Abstract
The purpose of this study was to determine whether chronic administration of Δ9-tetrahydrocannabinol (THC) during adolescence would (1) modify any sex-specific effects of THC on learning and (2) affect the development of tolerance to THC as an adult. Male and female rats received daily injections of saline or 5.6 mg/kg of THC from postnatal day 35–75, yielding four groups (female/saline, female/THC, male/saline, and male/THC). Rats were then trained on a procedure that assayed both learning and performance behavior and administered 0.32–18 mg/kg of THC acutely as adults (experiment 1). THC produced rate-decreasing and error-increasing effects in both sexes; however, female rats were more sensitive than male rats were to the rate-decreasing effects. Rats were then chronically administered 10 mg/kg of THC (experiment 2). Rats that received THC during adolescence developed tolerance to the rate-decreasing effects more slowly and less completely than did rats that received saline; in addition, females developed tolerance to the error-increasing effects of THC slower than males did. Western blot analysis of brain tissue indicated long-term changes in hippocampal and striatal cannabinoid type-1 receptor (CB1R) levels despite levels that were indistinguishable immediately after chronic treatment during adolescence. Striatal CB1R levels were increased in adult rats that received THC during adolescence; hippocampal CB1R levels varied by sex. In summary, female rats were more sensitive than male rats were to the acute and chronic effects of THC, and chronic administration of THC during adolescence produced long-term changes in CB1R levels that correlated with decreased tolerance development to the rate-decreasing effects of THC.
Introduction
Marijuana is one of the most commonly used illicit substances in the United States, especially among adolescents and young adults. According to the Youth Risk Behavior Study (Centers for Disease Control and Prevention, 2011), 39.9% of U.S. high school students had used marijuana at least once in their lifetime, and 23.1% had used it during the month before the survey. Furthermore, 67% of persons older than 12 claimed that marijuana was the first illicit substance they used, and the average age of drug initiation was 17.5 years (Substance Abuse and Mental Health Services Administration, 2012). Along with these epidemiologic data are data indicating that marijuana use during adolescence may result in long-lasting changes in the brain or behavior (Ehrenreich et al., 1999; Wilson et al., 2000; Pope et al., 2003; Meier et al., 2012).
Researchers have typically used rodents to characterize the long-term effects of chronic cannabinoid administration during adolescence in both males and females (Biscaia et al., 2003; Rubino et al., 2008, 2009; Harte and Dow-Edwards, 2010; Llorente-Berzal et al., 2011; Mateos et al., 2011; Winsauer et al., 2011, 2012; Harte-Hargrove and Dow-Edwards, 2012). Male rats that received Δ9-tetrahydrocannabinol (THC) during adolescence made more errors during the acquisition of a radial-arm maze task than males that received vehicle (Rubino et al., 2009). In addition, adult female rats chronically administered THC during adolescence were differentially sensitive to the rate-decreasing and error-increasing effects of THC on responding under a complex learning task, and these effects depended on ovarian hormone status (Winsauer et al., 2011; Winsauer and Sutton, 2014). Studies investigating the long-term effects of THC administration have also correlated these behavioral changes with changes in cannabinoid type-1 receptor (CB1R) expression in regions of the brain such as the hippocampus and striatum (Burston et al., 2010; Winsauer et al., 2011; Lopez-Gallardo et al., 2012). For example, Lopez-Gallardo et al. (2012) found that the administration of CP 55,940 (a cannabinoid receptor agonist) to adolescent rats produced sexually dimorphic changes in CB1R expression in several regions of the hippocampus. In their study specifically, receptor levels were decreased in male rats but were either increased or unchanged in females.
One mechanism controlling the cell surface regulation and expression of CB1R is intracellular transport. In a previous study, interactions between CB1R and the activator of heat-shock 90-kDa protein ATPase homolog 1 (AHA1) promoted CB1R transport to the plasma membrane and enhanced CB1R-mediated inhibition of adenylyl cyclase by THC (Filipeanu et al., 2011). AHA1 levels were also increased in the cerebellum of adult female rats that were ovariectomized (OVX) and received THC as adolescents, suggesting this protein is relevant to CB1R expression in vivo. Beyond that initial report, however, no studies have investigated the interaction between CB1R and AHA1.
Many of the behavioral disruptions produced by THC are reduced during chronic administration, both in human (Jones et al., 1976; Hunt and Jones, 1980) and nonhuman (McMillan et al., 1970; Ferraro and Grisham, 1972; Grilly et al., 1973; Ferraro and Grilly, 1974; Branch et al., 1980; Pertwee et al., 1993; Delatte et al., 2002) subjects. Although the development of tolerance to cannabinoids has been well characterized, few studies have specifically studied sex differences in the development of tolerance (Wakley et al., 2014). In addition, only one study has examined how chronic THC administration during adolescence can alter future tolerance development, and it was limited to the disruptive effects of THC on rotarod coordination in rats (Barnes and Fried, 1974).
The purpose of the present study was to determine whether chronic THC administration to adolescent male and female rats would: (1) modify any sex-specific effects of THC on learning and (2) affect the development of tolerance during subsequent chronic administration. To do this, both sexes of rat received daily injections of THC from postnatal day (PD) 35–75 as their chronic adolescent regimen. Experiment 1 determined the acute effects of THC on learning in both sexes as an adult and the relative potency of THC after this chronic adolescent regimen. For experiment 2, these same rats were chronically administered THC again with a higher dose to characterize tolerance development. The magnitude of tolerance was also determined by establishing dose-effect curves for THC during administration of the chronic dose. Finally, brain tissue was obtained from small cohorts of subjects after chronic administration of THC during adolescence, the acute dose-effect curves as adults, and chronic administration as adults to determine the CB1R and AHA1 protein levels in the hippocampus and striatum.
Materials and Methods
Subjects.
Fifty-six Long-Evans hooded rats (28 male and 28 female) served as subjects for this two-experiment study. All subjects were purchased from a commercial vendor (Harlan Laboratories, Indianapolis, IN) as pups and arrived at the animal care facility at 21 days of age. After their arrival, subjects were group housed until PD 30, when each subject was housed individually in polypropylene plastic cages with hardwood chip bedding. A diet of standard rodent chow (Harlan Laboratories) was available ad libitum until PD 75. Food restriction was instituted at this time to maintain subjects at approximately 90% of their free-feeding weight for behavioral experiments. Water was always available in the home cages.
The colony room was maintained at 21 ± 2°C with 50% ± 10% relative humidity on a 14/10 light/dark cycle (lights on 6:00 AM; lights off 8:00 PM). Rats responded for 45-mg food pellets (Purina Mills Test Diet, Richmond, VA) during the experimental sessions and were provided standard rodent chow in their home cage after the test sessions when necessary. This study was carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institutes of Health.
Adolescent Administration of Saline or THC.
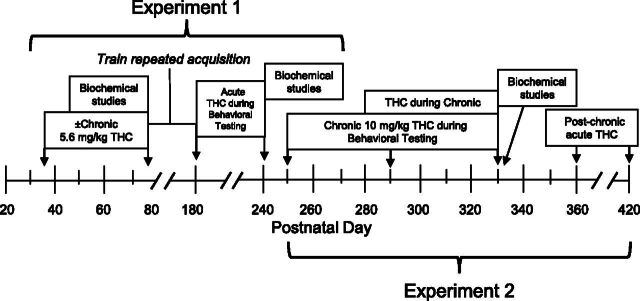
As diagramed in Fig. 1, from PD 35–75, all subjects received either a single injection of saline or 5.6 mg/kg THC i.p. at the same time every day (approximately 1:00 PM), yielding four treatment groups with respect to sex and adolescent treatment (i.e., female/saline, female/THC, male/saline, male/THC). This dose of THC and the period of administration have been used in several studies from this laboratory and have also been determined to produce long-term effects in female rats (Winsauer et al., 2011). The primary advantage of this dose is that it is a behaviorally effective dose for which the development of tolerance has been characterized (Delatte et al., 2002) and one that does not appear to induce marked dependence. The THC was obtained from National Institute on Drug Abuse (Research Technical Branch, Rockville, MD) and arrived in a 100% ethanol solution at a concentration of 200 mg/ml. The THC was stored at −20°C after these solutions were partitioned into smaller aliquots and the ethanol was removed by high-speed vacuum. When needed, an aliquot was reconstituted for injection as an emulsion using ethanol, emulphor (Alkamuls EL-620; Rhodia Inc., Cranbury, NJ), and saline in a 1:1:18 ratio. The volume for both saline and THC injections was 0.1 ml/100 g body weight. On PD 76, 24 hours after the final chronic injection during adolescence, 16 subjects (four subjects from each treatment group) were euthanized for Western blot analysis (described later herein). The remaining subjects began training to respond under the behavioral procedure.
Fig. 1.
Diagram showing the timeline of manipulations for the subjects in the male and female adolescent rat groups during the two experiments.
Apparatus.
Twelve identical modular test chambers (model E10-10TC; Coulbourn Instruments, Allentown, PA) configured specifically for rodents were used. Located on the front wall of each chamber were a house light, auditory feedback relay, pellet trough (5.5 cm above the floor and centered), and three response keys aligned horizontally (8 cm apart, center to center, and 14.5 cm above the floor). Each response key could be transilluminated by three miniature 28ESB indicator lamps (General Electric Company, Cleveland, OH): one with a green cap, one with a red cap, and one with a yellow cap. Correct responses produced an audible click of an electromechanical relay that was used to provide feedback. A food-pellet dispenser was located behind the front wall of the chamber and connected to the pellet trough. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise to mask extraneous sounds. All test chambers were connected to and controlled by a computer programmed with MED-PC for Windows, version IV software (MED Associates Inc., St. Albans, VT), and to cumulative recorders (Gerbrands, Arlington, MA) located within the same room.
Behavioral Training and Procedure.
The behavioral procedure for the two experiments was a two-component procedure that required both the repeated acquisition and performance of three-response sequences. In the repeated-acquisition component, subjects acquired a different sequence each session, whereas in the performance component, subjects completed the same sequence each session.
Preliminary training under the multiple schedules of repeated acquisition and performance occurred in a fashion that was modified from that described previously (Gerak et al., 2004). Briefly, subjects initially underwent magazine training, shaping of the response (nose press) on the center key by food presentation, and reinforcement over multiple days for responding on each of the three keys when individually illuminated after shaping. After subjects reliably responded on each key, they were introduced to the stimuli of the three-response sequence over 3 consecutive days. On the first day, subjects were allowed to respond on any key when all three keys were transilluminated yellow, the terminal stimulus of the response sequence. On the second and third day, subjects were still allowed to respond on any key, but the sessions began with the stimuli for the second (red) and first (green) links of the response sequence, respectively. Each response resulted in an audible click of the feedback relay, momentary offset of the key lights, and a change in key color. When the three-response sequence was completed, the key lights were extinguished, a stimulus light in the pellet trough was illuminated for 0.4 seconds, and a 45-mg food pellet was dispensed from the feeder. After reinforcement, the key lights were illuminated again. Key color presentation followed a set order (i.e., green, red, yellow), and this order did not change between sessions. Thus, at the end of this preliminary training, three responses could be emitted on any key to obtain a food reinforcer, and there was no restriction on the ordering (e.g., R-R-R, C-C-C, L-L-L, or any combination of responses was acceptable).
Training for repeated acquisition began by restricting the ordering of responses to each of the colored stimuli. More specifically, each subject’s task was to respond on the correct key in the presence of each color, such that a correct response in the presence of one color changed the color of the stimuli, as well as the position for the next correct response (e.g., keys green, center correct; keys red, left correct; keys yellow, right correct). When the response sequence was completed by emitting three correct responses (i.e., one correct response for each color), the key lights were extinguished and the stimulus light in the pellet trough was illuminated for 0.4 seconds. Subsequently, the response keys were illuminated with the first stimulus (i.e., green), and the sequence was reset. Within a given session, the correct response that was associated with a particular color did not change, and the same sequence (in this case, center-left-right or C-L-R) was repeated during all acquisition components of a given session. When rats responded on an incorrect key (in this example, the left or right key when the center key was correct), the error was followed by a 5-second timeout. Responding during this period had no programmed consequence, and an incorrect response did not reset the three-response sequence (i.e., the position of the correct response was the same before and after a timeout). Responding was initially maintained by food presentation under a second-order, fixed-ratio (FR) 1 schedule (i.e., completion of each three-response sequence resulted in presentation of a food pellet); however, the second-order FR schedule was changed gradually from 1 to 3 during training, such that every three completions of the sequence resulted in presentation of a food pellet.
To establish a steady state of repeated acquisition, the sequence was changed from session to session. An example of a typical set of sequences for five consecutive sessions was R-L-C, L-C-R, C-R-L, L-R-C, and C-L-R. The sequences were carefully selected to be equivalent in several ways, and there were restrictions on their ordering across sessions (Thompson, 1973).
During performance components, the house light and the response keys were illuminated. The house light served as a discriminative stimulus for responding during this component, and unlike the acquisition component, the sequence in this component remained the same from session to session (i.e., R-C-L, which was reserved for performance components after the introduction of the two components). In all other aspects (colored stimuli for each response in the sequence, second-order FR-3 schedule of food presentation, 5-second timeout, etc.), the performance components were identical to the acquisition components.
Experimental sessions always began with an acquisition component, which then alternated without a delay to a performance component after 40 food reinforcers or 20 minutes, whichever occurred first. Components alternated after 40 food reinforcers or 20 minutes throughout the session, and each session terminated after 160 food reinforcers or 80 minutes. During experiment 1, sessions were generally conducted 6 days per week, Monday through Saturday, and during experiment 2 (i.e., during chronic drug testing), sessions were conducted 7 days per week. Subjects were considered to have stable responding under the two-component procedure when response rates and the percentage of errors did not vary by more than 15% of each subject’s mean for 10 consecutive days.
Post-training Acute Administration of THC during Adulthood.
All but two female subjects (one from each treatment group) achieved stable responding under the behavioral procedure between PD 76–180, so they were excluded from the study. After completion of training, each group was administered THC acutely during PD 181–240. During these 60 days of acute testing, doses of THC were administered in a mixed order every 3 or 4 days. THC was administered i.p. 30 minutes before the start of these sessions, and the dose-effect curves for each subject ranged from an ineffective dose to a dose that substantially decreased response rate or eliminated responding entirely (0.32–18 mg/kg of THC). Most injection volumes were 0.1 ml/100 g body weight. Because of limitations in solubility, the injection volume for THC had to be increased for doses larger than 10 mg/kg. As a control for these acute THC injections, all subjects received vehicle injections every 7 days, 30 minutes before the start of the session. On days when subjects were not receiving THC or vehicle, baseline sessions (no injections) were conducted to maintain stable responding under the behavioral procedure.
On completion of these acute THC dose-effect curves, 12 subjects (three for each treatment group) were euthanized in pairs for analysis of protein levels in specific brain regions. To control for the circulating levels of endogenous hormones across the estrus cycle, each female was euthanized with one male from their respective adolescent treatment group (saline or THC) when the female was in proestrus.
Chronic Aministration of THC to Adult Rats.
As diagramed in Fig. 1, after completion of experiment 1 (PD 250), the 26 remaining subjects (six female/saline, six female/THC, seven male/saline, and seven male/THC) began receiving 10 mg/kg of THC i.p. 30 minutes before the start of each behavioral session (i.e., daily). Subjects received 5.6 mg/kg of THC on the first day of chronic treatment and 10 mg/kg thereafter until 40 days had elapsed. From this point forward, varying doses of THC (18–100 mg/kg) or vehicle were substituted for the chronic dose of THC twice a week on Tuesdays and Fridays. Otherwise, the chronic regimen was maintained until the end of the experiment.
On completion of the THC dose-effect curves, 16 subjects (four from each treatment group) were euthanized in male-female pairs. Ovarian cycle was not tracked during this phase of the experiment, and, thus, females were not euthanized during proestrus.
On the final day of the chronic regimen, 1 mg/kg of rimonabant (Cayman Chemical, Cayman, MI), a selective CB1R antagonist, was substituted for the chronic dose of THC for the remaining subjects to determine whether they were physically dependent on THC. Rimonabant was dissolved in a vehicle of ethanol, emulphor, and saline in a 1:1:1 ratio and administered 30 minutes before the behavioral session.
Western Blot Analysis.
After subjects were euthanized by decapitation for biochemical studies (Fig. 1), the brains were rapidly removed, and the hippocampus and striatum were separated using the procedure described in Glowinski and Iversen (1966). The levels of CB1R and AHA1 were determined by Western blotting of brain homogenates as described previously (Filipeanu et al., 2004, 2011). When all the samples could not be loaded on a single gel, samples from each treatment group were evenly divided between gels, and a sample was included in a separate lane for each gel and used as a loading control between blots.
The primary antibodies used were anti-CB1R (item no. 101500; Cayman Chemical, Ann Arbor, MI), anti-AHA1 (catalog no. H00010598-M01, AbNova, Taipei City, Taiwan), and anti-β-actin (SC-47778; Santa Cruz Biotechnology, Dallas, TX). The secondary antibodies used were anti-rabbit and anti-mouse, both of which were HRP-labeled (PerkinElmer, Waltham, MA).
Data Analyses.
The data collected for the acquisition and performance components of the behavioral procedure were analyzed in terms of: (1) the overall response rate (total responses/min, excluding timeouts) and (2) the overall accuracy, expressed as a percentage of errors [incorrect responses/total responses) × 100]. When response rates were less than 5 responses per minute, however, the percentage of errors was not calculated owing to the small number of responses involved. Significant differences in response rate or the percentages of errors for both components were determined using two-way analysis of variance (ANOVA) with repeated measures (Sigmaplot for Windows Version 12.5; Systat Software, Inc., Point Richmond, CA). To determine whether THC administration during adolescence altered adult sensitivity to the disruptive effects of THC for either sex, male and female subjects were analyzed separately using adolescent treatment and dose of THC as the factors. Significant main effects were determined by Holm-Sidak post hoc tests. After this analysis indicated there was no significant effect of adolescent treatment within each sex, the effect of sex on the acute effects of THC was examined using a two-way ANOVA with repeated measures, with sex and dose of THC as the factors. Significance was accepted at an α level of ≤ 0.05 for all statistical tests.
Sensitivity to the effects of THC was also quantified by comparing the ED50 values of the dose-effect curves. These ED50 values were determined for each subject by linear regression using two or more data points reflecting the slopes of the descending curve for the response rate or the ascending curve for the percentage of errors. For the response rate, the ED50 represented the estimated dose of THC that decreased responding from control levels by 50%. For the percentage of errors, the ED50 represented the estimated dose of THC that increased the percentage of errors to 150%, or 50% above control levels.
The first 41 days of chronic THC administration during adulthood were averaged across 3 days for each subject and then plotted as means for each treatment group. In addition, tolerance was determined for each individual subject by the following criteria: (1) the subject’s response rate and percent errors in the acquisition component did not vary by more than 10% of the mean for five consecutive days, or (2) the subject’s response rate and percent errors during acquisition were within the prechronic control range for 5 consecutive days. During the chronic phase of the experiment, these criteria helped capture the highest level of tolerance achieved by each subject, as not all subjects were able to respond at control levels emitted during the first experiment. Development of tolerance to the rate-decreasing and error-increasing effects of THC was also characterized using an index of curvature (Fry et al., 1960; Thompson, 1975), which was calculated from the group means rather than from individual subject data; thus, confidence intervals were not calculated.
The effects of rimonabant were compared with the effects of vehicle and 10 mg/kg of THC during the chronic phase using a two-way ANOVA with repeated measures. Because there was no discernable effect of adolescent treatment, and few subjects remained when rimonabant was tested (i.e., 2 female/saline, 2 female/THC, 3 male/saline, and 3 male/THC), the factors used were sex and drug administered.
Western blot quantifications were analyzed by two-way ANOVA tests for each brain region and experimental phase, with sex and adolescent treatment as the factors. Significant main effects for each factor were analyzed by Holm-Sidak post hoc tests. When a significant interaction occurred, one-way ANOVA tests were performed between each group to determine significant differences.
Results
Experiment 1
Chronic administration of 5.6 mg/kg during adolescence decreased the respective body weights of female and male rats (Table 1). The difference in weight produced by chronic administration of THC had diminished for males, however, or disappeared entirely for females, by the end of the first experiment (PD 230).
TABLE 1.
Mean (± S.E.M.) body weight for each treatment groupa at three different times during the study
| Group | Pre-THC (PD 35) | Post-THC (PD 74) | Adult (PD 230) |
|---|---|---|---|
| Female/saline | 111.7 ± 3.3 | 221.9 ± 4.7 | 225.1 ± 4.0 |
| Female/THC | 109.4 ± 3.2 | 200.1 ± 3.4 | 226.0 ± 3.7 |
| Male/saline | 116.3 ± 1.6 | 332.4 ± 4.9 | 352.7 ± 3.2 |
| Male/THC | 114.7 ± 2.8 | 312.1 ± 6.4 | 342.8 ± 4.2 |
Includes only subjects that were behaviorally trained.
Effects of THC Administration during Adolescence on Adult Sensitivity to THC.
In the female rats (Fig. 2A), THC significantly decreased the response rate and increased percent errors in both components of the behavioral procedure in a dose-dependent manner. Administration of 5.6 mg/kg of THC or saline during adolescence did not affect either measure of responding, as the dose-effect curves for the two groups largely overlapped. This was also evident from the statistical analyses, which indicated there was a main effect of THC dose on response rate [acquisition, F(7,94) = 23.549, P < 0.001; performance, F(7,94) = 22.930, P < 0.001] and the percent errors [acquisition, F(6,76) = 5.967, P < 0.001; performance, F(6,76) = 3.027, P = 0.010] but no effect of adolescent treatment on response rate [acquisition, F(1,94) = 0.421, P > 0.05; performance, F(1,94) = 0.049, P > 0.05] or percent errors [acquisition, F(1,76) = 0.639, P > 0.05; performance, F(1,76) = 1.737, P > 0.05] in both behavioral components. There were no dose X adolescent treatment interactions for any measure [acquisition, F(7,94) = 1.255, P > 0.05; performance, F(7,94) = 0.736, P > 0.05]; and errors [acquisition, F(6,76) = 0.439, P > 0.05; performance, F(6,76) = 0.248, P > 0.05]. Response rate was also more sensitive to disruption than percent errors in both components. For example, response rate was significantly reduced by 1 mg/kg of THC in the acquisition component, but a dose of 3.2 mg/kg of THC was required to significantly increase percent errors.
Fig. 2.
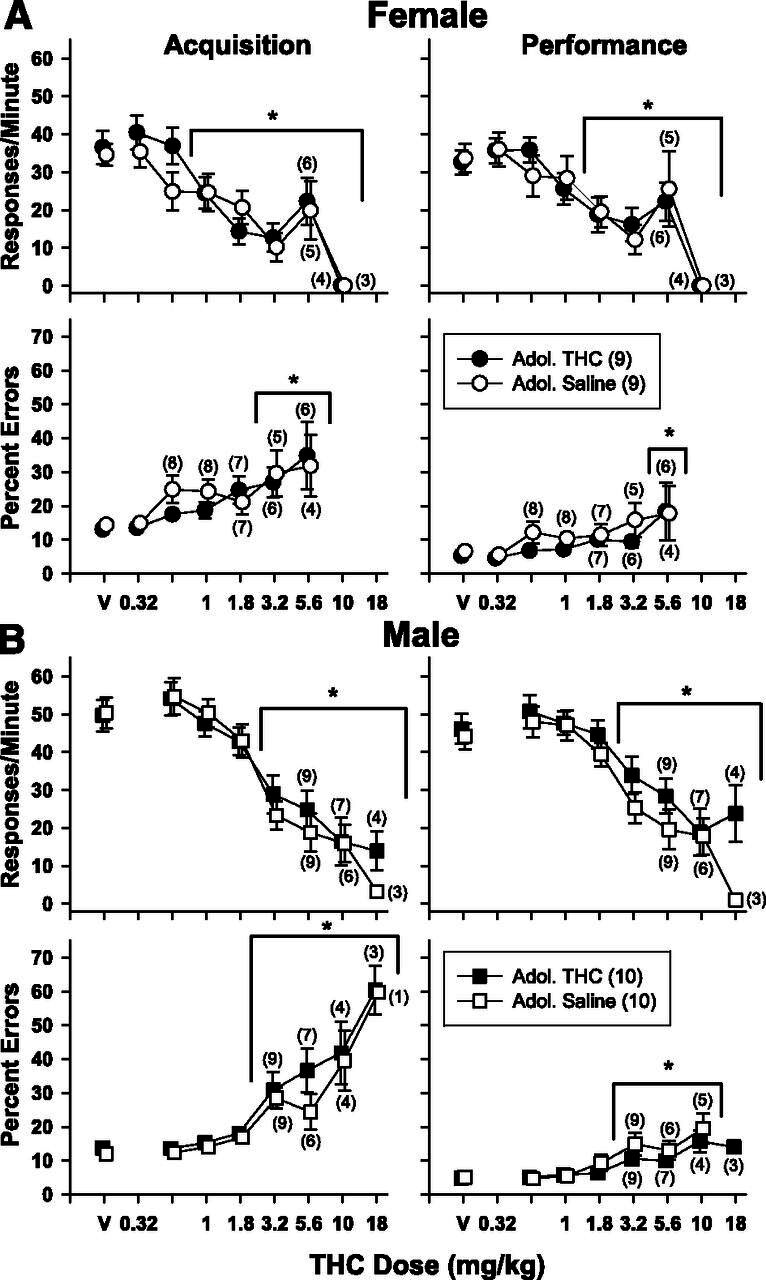
Mean effects of acute THC administration on response rate (upper panels) and percent errors (lower panels) in subjects responding under a repeated acquisition and performance procedure. Top panels (A) show the effects of THC on female subjects, and bottom panels (B) show the effects of THC on male subjects. Circles represent female subjects, and squares represent male subjects. Unfilled symbols represent subjects that received saline daily during adolescence, whereas filled symbols represent subjects that received 5.6 mg/kg daily of THC during adolescence. Asterisks and brackets represent a significant difference between a given drug dose and vehicle as determined by two-way repeated-measures ANOVA tests.
In addition, THC produced significant dose-dependent decreases in the response rate and increases in percent errors in the male rats (Fig. 2B). In contrast to the female subjects, however, THC was equipotent in disrupting response rate and percent errors in the male subjects. More specifically, 3.2 mg/kg significantly reduced the response rate and increased percent errors in both components of the procedure. There was a main effect of THC dose on response rate in both the acquisition [F(7,104) = 31.716, P < 0.001] and performance [F(7,104) = 26.119, P < 0.001] components, with no main effect of adolescent treatment [acquisition, F(1,104) = 0.003, P > 0.05; performance, F(1,104) = 0.592, P > 0.05] and no dose X adolescent treatment interaction [acquisition, F(7,104) = 0.346, P > 0.05; performance, F(7,104) = 0.605, P > 0.05]. Likewise, there were main effects of dose of THC for the percentage of errors in both components [acquisition, F(7,89) = 21.871, P < 0.001; performance, F(6,89) = 11.233, P < 0.001] but no main effect of adolescent treatment [acquisition, F(1,89) = 1.091, P > 0.05; performance, F(1,89) = 1.260, P > 0.05], and no interaction [acquisition, F(7,89) = 0.596, P > 0.05; performance, F(6,89) = 0.537, P > 0.05].
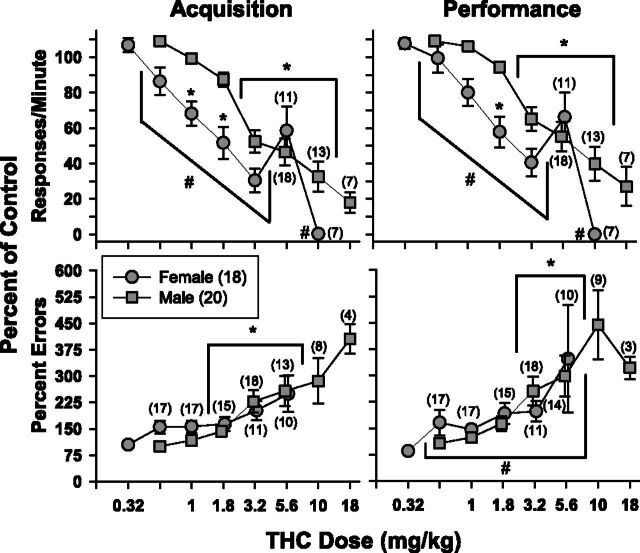
Given the lack of significant behavioral effects produced by THC during adolescence in both sexes, the adolescent treatment groups were collapsed to analyze the data for an overall effect of sex. Because of differences in baseline response rates between female and male rats, these data are presented as percentage of control. As shown (Fig. 3), females were markedly more sensitive than males to the rate-decreasing effects of THC in both components but only marginally more sensitive to the error-increasing effects. The findings for response rate were supported statistically by a main effect of sex [acquisition, F(1,189) = 34.162, P < 0.001; performance, F(1,189) = 23.599, P < 0.001], a main effect of THC dose [acquisition, F(6,189) = 44.602, P < 0.001; performance, F(6,189) = 42.359, P < 0.001] and a significant interaction between dose and sex [acquisition, F(6,189) = 3.768, P = 0.001; performance, F(6,189) = 3.351, P = 0.004]. In contrast, there was only a main effect of dose for percent errors in the acquisition component [F(5,151) = 18.099, P < 0.001], and main effects for dose and sex in the performance component [dose, F(5,152) = 7.603, P < 0.001; sex, F(1,152) = 5.486, P = 0.023]. There was no interaction in either component [acquisition, F(5,151) = 1.219, P > 0.05; performance, F(5,152) = 1.019, P > 0.05] and no effect of sex for acquisition errors [F(1,151) = 2.763, P > 0.05].
Fig. 3.
Mean effects of THC on response rate (upper panels) and percent errors (lower panels) in 38 subjects responding under a repeated acquisition and performance procedure. Circles represent data from female subjects, whereas squares represent data from male subjects. Two-way ANOVA tests were used to determine statistical significance. Asterisks and brackets represent doses that were significantly different than vehicle injections; pound signs and brackets represent doses that were significantly different between male and female subjects.
The marked difference in sensitivity to the rate-decreasing effects between sexes was also reflected in the ED50 values (Table 2). For example, the ED50 values for the female/saline and female/THC groups were 2.89 and 3.39 mg/kg for response rate in the acquisition component, whereas the ED50 values for the male/saline and male/THC groups were 5.57 and 5.71 mg/kg, respectively. The ED50 values for the male groups also fell outside of the 95% confidence intervals calculated for both female groups, further indicating a significant difference between the sexes. Although the male groups also had larger ED50 values than the female groups for percent errors in both acquisition (1.60 and 2.15 mg/kg vs. 1.02 and 1.28 mg/kg, respectively) and performance components (1.86 and 2.60 mg/kg vs. 1.15 and 1.16 mg/kg), the values for the male groups fell within the confidence intervals for the female groups.
TABLE 2.
Effective dose for decreasing response rate by 50% or increasing the percentage of errors by 50% in rats responding under a repeated acquisition and performance procedure
Numbers in parentheses are the 95% confidence intervals for the means.
| Group | Resp. Rate (A) | % Errors (A) | Resp. Rate (P) | % Errors (P) |
|---|---|---|---|---|
| Female/saline | 2.89 (1.16–4.62) | 1.02 (0.37–1.66) | 3.31 (1.29–5.33) | 1.15 (0.47–1.82) |
| Female/THC | 3.39 (1.25–5.52) | 1.28 (0.52–2.04) | 3.92 (1.81–6.03) | 1.16 (0.35–1.96) |
| Male/saline | 5.57 (2.73–8.41) | 1.60 (1.07–2.14) | 5.82 (2.43–9.20) | 1.86 (0.78–2.95) |
| Male/THC | 5.71 (3.38–8.04) | 2.15 (1.39–2.91) | 6.17 (3.51–8.84) | 2.60 (1.20–3.99) |
Biochemical Changes in the Hippocampus and Striatum after Chronic THC Administration during Adolescence.
Immediately after 40 days of chronic saline or THC administration during adolescence, protein levels of CB1R and AHA1 were quantified for each treatment group by Western blot (Fig. 4). CB1R was readily detected as a major band at approximately 45 kDa in both the hippocampus and striatum. After normalization to β-actin, no differences were seen between treatment groups for either brain area. More specifically, no main effects were seen for sex [hippocampus, F(1,12) = 0.940, P > 0.05; striatum, F (1,12) = 1.428, P > 0.05] or adolescent treatment [hippocampus, F(1,12) = 2.371, P > 0.05; striatum, F(1,12) = 0.421, P > 0.05] and no interaction between these two factors [hippocampus, F(1,12) = 0.043, P > 0.05; striatum, F(1,12) = 0.337, P > 0.05].
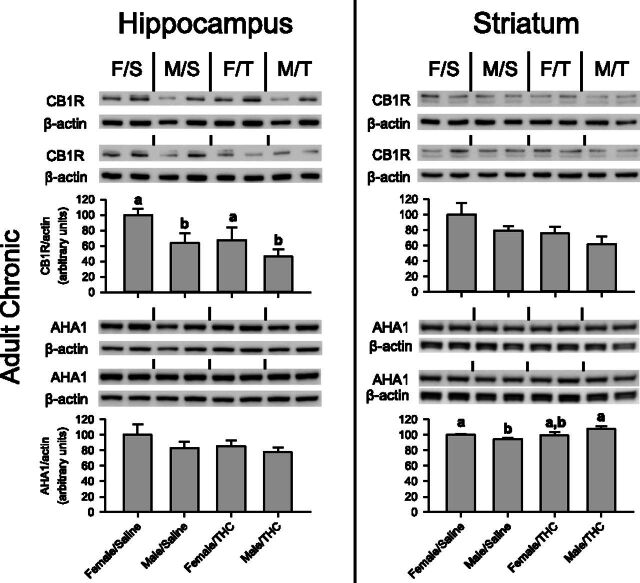
Fig. 4.
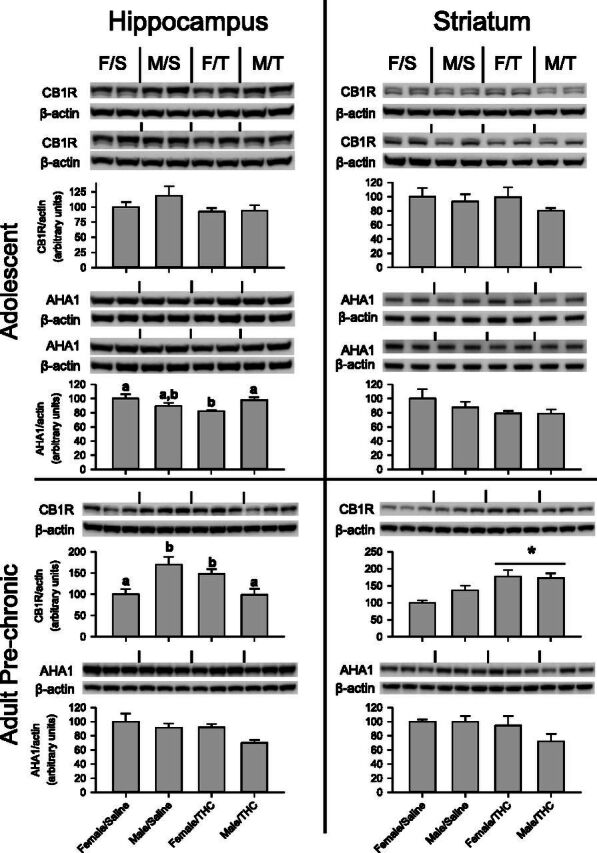
Immediate and long-term effects of chronic THC administration during adolescence on CB1R and AHA1 protein levels in the hippocampus (left) and striatum (right) of male and female rats. The images are Western blots of the corresponding protein and the actin for each treatment group; bar graphs represent quantification of the protein in each brain region. Each group is identified by a two-letter designation above the images indicating sex and adolescent treatment (F/S = female/saline, F/T = female/THC, M/S = male/saline, M/T = male/THC). Upper panels represent protein levels in adolescent rats immediately after 40 days of chronic saline or THC administration, and the lower panels represent protein levels in adult rats after acute administration of THC. Letters above the bars represent a significant difference between groups as determined by two-way ANOVA tests (e.g., “a” is significantly different from “b” but not different from “ab”). An asterisk and bar represent a main effect of adolescent treatment.
In the hippocampus, a significant interaction was noted between adolescent treatment and sex on AHA1 levels [F(1,12) = 8.587, P = 0.013], with no main effects of sex [F(1,12) = 0.370, P > 0.05] or adolescent treatment [F(1,12) = 1.246, P > 0.05]. Subsequent one-way ANOVA tests revealed that the female/THC group had significantly lower AHA1 levels in the hippocampus than both the female/saline and male/THC groups, but not the male/saline group. AHA1 levels did not differ between groups in the striatum [sex, F(1,12) = 0.566, P > 0.05; adolescent treatment, F(1,12) = 3.054, P > 0.05; sex X adolescent treatment, F(1,12) = 0.495, P > 0.05].
The lower panels of Fig. 4 show the Western blot analysis of CB1R and AHA1 protein levels obtained from the brain tissue of rats that received chronic saline or THC during adolescence followed by acute THC during adulthood. At this time point, significant differences were noted between treatment groups in both brain regions. In the hippocampus, CB1R levels varied with sex and adolescent treatment. Female rats that received chronic THC had more CB1R than females that received saline; however, the opposite occurred in male rats, with the male/THC group having lower CB1R levels than the male/saline group. These effects were verified by a significant interaction of sex and adolescence treatment [F(1,8) = 18.038, P = 0.003), whereas there were no main effects of adolescent treatment [F(1,8) = 0.671, P > 0.05] or sex [F(1,8) = 0.511, P > 0.05].
The effects on CB1R in striatum differed from those in hippocampus, as chronic THC during adolescence increased adult striatal CB1R levels compared with saline in male and female rats. This was supported by a main effect of adolescent treatment of CB1R [F(1,8) = 15.683, P = 0.004] and no main effect of sex [F(1,8) = 1.318, P > 0.05] or sex X adolescent treatment interaction [F(1,8) = 2.201, P > 0.05].
Unlike CB1R levels, AHA1 levels during this phase of the experiment were unaffected in the hippocampus [sex, F(1,8) = 4.423, P > 0.05; adolescent treatment, F(1,8) = 4.216, P > 0.05; sex X adolescent treatment, F(1,8) = 0.851, P > 0.05] and striatum [sex, F(1,8) = 1.337, P > 0.05; adolescent treatment, F(1,8) = 3.076, P > 0.05; sex X adolescent treatment, F(1,8) = 1.401, P > 0.05].
Experiment 2
Development of Tolerance to the Disruptive Effects of 10 mg/kg of THC.
When 10 mg/kg of THC was administered daily, some level of tolerance to the rate-decreasing and error-increasing effects of THC was evident for all groups within 40 days (Fig. 5). Tolerance took longer to develop in the females than in males, however, and was less complete in the groups that received THC as adolescents as indicated by the number of days to tolerance (Table 3) and the index of curvature values (Table 4). For example, tolerance took at least 5 days longer to develop in the female groups than in the male groups. In addition, four female subjects (two from each adolescent treatment group) failed to develop tolerance, and this was due to highly variable response accuracy for three of the subjects and an inability to develop tolerance to the rate-decreasing effects in one subject. Response rate also recovered more slowly in the male and female groups that received THC as adolescents. For the female/saline and male/saline groups, the indices of curvature were −0.392 and −0.338 for the acquisition component and −0.324 and −0.338 for the performance component, respectively. In contrast, the female/THC and male/THC groups had indices of curvature of −0.003 and −0.142 for the acquisition component and −0.039 and −0.131 for the performance component.
TABLE 3.
Development of tolerance to 10 mg/kg of THC
If a subject did not meet the tolerance criteria, 40 days was used for the calculation of the group mean.
| Group | Days (Mean ± S.E.M.) |
|---|---|
| Female/saline | 25.3 ± 4.4 |
| Female/THC | 27.3 ± 4.8 |
| Male/saline | 19.7 ± 1.6 |
| Male/THC | 19.4 ± 3.3 |
TABLE 4.
Mean index of curvature for the development of tolerance
| Subject | Response Rate (A) | % Errors (A) | Response Rate (P) | % Errors (P) |
|---|---|---|---|---|
| Female/saline | −0.392 | 0.101 | −0.324 | 0.547 |
| Female/THC | −0.003 | 0.259 | −0.039 | 0.575 |
| Male/saline | −0.338 | 0.631 | −0.338 | 0.502 |
| Male/THC | −0.142 | 0.545 | −0.131 | 0.611 |
Fig. 5.
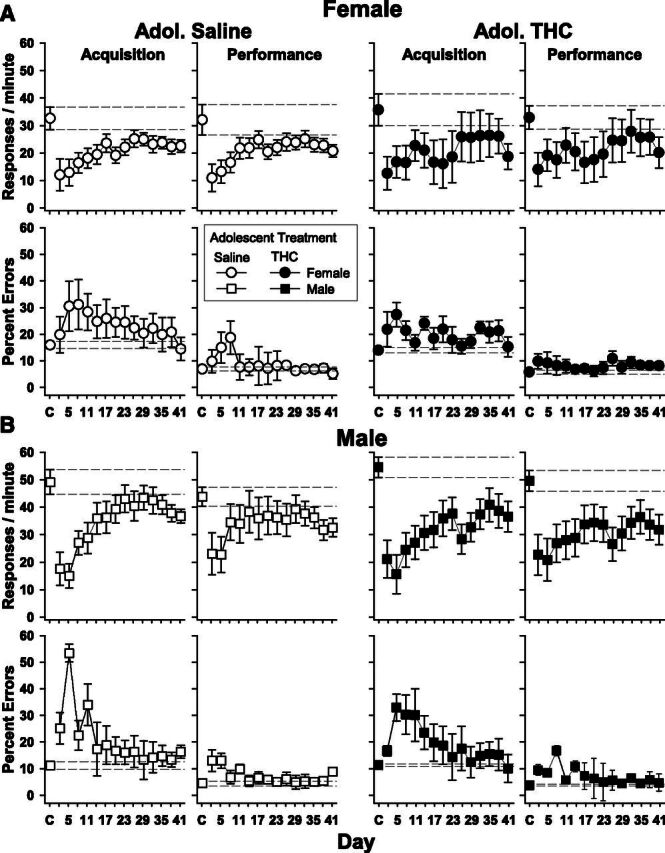
Mean effects of 41 consecutive days of 10 mg/kg of THC on response rate and percent errors in the acquisition and performance components of the procedure. The points and vertical lines above C represent the mean and S.E. for at least seven control (vehicle) injections during the first experiment. Dashed lines represent an extension of the control range for easy comparison with the chronic THC data. All other data points and vertical lines represent the grouped mean and standard error of the mean for 3 consecutive days. Unfilled symbols represent subjects that received saline daily during adolescence; filled symbols represent subjects that received 5.6 mg/kg daily of THC during adolescence. Squares represent males, and circles represent females.
A different pattern emerged for the percentage of errors. In the acquisition component, the female subjects recovered their accuracy more slowly, whereas in the performance component, both male and female subjects recovered their accuracy over a similar time frame. Ultimately, the difference in tolerance development between sexes was evident from the fact that the percentage of errors during the acquisition components for the male subjects rapidly returned close to prechronic levels after a sharp increase during the first 5 days of chronic THC. The indices of curvature were 0.101 and 0.259 for the female/saline and female/THC groups, respectively; for the male/saline and male/THC groups, the values were 0.631 and 0.545. All groups returned to control levels of responding during performance components at relatively similar rates and within 17 days.
THC Dose-Effect Curves after Developing Tolerance to 10 mg/kg of THC.
As shown in Fig. 6, the dose-effect curves for all groups were shifted rightward in a remarkably similar manner after tolerance developed. This was clearly evident from the small effects on response rate after 56 mg/kg and the absence of effects on either measure after 100 mg/kg. Because of the small effects at 56 mg/kg, there were significant main effects of dose on response rate for both sexes and both behavioral tasks but none for percent errors and no main effects for adolescent administration for either dependent measure or sex; there were also no significant interactions between adolescent administration and THC after tolerance developed (Table 5). The lone exception was the significant interaction that occurred between factors in the performance component for the females. In this behavioral component, 56 mg/kg only decreased response rate significantly from vehicle levels in the THC-treated group.
TABLE 5.
Statistical analyses for the THC dose-effect curves after developing tolerance to 10 mg/kg of THC
| Sex | Measure | Response Rate (A) | % Errors (A) | Response Rate (P) | % Errors (P) |
|---|---|---|---|---|---|
| Females | Dose | F(5,45) = 3.356 | F(5,41) = 1.649 | F(5,45) = 2.812 | F(5,43) = 1.889 |
| P = 0.012* | P > 0.05 | P = 0.027* | P > 0.05 | ||
| Adolescent treatment | F(1,45) = 0.065 | F(1,41) = 0.010 | F(1,45) = 0.158 | F(1,43) = 0.050 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||
| Interaction | F(5,45) = 1.871 | F(5,41) = 0.476 | F(5,45) = 2.506 | F(5,4) = 0.219 | |
| P > 0.05 | P > 0.05 | P = 0.044* | P > 0.05 | ||
| Males | Dose | F(5,59) = 4.920 | F(5,59) = 2.250 | F(5,59) = 3.545 | F(5,59) = 2.165 |
| P < 0.001* | P > 0.05 | P = 0.007* | P > 0.05 | ||
| Adolescent treatment | F(1,59) = 0.459 | F(1,59) = 0.126 | F(1,59) = 0.320 | F(1,59) = 0.854 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||
| Interaction | F(5,59) = 0.349 | F(5,59) = 0.504 | F(5,59) = 0.510 | F(5,59) = 1.170 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
Fig. 6.
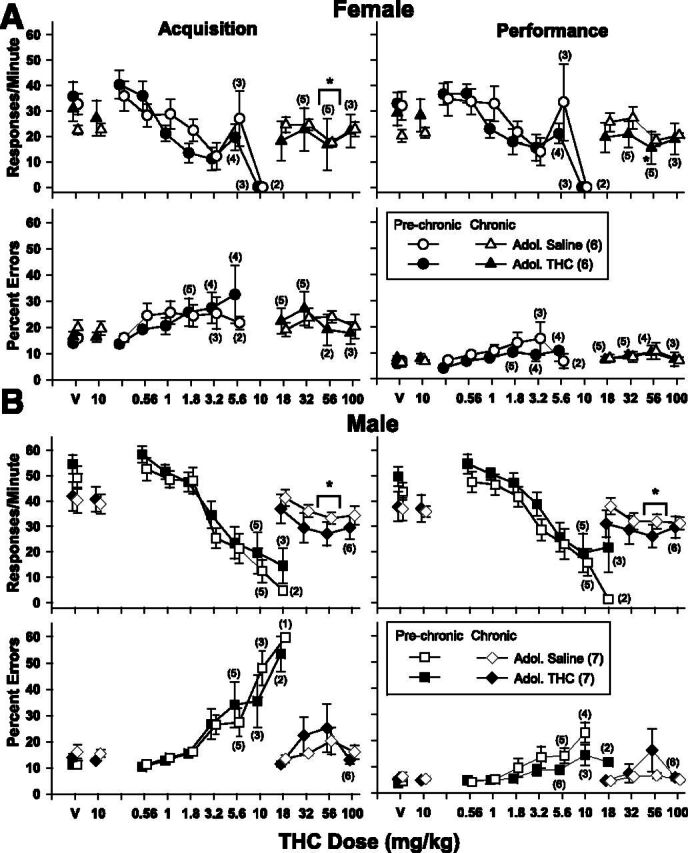
THC dose-effect curves for response rate (upper panels) and percent errors (lower panels) in the acquisition and performance components of the procedure after 40 or more days of 10 mg/kg of THC in 26 subjects. Top panels (A) show dose-effect curves for female subjects with (filled symbols) and without (unfilled symbols) a history of THC administration during adolescence. Bottom panels (B) show dose-effect curves for male subjects with and without a history of THC administration during adolescence. Circles (females) and squares (males) represent the effects of THC before chronic administration; triangles (females) and diamonds (males) represent the effects of THC after developing tolerance to 10 mg/kg of THC. Unfilled symbols represent subjects that received saline daily during adolescence; filled symbols represent subjects that received 5.6 mg/kg daily of THC during adolescence. Asterisks and brackets represent a significant difference between a given dosage of THC and vehicle as determined by two-way repeated-measures ANOVA tests.
Biochemical Changes in the Hippocampus and Striatum after Development of Tolerance to 10 mg/kg of THC.
At the end of experiment 2, females had significantly greater CB1R levels than males in the hippocampus but not the striatum (Fig. 7). In the hippocampus, there was a main effect of sex [F(1,12) = 5.902, P = 0.032], but no main effect of adolescent treatment [F(1,12) = 4.442, P > 0.05] and no interaction between sex and adolescent treatment [F(1,12) = 0.398, P > 0.05]. In the striatum, there was no main effect of sex [F(1,1) = 2.766, P > 0.05] or adolescent treatment [F(1,12) = 3.863, P > 0.05] and no interaction between sex and adolescent treatment [F(1,12) = 0.110, P > 0.05].
Fig. 7.
Effects of chronic administration of 10 mg/kg of THC on CB1R and AHA1 protein levels in the hippocampus (left) and striatum (right) of male and female subjects as adults. The images are Western blots of the corresponding protein and actin for each treatment group; bar graphs represent quantification of each protein normalized to actin in each brain region. Each group is identified by a two-letter designation above the images indicating sex and adolescent treatment (F/S = female/saline, F/T = female/THC, M/S = male/saline, M/T = male/THC). Letters are used to represent significant differences between groups as determined by two-way ANOVA tests (i.e., “a” is significantly different from “b”, but not “a,b”).
In contrast, AHA1 protein levels in the hippocampus were not different among the groups [sex, F(1,12) = 1.158, P > 0.05; adolescent treatment, F(1,12) = 1.685, P > 0.05; sex X adolescent treatment, F(1,12) = 0.253, P > 0.05], but they were different in the striatum. AHA1 levels were significantly decreased in the male/saline group compared with the female/saline and male/THC groups. There was no main effect of sex [F(1,12) = 4.447, P > 0.05] or adolescent treatment [F(1,12) = 0.277, P > 0.05], but there was a significant sex X adolescent treatment interaction [F(1,12) = 5.327, P = 0.040].
Determination of THC Dependence in Subjects Chronically Administered 10 mg/kg of THC.
When 1 mg/kg of rimonabant was substituted for 10 mg/kg of THC at the end of the chronic phase (Fig. 8), a small number of overt withdrawal signs, such as facial rubs, wet-dog shakes, and front paw tremors, were observed before the behavioral session. During the session, rimonabant administration significantly decreased response rate in both components and significantly increased percent errors in the performance component within each sex. For the response rate, in particular, females had lower rates than males; therefore, there were main effects of the drug administered [acquisition, F(2,16) = 27.071, P < 0.001; performance, F(2,16) = 19.711, P < 0.001] and sex [acquisition, F(1,16) = 8.040, P = 0.022; performance, F(1,16) = 5.324, P = 0.050]. There was no interaction between drug and sex [acquisition, F(2,16) = 1.233, P > 0.05; performance, F(2,16) = 0.479, P > 0.05].
Fig. 8.
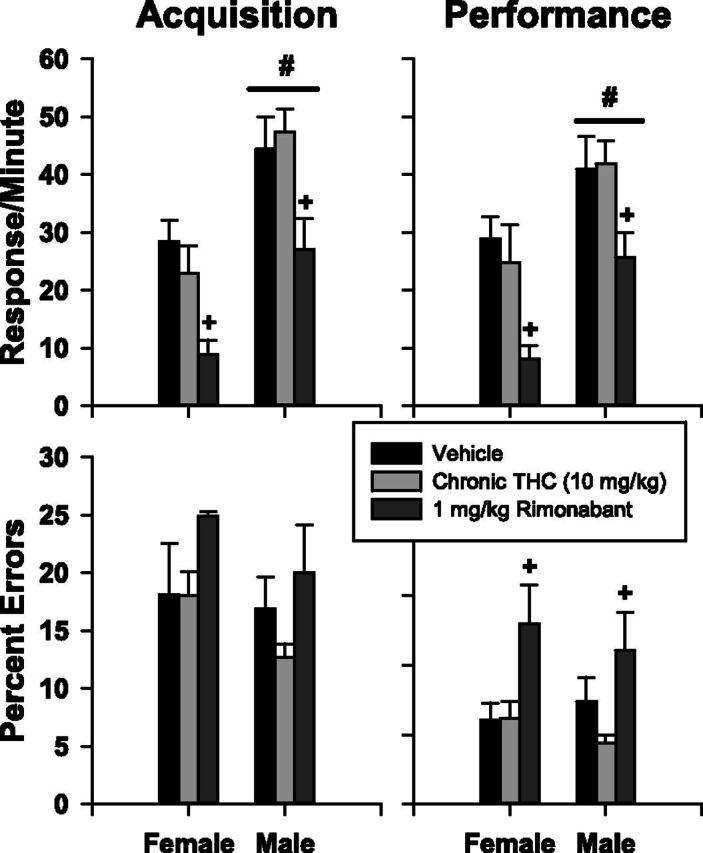
Mean effects of vehicle, 10 mg/kg of THC, or 1 mg/kg of rimonabant on response rate and the percentage of errors in four female and six male subjects chronically administered 10 mg/kg of THC for 80 days. Plus signs indicate that rimonabant is significantly different from vehicle and THC as determined by two-way repeated-measures ANOVA tests. Pound signs and bars represent a main effect of sex.
For the percentage of errors during acquisition, there were no main effects of drug administered [F(2,15) = 3.371, P > 0.05], sex [F(1,15) = 0.599, P > 0.05], and no interaction between factors [F(2,15) = 0.424, P > 0.05]. In the performance component, there was a main effect of the drug administered on percent errors [F(2,15) = 8.821, P = 0.003]. There was no main effect of sex [F(1,15) = 0.030, P > 0.05] and no interaction between factors [F(2,15) = 0.635, P > 0.05].
Discussion
The primary findings from the first experiment were that adult female rats were more sensitive than were males to the rate-decreasing effects of THC in both a learning and performance task. This sexually dimorphic effect was not altered by chronic administration of THC during adolescence in either male or female rats, as these subjects had almost identical responses to acute THC as their saline-treated counterparts. In the second experiment, however, chronic THC during adolescence clearly inhibited the development of tolerance as an adult in both sexes of rat. There were also sex differences with female rats developing tolerance to the error-increasing effects of THC during acquisition components more slowly than males.
To our knowledge, this is the first study designed to directly compare the effects of THC in male and female rats responding under a procedure with repeated acquisition and performance components. In a prior study using an identical 40-day period of chronic THC administration during adolescence, the long-term effects of THC and ovarian hormones were examined in female rats (Winsauer et al., 2011). One of the major findings was that female rats OVX at PD 30 were less sensitive to the rate-decreasing effects and more sensitive to the error-increasing effects of THC than their gonadally intact counterparts. With respect to the rate-decreasing effects, the OVX females were more similar to the male rats of the current study than to the intact females. Two studies that focused on the reinforcing effects of cannabinoids found that OVX rats responded for a similar number of WIN 55,212-2 infusions as male rats in a drug self-administration procedure and emitted extinction responses more comparable to males than intact females (Fattore et al., 2007; Fattore et al., 2010). Although these studies examined different effects of cannabinoids (reinforcing versus disruptive), they strongly support the notion that ovarian hormones are responsible for the increased sensitivity of females to the effects of cannabinoids.
The purpose of the second experiment was to determine whether chronic administration of THC during adolescence would affect an adult subject’s capacity to develop tolerance to the disruptive effects of THC in a sex-dependent manner. In general, both male and female rats that received THC as adolescents did not develop the same magnitude of tolerance to the rate-decreasing effects, and female rats developed tolerance to the error-increasing effects of THC more slowly. This was evident from both the number of days necessary to develop tolerance in each sex and the index of curvature data, which characterized the rate of tolerance development. In addition, unlike the males, some female rats were unable to develop tolerance to 10 mg/kg of THC in this experiment.
Another study has reported changes in adult tolerance to THC based on administration of THC during adolescence (Barnes and Fried, 1974). Male rats that received 4 mg/kg of THC for 41 days during adolescence, and then the same dose chronically as adults, developed tolerance to a THC-induced disruption of balance on a rotarod faster than rats that received saline during adolescence. In contrast, male and female rats that received 5.6 mg/kg THC during adolescence in the current study had more difficulty developing tolerance to the rate-decreasing effects of THC during both components of the procedure than rats that received saline during adolescence. These data were somewhat unexpected in that a prior history of drug administration might be expected to reduce overall (adult) sensitivity to that drug. This notion would also be consistent with data from our laboratory showing that THC administration during adolescence reduced the potency of its acute rate-decreasing effects in female rats as adults (Winsauer et al., 2011); however, the present data are consistent with a more recent paper showing that both intact and OVX female rats administered chronic THC from adolescence through adulthood were less tolerant than rats that initiated chronic administration during adulthood (Winsauer et al., 2015). Together, these studies would suggest that chronic adolescent administration of THC may compromise some of the signaling mechanisms required for the development of tolerance to THC in both males and females.
In previous rodent studies where THC was chronically administered, physical dependence was assessed by administering rimonabant, a CB1R antagonist (Aceto et al., 1996; Beardsley and Martin, 2000; Delatte et al., 2002). Rimonabant precipitates withdrawal and disrupts food-maintained behaviors when administered to THC-dependent rats responding under operant schedules of reinforcement. For example, administration of 1 mg/kg of rimonabant produced rate-decreasing and error-increasing effects in male rats that were chronically administered 5.6 mg/kg of THC (Delatte et al., 2002). Thus, the fact that all subjects showed signs of a precipitated withdrawal after 10 mg/kg in the current study was not surprising and suggests that these subjects were physically dependent on THC. Unfortunately, rimonabant was not tested in these subjects before the chronic phase so that its prechronic and postchronic potency could be compared. This may be important for determining whether its potency is different in the two sexes. Across studies, female rats were particularly sensitive to the disruptive effects of rimonabant as doses as small as 0.32 mg/kg increased percent errors under the repeated acquisition and performance procedure (Winsauer et al., 2015).
In the current study, there were no sex- or treatment-dependent changes in CB1R immediately after cessation of chronic THC during adolescence. This result contrasts with those of other studies in which chronic administration of a cannabinoid agonist decreased CB1R levels in the hippocampus and striatum (Burston et al., 2010; Lopez-Gallardo et al., 2012). There were, however, several differences between the studies that could account for the difference. First, the aforementioned studies determined CB1R binding in the brain by using radiolabeled CP 55,940 in either whole tissue or brain slices, whereas the present experiment determined CB1R levels by Western blot. The difference is that receptor binding assesses CB1R at the cell membrane, whereas Western blot assesses total receptor levels, which include intracellular pools. Second, cannabinoid agonists with different efficacies for CB1R could differentially affect tolerance development (Hruba et al., 2012).
One hypothesis regarding the development of tolerance to the cannabinoids is that CB1R desensitization is the predominant mechanism (Daigle et al., 2008; McKinney et al., 2008; Wu et al., 2008). This hypothesis is based on data showing that (1) receptor desensitization after chronic administration of an agonist is of greater magnitude than receptor downregulation (Breivogel et al., 1999; Rubino et al., 2008); 2) tolerance can occur in the absence of receptor downregulation (Rubino et al., 2000); and 3) the fact that CB1R mRNA expression returned to near-control levels within 21 days of an initial decrease in hippocampus and striatum (Zhuang et al., 1998). As the current study used a chronic regimen of THC administration for 40 days, mRNA expression may have returned to control levels in the THC groups and thereby restored protein levels to the prechronic state.
When CB1R levels were determined in the hippocampus and striatum of adult rats after establishing dose-effect curves for THC, there were differences depending on sex and brain region. In the hippocampus, CB1R levels were increased in female rats that received 5.6 mg/kg of THC during adolescence, whereas the opposite was true in the male rats. This finding systematically replicates the previous findings of Winsauer et al. (2011) for gonadally intact females. In the striatum, both male and female rats that received this dose during adolescence had more CB1R than rats that received saline. Finding these changes in receptor levels was somewhat surprising given there were no differences in the subjects’ acute sensitivity to THC. They could, however, have contributed directly to the differential development of tolerance that was obtained. For example, increased CB1R levels in the striatum of the male/THC and female/THC groups may have contributed to their slower adaptation to the rate-decreasing effects of chronic THC as an adult. These findings demonstrate that administration of THC during adolescence can result in long-term changes to cannabinoid receptor expression that do not necessarily alter the acute sensitivity of adults, but may affect their sensitivity to chronic administration.
One disappointing finding was that AHA1 levels varied unsystematically in the current study, and there was no readily identifiable correlation between its levels and behavior in either experiment. Moreover, these data did not replicate the increases in AHA1 observed in the cerebellum of female rats after chronic administration of 5.6 mg/kg of THC during adolescence (Filipeanu et al., 2011). These differences among brain regions may mean that any long-term effects of THC administration on AHA1 levels are region specific and may depend on a variety of other pharmacologic and behavioral variables. Despite the present data, examining chaperones involved in receptor trafficking remains essential because CB1R function can be affected by receptor internalization (Wu et al., 2008).
In summary, female rats were markedly more sensitive than male rats to the acute rate-decreasing effects of THC and developed tolerance to the disruptive effects of THC slower on an operant procedure of learning and performance. In addition, administration of 5.6 mg/kg of THC during adolescence to both female and male rats produced long-term changes in CB1R levels in the hippocampus and striatum that correlated with decreased tolerance development to the rate-decreasing effects of THC. These findings extend previous reports of the sexually dimorphic effects of cannabinoids to a complex learning procedure and provide further evidence that chronic THC during adolescence can produce long-term changes in the sensitivity of adults to its disruptive effects.
Abbreviations
- AHA1
activator of heat-shock 90-kDa protein ATPase homolog 1
- ANOVA
analysis of variance
- CB1R
cannabinoid type-1 receptor
- THC
Δ9-tetrahydrocannabinol
- PD
postnatal day
- OVX
ovariectomy/ovariectomized
Authorship Contributions
Participated in research design: Weed, Winsauer.
Conducted experiments: Weed, Ketchum.
Performed data analysis: Weed, Filipeanu.
Wrote or contributed to the writing of the manuscript: Weed, Filipeanu, Ketchum, Winsauer.
Footnotes
These data were adapted from the dissertation of Peter F. Weed: Effects of Chronic Δ9-Tetrahydrocannabinol during Adolescence on Adult Repeated Acquisition and Performance Behavior, Tolerance, and CB1 Receptor Expression in Male and Female Rats. In addition, some of these data were previously presented as a poster at the poster presentation: Weed PF (2015) ASPET meeting in Boston, MA, entitled, “Effects of Chronic D9-tetrahydrocannabinol during Adolescence on Adult Repeated Acquisition and Performance Behavior and CB1 Receptor ExpresQ: 2 sion in Male and Female Rats.” 2015 ASPET Conference; Mar 28-Apr 1; Boston, MA. abstract 101699, American Society of Pharmacology and Experimental Therapeutics, Bethesda.
References
- Aceto MD, Scates SM, Lowe JA, Martin BR. (1996) Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther 278:1290–1295. [PubMed] [Google Scholar]
- Barnes C, Fried PA. (1974) Tolerance to delta9-THC in adult rats with differential delta9-THC exposure when immature or during early adulthood. Psychopharmacology (Berl) 34:181–190. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Martin BR. (2000) Effects of the cannabinoid CB(1) receptor antagonist, SR141716A, after Delta(9)-tetrahydrocannabinol withdrawal. Eur J Pharmacol 387:47–53. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Marín S, Fernández B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. (2003) Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 170:301–308. [DOI] [PubMed] [Google Scholar]
- Branch MN, Dearing ME, Lee DM. (1980) Acute and chronic effects of delta 9-tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology (Berl) 71:247–256. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. (1999) Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73:2447–2459. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. (2010) Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol 161:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) Youth Risk Behavior Surveillance, CDC, Atlanta.
- Daigle TL, Kearn CS, Mackie K. (2008) Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. (2002) Tolerance to the disruptive effects of Delta(9)-THC on learning in rats. Pharmacol Biochem Behav 74:129–140. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. (1999) Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 142:295–301. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. (2007) Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. (2010) Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol 160:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro DP, Grilly DM. (1974) Effects of chronic exposure to delta9-tetrahydrocannabinol on delayed matching-to-sample in chimpanzees. Psychopharmacology (Berl) 37:127–138. [DOI] [PubMed] [Google Scholar]
- Ferraro DP, Grisham MG. (1972) Tolerance to the behavioral effects of marihuana in chimpanzees. Physiol Behav 9:49–54. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Guidry JJ, Leonard ST, Winsauer PJ. (2011) Δ9-THC increases endogenous AHA1 expression in rat cerebellum and may modulate CB1 receptor function during chronic use. J Neurochem 118:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. (2004) Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem 279:41077–41084. [DOI] [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L. (1960) A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav 3:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Stevenson MW, Winsauer PJ, Moerschbaecher JM. (2004) Effects of pregnanolone alone and in combination with other positive GABAA modulators on complex behavior in rats. Psychopharmacology (Berl) 173:195–202. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. (1966) Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 13:655–669. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Ferraro DP, Marriott RG. (1973) Long-term interactions of marijuana and behaviour in chimpanzees. Nature 242:119–120. [DOI] [PubMed] [Google Scholar]
- Harte LC, Dow-Edwards D. (2010) Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicol Teratol 32:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. (2012) Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 231:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. (2012) Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ⁹-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther 342:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. (1980) Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther 215:35–44. [PubMed] [Google Scholar]
- Jones RT, Benowitz N, Bachman J. (1976) Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci 282:221–239. [DOI] [PubMed] [Google Scholar]
- Llorente-Berzal A, Fuentes S, Gagliano H, López-Gallardo M, Armario A, Viveros MP, Nadal R. (2011) Sex-dependent effects of maternal deprivation and adolescent cannabinoid treatment on adult rat behaviour. Addict Biol 16:624–637. [DOI] [PubMed] [Google Scholar]
- López-Gallardo M, López-Rodríguez AB, Llorente-Berzal Á, Rotllant D, Mackie K, Armario A, Nadal R, Viveros MP. (2012) Maternal deprivation and adolescent cannabinoid exposure impact hippocampal astrocytes, CB1 receptors and brain-derived neurotrophic factor in a sexually dimorphic fashion. Neuroscience 204:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros MP. (2011) Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. J Psychopharmacol 25:1676–1690. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. (2008) Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 324:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE, Harris LS, Frankenheim JM, Kennedy JS. (1970) l-delta-9-trans-tetrahydrocannabinol in pigeons: tolerance to the behavioral effects. Science 169:501–503. [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 109:E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. (1993) Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol 110:1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. (2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69:303–310. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, et al. (2009) Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 19:763–772. [DOI] [PubMed] [Google Scholar]
- Rubino T, Viganò D, Costa B, Colleoni M, Parolaro D. (2000) Loss of cannabinoid-stimulated guanosine 5′-O-(3-[(35)S]Thiotriphosphate) binding without receptor down-regulation in brain regions of anandamide-tolerant rats. J Neurochem 75:2478–2484. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, et al. (2008) Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33:2760–2771. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2012) Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. SAMHSA, Rockville.
- Thompson DM. (1973) Repeated acquisition as a behavioral base line for studying drug effects. J Pharmacol Exp Ther 184:506–514. [PubMed] [Google Scholar]
- Thompson DM. (1975) Repeated acquisition of response sequences: stimulus control and drugs. J Exp Anal Behav 23:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. (2014) Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 143:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. (2000) Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 19:1–22. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, Lassen-Greene CL, Sutton JL. (2011) Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addict Biol 16:64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Filipeanu CM, Bailey EM, Hulst JL, Sutton JL. (2012) Ovarian hormones and chronic administration during adolescence modify the discriminative stimulus effects of delta-9-tetrahydrocannabinol (Δ⁹-THC) in adult female rats. Pharmacol Biochem Behav 102:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Filipeanu CM, Weed PF, Sutton JL. (2015) Hormonal status and age differentially affect tolerance to the disruptive effects of delta-9-tetrahydrocannabinol (Δ(9)-THC) on learning in female rats. Front Pharmacol 6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Sutton JL. (2014) Chronic administration during early adulthood does not alter the hormonally-dependent disruptive effects of delta-9-tetrahydrocannabinol (Δ9-THC) on complex behavior in female rats. Pharmacol Biochem Behav 117:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, Höllt V, Koch T. (2008) Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem 104:1132–1143. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Kittler J, Grigorenko EV, Kirby MT, Sim LJ, Hampson RE, Childers SR, Deadwyler SA. (1998) Effects of long-term exposure to delta9-THC on expression of cannabinoid receptor (CB1) mRNA in different rat brain regions. Brain Res Mol Brain Res 62:141–149. [DOI] [PubMed] [Google Scholar]