Abstract
Background:
A novel nanosomal paclitaxel lipid suspension (NPLS), free from Cremophor EL (CrEL) and ethanol, was developed to address the solvent-related toxicities associated with conventional paclitaxel formulation.
Objective:
To evaluate the efficacy and safety of NPLS versus CrEL-based paclitaxel (conventional paclitaxel) in patients with metastatic breast cancer (MBC).
Design:
A prospective, open-label, randomized, multiple-dose, parallel, phase II/III study.
Methods:
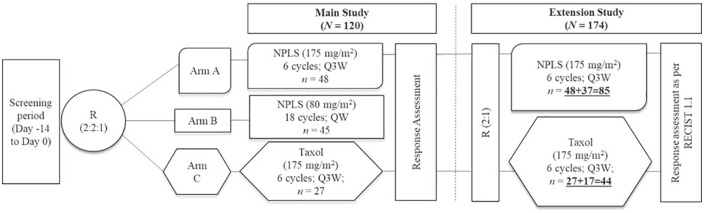
Adult (18–65 years) female patients with MBC who had previously failed at least one line of chemotherapy were randomized (2:2:1) to NPLS 175 mg/m2 every 3 weeks (Q3W, n = 48, arm A), NPLS 80 mg/m2 every week (QW, n = 45, arm B) without premedication or conventional paclitaxel (Taxol®, manufactured by Bristol-Myers Squibb, Princeton, NJ, USA) 175 mg/m2 Q3W (n = 27, arm C) with premedication. In the extension study, an additional 54 patients were randomized (2:1) to arm A (n = 37) or arm C (n = 17).
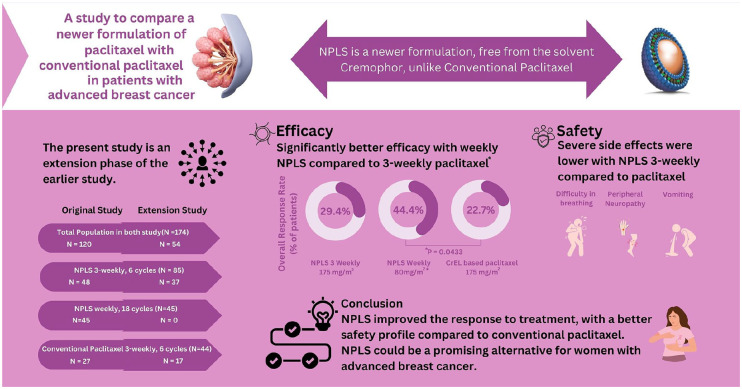
Results:
Pooled data from the primary study and its extension phase included 174 patients. The primary endpoint was the overall response rate (ORR). As per intent-to-treat analysis, ORR was significantly better in the NPLS QW arm as compared to conventional paclitaxel [44.4% (20/45) versus 22.7% (10/44), (p = 0.04)]. An improvement in ORR with NPLS Q3W versus conventional paclitaxel arm [29.4% (25/85) versus 22.7% (10/44)] (p = 0.53) was observed. Disease control rates observed were improved with NPLS Q3W versus conventional paclitaxel Q3W (77.7% versus 72.7%, p = 0.66) and with NPLS QW versus conventional paclitaxel Q3W (84.4% versus 72.7%, p = 0.20), although not significant. A lower incidence of grade III/IV peripheral sensory neuropathy, vomiting, and dyspnea was reported with NPLS Q3W versus conventional paclitaxel Q3W arms.
Conclusion:
NPLS demonstrated an improved tumor response rate and a favorable safety profile versus conventional paclitaxel. NPLS 80 mg/m2 QW demonstrated a significantly better response versus conventional paclitaxel 175 mg/m2 Q3W.
Trial registration:
Clinical Trial Registry-India (CTRI), CTRI/2010/091/001344 Registered on: 18 October 2010 (https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MjEzNQ==&Enc=&userName=CTRI/2010/091/001344), CTRI/2015/07/006062 Registered on: 31 July 2015 (https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MTE2Mjc=&Enc=&userName=CTRI/2015/07/006062)
Keywords: breast cancer, metastatic, nanosomal paclitaxel lipid suspension, NPLS, PacliAqualip, paclitaxel
Graphical abstract.
Plain language summary
Role of nanosomal paclitaxel lipid suspension (NPLS) in the treatment of patients with metastatic breast cancer (MBC)
Why was the study done?
Paclitaxel is a commonly used drug for the treatment of breast cancer. Conventional formulation of paclitaxel is known to cause side effects like injection site reactions. A newer formulation named NPLS was developed to overcome the limitations of the conventional paclitaxel. The current study was done to compare the safety and effectiveness of NPLS and conventional paclitaxel in patients with advanced breast cancer.
What did the researchers do?
The research team conducted a large study in multiple hospitals across India, involving women with advanced breast cancer who had experienced treatment failure with previous chemotherapy. A total of 174 patients were randomly assigned to receive either of the three treatment schedules: (1) NPLS every 3 weeks, (2) NPLS every week, (3) conventional paclitaxel every 3 weeks.
What did the researchers find?
The results showed that NPLS, in a weekly schedule, led to better tumor response rates compared to conventional paclitaxel given every 3 weeks. Additionally, NPLS demonstrated a favorable safety profile, as compared to conventional paclitaxel.
What do the findings mean?
These findings suggest that NPLS could be a promising alternative for women with advanced breast cancer. NPLS improved the response to treatment, with a better safety profile compared to conventional paclitaxel.
Introduction
In the last three decades, breast cancer has moved from fourth to first place in the category of most common cancers. 1 Globally, breast cancer is the most common cancer with an estimated 11.7% of all new cancer cases and a mortality rate of 6.9% as reported in the GLOBOCAN 2020 data. 2 In India, breast cancer is the most common cancer accounting for 13.5% (178,361) cases of all cancers in 2020. 3 The mortality rate of breast cancer in India is highest among other cancers at 10.6%, 3 which is attributed to a late diagnosis of disease at locally advanced or metastatic stage. 4
Metastatic breast cancer (MBC) is incurable and its treatment is directed toward symptom palliation and improving the patient’s survival. 5 Paclitaxel is a preferred chemotherapeutic agent for the treatment of recurrent, unresectable, or stage IV metastatic disease. 6 Paclitaxel is a highly hydrophobic molecule and hence, in the conventional commercial formulation, a combination of polyethoxylated castor oil [Cremophor EL (CrEL)] and ethanol is used as a vehicle.7,8 Studies have indicated that CrEL is an active solvent with biological activities and is known to be associated with toxicities such as severe anaphylactoid hypersensitivity reactions despite corticosteroid premedication; and prolonged, sometimes irreversible, peripheral neuropathy. 9 Furthermore, CrEL entraps the paclitaxel in plasma through micelle formation leading to a nonlinear pharmacokinetics and thus, causing increased drug levels, decreased drug elimination, and, in turn, diminishing the dose-dependent anticancer effects.8,10 Researchers have indicated a need for alternative paclitaxel formulations that are devoid of CrEL to minimize these toxicities. 9
A novel nanosomal paclitaxel lipid suspension (NPLS, PacliAqualip, manufactured by Intas Pharmaceuticals Ltd., Ahmedabad, India), devoid of CrEL and ethanol, was developed using lipid excipients, Generally Recognized As Safe (GRAS) by the United States Food and Drug Administration (USFDA), to overcome solvent-related toxicity challenges with conventional paclitaxel.11,12 NPLS has shown a relatively higher response rate versus conventional paclitaxel in the treatment of patients with MBC in a phase II/III study. The initial findings of this study demonstrated a better safety and efficacy [higher overall response rate (ORR) and disease control rate (DCR)] of 3-weekly and weekly NPLS versus conventional paclitaxel. There were no severe hypersensitivity reactions with NPLS, despite the absence of premedication.11,12 The current report presents the result of an extension study with updated data from 174 patients (main study: n = 120; extended study: n = 54).
Methods
This prospective, open-label, randomized, multiple-dose, parallel, phase II/III study enrolled patients from November 2010 to January 2017 (extension study: October 2015–January 2017) and was conducted at 14 sites across India. The study protocol and related documents were reviewed and approved by the institutional review boards or independent ethics committees. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and in accordance with the International Conference on Harmonization’s Good Clinical Practice (ICH-GCP) guidelines, applicable regulatory requirements, and compliance with the protocol. All participants provided written informed consent form to participate in the study. The reporting of our study adhered rigorously to the guidelines set forth by Equator Network, following the CONSORT statement (Supplemental Material), which ensures transparency and completeness in reporting our research.
Patient population
Nonpregnant, non-lactating females aged between 18 and 65 years with histologically or cytologically confirmed measurable MBC, who had failed previous combination chemotherapy or relapse within 6 months of adjuvant chemotherapy, and who had an expected survival of more than 6 months were eligible for participation. Patients were included if they had at least one measurable lesion as per the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (version 1.1), 13 Eastern Cooperative Oncology Group (ECOG) performance status ⩽2, left ventricular ejection fraction ⩾50%, no previous radiotherapy to >25% of marrow-containing bones, and who had completed previous chemotherapy or radiotherapy 4 weeks prior to study start. Patients were excluded from participation if they had preexisting motor or sensory neurotoxicity of ⩾grade 2 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), brain metastasis, carcinomatous meningitis or a clinically serious illness, history of hypersensitivity reactions to drugs formulated with CrEL, previous exposure to a taxane injection, or a history of cardiac disease with New York Heart Association class ⩾2.
Treatment
In the main study (n = 120), patients were randomized (2:2:1) to NPLS 175 mg/m2 every 3 weeks (Q3W) for a maximum of 6 cycles (n = 48, arm A), NPLS 80 mg/m2 every week (QW) for a maximum of 18 cycles (n = 45, arm B), or conventional paclitaxel (Taxol®) 175 mg/m2 Q3W for a maximum of 6 cycles (n = 27, arm C). The NPLS 175 mg/m2 and conventional paclitaxel 175 mg/m2 were administered as an intravenous infusion for 3 h (+30 min), whereas NPLS 80 mg/m2 was administered as an intravenous infusion for 1 h (+10 min). NPLS was administered without premedication with corticosteroids and antihistamines, whereas conventional paclitaxel was administered with premedication. The prophylactic granulocyte colony-stimulating factors were administered as per institutional practice. A reduction in treatment dose was allowed according to the package insert or investigator’s discretion.
Study design and sample size
At the screening visit, patients enrolling in the study were assigned a unique patient identification number according to the site. This number was serially allocated as per the chronological sequence of patients entering the trial at each site and it also incorporated the site identity. The randomization process was simple, with a block size of 3 (2:1 treatment allocation). For a comparison of two independent binomial proportions using Pearson’s chi-square statistic with a normal approximation with a one-sided significance level of 0.0294, a total of 108 completers, assuming an allocation ratio of 2:1 (72:36 in arm A:arm C) was expected to achieve a power of ⩾80% when the proportions were 20.8% and 36.4% and the non-inferiority margin was −10%.
In the main study, there were a total of 69 completers (44 and 25 completers in arms A and C respectively). Hence, as per guidance from the Drugs Controller General of India, an additional 54 patients were enrolled in a 2:1 ratio (36 and 18 patients) in arms A and C, respectively, in the extension study to achieve a power of 80%, a non-inferiority limit of 10%, and an α-error of 5% for analysis. However, of these additional 54 patients, 37 patients in arm A and 17 patients in arm B were enrolled due to site-specific randomization and it did not have any significant impact on the objectives of the study (Figure 1).
Figure 1.
Study design.
NPLS, nanosomal paclitaxel lipid suspension; QW, every week; Q3W, every 3 weeks; R, randomization; RECIST, Response Evaluation Criteria in Solid Tumors.
Study assessments
The primary study endpoint was ORR, defined as the proportion of patients whose best overall response was complete response (CR) or partial response (PR) after receiving at least two cycles of study treatment of NPLS or Taxol®. The secondary endpoint was DCR, defined as the proportion of patients with CR, or PR or stable disease (SD) (DCR = CR + PR + SD). The safety variables included adverse events (AEs), laboratory tests, vital signs, physical examination findings, concomitant medications, and electrocardiograms.
The efficacy evaluations were performed after 2, 4, and 6 cycles each in arms A and C, whereas in arm B, these were performed after 6, 12, and 18 cycles. Treatment response was assessed by an independent radiologist and analyzed using the RECIST version 1.1. 13 The incidence of AEs documented in the treatment charts was recorded and graded according to the CTCAE criteria version 4.3. 14
Statistical analysis
The statistical analysis was performed on the pooled data of the main study and the extension study. The efficacy population was defined as all patients who were evaluated for a response (computed tomography scan/magnetic resonance imaging) after receiving at least two treatment cycles, with no major protocol violations. The safety assessments were performed on a safety population that included all randomized patients who received at least one dose of study medication. Continuous variables were summarized using summary statistics (number of observations, mean, standard deviation, median, minimum and maximum, etc.) as applicable. Categorical values were summarized by dose group using frequencies and percentages. A point estimate and a two-sided 95% confidence interval (95% CI) were calculated for efficacy endpoints. Fisher’s exact test was used to compare the groups for significance. All statistical comparisons were performed using a two-sided significance level α = 0.05. The AEs were summarized as frequencies and percentages by type of reactions. The randomization and statistical analyses were performed by a biostatistician using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient disposition and demographics
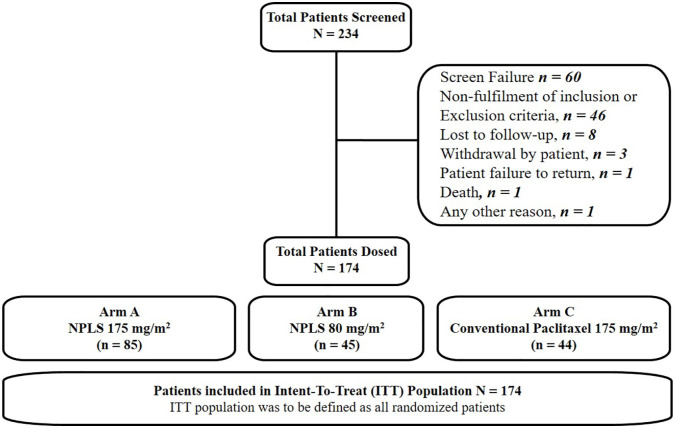
All patients from the main study were included in the study population and respective analyses without any changes in this extension study. A total of 234 female patients were screened, of whom, 174 (120 from the main study and 54 from the extension study) were randomized to arm A (n = 85), arm B (n = 45), and arm C (n = 44), respectively. Efficacy and safety endpoints were analyzed with data from the intent-to-treat (ITT) population (n = 174) (Figure 2).
Figure 2.
Patient disposition. ITT population included all randomized patients who received at least one dose of study medication.
CT, computed tomography; ITT, intent-to-treat; MRI, magnetic resonance imaging; NPLS, nanosomal paclitaxel lipid suspension.
The mean (SD) age of the patients was 48.2 (8.8) years, and the mean body surface area (BSA) was 1.5 (0.2) kg/m2. The racial makeup of the study was 100% Asian. The majority (>90%) of the patients had received prior anthracycline-based regimens in the adjuvant or metastatic setting. Overall, the demographic and baseline characteristics were similar among the treatment groups (Table 1).
Table 1.
Demographics and baseline characteristics (safety population, N = 174).
| Parameter | NPLS (175 mg/m2) (N = 85) Arm A |
NPLS (80 mg/m2) (N = 45) Arm B |
Conventional paclitaxel (175 mg/m2) (N = 44) Arm C |
|---|---|---|---|
| Age in years, mean (SD) | 48.2 (8.8) | 47.5 (9.7) | 49.5 (8.3) |
| Gender, female, n (%) | 85 (100) | 45 (100) | 44 (100) |
| BSA (m²), mean (SD) | 1.5 (0.2) | 1.4 (0.2) | 1.5 (0.2) |
| BMI (kg/m2) | 23.4 (4.8) | 22.7 (4.8) | 23.9 (4.9) |
| ECOG performance score, n (%) | |||
| 0 | 26 (30.6) | 22 (48.9) | 15 (34.1) |
| 1 | 52 (61.2) | 20 (44.4) | 23 (52.3) |
| 2 | 7 (8.2) | 3 (6.7) | 6 (13.6) |
| Menopausal status, n (%) | |||
| Pre-menopausal | 40 (47.1) | 18 (40) | 17 (38.6) |
| Post-menopausal | 45 (52.9) | 27 (60) | 27 (61.4) |
| Prior chemotherapy regimens in the metastatic setting, n (%) | |||
| 0 a | 45 (52.9) | 16 (35.6) | 26 (59.1) |
| 1 | 40 (47.1) | 29 (64.4) | 18 (40.9) |
Patients receiving study drugs as first-line therapy in metastatic settings included those who relapsed within 6 months of adjuvant chemotherapy.
BMI, body mass index; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; NPLS, nanosomal paclitaxel lipid suspension; SD, standard deviation.
Efficacy
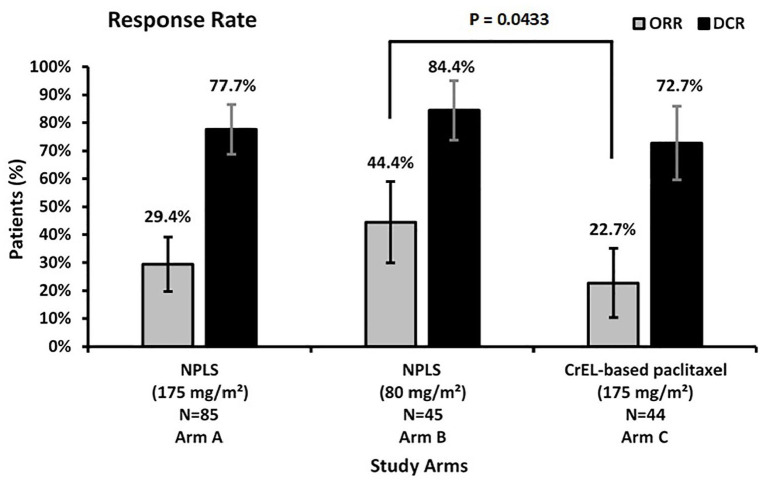
The ORR was significantly higher for NPLS QW versus conventional paclitaxel Q3W (44.4% versus 22.7%, p = 0.04). An improvement in ORR with NPLS Q3W versus conventional paclitaxel Q3W was observed (29.4% versus 22.7%, p = 0.53). An improved DCR was observed with NPLS Q3W versus conventional paclitaxel Q3W arm (77.7% versus 72.7%, p = 0.66) and NPLS QW versus conventional paclitaxel Q3W arm (84.4% versus 72.7%, p = 0.20), although not significantly different. A CR was achieved in three patients in the NPLS Q3W arm, whereas no CR was reported in other treatment arms (Figure 3 and Table 2). A per-protocol analysis was also done for 168 patients with no major protocol violation and with available efficacy data after at least two cycles of chemotherapy. Improved responses in ORR or DCR were observed, although not significant.
Figure 3.
Response rates.
DCR, disease control rate; NPLS, nanosomal paclitaxel lipid suspension; ORR, overall response rate.
Table 2.
Response rates (N=174).
| Response | NPLS (175 mg/m2) (N = 85) Arm A, n (%) |
NPLS (80 mg/m2) (N = 45) Arm B, n (%) |
Conventional paclitaxel (175 mg/m2) (N = 44) Arm C, n (%) |
|---|---|---|---|
| CR | 3 (3.5) | 0 (0) | 0 (0) |
| PR | 22 (25.9) | 20 (44.4) | 10 (22.7) |
| SD | 41 (48.2) | 18 (40) | 22 (50) |
| ORR (CR + PR) | 25 (29.4) | 20 (44.4) | 10 (22.7) |
| DCR (CR + PR + SD) | 66 (77.6) | 38 (84.4) | 32 (72.7) |
CR, complete response; DCR, disease control rate; NPLS, nanosomal paclitaxel lipid suspension; ORR, overall response rate; PR, partial response; SD, stable disease.
Safety
The proportion of patients with at least one AE was not statistically different between the three study arms (72.9% in the 3-weekly NPLS arm, 82.2% in the weekly NPLS arm, and 70.5% in the paclitaxel arm; p = 0.3855). Table 3 provides the AEs occurring in ⩾5% of patients in any treatment arm. A general trend toward a lesser incidence of all grade AEs for peripheral sensory neuropathy, diarrhea, vomiting, and anorexia was observed with NPLS versus conventional paclitaxel. A lower incidence of grade III/IV AEs in the NPLS Q3W versus conventional paclitaxel Q3W arms in terms of peripheral sensory neuropathy, vomiting, dyspnea, and a higher incidence of neutropenia was observed.
Table 3.
Adverse events occur in ⩾5% of patients in any treatment arm (N=174).
| AEs | NPLS (175 mg/m2) (N = 85) Arm A, n (%) |
NPLS (80 mg/m2) (N = 45) Arm B, n (%) |
Conventional paclitaxel (175 mg/m2) (N = 44) Arm C, n (%) |
|||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade ⩾3 | Grade 1–2 | Grade ⩾3 | Grade 1–2 | Grade ⩾3 | |
| Alopecia | 18 (21) | 0 | 8 (18) | 0 | 9 (20) | 0 |
| Anemia | 6 (7) | 2 (2) | 9 (20) | 4 (9) | 4 (9) | 0 |
| Asthenia | 11 (13) | 0 | 2 (4) | 0 | 4 (9) | 0 |
| Back pain | 9 (11) | 1 (1) | 5 (11) | 2 (4) | 0 | 0 |
| Chest discomfort | 1 (1) | 0 | 0 | 0 | 3 (7) | 0 |
| Chills | 18 (21) | 1 (1) | 8 (18) | 3 (7) | 1 (2) | 0 |
| Cough | 4 (5) | 0 | 6 (13) | 0 | 3 (7) | 0 |
| Decreased appetite | 5 (6) | 0 | 0 | 0 | 5 (11) | 0 |
| Diarrhea | 5 (6) | 0 | 4 (9) | 0 | 5 (11) | 0 |
| Dyspnea | 4 (5) | 1 (1) | 7 (16) | 0 | 0 | 1 (2) |
| Edema peripheral | 0 | 0 | 4 (9) | 0 | 0 | 0 |
| Headache | 4 (5) | 1 (1) | 1 (2) | 3 (7) | 2 (4) | 0 |
| Hypertension | 2 (2) | 0 | 4 (9) | 2 (4) | 1 (2) | 0 |
| Nausea | 6 (7) | 0 | 3 (7) | 0 | 3 (7) | 0 |
| Neutropenia | 11 (13) | 7 (8) | 8 (18) | 3 (7) | 2 (5) | 2 (4) |
| Pain | 11 (13) | 0 | 1 (2) | 1 (2) | 4 (9) | 0 |
| Peripheral sensory neuropathy | 10 (12) | 1 (1) | 1 (2) | 2 (4) | 9 (20) | 1 (2) |
| Pruritus | 3 (3) | 0 | 1 (2) | 0 | 3 (7) | 0 |
| Pyrexia | 14 (16) | 1 (1) | 4 (9) | 1 (2) | 5 (11) | 0 |
| Tachycardia | 3 (3) | 0 | 3 (7) | 0 | 0 | 0 |
| Vomiting | 6 (7) | 0 | 5 (11) | 0 | 3 (7) | 1 (2) |
The proportion of patients experiencing serious adverse events (SAEs) was 7.1% (n = 6/85) for NPLS Q3W, 11.1% (n = 5/45) for NPLS QW, and 9.1% (n = 4/44) for conventional paclitaxel Q3W arms (p = 0.6877). The SAEs in the NPLS Q3W arm were febrile neutropenia, pleural effusion, pyrexia, and dyspnea in one patient each, those in the NPLS QW arm were urinary tract infection, lung infection, pyrexia, food poisoning, and lymphedema in one patient each, and in conventional paclitaxel Q3W arm were dyspnea and vomiting in one patient each.
There were four deaths reported [two patients (2.4%) in the NPLS Q3W arm and two patients (4.5%) in the conventional paclitaxel Q3W arm]. In the NPLS Q3W arm, one death each (n = 2) was reported due to febrile neutropenia and pyrexia, which were considered by the investigator to be probably and unlikely related to treatment, respectively. In the conventional paclitaxel Q3W arm, one death each (n = 2) was reported due to respiratory distress and cardio-respiratory failure, both were considered by the investigator to be unlikely related to treatment. Notably, no death was reported in the NPLS QW arm.
Discussion
In this phase II/III study with an extension phase, NPLS, a novel CrEL-free formulation of paclitaxel, in a weekly regimen demonstrated a higher ORR compared to conventional paclitaxel. NPLS demonstrated an acceptable safety profile as compared with conventional paclitaxel formulation. DCR data were comparable between the three study arms. The ORR and DCR results presented here are consistent with the previous publication of the aforementioned parameters from this trial.11,12 NPLS was well tolerated overall and could be administered without corticosteroid and antihistaminic premedication in patients with MBC.
NPLS was developed using ‘NanoAqualip’ technology.15,16 Nano-sized (~100 nm) drug particles formulated with this technology may facilitate penetration of the drug to the tumor vasculature, and due to the decreased lymphatic drainage in the tumor, the retention and extravasation of NPLS in the tumor bed may increase and exert enhanced permeability and retention (EPR) effects possibly leading to enhanced antitumor activity.17,18 Consistent with this hypothesis, NPLS in the current study demonstrated an improved activity in terms of response rates.
The dose regimens of NPLS 175 mg/m2 Q3W and 80 mg/m2 QW used in the current study were in line with the prescribing information, treatment guidelines, and clinical practice.6,7,19 Paclitaxel is approved at a dose of 175 mg/m2 Q3W for the management of MBC 7 and reported an ORR of 29% in the landmark phase III trial by Siedman et al. (n = 735). 20 Another study by Winer et al. (n = 472) reported an ORR of 23% with a paclitaxel 175 mg/m2 Q3W regimen. 21 In our study, the conventional paclitaxel at 175 mg/m2 Q3W regimen reported an ORR of 22.7% in line with previous reports, whereas the NPLS 175 mg/m2 Q3W regimen reported an ORR of 29.4%.
Paclitaxel in a weekly regimen decreases myelosuppression and toxicity but at the same time maintains a higher dose intensity for improved efficacy outcomes as compared with standard Q3W regimens. 22 The efficacy and safety of weekly paclitaxel in MBC have been reported in several studies with an ORR ranging from 30% to 50%.19,23 –26 In the current study, the NPLS 80 mg/m2 QW arm demonstrated a significantly better ORR as compared with conventional paclitaxel 175 mg/m2 Q3W arm (44.4% versus 22.7%, p = 0.0433).
Another solvent-free formulation approved for MBC is nab-paclitaxel. In the phase III trial of nab-paclitaxel at a 3-weekly dose of 260 mg/m2, it demonstrated an ORR of 33% versus 19% with conventional paclitaxel at a 3-weekly dose of 175 mg/m2. 8 Conceptually, both NPLS and nab-paclitaxel are solvent-free formulations and hence these can overcome the shortcomings of conventional paclitaxel – namely, hypersensitivity, requirement of pre-medications like steroids, non-linear pharmacokinetics, etc. although both formulations have different dosing recommendations. While NPLS is dosed at an equivalent dose of conventional paclitaxel at the 3-weekly regimen of 175 mg/m2, nab-paclitaxel is dosed at ~50% higher dose at the 3-weekly regimen of 260 mg/m2. 27 A head-to-head study between NPLS and nab-paclitaxel in future can provide further data about the differences between efficacy and safety of these novel formulations.
NPLS is approved in India for the treatment of MBC, metastatic ovarian cancer, metastatic non-small-cell lung cancer, and acquired immune deficiency syndrome-related Kaposi’s sarcoma. 28 An Indian expert panel (funded by Intas Pharmaceuticals Limited) opined that novel CrEL-free formulations of paclitaxel like NPLS add value in the management of MBC and has recommended their usage in MBC patients who are at risk of hypersensitivity, diabetes, or patients with conditions that preclude the use of corticosteroids. 29
While our investigation revealed encouraging findings concerning the effectiveness and safety of NPLS in treating MBC, it is crucial to acknowledge certain limitations that merit consideration. These limitations include a short follow-up duration, age restriction of up to 65 years of age, lack of survival outcomes, a single country setting, and the absence of a weekly conventional paclitaxel arm as control, which may impact the generalizability of the study findings. Future studies with a larger sample size and an extended follow-up period to capture long-term outcomes and to assess potential late-onset AEs are warranted.
Conclusion
NPLS administered at a dose of 175 mg/m2 Q3W or 80 mg/m2 QW demonstrated a relatively improved tumor response versus conventional paclitaxel 175 mg/m2 administered Q3W in patients with MBC. NPLS 80 mg/m2 QW demonstrated a significantly better ORR versus conventional paclitaxel 175 mg/m2 Q3W. Overall, NPLS was well-tolerated without any significant safety concerns. In patients with MBC, NPLS may be an alternative treatment option as it precludes the need for premedication.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359241236442 for Treatment with nanosomal paclitaxel lipid suspension versus conventional paclitaxel in metastatic breast cancer patients – a multicenter, randomized, comparative, phase II/III clinical study by Chiradoni Thungappa Satheesh, Rakesh Taran, Jitendra Kumar Singh, Shanti Prakash Shrivastav, Nikunj K. Vithalani, Kalyan Kusum Mukherjee, Rajnish Vasant Nagarkar, Tanveer Maksud, Ajay Omprakash Mehta, Krishnan Srinivasan, Mummaneni Vikranth, Satish Ramkrishna Sonawane, Ateeq Ahmad, Saifuddin Sheikh, Shoukath M. Ali, Ronak Patel, Mahesh Paithankar, Lav Patel, Anil Rajani, Deepak Bunger, Alok Chaturvedi and Imran Ahmad in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to thank Dr Mehul R. Chorawala and Ms. Sakshi Srivastava (Intas Pharmaceuticals Limited, Ahmedabad, India) for providing scientific writing assistance and follow-up with Editorial office of Journal. The authors are also grateful to Chintan Bodiwala and the clinical data management and biostatistics department of Lambda Therapeutic Research Ltd, Ahmedabad, India for statistical analysis.
Footnotes
ORCID iDs: Chiradoni Thungappa Satheesh  https://orcid.org/0000-0003-0822-7747
https://orcid.org/0000-0003-0822-7747
Anil Rajani  https://orcid.org/0000-0002-2477-9499
https://orcid.org/0000-0002-2477-9499
Imran Ahmad  https://orcid.org/0000-0001-6383-0093
https://orcid.org/0000-0001-6383-0093
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chiradoni Thungappa Satheesh, Sri Venkateshwara Hospital, Bangalore, Karnataka, India.
Rakesh Taran, Convenient Hospital Ltd, CHL-Hospital and CHL-CBCC Cancer Center, Indore, Madhya Pradesh, India.
Jitendra Kumar Singh, Mahavir Cancer Sansthan, Patna, Bihar, India.
Shanti Prakash Shrivastav, Kiran Multi Super Specialty Hospital and Research Centre, Surat, Gujarat, India.
Nikunj K. Vithalani, Unique Hospital Multispecialty and Research Institute, Surat, Gujarat, India
Kalyan Kusum Mukherjee, Chittaranjan National Cancer Institute, Kolkata, West Bengal, India.
Rajnish Vasant Nagarkar, Curie Manavata Cancer Centre, Nashik, Maharashtra, India.
Tanveer Maksud, Bharat Cancer Hospital and Research Institute, Surat, Gujarat, India.
Ajay Omprakash Mehta, Central India Cancer Research Institute, Nagpur, Maharashtra, India.
Krishnan Srinivasan, Dr. Rai Memorial Medical Centre, Chennai, Tamil Nadu, India.
Mummaneni Vikranth, City Cancer Center, Vijayawada, Andhra Pradesh, India.
Satish Ramkrishna Sonawane, Anandrishiji Hospital and Medical Research Centre, Ahmednagar, Maharashtra, India.
Ateeq Ahmad, Jina Pharmaceuticals, Libertyville, IL, USA.
Saifuddin Sheikh, Jina Pharmaceuticals, Libertyville, IL, USA.
Shoukath M. Ali, Jina Pharmaceuticals, Libertyville, IL, USA
Ronak Patel, Lambda Therapeutic Research Limited, Ahmedabad, Gujarat, India.
Mahesh Paithankar, Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India; Jitendra Kumar Singh is currently affiliated to S. S. Hospital and Research Institute, Patna, Bihar, India.
Lav Patel, Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India; Jitendra Kumar Singh is currently affiliated to S. S. Hospital and Research Institute, Patna, Bihar, India.
Anil Rajani, Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India; Jitendra Kumar Singh is currently affiliated to S. S. Hospital and Research Institute, Patna, Bihar, India.
Deepak Bunger, Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India; Jitendra Kumar Singh is currently affiliated to S. S. Hospital and Research Institute, Patna, Bihar, India.
Alok Chaturvedi, Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India; Jitendra Kumar Singh is currently affiliated to S. S. Hospital and Research Institute, Patna, Bihar, India.
Imran Ahmad, Jina Pharmaceuticals Inc., 28100 N Ashley Circle, Suite 103, Libertyville, IL 60048, USA.
Declarations
Disclosure: Dr Ateeq Ahmad, Dr Saifuddin Sheikh, Dr Shoukath M Ali, and Dr Imran Ahmad are employees of Jina Pharmaceuticals, Libertyville, IL, USA. Mr Ronak Patel is an employee of Lambda Therapeutic Research Limited, Ahmedabad India. Mr Mahesh Paithankar, Dr Lav Patel, Dr Anil Rajani, Dr Deepak Bunger, and Dr Alok Chaturvedi are employees of Intas Pharmaceuticals Limited, Ahmedabad, India.
Previous presentation: The study data were presented as a poster at the European Society of Medical Oncology (ESMO) Congress, held in Paris, from 9 to 13 September 2022.
Ethics approval and consent to participate: Ethical approval for this study was obtained from the Institutional Ethics Committee (IEC) of the respective trial sites. This study is registered on Clinical Trial Registry-India (CTRI) vide registration numbers CTRI/2010/091/001344 (registered on 18 October 2010) and CTRI/2015/07/006062 (registered on 31 July 2015). The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and in accordance with the International Conference on Harmonization’s Good Clinical Practice (ICH-GCP) guidelines, applicable regulatory requirements, and in compliance with the protocol. All participants provided a written informed consent form to participate in the study.
Consent for publication: Consent for publication was obtained from all authors, participants, or legally authorized representatives involved in this study.
Author contributions: Chiradoni Thungappa Satheesh: Investigation; Project administration; Resources; Supervision.
Rakesh Taran: Investigation; Project administration; Resources; Supervision.
Jitendra Kumar Singh: Investigation; Project administration; Resources; Supervision.
Shanti Prakash Shrivastav: Investigation; Project administration; Resources; Supervision.
Nikunj K. Vithalani: Investigation; Project administration; Resources; Supervision.
Kalyan Kusum Mukherjee: Investigation; Project administration; Resources; Supervision.
Rajnish Vasant Nagarkar: Investigation; Project administration; Resources; Supervision.
Tanveer Maksud: Investigation; Project administration; Resources; Supervision.
Ajay Omprakash Mehta: Investigation; Project administration; Resources; Supervision.
Krishnan Srinivasan: Investigation; Project administration; Resources; Supervision.
Mummaneni Vikranth: Investigation; Project administration; Resources; Supervision.
Satish Ramkrishna Sonawane: Investigation; Project administration; Resources; Supervision.
Ateeq Ahmad: Conceptualization; Resources; Visualization; Writing – review & editing.
Saifuddin Sheikh: Conceptualization; Resources; Visualization; Writing – review & editing.
Shoukath M. Ali: Conceptualization; Resources; Visualization; Writing – review & editing.
Ronak Patel: Data curation; Formal analysis; Software; Validation.
Mahesh Paithankar: Conceptualization; Methodology; Validation; Visualization.
Lav Patel: Validation; Visualization; Writing – review & editing.
Anil Rajani: Validation; Visualization; Writing – review & editing.
Deepak Bunger: Validation; Visualization; Writing – review & editing.
Alok Chaturvedi: Methodology; Supervision; Validation; Visualization; Writing – review & editing.
Imran Ahmad: Conceptualization; Data curation; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India.
Dr Ateeq Ahmad, Dr Saifuddin Sheikh, Dr Shoukath M Ali, and Dr Imran Ahmad are employees of Jina Pharmaceuticals, Libertyville, IL, USA. Mr Ronak Patel is an employee of Lambda Therapeutic Research Limited, Ahmedabad India. Mr Mahesh Paithankar, Dr Lav Patel, Dr Anil Rajani, Dr Deepak Bunger, and Dr Alok Chaturvedi are employees of Intas Pharmaceuticals Limited, Ahmedabad, India.
Availability of data and materials: The data that support the findings of this research are not publicly available due to institutional restrictions.
References
- 1. Mehrotra R, Yadav K. Breast cancer in India: present scenario and the challenges ahead. World J Clin Oncol 2022; 13: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Cancer Prevention and Research. GLOBOCAN 2020: India factsheet, https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf (2020, accessed 25 April 2022).
- 4. Malvia S, Bagadi SA, Dubey US, et al. Epidemiology of breast cancer in Indian women. Asia-Pac J Clin Oncol 2017; 13: 289–295. [DOI] [PubMed] [Google Scholar]
- 5. Anampa J, Sparano JA. New agents for the management of resistant metastatic breast cancer. Expert Opin Pharmacother 2017; 18: 1815–1831. [DOI] [PubMed] [Google Scholar]
- 6. Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20: 691–722. [DOI] [PubMed] [Google Scholar]
- 7. Taxol® (Paclitaxel) Prescribing Information. Bristol-Myers Squibb Company, Princeton, NJ, USA. Revised April 2011. [Google Scholar]
- 8. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23: 7794–7803. [DOI] [PubMed] [Google Scholar]
- 9. Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001; 37: 1590–1598. [DOI] [PubMed] [Google Scholar]
- 10. van Tellingen O, Huizing MT, Panday VR, et al. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br J Cancer 1999; 81: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmad A, Sheikh S, Ali SM, et al. Nanosomal paclitaxel lipid suspension demonstrates higher response rates compared to paclitaxel in patients with metastatic breast cancer. J Cancer Sci Ther 2015; 7: 116–120. [Google Scholar]
- 12. Ahmad A, Sheikh S, Mehta AO, et al. Cremophor EL-free formulation of paclitaxel: a randomized, multicenter, safety, and efficacy study of nanosomal paclitaxel lipid suspension (NPLS) versus paclitaxel in women with metastatic breast cancer (MBC). J Clin Oncol 2014; 32: 573. [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v5.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (2017, accessed 25 April 2022).
- 15. Ali SM, Ahmad M, Ahmad A, et al.; Jina Pharmaceuticals Inc., Assignee. Aqueous systems for the preparation of lipid-based pharmaceutical compounds; compositions, methods, and uses thereof. International patent PCT/US2007/080984, 2008. [Google Scholar]
- 16. Subramanian S, Majumdar SKD, Biswas G, et al. Efficacy and safety of nanosomal docetaxel lipid suspension based chemotherapy in gastric and gastroesophageal junction adenocarcinoma. Mol Clin Oncol 2020; 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura Y, Mochida A, Choyke PL, et al. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 2016; 27: 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med 2021; 11: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uno Y, Hirano M, Murakami N, et al. [Weekly administration of low-dose paclitaxel for advanced or metastatic breast cancer]. Gan To Kagaku Ryoho 2002; 29: 227–232. [PubMed] [Google Scholar]
- 20. Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008; 26: 1642–1649. [DOI] [PubMed] [Google Scholar]
- 21. Winer EP, Berry DA, Woolf S, et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J Clin Oncol 2004; 22: 2061–2068. [DOI] [PubMed] [Google Scholar]
- 22. Horiguchi J, Rai Y, Tamura K, et al. Phase II study of weekly paclitaxel for advanced or metastatic breast cancer in Japan. Anticancer Res 2009; 29: 625–630. [PubMed] [Google Scholar]
- 23. Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 2005; 23: 5983–5992. [DOI] [PubMed] [Google Scholar]
- 24. Perez EA, Vogel CL, Irwin DH, et al. Weekly paclitaxel in women age 65 and above with metastatic breast cancer. Breast Cancer Res Treat 2002; 73: 85–88. [DOI] [PubMed] [Google Scholar]
- 25. Gori S, Mosconi AM, Basurtol C, et al. Weekly paclitaxel in metastatic breast cancer patients: a phase II study. Tumori 2002; 88: 470–473. [DOI] [PubMed] [Google Scholar]
- 26. Lombardi D, Crivellari D, Scuderi C, et al. Long-term, weekly one-hour infusion of paclitaxel in patients with metastatic breast cancer: a phase II monoinstitutional study. Tumori 2004; 90: 285–288. [DOI] [PubMed] [Google Scholar]
- 27. Martín M. Nab-Paclitaxel dose and schedule in breast cancer. Breast Cancer Res 2015; 17: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar Das Majumdar S, Kumar Muduly D, Mishra S, et al. Management of primary squamous cell carcinoma of the pancreas with a nanosomal paclitaxel lipid suspension-based regimen: a case report. Mol Clin Oncol 2019; 10: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajappa S, Joshi A, Doval DC, et al. Novel formulations of docetaxel, paclitaxel and doxorubicin in the management of metastatic breast cancer. Oncol Lett 2018; 16: 3757–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359241236442 for Treatment with nanosomal paclitaxel lipid suspension versus conventional paclitaxel in metastatic breast cancer patients – a multicenter, randomized, comparative, phase II/III clinical study by Chiradoni Thungappa Satheesh, Rakesh Taran, Jitendra Kumar Singh, Shanti Prakash Shrivastav, Nikunj K. Vithalani, Kalyan Kusum Mukherjee, Rajnish Vasant Nagarkar, Tanveer Maksud, Ajay Omprakash Mehta, Krishnan Srinivasan, Mummaneni Vikranth, Satish Ramkrishna Sonawane, Ateeq Ahmad, Saifuddin Sheikh, Shoukath M. Ali, Ronak Patel, Mahesh Paithankar, Lav Patel, Anil Rajani, Deepak Bunger, Alok Chaturvedi and Imran Ahmad in Therapeutic Advances in Medical Oncology