = 6) for lumacaftor and tezacaftor, respectively. The relative efficacies from these experiments were 141%, 136%, and 105% normalized to the on-plate compound 15 for ABBV-2222, lumacaftor, and tezacaftor, respectively. Efficacy drop-off was observed for ABBV-2222 above 2.22 µM and lumacaftor above 6.67 µM (data not shown in the plot and not used in the EC50 calculations).
Biochemical and Functional Characterization of ABBV-2222
ABBV-2222 Promotes Maturation of F508delCFTR as Assessed by Western Blot.
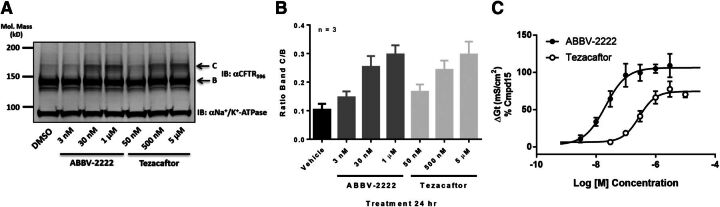
Biochemical characterization to assess the effects of ABBV-2222 on the maturation of F508delCFTR-mutated protein was performed in the CFBE41o- cells stably expressing F508del CFTR (CFBE clone DG3) that were obtained from the laboratory of Dr. Robert Bridges (Rosalind Franklin University of Medical Sciences). Western immunoblots are presented in Fig. 4, A and B and α1 Na+/K+ ATPase was used as a loading control. Consistent with the observations in the CSE-HRP assay, ABBV-2222 promoted maturation of F508delCFTR as measured by the accumulation of a higher molecular weight glycosylated band (band C) by western blot analysis (Fig. 4A). Figure 4B illustrates the observed concentration-dependent increase of the mature band C, which was similar to the one observed with tezacaftor albeit at lower concentrations; the bar graph shows the mean and S.D. values obtained from three independent experiments (n = 3) of the band C/B ratio for the representative western blot experiment shown in Fig. 4A.
Fig. 4.
(A) Three-point concentration response of ABBV-2222 and tezacaftor on F508delCFTR maturation in the CFBE cells as reflected by band C. Compounds were incubated for 24 hours with the cells prior to harvesting and lysis. (B) Bar graph showing mean with S.D. from n = 3 replicates of the band C/B ratio including the representative western blot experiment shown in (A). (C) Concentration response of ABBV-2222 and tezacaftor on F508delCFTR function in the CFBE-DG3 cells measured in the TECC assay in the presence of the potentiator GLPG1837. The EC50 value for ABBV-2222 was 20.8 ± 2.7 nM compared with 266.9 ± 38.6 nM for tezacaftor (n = 6 replicates at each concentration tested).
Functional IEQ Measurement Using TECC-24 in CFBE and Primary HBE Cells and Po Measurement Using Single Channel Excised Patch Clamp.
To determine if restoration of surface expression is translated to a functional response, we measured the forskolin-activated Cl− conductance in these CFBE clone DG3 cells using the semi-automated TECC-24 instrument. As shown in Fig. 4C, the increase in F508del-CFTR rescue by ABBV-2222 as monitored by the function, measured as an increase in conductance (Gt) due to CFTR channel opening on the membrane facilitated by a potentiator, was concentration dependent, with an EC50 value of 20.8 ± 2.7 nM (S.E.M.; n = 6 at each concentration). Analogous to the efficacy difference observed in the western blot results, tezacaftor was ∼14-fold less potent than ABBV-2222, with an EC50 value of 266.9 ± 38.6 nM (S.E.M.; n = 6 at each concentration).
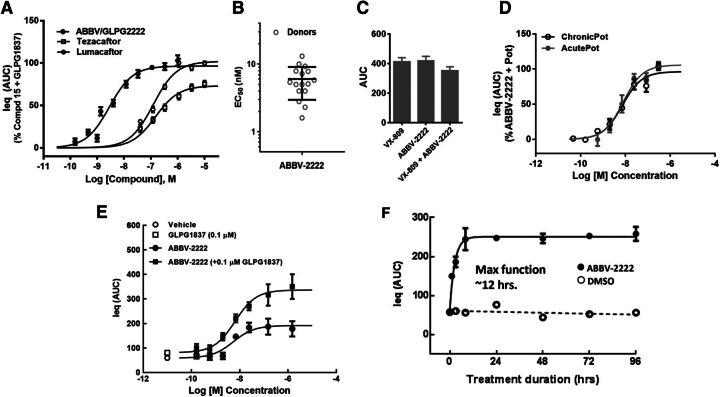
Further functional characterization of ABBV-2222 was extended to more biologically and clinically relevant primary human bronchial cells derived from the CF patients homozygous for the F508del variant. The cultured F508del-HBE cells were incubated with ABBV-2222 for 24 hours prior to the electrophysiological readout and are referred to as a chronic treatment with the compounds at the indicated test concentrations and treatment duration. The CFTR-mediated equivalent current from HBE cells was measured using TECC-24 in the continuous presence of corrector or potentiator compounds. Figure 5A shows representative concentration-response curves for ABBV-2222 in comparison with tezacaftor and lumacaftor, with EC50 values of 2.85 ± 0.4 nM (S.E.M.; n = 5 different donors), 148.2 ± 25.7 nM (S.E.M.; n = 4 different donors), and 122.8 ± 13.8 nM (S.E.M.; n = 1 donor), respectively. GLPG1837 was used as the potentiator in these experiments and was added acutely in combination with forskolin (10 µM). ABBV-2222 also exhibited equivalent efficacy to lumacaftor, and ∼20% improvement in efficacy compared with tezacaftor as measured with this functional assay in a dual format. Taken together, ABBV-2222 differentiates itself at the level of in vitro potency (from both lumacaftor and tezacaftor) and efficacy (from tezacaftor). The potency ranges of ABBV-2222 were determined in the HBE TECC assay from 16 different CF patient–derived donor cells that were homozygous for the F508delCFTR variant. As shown in Fig. 5B, the TECC functional potencies ranged from 1.6 to 13.1 nM across these homozygous 16 F508delCFTR variant donors’ cells with the median potency around 6 nM. Enantiomeric selectivity was demonstrated by A-9433, an enantiomer of ABBV-2222, which was ∼160-fold less potent and ∼70% less efficacious than ABBV-2222 (data not shown). ABBV-2222 does not show additivity with lumacaftor or tezacaftor in the HBE TECC functional assay and likely works via a similar mechanism of action. A representative example of lack of functional additivity for lumacaftor and ABBV-2222 is shown in Fig. 5C. The maximum percentage of activity concentrations for both lumacaftor and ABBV-2222 were chronically incubated along with GLPG1837 (0.1 µM) as a potentiator for this experiment (n = 8 replicates for each condition). As shown in Fig. 5D, there was no difference in potency of ABBV-2222 in both acute and chronic modes of addition of the potentiator (EC50 value of 7.9 vs. 7.3 nM, respectively). The potencies were determined using one primary HBE donor with the F508delCFTR variant, with n = 3 replicate values at each tested concentration. Potentiator GLPG1837 did not have any nonproductive interactions with the correctors for their relative efficacy in the western blot protein measurement assay or the TECC functional assay. To have a more clinically relevant format, all of the compounds were added chronically and were added back; therefore, they were also present during all of the TECC experimental runs. There was no change in potency of ABBV-2222 in the absence or presence of GLPG1837 potentiator (0.1 µM) (EC50 value of 6.4 vs. 5.4 nM, respectively) as shown in the concentration-response curves in Fig. 5E; the potencies were determined using one primary HBE donor with the F508delCFTR mutation, with n = 2 replicate values at each tested concentration. We also determined the onset of F508delCFTR activity in the functional HBE TECC assay. Similar to previous report (Lukacs et al., 1993), the functional response showed rapid onset with maximal effect achieved within 12 hours of treatment, as well as sustainability up to 96 hours in the TECC system (Fig. 5F).
Fig. 5.
Characterization of functional F508delCFTR correction by ABBV-2222 in HBE TECC assay. (A) Concentration-response curve of ABBV-2222, lumacaftor, and tezacaftor in TECC assay using primary HBE cells homozygous for F508delCFTR and potentiator GLPG1837. Compounds were added to the cells for 24 hours prior to the electrophysiological readout using TECC. The percentage of activity was compared with the activity of the control (compound 15) in the presence of potentiator GLPG1837. (B) Scatter plot of HBE TECC potency of ABBV-2222 across 16 different F508del/F508delCFTR homozygous donors. The potencies ranged from 1.6 to 13.1 nM across 16 different primary HBE donor cells with the median potency around 6 nM. (C) Bar graph showing lack of functional additivity for lumacaftor and ABBV-2222 in the TECC functional assay on F508del/F508delCFTR homozygous donor day (mean with S.E.M. from n = 8 replicates). (D) Concentration-response curve of ABBV-2222 to compare the potency of ABBV-2222 with the acute addition of the GLPG1837 potentiator (the potentiator was added together with forskolin at the time of the TECC assay readout) or the chronic addition of the GLPG1837 potentiator (coincubation of GLPG1837 with ABBV-2222 during 24 hours prior to addition of forskolin for channel activation). The EC50 values determined in the two conditions were similar: 7.9 nM (acute incubation) compared with 7.3 nM (chronic incubation). (E) HBE TECC potency of ABBV-2222 was determined with or without chronic addition of the potentiator GLPG1837. (F) Onset of rescued F508delCFTR protein function was determined using the TECC assay after correction with ABBV-2222 in the presence of GLPG1837 at the indicated times (n = 4; representative data from a single donor that was homozygous with F508delCFTR mutation). DMSO condition represents cells incubated with the potentiator GLPG1837 but without any corrector ABBV-2222.
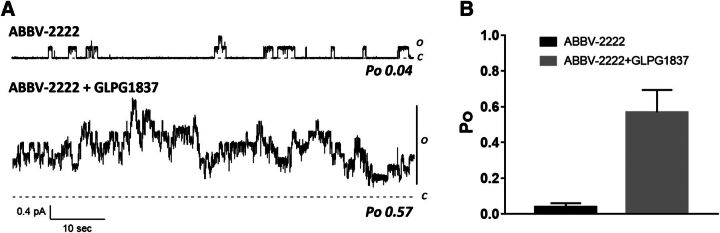
To assess the channel function of F508delCFTR pretreated with ABBV-2222, patch-clamp experiments in the inside-out mode were performed in CHO cells expressing F508delCFTR. Figure 6 shows a real-time current recording of F508delCFTR that was activated by protein kinase A and ATP (upper trace). There were only five distinguishable openings in >1 minute of recording in the presence of ABBV-2222, suggesting a persistent gating defect not corrected by ABBV-2222. A similar recording was obtained in the absence of ABBV-2222 (data not shown). However, in the same patch, the addition of the CFTR potentiator GLPG1837 (Yeh et al., 2017, 2019) dramatically increased the activity to reveal the presence of at least eight F508delCFTR channels in the patch (lower trace in Fig. 6). Single channel kinetic analysis of data like these revealed an apparent Po value of 0.57 in in the presence of GLPG1837, whereas the Po value of ABBV-2222–treated F508delCFTR was 0.04, which was similar to the vehicle-treated control reported previously (Kopeikin et al., 2014).
Fig. 6.
Single channel Po of F508delCFTR protein corrected with ABBV-2222 in the absence and presence of potentiator GLPG1837. (A) Patch-clamp experiments were performed in CHO cells expressing F508delCFTR (Lin et al., 2016) to determine the Po of the F508delCFTR protein in the absence (0.04) and presence (0.57) of potentiator GLPG1837. (B) Bar graph representation of the data shown in (A) (n = 4 replicates). ABBV-2222 does not have direct affect the Po of the corrected F508delCFTR channel until activated by GLPG1837 potentiator.
ABBV-2222 Stabilizes the Nucleotide Binding Domain 1/Membrane-Spanning Domain Interface.
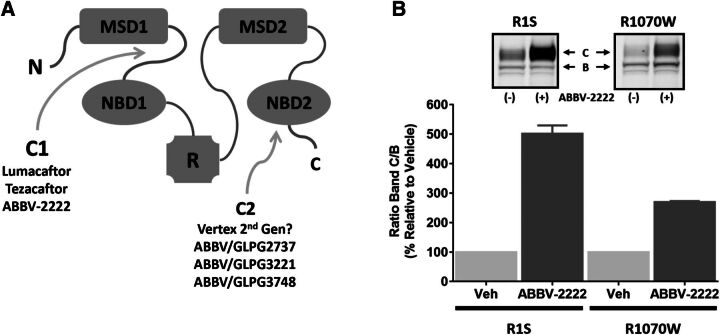
Several investigators have successfully demonstrated that lumacaftor targets F508del-CFTR during endoplasmic reticulum biogenesis and engages in undefined interactions to facilitate protein maturation. As well documented in literature, and shown in Fig. 7A, F508del mutation in CFTR causes severe dysregulations of NBD1 energetics as well as domain-domain interactions, mainly the NBD1-MSD1/2 interface. It has been previously shown that stabilization of NBD1 or the NBD1-MSD2 interface by second site suppressor mutations such as R1S or R1070W only modestly improves CFTR folding (<20%). However, simultaneous stabilization of these primary structural defects can lead to robust rescue of CFTR folding (∼80%) (Okiyoneda et al., 2013). CFTR-F508del integrated with the R1070W suppressor mutation has been shown to stabilize the NBD1-MSD2 interface, and hence serves as a reference for how type C1 correctors function; type C2 correctors promote the stabilization of the NBD2 interactions, and type C3 correctors stabilize NBD1 directly (Okiyoneda et al., 2013). CFTR-F508del integrated with the R1S suppressor mutations has been shown to stabilize NBD1 directly and replicate the actions of a type 3 corrector. Okiyoneda et al. (2013) demonstrated that compounds like lumacaftor preferentially target the NBD1-MSD1/2 interface, and hence these authors classified them as a type C1 corrector.
Fig. 7.
ABBV-2222 functions as a type C1 corrector. (A) Proposed model categorizing different types of CFTR correctors. (B) Western blot analysis of CFTR ± 1 µM ABBV-2222 in BHK cells expressing either R1S-CFTR-F508del (R1S) or R1070W-CFTR-F508del (R1070W) suppressor mutations. BHK cells overexpressing either R1S or R1070 were incubated with ABBV-2222 for 24 hours. Cells were harvested, lysed, and loaded onto a 3%–8% Tris-acetate gel. CFTR was detected using CFTR antibody 596.
Suppressor mutants were used in our study as a tool for structural-based characterization of our C1 corrector, ABBV-2222, to determine if it stabilizes the NBD1 domain and/or the NBD1-MSD2 interface, based on possible synergism with R1S or R1070W. As shown in our western blot data, ABBV-2222 substantially rescued the folding of F508del-CFTR-R1S and it had a lesser impact on F508del-CFTR-R1070W. This suggests that ABBV-2222 preferentially impacts the NBD1/MSD2 interface restoration over the NBD1 structural defects, and hence can be classified as a type C1 corrector that functions by promoting stabilization of the interface between NBD1 and MSD2. ABBV-2222 exhibits more efficiency in restoring band C formation with BHK cells expressing the R1S suppressor mutation compared with those expressing the R1070W suppressor mutation. The pattern of the band C/B ratio is similar to that reported for lumacaftor, supporting the idea that ABBV-2222 stabilizes the NBD1-MSD2 interface as a type C1 corrector.
In analogy with this suppressor mutant model, corrector pairs acting on different primary structural defects are needed to restore F508del-CFTR folding synergistically and those will be the ones that will yield better efficacy. Since both primary structural defects need to be corrected to notice a substantial rescue of F508del-CFTR folding, a small molecule acting on the same prestabilized intramolecular interdomain interaction (by R1S suppressor mutation) is unlikely to produce the level of correction observed in our western blot data for R1S.
ABBV-2222 was selective for correction of F508delCFTR processing and was unable to improve the processing of other misfolded mutant proteins like P-glycoprotein (G268V-PgP) and hERG K+ channel (G601S-hERG) (data not shown).
ABBV-2222 Corrects F508delCFTR through Action on Membrane-Spanning Domain 1.
Another set of experiments was conducted to further explore whether ABBV-2222 behaved in a similar fashion as lumacaftor and tezacaftor, which correct the folding defects in CFTR protein via direct action on a specific region of CFTR, i.e., on MSD1, and this correction does not require the presence of NBD1. Biochemical assays monitoring the effects of CFTR modulators on the steady-state accumulation and half-life durations of different lengths of CFTR fragments have been used to study CFTR biogenesis. Ren et al. (2013) demonstrated that the shortest-length CFTR requirement for lumacaftor action is on the N-terminal CFTR fragment that only contained MSD1 (Fig. 8A). We saw a similar protein fragment correction pattern with ABBV-2222, in that the shortest length of CFTR fragment affected by ABBV-2222 was CFTR 375, which contains only MSD1 (Fig. 8B). Like lumacaftor, ABBV-2222 did not increase the stability of CFTR 837–1480 (data not shown). As shown in Fig. 8B, ABBV-2222, lumacaftor, and tezacaftor were all unable to increase the accumulation of CFTR 370, suggesting that they do not act as a proteasome inhibitor, whereas bortezomib was able to increase the stability of this CFTR fragment (Ren et al., 2013). Cycloheximide-chase experiments were carried out to determine the half-life of the CFTR 380 fragment in the presence of protein synthesis inhibitor cycloheximide (10 µg/ml), and the changes in CFTR 380 signals over the course of a 2-hour chase reaction were normalized to the CFTR 380 signal at T = 0, as shown in Fig. 8C. The half-life of CFTR 380 was increased in the presence of ABBV-2222, lumacaftor, and tezacaftor compared with the untreated control, indicating that the increase in the steady-state accumulation caused by these compounds is associated with an increase in the stability of the CFTR 380 fragment. In summary, ABBV-2222 acts similarly to lumacaftor and tezacaftor, with improved stabilization of CFTR 375.
Fig. 8.
The impact of CFTR modulators on the accumulation of short CFTR fragments that contain MSD1 and different lengths of C-terminal extensions. (A) Schematic diagram of CFTR fragments whose accumulation becomes sensitive to modulators upon inclusion of residues that are located between amino acid 375 and 380. (B) Comparison of ABBV-2222, lumacaftor, and tezacaftor on the steady-state accumulation of the indicated CFTR fragment. Indicated modulators were added at 5 µM to cultures of human embryonic kidney (HEK) 293 cells 14 hours prior to harvest and detection of the fragment. Modulators were added to cells that expressed 380X-CFTR at T = 0 of the chase reaction in the same media with the protein synthesis inhibitor cycloheximide (10 µg/ml). The pCDNA3.1 expression plasmid (0.25 µg) that harbored the indicated form of CFTR was transfected into HEK293 cells at 18 hours prior to harvest. CFTR fragment expression was detected by western blot with the CFTR N-terminal tail antibody (MM13-4). In (B), the level of respective CFTR fragment was quantitated with a GE Healthcare LAS 4000 and compared with levels detected in the presence of the proteasome inhibitor bortezomib (10 µM), which was added to cultures 4 hours prior to harvest. This was done because CFTR fragments 370X, 373X, and 375X have a short half-life and do not accumulate to detectable levels unless they are stabilized by folding modulators or inhibition of the proteasome. In (C), changes in CFTR signals over the course of a 2-hour chase reaction were normalized to the CFTR signal at T = 0.
Discussion
The molecular defect caused by different CFTR variants, especially F508delCFTR, has been a focus of studies, which have shown that small molecules are able to enhance the amount and function of CFTR at the cell surface in different cell systems, including the primary HBE, hNE (human nasal epithelia), and intestinal organoids (Awatade et al., 2018). The past two decades have seen exponential growth in the discovery and development of these small molecule CFTR modulators, namely, the CFTR potentiators and correctors. Indeed, one of the biggest breakthroughs in the field of CF this decade has been the development and marketing of CFTR potentiators and correctors that rectify the underlying defects in the CFTR protein (Gentzsch and Mall, 2018; Li et al., 2018). Referred to as first-generation CFTR C1 correctors, both lumacaftor and tezacaftor restored F508delCFTR folding and increased the CFTR function in preclinical in vitro testing in combination with the CFTR potentiator ivacaftor to ∼25% normal CFTR activity (Van Goor et al., 2011). Monotherapy in CF carrying the homozygous F508delCFTR variant with both of these C1 correctors did not show marked improvement in lung functions as measured by FEV1 (Clancy et al., 2012). Even the dual therapy of either of these C1 correctors in combination with the potentiator ivacaftor only resulted in significant but marginal improvement in lung function, in the range of ∼3% to 4% FEV1 (Wainwright et al., 2015; Ratjen et al., 2017). Based on their objective of meeting the primary endpoint of statistically significant FEV1 improvement, both ORKAMBI (lumacaftor plus ivacaftor) and SYMDEKO (tezacaftor plus ivacaftor) have been approved by the Food and Drug Administration for the treatment of CF patients with the homozygous F508delCFTR variant. However, adverse respiratory events resulting in a discontinuation rate of 25%–30% in the first 3 months that is largely attributed to lumacaftor have been reported for the patients on ORKAMBI (Hubert et al., 2017; Jennings et al., 2017; Labaste et al., 2017). In addition, a noteworthy drawback of lumacaftor is its ability to cause strong cytochrome P450-3A induction that limits its use in patients with ivacaftor-responsive variants, even F508delCFTR ( ). Tezacaftor was developed closely behind lumacaftor to address the liabilities described previously (Van Goor et al., 2016). ABBV-2222 represents a novel C1 CFTR corrector discovered by AbbVie in collaboration with Galapagos NV, distinguished from other C1 correctors on the market, with significant improvements in potency and drug-drug interaction (Wang et al., 2018, Vu et al., 2017) profile compared with lumacaftor and improvements in potency and efficacy compared with tezacaftor. Based upon these characteristics, ABBV-2222 has been advanced into clinical trials.
The data presented here indicate that ABBV-2222 acts via a similar mechanism of action as lumacaftor and tezacaftor in promoting proper folding of the F508delCFTR variant in the endoplasmic reticulum to facilitate trafficking to the cell surface. Although the structure of ABBV-2222 resembles lumacaftor and tezacaftor, the difluoromethoxy chromane with the phenyl group is a unique chemical entity that endows the high potency not achieved by the other reported C1 correctors (). Several lines of investigation were used to demonstrate the effectiveness of ABBV-2222 in correcting the misfolded F508delCFTR protein to produce a functional protein on the membrane. The assays used with the background of the CFBE41o- epithelial cells were correlative of the corrector activities observed as measured with the different readouts, e.g., the potencies in the CSE cellular assay were correlative with the subsequent western blot band C and the TECC experiments of ABBV-2222 (as shown in Fig. 4). The western blot experiments demonstrated that ABBV-2222 promoted maturation of F508delCFTR as measured by the accumulation of a higher molecular weight glycosylated band (band C) in the CFBE cells, and this maturation was not affected by chronic coincubation with the clinically relevant concentrations of the potentiator (data not shown). Similar observations were made with the functional readouts in the TECC assay, whereby the corrected F508delCFTR in the primary HBE cells homozygous for the same variant showed no difference in the potency of ABBV-2222 with either acute or chronic presence of clinically relevant potentiator concentrations. This characteristic of ABBV-2222 uniquely separates it from lumacaftor, for which several in vitro studies have shown a nonproductive interaction in the presence of the potentiator ivacaftor (Cholon et al., 2014; Veit et al., 2014). However, the high nonphysiologically relevant concentrations used in these studies do question the validity or significance for such interactions in a clinical setting (Matthes et al., 2016). We did not observe any such interactions for ABBV-2222 with any of the potentiators at their clinically relevant concentrations. ABBV-2222 appears to work purely as a corrector/trafficking enhancer. ABBV-2222 promotes an increase in the amount of F508delCFTR protein at the plasma membrane as measured by western blot, and this corrected protein was functional, albeit minor, in the TECC assay in the absence of a potentiator. However, there was no observed increase in the open probability of corrected CFTR as measured by the single channel excised patch experiments after the overnight treatment (as shown in Fig. 6), suggesting that the observed increase in activity in the functional HBE TECC measurements is due to the presence of increased amounts of F508delCFTR at the plasma membrane and not due to its improved channel activity. Subsequent acute addition of a potentiator in these experiments resulted in a Po value similar to that of wild-type CFTR protein (Lin et al., 2016), suggesting that ABBV-2222 promotes a properly folded channel gating activity for F508del-CFTR in the presence of a potentiator. Therefore, it appears that the fraction of F508delCFTR protein that ABBV-2222 is able to export out of the endoplasmic reticulum attains a more stable protein conformation and suggests it is not recognized as improperly folded by the membrane peripheral quality control pathways (Okiyoneda et al., 2010). This is further supported by the normal functional onset of the corrected F508delCFTR in the primary HBE TECC assay (as shown in Fig. 5F). The relative functional efficacy of our dual combination (ABBV-2222 plus potentiator GLPG1837) is equivalent to that of lumacaftor plus KALYDECO, and approximately 30% better than that of the tezacaftor plus KALYDECO combination, in side-by-side comparison in the HBE TECC assay using homozygous F508delCFTR HBE cells (as shown in Fig. 5A).
Using the targeted biosensor technology of Sharpedge Laboratories (Gentzsch and Mall, 2018) to study the cellular trafficking of misfolded proteins, we were able to demonstrate the selectivity of ABBV-2222 for the processing of F508delCFTR. Compared with the efficient folding detected for F508delCFTR, ABBV-2222 was unable to process mutant hERG (G601S) and mutant PgP (G268V). VRT-325, a positive control in both of these assays was not selective and it increased the processing of all of the proteins tested (Van Goor et al., 2011).
Several investigators have successfully shown the discovery and development of novel CFTR modulators (Grootenhuis et al., 2017; Gentzsch and Mall, 2018; Li et al., 2018) that are biochemically and functionally additive to the C1 corrector class of F508delCFTR modulators, e.g., lumacaftor, tezacaftor, and ABBV-2222. Development of ABBV-2222 represents a novel C1 corrector as the first component of the triple combination therapy being developed by AbbVie. It remains a challenge to attain a clear understanding of the causal relationship that exists between the three components of this triple combination therapy. Ongoing efforts (Li et al., 2018) are focused on understanding the mechanism of action and drug-drug interactions of these three components to produce a best-in-class, clinically safe, and efficacious triple combination therapy for CF patients.
Using C1 correctors as the foundation for triple agent combination therapies, coupled with the recent proof-of-concept studies demonstrated by Vertex (Davies et al., 2018b; Taylor-Cousar et al., 2018), there is the potential to treat all patients who harbor at least one copy of the F508del mutation (∼90% of patients). However, there are still patients with known heterozygous missense variants that are not responsive to this triple combination therapy. This includes CF patients with nonsense mutations, leading to premature termination as well as the less characterized RNA splicing mutations. These variants have been the focus of a major discovery effort led by the North American CF Foundation at several laboratories (Clancy et al., 2019) and also by other individuals.
In summary, there has been tremendous progress in the field that continues in the pursuit of providing disease-changing therapies for all CF patients, regardless of the different variants they carry. The data presented in the current work lead us to propose that ABBV-2222 has distinct advantages over existing C1 correctors and could serve as a foundation for another potential triple combination therapy option for CF patients.
Acknowledgments
The authors acknowledge Elzbieta Indyk for cell culture work. The authors thank Dr. Robert Bridges and his group and Dr. Neil Bradbury at Rosalind Franklin University of Medical Sciences (North Chicago, IL) for providing support with the HBE-TECC assay and other discussions.
Abbreviations
- BHK
baby hamster kidney
- CF
cystic fibrosis
- CFBE
cystic fibrosis bronchial epithelial
- CFTR
cystic fibrosis transmembrane conductance regulator
- compound 15
3-[(2R,4R)-4-([[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl]amino)-7-methoxy-3,4-dihydro-2H-chromen-2-yl]benzoic acid
- CSE
cell surface expression
- FEV1
forced expiratory volume in one second
- GLPG1837
N-(3-carbamoyl-5,5,7,7-tetramethyl-4,7-dihydro-5H-thieno[2,3-c]pyran-2-yl)-1H-pyrazole-5-carboxamide
- Gt
transepithelial conductance
- HBE
human bronchial epithelial
- HRP
horseradish peroxidase
- I EQ
equivalent current
- ivacaftor
N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide
- lumacaftor
3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
- MSD
membrane-spanning domain
- NBD
nucleotide binding domain
- P o
open probability
- R T
transepithelial resistance
- SAR
structure-activity relationship
- TECC
transepithelial current clamp
- tezacaftor
1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-[1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide
- V T
transepithelial voltage
Authorship Contributions
Participated in research design: Singh, Balut, Swensen, Vortherms, Wang, Gao, Hwang, Cyr, Kym, Conrath, Tse.
Conducted experiments: Singh, Fan, Balut, Alani, Manelli, Jia, Neelands, Vortherms, Liu, Searle, Hwang, Ren.
Performed data analysis: Singh, Balut, Swensen, Vortherms, Hwang, Ren, Cyr.
Wrote or contributed to the writing of the manuscript: Singh, Hwang, Cyr, Conrath, Tse.
Footnotes
Financial support for this work was provided by AbbVie Inc. and Galapagos NV.
A.K.S., Y.F., C.B., S.A., A.M.M., A.M.S., Y.J., T.A.V., B.L., X.B.S., X.W., W.G., P.R.K., and C.T. are employees of AbbVie Inc.; K.C. is an employee of Galapagos NV and may own stock in the company. AbbVie Inc. and Galapagos NV participated in the design and study content. AbbVie Inc. participated in the interpretation of data, review, and approval of the publication.
References
- Younger JM, Chen L, Ren H-Y, Rosser MFN, Turnbull EL, Fan C-Y, Patterson C, Cyr DM (2006) Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126 (3):571–582. [DOI] [PubMed] [Google Scholar]
- (2018) Tezacaftor/ivacaftor (SYMDEKO) for cystic fibrosis. Med Lett Drugs Ther 60:174–176. [PubMed] [Google Scholar]
- Altenbach R, Bogdan A, Greszler S, Koenig J, Kym P, Liu B, Searle X, Voight E, Wang X, Yeung C (2016) Preparation of substituted chromanes as CFTR modulators useful in treatment of diseases, United States Patent US9642831B2 [Google Scholar]
- Awatade NT, Wong SL, Hewson CK, Fawcett LK, Kicic A, Jaffe A, Waters SA (2018) Human primary epithelial cell models: promising tools in the era of cystic fibrosis personalized medicine. Front Pharmacol 9:1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balut CM, Akkari R, Alani S, Bock X, Bogdan A, Claes P, Cowart MD, Couty S, De Lemos E, Desroy N, et al. (2017) Development and characterization of next-generation correctors as part of a triple CF therapy. Pediatr Pulmonol 52 Abstract 198: 286
- Cai ZW, Liu J, Li HY, Sheppard DN (2011) Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis. Acta Pharmacol Sin 32:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827–834. [DOI] [PubMed] [Google Scholar]
- Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M (2014) Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 6:246ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JP, Cotton CU, Donaldson SH, Solomon GM, VanDevanter DR, Boyle MP, Gentzsch M, Nick JA, Illek B, Wallenburg JC, et al. (2019) CFTR modulator theratyping: current status, gaps and future directions. J Cyst Fibros 18:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, et al. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting GR (2015) Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M (1991) Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature 354:526–528. [DOI] [PubMed] [Google Scholar]
- Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, Plant BJ, Prais D, Ramsey BW, Taylor-Cousar JL, et al. VX16-659-101 Study Group (2018a) VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 379:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JC, Moskowitz S, Brown CD, Horsley AR, Mall MA, McKone EF, Plant BJ, Prais D, Taylor-Cousar JL, Tullis E, et al. (2018b) Phase 2 safety and efficacy of the triple-combination CFTR modulator regimen VX-659/TEZ/IVA in CF. Pediatr Pulmonol 228. [Google Scholar]
- Du K, Sharma M, Lukacs GL (2005) The ΔF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol 12:17–25. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, Lehr CM (2006) Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o-. Cell Tissue Res 323:405–415. [DOI] [PubMed] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, Waltz D, Wainwright CE, VX-809 TRAFFIC and TRANSPORT study groups (2016) Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med 4:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch M, Mall MA (2018) Ion channel modulators in cystic fibrosis. Chest 154:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootenhuis PDJ, Van Goor F, Hadida S, Burton B, Young T, Selkirk J, Chen W, Zhou J, Yu H, Negulescu P (2017) Discovery and biological profile of next-generation CFTR correctors. Pediatr Pulmonol 51:S194–S485. [Google Scholar]
- Haardt M, Benharouga M, Lechardeur D, Kartner N, Lukacs GL (1999) C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem 274:21873–21877. [DOI] [PubMed] [Google Scholar]
- Hubert D, Chiron R, Camara B, Grenet D, Prévotat A, Bassinet L, Dominique S, Rault G, Macey J, Honoré I, et al. (2017) Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros 16:388–391. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Sheppard DN (2009) Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol 587:2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TC, Yeh JT, Zhang J, Yu YC, Yeh HI, Destefano S (2018) Structural mechanisms of CFTR function and dysfunction. J Gen Physiol 150:539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- info VOp (2015) Highlights of prescribing information. www.ema.europa.eu/en/documents/annual-report/2015-annual-report-european-medicines-agency_en.pdf
- Jennings MT, Dezube R, Paranjape S, West NE, Hong G, Braun A, Grant J, Merlo CA, Lechtzin N (2017) An observational study of outcomes and tolerances in patients with cystic fibrosis initiated on lumacaftor/ivacaftor. Ann Am Thorac Soc 14:1662–1666. [DOI] [PubMed] [Google Scholar]
- Kalydeco (2019) www.kalydeco.com
- Kartner N, Hanrahan JW, Jensen TJ, Naismith AL, Sun SZ, Ackerley CA, Reyes EF, Tsui LC, Rommens JM, Bear CE, et al. (1991) Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell 64:681–691. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073–1080. [DOI] [PubMed] [Google Scholar]
- Kopeikin Z, Yuksek Z, Yang HY, Bompadre SG (2014) Combined effects of VX-770 and VX-809 on several functional abnormalities of F508del-CFTR channels. J Cyst Fibros 13:508–514. [DOI] [PubMed] [Google Scholar]
- Labaste A, Ohlmann C, Mainguy C, Jubin V, Perceval M, Coutier L, Reix P (2017) Real-life acute lung function changes after lumacaftor/ivacaftor first administration in pediatric patients with cystic fibrosis. J Cyst Fibros 16:709–712. [DOI] [PubMed] [Google Scholar]
- Li H, Pesce E, Sheppard DN, Singh AK, Pedemonte N (2018) Therapeutic approaches to CFTR dysfunction: from discovery to drug development. J Cyst Fibros 17:S14–S21. [DOI] [PubMed] [Google Scholar]
- Lin WY, Sohma Y, Hwang TC (2016) Synergistic potentiation of cystic fibrosis transmembrane conductance regulator gating by two chemically distinct potentiators, ivacaftor (VX-770) and 5-nitro-2-(3-phenylpropylamino) benzoate. Mol Pharmacol 90:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S (1993) The ΔF508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem 268:21592–21598. [PubMed] [Google Scholar]
- Lukacs GL, Verkman AS (2012) CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol Med 18:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigowda G, Liu F, Waltz D (2017) Effect of bronchodilators in healthy individuals receiving lumacaftor/ivacaftor combination therapy. J Cyst Fibros 16:246–249. [DOI] [PubMed] [Google Scholar]
- Matthes E, Goepp J, Carlile GW, Luo Y, Dejgaard K, Billet A, Robert R, Thomas DY, Hanrahan JW (2016) Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX-770 (ivacaftor). Br J Pharmacol 173:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM (2001) The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3 (1):100–105. [DOI] [PubMed] [Google Scholar]
- Neuberger T, Burton B, Clark H, Van Goor F (2011) Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol 741:39–54. [DOI] [PubMed] [Google Scholar]
- Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL (2010) Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, et al. (2013) Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol 9:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkambi (2018) https://www.orkambi.com
- Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, Robinson PD, Waltz D, Davies JC, VX14-809-109 investigator group (2017) Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 5:557–567. [DOI] [PubMed] [Google Scholar]
- Ren HY, Grove DE, De La Rosa O, Houck SA, Sopha P, Van Goor F, Hoffman BJ, Cyr DM (2013) VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell 24:3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073. [DOI] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. (1989) Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245:1059–1065. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, et al. (2017) Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 377:2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, et al. (2004) Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 164:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Cousar JL, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass L, Tullis E, et al. (2018) Phase 2 safety and efficacy of the triple-combination CFTR modulator regimen VX-445/TEZ/IVA in CF. Pediatr Pulmonol 227. [Google Scholar]
- Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, et al. (2017) Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 377:2013–2023. [DOI] [PubMed] [Google Scholar]
- Van der Plas SE, Kelgtermans H, De Munck T, Martina SLX, Dropsit S, Quinton E, De Blieck A, Joannesse C, Tomaskovic L, Jans M, et al. (2018) Discovery of N-(3-carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a novel potentiator which can open class III mutant cystic fibrosis transmembrane conductance regulator (CFTR) channels to a high extent. J Med Chem 61:1425–1435. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Grootenhuis P, Hadida S, Burton B, Young T, Selkirk J, Powe A, De La Rosa O, Jiang L, Zhou J, et al. (2016) Nonclinical profile of the CFTR corrector VX-661. Pediatr Pulmonol 51:274. [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106:18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 108:18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, et al. (2014) Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 6:246ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu CB, Bridges RJ, Pena-Rasgado C, Lacerda AE, Bordwell C, Sewell A, Nichols AJ, Chandran S, Lonkar P, Picarella D, et al. (2017) Fatty acid cysteamine conjugates as novel and potent autophagy activators that enhance the correction of misfolded F508del-cystic fibrosis transmembrane conductance regulator (CFTR). J Med Chem 60:458–473. [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, et al. TRAFFIC Study Group; TRANSPORT Study Group (2015) Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu B, Searle X, Yeung C, Bogdan A, Greszler S, Singh A, Fan Y, Swensen AM, Vortherms T, et al. (2018) Discovery of 4-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic acid (ABBV/GLPG-2222), a potent cystic fibrosis transmembrane conductance regulator (CFTR) corrector for the treatment of cystic fibrosis. J Med Chem 61:1436–1449. [DOI] [PubMed] [Google Scholar]
- Yeh HI, Qiu L, Sohma Y, Conrath K, Zou X, Hwang TC (2019) Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770. J Gen Physiol 151:912–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HI, Sohma Y, Conrath K, Hwang TC (2017) A common mechanism for CFTR potentiators. J Gen Physiol 149:1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]