Abstract
Behcet’s disease is a multisystemic vasculitis. It can affect the pulmonary artery in 2% to 5% cases. We discuss a case of a young male diagnosed with Behcet's disease on immunosuppressive therapy who presented with bilateral pulmonary artery aneurysms which were closed with covered stent and other devices.
Key Words: autoimmune, imaging, occluder, pulmonary circulation, stents, vascular disease
Graphical Abstract

History of Presentation
A 29-year-old male presented with complaints of recurrent episodes of cough with hemoptysis of 6-month duration. General physical examination was within normal limits — heart rate was 100 beats/min, blood pressure was 110/70 mm Hg, and oxygen saturation was 98% on room air. The systemic examination was unremarkable.
Learning Objectives
-
•
To diagnose PAAs which are rare in Behcet’s syndrome.
-
•
To evaluate differential diagnosis of PAAs.
-
•
To understand the feasibility for percutaneous closure of PAAs.
Past Medical History
The patient had a history of recurrent painful oral and genital ulcers over the past 5 years and erythematous rash on bilateral legs of 6-month duration.
Investigations
The patient was anemic with elevated inflammatory markers (erythrocyte sedimentation rate 105 mm, C-reactive protein of 119 mg/L) and normal coagulation parameters.
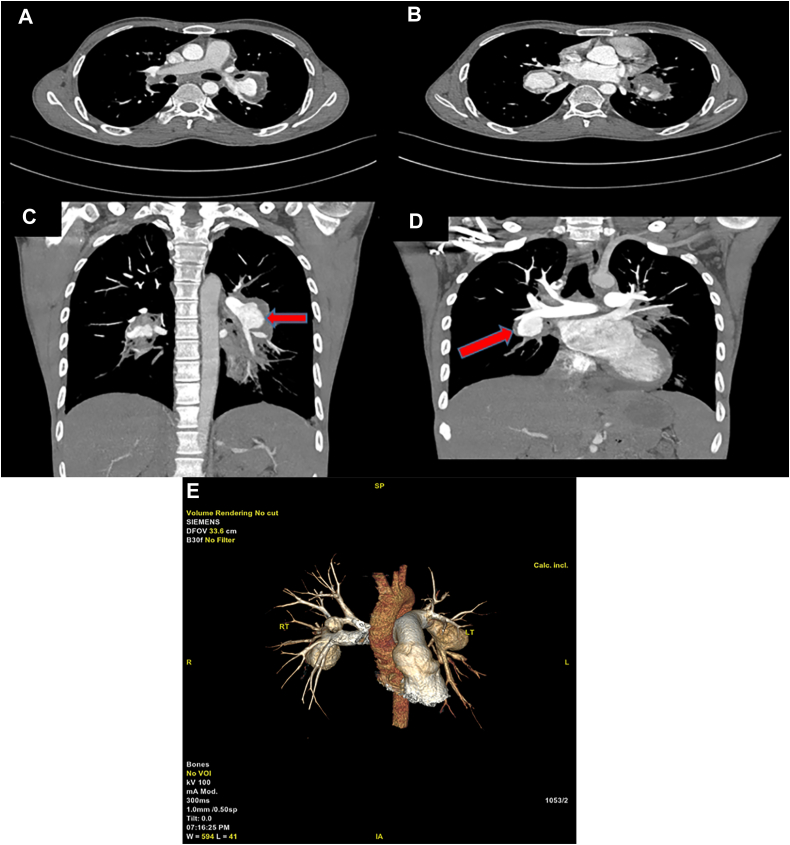
Autoimmune workup of antinuclear antibodies, antineutrophil cytoplasmic antibodies was negative. Chest x-ray revealed bilateral perihilar enlargements suggestive of pulmonary artery aneurysms (Figure 1). Echocardiogram showed mildly dilated right atrium and right ventricle with mild pulmonary artery hypertension. According to international study group criteria, he was diagnosed with Behcet’s disease. A multidetector computed tomography (CT) of the chest with pulmonary angiogram (Figures 2A to 2E) showed a saccular aneurysm of 3 × 3 cm in the right lower lobar pulmonary artery, 3.3 × 2.5 cm in left lower branch of the pulmonary artery (PA) with partial thrombosis, and 1.5 × 1.6 cm in segmental branch of right upper lobe. Human leucocyte antigen testing was positive for B51.
Figure 1.
Bilateral Perihilar Enlargements Suggestive of the Pulmonary Artery Aneurysms
Arrows indicate the enlargements.
Figure 2.
Chest Computed Tomography Scans
(A, C) Left lower lobar branch of pulmonary artery (PA) with saccular aneurysm with partial thrombosis. (B, D) Pulmonary artery aneurysm (PAA) in right lower branch of PA. (E) three-dimensional reconstruction images of bilateral PAAs.
Differential Diagnosis
Interferon gamma release assay for tuberculosis and VDRL testing for syphilis were negative. Behcet’s disease is the clinical diagnosis; our patient satisfied 1 major and 2 minor international study group criteria.
Management
Medical
The patient received 6 doses of intravenous adalimumab 40 mg subcutaneously biweekly and 2 doses of intravenous cyclophosphamide 500 mg along with oral steroids and colchicine. Although the mucocutaneous manifestations healed and inflammatory markers showed decreasing trend, he continued to have recurrent bouts of hemoptysis. The patient was referred to our hospital in view of recurrent hemoptysis with bilateral pulmonary artery aneurysms (PAAs) for interventional management.
Interventional
A CT pulmonary angiogram was reviewed and thought feasible for endovascular management with covered stent to left lower branch PAA and device for right lower branch PAA. Under aseptic precautions, a 6-F sheath was introduced into the right femoral vein. A 6-F Judkins right catheter was passed from the inferior vena cava to the right atrium to the right ventricle to the main pulmonary artery; it was used to engage the left lower PA branch; then a contrast injection was administered that showed a saccular aneurysm of the left interlobar artery, with the mouth of aneurysm measuring 27 mm (Figure 3, Videos 1 and 2). A straight tip exchange length wire was parked deep into the left lower PA branch. Flexible wire was exchanged with a 0.035-inch Teflon wire. A 10-F Lifetech sheath was introduced over this wire. A 13.5 × 40–mm Fluency plus vascular stent graft was taken over the Teflon wire and positioned across the aneurysm. The size of vascular stent graft was determined according to proximal left lower lobar artery diameter (13 mm). After confirming position, a stent was deployed (Figure 4, Video 3). After deployment, it was noted that the distal end of the aneurysm was inadequately covered with residual flow into the aneurysm (Figure 5, Video 4). An 8/6 Lifetech Konar MFO device was used to seal the residual defect at the lower end of the aneurysm (Figure 6, Video 5).
Figure 3.
Left Pulmonary Angiogram
Saccular aneurysm arising from the left interlobar artery with the mouth of the aneurysm measuring 27 mm and a proximal left interlobar artery diameter of 13 mm.
Figure 4.
Vascular Stent
A 13.5 × 40–mm fluency plus vascular stent graft (arrow) was placed across the left PA aneurysm and deployed. Abbreviation as in Figure 2.
Figure 5.
Re-Check Angiogram After Stent Deployment
Residual leak is shown at the distal end of the aneurysm.
Figure 6.
Residual Leak Closure
An 8/6 Lifetech Konar MFO device (red circle) was deployed at the lower end of the aneurysm to close the residual leak.
A re-check angiogram after stenting and device deployment showed minimal residual flow into the aneurysm (Figure 7, Video 6) on the left side.
Figure 7.
Final Left PA Angiogram
Minimal residual flow into aneurysm is shown with unobstructed flow in the PA branches. Abbreviation as in Figure 2.
A 6-F Judkins right catheter guide and 0.032-inch straight tip exchange length wire was advanced into the right pulmonary artery and parked in the aneurysm arising from right lower lobar artery (Video 7). Contrast injection was administered that showed the saccular aneurysm arising from right lower branch of the PA, measuring 10 mm at the mouth (Figure 8, Video 8). A straight tip wire was then exchanged for 0.035-inch Teflon wire. A 10F Lifetech sheath was advanced over the Teflon wire. A 16/14 patent ductus arteriosus Occlunix device was selected for closing this aneurysm. It was loaded on delivery cable, positioned into the aneurysm, a larger disc was opened into the aneurysm and then pulled back to position a smaller disc across the mouth of the aneurysm (Figure 9, Video 9). The final angiogram showed the device in place with complete obliteration of the aneurysm (Figure 10, Video 10). The patient was hemodynamically stable and was discharged after 2 days on an immunosuppressive regimen.
Figure 8.
Right PA Angiogram
A saccular aneurysm is shown arising from the lower lobar branch with the mouth of the aneurysm measuring 10 mm.
Figure 9.
Patent Ductus Arteriosus Device Deployment
A 16/14 Occlunix patent ductus arteriosus device (arrow) was used to close the right-sided PAA. Abbreviation as in Figure 2.
Figure 10.
Final Right PA Angiogram
The aneurysm was cut off successfully with no flow and unobstructed flow into right PA branches. Abbreviation as in Figure 2.
Discussion
PAAs can be congenital (50%) or acquired. Behcet’s disease can involve any vessel (arteries and veins) of any size. Vascular involvement in Behcet’s disease was identified in 15% cases, and venous involvement is more common than arterial. The PA is the second most common site of arterial involvement (<5%) after the aorta.1 Peculiar characteristics of PAA in Behcet’s disease are that they are predominantly right sided, multiple, with lobar artery involvement, sized 4 ± 2.4 cm with intramural thrombus, and present with hemoptysis in 78% of cases.2 CT angiography has emerged as a noninvasive and excellent method for detecting pulmonary aneurysms in Behcet’s disease compared to invasive pulmonary angiography; it also aids in assessing feasibility of percutaneous closure.
The European Alliance of Associations for Rheumatology guidelines for managing PAAs in Behcet’s disease recommend glucocorticoid and cyclophosphamide as first-line treatment.3 Hamuryudan et al4 reported in a case series of 26 patients that intravenous pulses of corticosteroids and cyclophosphamide followed by long-term oral corticosteroids with oral cyclophosphamide or azathioprine improved 5-year survival from approximately 40% to 80%. Kage et al5 showed that immunosuppressive treatment for 5 months lead to resolution of PAAs and thrombosis in Behcet’s syndrome in his case report. However, in our patient, the aneurysms did not regress with medical management and he continued to have hemoptysis; therefore, we decided to close the PAAs percutaneously.
Surgical methods for PAAs are less preferred because: 1) there can be increased morbidity and mortality; 2) steroids can impede postoperative healing; 3) the lobectomy increases the size of other PAAs; and 4) there can be anastomotic site dehiscence following graft interposition.6 Hence, methods such as lobectomy, pneumonectomy, aneurysmorrhaphy, and aneurysmectomy should only be used as salvage therapy.7 However, Kreibich et al8 recommend surgery for all PAAs with a diameter ≥6 cm.
In the current era, endovascular treatment is the preferred option over surgery. For peripheral types of PAA, embolization of the affected PAs with coils or acrylic glue is recommended. For central types, plugs, Amplatzer occluders, or stent grafts could be attempted.7
In our case, the neck of the left-sided aneurysm being wide, the use of coils was deferred because of the risk of embolization. n-Butyl cyanoacrylate glue is another option, but limitations of this technique include requirement of high glue concentrations, operator experience, risk of embolization, and occlusion of distal branches; therefore, it was not preferred. Vascular plugs were not used because the neck of aneurysm was >10 mm and the maximum available sized plug was 8 mm. Covered stents for PAAs have been studied in literature for indications such as lung malignancy and mycotic aneurysm. There are no clear guidelines on when and how to use each endovascular approach. This case presents a novel approach of successfully using a covered stent and patent ductus arteriosus/ventricular septal defect device to close a PAA. The Lifetech Konar MFO was used in our case to close residual leak into the aneurysm noted after stent deployment.
Follow-Up
The patient was asymptomatic and had no further episodes of hemoptysis at 3-month follow-up.
Conclusions
There are no clear guidelines on the optimal treatment for patients with PAAs, which are the most common cause of mortality in Behcet’s disease. Immunosuppressive therapy is the first-line treatment; however, if that fails, endovascular treatment can be tried. Imaging using CT pulmonary angiography to size the devices/stents in PAAs closure is of utmost importance.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Through a 6-F sheath in RFV, 6-F JR and 0.018-inch straight tip roadrunner wire was introduced IVC→RA→RV→MPA, and parked into lower branch of LPA.
Contrast injection given through sheath shows saccular aneurysm arising from left lower PA branch, mouth of aneurysm 27 mm, proximal left lower lobar artery diameter, 13 mm.
13.5 × 40 mm Fluency plus Vascular stent graft (COVERED STENT) was taken over stiff wire and positioned across LPA aneurysm and deployed.
Check angiogram after stent deployment showed uncovered distal end of aneurysm with residual flow.
With help of 6-F JR and 0.032-inch Terumo wire, a 8/6 Lifetech Konar MFO device was taken across residual defect into aneurysm and deployed.
Final angiogram showed minimal residual flow into the aneurysm and unobstructed flow into left upper and lower lobe branches of PA.
6-F JR and 0.032-inch straight tip Terumo wire exchange length was advanced into RPA and parked into aneurysm arising from right lower branch of PA.
RPA Angiogram showed saccular aneurysm arising from right lower PA branch (10 mm diameter).
16/14 mm OCCLUNIX PDA device was positioned into the aneurysm such that smaller disc was at the mouth of aneurysm and device was deployed.
Final angiogram showed no flow into the aneurysm and unobstructed flow into right upper, middle, and lower lobar branches.
References
- 1.Modaghegh M.H.S., Kazemzadeh G.H., Jokar M.H. A case of Behçet disease with pulmonary artery pseudoaneurysm: long term follow-up. East Mediterr Heal J. 2010;16(3):346–349. [PubMed] [Google Scholar]
- 2.Yuan S.M. Aneurismas de artérias pulmonares na doença de Behçet. J Vasc Bras. 2014;13(3):217–228. [Google Scholar]
- 3.Samreen I., Darji P., Genobaga S., et al. Pulmonary artery aneurysm in Behcet disease: medical, endovascular or surgical intervention. Cureus. 2023;15(11) doi: 10.7759/cureus.49368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamuryudan V., Er T., Seyahi E., et al. Pulmonary artery aneurysms in Behçet syndrome. Am J Med. 2004;117(11):867–870. doi: 10.1016/j.amjmed.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Kage H., Goto Y., Amano Y., et al. Development of pulmonary artery aneurysms due to Behçet’s disease and resolution after treatment. Intern Med. 2016;55(22):3337–3340. doi: 10.2169/internalmedicine.55.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaifel M., Suresh Daniel R., Nakanishi H. Approach for the treatment of pulmonary artery aneurysm repair using inclusion technique: a case report. Cureus. 2023;15(3):1–7. doi: 10.7759/cureus.36456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie D., Chen C., Wang H., et al. Refractory pulmonary artery aneurysm in Behçet’s disease. Ann Transl Med. 2015;3(16):2–4. doi: 10.3978/j.issn.2305-5839.2015.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreibich M., Siepe M., Kroll J., et al. Aneurysms of the pulmonary artery. Circulation. 2015;131(3) doi: 10.1161/CIRCULATIONAHA.114.012907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Through a 6-F sheath in RFV, 6-F JR and 0.018-inch straight tip roadrunner wire was introduced IVC→RA→RV→MPA, and parked into lower branch of LPA.
Contrast injection given through sheath shows saccular aneurysm arising from left lower PA branch, mouth of aneurysm 27 mm, proximal left lower lobar artery diameter, 13 mm.
13.5 × 40 mm Fluency plus Vascular stent graft (COVERED STENT) was taken over stiff wire and positioned across LPA aneurysm and deployed.
Check angiogram after stent deployment showed uncovered distal end of aneurysm with residual flow.
With help of 6-F JR and 0.032-inch Terumo wire, a 8/6 Lifetech Konar MFO device was taken across residual defect into aneurysm and deployed.
Final angiogram showed minimal residual flow into the aneurysm and unobstructed flow into left upper and lower lobe branches of PA.
6-F JR and 0.032-inch straight tip Terumo wire exchange length was advanced into RPA and parked into aneurysm arising from right lower branch of PA.
RPA Angiogram showed saccular aneurysm arising from right lower PA branch (10 mm diameter).
16/14 mm OCCLUNIX PDA device was positioned into the aneurysm such that smaller disc was at the mouth of aneurysm and device was deployed.
Final angiogram showed no flow into the aneurysm and unobstructed flow into right upper, middle, and lower lobar branches.