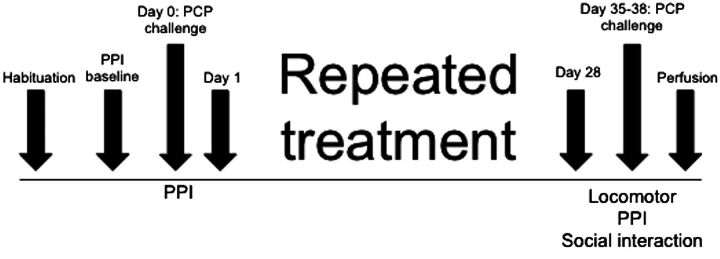Abstract
Phencyclidine (PCP), a noncompetitive N-methyl d-aspartate (NMDA) receptor antagonist, provides the most complete pharmacologic model of schizophrenia in humans and animals. Acute PCP causes hyperlocomotion, disrupts prepulse inhibition (PPI), and increases social avoidance in rats. We have previously shown that repeated treatment with the dopamine (DA) D2-like receptor agonists, quinpirole or ropinirole, prevents agonist-induced PPI disruption. In the present study, we examined whether repeated ropinirole treatment similarly attenuates the effects of PCP in a more complete model of schizophrenia symptoms and examined the effect of repeated D2-like agonist treatment on locomotion, PPI, and social interaction after acute PCP challenge. The acute effect of PCP (3.0 or 6.0 mg/kg) on locomotor activity was examined to establish a minimum effective dose. Thereafter, the effect of PCP challenge (3.0 mg/kg) on locomotor activity, PPI, and social interaction was assessed in adult male rats before or 7–10 days after termination of repeated daily treatment with ropinirole (0.1 mg/kg) or saline vehicle (0.1 ml/kg) for 28 days. Repeated ropinirole treatment attenuates PCP-induced hyperlocomotion, PPI deficits, and social avoidance. These findings suggest that repeated ropinirole treatment might affect a final common pathway that is vulnerable to both PCP- and dopamine agonist–induced behavioral disruption, thereby providing an alternative approach to block the effects of PCP.
Introduction
Phencyclidine (PCP), a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, is widely used in experimental animal models to study the underlying neurobiology of schizophrenia. PCP was first identified as a possible pharmacologic model in rodents after it was noted that the drug exacerbated symptoms in patients with schizophrenia (Itil et al., 1967). Compared with other pharmacologic animal models of schizophrenia, PCP is considered more complete because of its ability to induce positive, negative, and cognitive symptoms (Angrist and Gershon, 1970; Goldmann et al., 1999). In rodents, acute PCP treatment reduces cortical functioning and impairs behavioral tasks associated with schizophrenia symptoms, such as social behavior and prepulse inhibition (PPI) of the acoustic startle response (Rosenbaum et al., 1959; Hoehn-Saric et al., 1991; Aguado et al., 1994; Pallares et al., 1995; Sams-Dodd, 1996). PCP also increases locomotion in a dose-dependent manner (Sams-Dodd, 1995), which has been used as an indicator of its ability to induce or exacerbate psychotic symptoms (Ogren and Goldstein, 1994; Steinpreis, 1996).
Acute administration of dopamine D2-like receptor agonists is also used to model symptoms of schizophrenia in rodents. Specifically, PPI deficits are observed after acute infusion of D2-like agonists either systemically (Chen et al., 1991) or directly into the nucleus accumbens (NAc) (Wan and Swerdlow, 1993). In contrast to acute treatment, repeated treatment with the indirect dopamine agonist cocaine (Collins et al., 2000) or the direct dopamine agonists quinpirole or ropinirole (Culm and Hammer, 2004) alleviates prior PPI deficits. Such behavioral tolerance, which we have termed PPI recovery, is observable immediately after repeated ropinirole treatment and even 28 days later in the absence of further ropinirole treatment (Berger et al., 2011). This effect of repeated D2-like agonist treatment on PPI, however, has never been assessed after an acute PCP challenge.
In the present study, we examined the hypothesis that repeated ropinirole treatment would block PCP-induced PPI deficits representing a cognitive symptom associated with schizophrenia. We also investigated the effect of repeated ropinirole on PCP-induced social avoidance to establish whether repeated ropinirole treatment could affect a behavior representing a negative symptom of schizophrenia. Finally, we measured PCP-induced hyperlocomotion after repeated ropinirole, which provides additional evidence of repeated ropinirole effects on PCP-induced behavior.
Materials and Methods
Subjects
Adult male naive Sprague-Dawley rats (approximately 250 g at the start of the experiment, Harlan Laboratories, Indianapolis, IN) were triple-housed for the duration of the experiment and provided with ad libitum food and water. Rats were housed with the same cage mates in their home cage (L, W, H: 19 × 10.25 × 8 inches) throughout the experiment regardless of group assignment. Behavioral testing occurred from 1000 to 1400 h during the dark phase of a 12-hour reversed light/dark cycle (lights off at 0900 h). All studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and were approved by the institution’s Animal Care and Use Committee.
Behavioral Testing and Drug Treatment
Experiment 1: Acute PCP Dose-Response Locomotor Testing.
Each rat was placed in a locomotor testing cage, which was the same size as the home cage and contained clean bedding, and was injected with saline (1.0 ml/kg, i.p.) before a 30-minute baseline locomotion period. Locomotor activity was assessed after saline and subsequent drug exposure using VideoTrack (Viewpoint Life Sciences, Montreal, QC). After baseline testing, acute PCP (0, 3.0, or 6.0 mg/kg, i.p.) was administered, and the software was characterized and recorded total movements for each animal within 10-minute time intervals for 60 minutes. For each time interval, total distance traveled measured in centimeters was recorded.
Experiment 2: Locomotor Testing after Repeated Ropinirole Treatment and PCP Challenge.
Rats were injected for 28 days with repeated ropinirole HCl (0.1 mg/kg, s.c.) or saline vehicle (1.0 ml/kg, s.c.; Sigma-Aldrich, St. Louis, MO) (Fig. 1). Seven to 10 days after termination of repeated treatment, rats were injected with saline (i.p.), and locomotor activity was recorded in a locomotor testing cage for 30 minutes to establish their baseline activity. Rats then received PCP (3.0 mg/kg, i.p.) or saline vehicle (1.0 ml/kg, i.p.), and were immediately returned to the testing cage for an additional 60 minutes of testing. PCP was graciously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD).
Fig. 1.
Timeline and design of chronic treatment experiments. In each experiment, rats received 28 days of repeated daily ropinirole (0.0 or 0.1 mg/kg, s.c.) treatment followed by an acute PCP (3.0 mg/kg, i.p.) challenge 7–10 days after termination of treatment.
Experiment 3: Prepulse Inhibition Testing.
Startle amplitude was measured using the Startle Monitor behavioral testing system (Kinder Scientific, Poway, CA). After 2 days of saline injections (1.0 ml/kg, i.p.) followed by 5 minutes of acclimation to the PPI chamber each day, baseline PPI was determined by averaging the results of testing on both days. PPI testing included the rat being placed into a PPI chamber and exposed to a 70-dB ambient sound for 5 minutes, followed by a PPI baseline or test session. A PPI baseline session consisted of four consecutive pulse trials (120-dB, 40-ms pulses), a randomized presentation of 16 pulses, 15 prepulses (10 each of 73, 76, and 82-dB, 20-ms prepulses, followed 100 ms later by a pulse), and eight trials without stimulation, ending with four pulse trials. Based on the average of their PPI baseline tests, the rats were ranked from highest to lowest; then each of four rats within clusters having the same average PPI response were randomly assigned to one of the four treatment groups. Thus, each treatment group contained subjects with the same average PPI response before experimental intervention.
The first PPI test session occurred 1 day after the PPI baseline sessions (Fig. 1), when rats were given an acute PCP (3.0 mg/kg, i.p.) or saline vehicle (1.0 ml/kg, i.p.) challenge, and then they were immediately placed into PPI chambers and exposed to 5 minutes of chamber acclimation (65-dB white noise), followed by four consecutive pulse trials, a randomized presentation of 16 pulses, 30 prepulses (10 each of 73, 76, and 82-dB, 20-ms prepulses, followed 100 ms later by a pulse), and 10 trials without stimulation, ending with four pulse trials. The final PPI test session took place 7–10 days after termination of 28 daily ropinirole HCl (0.1 mg/kg, s.c.) or saline vehicle (1.0 ml/kg, s.c.) treatments, when rats received PCP (3.0 mg/kg, i.p.) or saline vehicle (1.0 ml/kg, i.p.) challenge, and were immediately placed in the PPI chambers for testing, as described already. This dose of ropinirole has been previously demonstrated to reliably induce PPI tolerance on repeated treatment (Culm and Hammer, 2004; Culm et al., 2004; Berger et al., 2011).
Mean startle amplitude was measured over 100 ms after presentation of the pulse stimulus in units of newton (N). Percent PPI was calculated using the following equation: 1 − [(mean prepulse response/mean pulse response) × 100]; a higher percent PPI implies greater inhibition of startle response owing to presentation of the prepulse. Intertrial intervals ranged from 5 to 30 seconds and averaged 15 seconds for both PPI baseline and test sessions.
Experiment 4: Social Interaction.
Rats received 28 days of repeated ropinirole HCl (0.1 mg/kg, s.c.) or saline vehicle (1.0 ml/kg, s.c.) treatment; 7–10 days later, they received a PCP (3.0 mg/kg, i.p.) or saline vehicle (1.0 ml/kg, i.p.) challenge as described already (Fig. 1). Treatment groups were randomly assigned, and two rats taken from different home cages and naive to any prior behavioral testing (i.e., novel conspecific exposure) were placed simultaneously at opposite corners of a clean open arena (3 feet × 3 feet) 10 minutes after either PCP or vehicle treatment. These rats received the same acute challenge; the only criteria were that they were not cage mates. One rat of the pair was identified by a black stripe placed on the back with a permanent marker, and social activity for each rat was recorded individually. Interaction between rats was recorded for 5 minutes using Top Scan (Clever Sys, Reston, VA) under red light using a camera (Panasonic WV-CP284 Camera-540 TVL-Day/Night (Uemura et al., 2015), which detected the location of each subject within either a center interaction zone (2 feet × 2 feet) or the remaining peripheral interaction zone. Social contact was detected when subjects were within 2 cm of each other. Additionally, the velocity of approach before contact determined active or passive contact. For example, if one rat was stationary and the other rat moved toward it, the activity of the animal in motion was labeled active contact; the stationary animal’s activity was labeled passive contact. Identification of contacts between rats was defined by the velocity of the animal’s approach; Active contacts required greater than 80 mm/s per 15 frames approach speed, whereas passive contacts were less than 20 mm/s per 15 frames.
Statistical Analysis
Changes in locomotor activity between trials were analyzed using two-way repeated-measures analyses of variance (ANOVA) followed by Tukey’s post hoc test for the cumulative postinjection activity to identify between-group differences at specific time intervals. Percent PPI data were calculated for each prepulse and then combined to determine the mean pulse response on the first and the last day of PCP challenge, analyzed using a repeated-measures ANOVA with drug treatment as the between-subject factor and Tukey’s post hoc test. Startle (pulse only) and no stimulation responses were analyzed on last day of PCP challenge. Differences in social interaction between drug treatments were analyzed using between subjects ANOVA followed by Tukey’s post hoc test. Statistical significance was determined using GraphPadPrism (GraphPad Software Inc., San Diego, CA), and the researcher was blinded to the animal’s prior experimental condition.
Results
Experiment 1: Locomotion after Acute PCP Challenge.
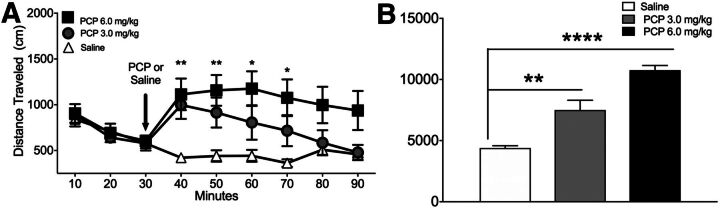
PCP (3.0 or 6.0 mg/kg, i.p.) increased distance traveled (centimeters) in a dose-dependent manner at 50 and 60 minutes after PCP challenge over the observation period, as indicated by a significant interaction between PCP and time (F16,232 = 4.545, P < 0.0001; Fig. 2A). The higher PCP dose produced extended hyperlocomotion beyond the end of the observation period (P < 0.001), and the lower PCP dose increased locomotion for 30 minutes after the challenge injection (P = 0.018) compared with rats that received saline treatment. Distance traveled collapsed over time revealed a significant effect of PCP (F 2,15 = 36.82, P < 0.0001; Fig. 2B) when the higher PCP dose (P = 0.02) and the lower PCP dose (P < 0.0001) were compared with saline treatment. The lower dose of PCP (3.0 mg/kg) was used for all subsequent challenge studies because distance traveled had normalized to baseline levels by the end of the test.
Fig. 2.
(A) Distance traveled over time before and after acute PCP (3.0 and 6.0 mg/kg) or saline injection. Injection time is indicated by the vertical arrow. (B) Total distance traveled during 60 minutes after PCP or saline injection. *P < 0.05; **P < 0.01; ****P < 0.0001 versus saline treatment by two-way ANOVA followed by Tukey’s test. Data are expressed as the amount of distance traveled (mean ± S.E.M.). n = 11 rats/group.
Experiment 2: Locomotion after Acute PCP Challenge after Repeated Ropinirole Treatment.
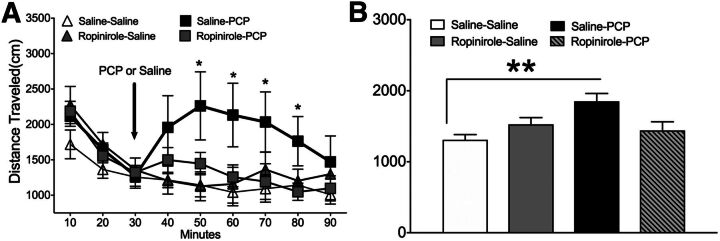
PCP challenge after repeated saline treatment significantly increased distance traveled over time as indicated by a significant interaction between PCP and time (F24,232 = 1.741, P = 0.026; Fig. 3A). This acute PCP challenge significantly (P = 0.037) increased total distance traveled compared with all other drug treatment groups 10–40 minutes after injection. Distance traveled collapsed over time revealed a significant main effect of PCP (F3,32 = 5.091, P = 0.005; Fig. 3B) wherein acute PCP induced greater locomotion compared with acute saline challenge (P = 0.038, post hoc comparison). By contrast, PCP did not increase locomotion in rats that were treated with repeated ropinirole, as there was no significant effect of PCP challenge in those rats. Neither distance traveled nor the number of ambulations was affected by saline challenge after either repeated saline or repeated ropinirole treatment (Fig. 3, A and B).
Fig. 3.
(A) Distance traveled over time before and after PCP (3.0 mg/kg) or saline challenge 10 days after repeated saline or ropinirole (0.1 mg/kg) treatment. Injection time is indicated by the vertical arrow. (B) Total distance traveled during 60 minutes after PCP or saline injection. **P < 0.01 versus repeated saline treatment and challenge by two-way ANOVA followed by Tukey’s test. Data are expressed as amount of distance traveled (mean ± S.E.M.). n = 8 rats/group.
Experiment 3: Prepulse Inhibition after Acute PCP Challenge after Repeated Ropinirole Treatment.
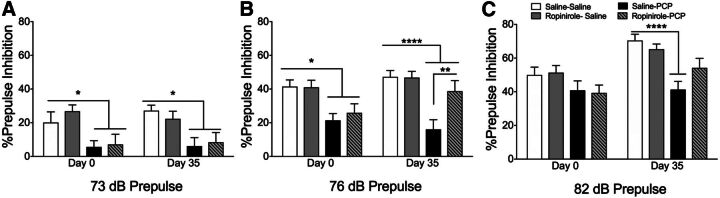
Independent analyses of individual prepulse levels revealed a similar trend as combined PPI prepulse intensities. At 73-dB prepulse PPI, a main effect of drug treatment (F3,124 = 16.84, P < 0.0001; Fig. 4A) was detected and PCP reduced PPI on both first (P = 0.025) and final (P = 0.014) PPI tests. At 76-dB prepulse PPI, a main effect of drug treatment (F3, 124 = 23.88, P < 0.0001; Fig. 4B) was detected, and PCP reduced PPI in rats repeatedly treated with saline on the first (P = 0.015) and final (P < 0.0001) PPI tests. By contrast, PCP failed to have the same effect on PPI 7 days after 28 days of repeated ropinirole compared with repeated vehicle treatment (P = 0.0058). At 82-dB prepulse PPI, the main effects of drug treatment (F3, 124 = 14.43, P < 0.0001; Fig. 4C) and day of testing (F3, 124 = 8.81, P < 0.001; Fig. 4C) were detected, and PCP reduced PPI on day 35 (P < 0.0001). Thus, repeated ropinirole treatment attenuated the effect of acute PCP-induced PPI deficits.
Fig. 4.
Percent PPI data determined at prepulse levels 3 (A), 6 (B), or 12 (C) dB above ambient noise (70 day (B); PCP challenge 1 day before repeated treatment (day 0) and 7 days after repeated treatment (day 35). *P < 0.05; **P < 0.01 ****P < 0.0001 on day 0 or day 35. PCP challenge after repeated saline treatment by two-way ANOVA followed by Tukey’s test. Data are expressed as percentage of PPI (mean ± S.E.M.). n = 16 rats/group.
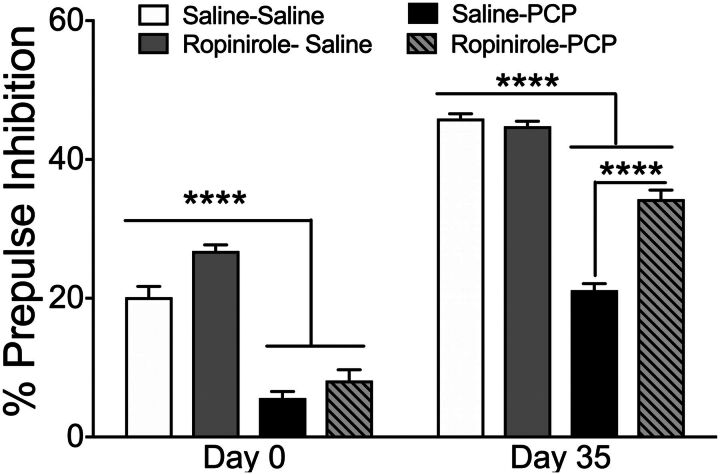
After combining all PPI intensities and days of testing, repeated-measures ANOVA with PPI intensities (73, 76, and 82 dB) and day of testing (day 0 and day 35) as within-subject factors and drug treatment (saline-saline, ropinirole-saline, saline-PCP, ropinirole-PCP) as the between-subject factor detected an interaction between the day of testing and decibel level (F3,62 = 4.02, P = 0.019), main effect of day of testing (F1,62 = 4.853, P = 0.031), decibel level (F1,62 = 296.661, P < 0.0001), and drug treatment (F1,62 = 10.36, P < 0.0001; Fig. 5). Acute PCP challenge before repeated ropinirole treatment induced a PPI deficit on day 0 (P < 0.0001), but rats that had received 28 days of repeated ropinirole treatment attenuated the PCP-induced PPI deficit, as observed by a significant difference between rats repeatedly treated with ropinirole versus saline (P < 0.0001). Neither repeated treatment nor PCP challenge affected behavior during the no-stimulation trials before and after repeated ropinirole treatment; there was a significant effect of PCP on the startle response during the final PPI test (P = 0.05), but this parameter was unaffected by repeated treatment (Table 1).
TABLE 1.
Average raw Newtons (N) response to pulse (120 dB) and no stimulus trials (mean ± S.E.M.) on challenge day (day 0 or 35)
| Drug Treatment Repeated-Challenge | 120-dB Pulse Day 0 | No Stimulus Day 0 | 120-dB Pulse Day 35 | No Stimulus Day 35 |
|---|---|---|---|---|
| Saline-saline | 0.466 ± 0.090 | 0.087 ± 0.049 | 0.549 ± 0.080 | 0.047 ± 0.005 |
| Ropinirole-saline | 0.688 ± 0.148 | 0.081 ± 0.038 | 1.018 ± 0.214 | 0.049 ± 0.003 |
| Saline-PCP | 0.854 ± 0.136 | 0.047 ± 0.005 | 1.509 ± 0.346* | 0.051 ± 0.005 |
| Ropinirole-PCP | 0.672 ± 0.152 | 0.041 ± 0.004 | 1.326 ± 0.238* | 0.048 ± 0.006 |
P < 0.05 compared with saline-saline.
Fig. 5.
Percent PPI data collapsed across prepulse levels 3, 6, or 12 days (B) above ambient noise (70 day) (B); PCP challenge 1 day before repeated treatment (day 0) and 7 days after repeated treatment (day 35). ****P < 0.0001 on day 0 versus saline acute challenges, ****P < 0.0001 on day 35 versus repeated saline treatment and challenge; also, PCP challenge after repeated ropinirole treatment versus PCP challenge after repeated saline treatment by two-way ANOVA followed by Tukey’s test. Data are expressed as percentage of PPI (mean ± S.E.M.). n = 16 rats/group.
Experiment 4: Social Interaction after Acute PCP Challenge after Repeated Ropinirole Treatment.
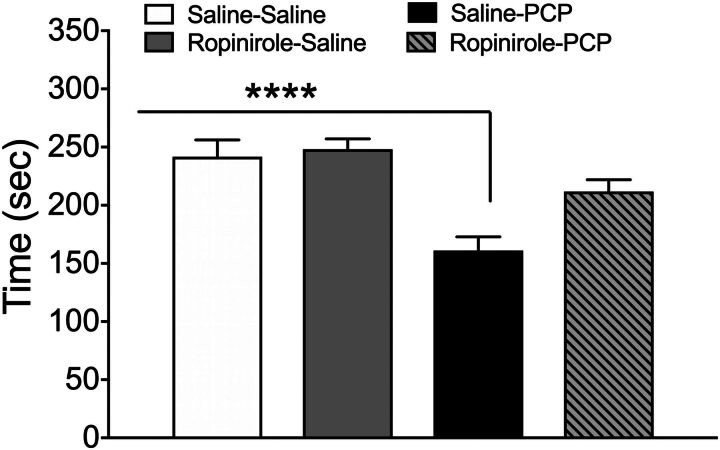
The amount of time rats engaged in active social interaction was reduced by acute PCP challenge 10 days after repeated saline treatment (F3,61 = 12.27, P < 0.001; Fig. 5 and Fig. 6); however, this effect did not occur in rats treated repeatedly with ropinirole before PCP challenge (P < 0.0001). Repeated treatment or PCP challenge had no significant effect on the amount of time in passive contact (P = 0.9; Table 2). Locomotion was unaffected by repeated treatment or drug challenge as no significant difference in total distance traveled was detected (F3,61 = 1.389, P = 0.254). Active contact, as determined by the velocity of the approach before social interaction, was also assessed and replicated the ropinirole-induced reduction of PCP’s effect on social contact (data not shown).
TABLE 2.
Average amount of time (mean seconds ± S.E.M.) spent in passive interaction on challenge day
| Drug Treatment | Time (Mean Seconds ± S.E.M.) |
|---|---|
| Saline-saline | 32.74 ± 5.27 |
| Ropinirole-saline | 37.04 ± 8.71 |
| Saline-PCP | 31.60 ± 6.59 |
| Ropinirole-PCP | 38.78 ± 10.71 |
No significant effect of repeated drug treatment or PCP challenge on time during passive interaction.
Fig. 6.
Time (mean ± S.E.M.) the rats spent engaged in social interaction after acute PCP challenge 7 days after repeated treatment. ****P < 0.0001 compared with saline challenge by two-way ANOVA followed by Tukey’s test. Data are expressed as the average of seconds (mean ± S.E.M.). n = 16 rats/group.
Discussion
The focus of the present study was to determine whether repeated ropinirole treatment could block the effects of PCP on various behaviors associated with symptoms of schizophrenia. After determining that acute PCP dose dependently increased locomotion, we then used in subsequent experiments the minimal PCP dose whose effect normalized within the test period (3.0 mg/kg). We observed that repeated ropinirole treatment blocked hyperlocomotion caused by acute PCP challenge without affecting locomotion after saline challenge. Furthermore, acute PCP challenge produced PPI deficits before and after repeated saline treatment, whereas repeated ropinirole treatment attenuated PCP-induced PPI deficits and significantly increased PPI. Similarly, acute PCP challenge after repeated saline treatment increased social avoidance, whereas repeated ropinirole treatment led to recovery of social interaction after PCP challenge.
Acute PCP challenge dose dependently increases regional local cerebral glucose utilization in the NAc and pallidum (Weissman et al., 1987), defining a brain circuit that may be responsible for its effects on locomotion and PPI (Ogren and Goldstein, 1994; Swerdlow et al., 2001). The observed attenuation of these behavioral effects suggests that the effect of PCP on this circuitry may be reduced after repeated ropinirole treatment. We have shown that repeated treatment with quinpirole or ropinirole produced recovery of agonist-induced PPI deficits, which requires activation of cAMP response element binding protein (CREB) in NAc neurons as overexpression of mutant CREB in this region prevents repeated ropinirole-induced PPI recovery (Culm and Hammer, 2004; Culm et al., 2004; Berger et al., 2011). The reversal of both PCP- and D2-like agonist-induced deficits suggests that repeated ropinirole treatment might have altered a final common pathway for both PCP and D2-like agonist effects.
Acute PCP challenge (5.0 mg/kg, ip) is known to increase extracellular levels of dopamine and glutamate in both NAc and medial prefrontal cortex (Adams and Moghaddam, 1998), which may be due to disinhibition of VTA dopamine neurons projecting to the forebrain and ventral striatum owing to reduced local GABAergic function (Deutch et al., 1987; Moghaddam et al., 1997; Yonezawa et al., 1998; Goldmann et al., 1999). Whereas PCP targets its receptors on the NMDA channel complex, which are present on both NAc and cortical pyramidal neurons, there is also evidence that it may bind directly to D2 receptors in their high affinity state (Seeman and Guan, 2008). Despite these potential relationships of PCP with D2 receptors, haloperidol did not block the effect of PCP (5.0 mg/kg) on either PPI or social interaction (Keith et al., 1991; Steinpreis et al., 1994), although haloperidol did block the effects of PCP (3.0 mg/kg) on locomotor activity (Ogren and Goldstein, 1994), even though PCP was observed to induce locomotion in dopamine-deficient mice (Chartoff et al., 2005). Thus, dopamine binding to dopamine receptors after PCP challenge might not underlie ropinirole-induced recovery of PCP effects. Instead, repeated ropinirole-induced alteration of function in a final common neuronal pathway from the NAc might be responsible for attenuation of both PCP and dopamine agonist effects on locomotion, PPI, and social behavior.
An interesting corollary of our findings on repeated ropinirole treatment is that PPI recovery is present up to 30 days after termination of ropinirole treatment (Berger et al., 2011). In the present study, repeated ropinirole attenuated PCP effects on PPI, locomotion, and social behavior 7–10 days after termination of ropinirole treatment, demonstrating that long-lasting effects on the final common pathway underlying certain symptoms of schizophrenia may be present. The mechanism of such long-lasting effects is unknown. We have previously shown that acute quinpirole reduces, whereas repeated treatment increases, NAc CREB phosphorylation (Culm et al., 2004). Similarly, we have observed that acute quinpirole reduces, whereas repeated treatment increases, expression of the long-lasting transcription factor ΔFosB in NAc neurons (Maple, submitted). We speculate that transcriptional regulation by ΔFosB within NAc circuits may underlie the long-lasting behavioral response to repeated D2-like agonist treatment, which is opposite that caused by acute treatment.
It should be noted that chronic ropinirole treatment as used herein could exacerbate existing sensorimotor gating deficits in patients with schizophrenia (Braff et al., 2001); however, dopamine agonist effects on sensorimotor gating are dose-dependent, suggesting that an escalating treatment paradigm starting with minimal doses might avoid initial disruption, while ultimately reversing PCP- or schizophrenia-induced sensorimotor gating deficits. The present study did not examine effects on working memory, however, which may be more closely related to glutamatergic effects of PCP in frontal cortex (Adams and Moghaddam, 1998). Future studies are needed to determine the effect of repeated dopamine agonist treatment on the frontal cortical function and dysfunction.
Here we showed that repeated D2-like agonist treatment blocked PCP-induced hyperlocomotion, PPI deficits, and social avoidance 7 days after termination of treatment. This study is the first to examine the effects of repeated D2-like agonist treatment on acute PCP deficits. These findings suggest that changes induced by repeated ropinirole treatment may alter the function of the neuronal circuitry that regulates locomotion, PPI, and social interaction, perhaps stemming from the NAc. The use of PCP challenge providing a more complete model of schizophrenia symptoms may be a more reasonable alternative to assess the behavioral effects of repeated dopamine agonists.
Acknowledgments
The authors thank the National Institute of Drug Abuse, Drug Supply Program for providing the PCP for this study.
Abbreviations
- ANOVA
analysis of variance
- CREB
cAMP response element binding protein
- NAc
nucleus accumbens
- NMDA
N-methyl d-aspartate
- PCP
phencyclidine
- PPI
prepulse inhibition
Authorship Contributions
Developed the concept and the designed experiments: Maple, Hammer.
Completed the drug administrations and behavioral testing: Maple, Call, Kimmel.
Conducted statistical analysis: Maple.
Interpreted the results and prepared the manuscript: Maple, Call, Hammer.
Footnotes
This research was supported by the United States Public Health Service Award RO1 [Grant MH073930] and Science Foundation of Arizona Bisgrove Scholarship.
References
- Adams B, Moghaddam B (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado L, San Antonio A, Pérez L, del Valle R, Gómez J (1994) Effects of the NMDA receptor antagonist ketamine on flavor memory: conditioned aversion, latent inhibition, and habituation of neophobia. Behav Neural Biol 61:271–281. [DOI] [PubMed] [Google Scholar]
- Angrist BM, Gershon S (1970) The phenomenology of experimentally induced amphetamine psychosis--preliminary observations. Biol Psychiatry 2:95–107. [PubMed] [Google Scholar]
- Berger AK, Green T, Siegel SJ, Nestler EJ, Hammer RP Jr (2011) cAMP response element binding protein phosphorylation in nucleus accumbens underlies sustained recovery of sensorimotor gating following repeated D2-like receptor agonist treatment in rats. Biol Psychiatry 69:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR (2001) Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156:234–258. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD (2005) Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology 30:1324–1333. [DOI] [PubMed] [Google Scholar]
- Chen DY, Swerdlow HP, Harke HR, Zhang JZ, Dovichi NJ (1991) Low-cost, high-sensitivity laser-induced fluorescence detection for DNA sequencing by capillary gel electrophoresis. J Chromatogr A 559:237–246. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, Thaler HT, Polyak T, Rapkin B, Portenoy RK (2000) The measurement of symptoms in children with cancer. J Pain Symptom Manage 19:363–377. [DOI] [PubMed] [Google Scholar]
- Culm KE, Hammer RP Jr (2004) Recovery of sensorimotor gating without G protein adaptation after repeated D2-like dopamine receptor agonist treatment in rats. J Pharmacol Exp Ther 308:487–494. [DOI] [PubMed] [Google Scholar]
- Culm KE, Lugo-Escobar N, Hope BT, Hammer RP Jr (2004) Repeated quinpirole treatment increases cAMP-dependent protein kinase activity and CREB phosphorylation in nucleus accumbens and reverses quinpirole-induced sensorimotor gating deficits in rats. Neuropsychopharmacology 29:1823–1830. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Tam SY, Freeman AS, Bowers MB Jr, Roth RH (1987) Mesolimbic and mesocortical dopamine activation induced by phencyclidine: contrasting pattern to striatal response. Eur J Pharmacol 134:257–264. [DOI] [PubMed] [Google Scholar]
- Goldmann C, Petry H, Frye S, Ast O, Ebitsch S, Jentsch KD, Kaup FJ, Weber F, Trebst C, Nisslein T, et al. (1999) Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J Virol 73:4465–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Glowa JR (1991) The effects of NMDA receptor blockade on the acquisition of a conditioned emotional response. Biol Psychiatry 30:170–176. [DOI] [PubMed] [Google Scholar]
- Itil TM, Rizzo AE, Shapiro DM (1967) Study of behavior and EEG correlation during treatment of disturbed children. Dis Nerv Syst 28:731–736. [PubMed] [Google Scholar]
- Keith VA, Mansbach RS, Geyer MA (1991) Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle. Biol Psychiatry 30:557–566. [DOI] [PubMed] [Google Scholar]
- Maple AMH R, Britton MJ, Nikulina EM, Hammer RP Jr. Repeated quinpirole treatment induces tolerance of prepulse inhibition and conditioned avoidance responding, with concurrent FosB expression in the rat nucleus accumbens. Pharmacol Biochem Behav (submitted). [Google Scholar]
- Moghaddam PH, Zwinderman AH, de Knijff P, Roep BO, Schipper RF, Van der Auwera B, Naipal A, Gorus F, Schuit F, Giphart MJ (1997) TNFa microsatellite polymorphism modulates the risk of IDDM in Caucasians with the high-risk genotype HLA DQA1*0501-DQB1*0201/DQA1*0301-DQB1*0302. Belgian Diabetes Registry. Diabetes 46:1514–1515. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Goldstein M (1994) Phencyclidine- and dizocilpine-induced hyperlocomotion are differentially mediated. Neuropsychopharmacology 11:167–177. [DOI] [PubMed] [Google Scholar]
- Pallarés MA, Nadal RA, Silvestre JS, Ferré NS (1995) Effects of ketamine, a noncompetitive NMDA antagonist, on the acquisition of the lever-press response in rats. Physiol Behav 57:389–392. [DOI] [PubMed] [Google Scholar]
- Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D (1959) Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. AMA Arch Gen Psychiatry 1:651–656. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F (1995) Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol 6:55–65. [PubMed] [Google Scholar]
- Sams-Dodd F (1996) Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol 7:3–23. [PubMed] [Google Scholar]
- Seeman P, Guan HC (2008) Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse 62:819–828. [DOI] [PubMed] [Google Scholar]
- Steinpreis RE (1996) The behavioral and neurochemical effects of phencyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res 74:45–55. [DOI] [PubMed] [Google Scholar]
- Steinpreis RE, Sokolowski JD, Papanikolaou A, Salamone JD (1994) The effects of haloperidol and clozapine on PCP- and amphetamine-induced suppression of social behavior in the rat. Pharmacol Biochem Behav 47:579–585. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156:194–215. [DOI] [PubMed] [Google Scholar]
- Uemura H, Katsuura-Kamano S, Yamaguchi M, Arisawa K, Hamajima N, Hishida A, Kawai S, Oze I, Shinchi K, Takashima N, Japan Multi-institutional Collaborative Cohort Study Group et al. (2015) Variant of the clock circadian regulator CLOCK gene and related haplotypes are associated with the prevalence of type 2 diabetes in the Japanese population. J Diabetes 8:661–676. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR (1993) Intra-accumbens infusion of quinpirole impairs sensorimotor gating of acoustic startle in rats. Psychopharmacology (Berl) 113:103–109. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Dam M, London ED (1987) Alterations in local cerebral glucose utilization induced by phencyclidine. Brain Res 435:29–40. [DOI] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H (1998) Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol 341:45–56. [DOI] [PubMed] [Google Scholar]