Significance
The oil palm boom in Indonesia has improved livelihoods but has also led to biodiversity loss and ecosystem degradation. We explored economic, ecological trade-offs, and synergies of oil palm cultivation and quantified the performance of a wide range of management types, including high- and low-intensity cropping systems, as well as the innovative diversification of plantations. We identify key components for economic–ecological win–win outcomes and outline pathways for the future of oil palm cultivation that may better align economic prosperity with ecological sustainability.
Keywords: oil palm, trade-offs, biodiversity, ecosystem functions, forest transformation
Abstract
The expansion of the oil palm industry in Indonesia has improved livelihoods in rural communities, but comes at the cost of biodiversity and ecosystem degradation. Here, we investigated ways to balance ecological and economic outcomes of oil palm cultivation. We compared a wide range of production systems, including smallholder plantations, industrialized company estates, estates with improved agronomic management, and estates with native tree enrichment. Across all management types, we assessed multiple indicators of biodiversity, ecosystem functions, management, and landscape structure to identify factors that facilitate economic–ecological win–wins, using palm yields as measure of economic performance. Although, we found that yields in industrialized estates were, on average, twice as high as those in smallholder plantations, ecological indicators displayed substantial variability across systems, regardless of yield variations, highlighting potential for economic–ecological win–wins. Reducing management intensity (e.g., mechanical weeding instead of herbicide application) did not lower yields but improved ecological outcomes at moderate costs, making it a potential measure for balancing economic and ecological demands. Additionally, maintaining forest cover in the landscape generally enhanced local biodiversity and ecosystem functioning within plantations. Enriching plantations with native trees is also a promising strategy to increase ecological value without reducing productivity. Overall, we recommend closing yield gaps in smallholder cultivation through careful intensification, whereas conventional plantations could reduce management intensity without sacrificing yield. Our study highlights various pathways to reconcile the economics and ecology of palm oil production and identifies management practices for a more sustainable future of oil palm cultivation.
The oil palm (Elaeis guineensis) is the world’s most important vegetable oil plant (1). Due to its unrivaled high yield compared to other oil crops like soybeans and rapeseed, it has a much smaller land-use footprint than these alternatives (1). Driven by high global demand for vegetable oil, the area under oil palm cultivation has expanded massively in the last decades (2), often at the expense of natural forests (3, 4). This “oil palm boom” improved living standards and welfare in the growing regions which are mainly located in SE Asia (5) but also caused widespread transformation of megadiverse, complex rainforests into species-poor, structurally simplified oil palm monocultures. This transformation has led to the loss of key ecological functions with serious repercussions on human health and regional ecological processes (6). As such, there is an urgent need to solve the resulting trade-offs between private economic benefits and public environmental goods (7). However, strategies to balance ecological and economic needs are lacking. Most conservation efforts have focused on the creation and maintenance of forest reserves (8, 9). Although primary forests are irreplaceable for tropical biodiversity conservation (10), huge amounts of tropical forests have already been cleared and transformed into monoculture production systems. The few forest patches remaining in tropical production landscapes are unlikely to sustain biodiversity and ecosystem functions (11, 12) making it imperative to also explore options that target the oil palm matrix itself (13) and that can maximize ecological outcomes, while preserving economic benefits (14).
Locally, at the plantation level, negative impacts can be balanced by promoting structural vegetation complexity (15). One effective method is to enrich the oil palm matrix by incorporating tree plantings (16). Other studies emphasize the ecological benefits of reduced management intensity, such as reduced fertilization or mechanical weeding, instead of chemical herbicide application (17–19). At the landscape level, the retention of forest patches can benefit biodiversity and ecosystem functions such as pest control or pollination within plantations (20). Notably, around 50% of the world’s oil palm acreage is cultivated by smallholders (1, 5). Smallholder oil palm systems can support greater biodiversity than larger estates (21, 22); however, they typically have much reduced crop yields (23). As such, when exploring optimization options, it is important to assess the efficiency of production systems as well.
Here, we evaluate the drivers of synergies and trade-offs in economic and ecological functions among the major oil palm production systems on Sumatra, Indonesia, a epicenter of the oil palm boom. Specifically, we identify win–win cases or “bright spots” (24) in which high yields coincide with relatively high levels of biodiversity and/or ecosystem functioning. In doing so, we aim to identify concrete management practices or levers that can improve the ecological value of oil palm plantations without compromising their economic benefits. To this end, we assembled an extensive dataset combining oil palm yields, which we used as a proxy for economic performance, with observations of 11 ecological indicators measuring biodiversity and ecosystem functioning, covering a total of 42 study plots. Specifically, we consider four oil palm production systems: 1) low-input smallholder plantations; 2) “enriched” estates, in which oil palms have been intercropped with native trees; 3) extensive company estates with reduced management regimes (reduced fertilization and replacement of chemical with mechanical weeding); and 4) high-input, conventional estates. Our study is unique because we evaluate both widely used oil palm cropping systems and cutting-edge experiments designed to improve the sustainability of palm oil production (25, 26), comprehensively comparing these systems alongside each other.
We first i) compare all production systems in terms of yields, ecological outcomes (biodiversity and ecosystem function indicators), agronomic management, vegetation structure, and landscape context. We then ii) assess the ecological and economic performance of the four systems by describing the covariation between standardized ecological indicators and yields, with four dual outcomes (high-high, high-low, low-high, and low-low), and counting the number of observations per outcome. To explore potential management levers to reduce economic–ecological trade-offs and create synergies, we iii) identify environmental and management variables at plot-, plantation- and landscape-level that correlate with situations of high yield and high ecological value.
Among others, we find that ecological indicators of biodiversity and ecosystem functions exhibited great variability across all systems, independent of covariations in oil palm yield, indicating the potential for win–win situations combining high biodiversity with high yields. Furthermore, greater landscape-level forest cover generally benefited biodiversity and ecosystem functions within plantations.
Results
Characterization of Production Systems.
The studied cultivation systems encompassed a range of oil palm yields, from 6.8 to 35.9 t ha−1 a−1 fresh fruit bunch weight. Annual yields in industrialized estates, on average, were double those of smallholder plantations (Fig. 1E) [Smallholder plantations: 12.8 t ha−1 a−1 (±3.9 t ha−1 a−1), Estate enriched: 20.8 t ha−1 a−1 (±4.0 t ha−1 a−1), Estate extensive: 31.7 t ha−1 a−1 (±3.9 t ha−1 a−1), and Estate conventional: 25.8 t ha−1 a−1 (±5.8 t ha−1 a−1)]. We recorded the highest yields in the extensively managed estates that had 19% greater yields than the conventional estates. Lastly, oil palm estates that had been enriched with native trees achieved yields that were not significantly different from both smallholders and conventional estates (Fig. 1E), despite lower palm densities: 86 palm/ha−1 enriched estates; 131 palm/ha−1 conventional estates; 142 palm/ha−1 extensive estates and 136 palm/ha−1 for smallholders. Note that on average plantation ages were similar between systems [smallholder plantations: 21.4 y (±2.0), estate enriched: 18 y (±0), estate extensive: 23 y (±0), and estate conventional: 20.5 y (±2.7)].
Fig. 1.
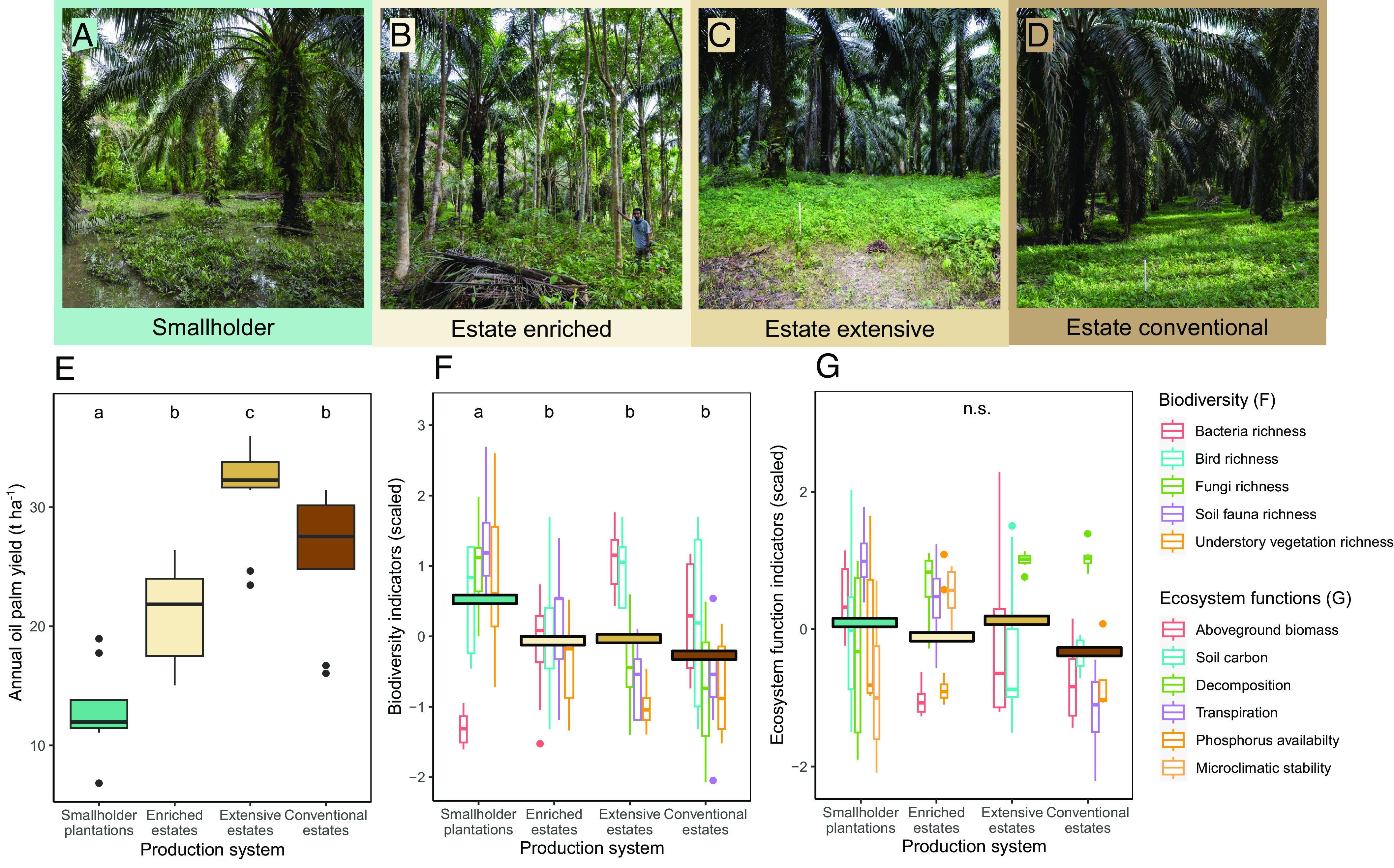
Oil palm production systems considered in this study: smallholder plantations (A), estates enriched with trees (B), estates with extensive management (C) and conventionally managed estates (D). Boxplots of mean annual palm oil yields per hectare for each system (E), boxplots of standardized biodiversity indicators (F), and ecosystem function indicators per cultivation system (G). Boxplots represent the median (bars), the 25 to 75% intervals (box edges) and the 1.5 interquartile range (whiskers) of the raw data (n = 355). Boxplot colors in F and G indicate ecological indicator identity. Colored horizontal bars represent the mean indicator scores per system. Distinct letter combinations indicate significant differences between systems assessed through linear models with production system identity as the only predictor (Tukey test, P < 0.05).
Analyses of five biodiversity indicators (the species richness of birds, soil fauna, understory vegetation, root fungi, soil bacteria) and six ecosystem function indicators (soil carbon, within-canopy microclimatic stability, phosphorus availability, decomposition rate, aboveground biomass, and transpiration) revealed that differences in the average ecological performance between systems were small. When comparing the average biodiversity indicators across oil palm systems (Fig. 1F), smallholder plantations had significantly higher scores than all other systems. For indicators of ecosystem functioning (Fig. 1G), no significant differences were observed between systems. Nonetheless, high variation in ecological outcomes was observed in all systems.
Increasing plantation-level yields were related to thicker and structurally more complex vegetation (SI Appendix, Fig. S1A), as indicated by a doubling in the “effective number of layers,” an airborne light detection and ranging (LiDAR) (27) metric that describes vegetation complexity and vertical stratification (28) (see SI Appendix, Table S1 for an overview of all LiDAR metrics). There were also fewer horizontal canopy gaps (SI Appendix, Fig. S1B), while palms grew overall taller with an average increase in maximum height from 14.5 m to 17.8 m (SI Appendix, Fig. S1C). Landscape composition correlated with yields: comparing the lowest-yielding (6.8 t ha−1 a−1) and highest-yielding (35.9 t ha−1 a−1) oil palm plantations, the proportion of surrounding forest cover within a 1 km buffer decreased from 4.8 to 0.8% (smallholders were on average surrounded by 13.6% of forest vs. only 0.6% for all three estate systems; SI Appendix, Fig. S2A and Table S5), while the surrounding oil palm cover increased from 72 to 92% along the yield gradient (SI Appendix, Fig. S2B). Finally, yields increased with management intensity. Along the yield gradient, average nitrogen inputs increased from 0 kg to 239 kg per hectare (SI Appendix, Fig. S3A, also correlated to phosphorus and potassium inputs), and mechanical weeding effort from 1.3 h to 81.3 h per hectare annually (SI Appendix, Fig. S3B). The latter is driven by the extensively managed estates, in which chemical herbicides were experimentally replaced by mechanical weeding to enhance ecological sustainability in oil palm estates (17).
Economic–Ecological Performance of Systems.
To simultaneously assess the ecological and economic dimensions of the production system performance, we standardized all data. First, we computed a weighted mean for each indicator and yields, which was consequently used as a threshold to define low and high outcomes. Next, we scaled the data by centering each variable around its weighted mean set to zero. We then plotted all scaled indicators against the scaled yield and split the two-dimensional ordination into four dual outcome quadrants with the respective zero as thresholds (Top Right: win–win, Lower Right and Top Left: trade-offs, Lower Left: lose-lose). Last, we counted the number of observations falling within each quadrant (Fig. 2).
Fig. 2.
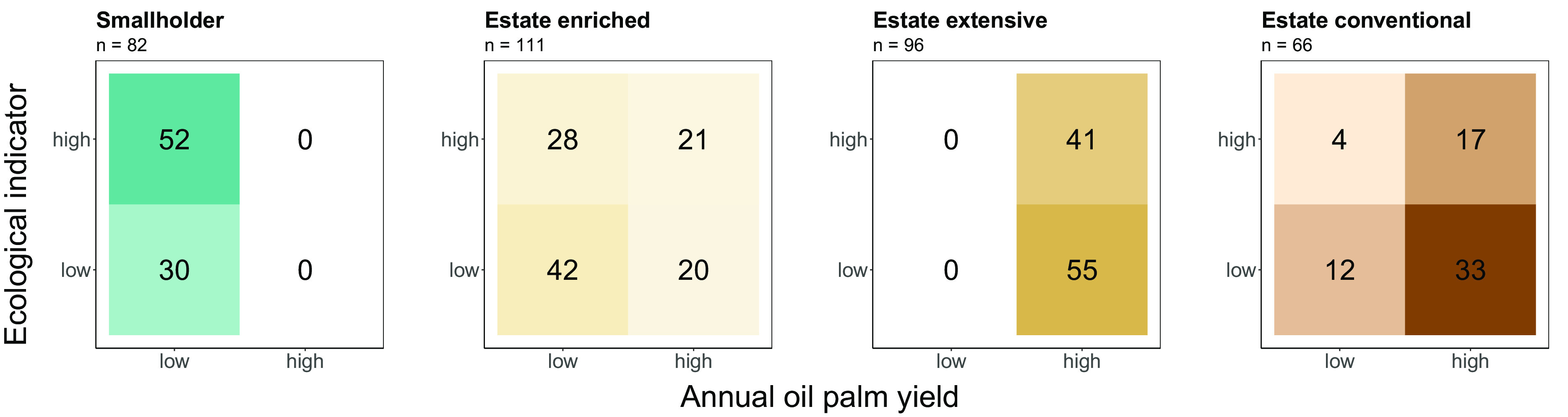
Contingency tables of biodiversity/ecosystem functioning indicators and oil palm yield. The tables show the number of observations of standardized indicators that fall above or below a threshold value that indicates high (i.e., win) or low (i.e., lose) outcomes for indicators (y axis) and yield (x axis), resulting in four quadrants of possible dual outcomes (win–win, win-lose, lose-win, lose-lose). The respective thresholds were based on balanced means calculated for each individual indicator and oil palm yield. Colors indicate cultivation systems, while color intensity corresponds to the number of observations per quadrant. The total number of observations is displayed for each system.
Smallholders, limited by their low productivity, did not achieve economic–ecological win–wins as all points fell into the two Left-sided quadrants, i.e., below the “yield-win” threshold (Fig. 2). In contrast, for extensive and conventional estates, the majority of observations fell into the Right Quadrants, i.e., above the yield-win threshold (100 and 76% of observations respectively; Fig. 2). Estates did also achieve economic–ecological win–wins, with extensified estates having the most win–win cases (43% of the observations). We found the highest number of “ecological wins,” i.e., points falling within the Top-Left Quadrant, in smallholder plantations (63% of observations; Fig. 2). Finally, tree-enriched estates had a high share of lose-lose cases (38% of observations), but also ecological wins (25%) and win–wins (19%) (Fig. 2).
Modeling the probability of a win–win outcome across systems revealed similar patterns, with a predicted probability of a win–win to occur for extensive estates of 43%, followed by conventional estates with 24% and enriched estates with 16%, while the chance of a win–win for smallholders was estimated at 0% (SI Appendix, Table S2).
Predictors of Economic–Ecological Outcomes.
To better understand the drivers behind the patterns of ecological-economic outcomes, we investigated their correlation with environmental and management variables by overlaying the variables as vectors onto the aforementioned two-dimensional ordination of the standardized data. The first analysis included all observations of the standardized indicators of biodiversity and ecosystem functioning. A set of uncorrelated LiDAR vegetation metrics, management inputs, and landscape composition (land cover shares within a 1 km radius) were used as predictors. In the resulting ordination (Fig. 3), none of the variables showed a strong association with gains in both dimensions simultaneously, i.e., the Top Right Quadrant (r < 0.6; SI Appendix, Table S3). However, several predicting variables were strongly correlated (r > 0.6; SI Appendix, Table S3) with the yield dimension. Particularly, maximum vegetation height and vegetation structural complexity, indicating larger palms and more structurally complex systems, respectively, were strongly positively correlated with yields. Additionally, increasing weeding effort, either through chemical or mechanical means, was associated with high yields (Fig. 3). Conversely, a higher cover of surrounding forests, young oil palm plantations, and an increase in vegetation gaps were correlated with low yields. Phosphorus availability and surrounding rubber cover were the only variables that exhibited correlations with the ecological dimension, but goodness-of-fit statistics were very small for both variables (r2 < 0.03; SI Appendix, Table S3). None of the variables were correlated with declines in the ecological dimension.
Fig. 3.
Variation in ecological indicators of biodiversity/ecosystem functions and oil palm yield across the studied oil palm systems. Each point shows a plot-level yield—biodiversity/ecosystem function combination. Scaled values for all indicators included in the dataset are shown. Colors indicate oil palm production systems. Arrows indicate plot-level structural complexity, management, and landscape variables that significantly (P < 0.05) explain the two-dimensional variation in biodiversity/functions and yield. Arrow tips indicate the direction of steepest increase. Dotted lines separate win–win situations (i.e., Top-Right Quadrant) from trade-offs (Top-Left, Lower-Right) and lose-lose situations (Lower-Left). Considered variables are: Management variables: Phosphorus inputs, herbicide weeding intensity, mechanical weeding intensity, LiDAR metrics: Maximum canopy height, number of gaps, vegetation layer (structural complexity, “enl”); Landscape variables: Forest, rubber and young oil palm cover in a 1 km radius around site.
In a next step, we analyzed each biodiversity and ecosystem function indicator separately, i.e., created a two-dimensional ordination for each pairwise indicator-yield combination (Fig. 4 and SI Appendix, Table S4). Within these individual ordinations, some variables were associated with win–win scenarios. Examples included vegetation height in the case of bird richness (Fig. 4E), structural complexity for soil carbon (Fig. 4G), or mechanical weeding effort in the case of aboveground biomass (Fig. 4K). Among all considered variables, mechanical weeding and structural complexity were most frequently correlated with win–win observations, leading to gains in both dimensions across five indicators (Table 1 and SI Appendix, Table S4). While the variables associated with yield gains were the same as those in the global ordination (i.e., maximum vegetation height, vegetation complexity, mechanical weeding effort), forest cover was the variable most frequently correlated with gains in ecological indicators, increasing biodiversity or ecosystem functioning across eight indicators (Table 1).
Fig. 4.
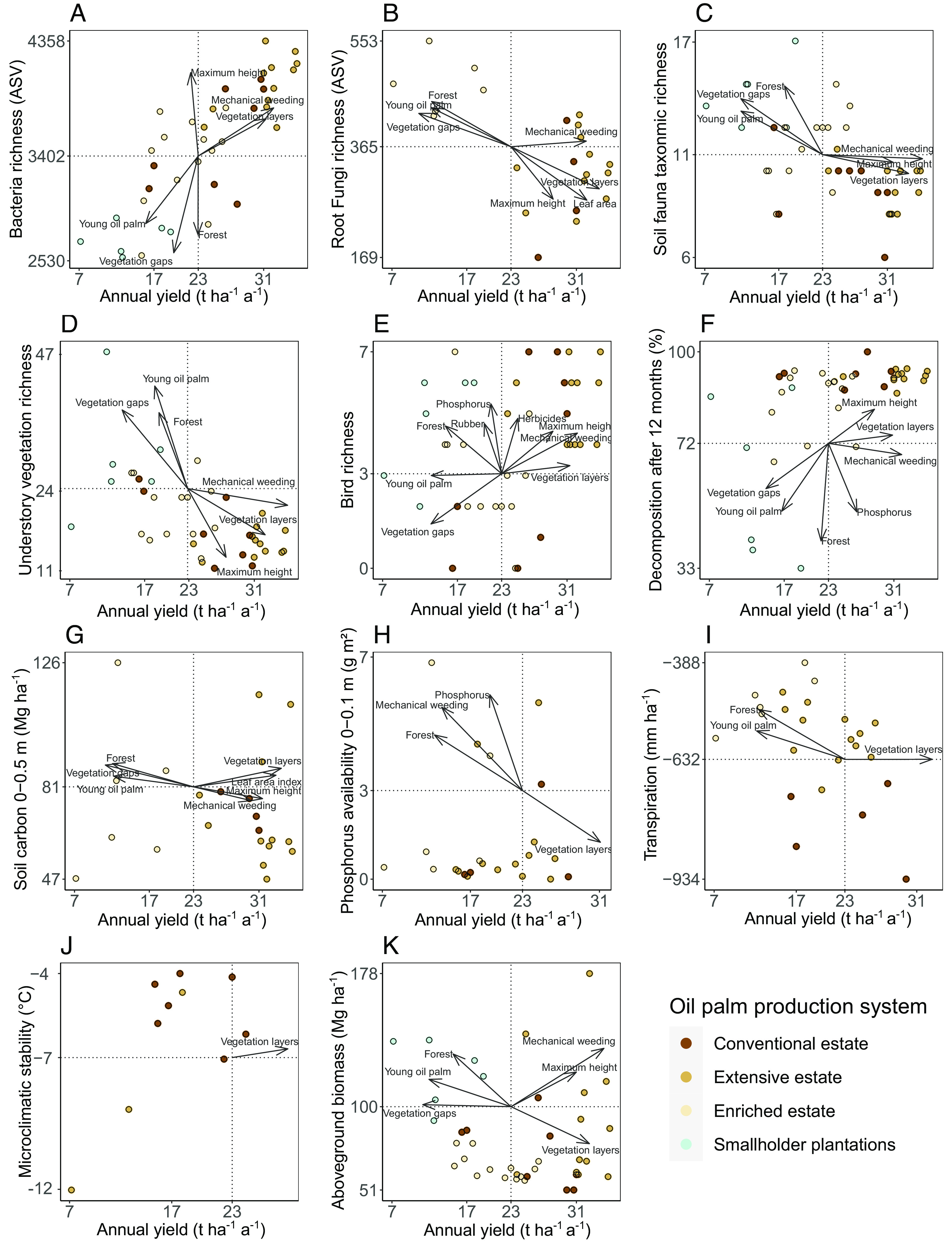
Variation in individual ecological indicators of biodiversity/ecosystem functions and oil palm yield across the studied oil palm systems (identified by letters A–K). Individual ordinations are shown for all indicators included in the dataset. Colors indicate oil palm production systems. Arrows indicate plot-level structural complexity, management and landscape variables that significantly (P < 0.05) explain the two-dimensional variation in biodiversity/functions and yield. Arrow tips indicate the direction of steepest increase. Dotted lines separate win–win situations (i.e., Top Right Quadrant) from trade-offs (Top Left, Lower Right) and lose-lose situations (Lower Left). Note that observations of transpiration (I) and microclimatic stability (J) have been inverted (*−1), so that high values indicate desirable outcomes. Considered predictor variables were: LiDAR metrics: Maximum canopy height (m), number of gaps, effective number of layers, i.e., structural complexity; Landscape metrics: Forest, rubber and young oil palm cover (% in a 1 km radius); and management variables: Phosphorus inputs (kg ha−1 a−1), herbicide weeding intensity (l ha−1 a−1), mechanical weeding intensity (h ha−1 a−1).
Table 1.
Summary table of individual ordination results
| Win–win | Ecological-win | Yield-win | |
|---|---|---|---|
| Variable |
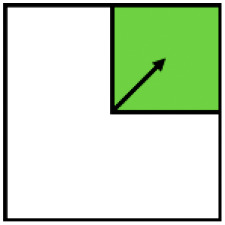
|
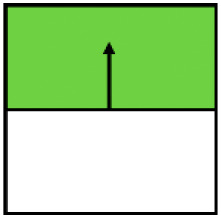
|
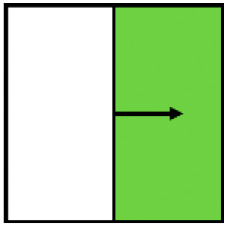
|
| Herbicides | 1 | 1 | 1 |
| Mechanical weeding | 4 | 5 | 8 |
| Phosphorus inputs | 0 | 1 | 1 |
| Leaf area index | 1 | 1 | 2 |
| Maximum height | 3 | 4 | 7 |
| Vegetation gaps | 0 | 5 | 0 |
| Vegetation complexity | 5 | 5 | 10 |
| Forest % | 0 | 8 | 0 |
| Rubber % | 0 | 1 | 0 |
| Young oil palm % | 0 | 6 | 0 |
Displayed is how often the 10 environmental variables that describe management (three variables), vegetation structure (four variables), or landscape context (three variables) were significantly (P < 0.05) correlated to the Top Right Quadrant (win–wins), Top Quadrants (ecological wins) or the Right Quadrants (yield-wins) in a total of 11 individual ordination plots fitted for each ecological indicator dataset.
Discussion
The oil palm boom in Southeast Asia has improved livelihoods in many rural regions (5), but has also caused widespread biodiversity loss and ecosystem degradation (6, 29, 30). Ecological restoration strategies, to be successful, need to acknowledge the economic dimensions of oil palm cultivation (14). Here, we explored options to reconcile ecological and economic dimensions of oil palm cultivation, covering a wide range of production systems such as smallholder plantations, industrialized company estates, as well as oil palm plantations with improved agronomic management or native tree enrichment. We observed a clear yield gap between smallholder systems and company estates, but at the same time, differences in ecological indicators between the studied systems were small with great variation present within all production systems. Consequently, win–win opportunities exist in all the studied production systems, suggesting that more intensive forms of oil palm cultivation may not necessarily result in worse ecological outcomes at the plantation level, provided appropriate management practices and conservation schemes are implemented. To put the potential for ecological “wins” into scale, consider the case of understory vegetation and birds, where the best-performing oil palm plots in our study, achieved 15% and 28% of the respective species richness found in adjacent forests (SI Appendix, Table S6). Smallholder systems in our study, situated at the forest frontier, may also face the risk of experiencing further biodiversity declines due to extinction debts. Based on these findings, we propose the below measures to improve the sustainability of oil palm cultivation.
Intensifying Management in Smallholder Systems.
First, we regard closing yield gaps and the intensification of management in low-yielding cases as a promising course of action, especially in smallholder systems. Currently, smallholders do not achieve synergies between ecological and economic outcomes due to their low yields (Fig. 1E and SI Appendix, Table S2), resulting in ecological-economic win-lose or even lose-lose outcomes (Fig. 2). Increasing management intensity in smallholder systems might not necessarily lead to lower ecological value at the plantation-level, as no management variable was negatively correlated with the ecological dimension in the global ordination (Fig. 3). Furthermore, mean differences in biodiversity and ecosystem function indicators were small between smallholders and high-input estate systems (Fig. 1 F and G). Increasing output efficiency is also important to satisfy the increasing demand for vegetable oil (1, 7), which could otherwise lead to further deforestation. Higher profits through higher yields could also allow for setting aside more areas for conservation or restoration (31, 32). However, there is also evidence from Indonesia that this could be the opposite (33, 34). One option to increase yields that emerged from our analysis is the promotion of large and structurally complex palms (Fig. 3, correlation of high yield with maximum palm heights, leaf area index, and effective numbers of vegetation layers) that effectively utilize the available horizontal and vertical space. However, smallholders often struggle to provide sufficient fertilization, which limits palm growth, and face financial and logistic constraints in accessing quality seedlings, leading to suboptimal planting densities and inefficient space utilization (35, 36). Mechanical and chemical weeding were further factors correlated with high yields (Fig. 3). Controlling weeds facilitates access to palms for harvesting, allowing for more frequent harvest intervals, while also potentially reducing competition. However, mechanical weeding appears to be more advantageous than chemical weeding, as it not only improves ecological value (29, 30), but it also exhibited a stronger correlation with yields than herbicide weeding in our analysis (Fig. 2 and SI Appendix, Table S3). Mechanical weeding also promotes understory plants as it facilitates rapid regrowth of vegetation by preserving the root biomass (in contrast to chemical herbicides), with cascading positive effects on associated taxa, such as flower-visiting insects, soil biodiversity or mammals (18, 37–39), while also avoiding non-target effects (40). However, employing mechanical weeding increases labor costs. For a company estate, this increase was 10% (23) and could be higher for smallholder farming. As such, boosting yields through chemical means may be a more favorable option for smallholders, in particular if extra costs are not offset by subsidies. Overall, limited access to capital, labor, inputs, high-yielding cultivars, and technical knowledge are considered as key factors limiting smallholder productivity (23). Therefore, policies should target these areas through education, extension services, and subsidies (41).
Informed Extensification of Company Estates.
Second, we regard the extensification of conventional high-input company estates as another set of easy to implement measures. Recent studies highlight that fertilizer rates in conventionally managed oil palm estates (260 kg N; 50 kg P; 220 kg K; average values ha−1 y−1) are far above the needed rates based on quantified nutrient exported through harvest (136 kg N; 17 kg P; 187 kg K; average values ha−1 y−1) (17, 25). Reducing fertilization rates not only decreases greenhouse gas emissions and nutrient leaching but also increases the abundance of beneficial root fungi (42–44), all the while reducing management costs in industrial plantations. Our analysis showed that extensive estates, characterized by fertilizer rates equal to the quantified nutrient export from harvest products and the replacement of chemical herbicides with mechanical weeding, emerged as the most successful in achieving win–win outcomes (Fig. 2 and SI Appendix, Table S2). In addition, even with the reduction in management intensity, the extensive estates achieved similar plantation-level yields to high-input conventional estates, while outperforming the other studied production systems (Fig. 1E, see also ref. 25).
Overall, the probability of achieving economic–ecological win–wins was higher in the extensive estates (55%) than in any other production system (SI Appendix, Table S2). These results are in line with previous studies showing that a reduction in management not only enhances biodiversity and multifunctionality, i.e., improves ecological outcomes (17, 18), but also enhances economic functions through profit gains from reduced production costs (25). Social-media campaigns and participatory programs can play a role in reducing excessive use of agrochemical inputs, which are often heavily promoted by the agroindustry (45). Adapting management schemes to local growing conditions, such as soil quality and acidity could benefit both economic and ecological interests. Notably, the “extensive” management in extensified estates was still substantially higher (260% more NPK fertilization) than in any smallholder plantations (SI Appendix, Fig. S3). Nevertheless, the oil palm sector is a diverse industry, and we need to acknowledge that our study does not cover all the ways in which oil palm can be cultivated. For instance, some companies have adopted biocontrol methods for pest management to reduce their reliance on chemical inputs (18). Moreover, rising fertilizer costs have already prompted a wider utilization of compost and other organic fertilizers (43). These developments hold further potential for reducing the negative consequences of oil palm cultivation.
Ecological Restoration through Native Tree Enrichment.
Ecological restoration in oil palm plantations can also be achieved by enriching plantations with native trees (16, 46). This strategy has been shown to promote ecosystem functioning and biodiversity of many taxa at the local scale (26), without affecting plantation yields (26, 47). Indeed, the per hectare yields of enriched plantations were not significantly different from conventional estates (Fig. 1F), despite the considerably lower palm tree density (on average, 86 palms/ha–1 vs. 136 palms/ha–1 in conventional estates) and without any application of fertilizers or herbicides. This reflects the high variability within the production systems that overcomes the differences across systems. Furthermore, we did not consider changes in yield of oil palms adjacent to the enrichment plots (i.e., tree island effect), and thereby underestimated the yield at the plantation scale (26, 47). In conclusion, we strongly advocate for the enrichment of oil palm plantations by incorporating larger islands of native trees (32), as a promising strategy to enhance the ecological value of oil palm landscapes without compromising agricultural productivity.
Considering Landscape Factors for Ecological Value.
The absence of local or landscape variables that are strongly correlated with the ecological dimension (Fig. 3) suggests that there is no one-size-fits-all solution to improve the ecological value of oil palm production. Instead, the specific environmental and management variables associated with ecological wins were strongly taxon- and function-specific (Fig. 4). However, some variables were more commonly correlated to ecological wins than others and in particular, forest cover at landscape-level (Table 1).
Previous studies found more diverse species communities when the agricultural landscape still had forest patches (48), also in oil palm landscapes (49). Forest patches can also act as stepping stones, connecting habitats across the agricultural matrix (50). Additionally, it has been observed that oil palm fruit set tends to be higher in areas adjacent to forests due to better pollination (51). The importance of forest patches also emphasizes the potential advantages that come with enhancing oil palm plantations through the inclusion of native trees. Notably, smallholders in our study region had a significantly higher forest and young oil palm cover in the surrounding landscape than estate systems (SI Appendix, Fig. S2 and Table S5). In the studied region, smallholders usually operate at the deforestation frontier, while estates are embedded in simplified landscapes that are already composed mostly of oil palm.
Bringing It all Together: How to Create Synergies.
The three variables that were most frequently correlated with win–wins across individual indicators of biodiversity and ecosystem services were mechanical weeding, maximum canopy height and structural complexity (Table 1). Their frequent correlation highlights the advantages of mechanical weeding and the benefits of promoting a complex vegetation, which can offer additional niches, habitats, and resources (52), promoting ecological outcomes, while larger palms also produce more fruits. Larger palms in combination with their larger root systems can promote many ecosystem processes, such as soil carbon content (53) or temperature stability (54). Recognizing that vegetation requires time to mature, both the economic and ecological value of palm oil plantations should therefore be considered as a function of palm tree age (54). Old plantations are known to have a deeper leaf litter depth, a more complex understory vegetation, and an abundant epiphyte community (54, 55). Therefore, we consider the common practice of clear-cutting the entire plantation once palms have reached the end of their productive cycle as problematic. This effectively resets habitat and biodiversity complexity (56), and leads to additional issues such as erosion and nutrient leaching (57). Potentially, mixed-age stands, underplantings, or staggered replantings could be solutions, but require further research (56).
Limitations of Our Analysis.
We only focused on plantation-level outcomes and all studied systems were plantations with low ecological value in comparison to natural forests. Furthermore, we explored relative performance between investigated cultivation systems and defined satisfactory levels (i.e., wins) out of the available data. As such, levels of biodiversity or ecosystem functioning we report as wins are only high in comparison to other oil palm plantations and not to natural habitats or benchmark values. While we focused on species richness as biodiversity indicator in our analysis, further research should analyze species-specific responses and community compositions. As such, we might overestimate the biodiversity value of communities, where common or invasive species might have replaced species of higher conservation concern, such as forest specialists. In fact, rainforest transformation landscapes are known to be particularly beneficial to generalistic and alien species (58), while forest specialists are lost (7, 59).
All studied farms were mature plantations of similar ages. Although this allows for an unbiased comparison it also restricts our understanding of how the described trade-offs might change with plantation age.
Furthermore, we valued all available ecological indicators as equally important. Thus, the reduction of some indicators might be offset by increases in others. For instance, the higher amount of soil amendment and inputs in more intensive systems is known to promote some soil processes (60), such as decomposition or the biodiversity of soil bacteria and might have concealed losses in other taxa or functions with increasing yields.
Finally, some datasets were collected at different times, and we cannot exclude that comparisons between production systems are influenced by temporal or weather changes. Nevertheless, the regional microclimate was relatively stable within and between years (SI Appendix, Table S7). We also assume that temporal turnover of species communities in oil palm plantations, which are usually dominated by widespread generalist species (55), should be smaller than in more specialized forest communities.
Overall, we believe that our study provides valuable insights into general patterns of oil palm cultivation and how a minimum of ecological value can be maintained in profitable situations. The pathways and suggestions we synthesized from our results are widely applicable to various localities and growing contexts. As such, our results contribute to the broader conversation on sustainable oil palm cultivation.
Future Directions for Science in Indonesia.
Future directions for science in Indonesia concerning oil palm cultivation could build upon our findings. Researchers could further investigate and refine the strategies identified, such as implementing careful intensification techniques in smallholder cultivation to bridge yield gaps, exploring ways to reduce management intensity in conventional systems while prioritizing biodiversity restoration within plantations. We also see the need to study dynamics, i.e., oil palm’s growth and replanting cycle and how to prevent a second wave of biodiversity loss (58). Future research should address recent innovations in the oil palm sector that have the potential to enhance both economic and ecological outcomes. For instance, mechanization could reduce labor costs and improve working conditions (1), while also reducing labor shortages, which are currently a major problem in the industry. Additionally, the introduction of new high-yielding oil palm varieties, potentially doubling current yields (1), could significantly boost incomes and overall land-use efficiency (7). This is especially relevant as many oil palm plantations in Indonesia are exceeding their optimal production age and will soon require replanting (60), presenting an opportunity to dramatically boost yields without expanding the overall cultivated area. Critically, also smallholder producers need access to these high-yielding varieties (61). Last, considering the challenges posed by climate change, which also include biotic factors such as heightened pest pressure (1), it is imperative to target climate resilience measures as well.
Conclusion
The greatest negative ecological impact of conventional oil palm cultivation comes from the conversion of biodiverse native forests to monocultural plantations, and once forests are converted, it is difficult to reverse the negative ecological consequences. The current reality is that millions of hectares have already been transformed into oil palm plantations. Our results shed light on how one can enhance the ecological value of oil palm plantations and at the same time fulfill economic interests. Smallholders should seek to increase production efficiency through careful intensification, while conventional farms should adopt reduced management practices tailored to local needs. Elements of more ecologically sustainable management include increasing vegetation complexity and using mechanical rather than chemical weed control. Moreover, enriching oil palm plantations with native trees did not reduce yields compared to conventional plantations and might be a promising strategy to further increase the ecological value of oil palm cultivation. Finally, maintaining forested areas in the landscape is essential for sustaining biodiversity and ecosystem functions in oil palm plantations. Our proposals are not a substitute for effective forest conservation policies, but can help improve the economic and ecological sustainability of existing and future oil palm plantations.
Materials and Methods
Study Region.
All study plots were located in Jambi province and were sampled within the framework of the EFForTS project (Ecological and socioeconomic Functions of tropical lowland rainforest Transformation Systems) (62). In the past, the region was dominated by forest and traditional agricultural practices, including small-scale farming and shifting cultivation. However, over the last few decades, the region has witnessed a significant transformation in land use, driven primarily by the rapid expansion rubber and oil palm monoculture plantations (60). This transformation was facilitated by favorable policies and subsidies, such as the transmigration programs and incentives for large-scale land acquisitions by agribusinesses (63).
Study Plots.
Smallholder plots (n = 8) measured 50 m × 50 m, and their design is described in ref. 62. Enriched estate plots (n = 13) considered in this study measured 40 m × 40 m and were part of EFForTS’ Biodiversity Enrichment Experiment (EFForTS-BEE) (16). Oil palms were removed before enrichment (removed palms: min = 4, max = 8, median = 7). In 12 plots, gaps were interplanted with trees with either 1 (n = 6), 2 (n = 3), 3 (n = 2) or 6 (n = 1) different tree species. One plot was only thinned, but no trees were planted. Extensive estate plots (n = 12) measured 50 × 50 m and were part of EFForTS’s Oil Palm Management Experiment (EFForTS-OPMX) (17), which consisted of a cross-factorial design of four treatments: conventional fertilization, reduced fertilization, herbicide spraying, and mechanical weeding. Finally, as conventional estate plots (n = 8), we considered the control plots of EFForTS-BEE and EFForTS-OPMX, i.e., plots that have not been thinned, enriched, or under conventional fertilization plus herbicide spraying management.
Yield Data.
Yield data on fresh fruit bunch weight across all systems were averaged at the plot-level across the available timespan of recording and standardized to mean annual yield per hectare and year. For smallholder plots, total yield per plot was recorded monthly from 2016 to 2022. For EFForTS-BEE (estate enriched and conventional), yield data were measured on the palm level from November 2017 to October 2020. We scaled the mean annual yield per palm up to one hectare (per area yield) by multiplying it by the number of remaining palms inside the plots when plot sizes would be one hectare, i.e., under consideration of the respective palm densities of the individual enrichment plots (ranging from 76 to 93 palms/ha−1, average: 86 palms/ha−1) (26). In case of the EFForTS-BEE control plots (included in the conventional estates group), mean per palm yields were multiplied by 120, the usual palm density of the plantation where the experiment was conducted (26). Here, the calculation of per area yield does not account for spillover effects, i.e., changes in per palm yield adjacent to the enrichment plots (26, 47). In the EFForTS-OPMX (estate extensive and conventional), all yield events were measured per individual palm from 2016 to 2022. Here, we first calculated mean annual per palm yield and then upscaled it to hectare by multiplying the mean palm yield by 142, the planting density of the plantation where the experiment was located (25). We refrained from standardizing yields to the level of the individual palm, as we considered the respective palm tree density as an important component of the investigated systems.
Airborne LiDAR Data.
Between January 24 and February 5, 2020, airborne laser scanning data were gathered over a total area of 434.14 km2. The acquisition flights were conducted using a BN2T fixed-wing aircraft, flying up to 4,750 m above ground level. LiDAR data were collected using a Riegl LMS-Q780 full waveform scanner operating at near-infrared wavelength, with a 60-degree field of view and a laser pulse repetition rate of up to 400 kHz. Point cloud density varied within and between study areas, ranging from 16.6 to 40.5 points m2. The LiDAR dataset was clipped to an area of 40 × 40 m for each of the 45 oil palm plots, and a suite of LiDAR-derived metrics was computed for each plot, quantifying different aspects of vegetation structure. These metrics included traditional stand summary measures, complexity/heterogeneity measures, measures of vertical and horizontal structure, and more (27). In total, we selected a set of 21 LiDAR metrics for this study based largely on their biological interpretability (SI Appendix, Table S1). To reduce this large set of LiDAR metrics we plotted the principal components, which allowed for visual assessment of correlated groups and showed how metrics related to study plots, from which we selected 11 variables with high loading scores in each direction (SI Appendix, Fig. S4).
Landscape Data.
Land cover data were derived from a supervised classification of Sentinel-1 and -2 imagery from 2019. In total, seven land use classes were distinguished: Oil palm (mature plantations), young oil palm (recently established or replanted oil palm), rubber, forest, barren land, water, and settlements. Slope corrected Sentinel-1 data were aggregated to monthly median composites. Clouds were masked out from Sentinel-2 with the cloud masks of the Level-2A data and yearly composites of the different bands were retrieved. Random forest classification was used to create a map of land use classes for the study area in Jambi province. Isolated land use pixels were removed with the Sieve filter. Finally, using circular buffers with a radius of 1,000 m, the resulting land use map was clipped for each of the 42 study plots and the percentage share of all land use classes within the buffers was computed. Additionally, we calculated landscape diversity within the buffers using the Simpson index (64).
Management Data.
Data on management practices were collected for smallholders through household surveys in 2015 and 2018, as described in ref. 65. The management of the control plots in EFForTS-BEE (conventional management) was obtained through an interview with the plantation manager in 2018 (66). For the enriched plots in EFForTS-BEE, no additional management was performed other than thinning and subsequent tree planting (16). In EFForTS-OPMX, where extensive management was implemented, the management followed the specific requirements of the experimental design (17). In the control plot of EFForTS-OPMX (i.e., conventional estates), the management practices followed the business-as-usual approach of the estate owning company. We standardized all management data to the mean annual inputs per hectare. The five management variables available throughout all systems were nitrogen, phosphorus, and potassium inputs (kg applied), mechanical weeding effort (labor hours), and herbicide weeding effort (liters of herbicides, mostly Glyphosate, applied).
Standardization of Indicator Data and Dual Outcomes.
We standardized all 11 indicators by subtracting observations with a weighted mean of the corresponding variable divided by its SD. The weighted mean was calculated by averaging the five highest and five lowest observations of the respective variable. This method of scaling around a weighted mean as normalized zero, gave a more balanced center that accounted for the extreme values of the indicator (i.e., win or lose cases). We employed the same procedure also for palm oil yields. When small indicator values indicated ecologically more desirable outcomes (microclimatic stability or transpiration), we inverted their sign. For both ecological indicators and yield, we classified all observations above the weighted mean as wins for the respective variables. Conversely, observations below this threshold were categorized as lose cases. Note that not all of the indicators were consistently measured across all of the considered cultivation systems, and some data were missing in some systems (see for example Fig. 1G).
Selection of Predictors.
For all potential explanatory parameters, we first examined all pairwise correlations to identify strongly correlated pairs (r > 0.7). From each set of highly correlated variables, we then selected one variable that best represented the group based on factors such as ease of interpretation and the distribution of data. We applied this process separately to the management (SI Appendix, Fig. S5), landscape (SI Appendix, Fig. S6), and LiDAR metrics (SI Appendix, Fig. S7), which reduced the number of variables from 5 to 3 for management, 6 to 3 for landscape, and 11 to 5 for LiDAR metrics. Finally, we repeated the process for all three variables groups together, while also checking their correlation to system levels (SI Appendix, Fig. S8). Subsequently, we dropped one further LiDAR variable for a final of 10 selected predictor variables (Table 1).
Ordination and Fitting Environmental Vectors.
To assess the relationship between the selected set of environmental and management variables with oil palm yields and ecological indicators at the same time, we used the R function envfit() (67) calculates the magnitude and direction of the correlation between each variable and the ordination axes. Statistical significance of these correlations was assessed using permutation tests. In the first step, we did this for a global ordination, using the data across all available ecological indicators at the same time. In a second step, we repeated the analysis but for individual ordinations of single ecological indicators.
Logistic Models.
To predict the occurrence of win–win cases in relation to cultivation system identity we used a logistic model, using the presence or absence of win–win outcomes as response variable and the system identity as predictor.
Microclimatic Stability.
Below canopy air temperature range (difference between the minimum and maximum measured temperature) was used to assess microclimatic stability. In EFForTS-BEE, temperature was measured through dataloggers (iButtons) installed 1.5 m above and 10 cm below ground (68). Loggers were installed for 6 d (20.09.2017 to 26.09.2017) with measurements available between 10 am and 3 pm local time. Air and soil temperature in smallholder plantations was compiled from sensors installed at 2 m above and 30 cm below ground, respectively, within each smallholder plantation (69). The data from smallholder locations was matched to the data of the EFForTS-BEE, using the same 6 d in 2017 and the same timeframe. In a few cases, data were missing within this period due to sensor failure, so we used averaged data over a complete period of one month (10.09.2017 to 10.10.2017) instead.
Bird Diversity.
We recorded stereo sound at 22.05 kHz sampling frequency (SMX-II microphones, SM2+ recorder, Wildlife acoustics) for 15 min starting at sunrise. Recorders were attached to a central tree of the plot (in EFForTS-BEE) or on a central pole at 2.0 to 2.5 m height (EFForTS-OPMX and smallholder plantations). Recordings in EFForTS-BEE (estate enriched and estate conventional) took place in March 2017, in EFForTS-OPMX (estate extensive and estate conventional) in September 2017 and in the smallholder plots in November and December 2016. We uploaded all recordings to a website (70) where ornithologists could identify all audible and visible bird calls (within an estimated 35 m radius to recorders) to species. Only identifications to the species levels were considered. For future details on methodology see refs. 17 and 71.
Soil Fauna.
Soil invertebrates (meso- and macrofauna) were assessed with the heat extraction method. Using a spade, we collected soil and litter samples in October to November 2013 and 2016 in smallholder plots (3 samples/subplots per plot each year), in November 2016 in EFForTS-BEE (4 samples/subplots per plot) and in October to November 2017 in EFForTS-OPMX (5 samples/subplots per plot). Each soil sample measured 16 cm × 16 cm and included litter (if present) and underlying soil down to a depth of 5 cm. Animals from litter and soil were extracted using a gradient heat extractor (72) and collected in dimethyleneglycol–water solution (1:1) and thereafter transferred into 70% ethanol. All extracted animals were counted and sorted into 28 taxonomic groups (in most cases orders) under a dissecting microscope (73). In total, 152,430 individuals were collected and sorted. Taxonomic diversity was calculated as the number of taxonomic groups present in each sample; data from individual samples/subplots were averaged per plot.
Decomposition.
Decomposition of leaf litter was explored by placing litterbags (20 cm × 20 cm polyester bags with a 4 mm mesh). The material consisted of freshly cut and air-dried (25 °C) fronds of oil palm leaves. Each of the litterbags contained 10 g material for smallholder coreplots and EFForTS-OPMX plots and 12 g for EFForTS-BEE plots; for details of the setup and litter preparation see refs. 17, 26, and 74. In each plot, one litterbag was installed, i.e., in October 2013 in the smallholder plantations, in December 2016 in EFForTS-OPMX plots and in November 2017 in EFForTS-BEE plots. Note that the climatic conditions were comparable between the sampling years (SI Appendix, Table S7), as such the impact of different sampling years on measured decomposition rates should be marginal. The litterbags were placed in the field for 12 mo. The decomposition, i.e., litter mass loss, was determined by comparing the initial dry mass of the litter with the remaining dry mass (25 °C) after 12 mo in the field. The decomposition rate was expressed as a percentage of the decomposed material.
Bacteria.
Soil samples from EFForTS-OPMX and B11 were mixed with an equal volume of RNAprotect Bacteria Reagent (Qiagen, Hilden, Germany) after sampling. Bulk soil DNA was extracted from three subplots in smallholder plantation plots, five subplots in EFForTS-OPMX and EFForTS-BEE plots. The extracted DNA was then used for amplification of 16S rRNA genes targeting the V3-V5 (smallholders) or V3-V4 (EFForTS-OPMX and EFForTS-BEE) variable regions. Amplification was performed using the Phusion hot start high-fidelity DNA Polymerase (Finnzymes and Thermo Scientific). The EFForTS-BEE dataset was sequenced using a 454 GS-FLX sequencer and Titanium chemistry (Roche, Mannheim, Germany), while the EFForTS-OPMX and EFForTS-BEE datasets were sequenced using a MiSeq instrument with v3 chemistry (Illumina, San Diego, CA, USA). All datasets underwent quality filtering, primer and singleton removal, chimera checking, denoising, and length filtering. ASVs were classified using the SILVA database version 138.1 with BLCA v2.1. ASVs classified as eukaryotes, chloroplasts, mitochondria, and unclassified domains were removed. The ASV tables were rarefied to 9,183 sequences per sample. All sequences are available at the National Center for Biotechnology Information (NCBI) under the accession numbers SRP239591 and SRP056374.
Fungi.
In each plot, five soil cores (15 cm depth, 4 cm diameter) were extracted, mixed, and separated into soil and roots. The samples were freeze-dried, used for DNA extraction and high-throughput sequencing on the MiSeq platform, after amplification of the fungal barcoding ITS1 marker region (ITS1f-KYO2 and ITS2) as reported previously (75). Raw reads were processed, clustered at 97% genetic identity (yielding virtual fungal species) and searched against the UNITE v.7.2 public database following a published bioinformatic pipeline (75).
Understory Vegetation.
Understory vegetation was assessed in smallholder plantations between March 2013 and March 2014, in EFForTS-OPMX in September 2017, and in EFForTS-BEE from February to March 2018. Within 5 m × 5 m subplots, all terrestrial vascular plants, except woody plants with a diameter of ≥1 cm and a height of ≥1.3 m, were recorded and assigned to species or morphospecies. Of each morphospecies, field pictures were taken and, if possible, herbarium specimens, which were deposited (Herbarium Bogoriense, BIOTROP Herbarium, Herbarium of the University of Jambi, Harapan Rainforest Herbarium). In the smallholder plantations, epiphytes growing on the oil palm trunks were originally included in the inventory, but were later filtered out in order to harmonize the smallholder data with that of the other systems. Species richness was calculated as the total number of (morpho-)species per subplot. Species richness per plot was calculated as the mean across all five subplots in smallholder plantations and EFForTS-OPMX, whereas in EFForTS-BEE plants were only sampled in a single subplot.
Aboveground Biomass.
Total aboveground biomass per plot area was estimated based on structural inventories of all trees and palms with a diameter at breast height ≥10 cm (dbh at 1.3 m) using specific allometric equations. The inventories took place in 2017 and 2019 in smallholder plantations, 2017 in EFForTS-BEE and 2019 in EFForTS-OPMX. In the case of smallholders we average the biomass over the two available sampling years. For the smallholder plots all trees and palms within each 50 m × 50 m plot were tagged, the dbh measured with a measuring tape (Richter Measuring Tools, Speichersdorf, Germany), and total height recorded using a Vertex III height meter (Haglöf, Langsele, Sweden). Wood density values for different species were based on Pilodyn measurements (76), obtained from the global wood density database (77) or plot measurements. Palm stem height was defined as the distance from the ground to the base of the youngest leaf (the meristem point). The structural data were converted to biomass estimates using the allometric equations of (78) for trees and (79) for oil palms and summed on a plot area basis. Necromass, leaf litter, understory vegetation, and spontaneously established trees <10 cm dbh were deemed negligible in the estimations.
Soil Carbon and Phosphorus Availability.
SOC was measured in 2013 in the smallholder core plots at depth intervals of 0 to 50 cm in five subplots per plot. For the EFForTS-OPMX, SOC was measured in March 2018 at the same depth. Soil samples were air-dried, finely grounded and analyzed for SOC using a CN analyzer (Vario EL Cube, Elementar Analysis Systems). Extractable P was determined using both the Bray-1 and Bray-2 methods (80, 81).
Transpiration.
The presented transpiration (Et) values are annual rates derived from sap flux and micrometeorological measurements. Daily Et rates of oil palms and trees were put in relation to potential evapotranspiration (E0) and the ratios were multiplied by the annual E0 in 2016 to obtain annual Et. For smallholder oil palm plantations data from 2013 and 2014 were used, and for oil palm estates data from 2014 and 2016 (82). In the EFForTS-BEE plots, sap flux measurements were conducted in one plot in 2016 (83), and transpiration at the remaining plots was estimated through linear relationships with canopy cover as derived from drone photogrammetry (84), separately for oil palms and trees. Total Et for each plot was calculated by adding palm and tree Et. These methods provided reliable annual Et estimates for the study plots.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank village leaders, respondents, farmers, PT REKI, PT Humusindo, PT Perkebunan Nusantara VI company, and Bukit Duabelas National Park for their support and cooperation, granting us access to the research sites and providing information. We also thank our dedicated staff at the EFForTS offices in Jambi and Göttingen for their exceptional coordination. Special thanks are extended to our hard working local field assistants, whose contributions were essential to the successful completion of this research. We are also grateful to Ristekdikti for granting us the necessary research permits. This study was financed by the Deutsche Forschungsgemeinschaft (DFG), project number 192626868, in the framework of the collaborative German–Indonesian research center CRC990. Catrin Westphal is grateful for being funded by the DFG, project number 493487387.
Author contributions
A.W., C.W., and I.G. designed research; A.W., J.B., D. Berkelmann, F.B., R.D., K.D., G.F., N.A.-A.I., M.M.K., V.K., A. Potapov, A.R., K.T.S., C.S., A.T., P.-A.W., and D.C.Z. performed research; A.W., J.B., D. Berkelmann, F.B., N.C., R.D., G.F., N.A.-A.I., M.M.K., V.K., G.B.P., A. Potapov, A.R., D.S., K.T.S., C.S., D.C.Z., and I.G. analyzed data; and A.W., C.W., J.B., D. Berkelmann, F.B., D. Buchori, N.C., M.D.C., R.D., K.D., S.E., G.F., D.H., N.A.-A.I., B.I., A.K., M.M.K., V.K., H.K., Y.M., O.M., G.B.P., A. Polle, A. Potapov, A.R., S.S., M.S., D.S., K.T.S., C.S., L.S., A.T., T.T., E.V., P.-A.W., M.W., D.C.Z., and I.G. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. S.M. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
The data utilized for the analysis presented in this manuscript has been deposited and is accessible at the following link: https://doi.org/10.25625/NAJYVI (85).
Supporting Information
References
- 1.Murphy D. J., Goggin K., Paterson R. R. M., Oil palm in the 2020s and beyond: Challenges and solutions. CABI Agric. Biosci. 2, 39 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FAO, FAOSTAT, Online Database. http://faostat.fao.org/. Accessed 22 February 2023.
- 3.Chrisendo D., Siregar H., Qaim M., Oil palm and structural transformation of agriculture in Indonesia. Agric. Econ. 52, 849–862 (2021). [Google Scholar]
- 4.Vijay V., Pimm S. L., Jenkins C. N., Smith S. J., The impacts of oil palm on recent deforestation and biodiversity loss. PLoS One 11, e0159668 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qaim M., Sibhatu K. T., Siregar H., Grass I., Environmental, economic, and social consequences of the oil palm boom. Annu. Rev. Resour. Econ. 12, 321–344 (2020). [Google Scholar]
- 6.Teng S., Khong K. W., Che Ha N., Palm oil and its environmental impacts: A big data analytics study. J. Clean. Prod. 274, 122901 (2020). [Google Scholar]
- 7.Meijaard E., et al. , The environmental impacts of palm oil in context. Nat. Plants 6, 1418–1426 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Edwards D. P., et al. , Wildlife-friendly oil palm plantations fail to protect biodiversity effectively. Conserv. Lett. 3, 236–242 (2010). [Google Scholar]
- 9.Scriven S. A., et al. , Testing the benefits of conservation set-asides for improved habitat connectivity in tropical agricultural landscapes. J. Appl. Ecol. 56, 2274–2285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson L., et al. , Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Curran L. M., et al. , Lowland forest loss in protected areas of Indonesian Borneo. Science 303, 1000–1003 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Mardiastuti A., Mulyani Y. A., Hasan M., Kaban A., Is forest remnants able to support bird community? Case in tropical lowland forest of West Java, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 399, 012034 (2019). [Google Scholar]
- 13.Foster W. A., et al. , Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philos. Trans. R. Soc. B Biol. Sci. 366, 3277–3291 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai K., Hassan M. A., Vairappan C. S., Shirai Y., Promotion of a green economy with the palm oil industry for biodiversity conservation: A touchstone toward a sustainable bioindustry. J. Biosci. Bioeng. 133, 414–424 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Ganser D., Denmead L. H., Clough Y., Buchori D., Tscharntke T., Local and landscape drivers of arthropod diversity and decomposition processes in oil palm leaf axils. Agric. For. Entomol. 19, 60–69 (2017). [Google Scholar]
- 16.Teuscher M., et al. , Experimental biodiversity enrichment in oil-palm-dominated landscapes in indonesia. Front. Plant Sci. 7, 1538 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darras K. F. A., et al. , Reducing fertilizer and avoiding herbicides in oil palm plantations—Ecological and economic valuations. Front. For. Glob. Change 2, 65 (2019). [Google Scholar]
- 18.Luke S. H., et al. , Effects of understory vegetation management on plant communities in oil palm plantations in Sumatra, Indonesia. Front. For. Glob. Change 2, 33 (2019). [Google Scholar]
- 19.Formaglio G., Veldkamp E., Damris M., Tjoa A., Corre M. D., Mulching with pruned fronds promotes the internal soil N cycling and soil fertility in a large-scale oil palm plantation. Biogeochemistry 154, 63–80 (2021). [Google Scholar]
- 20.Nurdiansyah F., Denmead L. H., Clough Y., Wiegand K., Tscharntke T., Biological control in Indonesian oil palm potentially enhanced by landscape context. Agric. Ecosyst. Environ. 232, 141–149 (2016). [Google Scholar]
- 21.Lee J. S. H., et al. , Environmental impacts of large-scale oil palm enterprises exceed that of smallholdings in Indonesia. Conserv. Lett. 7, 25–33 (2014). [Google Scholar]
- 22.Razak S. A., Saadun N., Azhar B., Lindenmayer D. B., Smallholdings with high oil palm yield also support high bird species richness and diverse feeding guilds. Environ. Res. Lett. 15, 094031 (2020). [Google Scholar]
- 23.Euler M., Hoffmann M. P., Fathoni Z., Schwarze S., Exploring yield gaps in smallholder oil palm production systems in eastern Sumatra, Indonesia. Agric. Syst. 146, 111–119 (2016). [Google Scholar]
- 24.Frei B., et al. , Bright spots in agricultural landscapes: Identifying areas exceeding expectations for multifunctionality and biodiversity. J. Appl. Ecol. 55, 2731–2743 (2018). [Google Scholar]
- 25.Iddris N. A.-A., et al. , Mechanical weeding enhances ecosystem multifunctionality and profit in industrial oil palm. Nat. Sustain. 6, 683–695 (2023). [Google Scholar]
- 26.Zemp D. C., et al. , Tree islands enhance biodiversity and functioning in oil palm landscapes. Nature 618, 316–321 (2023), 10.1038/s41586-023-06086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camarretta N., et al. , Using airborne laser scanning to characterize land-use systems in a tropical landscape based on vegetation structural metrics. Remote Sens. 13, 4794 (2021). [Google Scholar]
- 28.Ehbrecht M., Effective number of layers: A new measure for quantifying three-dimensional stand structure based on sampling with terrestrial LiDAR. For. Ecol. Manag. 380, 212–223 (2016). [Google Scholar]
- 29.Clough Y., et al. , Land-use choices follow profitability at the expense of ecological functions in Indonesian smallholder landscapes. Nat. Commun. 7, 13137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh L. P., Miettinen J., Liew S. C., Ghazoul J., Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. U.S.A. 108, 5127–5132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bicknell J. E., et al. , Enhancing the ecological value of oil palm agriculture through set-asides. Nat. Sustain. 6, 513–525 (2023). [Google Scholar]
- 32.Grass I., Batáry P., Tscharntke T., “Combining land-sparing and land-sharing in European landscapes” in Advances in Ecological Research (Elsevier, 2021), pp. 251–303. [Google Scholar]
- 33.Angelsen A., Policies for reduced deforestation and their impact on agricultural production. Proc. Natl. Acad. Sci. U.S.A. 107, 19639–19644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelsen A., Kaimowitz D., Rethinking the causes of deforestation. World Bank Res. Obs. 14, 73–98 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Woittiez L. S., Slingerland M., Rafik R., Giller K. E., Nutritional imbalance in smallholder oil palm plantations in Indonesia. Nutr. Cycl. Agroecosyst. 111, 73–86 (2018). [Google Scholar]
- 36.Woittiez L. S., van Wijk M. T., Slingerland M., van Noordwijk M., Giller K. E., Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 83, 57–77 (2017). [Google Scholar]
- 37.Hood A. S. C., et al. , Understory vegetation in oil palm plantations promotes leopard cat activity, but does not affect rats or rat damage. Front. For. Glob. Change 2, 51 (2019). [Google Scholar]
- 38.Ashton-Butt A., et al. , Understory vegetation in oil palm plantations benefits soil biodiversity and decomposition rates. Front. For. Glob. Change 1, 10 (2018). [Google Scholar]
- 39.Li K., et al. , Tree identity and canopy openness mediate oil palm biodiversity enrichment effects on insect herbivory and pollination. Ecol. Appl. 33, e2862 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Qi Y., et al. , Effects of herbicides on non-target plant species diversity and the community composition of fallow fields in northern China. Sci. Rep. 10, 9967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenneis K., Irawan B., Wollni M., Promoting agricultural technologies with positive environmental effects: Evidence on tree planting in Indonesia. Ecol. Econ. 204, 107666 (2023). [Google Scholar]
- 42.Hassler E., Corre M. D., Kurniawan S., Veldkamp E., Soil nitrogen oxide fluxes from lowland forests converted to smallholder rubber and oil palm plantations in Sumatra, Indonesia. Biogeosciences 14, 2781–2798 (2017). [Google Scholar]
- 43.Formaglio G., Veldkamp E., Duan X., Tjoa A., Corre M. D., Herbicide weed control increases nutrient leaching compared to mechanical weeding in a large-scale oil palm plantation. Biogeosciences 17, 5243–5262 (2020). [Google Scholar]
- 44.Meijide A., et al. , Measured greenhouse gas budgets challenge emission savings from palm-oil biodiesel. Nat. Commun. 11, 1089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westphal C., et al. , Promoting multiple ecosystem services with flower strips and participatory approaches in rice production landscapes. Basic Appl. Ecol. 16, 681–689 (2015). [Google Scholar]
- 46.Teuscher M., et al. , Trade-offs between bird diversity and abundance, yields and revenue in smallholder oil palm plantations in Sumatra, Indonesia. Biol. Conserv. 186, 306–318 (2015). [Google Scholar]
- 47.Gérard A., et al. , Oil-palm yields in diversified plantations: Initial results from a biodiversity enrichment experiment in Sumatra, Indonesia. Agric. Ecosyst. Environ. 240, 253–260 (2017). [Google Scholar]
- 48.Morante-Filho J. C., Benchimol M., Faria D., Landscape composition is the strongest determinant of bird occupancy patterns in tropical forest patches. Landsc. Ecol. 36, 105–117 (2021). [Google Scholar]
- 49.Lucey J. M., et al. , Tropical forest fragments contribute to species richness in adjacent oil palm plantations. Biol. Conserv. 169, 268–276 (2014). [Google Scholar]
- 50.Seaman D. J. I., et al. , Densities of Bornean orang-utans (Pongo pygmaeus morio) in heavily degraded forest and oil palm plantations in Sabah, Borneo. Am. J. Primatol. 81, e23030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K., et al. , Adjacent forest moderates insect pollination of oil palm. Agric. Ecosyst. Environ. 338, 108108 (2022). [Google Scholar]
- 52.MacArthur R. H., MacArthur J. W., On bird species diversity. Ecology 42, 594–598 (1961). [Google Scholar]
- 53.Frazão L. A., Paustian K., Cerri C. E. P., Cerri C. C., Soil carbon stocks under oil palm plantations in Bahia State, Brazil. Biomass Bioenergy 62, 1–7 (2014). [Google Scholar]
- 54.Luskin M. S., Potts M. D., Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic Appl. Ecol. 12, 540–551 (2011). [Google Scholar]
- 55.Böhnert T., et al. , Effects of land-use change on vascular epiphyte diversity in Sumatra (Indonesia). Biol. Conserv. 202, 20–29 (2017). [Google Scholar]
- 56.Potapov A., et al. , Aboveground soil supports high levels of biological activity in oil palm plantations. Front. Ecol. Environ. 18, 181–187 (2020). [Google Scholar]
- 57.Snaddon J. L., Willis K. J., Macdonald D. W., Biodiversity: Oil-palm replanting raises ecology issues. Nature 502, 170–171 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Rembold K., Mangopo H., Tjitrosoedirdjo S. S., Kreft H., Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 213, 234–242 (2017). [Google Scholar]
- 59.Prabowo W. E., et al. , Bird responses to lowland rainforest conversion in Sumatran Smallholder Landscapes, Indonesia. PLoS One 11, e0154876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grass I., et al. , Trade-offs between multifunctionality and profit in tropical smallholder landscapes. Nat. Commun. 11, 1186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J., et al. , Replanting and yield increase strategies for alleviating the potential decline in palm oil production in Indonesia. Agric. Syst. 210, 103714 (2023). [Google Scholar]
- 62.Drescher J., et al. , Ecological and socio-economic functions across tropical land use systems after rainforest conversion. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunz Y., et al. , ‘The fridge in the forest’: Historical trajectories of land tenure regulations fostering landscape transformation in Jambi Province, Sumatra, Indonesia. For. Policy Econ. 81, 1–9 (2017). [Google Scholar]
- 64.Simpson E. H., Measurement of diversity. Nature 163, 688–688 (1949). [Google Scholar]
- 65.Sibhatu K. T., Oil palm boom and farm household diets in the tropics. Front. Sustain. Food Syst. 3, 75 (2019). [Google Scholar]
- 66.Lorenz H., Competition and Yield in Oil Palm Agroforestry: Examining the ‘Yield Penalty’ of Biodiversity (University of Göttingen, Göttingen, 2018). [Google Scholar]
- 67.Oksanen J., Vegan: Ecological diversity package. R package version 2.6-4. https://CRAN.R-project.org/package=vegan (2022).
- 68.Donfack L. S., et al. , Microclimate and land surface temperature in a biodiversity enriched oil palm plantation. For. Ecol. Manag. 497, 119480 (2021). [Google Scholar]
- 69.Meijide A., et al. , Impact of forest conversion to oil palm and rubber plantations on microclimate and the role of the 2015 ENSO event. Agric. For. Meteorol. 252, 208–219 (2018). [Google Scholar]
- 70.Darras K. F. A., et al. , ecoSound-web: An open-source, online platform for ecoacoustics. F1000Research 9, 1224 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darras K., et al. , Autonomous sound recording outperforms human observation for sampling birds: A systematic map and user guide. Ecol. Appl. 29, e01954 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Lloyd D., Ghelardi R., A new extractor for woodland litter. Pedobiol. Jena 3, 1–21 (1963). [Google Scholar]
- 73.Potapov A. M., Klarner B., Sandmann D., Widyastuti R., Scheu S., Linking size spectrum, energy flux and trophic multifunctionality in soil food webs of tropical land-use systems. J. Anim. Ecol. 88, 1845–1859 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Krashevska V., et al. , Micro-decomposer communities and decomposition processes in tropical lowlands as affected by land use and litter type. Oecologia 187, 255–266 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Ballauff J., et al. , Shifts in root and soil chemistry drive the assembly of belowground fungal communities in tropical land-use systems. Soil Biol. Biochem. 154, 108140 (2021). [Google Scholar]
- 76.Kotowska M. M., Leuschner C., Triadiati T., Meriem S., Hertel D., Quantifying above- and belowground biomass carbon loss with forest conversion in tropical lowlands of Sumatra (Indonesia). Glob. Change Biol. 21, 3620–3634 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Chave J., et al. , Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Chave J., et al. , Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Khasanah N., van Noordwijk M., Ningsih H., Aboveground carbon stocks in oil palm plantations and the threshold for carbon-neutral vegetation conversion on mineral soils. Cogent Environ. Sci. 1, 1119964 (2015). [Google Scholar]
- 80.Sarker A., Kashem M. A., Osman K. T., Hossain I., Ahmed F., Evaluation of available phosphorus by soil test methods in an acidic soil incubated with different levels of lime and phosphorus. Open J. Soil Sci. 4, 103–108 (2014). [Google Scholar]
- 81.Ballauff J., et al. , Legacy effects overshadow tree diversity effects on soil fungal communities in oil palm-enrichment plantations. Microorganisms 8, 1577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Röll A., et al. , Transpiration on the rebound in lowland Sumatra. Agric. For. Meteorol. 274, 160–171 (2019). [Google Scholar]
- 83.Ahongshangbam J., et al. , Drone-based photogrammetry-derived crown metrics for predicting tree and oil palm water use. Ecohydrology 12, e2115 (2019). [Google Scholar]
- 84.Khokthong W., et al. , Drone-based assessment of canopy cover for analyzing tree mortality in an oil palm agroforest. Front. For. Glob. Change 2, 12 (2019). [Google Scholar]
- 85.Wenzel A., et al. , Data used for the analysis of Balancing economic and ecological functions in smallholder and industrial oil palm plantations. GRO.data. 10.25625/NAJYVI. Deposited 17 February 2024. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The data utilized for the analysis presented in this manuscript has been deposited and is accessible at the following link: https://doi.org/10.25625/NAJYVI (85).