Abstract
Intraocular pressure (IOP) is the primary risk factor for glaucoma, a blinding eye disease. Cannabinoid agonists have long been known to decrease IOP, suggesting they may be useful in glaucoma treatment. However, the specific mechanism by which cannabinoids generate this ocular hypotensive effect remains unknown. The current evidence suggests the cannabinoids reduce IOP through actions at cannabinoid 1 (CB1) receptors within the eye, and adrenergic receptors (ARs) may also contribute to this action of cannabinoids. Considering this, the present study aimed to elucidate the mechanism behind the ocular hypotensive properties of cannabinoids through the use of mice genetically lacking either cannabinoid receptors or βARs. Cannabinoid agonists, βAR antagonists, and βAR agonists decreased IOP in wild-type mice and CB2(−/−) mice. In contrast, none of these compounds were found to reduce IOP in βAR(−/−) or CB1(−/−) mice. Desensitization of the βARs and depletion of catecholamines in wild-type mice also eliminated the ability of the cannabinoid agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN 55,212-2) to reduce IOP, strongly implicating a role for both βARs and catecholamines in the ocular hypotensive properties of cannabinoids. Finally, CB1 receptors were shown to colocalize with tyrosine hydroxylase, a marker for adrenergic neurons. Taken together, these findings suggest that βARs are required for the ocular hypotensive properties of cannabinoids, and cannabinoids reduce IOP by acting as indirect sympatholytics and inhibiting norepinephrine release within the eye.
Introduction
Cannabinoid agonists and antagonists have received significant interest for use as therapeutics in the treatment of a variety of conditions including obesity and diabetes, pain, neurodegenerative disorders, and immune dysfunction among many others (Piomelli et al., 2000). One property of cannabinoid agonists that has received attention is their ability to reduce intraocular pressure (IOP) (Hepler and Frank, 1971). IOP is the primary risk factor for glaucoma, a blinding neurodegenerative eye disease, and drugs that reduce IOP, so-called ocular hypotensives, are used in the treatment of glaucoma. Although the IOP-lowering ability of cannabinoid agonists is well established (Hepler and Frank, 1971; Liu and Dacus, 1987; Chien et al., 2003; Szczesniak et al., 2006), none have been developed for the treatment of glaucoma, largely because of a limited understanding of the mechanisms by which cannabinoids produce their effect on IOP.
IOP is maintained in the eye by the balance between aqueous humor (AH) secretion and outflow. AH is secreted by the cells of the ciliary body (Civan and Macknight, 2004), whereas outflow occurs through one of two distinct pathways: 1) the trabecular meshwork pathway (Ferrer, 2006) and 2) the uveoscleral pathway (Alm and Nilsson, 2009). Ocular hypotensive actions of cannabinoids may include effects on both AH production and outflow, because CB1 receptors are expressed in the ciliary body and on trabecular meshwork cells (Straiker et al., 1999). There is also in vitro and in vivo evidence suggesting that cannabinoids affect both AH secretion (Chien et al., 2003) and outflow, specifically through the trabecular meshwork outflow pathway (Stumpff et al., 2005; McIntosh et al., 2007). Although the specific sites of the cannabinoid-mediated reduction in IOP may still be unclear, there is general consensus that the effects are not mediated via central nervous system receptors, but instead by CB1 receptors present within the eye (Liu and Dacus, 1987; Pate et al., 1998).
Drugs targeting ocular adrenoceptors (ARs) also reduce IOP, and βAR antagonists are first-line therapeutics in the treatment of glaucoma, because of their ability to reduce AH secretion (Watanabe and Chiou, 1983). βAR agonists somewhat paradoxically also reduce IOP; however, they do so by increasing AH outflow through the uveoscleral and trabecular meshwork pathways (Alvarado et al., 1998). In addition to the βARs, α2AR agonists are ocular hypotensive, reducing AH secretion, while at the same time increasing uveoscleral outflow (Woodward and Gil, 2004).
Given the importance of the various ARs in IOP regulation, it is not surprising that several lines of evidence suggest adrenergic receptors contribute to the ocular hypotensive actions of cannabinoids (Green, 1979). In several studies where βAR antagonists have been coadministered with cannabinoid agonists additive effects on IOP were not observed (Green et al., 1977; Oltmanns et al., 2008). This lack of additive effect suggests that there may be an overlap in the IOP-lowering mechanisms for these two drug classes. Further support for the involvement of ARs in the ocular hypotensive properties of cannabinoids comes from the observation that when rabbits were gangliectomized to eliminate sympathetic input cannabinoids agonists had a reduced ability to lower IOP (Green and Kim, 1976; Green et al., 1977; Green, 1979), although this seems to be species-dependent because similar findings were not observed in gangliectomized cats (Colasanti and Powell, 1985). To date, no study has definitively examined whether ARs are involved in the ocular hypotensive properties of cannabinoids in mice or considered the specific mechanism by which individual AR subtypes may be involved in the cannabinoid-mediated reduction in IOP.
In several other tissues presynaptic CB1 receptors inhibit norepinephrine (NE) release (Ishac et al., 1996). If this were also the case in the eye, it suggests that cannabinoid agonists could reduce IOP by inhibiting NE release in the ciliary body, thus acting as indirect sympatholytics. The present study is the first to examine this possibility through the use of βAR(−/−), CB1(−/−), and CB2(−/−) mice. We demonstrate that the ocular hypotensive properties of cannabinoids are absent in mice genetically lacking either CB1 or the βARs, but remain intact in mice lacking CB2. In addition, we demonstrate that treating wild-type mice with the catecholamine-depleting agent reserpine eliminates the ability of cannabinoid agonists to reduce IOP. These findings indicate that cannabinoids reduce IOP through the activation of the CB1 receptor, in a mechanism that also depends on the presence of both the βARs and catecholamines.
Materials and Methods
Animals.
All C57BL6 mice used for IOP experiments were handled according to Canadian Council for Animal Care guidelines (http://www.ccac.ca/), and all experimental procedures were approved by the Dalhousie University Committee on Laboratory Animals. Mice were kept on a 12-h (7:00 AM–7:00 PM) light/dark cycle and fed ad libitum. C57BL/6J mice were obtained from Charles River Laboratories Inc. (Wilmington, MA). C57BL/6J mice were obtained at 6 to 8 weeks of age and allowed to acclimatize to the animal-care facility for at least 1 week before their use in experiments. Mice genetically lacking both β1AR and β2AR [βAR(−/−)] were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in homozygous breeding pairs to produce all animals used in this study. Mice genetically lacking the CB1 receptor [CB1(−/−)] were kindly provided by Dr. Carl Lupica (National Institute on Drug Abuse, Bethesda, MD). Heterozygous mice were obtained and bred with the resulting offspring genotyped by polymerase chain reaction using a CB1 forward primer (5′-GTACCATCACCACAGACCTCCT-3′), in combination with a CB1 wild-type reverse primer (5′-GGATTCAGAATCATGAAGCACTC-3′) or a CB1 knockout reverse primer (5′-AAGAACGAGATCAGCAGCCTCT-3′).
CD1 strain animals [wild type and CB1(−/−)] used for immunohistochemistry and IOP experiments were kindly provided by Dr. Ken Mackie (Indiana University, Bloomington, IN) and handled according to the Guidelines of the National Institutes of Health on the Care and Use of Animals (Institute of Laboratory Animal Resources, 1996); all procedures used in this study were approved by the Animal Care Committee of Indiana University, Bloomington Campus. Adult mice (CD1 strain, from breeding colony) were housed under a 12-h day/night cycle then killed (during the light cycle) by rapid cervical dislocation for immunohistochemistry.
Intraocular Pressure Measurements.
IOP was measured in mice by rebound tonometry, using a Tonolab (Icare Finland Oy, Helsinki, Finland). This instrument involves a light plastic-tipped probe briefly making contact with the cornea; after the probe hits the eye the instrument measures the speed at which it rebounds to calculate IOP (Cervino, 2006).
To obtain reproducible IOP measurements, mice were anesthetized with isoflurane (4% induction). The anesthetized mouse was then placed on a platform in a prone position, where anesthesia was maintained with isoflurane (2% maintenance). IOP measurements were then made with at least six individual pressure readings taken from each eye. The pressure from each eye was then recorded as the average of these measurements.
For diurnal IOP experiments, IOP was measured on the same day and from the same animal both early in the light cycle, between 9:00 AM and 9:30 AM (reported as 9:00 AM), and early in the dark cycle, between 9:00 PM and 9:30 PM (reported as 9:00 PM). Statistical analysis of these data was carried out for each eye independently by matched-pair t test, comparing the 9:00 AM and 9:00 PM measurements.
All IOP measurements after drug administration were recorded between 4:00 PM and 6:00 PM to reduce any variability in IOP resulting from diurnal changes. Drugs were applied topically to one randomly assigned eye, whereas the other eye received the appropriate vehicle. IOP measurements after drug administration were analyzed by matched-pair t tests comparing the drug-treated eye to the vehicle-treated eye of the same animal.
In the chronic isoprenaline (ISO; 1-(3′,4′-dihydroxyphenyl)-2-isopropylaminoethanol hydrochloride, N-isopropyl-dl-noradrenaline) desensitization experiments, either ISO (2.5%), or saline vehicle was administered twice daily for the 1-week experiment (9:00 AM and 9:00 PM) or three times daily (5%) for the 2-week experiment to both eyes of the animal. After 7 or 14 days, the ability of the βAR antagonist timolol [S-(−)-1-(t-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol] to reduce IOP was tested by applying timolol to the right eye and saline vehicle to the left eye and comparing the IOP from the two eyes. ISO treatment was again resumed for an additional 3 days, before the ability of (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN 55,212-2; WIN) to reduce IOP in these animals was examined.
Immunohistochemistry.
After the animals were killed, their eyes were removed, and the anterior or posterior eye section was cut away to form a posterior or anterior eyecup. The eyecup was fixed in 4% paraformaldehyde followed by a 30% sucrose immersion for 24 to 72 h at 4°C. Tissue was then frozen in OCT compound and sectioned (15–25 μm) using a Leica (Wetzlar, Germany) CM1850 cryostat. Tissue sections were mounted onto Superfrost-plus slides, washed, treated with a detergent (0.3% Triton X-100 or 0.1% saponin) and milk (5%), followed by primary antibodies overnight at 4°C. Secondary antibodies (Alexa 594 or Alexa 488, 1:500; Invitrogen, Carlsbad, CA) were subsequently applied at room temperature for 1.5 h. The tyrosine hydroxylase (TH) antibody (Sigma-Aldrich, St. Louis, MO; 1:500) has been well characterized (Whitney et al., 2009). TH retinal staining was limited to a characteristic population of amacrine cells and their processes in the distal inner plexiform layer (data not shown). The CB1 antibody was the generous gift of Dr. Ken Mackie. The specificity of the CB1 antibody (1:300) was characterized by the use of knockout mouse models (Hu et al., 2010), including anterior eye (data not shown). Images were acquired with a Leica TCS SP5 confocal microscope using Leica LAS AF software and a 63× oil objective. Images were processed using ImageJ (available at http://rsbweb.nih.gov/ij/) and/or Photoshop (Adobe Systems, San Jose, CA). Images were modified only in terms of brightness and contrast.
Reserpine Depletion of Catecholomines.
Reserpine treatment was used to deplete catecholomines in mice, according to the previously described protocol (Olfe et al., 2010). In brief, reserpine (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada) was dissolved in 5% glacial acetic acid at 2 μg/μl. The dose given was μg/g body weight, and the resulting solution was combined with 100 μl of saline. Reserpine solution, or saline vehicle, was then injected subcutaneously 24 h before experimental manipulation. After 24 h, animals were anesthetized using pentobarbital at 60 mg/kg for induction and 5 mg/kg for maintenance of anesthesia if needed. Baseline IOP measurements were then taken by rebound tonometry (Tonolab) before any further treatment to determine basal IOP under pentobarbital anesthesia. After basal IOP measurement, one eye was treated topically with drug, whereas the other was treated with the appropriate vehicle. IOP was then measured 20 min after drug administration.
Drugs.
Timolol, ISO, and metoprolol [(±)1-(isopropylamino)-3-[p-(β-methoxyethyl)phenoxy]-2-propanol] were obtained from Sigma-Aldrich Canada Ltd. and prepared in 0.9% sterile saline for topical applications. WIN, 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM281), 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl(4-methoxyphenyl)methanone (AM630), and N-(2-chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) were obtained from Tocris Bioscience (Ellisville, MO) and prepared in Tocrisolve 100 (Tocris Bioscience) for topical application. Latanoprost [isopropyl(Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2- [(3R)3-hydroxy-5-phenylpentyl]-cyclopentyl]hept-5-enoate] was obtained as an ophthalmic solution (Xalatan; Pfizer, New York, NY).
Results
Diurnal Variations in IOP.
Diurnal variation in the IOP of rodents is well established, such that IOP is lowest early in the light cycle and highest at the beginning of the dark cycle (Savinova et al., 2001). Therefore, to validate our experimental method of measuring the IOP in the mouse eye, diurnal changes in IOP were measured first in wild-type mice (Fig. 1A), and then in βAR(−/−), C57BL6-CB1(−/−), and CB2(−/−) mice to determine what effects genetic disruption of these receptors has on the diurnal regulation of IOP in mice (Fig. 1, B–D).
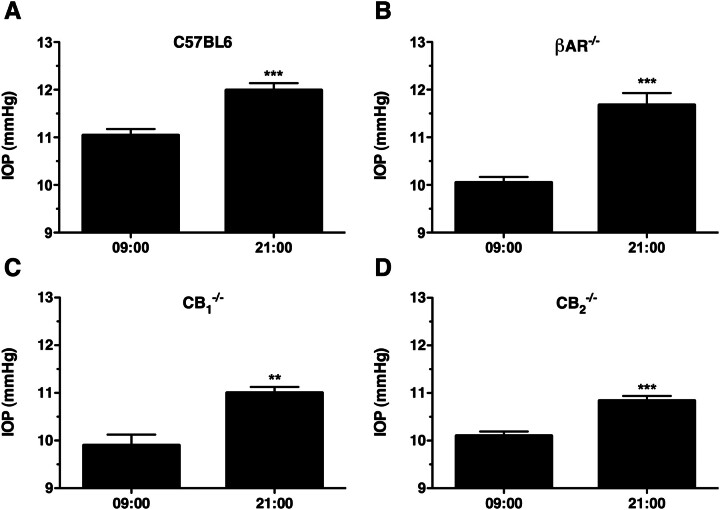
Fig. 1.
Diurnal variation in the IOP of C57BL6, βAR(−/−), CB1(−/−), and CB2(−/−) mice. A, mean IOP data recorded from wild-type male C57BL6 mice at 9:00 AM (left) and 9:00 PM (right) of the same day. ***, p < 0.001; n = 9. B, mean IOP data recorded from male βAR(−/−) mice at 9:00 AM (left) and 9:00 PM (right) of the same day. ***, p < 0.001; n = 8. C, mean IOP measured from C57BL6-CB1(−/−) mice at 9:00 AM (left) and 9:00 PM (right) of the same day. **, p < 0.01; n = 6. D, IOP measured from CB2(−/−) mice at 9:00 AM (left) and 9:00 PM (right) of the same day. ***, p < 0.001; n = 8.
In wild-type mice, the expected diurnal variation in IOP was observed with significantly higher IOP (0.95 ± 0.20 mm Hg) recorded at 9:00 PM compared with that recorded at 9:00 AM (Fig. 1A; p < 0.001). Likewise, significantly higher IOPs were measured from βAR(−/−) mice at 9:00 PM compared with 9:00 AM (Fig. 1B; 1.62 ± 0.28 mm Hg higher; p < 0.001), C57BL6-CB1(−/−) mice (Fig. 1C; 1.10 ± 0.26 mm Hg higher at 9:00 PM; p < 0.001), and CB2(−/−) mice (Fig. 1D; 0.74 ± 0.14 mm Hg higher at 9:00 PM; p < 0.001). These findings demonstrate our ability to measure changes in IOP in each of the strains of mice tested. The diurnal regulation of IOP remains intact in mice genetically lacking the βARs, the CB1 receptor, or the CB2 receptor. It is noteworthy that there did seem to be significant differences in the basal IOPs (measured at 9:00 AM) across the four different mouse genotypes, specifically with each of the βAR(−/−) (10.0 ± 0.12 mm Hg), C57BL6-CB1(−/−) (10.1 ± 0.21 mm Hg), and CB2(−/−) (10.1 ± 0.09 mm Hg) mice having significantly lower (p < 0.001) IOP at 9:00 AM compared with the wild-type mice (11.0 ± 0.13 mm Hg).
βAR Antagonists and Agonists Reduce IOP in C57BL6 Mice.
Before examining interactions between the cannabinoid and adrenergic systems in their regulation of IOP, the ability of various βAR ligands to lower IOP was first examined. The nonselective βAR antagonist timolol, a βAR antagonist commonly used clinically to reduce IOP, was examined (Fig. 2A). The baseline IOP from the vehicle-treated control eye displayed some variation among animals, with control IOP values ranging from 10.40 to 11.33 mm Hg (mean 10.88 ± 0.31 mm Hg). However, in each animal tested the IOP measured from the timolol-treated eye was less than that of the vehicle-treated eye of the same animal. Based on this, all subsequent statistical analyses of drug treatments were carried out using matched-pairs t tests, comparing the drug-treated eye to the vehicle-treated eye of the same animal.
Fig. 2.
Topical application of βAR antagonists and agonists both reduce IOP in C57BL6 mice. A, IOP data from wild-type C57BL6 mice treated topically with either vehicle or 0.5% timolol. Data are plotted as individual points showing the difference between vehicle and treated eyes of the same animal. ***, p < 0.001 B, mean IOP data recorded from wild-type male C57BL6 mice treated with vehicle, the nonselective βAR antagonist timolol (0.5%; n = 9), or the β1AR-selective antagonist metoprolol (1.0%; n = 5). ***, p < 0.001; *, p < 0.01 compared with the appropriate vehicle control. C, IOP measured from wild-type male C57BL6 mice treated with vehicle or the nonselective βAR agonist ISO (1.0%; n = 5). **, p < 0.01.
To establish the role of specific βARs in the ocular hypotensive effects of βAR antagonists, the IOP-lowering effect was compared between the nonselective antagonist timolol and the β1AR-selective antagonist metoprolol (Fig. 2B). In these experiment, both timolol (0.5%) and metoprolol (1.0%) produced statistically significant reductions in IOP (p < 0.001 and < 0.05, respectively). However, the magnitude of IOP decrease for timolol treatment (1.056 ± 0.18 mm Hg) seemed to be greater than for metoprolol treatment (0.62 ± 024 mm Hg). This greater efficacy of the nonselective antagonist suggests that the IOP-lowering effect of βAR antagonists is mediated in part by both the β1AR and the β2AR. Finally, the effect of the βAR agonist ISO on the IOP of C57BL6 mice was also considered (Fig. 2C). In these experiments, ISO (1.0%) resulted in a significant reduction in IOP of 0.98 ± 0.23 mm Hg (p < 0.01).
Cannabinoid Agonist-Mediated Reduction in IOP Is Additive with the Prostaglandin Analog Latanoprost, But Not with the βAR Antagonist Timolol.
The ability of the nonselective cannabinoid agonists WIN and (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol (CP 55,940; CP) to reduce IOP in C57BL6 mice was determined next (Fig. 3A). Treatment with either cannabinoid agonist resulted in a significant decrease in IOP, with WIN (1.0%) producing a 0.86 ± 0.23 mm Hg (p < 0.01) drop in pressure, whereas CP (1.0%) treatment resulted in a drop in IOP of 1.02 ± 0.21 mm Hg (p < 0.001). To establish the ideal concentration of WIN to use for subsequent experiments, a WIN dose-response experiment was conducted (Fig. 3B). WIN significantly reduced IOP at concentrations of 0.5, 1.0, and 2.0%, and the maximum response was achieved with concentrations equal to or more than 1.0%. Therefore, 1.0% WIN was selected as the optimal concentration to use in all subsequent experiments.
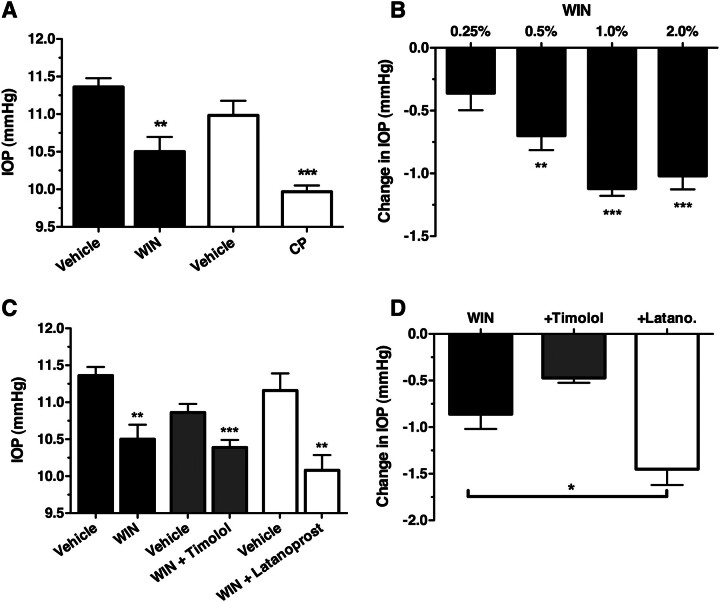
Fig. 3.
Cannabinoid agonists reduce IOP in C57BL6 mice, but are not additive with βAR antagonists. A, mean IOP data recorded from wild-type male C57BL6 mice treated for 1 h with vehicle or WIN (1.0%; n = 6) or with vehicle or CP (1.0%; n = 6). **, p < 0.01; ***, p < 0.001 compared with appropriate vehicle control. B, mean change in IOP between vehicle- and WIN-treated (0.25, 0.5, 1.0, and 2.0%) eyes of the same animal. Statistically significant changes in IOP between vehicle- and WIN-treated eyes are indicated as **, p < 0.01 and ***, p < 0.001, respectively; n = 5. C, mean IOP measured from wild-type male C57BL6 mice treated with vehicle or WIN (1%; solid bars; n = 6) or a combination of WIN (1%) and timolol (0.5%; dark gray bars; n = 6) or latanoprost (0.005%; open bars; n = 5). ***, p < 0.001; **, p < 0.01 compared with the appropriate vehicle control. D, mean change in IOP after treatment with WIN, WIN + timolol (+Timolol), and WIN + latanoprost (+Latano). *, p < 0.05.
It was next tested whether WIN has an additive effect on IOP when given in combination with a βAR antagonist or the prostaglandin analog latanoprost, another agent that is commonly used clinically to reduce IOP (Fig. 3, C and D). Treatment with all drug combinations resulted in significant reductions in IOP: 0.86 ± 0.39 mm Hg for WIN alone (p < 0.01), 0.47 ± 0.13 mm Hg for WIN and timolol (p < 0.001), and 1.45 ± 0.34 mm Hg for WIN and latanoprost (p < 0.01). Statistical analysis by one-way analysis of variance of these results demonstrates that the magnitude of IOP reduction is significantly greater when WIN is given in combination with latanoprost (p < 0.05) compared with WIN treatment alone. In contrast, the combination of WIN and timolol produced an IOP reduction that was not significantly different from WIN treatment alone (p > 0.05). These data demonstrate that cannabinoid agonists reduce IOP and the effect of WIN on IOP is additive with the prostaglandin analog latanoprost but is not additive with the βAR antagonist timolol.
CB1 Colocalizes with a Marker of Adrenergic Neurons in the Anterior Chamber of the Eye.
To examine the possibility that CB1 lowers IOP by inhibiting NE release in the anterior chamber of the eye, thus acting as an indirect adrenergic antagonist, we first examined whether CB1 receptors colocalize with the catecholamine synthetic enzyme TH. We have previously shown that CB1 is prominently expressed throughout the anterior chamber of the human eye (Straiker et al., 1999) including the ciliary epithelium and the angle, sites consistent with a role in regulation of IOP. Therefore, if CB1 is affecting IOP by inhibiting NE release, it should be expected that some level of colocalization will be seen between TH and CB1 in this tissue. To test this hypothesis, we used immunohistochemistry to costain for these two proteins in frozen sections of anterior murine eye. We found that CB1 costained with TH in portions of the ciliary epithelium, the angle, the iris, and the cornea. In the ciliary epithelium TH and CB1 overlap near presumed blood vessels (Fig. 4A, arrows). We also observed double-labeling in presumed neuronal projections in the angle (Fig. 4B, arrow). CB1 also colocalized with TH in cornea sections; costaining was restricted to the corneal periphery (Fig. 4C). Finally, CB1 and TH both prominently stained the murine iris with some overlap (Fig. 4D).
Fig. 4.
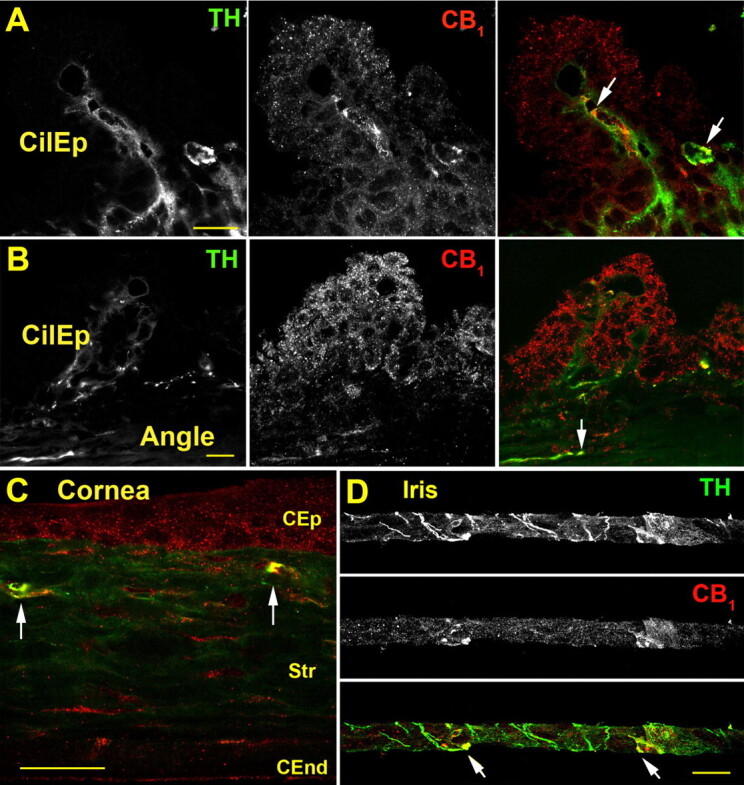
CB1 colocalizes with TH in the murine anterior eye. A, TH (left) and CB1 staining (center) in the ciliary epithelium (CilEp), and TH (green) and CB1 (red), with overlapping staining in yellow (arrows) (right). B, overview of ciliary epithelium (CilEp); including the angle shows TH and CB1 staining in the angle (arrow). C, in the cornea, CB1 is present in corneal epithelium (CEP), stroma (Str), and corneal endothelium (CEnd) and colocalizes with TH in the peripheral distal stroma (arrows). D, CB1 exhibits partial overlap with TH in the iris (arrows). Scale bars, 25 μm.
βAR and Cannabinoid Ligands Do Not Reduce IOP in βAR(−/−) Mice.
The ability of βAR agonists and antagonists to reduce IOP in mice genetically lacking both βARs [βAR(−/−)] was considered next (Fig. 5A). For these experiments, mice lacking both β1AR and β2AR were chosen because our previous data using the nonselective βAR antagonist timolol and the β1AR-selective antagonist metoprolol (Fig. 2B) indicate that both β1ARs and β2ARs are involved in the ocular hypotensive properties of βAR antagonists. Not surprisingly, in the βAR(−/−) mice, neither the βAR antagonists, timolol (0.5%) and metoprolol (1.0%), nor the βAR agonist, ISO (1.0%), significantly decreased IOP (p > 0.05). It is noteworthy that ISO treatment actually produced a statistically significant (p < 0.05) small increase in IOP of 0.42 ± 0.26 mm Hg, suggesting that ISO may also act on other targets in addition to β1AR and β2AR. To verify that pharmacological manipulations of IOP could still be measured in βAR(−/−) mice, the effect of the prostaglandin analog latanoprost on the IOP of these mice was examined (Fig. 5B). In these experiments, as expected, latanoprost was able to produce a significant 1.00 ± 0.45 mm Hg reduction in IOP (p < 0.05), demonstrating that IOP can be manipulated by βAR-independent pathways in βAR(−/−) mice.
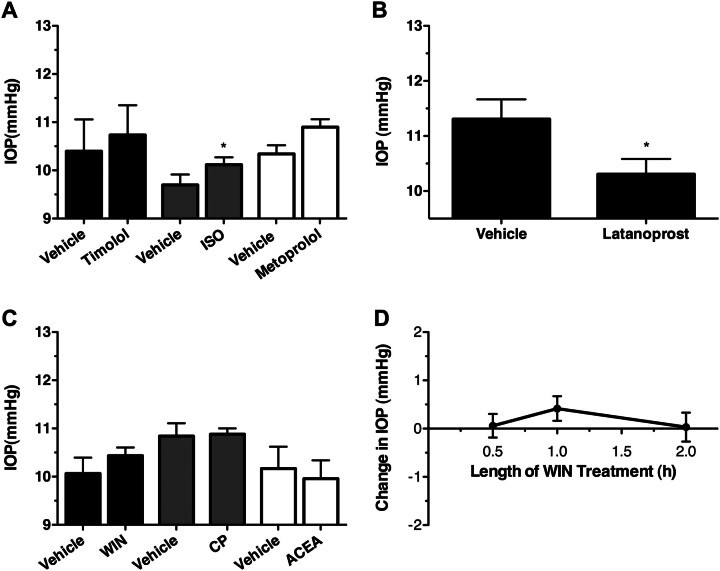
Fig. 5.
βAR antagonists, βAR agonists, and cannabinoid agonists do not reduce the IOP of βAR(−/−) mice. A, mean IOP data recorded from βAR(−/−) mice after the topical administration of vehicle with the βAR antagonists timolol (0.5%; solid bars; n = 5) and metoprolol (1.0%; gray bars; n = 5) or the βAR agonist ISO (1.0%; open bars; n = 5). *, p < 0.05 compared with appropriate vehicle. B, mean IOP data recorded from βAR(−/−) mice after the topical administration of either vehicle or latanoprost. *, p < 0.05; n = 6. C, mean IOP data obtained from βAR(−/−) mice after 1-h topical administration of vehicle, the nonselective cannabinoid agonists WIN (1.0%; n = 13) and CP (1.0%; n = 5), or the CB1-selective agonist ACEA (1%; n = 4). D, time course for change in IOP in βAR(−/−) mice after administration of WIN (1.0%; n = 5).
To establish whether the βARs were also involved in the ocular hypotensive effects of cannabinoid agonists, the ability of various cannabinoids to lower IOP in βAR(−/−) mice was examined (Fig. 5C). In these experiments, neither the nonselective cannabinoid agonists, WIN (1.0%) and CP (1.0%), nor a CB1-selective cannabinoid agonist, ACEA (1.0%), were able to significantly affect IOP in βAR(−/−) mice (p > 0.05). To further confirm that cannabinoids did not significantly affect the IOP of βAR(−/−) mice, additional experiments were carried out with both shorter (30 min) and longer (2 h) WIN treatments (Fig. 5D), none of which resulted in significant changes in the IOP of these animals (p > 0.05). These findings demonstrate that βAR antagonists and agonists, as well as cannabinoid agonists, reduce IOP through mechanisms that depend on the presence of βARs.
Cannabinoid Agonists and βAR Ligands Do Not Reduce IOP in CB1(−/−) Mice That Are on a C57BL6 Genetic Background.
Having examined the effect on IOP of cannabinoid agonists on βAR(−/−) mice, these compounds were then examined in CB1(−/−) and CB2(−/−) mice, each on a C57BL6 genetic background, to establish which cannabinoid receptor was responsible for their IOP-lowering effects. First, the effect of WIN on the IOP of CB1(−/−) mice was compared with its effect on CB1(+/+) littermates (Fig. 6A). In these experiments, WIN significantly decreased IOP in the CB1(+/+) mice (p < 0.05) but was found to increase IOP in the CB1(−/−) mice (p < 0.05). These findings demonstrate that not only is the IOP-lowering effect of WIN mediated by the CB1 receptor, but also that WIN may have an additional non-CB1 site of action that increases IOP.
Fig. 6.
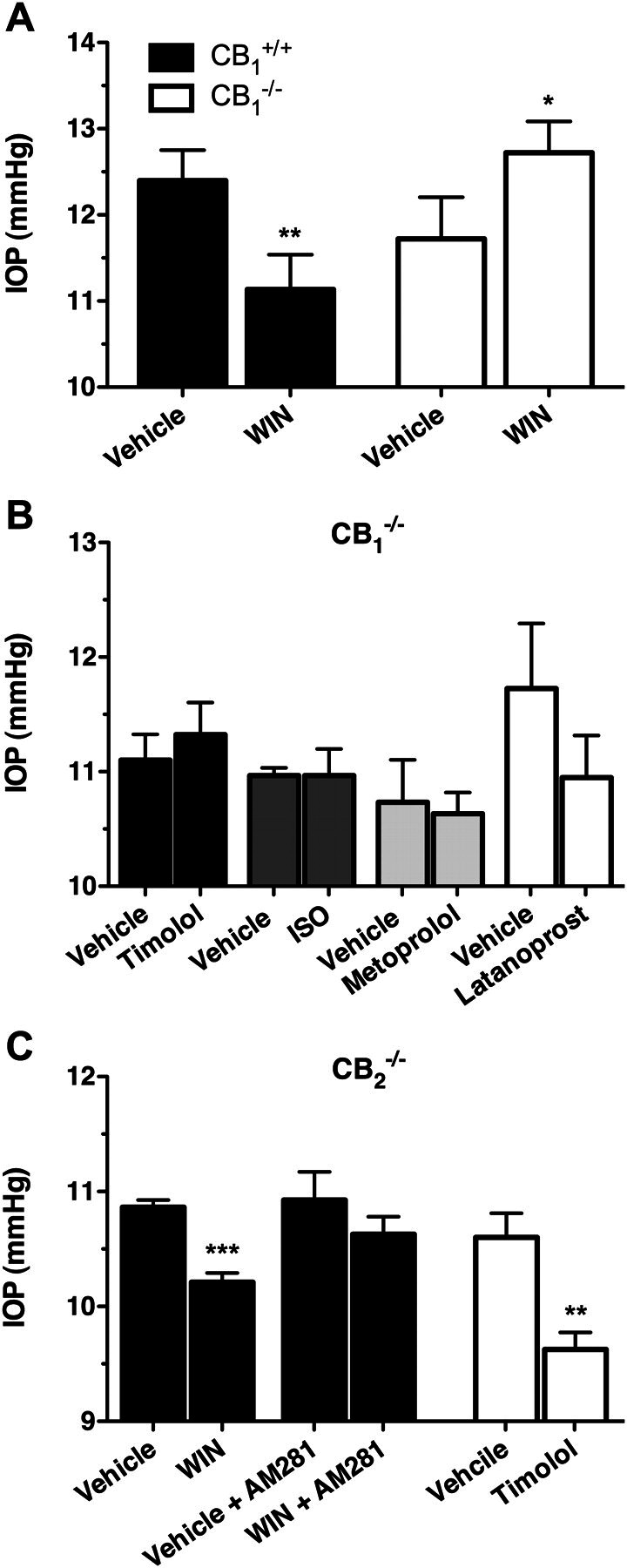
The effect of cannabinoid and βAR ligands on IOP in CB1(−/−) and CB2(−/−) mice on a C57BL6 genetic background. A, mean IOP data recoded from either C57BL6 CB1(−/−) (n = 3) mice or CB1(+/+) (n = 5) littermates after the topical application of either vehicle or WIN (1%). **, p < 0.01; *, p < 0.05 compared with the appropriate vehicle control. B, mean IOP measured in CB1(−/−) mice after treatment with vehicle and timolol (0.5%; n = 4), ISO (1%; n = 3), or latanoprost (0.005%; n = 4). C, IOP measured in CB2(−/−) mice after 1-h topical administration of WIN (1.0%; solid bars) or timolol (0.5%; open bars). ***, p < 0.001; **, p < 0.01 compared with the appropriate vehicle control.
The βAR antagonists, timolol (0.5%) and metoprolol (1.0%), as well as the βAR agonist, ISO (1.0%), were then examined in CB1(−/−) mice (Fig. 6B). It is noteworthy that none of these βAR ligands produced a significant reduction in IOP when administered to CB1(−/−) mice (p < 0.05). Latanoprost was then tested in these mice to verify that pharmacological reductions in IOP could still be measured in CB1(−/−) mice. Latanoprost still reduced IOP in CB1(−/−) mice, producing a drop in IOP of 0.78 ± 0.68 mm Hg, although it did not quite achieve statistical significance (p = 0.058).
To verify that the IOP-lowering effect of cannabinoid agonists are mediated by CB1 receptors, the IOP-lowering effect of WIN was also evaluated in CB2(−/−) mice (Fig. 6C). As expected, WIN significantly reduced IOP in the CB2(−/−) mice (0.65 ± 0.10 mm Hg reduction; p < 0.001), an effect that was blocked by pretreatment with the CB1 antagonist AM281. Timolol was also effective at reducing IOP in the CB2(−/−) mice, producing a 0.98 ± 0.25 mm Hg reduction (p < 0.01). Together, these findings demonstrate that CB2 does not seem to play a role in the ocular hypotensive properties of either cannabinoid or βAR ligands.
The Effect of Cannabinoid Agonists and βAR Antagonists on CB1(−/−) Mice on a CD1 Genetic Background.
We observed several unexpected findings in the C57BL6-CB1(−/−) mice, specifically that WIN increased IOP in these mice, and timolol had no effect on IOP. Therefore, to rule out that these observations may be mouse strain-dependent, we next tested these compounds in independently generated CB1(−/−) mice on a CD1 genetic background (Ledent et al., 1999). For this, we first demonstrated that timolol (0.5%), WIN (1.0%), and CP (1.0%) all significantly decreased IOP in wild-type CD1 mice (p < 0.001; Fig. 7A), producing decreases of 0.82 ± 0.20, 0.80 ± 0.30, and 0.96 ± 0.20, respectively. In contrast, as was observed in the C57BL6-CB1(−/−) mice, neither timolol nor WIN reduced IOP in the CD1-CB1(−/−) mice, whereas WIN again produced a small increase in IOP (p < 0.05; Fig. 7B). We next demonstrated that IOP could be reduced in these mice by using an unrelated ocular hypotensive agent, dorzolamide (Fig. 7B). This agent was chosen as the positive control in the CD1 background mice instead of latanoprost, which was used as in the C57BL6 mice, because latanoprost was found to have a lesser effect on IOP in wild-type CD1 mice than it had on wild-type C57BL6 mice (data not shown).
Fig. 7.
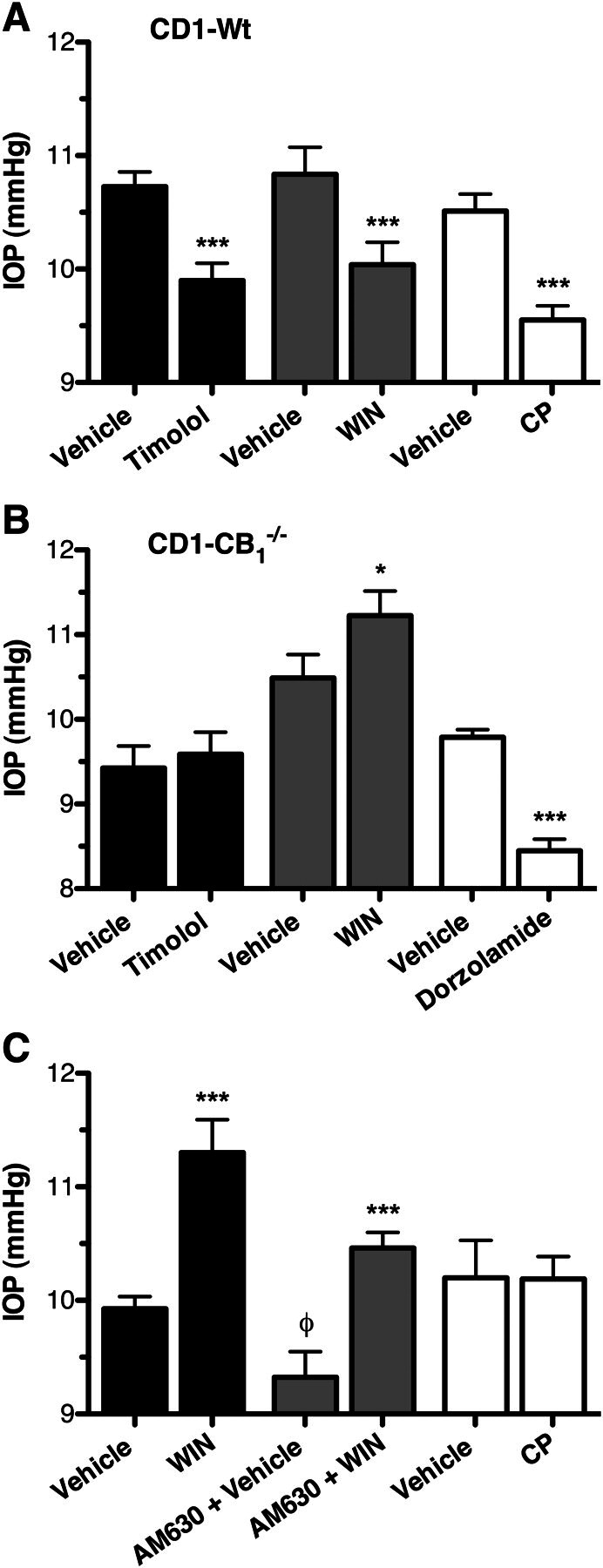
The effect of cannabinoid and βAR ligands on IOP in CB1(−/−) mice on a CD1 genetic background. A, mean IOP measured from male wild-type CD1 mice after topical application of the appropriate vehicle or timolol (0.5%), WIN (1.0%), or CP (1.0%). ***, p < 0.001 compared with appropriate vehicle control. B, mean IOP measured in male CD1-CB1(−/−) mice after treatment with vehicle and timolol (0.5%; n = 8), WIN (1%; n = 8), or dorzolamide (1.0%; n = 8). ***, p < 0.001; *, p < 0.05 compared with the appropriate vehicle control. C, IOP measured from male CD1-CB1(−/−) mice after topical treatment with vehicle, WIN (1.0%), or CP (1.0%) in the absence or presence of pretreatment with the CB2 antagonist AM630. ***, p < 0.001 compared with the appropriate vehicle control. φ, p < 0.05 compared with non-AM630-treated vehicle.
Because WIN is a nonselective CB1/CB2 agonist, to determine whether the WIN-mediated increase in IOP is mediated by CB2 we attempted to block the effect by pretreatment with AM630 (Fig. 7C). Somewhat surprisingly, treatment with AM630 seemed to decrease the basal IOP in these animals (0.74 mm Hg ± 0.29; p < 0.05); however, despite this, AM630 had no statistically significant effect on the WIN-mediated increase in IOP. Specifically, WIN increased IOP by 1.38 ± 0.31 mm Hg in the absence of AM630, compared with increasing IOP by 1.14 ± 0.26 mm Hg in the presence of AM630. Next, to establish whether the effect was specific to WIN, we tested another nonselective cannabinoid agonist, CP, for its effect on the IOP of CD1-CB1(−/−) mice. This compound did not significantly alter the IOP of these mice (p > 0.05), suggesting that the WIN-mediated increase in IOP observed in CB1(−/−) mice may be specific to this particular class of cannabinoid agonist.
Chronic Treatment with the βAR Agonist ISO Desensitizes the IOP Responses to Both Timolol and WIN.
The lack of IOP response to βAR agonists and antagonists in the CB1(−/−) mice may result from a compensatory increase in basal βAR tone caused by the elimination of presynaptic CB1 receptors; this in turn could lead to the desensitization of βARs, thus reducing the ability of adrenergic ligands to affect IOP. As a proof-of-principle experiment to demonstrate that increased adrenergic tone could result in βAR desensitization, wild-type C57BL6 mice were treated chronically with the βAR agonist ISO for 1 week, after which the ability of timolol to reduce IOP in these mice was assessed (Fig. 8A). In this experiment, timolol significantly reduced IOP in both the saline control-treated animals (p < 0.001) and ISO-treated animals (p < 0.001); however, although not significant, the magnitude of the timolol IOP decrease seemed to be somewhat diminished in the ISO-treated animals (0.78 ± 0.13 mm Hg) compared with the saline control-treated animals (1.06 ± 0.17 mm Hg). Based on this, a subsequent experiment was done where ISO treatment was carried out for 2 weeks before the effect of timolol on IOP was determined (Fig. 8B). In this case, after 2 weeks of ISO treatment timolol no longer produced a significant reduction in IOP (p > 0.05), while still reducing IOP in the saline control-treated animals (1.14 ± 0.63 mm Hg reduction; p < 0.05). These results indicate that chronic adrenergic activation by ISO results in desensitization of βARs.
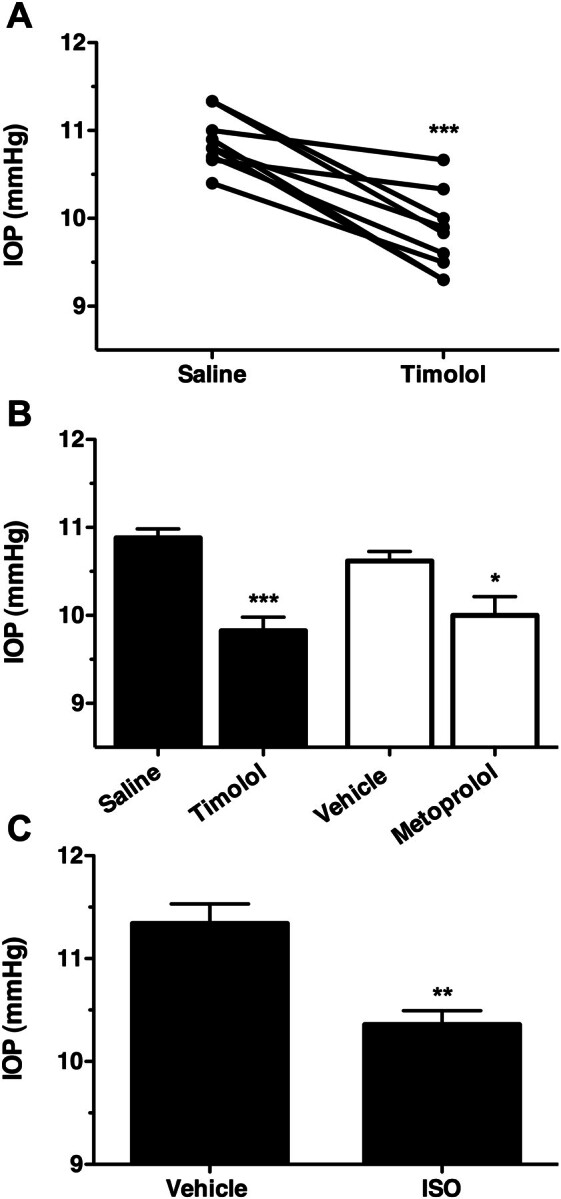
Fig. 8.
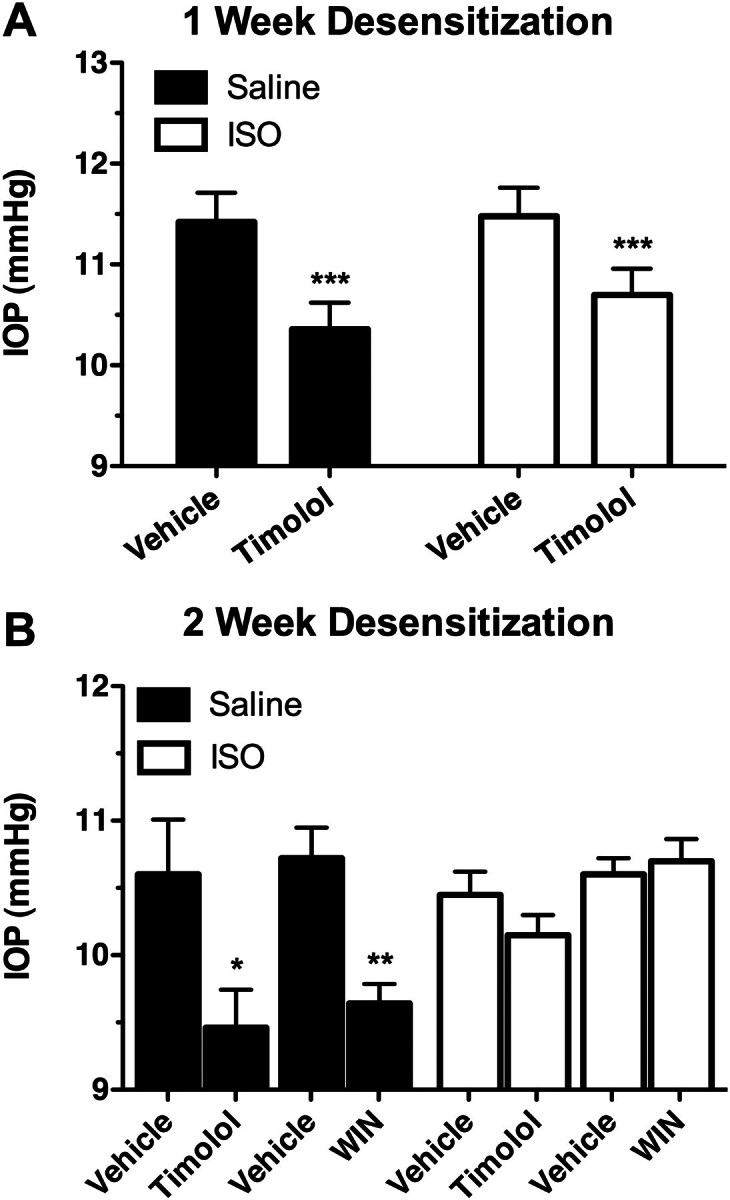
Chronic topical application of the βAR agonist ISO desensitizes the IOP response to timolol in C57BL6 mice. A, mean IOPs measured from wild-type C57BL6 mice treated for 1 week with saline vehicle (solid bars; n = 5) or ISO (2.5%; open bars; n = 5), followed by topical application of either vehicle or timolol (1%). ***, p < 0.001 compared with the appropriate vehicle control. B, mean IOPs measured from wild-type C57BL6 mice treated for 2 weeks with saline vehicle (solid bars; n = 5) or ISO (2.5%; open bars; n = 4), followed by topical application of vehicle, timolol (0.05%), or WIN (1%). **, p < 0.01; *, p < 0.05 compared with the appropriate vehicle control.
The fact that chronic ISO treatment in C57BL6 mice resulted in a desensitization of βARs provided an additional means to demonstrate the dependence of the IOP-lowering effects of the cannabinoid agonist WIN on βARs. To test this, the effect of WIN on C57BL6 mice was examined in mice that were treated for 2 weeks with either saline control or ISO (Fig. 8B). In the saline control mice, WIN produced a significant reduction (1.10 ± 0.49 mm Hg; p < 0.001) in IOP. In contrast, in the ISO-treated mice WIN had no significant effect on the measured IOP (p > 0.05). These observations demonstrate a proof of principle that chronic activation of the βARs leads to the desensitization of these receptors, and after this desensitization, the βAR antagonist, timolol, and the cannabinoid agonist, WIN, are no longer able to reduce IOP.
Depletion of Catecholamines with Reserpine Eliminates the IOP-Lowering Effects of Both Timolol and WIN.
Finally, to directly demonstrate the dependence on catecholamines of the IOP-lowering effects of both timolol and WIN, C57BL6 mice were pretreated with the catecholamine-depleting agent reserpine. For this, it was first established that reserpine treatment did not significantly affect the baseline IOP of these mice (Fig. 9A). Then, because timolol is a βAR antagonist and, therefore, produces its effect by blocking activation of βARs by catecholamines, the effect of timolol treatment was assessed in reserpine- and saline control-treated mice to demonstrate catecholamine depletion (Fig. 9B). Timolol significantly reduced IOP in saline control-treated mice (p < 0.05), but did not significantly affect IOP in the reserpine-treated animals (p > 0.05). Likewise, WIN treatment was found to reduce IOP in the saline control-treated animals (p < 0.01), but did not reduce IOP in reserpine-treated animals (Fig. 9C; p > 0.05). These findings demonstrate that catecholamines are required for the IOP-lowering effects of both the βAR antagonist, timolol, as well as the cannabinoid agonist, WIN.
Fig. 9.
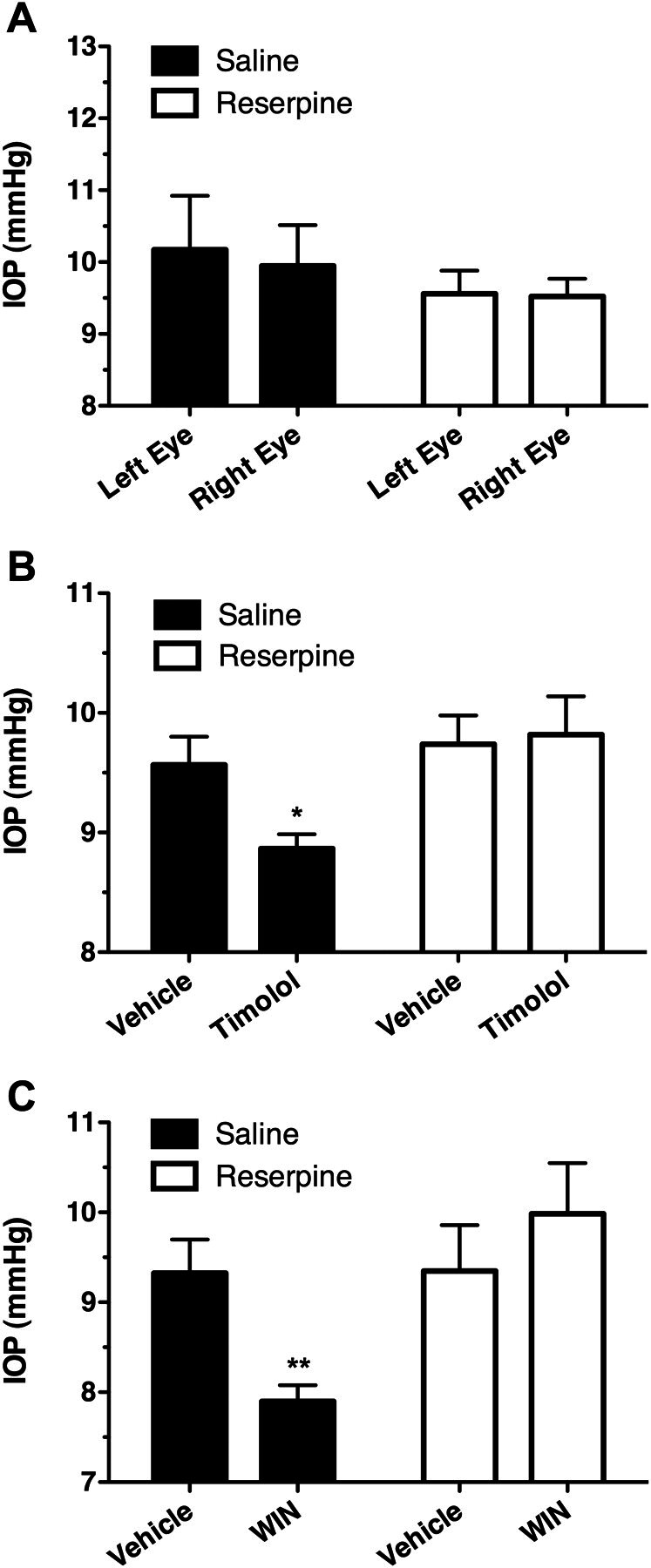
Pretreatment with reserpine to deplete catecholamines eliminates the ability of either timolol and WIN to reduce IOP. A, mean IOP recorded from each eye in C57BL6 mice pretreated with saline vehicle or reserpine to deplete catecholamines. B, mean IOP data for either vehicle or timolol treatment in mice pretreated with either saline (solid bars; n = 3), or reserpine (open bars; n = 5) to deplete catecholamines. *, p < 0.05 compared with the appropriate vehicle control. C, mean IOP data measured after topical application of either vehicle or WIN from mice pretreated with either saline (solid bars; n = 4) or reserpine (open bars; n = 6) to deplete catecholamines. **, p < 0.01 compared with the appropriate vehicle control.
Discussion
Cannabinoid agonists have long been known to lower IOP (Hepler and Frank, 1971; Merritt et al., 1980). Although the mechanism underlying this ocular hypotensive effect has remained unclear, several previous studies have suggested some involvement of the adrenergic nervous system (Green and Kim, 1976; Green et al., 1977; Oltmanns et al., 2008). The present study, using genetic receptor knockout models and pharmacology, demonstrates that cannabinoids reduce IOP primarily by acting as indirect sympatholytic agents. This mechanism involves CB1 receptors and depends on the presence of both catecholamines and βARs. Together with evidence that CB1 receptors colocalized with adrenergic inputs in the anterior eye, our findings indicate that cannabinoids produce their primary ocular hypotensive effect in the mouse eye by inhibiting NE release.
Intraocular pressure is maintained in the eye through the balance of AH secretion from the ciliary body and outflow through either the trabecular meshwork or the uveoscleral outflow pathways. Because both CB1 receptors and βARs are expressed in the tissues involved in each of these processes (Watanabe and Chiou, 1983; Alvarado et al., 1998; Straiker et al., 1999), the overall effects on IOP of drugs acting on these receptors are probably the result of the combination of their effects on each of these tissues. For example, both βAR antagonists and agonists can reduce IOP, and βAR antagonists decrease AH inflow from the ciliary body (Watanabe and Chiou, 1983), whereas βAR agonists increase AH outflow (Alvarado et al., 1998). Given that CB1 receptors are expressed in the tissues of both inflow and outflow pathways, it is also likely that these compounds, acting either indirectly or directly, have more than one site of action affecting IOP (Chien et al., 2003; Stumpff et al., 2005; McIntosh et al., 2007).
The results of this study are consistent with previous work demonstrating that when cannabinoid agonists and βAR antagonists are given in combination they do not produce an additive effect (Green et al., 1977; Oltmanns et al., 2008). This observation is noteworthy in that IOP-lowering agents acting through independent mechanisms are typically found to have additive effects in both rodents and humans (Akaishi et al., 2009). Whereas previous studies have suggested this lack of additive effect may indicate that the ocular hypotensive actions of cannabinoids involve βARs (Green et al., 1977), the present study provides the first evidence that cannabinoid agonists do not reduce IOP in βAR(−/−) mice, directly demonstrating for the first time the involvement of βARs in the ocular hypotensive actions of cannabinoids.
The most plausible mechanism accounting for this observation is that CB1 receptor agonists lower IOP primarily by activating presynaptic CB1 receptors to inhibit norepinephrine release, as has been shown in other tissues (Ishac et al., 1996), which is consistent with our observation that CB1 colocalizes with adrenergic inputs in the anterior eye. This presynaptic effect should decrease AH secretion from the ciliary epithelium, leading to reduce IOP. However, given that activation of βARs in the trabecular meshwork increases outflow, this same presynaptic effect of cannabinoids could conceivably also increase IOP, by decreasing trabecular outflow. Indeed the net adrenergic-dependent effect of cannabinoids on IOP probably results from the balance of these two actions. Considering the fact that there is substantially more adrenergic innervation to the subepithelial layer of ciliary body than there is to the trabecular meshwork (Akagi et al., 1976), it is not surprising that the IOP-lowering effect on AH secretion predominates. Indeed, such a mechanism is consistent with previous work demonstrating that, at least in primates, cannabinoids reduce IOP through actions on AH secretion (Chien et al., 2003). However, it should be noted that although CB1 was found to colocalize with adrenergic inputs in the anterior eye, the bulk of CB1 staining did not overlap with the inputs, suggesting that CB1 may have additional postsynaptic (or nonsynaptic) functions. Recent work has demonstrated that CB1 receptors heterodimerize with β2AR receptors (Hudson et al., 2010), and because both of these receptors are expressed within the ciliary body epithelium (Straiker et al., 1999; Stamer et al., 2001; Crider and Sharif, 2002), the possibility that CB1/β2AR heterodimerization within the ciliary epithelial cells contributes to the ocular hypotensive properties of cannabinoids cannot be completely ruled out.
In addition to a possible action at the ciliary epithelium on AH inflow, CB1/β2AR heterodimerization could affect AH outflow. Previous work has suggested cannabinoids may increase trabecular outflow (Stumpff et al., 2005), and CB1/β2AR heterodimerization modulates the function of β2ARs within trabecular meshwork cells (Hudson et al., 2010). Therefore this heterodimer, at least theoretically, could be one mechanism that explains the fact that cannabinoid and βAR agonists did not reduce IOP in CB1(−/−) and βAR(−/−) mice. However, the pharmacological properties of the CB1/β2AR heterodimer are such that this interaction seems to have more of a modulatory effect on CB1 and β2AR signaling (Hudson et al., 2010) and thus is not likely to account for the complete loss of ocular hypotensive function observed in the knockout mice used in the present study.
Our findings in both C57BL6 and CD1 genetic background CB1(−/−) mice confirm the involvement of CB1 receptors in the ocular hypotensive actions of cannabinoids. However, the fact that WIN not only failed to reduce the IOP of CB1(−/−) mice, but instead actually produced a small increase in IOP was surprising. Pharmacological evidence in rodents has reported that WIN mediates its IOP effects through the CB1 receptor (Pate et al., 1998; Song and Slowey, 2000; Oltmanns et al., 2008); however, this has never been directly tested in mice genetically lacking this receptor. Considering our findings, it seems that there is at least one additional, non-CB1 ocular target for WIN. Because the WIN-mediated IOP increase was not blocked by the CB2 antagonist AM630, nor mimicked by a chemically distinct cannabinoid agonist, CP55,940, the effect is probably specific to WIN or potentially to the aminoalkylindole class of cannabinoid agonists to which WIN belongs. The chemical structures of aminoalkylindoles are quite distinct from all other CB1 agonist classes (Howlett et al., 2002), so it is perhaps not surprising that this class would have actions at additional non-CB1/CB2 targets.
More difficult to account for, however, is the lack of IOP response to either timolol or ISO in the CB1(−/−) mice. The fact that timolol and ISO reduce IOP through completely independent mechanisms of action (Watanabe and Chiou, 1983; Alvarado et al., 1998) suggests that the only commonality shared by these ligands is that they both produce their effects through actions on the βARs. Based on this, the lack of effect for both timolol and ISO in CB1(−/−) mice could probably be explained by a compensatory desensitization of βARs that occurs in the CB1(−/−) mice. Such a desensitization would not be unexpected, because the elimination of inhibitory presynaptic CB1 receptors would increase basal noradrenergic tone, an effect seen in other tissues of CB1(−/−) mice (Schlicker et al., 2003), which in turn could produce desensitization of postsynaptic βARs. This hypothesis may also be supported by our observation that basal IOP at 9:00 AM was decreased in both CB1(−/−) and β2AR(−/−) mice compared with wild-type mice. Indeed, one might predict that desensitization or genetic elimination of βARs in the ciliary body could lead to decreased basal levels of AH secretion and as a result lower IOP. As a proof of principle, our demonstration that chronic ISO treatment does indeed desensitize the timolol IOP response, indicates that even after only 2 weeks of receptor overactivation desensitization of βARs can be observed in mice.
The overall findings of this study demonstrate that cannabinoid agonists reduce IOP through activation of the CB1 receptor, which in turn affects IOP through a mechanism that depends on the presence of both catecholamines and βARs. Considering this mechanism of action, it may be expected that cannabinoid agonists acting on this pathway are unlikely to produce better IOP-lowering agents than currently available βAR antagonists. Given this, the results presented in this study indicate CB1-targeting cannabinoid agonists may not to be useful therapeutics when used for their ocular hypotensive abilities alone. In addition, the results of the present study suggest that the aminoalkylindole class of cannabinoid agonists in particular are the least likely to be useful therapeutics, because they seem to produce additional non-CB1/CB2 effects on AH dynamics, which are likely to reduce their overall ability to lower IOP. Despite these limitations, cannabinoid compounds may still be of interest in the treatment of glaucoma, based on their neuroprotective properties (Järvinen et al., 2002; Nucci et al., 2008). Evidence has also suggested that cannabinoid compounds that act at receptor targets independent of CB1 and CB2 may also reduce IOP (Colasanti et al., 1984; Szczensiak et al., 2011) and therefore may represent alternative clinically useful drugs in the treatment of glaucoma. Based on the findings of the present study, we suggest that it is these non-CB1 cannabinoid targets, as well as the neuroprotective actions of cannabinoids, that are likely to be the most successful in producing therapeutically useful cannabinoids for the treatment of glaucoma.
Acknowledgments
We thank Suresh Viswanathan for assistance with this study.
This work was supported by studentships from the Killam Trusts and Natural Sciences and Engineering Research Council of Canada (to B.D.H.); and a Canadian Institutes of Health operating grant (to M.E.M.K.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.185769.
- IOP
- intraocular pressure
- CB
- cannabinoid
- AH
- aqueous humor
- AR
- adrenoceptor
- NE
- norepinephrine
- TH
- tyrosine hydroxylase
- ISO
- isoprenaline [1-(3′,4′-dihydroxyphenyl)-2-isopropylaminoethanol hydrochloride, N-isopropyl-dl-noradrenaline]
- WIN
- WIN 55,212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate]
- CP
- CP 55,940 [(1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol]
- AM281
- 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- AM630
- 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl(4-methoxyphenyl)methanone
- ACEA
- N-(2-chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide.
Authorship Contributions
Participated in research design: Hudson and Kelly.
Conducted experiments: Hudson, Beazley, Szczesniak, and Straiker.
Performed data analysis: Hudson, Beazley, and Straiker.
Wrote or contributed to the writing of the manuscript: Hudson, Straiker, and Kelly.
References
- Akagi Y, Ibata Y, Sano Y. (1976) The sympathetic innervation of the ciliary body and trabecular meshwork of the cat. Fluorescence histochemistry and electron microscopy. Cell Tissue Res 173:261–269. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. (2009) Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther 25:401–408. [DOI] [PubMed] [Google Scholar]
- Alm A, Nilsson SF. (2009) Uveoscleral outflow–a review. Exp Eye Res 88:760–768. [DOI] [PubMed] [Google Scholar]
- Alvarado JA, Murphy CG, Franse-Carman L, Chen J, Underwood JL. (1998) Effect of β-adrenergic agonists on paracellular width and fluid flow across outflow pathway cells. Invest Ophthalmol Vis Sci 39:1813–1822. [PubMed] [Google Scholar]
- Cervino A. (2006) Rebound tonometry: new opportunities and limitations of non-invasive determination of intraocular pressure. Br J Ophthalmol 90:1444–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien FY, Wang RF, Mittag TW, Podos SM. (2003) Effect of WIN 55212-2, a cannabinoid receptor agonist, on aqueous humor dynamics in monkeys. Arch Ophthalmol 121:87–90. [DOI] [PubMed] [Google Scholar]
- Civan MM, Macknight AD. (2004) The ins and outs of aqueous humour secretion. Exp Eye Res 78:625–631. [DOI] [PubMed] [Google Scholar]
- Colasanti BK, Craig CR, Allara RD. (1984) Intraocular pressure, ocular toxicity and neurotoxicity after administration of cannabinol or cannabigerol. Exp Eye Res 39:251–259. [DOI] [PubMed] [Google Scholar]
- Colasanti BK, Powell SR. (1985) Effect of Δ9-tetrahydrocannabinol on intraocular pressure after removal of autonomic input. J Ocul Pharmacol 1:47–57. [DOI] [PubMed] [Google Scholar]
- Crider JY, Sharif NA. (2002) Adenylyl cyclase activity mediated by β-adrenoceptors in immortalized human trabecular meshwork and non-pigmented ciliary epithelial cells. J Ocul Pharmacol Ther 18:221–230. [DOI] [PubMed] [Google Scholar]
- Ferrer E. (2006) Trabecular meshwork as a new target for the treatment of glaucoma. Drug News Perspect 19:151–158. [DOI] [PubMed] [Google Scholar]
- Green K. (1979) The ocular effects of cannabinoids. Curr Top Eye Res 1:175–215. [PubMed] [Google Scholar]
- Green K, Bigger JF, Kim K, Bowman K. (1977) Cannabinoid action on the eye as mediated through the central nervous system and local adrenergic activity. Exp Eye Res 24:189–196. [DOI] [PubMed] [Google Scholar]
- Green K, Kim K. (1976) Mediation of ocular tetrahydrocannabinol effects by adrenergic nervous system. Exp Eye Res 23:443–448. [DOI] [PubMed] [Google Scholar]
- Hepler RS, Frank IR. (1971) Marihuana smoking and intraocular pressure. JAMA 217:1392. [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202. [DOI] [PubMed] [Google Scholar]
- Hu SS, Arnold A, Hutchens JM, Radicke J, Cravatt BF, Wager-Miller J, Mackie K, Straiker A. (2010) Architecture of cannabinoid signaling in mouse retina. J Comp Neurol 518:3848–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BD, Hébert TE, Kelly ME. (2010) Physical and functional interaction between CB1 cannabinoid receptors and β2-adrenoceptors. Br J Pharmacol 160:627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC. [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. (1996) Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118:2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen T, Pate DW, Laine K. (2002) Cannabinoids in the treatment of glaucoma. Pharmacol Ther 95:203–220. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, et al. (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401–404. [DOI] [PubMed] [Google Scholar]
- Liu JH, Dacus AC. (1987) Central nervous system and peripheral mechanisms in ocular hypotensive effect of cannabinoids. Arch Ophthalmol 105:245–248. [DOI] [PubMed] [Google Scholar]
- McIntosh BT, Hudson B, Yegorova S, Jollimore CA, Kelly ME. (2007) Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br J Pharmacol 152:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. (1980) Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 87:222–228. [DOI] [PubMed] [Google Scholar]
- Nucci C, Bari M, Spanò A, Corasaniti M, Bagetta G, Maccarrone M, Morrone LA. (2008) Potential roles of (endo)cannabinoids in the treatment of glaucoma: from intraocular pressure control to neuroprotection. Prog Brain Res 173:451–464. [DOI] [PubMed] [Google Scholar]
- Olfe J, Domanska G, Schuett C, Kiank C. (2010) Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmanns MH, Samudre SS, Castillo IG, Hosseini A, Lichtman AH, Allen RC, Lattanzio FA, Williams PB. (2008) Topical WIN55212-2 alleviates intraocular hypertension in rats through a CB1 receptor mediated mechanism of action. J Ocul Pharmacol Ther 24:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate DW, Järvinen K, Urtti A, Mahadevan V, Järvinen T. (1998) Effect of the CB1 receptor antagonist, SR141716A, on cannabinoid-induced ocular hypotension in normotensive rabbits. Life Sci 63:2181–2188. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Giuffrida A, Calignano A, Rodríguez de Fonseca F. (2000) The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci 21:218–224. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Sugiyama F, Martin JE, Tomarev SI, Paigen BJ, Smith RS, John SW. (2001) Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Redmer A, Werner A, Kathmann M. (2003) Lack of CB1 receptors increases noradrenaline release in vas deferens without affecting atrial noradrenaline release or cortical acetylcholine release. Br J Pharmacol 140:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZH, Slowey CA. (2000) Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J Pharmacol Exp Ther 292:136–139. [PubMed] [Google Scholar]
- Stamer WD, Golightly SF, Hosohata Y, Ryan EP, Porter AC, Varga E, Noecker RJ, Felder CC, Yamamura HI. (2001) Cannabinoid CB1 receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol 431:277–286. [DOI] [PubMed] [Google Scholar]
- Straiker AJ, Maguire G, Mackie K, Lindsey J. (1999) Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 40:2442–2448. [PubMed] [Google Scholar]
- Stumpff F, Boxberger M, Krauss A, Rosenthal R, Meissner S, Choritz L, Wiederholt M, Thieme H. (2005) Stimulation of cannabinoid (CB1) and prostanoid (EP2) receptors opens BKCa channels and relaxes ocular trabecular meshwork. Exp Eye Res 80:697–708. [DOI] [PubMed] [Google Scholar]
- Szczesniak AM, Kelly ME, Whynot S, Shek PN, Hung O. (2006) Ocular hypotensive effects of an intratracheally delivered liposomal Δ9-tetrahydrocannabinol preparation in rats. J Ocul Pharmacol Ther 22:160–167. [DOI] [PubMed] [Google Scholar]
- Szczensiak AM, Maor Y, Hung O, Robertson H, Kelly MEM. (2011) Nonpsychotropic cannabinoids, abnormal cannabidiol and canabigerol-dimethyl heptyl, act at novel cannabinoid receptors to reduce intraocular pressure. J Ocul Pharmacol Ther 27:427–435. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Chiou GC. (1983) Action mechanism of timolol to lower the intraocular pressure in rabbits. Ophthalmic Res 15:160–167. [DOI] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Ciobanu DC, Williams RW, Reese BE. (2009) Multiple genes on chromosome 7 regulate dopaminergic amacrine cell number in the mouse retina. Invest Ophthalmol Vis Sci 50:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Gil DW. (2004) The inflow and outflow of anti-glaucoma drugs. Trends Pharmacol Sci 25:238–241. [DOI] [PubMed] [Google Scholar]