Abstract
Purpose of Review:
Chronic kidney disease (CKD)-associated pruritus is a common, persistent, and distressing itch experienced by patients across the CKD spectrum. Although the disorder is associated with adverse outcomes and poor health-related quality of life, it remains underdiagnosed and undertreated. The purpose of this narrative review is to offer health care providers guidance on how to effectively identify, assess, and treat patients with CKD-associated pruritus, with the goal of reducing symptom burden and improving patient-important outcomes, such as quality of life (QoL).
Sources of Information:
A panel of nephrologists and researchers from across Canada and the United States was assembled to develop this narrative review based on the best available data, current treatment guidelines, and their clinical experiences.
Methods:
A panel of nephrologists who actively care for patients with pruritus receiving dialysis from across Canada was assembled. Two researchers from the United States were also included based on their expertise in the diagnosis and management of CKD-associated pruritus. Throughout Spring 2023, the panel met to discuss key topics in the identification, assessment, and management of CKD-associated pruritus. Panel members subsequently developed summaries of the pertinent information based on the best available data, current treatment guidelines, and added information on their own clinical experiences. In all cases, approval of the article was sought and achieved through discussion.
Key Findings:
This narrative review provides pragmatic guidance addressing: (1) methods for screening CKD-associated pruritus, (2) assessing severity, (3) management of CKD-associated pruritus, and (4) suggested areas for future research. The panel developed a 3-pillar framework for proactive assessment and severity scoring in CKD-aP: systematic screening for CKD-associated pruritus (pillar 1), assessment of pruritus intensity (pillar 2), and understanding the impact of CKD-associated pruritus on the patient’s QoL (pillar 3). Management of CKD-associated pruritus can include ensuring optimization of dialysis adequacy, achieving mineral metabolism targets (ie, calcium, phosphate, and parathyroid hormone). However, treatment of CKD-associated pruritus usually requires additional interventions. Patients, regardless of CKD-associated pruritus severity, should be counseled on adequate skin hydration and other non-pharmacological strategies to reduce pruritus. Antihistamines should be avoided in favor of evidence-based treatments, such as difelikefalin and gabapentin.
Limitations:
A formal systematic review (SR) of the literature was not undertaken, although published SRs were reviewed. The possibility for bias based on the experts’ own clinical experiences may have occurred. Key takeaways are based on the current available evidence, of which head-to-head clinical trials are lacking.
Funding:
This work was funded by an arm’s length grant from Otsuka Canada Pharmaceutical Inc. (the importer and distributer of difelikefalin in Canada). LiV Medical Education Agency Inc. provided logistical and editorial support.
Keywords: assessment, chronic kidney disease-associated pruritus, dialysis, screening, CKD-associated pruritus treatment
Abrege
Motif de la revue:
Le prurit associé à l’insuffisance rénale chronique (IRC) est une démangeaison cutanée fréquente, persistante et invalidante que les patients de tout le specter de l’IRC peuvent ressentir. Bien que le prurit soit associé à des effets indésirables et à une mauvaise qualité de vie liée à la santé, il demeure sous-diagnostiqué et sous-traité. L’objectif de cette revue narrative est d’offrir des conseils aux professionnels de la santé sur la façon d’identifier, d’évaluer et de traiter efficacement les patients atteints de prurit associé à l’IRC; ceci dans le but de réduire la charge des symptômes et d’améliorer les résultats importants pour les patients, notamment leur qualité de vie (QdV).
Sources de l’information:
Un comité de néphrologues et de chercheurs de partout au Canada et des États-Unis a été constitué pour élaborer la présente revue narrative à partir des meilleures données disponibles, des lignes directrices actuelles pour le traitement et de leurs expériences cliniques.
Méthodologie:
Un groupe de néphrologues canadiens qui s’occupent activement de patients dialysés souffrant de prurit a été constitué. Deux chercheurs des États-Unis ont été inclus au groupe en raison de leur expertise dans le diagnostic et la prise en charge du prurit associé à l’IRC. Le comité s’est réuni tout au long du printemps 2023 pour discuter de sujets clés en lien avec l’identification, l’évaluation et la prise en charge du prurit associé à l’IRC. Les membres du comité ont par la suite rédigé des résumés des informations pertinentes en se basant sur les meilleures données disponibles et les lignes directrices actuelles pour le traitement, auxquels ils ont ajouté des informations issues de leurs propres expériences cliniques. Dans tous les cas, l’approbation du manuscrit a été sollicitée et obtenue par discussion.
Principaux résultats:
Cette revue narrative offre des conseils pragmatiques sur les points suivants: (1) les méthodes de dépistage du prurit associé à l’IRC; (2) l’évaluation de sa gravité; (3) sa prise en charge; et (4) les domaines suggérés pour de futures recherches. Le comité a développé un cadre à trois piliers pour l’évaluation proactive du prurit associé à l’IRC et l’établissement d’un score de gravité: le dépistage systématique du prurit associé à l’IRC (pilier 1), l’évaluation de son intensité (pilier 2) et la compréhension de son impact sur la QdV du patient (pilier 3). La prise en charge du prurit associé à l’IRC peut inclure l’optimisation de l’adéquation de la dialyse et l’atteinte des cibles du métabolisme minéral (c.-à-d. calcium, phosphate et hormone parathyroïdienne). Cependant, son traitement nécessite habituellement des interventions supplémentaires. Les patients, quelle que soit la gravité du prurit associé à l’IRC, devraient être avisés d’hydrater adéquatement leur peau et informés des autres stratégies non pharmacologiques afin de réduire le prurit. On devrait éviter les antihistaminiques et les remplacer par des traitements fondés sur des données probantes comme la difélikéfaline et la gabapentine.
Limites:
Aucune revue systématique de la littérature n’a été formellement entreprise, bien que les revues systématiques publiées aient été examinées. La possibilité d’un biais fondé sur les expériences cliniques des experts est envisageable. Les principales conclusions de cette étude sont fondées sur les données probantes actuellement disponibles, pour lesquelles il n’existe pas d’essais cliniques comparatifs.
Financement:
Ces travaux ont été financés par une subvention indépendante d’Otsuka Canada Pharmaceutical Inc. (l’importateur et distributeur de la difélikéfaline au Canada). Un soutien logistique et éditorial a été fourni par liV Medical Education Agency Inc.
Introduction
Chronic kidney disease (CKD)-associated pruritus is a common and distressing symptom experienced by patients with CKD for which scratching provides little or transient relief.1,2 Pruritus is present across the CKD spectrum 3 and becomes increasingly prevalent as CKD progresses.3,4 In the Dialysis Outcomes and Practice Patterns Study (DOPPS) international study of > 23 000 patients receiving hemodialysis (HD) from 21 countries, 37% of patients globally and 40% of patients in Canada suffered from moderate-to-severe pruritus.1,5
CKD-associated pruritus is associated with multiple adverse outcomes, including reduced health-related quality of life (HR-QoL), 6 poor sleep quality, 7 depression and mental health problems,8,9 and mortality.1,10 CKD-associated pruritus is also associated with increased dialysis treatment recovery time, missed dialysis sessions, and an increased likelihood of withdrawing from dialysis.1,11 Pruritus tends to cluster with other dialysis-related consequences, including restless leg syndrome, sleep disturbances, hospitalization, and mental health issues, which further negatively impact HR-QoL.1,12,13
Managing CKD-associated pruritus is an important research priority for patients, second only to optimizing dialysis treatment, 14 yet it remains underrecognized and undertreated. 15 Kidney care providers may be unaware of the presence and severity of CKD-associated pruritus 16 as is reflected in the reported discrepancy between physician-perceived versus patient-reported rates.2,15,17 Heterogeneity in CKD-associated pruritus measurement and lack of safe and effective treatment options are also barriers to appropriate management.15,18 A 2022 pan-Canadian needs assessment of 112 health care professionals (HCPs)—including nephrologists, nurses/nurse practitioners, pharmacists, and dieticians revealed numerous challenges in the identification, assessment, and treatment of CKD-associated pruritus (Supplemental Figure. Challenges in the identification and treatment of CKD-aP for Canadian HCPs).
The objective of this narrative review is to provide a practical summary of the current evidence on the identification, assessment, and treatment of CKD-associated pruritus with the goal of improving care and optimizing health outcomes for patients with CKD-associated pruritus.
Methods and Sources of Information
A panel of 13 nephrologists who actively care for patients with CKD-associated pruritus receiving dialysis from across Canada (British Columbia [2], Alberta [2], Saskatchewan [1], Manitoba [2], Ontario [3], Quebec [3], and Atlantic Canada [1]) was assembled. Panelists were selected by the lead author (CR), based upon their expressed interest and publication in the field. Two researchers from the United States were also included based on their published expertise in the diagnosis and management of CKD-associated pruritus. Throughout Spring 2023, the panel met virtually 2 times to discuss key topics in the identification, assessment, and management of CKD-associated pruritus. These meetings were led by CR. Panel members subsequently developed summaries of the pertinent information based on the best available data, current treatment guidelines, and added information drawn from their own clinical experiences. In teams, panel members led development of 1 of 4 sections of the review but were responsible for reviewing the entirety of the article. Topics and teams were: screening methods (RP, AL, AGV, and AK), severity assessment (KT, DS, NP, and PM), treatment approaches (CR, DC, LG, MS, and BP), and further research (CR, RS, and JH). The entirety of the review was developed by this panel, with no input from any other outside parties. Agreement with the article was sought and achieved through discussion at the virtual meetings and by email. This review was sponsored by an unrestricted education grant from Otsuka Canada Pharmaceutical Inc. (the importer and distributer of difelikefalin in Canada). All funds were used solely for the purposes of editorial and logistical support, which were provided by liV Medical Agency.
Review
What Is the Best Method to Screen for CKD-Associated Pruritus in a Dialysis Unit?
Key takeaway: Moderate-to-severe pruritus afflicts up to 40% of HD patients in Canada but remains underrecognized and undertreated.1,15 The panel thought this gap could be reduced by systematic screening for pruritus every 3 to 4 months in patients receiving dialysis, facilitating identification of patients suffering from pruritus.
CKD-associated pruritus usually presents without visible skin lesions, which contributes to delayed identification2,19 and potentially minimizes perception of severity by the health care provider. Patients seldom discuss pruritus with their HCP, attributable to a lack of awareness that the pruritus may be related to their CKD, symptom habituation, limited ability to communicate, or limited time with their HCP.19,20 On the provider side, HCPs may not routinely discuss pruritus with patients because of competing patient health priorities, lack of knowledge regarding CKD-associated pruritus, previous failed treatment attempts, and inertia resulting from a lack of effective therapies.
A single-question approach to routine screening is practical for both the physician and patient, since it is simple to implement, and has been found to be effective in identifying CKD-associated pruritus. 20 In a DOPPS cross-sectional analysis of patients receiving HD, identification of CKD-associated pruritus using only question 20 of the Kidney Disease Quality of Life 36-item survey (KDQOL-36)—“to what extent have you been bothered by itchy skin over the past 4 weeks?”—was highly correlated with the Skindex-10 score, a CKD-associated pruritus-specific measure of severity and impact on QoL. 20 This question is available in English and French, and validated as part of the translated KDQOL-36. 21
In practice, routine screening may be carried out by any clinician or allied health team member, but nurses or nurse practitioners often have the most contact time with patients and may be best able to screen and document pruritus in the patient’s electronic medical record (EMR) or physical chart. While implementation strategies will vary center to center, a systematic approach is preferred (ie, asking the same question after a set period of time). We rescreen our patients every 3 to 4 months to capture those who were previously asymptomatic but who may have newly developed CKD-associated pruritus.
Educating patients and their circle of care that pruritus is a common symptom of CKD may also improve identification of CKD-associated pruritus. Patients should be encouraged to discuss new onset or worsening pruritus with health care providers. 22
Diagnosis
Key takeaway: CKD-associated pruritus remains a diagnosis of exclusion. A physical exam and chart review should be done to exclude other potential causes of pruritus in a patient with CKD.
Once pruritus has been identified by screening, the diagnosis of CKD-associated pruritus requires exclusion of other potential causes, such as skin disease or comorbidities associated with pruritus. When visible skin lesions are present with pruritus in CKD, it is important to differentiate those secondary to scratching from primary lesions indicating a dermatological condition. Importantly, hyperphosphatemia has not been shown to be associated with CKD-associated pruritus and should be treated separately (see the “What Are the Best Treatment Approaches for CKD-Associated Pruritus?” section). 1
What Is the Best Way to Assess the Severity of CKD-Associated Pruritus Once Identified?
Once CKD-associated pruritus has been identified, an assessment of its severity and impact on other aspects of a patient’s life, such as sleep and mental health, will help guide subsequent treatment.23,24 The burden and impact of pruritus can be assessed with unidimensional and multidimensional tools. Unidimensional tools assess severity of pruritus and are simple and fast to administer. Multidimensional tools are more complex and generally more time-consuming but have the advantage of assessing the impact of pruritus on HR-QoL and the severity of pruritus with more granularity.
Unidimensional tools
Common unidimensional tools used in CKD-associated pruritus clinical trials are summarized in Table 1.
Table 1.
Unidimensional Patient-Reported Outcome Tools Used in the Assessment of CKD-Associated Pruritus Severity.
| PRO TOOL | SYMPTOM RECALL | STRENGTHS | LIMITATIONS | |
|---|---|---|---|---|
| WI-NRS 24 |
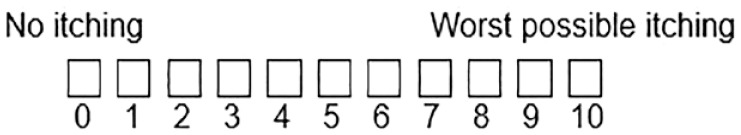
|
Worst itch in the previous 24 hours | Simple and fast Validated to analyze itch severity |
Not multidimensional, only measures severity |
| VAS24,25 |

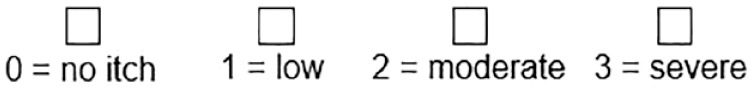
|
Worst itch in the previous 24 hours | Simple and fast Validated to analyze itch severity |
Not multidimensional, only measures severity |
| VRS 24 | ||||
| Q-20 KDQOL-361,20 |
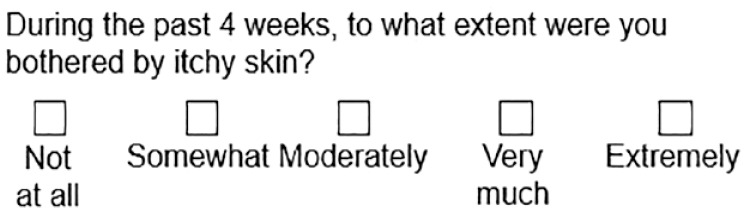
|
Evaluation of the last 4 weeks | Inclusion in KDQOL-36, used in large studies | Not validated as a severity measure |
CKD = chronic kidney disease; KDQOL = Kidney Disease Quality of Life Instrument; PRO = patient-reported outcome; VAS = Visual Analogue Scale; VRS = Verbal Rating Scale; WI-NRS = Worst Itch Numerical Rating Scale.
Key takeaway: Once CKD-associated pruritus has been identified, the panel thought a validated unidimensional tool, such as the Worst Itch Numerical Rating Scale (WI-NRS) should be used to assess severity and monitor response to treatment.
The Worst Itch Numerical Rating Scale (WI-NRS), Visual Analogue Scale (VAS), and Verbal Rating Scale (VRS) have all been validated for the assessment of pruritus intensity. 24 Question 20 of KDQOL-36 has been used in large studies to screen for pruritus,1,2,10 and has been shown to be strongly correlated with degree of CKD-associated pruritus severity measured using the WI-NRS, Average Itch Numeric Rating Scale (AI-NRS), Skindex-10, and 5-D itch instruments.20,26
Studies have shown that the WI-NRS, VAS, and single KDQOL-36 pruritus question can reliably quantify pruritus intensity in CKD-associated pruritus and can detect changes over time in patients, 23 with concordant scores on QoL measures.23,27
Worst Itch Numerical Rating Scale
The panel prefers the WI-NRS for assessing the severity of CKD-associated pruritus because it is simple to use, has been validated in randomized trials, is available in English and French, 28 and is reproducible, discriminating, and responsive to change. 29 Scores were well-correlated with multidimensional Skindex-10 and 5-D itch measures (covered in the next section). Patients found it to be straightforward, comprehensive, and relevant to CKD-associated pruritus. 29
A WI-NRS score of 1 to 2 corresponds to mild pruritus, 3 to 6 to moderate, and 7 to 8 to severe, and 9 to 10 to very severe pruritus. 30 Based on an analysis of pooled phase 3 cohorts of the KALM-1 and KALM-2 difelikefalin studies, an improvement of at least 3 points from baseline was found to be clinically meaningful, corresponding to much improved pruritus. 29 However, clinical improvements have also been shown with smaller WI-NRS changes. 29
Multidimensional tools
Key takeaway: The panel endorses the use of a validated multidimensional tool, such as the ESAS-r or SADS, to assess and monitor multidimensional impact of CKD-associated pruritus on other aspects of a patient’s life. A multidimensional tool may be used in place of a unidimensional tool if it is already implemented in the unit.
The symptoms of CKD-associated pruritus are subjective and multidimensional.31,32 Multidimensional tools can assess not just severity of pruritus, but also body distribution, hours per day bothered by pruritus, direction of pruritus severity, as well as impact on QoL, sleep, and life activities. The most commonly used multidimensional tools in CKD-associated pruritus clinical trials are the 5-D itch questionnaire, the Skindex-10, 33 and the Edmonton Symptom Assessment System-Revised: Renal (ESAS-r) (summarized in Table 2). From a practical perspective, the panel suggests using the ESAS-r if it is already implemented in a practice setting. For a new implementation in a setting where the ESAS-r is not being used, the Self-assessed Disease Severity (SADS) scale may be simpler to implement. While other pruritus-specific tools may be suitable for multidimensional assessment of CKD-associated pruritus, they are less practical for widespread implementation.
Table 2.
Multidimensional Patient-Reported Outcome Tools Validated for Use in the Assessment of CKD-Associated Pruritus.
| Dimensions assessed | Scoring | Recall time | Strengths | Limitations | |
|---|---|---|---|---|---|
| ESAS-r 34 | Pain Sleep/drowsiness/tiredness Nausea/appetite Shortness of breath Mood/emotional distress Itching |
12 symptoms scored 0 (none) to 10 (worst possible) | At administration | Validated for longitudinal HD symptom burden Commonly used and simple |
Not validated specifically for monitoring itch-related burden and response to therapy |
| SADS 23 | Disease Mood/emotional distress Sleep |
Self-categorization as most closely resembling patient A (absent/mild), B (moderate) or C (severe) | At administration | Fast Validated in CKD-aP |
Less used in studies |
| 5-D itch scale25,33 | Disability Distribution Duration Degree Direction |
5 item domains scored 1 (none/absent) to 5 (most severe) Total score: 5-25 |
14 days | Validated to analyze course of itch (including in CKD-aP patients) | 5 minutes to compete |
| Skindex-1020,23,33 | Disease Mood/emotional distress Social functioning |
10 items scored 0 (never bothered) to 6 (always bothered) Total score: 0-60 |
7 days | Validated to evaluate CKD-aP intensity | 5 minutes to compete |
| Itch MOS 23 | Sleep Sleep-related QoL |
10 items scored 1 (all of the time) to 6 (none of the time) + average sleep/night estimate Total score: 10-60 |
Previous 7 days | Validated to assess sleep disturbance in CKD-aP | Exclusively assesses sleep disturbance |
CKD-aP = chronic kidney disease-associated pruritus; ESAS-r = Edmonton Symptom Assessment System-Revised: Renal; HD = hemodialysis; MOS = medical outcomes study; PRO = patient-reported outcome; QoL = quality of life; SADS = Self-assessed Disease Severity.
Edmonton Symptom Assessment System-Revised: Renal
The ESAS-r assesses longitudinal physical and psychological symptom burden in patients receiving maintenance dialysis, and includes a question on pruritus that is similar to the WI-NRS. 35 Changes in overall ESAS-r scores have been associated with changes in QoL. 36 It has also been validated in English and French. 37 The ESAS-r is routinely used in many Canadian dialysis units, 38 and represents a straightforward and feasible tool for the routine assessment of CKD-aP, even in minimal resource settings.36,38 However, an important limitation of the ESAS-r is that while it assesses CKD-associated pruritus symptoms across several dimensions—such as psychosocial well-being and sleep—it does not assess how these dimensions specifically relate to pruritus severity.
A longitudinal study by Evans et al 34 assessed the impact of ESAS-r screening and found that incorporating the screening into existing workflows and leveraging technology to directly record data in EMRs resulted in more successful implementation. Other advantages included improved patient and provider awareness of symptoms—particularly of psychosocial symptoms—and standardization of symptom screening across HD sites and providers. Barriers identified by providers included difficulty improving psychosocial symptoms based on scores and a lack of standardized triggers for intervention; notably, some patients felt that their symptoms were not adequately managed despite having been identified upon screening. 34
Symptoms assessed with the ESAS-r 35
Patients self-report burden of the following symptoms at the time of measurement on a scale from 0 (not present) to 10 (worst possible):
• Pain
• Drowsiness
• Nausea
• Appetite
• Shortness of breath
• Depression
• Anxiety
• Well-being
• Itching
• Problem sleeping
• Restless legs
• Some versions include a space for patients to report burden of a symptom not listed
Self-assessed Disease Severity
The SADS asks patients to categorize themselves as 1 of 3 patient types with differing severity of signs and symptoms associated with CKD-associated pruritus. Execution time is quick (< 1 minute), consistently identifies patients who are adversely affected by pruritus, and has been validated against other measures of HR-QoL, sleep loss, and severity. 23 For example, intensity of pruritus associated with category A, B, and C patients strongly correlated with Skindex-10 severity scores and roughly corresponded to mild, moderate, and severe CKD-associated pruritus, respectively. 23 While it has been used less frequently in clinical trials and has not been validated for assessing changes in pruritus in response to treatment over time, it has been recommended for use in treatment algorithms and other recent reviews in CKD-associated pruritus.33,39
SADS patient types 23
Patients mark the patient type they are most like:
Patient A
• I do not generally have scratch marks on my skin
• I do not generally have a problem sleeping because of itching
• My itching does not generally make me feel agitated or sad
Patient B
• I sometimes have scratch marks on my skin
• I sometimes have problems sleeping because of itching
• My itching can sometimes make me feel agitated or sad
Patient C
• I often have scratch marks on my skin that may or may not bleed or get infected
• I often have a problem sleeping because of itching
• My itching often makes me feel agitated or sad
5-D itch questionnaire
The 5-D itch questionnaire is a validated measure of pruritus over time with high test-retest reliability. 25 It is simple to complete and score, detects changes in severity, and assesses several dimensions of pruritus and the impact of pruritus on QoL. While designed to be brief (< 5 minutes), it is still relatively time-consuming, with 8 items to complete. 25 The 14-day recall time of the 5-D itch scale may be more sensitive to the fluctuating nature of CKD-associated pruritus20,25; however, validated tools with shorter recall periods (ie, 24 hours) still have high agreement and correlation. 23
Skindex-10
The Skindex-10 is a 10-question instrument assessing disease, emotional, and social domains of pruritus in the previous week. It has been used extensively in research and has been specifically validated in CKD-associated pruritus, with good predictive value of disease severity and mental health diagnoses.20,23 However, it may not provide additional predictive value to determine physical, mental, or overall QoL compared with the single KDQOL-36 question 20, and is more time-consuming to administer. 20
Itch medical outcomes study
The Itch medical outcomes study (Itch MOS) assesses the impact of pruritus on sleep disturbance, which is commonly reported in individuals with CKD-associated pruritus. The Itch MOS is reliable and validated for use in CKD-associated pruritus; 23 however, it only assesses the impact of pruritus on sleep and no other domain.
Other tools
Comprehensive HR-QoL tools, such as the KDQOL-36, may be impractical to implement in clinical practice due to time burden.36,38 Other tools developed to assess the impact of pruritus on QoL may not be simple to implement, easily quantifiable, or sensitive to changes in pruritus over time. 25
Key takeaway: The panel thought that use of a unidimensional or multidimensional severity tool every 3 to 4 months in patients with CKD-associated pruritus to assess pruritus severity would be reasonable. In patients newly starting treatment for CKD-associated pruritus, the panel thought increasing the frequency of assessment (eg, every 4-6 weeks and prior to changes in treatment) would also be reasonable. Systematic use of any severity pruritus assessment tool was preferred over using no tool at all.
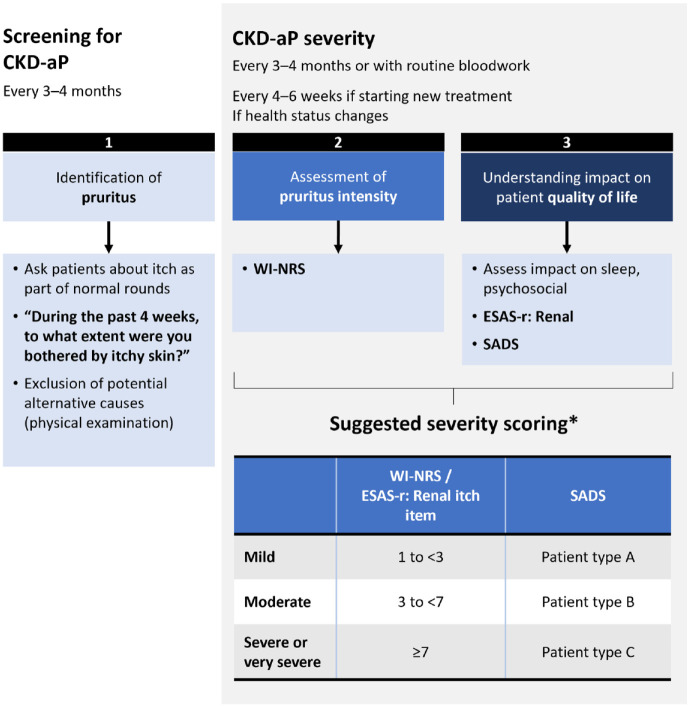
How should these tools be implemented in practice?
The panel created a 3-pillar framework for proactive assessment and severity scoring in CKD-associated pruritus (Figure 1): systematic screening for CKD-associated pruritus (pillar 1), assessment of intensity (pillar 2), and understanding the impact on the patient’s QoL (pillar 3). For patients with identified CKD-associated pruritus, we suggest screening for severity of pruritus every 3 to 4 months. Acknowledging that capacity for evaluation varies across programs, some sites may choose to do this more frequently if resources are available. When a CKD-associated pruritus intervention is initiated, assessment of severity should be done more frequently to monitor incremental changes and response to treatment. A unidimensional or multidimensional tool of choice can be administered to assess pruritus severity. While the choice of tool may vary across centers, systematic implementation of any tool is better than using no tool at all. In some regions, formal assessment of pruritus with a validated tool may be required to obtain access to reimbursement for some therapies.
Figure 1.
The 3 pillars of proactive assessment and severity scoring in CKD-associated pruritus.1,20,23,29,30,33,34
Proposed framework for proactive assessment and severity scoring in CKD-associated pruritus. Pillar 1: systematic screening for CKD-associated pruritus; Pillar 2: assessment of intensity; Pillar 3: understanding the impact on the patient’s QoL. CKD-aP = chronic kidney disease-associated pruritus; ESAS-r = Edmonton Symptom Assessment System-Revised: Renal; PRO = patient-reported outcomes; SADS = Self-assessed Disease Severity; WI-NRS = Worst-Itch Numerical Rating Scale.
aSeverity scoring ranges have not been validated and are based on limited evidence.
In a multicultural country like Canada, a significant proportion of patients read neither English nor French. In these cases, consider using a simple tool, such as the WI-NRS, VRS, or VAS paired with verbal instructions/explanation, if the provider can communicate in the same language as the patient. As needed, the provider or a family/friend can complete the assessment on behalf of the patient. 35
What Are the Best Treatment Approaches for CKD-Associated Pruritus?
Given the variety of potential drivers of CKD-associated pruritus, a diverse range of interventions have been investigated. Despite this, data supporting their use are sparse and direct comparisons of agents are lacking. 18 A summary of available interventions and the data that support their use is presented here.
Universal approaches (for mild, moderate, or severe CKD-associated pruritus)
Optimization of dialysis
Key takeaway: While dialysis, calcium, phosphate, and parathyroid hormone (PTH) targets should be achieved, the association between abnormalities of CKD-mineral bone disease (including hyperphosphatemia) and CKD-associated pruritus is inconsistent. Thus, management of CKD-associated pruritus will usually require additional interventions.
CKD-associated pruritus is more common in under-dialyzed patients and findings from a randomized trial suggest that symptoms may be improved by increasing the dialysis dose.40,41 However, the study only randomized 22 patients and the Kt/V urea values that improved pruritus were below current standards, thereby limiting the interpretation of targeting a certain Kt/V target. 41 While subsequent studies have yielded mixed results, current recommendations for optimizing CKD-associated pruritus care, based on inconsistent evidence, are to target a Kt/V of ≥ 1.4 per treatment using a single-compartment model.40,42,43 Use of medium cut-off dialyzers has been suggested to improve patient-reported outcomes, particularly the physical components of QoL and CKD-associated pruritus, in patients with high-flux dialyzers. 44
Some studies have described elevated calcium and phosphate levels in patients with CKD-associated pruritus;41,45,46 no association was observed between CKD-associated pruritus and CKD-related laboratory values, including parathyroid hormone (PTH), phosphate, and calcium levels, in a large DOPPS study. 1
The panel considers optimization of Kt/V, calcium, phosphate, and PTH levels as a reasonable initial approach to CKD-aP management. However, these measures alone will not be sufficient in the majority of patients with CKD-associated pruritus.2,47 The panel notes that no ideal CKD-associated pruritus target thresholds for these parameters have been identified.
Topical treatments
Key takeaway: The panel recommends counseling patients, regardless of CKD-associated pruritus severity, on adequate skin hydration and other non-pharmacological strategies to reduce pruritus.
Skin hydration is a cornerstone of CKD-associated pruritus management along with other non-pharmacological strategies and should be considered for all patients with CKD-associated pruritus regardless of severity.40,48,49 Xerosis (dry skin) is common in patients receiving kidney replacement therapy and may aggravate pruritus. 50 Hydration with emollients 50 and baby oil 51 reduces skin dryness and may improve QoL when applied 2 to 4 times daily.
Non-pharmacological treatment strategies. Adapted from Weisshaar and colleagues 48
• Wear soft, permeable clothing; dress in layers to avoid sweating
• Keep room temperature low at night
• Use mild, non-alkaline, perfume-free soaps, moisturizing syndets and shower/bath oils
• Apply emollients to skin twice a day, especially after bathing, and before bed
• Apply cooling wet or fat-moist wraps
• Limit baths to 20 minutes. Use lukewarm water ± oatmeal or potassium permanganate; dab skin dry.
• Avoid contact with allergenic and irritant substances
Examples of fragrance-free emollients:
• Cerave® cream
• Cetaphil® cream
• Lipikar® Baume AP & cream
• Aveeno® cream
• Glaxal base® cream
• Cliniderm® soothing cream
• Aquaphor® ointment
• Vaseline® ointment
• Uremol® cream
Beyond emollients, evidence for efficacy of other topical agents, such as menthol/capsaicin creams, gamma-linolenic acid (GLA), and tacrolimus ointment, is weak. 52 A meta-analysis of topical and systemic interventions for CKD-associated pruritus found that capsaicin creams are associated with a reduction in pruritus severity. 18 In a small study of 17 patients, topical GLA cream (2.2%) applied 3 times daily improved CKD-associated pruritus. 53 A meta-analysis of calcineurin inhibitor creams (eg, 0.1% tacrolimus and 1% pimecrolimus) showed that treatment resulted in a non-significant, but greater reduction in VAS compared with vehicle cream; however, quantitative analysis was inadequate precluding meaningful comparison. 18 Other topical agents, such as menthol/camphor, have demonstrated benefit in small uncontrolled studies.54,55
Acupuncture
Acupuncture has been used globally for the treatment of CKD-associated pruritus and may be an option for some patients. 56 Acupuncture is thought to increase local microcirculation, block transmission of pruritus signals to the brain, and reduce inflammatory mediators. 56 One systematic review (SR) and meta-analysis found that compared with certain conventional Western treatments (eg, HD, control of calcium and phosphorus metabolism, loratadine), acupuncture was more effective in treating CKD-associated pruritus (risk ratio [RR] = 1.28, 95% confidence interval [CI] = 1.09, 1.50; P = .003). 56 However, there was much heterogeneity in the data and further research is needed.
Phototherapy
Evidence for phototherapy in the management of CKD-associated pruritus is limited and existing data lacks consistency.18,57 Implementation of phototherapy can be challenging due to limited availability and the frequency required (3-4 times per week). 58 Thus, ultraviolet B (UVB) phototherapy may be considered as a last resort in the management of CKD-associated pruritus if it can be practically implemented.
Systemic therapies (for moderate-to-severe CKD-associated pruritus)
Gabapentin/pregabalin
Key takeaways:
• Gabapentin/pregabalin may be considered in the appropriate patient with moderate-to-severe CKD-associated pruritus. If used, begin with the lowest possible dose to minimize the risk of neurological side effects. Patients may be started with 100 mg gabapentin 3 times a week and titrated up by 100 mg every 2 weeks to maximum of 300 mg/day. Pregabalin may be initiated at 25 mg 3 times a week and titrate up by 25 mg every 2 weeks to max of 75 mg/day. The maximum dose should be adjusted based on kidney function and patient tolerance. Patients should be educated/monitored for potential side effects.
• After a 1- to 2-month trial of gabapentin/pregabalin, if the patient experiences burdensome side effects or has not experienced an improvement in pruritus severity, the dose should be adjusted or treatment should be discontinued.
Gabapentin and pregabalin are hypothesized to block nerve impulses crucial to the sensation of pruritus. Although they are not specifically indicated for use in CKD-associated pruritus, gabapentinoids are used off label for treatment of pruritus in many regions. A meta-analysis of 92 small, short duration studies (n = 4466) concluded that gabapentinoids produce meaningful reductions in pruritus on the 10-point VAS (4.95 cm reduction, 95% CI: 5.46, 4.44 lower in VAS compared with placebo). 18 Two other SRs concluded that of the historically available treatments for CKD-associated pruritus, use of gabapentinoids was the most robustly supported by the randomized trial evidence. Limitations of the data were also noted, including the small size and very short duration of most of the included studies.18,57
Gabapentin and pregabalin need to be used cautiously in dialysis patients. Both drugs are eliminated by the kidneys and their half-life is severely prolonged in dialysis, leading to a higher risk of adverse neurological events (eg, drowsiness, somnolence, dizziness, falls). 18 In a prospective study of over 140K patients on dialysis included in the United States Renal Data System (USRDS) in 2011, ~27K (19%) were on gabapentin and ~5600 on pregabalin (4%). Gabapentin was associated with 50%, 55%, and 38% higher hazards of altered mental status, fall, and fracture, respectively, in the highest dose category (> 300 mg/day), but even lower dosing was associated with a higher hazard of altered mental status (31%-41%) and fall (26%-30%). Similar trends were noted for pregabalin. 59 In a meta-analysis of trials of gabapentin use specifically in CKD-aP, adverse reactions were reported in 5.9% to 67.5% of patients on HD depending on the clinical trial. Gabapentin was also associated with a higher incidence of adverse drug events compared with other agents (ketotifen, dexchlorpheniramine, hydroxyzine, and pregabalin), although the results were not significant (RR = 1.3, 95% CI: 0.81, 2.11; P = .28, I 2 = 37%). 60
Despite these potential limitations, this panel considers gabapentinoids appropriate in patients with moderate-to-severe CKD-associated pruritus in whom education and monitoring of side effects are assured. While there have not been sufficient randomized controlled trials (RCTs) using different dosing regimens to give definitive recommendations about the doses of specific interventions, evidence suggests that a low dose of gabapentin or pregabalin should be used initially and then titrated up. 18 The panel recommends using low starting doses (eg, 100 mg of gabapentin or 25 mg of pregabalin 3 times a week) and titrating slowly in patients with CKD-associated pruritus.
Opioid receptor modifiers
While the physiological mechanisms underlying CKD-associated pruritus are not fully elucidated, opioid peptides and the opioid system have been implicated. 61 Thus, interventions targeting the µ- and κ-opioid systems have the potential to reduce pruritus severity. Systematic review and meta-analyses have found benefit with specific κ-opioid agonists, nalfurafine and difelikefalin.62,63
Difelikefalin
Key takeaway: Difelikefalin should be considered a valid option for the management of moderate-to-severe CKD-associated pruritus in patients on hemodialysis.
Difelikefalin is a highly selective, peripherally acting κ-opioid receptor agonist with antipruritic effects in patients on HD with moderate-to-severe CKD-associated pruritus.64 -66 Difelikefalin administered by intravenous (IV) bolus at the end of dialysis was approved by Health Canada in 2022 for the treatment of moderate-to-severe CKD-associated pruritus based on the KALM-1 and KALM-2 trials. 66 A phase 3 multicenter, randomized, placebo-controlled study is currently underway to evaluate the safety and efficacy of oral difelikefalin in patients with moderate-to-severe pruritus not on dialysis. 67
Pooled data from the KALM-1 and KALM-2 trials found a clinically significant change from baseline (≥ 3-point reduction) in WI-NRS score with difelikefalin vs placebo, with response seen in 66% of responders at 4 weeks and 90% of responders at 8 weeks. These results translated into clinically meaningful improvements in pruritus-related QoL (Skindex-10: P ≤ .05 and 5-D itch: P ≤ .001). Improvements in 5-D itch response with difelikefalin were maintained over a 52-week open-label extension and emerged in patients who switched from placebo; 20.4% and 17.4% of patients in the difelikefalin and placebo groups, respectively, reported baseline gabapentin use for any indication. Only 1.2% of patients in either group were on gabapentinoids specifically for treatment of pruritus. Difelikefalin benefit was consistent regardless of baseline gabapentinoid use. 64 A separate multicenter, open-label study of difelikefalin in patients with moderate-to-severe CKD-associated pruritus found a correlation between improvement in pruritus and sleep at week 12. 68
In a pooled safety analysis of the difelikefalin phase 3 clinical trial program, in patients with up to 64 weeks of exposure to difelikefalin, the most commonly reported treatment-emergent adverse events were diarrhea (9.0%), dizziness (6.8%), nausea (6.6%), gait disturbances (6.6%), hyperkalemia (4.7%), headache (4.5%), somnolence (4.2%), and mental status changes (3.3%). These were mostly mild or moderate, with few leading to discontinuation. 69 No euphoria, hallucinations, dysphoria, or signs of potential physical dependence were observed. 70
Despite strong evidence of the efficacy of difelikefalin, cost and drug plan reimbursement issues may limit adoption of this therapy. Despite stakeholder feedback from across Canada advocating for a positive reimbursement recommendation, in July 2023, the Canadian Agency for Drugs and Technologies in Health (CADTH) recommended that difelikefalin not be reimbursed for the treatment of moderate-to-severe CKD-associated pruritus in adult patients on HD, in part due to a perceived uncertainty in meaningful therapeutic benefit. 71 Our panel disagrees with the CADTH position that the primary outcome of the trials, a 3-point change in WI-NRS, is not clinically meaningful for patients or useful in clinical practice. A 3-point change in WI-NRS has been associated with clinically meaningful improvements in pruritus and WI-NRS is a validated instrument.24,29 Moreover, the panel notes that the CADTH decision is at variance with that of several other national regulatory agencies (including those in Germany, France, the United States, and National Institute for Health and Care Excellence [NICE]), which recommend reimbursing difelikefalin.72 -75 Review of difelikefalin by the Institut national d’excellence en santé et services sociaux (INESSS) in Quebec is pending. At present individual program funding is considered an option for access to difelikefalin.
Selecting gabapentin/pregabalin vs difelikefalin
To date, no head-to-head trials have been conducted comparing difelikefalin with gabapentin/pregabalin, and thus the relative merits of these therapies can only be assessed indirectly. The efficacy of both treatments is supported by scientific evidence; each agent has advantages and disadvantages.18,70 The main advantages of gabapentinoids are that they are widely available, inexpensive, and orally administered. The disadvantages are that half-life is severely prolonged in dialysis, dosing must be adjusted and then titrated slowly, and adverse side effects are common.59,76 With respect to difelikefalin, clinical trial and prospective data suggest a low frequency of side effects (6-9%) with a low frequency of discontinuation. 69 The main disadvantages of difelikefalin are higher cost, lack of public reimbursement mechanisms in Canada for difelikefalin, and the need to administer the medication by IV. This panel prioritizes efficacy and safety while recognizing that both patient and system factors must be considered, and therapy individualized. For example, difelikefalin may be preferred in patients for whom gabapentin-related side effects are of particular concern (eg, patients at high risk of delirium or falls, or with prior adverse reaction to gabapentinoids), whereas gabapentinoids may be preferred for patients with other indications (eg, concomitant neuropathy). The panel also observed that in clinical practice, not all patients respond adequately to a given therapy, and thus difelikefalin might be indicated in patients who fail gabapentinoids and vice versa.
Other approaches
Antihistamines
Key takeaway: Avoid antihistamines for the management of patients with CKD-associated pruritus.
Despite the lack of substantial evidence, antihistamines (eg, cetirizine, loratadine, hydroxyzine) are commonly used in first or second line to treat pruritic symptoms in patients with CKD.2,18 Studies of antihistamines in the treatment of CKD-associated pruritus have generally failed to demonstrate efficacy 18 with speculation that perceived benefits are a function of sedation rather than of antipruritic effect.48,77 Given the potential for side effects, such as confusion and sedation, particularly in an older population with multiple comorbidities, antihistamines are not recommended for CKD-associated pruritus.40,48
Selective serotonin reuptake inhibitors
The selective serotonin reuptake inhibitor (SSRI) sertraline was thought to reduce pruritus based on small, open-label, and uncontrolled studies,78,79 but was found to make little or no difference for the symptoms of CKD-associated pruritus when compared with placebo. 80
Cannabinoids
Medical marijuana is becoming more widely available and many patients with kidney disease express interest in its use for symptom control. 81 The endocannabinoid system plays an important role in skin homeostasis in addition to effects on neurogenic responses, such as pruritus. While open-label human studies have shown reductions in scratching and symptoms in chronic pruritus, these studies are limited by differences in the cannabinoids used, disease models, and delivery method. Nonetheless, these preliminary human studies suggest additional trials are warranted. 82
Management of CKD-associated pruritus in practice
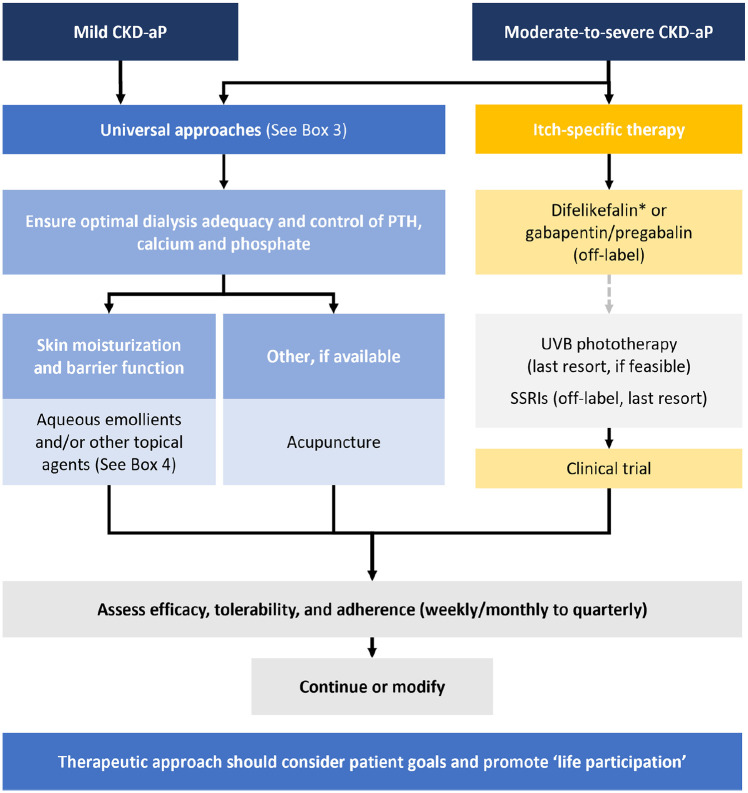
Management of CKD-associated pruritus should broadly follow the algorithm outlined in Figure 2 and be tailored to the individual patient’s goals and needs.40,47 Patient input and priorities should be solicited as pruritus management is a recognized patient priority. 14
Figure 2.
A modernized approach to CKD-associated pruritus.
Proposed algorithm for the management of CKD-associated pruritus. Treatment should be tailored to the individual patient’s goals and needs. CKD-aP = chronic kidney disease-associated pruritus; PTH = parathyroid hormone; SSRI = selective serotonin reuptake inhibitor; UVB = ultraviolet B.
aTreatment selection dependent on the availability of difelikefalin.
Regional variations and resources should be considered and implemented as appropriate.
Select resources on the management of CKD-associated pruritus
• Conservative Kidney Management (CKM) Uremic Pruritus Guideline: https://www.ckmcare.com/
• Ontario Renal Network: How to manage itchy skin: https://www.ontariorenalnetwork.ca/sites/renalnetwork/files/assets/HowToManageItchySkin.pdf
• BC Renal Agency Management of pruritus in patients with CKD: http://www.bcrenal.ca
• Uremic pruritus treatment algorithm and patient information: Ragazzo, et al. J Pain Symptom Manage. 2020;59(2):279-292.e5
• Systematic review and meta-analysis of randomized controlled trials (focused more broadly on the opioid pathway and provided GRADE recommendations): Bailey, et al. Br J Dermatol. 2022;186(3):575-577
What Are Key Issues Requiring Further Research or Efforts?
Ensuring that CKD-associated pruritus gets the attention it needs
Health care professionals should actively share information on appropriate identification, assessment, and management of pruritus in patients on HD, to ensure that CKD-associated pruritus is not overlooked. Each practice and center should incorporate a standardized approach that is simple to implement. Once these have been implemented (or if they have already been), seek feedback to optimize the approach within individual and center practices. Where possible, share your ongoing findings and best practices at regional or national exchanges to help strengthen Canadian CKD-associated pruritus care.
Seeking patient input
Patient priorities may differ from HCP priorities. 14 Although patient perspectives have been collected on some assessment tools, patient input on all aspects of assessment and management is critical to optimizing care. While a systematic approach should be undertaken for assessment, individual patient preferences remain important, and treatment choices should be made using shared decision making.
Research gaps, future directions, and precision medicine
Many patients will not respond to current therapies, and further research is needed into different mechanisms of pruritus. The pathogenesis of CKD-associated pruritus is incompletely understood, complex, and multifactorial. 47 Peripheral neuropathy, immune system dysregulation, opioid pathway dysregulation, and toxin deposition are all implicated 47 and a deeper understanding of these mechanisms may lead to the development of more targeted therapies.
Trials with new investigational compounds and alternative routes of administration are underway, including with MC2-25 cream, narrowband UVB, HSK21542 injection, nemolizumab.83 -86 In addition, more large-scale, well-designed studies of alternative approaches to managing CKD-associated pruritus, such as acupuncture, phototherapy, and exercise 87 are needed to provide clarity on their potential place in therapy. 88 Head-to-head comparisons to existing therapies should be prioritized.
Another area of research priority includes study of oral preparations of κ-receptor opioid agonists, particularly in peritoneal dialysis (PD) patients since IV therapy is not practical for these patients and they appear to experience similar rates of pruritus. 47 Another approach that could be investigated is short courses (eg, a few months) of IV κ-receptor opioid agonists treatment followed by an “off therapy” period to determine whether this can remove the pruritus stimulus and prevent immediate return of symptoms, which could help centers cope with any potential treatment availability challenges and cost. Further evaluation of incremental and more frequent dialysis, with more or less frequent κ-receptor opioid agonist doses, also needs further exploration.
Limitations
We conducted a narrative review of the identification, assessment, and management of patients with CKD-associated pruritus, through an expert-led process involving consultation, discussion, debate, and consensus. Although less rigorous than a formal guideline development process, our approach was meant to provide useful, pragmatic guidance. A formal SR of the literature was not undertaken, and the possibility for bias based on the experts’ own clinical experiences may have occurred. Furthermore, no framework (eg, GRADE [Grading of Recommendations, Assessment, Development, and Evaluations]) 89 was used to assess the quality of the evidence. The suggestions made in this report are based on the current available evidence, of which head-to-head clinical trials are lacking.
Conclusions
CKD-associated pruritus is a highly prevalent condition in patients with CKD that is associated with poor outcomes. However, it is commonly underdiagnosed and undertreated, highlighting a significant gap in clinical practice. The pathways presented in this review offer HCPs guidance on how to effectively identify, assess, and treat patients with CKD-associated pruritus, with the goal of helping to reduce symptom burden and improve patient outcomes and QoL.
Supplemental Material
Supplemental material, sj-tif-1-cjk-10.1177_20543581241238808 for Pathways for Diagnosing and Treating CKD-Associated Pruritus: A Narrative Review by Claudio Rigatto, David Collister, Alexandre Granger-Vallée, Louis Girard, Jay Hingwala, Angelo Karaboyas, Adeera Levin, Philip McFarlane, Ron Pisoni, Bhanu Prasad, Normand Proulx, Daniel Schwartz, Manish Sood, Rita Suri and Karthik Tennankore in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors thank Maryssa Canuel (Medical Director, liV Agency Inc.), David Haberl (Associate Medical Director, liV Agency Inc.), and Oksana Harasemiw (Seven Oaks General Hospital, Winnipeg, MB) for editorial support and liV Agency Inc. for logistical support organizing the virtual meetings between the authors.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Availability of Data and Materials: Not applicable.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Claudio Rigatto: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Boehringer Ingelheim, Astra Zeneca, Sanofi; Grants/Clinical trials: Sanofi. David Collister: Speaker’s bureau/honoraria: N/A; Grants/investigator: Canadian Institutes of Health Research: RESET-DIALYSIS, Canadian Institutes of Health Research: GAHT-KIDNEY, Kidney Foundation of Canada: RESET-DIALYSIS, KRESCENT post-doctoral fellowship, KRESCENT new investigator award, Research Manitoba/Boehringer Ingelheim: Virtual Kidney Check and Follow-Up; I am national leader for POSIBIL-6 which is sponsored by CSL-Behring but fees are directed to my research program. Alexandre Granger-Vallée: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, GSK. Louis Girard: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, Bayer, BI-Lilly, AstraZeneca, Janssen, Merck, Bausch Health, CPD Network, and Sanofi; Grants/investigator: Chemocentryx, Otsuka (the importer and distributer of difelikefalin in Canada) and Visterra. Jay Hingwala: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, GSK; Grants/investigator: Otsuka (the importer and distributer of difelikefalin in Canada). Angelo Karaboyas: Dr Karaboyas is an employee of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Program is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funds are made to Arbor Research Collaborative for Health and not directly to Dr Karaboyas. Adeera Levin: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, AZ, Gilead, GSK, Jansen; Grants/Clinical trials: KFOC/CIHR, Jansen, BI, AZ, GSK. Philip McFarlane: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, AMGEN, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Janssen, Lilly, Sanofi-Aventis, and Vifor; Grants/investigator: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, AstraZeneca, Bayer, Boehringer Ingelheim, Fresenius, GSK, Janssen, Novartis. Ron Pisoni: Dr Pisoni is an employee of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Program is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funds are made to Arbor Research Collaborative for Health and not directly to Dr Pisoni. Bhanu Prasad: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer; Grants/Clinical trials: Medtronic. Normand Proulx: Speaker’s bureau/honoraria: AMGEN, AstraZeneca, Bayer, Beigene, BMS, Eli Lilly, EMD Serono, Ipsen, Merck, Novartis, Pfizer, Roche, Sanofi, Servier, Takeda. Daniel Schwartz: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Janssen, BI, Lilly; Grants/investigator: CPD Network, Endocrine Research Society. Manish Sood: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), AstraZeneca, Bayer, and GlaxoSmithKline. Rita Suri: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Astra Zeneca, GSK, Amgen. Karthik Tennankore: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Baxter, GSK, Vifor Pharmaceuticals, Virtual Hallway; Grants/investigator: Multiple, but industry specific grant: unrestricted grant funding for an investigator-initiated project on CKD-aP severity measurement algorithm in hemodialysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was sponsored by an unrestricted education grant from Otsuka Canada Pharmaceutical Inc. (the importer and distributer of difelikefalin in Canada). All funds were used solely for the purposes of editorial and logistical support.
ORCID iDs: Claudio Rigatto  https://orcid.org/0000-0002-8306-8072
https://orcid.org/0000-0002-8306-8072
Philip McFarlane  https://orcid.org/0000-0003-2935-9618
https://orcid.org/0000-0003-2935-9618
Bhanu Prasad  https://orcid.org/0000-0002-1139-4821
https://orcid.org/0000-0002-1139-4821
Manish Sood  https://orcid.org/0000-0002-9146-2344
https://orcid.org/0000-0002-9146-2344
Rita Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Karthik Tennankore  https://orcid.org/0000-0002-7919-6709
https://orcid.org/0000-0002-7919-6709
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42-53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12:2000-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wulczyn KE, Rhee EP, Myint L, et al. Incidence and risk factors for pruritus in patients with nondialysis CKD. Clin J Am Soc Nephrol. 2023;18:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee D, Vassalotti JA, Torres G, et al. Burden of pruritus in advanced CKD and hemodialysis: results from national kidney foundation surveys. Kidney Med. 2023;5:100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sukul N, Speyer E, Tu C, et al. Pruritus and patient reported outcomes in non-dialysis CKD. Clin J Am Soc Nephrol. 2019;14:673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poku E, Harnan S, Rooney G, et al. The relationship between chronic kidney disease–associated pruritus and health-related quality of life: a systematic review. Clin Kidney J. 2022;15(3):484-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rehman IU, Chohan TA, Bukhsh A, et al. Impact of pruritus on sleep quality of hemodialysis patients: a systematic review and meta-analysis. Medicina (Mex). 2019;55:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res. 2012;21(4):603-612. [DOI] [PubMed] [Google Scholar]
- 9. van der Willik EM, Lengton R, Hemmelder MH, et al. Itching in dialysis patients: impact on health-related quality of life and interactions with sleep problems and psychological symptoms—results from the RENINE/PROMs registry. Nephrol Dial Transplant. 2022;37:1731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495-3505. [DOI] [PubMed] [Google Scholar]
- 11. Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahdoot RS, Kalantar-Zadeh K, Burton JO, et al. Novel approach to unpleasant symptom clusters surrounding pruritus in patients with chronic kidney disease and on dialysis therapy. Curr Opin Nephrol Hypertens. 2022;31:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhee CM, Edwards D, Ahdoot RS, et al. Living well with kidney disease and effective symptom management: consensus conference proceedings. Kidney Int Rep. 2022;7(9):1951-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burton JO, Walpen S, Danel S, et al. Current practices in chronic kidney disease-associated pruritus: international nephrologist survey. Kidney Int Rep. 2023;8:1455-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weisshaar E, Matterne U, Mettang T. How do nephrologists in haemodialysis units consider the symptom of itch? results of a survey in Germany. Nephrol Dial Transplant. 2009;24(4):1328-1330. [DOI] [PubMed] [Google Scholar]
- 17. Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960-967. [DOI] [PubMed] [Google Scholar]
- 18. Hercz D, Jiang SH, Webster AC. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12:CD011393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage. 2019;58(4):578-586.e2. [DOI] [PubMed] [Google Scholar]
- 20. Lopes MB, Karaboyas A, Sukul N, et al. Utility of a single itch-related question and the Skindex-10 questionnaire for assessing pruritus and predicting health-related quality of life in patients receiving hemodialysis. Kidney Med. 2022;4(6):100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boini S, Leplege A, Loos Ayav C, et al. Mesure de la qualité de vie dans l’insuffisance rénale chronique terminale. Néphrologie Thérapeutique. 2007;3:372-383. [DOI] [PubMed] [Google Scholar]
- 22. Jha CM, Dastoor HD, Gopalakrishnan N, Holt SG. Obstacles to early diagnosis and treatment of pruritus in patients with chronic kidney disease: current perspectives. Int J Nephrol Renovasc Dis. 2022;15:335-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol CJASN. 2010;5:1410-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502-507. [DOI] [PubMed] [Google Scholar]
- 25. Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karaboyas A, Tu C, Sukul N, et al. CKD-associated pruritus (CKD-aP) in hemodialysis (HD) patients: comparison of instruments used to measure self-reported itch severity. American Society of Nephrology. https://www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3765777. Published 2022. Accessed May 26, 2023. [Google Scholar]
- 27. Lai J-W, Chen H-C, Chou C-Y, et al. Transformation of 5-D itch scale and numerical rating scale in chronic hemodialysis patients. BMC Nephrol. 2017;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Storck M, Sandmann S, Bruland P, et al. Pruritus Intensity Scales across Europe: a prospective validation study. J Eur Acad Dermatol Venereol. 2021;35(5):1176-1185. [DOI] [PubMed] [Google Scholar]
- 29. Vernon MK, Swett LL, Speck RM, et al. Psychometric validation and meaningful change thresholds of the Worst Itching Intensity Numerical Rating Scale for assessing itch in patients with chronic kidney disease-associated pruritus. J Patient Rep Outcomes. 2021;5:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reich A, Chatzigeorkidis E, Zeidler C, et al. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97:759-760. [DOI] [PubMed] [Google Scholar]
- 31. Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30(2):231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manenti L, Leuci E. Do you feel itchy? a guide towards diagnosis and measurement of chronic kidney disease-associated pruritus in dialysis patients. Clin Kidney J. 2021;14(suppl 3):i8-i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans JM, Glazer A, Lum R, et al. Implementing a patient-reported outcome measure for hemodialysis patients in routine clinical care: perspectives of patients and providers on ESAS-r:Renal. Clin J Am Soc Nephrol. 2020;15:1299-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ontario Renal Network. ESAS-r: renal. https://www.ontariorenalnetwork.ca/sites/renalnetwork/files/assets/esasrrenal-english_0.pdf. Published 2023.
- 36. Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21(11):3189-3195. [DOI] [PubMed] [Google Scholar]
- 37. Pautex S, Vayne-Bossert P, Bernard M, et al. Validation of the French version of the Edmonton symptom assessment system. J Pain Symptom Manage. 2017;54(5):721-726.e1. [DOI] [PubMed] [Google Scholar]
- 38. Wen J, Jin X, Al Sayah F, et al. Mapping the Edmonton Symptom Assessment System-Revised: renal to the EQ-5D-5L in patients with chronic kidney disease. Qual Life Res. 2022;31(2):567-577. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal R, Burton J, Gallieni M, et al. Alleviating symptoms in patients undergoing long-term hemodialysis: a focus on chronic kidney disease-associated pruritus. Clin Kidney J. 2022;16:30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178(1):34-60. [DOI] [PubMed] [Google Scholar]
- 41. Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic pruritus. Am J Kidney Dis. 1995;25(3):413-419. [DOI] [PubMed] [Google Scholar]
- 42. Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS ONE. 2013;8(8):e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duque MI, Thevarajah S, Chan YH, Tuttle AB, Freedman BI, Yosipovitch G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol. 2006;66(3):184-191. [DOI] [PubMed] [Google Scholar]
- 44. Lim J-H, Park Y, Yook J-M, et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020;10:7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang CP, Lu YC, Tsai IT, et al. Increased levels of total p-cresylsulfate are associated with pruritus in patients with chronic kidney disease. Dermatology. 2016;232(3):363-370. [DOI] [PubMed] [Google Scholar]
- 46. Hu T, Wang B, Liao X, Wang S. Clinical features and risk factors of pruritus in patients with chronic renal failure. Exp Ther Med. 2019;18(2):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weisshaar E, Szepietowski JC, Dalgard FJ, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469-506. [DOI] [PubMed] [Google Scholar]
- 49. Balaskas E, Szepietowski JC, Bessis D, et al. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol. 2011;6(4):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morton CA, Lafferty M, Hau C, et al. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant. 1996;11:2031-2036. [DOI] [PubMed] [Google Scholar]
- 51. Karadag E, Kilic SP, Karatay G, Metin O. Effect of baby oil on pruritus, sleep quality, and quality of life in hemodialysis patients: pretest–post-test model with control groups. Jpn J Nurs Sci. 2014;11(3):180-189. [DOI] [PubMed] [Google Scholar]
- 52. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen YC, Chiu WT, Wu MS. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis. 2006;48(1):69-76. [DOI] [PubMed] [Google Scholar]
- 54. Westby EP, Purdy KS, Tennankore KK. A review of the management of uremic pruritus: current perspectives and future directions. Itch. 2020;5:e38. [Google Scholar]
- 55. Tennankore KK, Westby EP, McCarron KL, et al. Management of uremic pruritus in hemodialysis: effectiveness of a quality improvement treatment algorithm, ASN 2018 abstract TH-PO322. J Am Soc Nephro. 2018;29:197. [Google Scholar]
- 56. Zhang L, Li Y, Xiao X, et al. Acupuncture for uremic pruritus: a systematic review and meta-analysis. J Pain Symptom Manage. 2023;65:e51-e62. [DOI] [PubMed] [Google Scholar]
- 57. Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655. [DOI] [PubMed] [Google Scholar]
- 58. Świerczyńska K, Białynicki-Birula R, Szepietowski JC. Chronic intractable pruritus in chronic kidney disease patients: prevalence, impact, and management challenges—a narrative review. Ther Clin Risk Manag. 2021;17:1267-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eusebio-Alpapara KMV, Castillo RL, Dofitas BL. Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Int J Dermatol. 2020;59(4):412-422. [DOI] [PubMed] [Google Scholar]
- 61. Manenti L, Tansinda P, Vaglio A. Uraemic pruritus: clinical characteristics, pathophysiology and treatment. Drugs. 2009;69(3):251-263. [DOI] [PubMed] [Google Scholar]
- 62. Jaiswal D, Uzans D, Hayden J, Kiberd BA, Tennankore KK. Targeting the opioid pathway for uremic pruritus: a systematic review and meta-analysis. Can J Kidney Health Dis. 2016;3:2054358116675345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bailey AMJ, Li HO, Burns K, et al. Targeting the opioid pathway for the treatment of chronic kidney disease-associated pruritus: a systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2022;186(3):575-577. [DOI] [PubMed] [Google Scholar]
- 64. Topf J, Wooldridge T, McCafferty K, et al. Efficacy of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 phase 3 studies. Kidney Med. 2022;4(8):100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gardell LR, Spencer RH, Chalmers DT, Menzaghi F. Abstract PW-231: preclinical profile of CR845: a novel, long-acting peripheral kappa opioid receptor agonist. IASP 2008. https://ir.caratherapeutics.com/static-files/396df363-8031-47f6-b903-eca1df1df26f. [Google Scholar]
- 66. Vifor Fresenius Medical Care Renal Pharma Ltd. KORSUVA® (difelikefalin injection) product monograph. https://pdf.hres.ca/dpd_pm/00066996.PDF [Google Scholar]
- 67. ClinicalTrials.gov. A multicenter, randomized, double—blind, placebo—controlled 12—week study to evaluate the safety and efficacy of oral difelikefalin in advanced chronic kidney disease subjects with moderate—to—severe pruritus and not on dialysis with an up to 52—week long—term extension—NCT05342623. Clinical Trial Registration NCT05342623. https://clinicaltrials.gov/ct2/show/NCT05342623. Published May 2, 2023. Accessed May 25, 2023.
- 68. Weiner DE, Vervloet MG, Walpen S, et al. Safety and effectiveness of difelikefalin in patients with moderate-to-severe pruritus undergoing hemodialysis: an open-label, multicenter study. Kidney Med. 2022;4(10):100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fishbane S, Wen W, Munera C, et al. Safety and tolerability of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis from the phase 3 clinical trial program. Kidney Med. 2022;4(8):100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fishbane S, Jamal A, Munera C, et al. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382:222-232. [DOI] [PubMed] [Google Scholar]
- 71. CADTH final reimbursement recommendation —difelikefalin (KORSUVA). https://www.cadth.ca/sites/default//files/DRR/2023/SR0752%20Korsuva%20-%20CADTH%20Final%20Recommendation%20(with%20correction%20notice.pdf [Google Scholar]
- 72. KAPRUVIA (difelikefaline). Haute Autorité de Santé. https://www.has-sante.fr/jcms/p_3334049/fr/kapruvia-difelikefaline. Accessed November 29, 2023.
- 73. Centers for Medicare & Medicaid Services. ESRD PPS transitional drug add-on payment adjustment. https://www.cms.gov/medicare/payment/prospective-payment-systems/end-stage-renal-disease-esrd/esrd-pps-transitional-drug-add-payment-adjustment. Accessed November 29, 2023.
- 74. National Institute for Health and Care Excellence. Kapruvia® (difelikefalin) recommended by England’s NICE for the treatment of adults with moderate-to-severe CKD-associated pruritus. CSL Vifor. https://www.viforpharma.com/kapruviarv-difelikefalin-recommended-englands-nice-treatment-adults-moderate-severe-ckd-associated. Accessed November 29, 2023. [Google Scholar]
- 75. Gemeinsamer Bundesausschuss. Benefit assessment procedure for the active ingredient difelikefalin (pruritus in chronic kidney disease, hemodialysis patients). Federal Joint Committee. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/887/. Accessed November 29, 2023. [Google Scholar]
- 76. Cheikh Hassan HI, Brennan F, Collett G, Josland EA, Brown MA. Efficacy and safety of gabapentin for uremic pruritus and restless legs syndrome in conservatively managed patients with chronic kidney disease. J Pain Symptom Manage. 2015;49(4):782-789. [DOI] [PubMed] [Google Scholar]
- 77. Weisshaar E, Dunker N, Röhl FW, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13(5):298-304. [DOI] [PubMed] [Google Scholar]
- 78. Chan KY, Li CW, Wong H, et al. Use of sertraline for antihistamine-refractory uremic pruritus in renal palliative care patients. J Palliat Med. 2013;16(8):966-970. [DOI] [PubMed] [Google Scholar]
- 79. Shakiba M, Sanadgol H, Azmoude HR, Mashhadi MA, Sharifi H. Effect of sertraline on uremic pruritus improvement in ESRD patients. Int J Nephrol. 2012;2012:363901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pakfetrat M, Malekmakan L, Hashemi N, Tadayon T. Sertraline can reduce uremic pruritus in hemodialysis patient: a double blind randomized clinical trial from Southern Iran. Hemodial Int. 2018;22(1):103-109. [DOI] [PubMed] [Google Scholar]
- 81. Collister D, Herrington G, Delgado L, et al. Patient views regarding cannabis use in chronic kidney disease and kidney failure: a survey study. Nephrol Dial Transplant. 2023;38:922-931. [DOI] [PubMed] [Google Scholar]
- 82. Avila C, Massick S, Kaffenberger BH, Kwatra SG, Bechtel M. Cannabinoids for the treatment of chronic pruritus: a review. J Am Acad Dermatol. 2020;82(5):1205-1212. [DOI] [PubMed] [Google Scholar]
- 83. MC2 Therapeutics. A parallel-group (2-Arm), randomized, double-blind, 12-week trial to evaluate the efficacy and safety of MC2-25 cream and MC2-25 vehicle in subjects with chronic kidney disease-associated pruritus (CKD-aP). Clinical Trial Registration NCT05482698. clinicaltrials.gov. https://clinicaltrials.gov/study/NCT05482698. Published January 10, 2023. Accessed December 31, 2022.
- 84. Elmasry MF. Klotho and fibroblast growth factor 23 in chronic kidney disease-associated pruritus and their response to narrowband ultraviolet B. Clinical Trial Registration NCT03532568. clinicaltrials.gov. https://clinicaltrials.gov/study/NCT03532568. Published May 10, 2018. Accessed December 31, 2022.
- 85. Haisco Pharmaceutical Group Co., Ltd. A multi-center, randomized, double-blind, placebo-controlled phase III clinical study to evaluate the efficacy and safety of HSK21542 injection in maintenance hemodialysis patients with chronic kidney disease-associated pruritus. Clinical Trial Registration NCT05135390. clinicaltrials.gov. https://clinicaltrials.gov/study/NCT05135390. Published November 23, 2021. Accessed December 31, 2022.
- 86. Galderma R&D. A multicenter, double-blind, randomized, placebo-controlled study to evaluate the efficacy and safety of nemolizumab in subjects with chronic kidney disease with associated moderate to severe pruritus. Clinical Trial Registration NCT05075408. clinicaltrials.gov. https://clinicaltrials.gov/study/NCT05075408. Published November 15, 2023. Accessed December 31, 2022.
- 87. Wilkinson TJ, Watson EL, Gould DW, et al. Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: a secondary analysis of the “ExTra CKD” trial. Clin Kidney J. 2019;12(1):113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Prasad B, Gagarinova M, Sharma A. Five things to know about pruritus in patients on dialysis. Can J Kidney Health Dis. 2023;10:20543581221149620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-cjk-10.1177_20543581241238808 for Pathways for Diagnosing and Treating CKD-Associated Pruritus: A Narrative Review by Claudio Rigatto, David Collister, Alexandre Granger-Vallée, Louis Girard, Jay Hingwala, Angelo Karaboyas, Adeera Levin, Philip McFarlane, Ron Pisoni, Bhanu Prasad, Normand Proulx, Daniel Schwartz, Manish Sood, Rita Suri and Karthik Tennankore in Canadian Journal of Kidney Health and Disease