Abstract
Respiratory tract infections (RTIs) are the leading cause of antibiotic prescriptions, primarily due to the risk for secondary bacterial infections. In this study, we examined whether Echinacea could reduce the need for antibiotics by preventing RTIs and their complications, and subsequently investigated its safety profile. A comprehensive search of EMBASE, PubMed, Google Scholar, Cochrane DARE and clinicaltrials.gov identified 30 clinical trials (39 comparisons) studying Echinacea for the prevention or treatment of RTIs in 5652 subjects. Echinacea significantly reduced the monthly RTI occurrence, risk ratio (RR) 0.68 (95% CI 0.61–0.77) and number of patients with ≥1 RTI, RR = 0.75 [95% CI 0.69–0.81] corresponding to an odds ratio 0.53 [95% CI 0.42–0.67]. Echinacea reduced the risk of recurrent infections (RR = 0.60; 95% CI 0.46–0.80), RTI complications (RR = 0.44; 95% CI 0.36–0.54) and the need for antibiotic therapy (RR = 0.60; 95% CI 0.39–0.93), with total antibiotic therapy days reduced by 70% (IRR = 0.29; 95% CI 0.11–0.74). Alcoholic extracts from freshly harvested Echinacea purpurea were the strongest, with an 80% reduction of antibiotic treatment days, IRR 0.21 [95% CI 0.15–0.28]. An equal number of adverse events occurred with Echinacea and control treatment. Echinacea can safely prevent RTIs and associated complications, thereby decreasing the demand for antibiotics. Relevant differences exist between Echinacea preparations.
Keywords: Echinacea, prevention, respiratory tract infections, antibiotics, recurrent RTIs, complications
1. Introduction
Despite advances in pathological understanding, hygienic improvements and vaccination technology, respiratory tract infections (RTIs) are still the most frequent illnesses worldwide. They are divided into upper RTIs (URTIs), which affect the naso-pharynx and sinuses, and lower RTIs (LRTIs), which affect the trachea, bronchi and lungs [1]. A study performed by the Global Burden of Diseases, Injuries and Risk Factors (GBD) estimated that by 2019, 17.2 billion cases (or 42.8% of all worldwide diseases) were a consequence of URTIs, with a high prevalence in countries with high sociodemographic indices [2]. The same study attributed 291.7 million cases to LRTIs, of which approximately 1% were fatal [3]. In 2019, LRTIs were the leading infectious cause of death [4].
Approximately one-third of all RTIs affect children below five years of age, of which a disproportionally high number of 0.7 million cases are lethal. A higher fatality rate is also reported for elderly people and immunocompromised patients [5]. These numbers do not account for the recent COVID-19 pandemic that caused an estimated 677 million infections and 6.9 million deaths worldwide [6].
Containment measures like social distancing and hygiene not only curbed overall viral infections but also secondary bacterial respiratory infections and, importantly, the worldwide use of antibiotics—indicating a close correlation between those factors [7]. Suspension of those containment measures brought antibiotic use back to pre-pandemic levels and, although COVID-19 is understood as a viral illness that is rarely associated with bacteria (10%), up to 75% of infections were treated with antibiotics [8,9].
The prevention of RTIs may be achieved by taking Echinacea species, as antiviral and immune-modulatory actions have been reported [10,11]. Great heterogeneity exists between different preparations, but for alcoholic extracts, recent literature found a wide spectrum of activity against enveloped respiratory viruses, including influenza viruses, respiratory syncytial virus (RSV), coronaviruses and SARS-CoV-2 [10]. Activation of interferon signaling, chemotaxis and anti-inflammatory actions constitute the immune supportive effects of the medicinal plant [11,12]. Clinical benefits manifest not only in a reduced risk of RTIs but also of RTI relapses and secondary complications [13].
For the first time, a recent study in children demonstrated a benefit on the frequency of antibiotic prescriptions, showing a reduction by 76.3%, which was significant as a secondary outcome [14]. The aim of the current systematic review and meta-analysis was to test the hypothesis that taking Echinacea could reduce not only recurrent RTI episodes but also RTI complications and, further, that this reduction would lead to a reduced need for antibiotic prescriptions. In addition, we investigated the safety profile by studying the occurrence of adverse events (AEs) upon Echinacea therapy.
2. Results
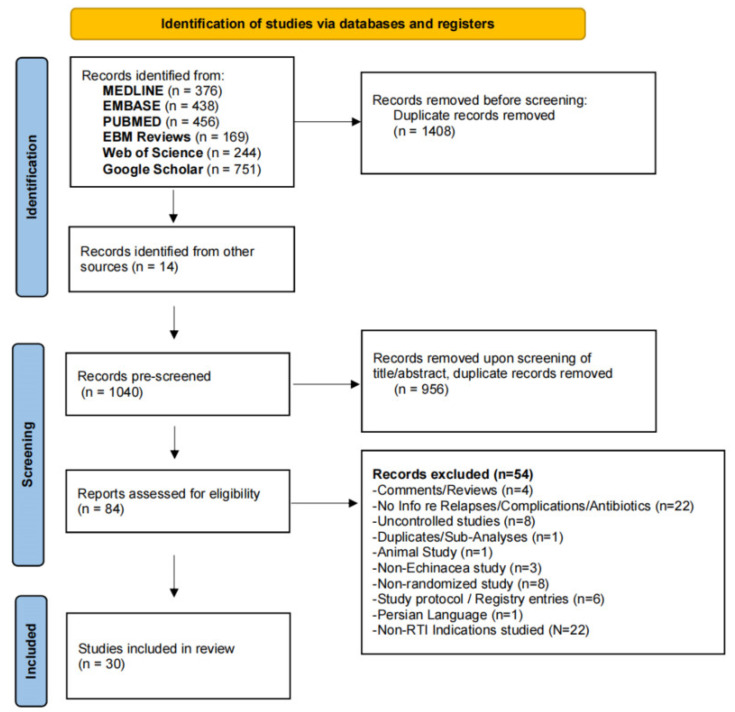
Our systematic literature search yielded a total of 2434 hits from screened databases, whereas another 14 were identified from reviewing reference lists of review articles and study registers (Figure 1).
Figure 1.
Flow chart of included and excluded studies.
After removing duplicates (n = 1408), records were selected based on title/abstract interpretation, leaving 84 articles overall, of which n = 54 did not describe original work, contained no information regarding RTIs (complications) or usage of antibiotics or were not controlled.
2.1. Study Characteristics
Overall, a total of 30 clinical studies were included in our analysis, reporting on 39 comparisons of Echinacea preparations with a control group. In 22 trials, Echinacea was investigated for prevention of RTIs (27 comparisons [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]) while in 8 trials, Echinacea was studied for the acute treatment (12 comparisons [36,37,38,39,40,41,42,43]). Taylor et al. (2003) [42] and Sumer et al. (2023) [37] allowed for a repetitive therapy of up to three episodes over a prolonged observational time [37,42]. Six prevention studies administered Echinacea for a shorter period of equal or less than one month [21,24,25], three of which employed an artificial inoculation method [16,31,33], whereas the remaining studies employed longer treatment periods between six weeks to five months [14,15,17,18,19,20,22,23,26,27,28,29,30,32,34,42]. Awad et al. applied an interval preventive therapy of 6 × 10 days throughout half a year [29]. Weber et al. [35] presented a sub-analysis of the work by Taylor et al. [42], giving information on recurrent infections under Echinacea or placebo therapy.
A total of 21 studies investigated an Echinacea mono-product, with nine containing further ingredients like vitamin C, Sambucus nigra, Nigella sativa, Thuja occidentalis, Baptisia tinctoria, propolis or homeopathic dilutions as additives [17,18,19,20,22,25,30,39,43]. The majority of the 39 comparisons involved lipophilic Echinacea purpurea extracts based on alcoholic extractions, glycerol or hypercritical CO2 extractions [14,15,17,18,19,22,25,26,27,30,32,36,37,38,39]. Seven preparations contained Echinacea purpurea pressed-juices (hydrophilic) [21,23,24,31,33,40,42], whereas four preparations contained dried, powdered or unspecified Echinacea [16,28,29,43]. As anticipated, a great variety of Echinacea preparations were included in this analysis with the aim to investigate overarching evidence of activity for the medicinal plant.
RTI was the studied indication, mostly detected as a patient-reported, physician/nurse-confirmed outcome [14,15,16,18,19,20,23,26,27,28,30,31,34,36,37,38,39,41,42,43]. This entity comprised the common cold, rhinitis, non-specified respiratory infections, flu-like infections or flu. More recent clinical studies also involved RT-PCR based confirmation of respiratory viruses [26,27,37,39] and three trials artificially induced infections through rhinovirus inoculation [16,31,33]. Seven studies included children below twelve years of age [14,18,22,25,29,41,42], whereas three trials researched Echinacea in children as young as one or two years [18,25,42].
With respect to safety, AEs were reported either as numbers of patients experiencing AEs or total number of AEs by 17 clinical studies [14,15,18,21,23,26,27,28,30,31,32,34,36,38,40,41,42]. Ogal et al. [14] reported a total of 105 AEs for 103 study subjects in the control group and we decided for this particular study to define the total number of AEs (105) rather than the sample size (103) as the denominator for assessment of the risk ratio (see Appendix A Table A3).
2.2. Risk of Bias
We employed the risk of bias tool by Cochrane (RoB2) to estimate the quality of included studies based on seven aspects addressing selection, performance and reporting biases. Our assessment of study quality was in principal agreement with results by David et al. [44], whereas additional literature was rated independently [45].
Some research was carried out before the implementation of the Consolidated Standards of Reporting Trials (CONSORT statement) in 1992 when reporting principles were still not elaborate yet. Where randomization was mentioned, details regarding sequence generation was sometimes missing [16,20,21,25,30,36]. In double-blind studies with low numbers of dropouts and principally healthy participants, we assumed a low risk for allocation concealment and performance bias (blinding of patients/personnel, attrition bias and incomplete outcomes). For open studies lacking placebo or using active control, a high risk for bias was principally suspected [17,19,20,22,25,29,41], unless blinding effectiveness was explicitly confirmed [15,27,37] and if an objective parameter was investigated (i.e., routine virus analytics from nasopharyngeal samples) [27]. Hence, high risk of bias was detected in at least one RoB2 domain in eleven studies, which consequently obtained inadequate quality ratings of <4 also according to Jadad [46] (see Appendix A Table A2) [17,19,20,22,25,27,29,34,36,40,41]. Those studies were dealt with separately in a sensitivity analysis.
Selected studies mostly included healthy subjects, thus, the risk for imbalanced allocation and selection bias was expected to be low, as evidenced by demographic data given for most trials. Despite randomization, Wahl et al. obtained significantly heterogeneous groups for comparison [47] The article by Rahmati et al. provided an abstract in English but the main article was written in Arabic and was therefore excluded [48].
2.3. Results from Individual Studies
Results from individual studies are summarized in Appendix A Table A3. Information regarding RTI incidence was available in form of patients experiencing ≥ 1 episode/infection and/or the number of episodes/infections occurring throughout the observation period [14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Since intervention durations varied greatly between studies (10 days–6 months), we normalized the latter parameter to monthly occurrence of RTI as well. Data pertaining to patients with recurrent infections/relapses or the number of recurrences/relapses was available from [14,15,18,23,25,26,30,42]. Those included classical prevention trials and acute therapy studies with appropriate follow-up periods. Finally, information on antibiotic use was gathered from 11 studies, either as number of patients treated with antibiotics, overall antibiotic treatment days or mean differences in antibiotic treatment days [14,18,25,26,27,36,37,39,41,42,43]. For all analyses, we conservatively commented on random rather than common/fixed effect model, while supplementing results for risk ratios (RR) by the odds ratio (OR), where appropriate.
2.4. Results of Meta-Analysis
2.4.1. Prevention of Respiratory Tract Infections (RTIs)
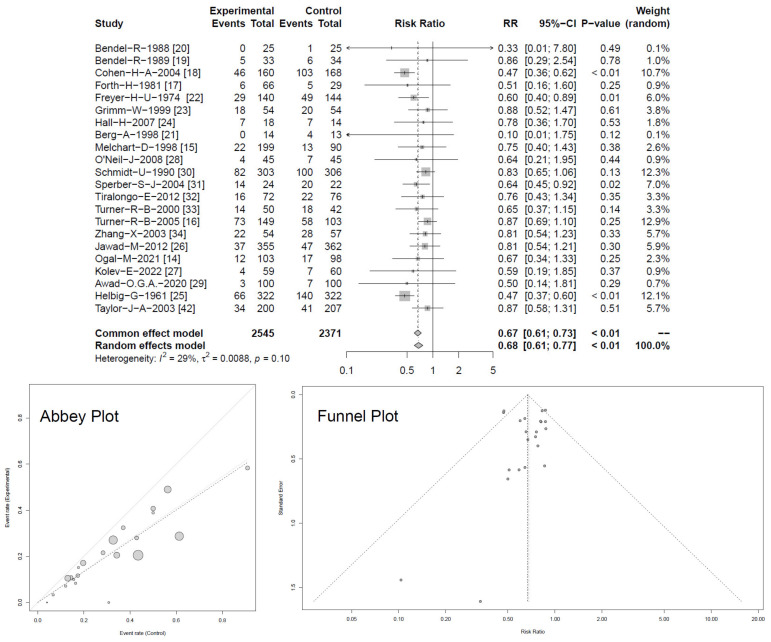
As a first objective, the prevention of RTIs through Echinacea use was tested. Figure 2 shows the risk of RTIs normalized per treatment month and patient for Echinacea and control, referring to 4916 study subjects included in 22 studies [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,42]. All included studies point towards the superiority of Echinacea over control treatment. Effect sizes ranged between RR 0.10 and 0.88, where four studies reached a p < 0.05. Pooled effect sizes of individual studies (random effect model) yielded a significant risk ratio of RR = 0.68 [95% CI 0.61–0.77; p < 0.01], while a heterogeneity of I2 = 29% was considered to be low (τ2 = 0.0088; p = 0.1).
Figure 2.
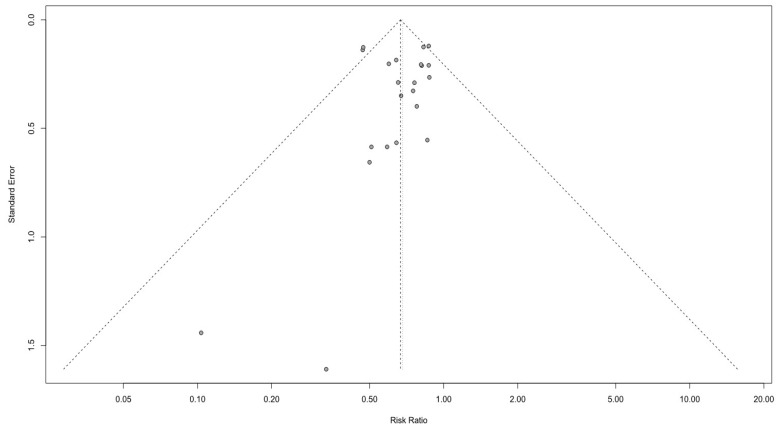
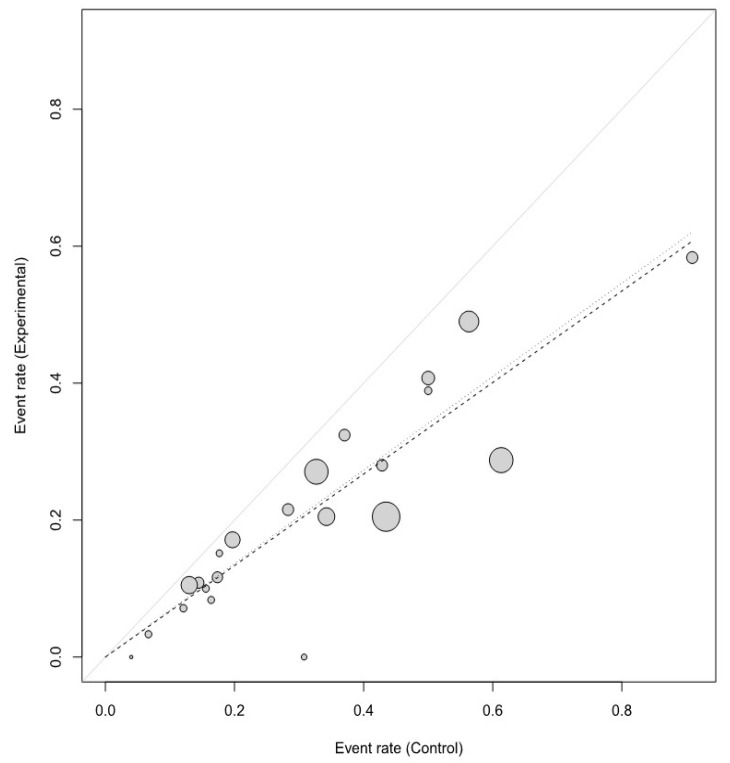
Forest plot showing meta-analysis of overall risk for occurrence of RTIs between groups with Abbey and Funnel plots, indicating low risk of publication bias (for clearer Abbey and Funnel plots see Appendix B Figure A2 and Figure A3). Shown are “events” (RTIs), “total” (participants) for Echinacea (“experimental”) and control, risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies.
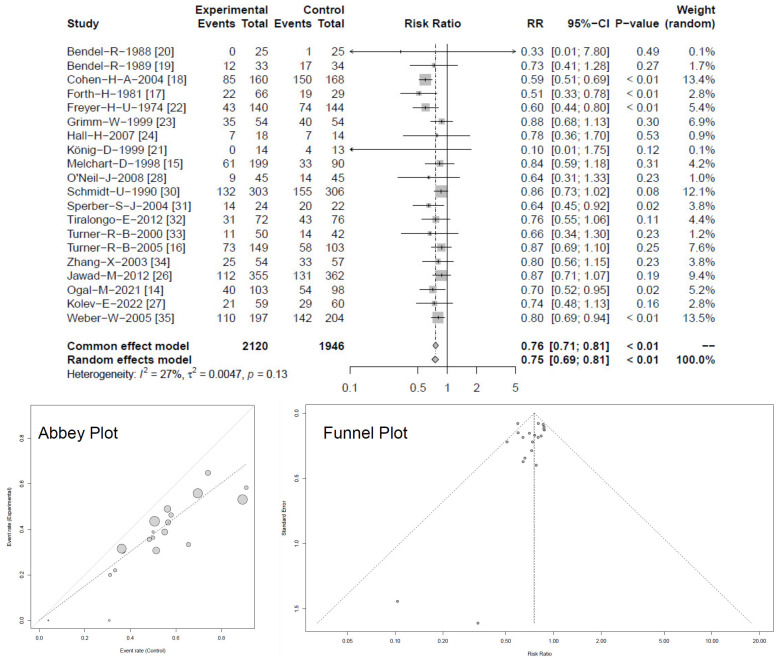
Twenty studies reported numbers of participants experiencing one or more RTIs. When data were pooled in meta-analyses, heterogeneity decreased to I2 = 27% (τ2 = 0.0047, p = 0.13) and RR yielded 0.75 [95% CI 0.69–0.81; p < 0.01], respectively OR = 0.53 [95% CI 0.42–0.67; p < 0.01], see Figure 3 [14,15,16,17,18,19,20,21,22,23,24,26,27,28,30,35].
Figure 3.
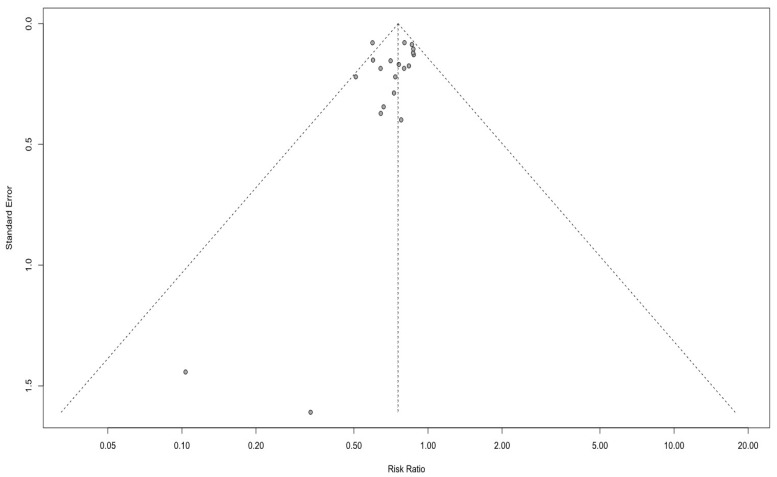
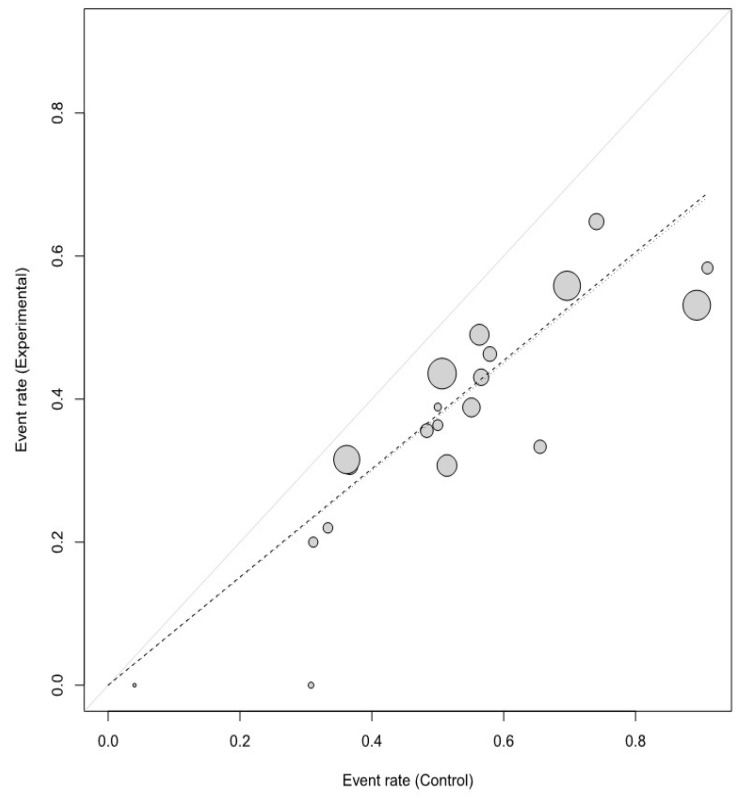
Forest plot showing meta-analysis of proportion of Echinacea-treated subjects with ≥1 RTI compared with control (for clearer Abbey and Funnel plots see Appendix B Figure A4 and Figure A5). Shown are “events” (pts with RTIs), “total” (participants) for Echinacea (“experimental”) and control, risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies.
Again, all studies indicated superiority for Echinacea, of which six trials reported significant benefits, with p < 0.05. For both analyses (Figure 2 and Figure 3), random and common effect models provided similar and consistent results. Both Abbey and Funnel plots described a rather natural scatter of large and smaller studies showing a typical variation (confidence interval and standard deviation) experienced in such trials. Selection bias due to unpublished or possibly negative studies is not indicated. The dispersion of standard errors against estimated effect size also indicates the absence of asymmetry for the parameter monthly risk for RTIs. A similar picture was observed for number of participants with RTIs.
The above analysis contained several studies with high risk of bias in at least one section of the Cochrane RoB analysis, thus scoring less than four points in Jadad’s assessment. Exclusion of these potentially high-risk studies provided a result based on more reliable evidence without changing the estimated effect with RR = 0.75 [95% CI 0.64–0.87; p < 0.01] for RTI’s. Unexpectedly, the heterogeneity increased to significance with I2 = 40% (τ2 = 0.0052, p = 0.08), indicating that excluded studies, though lower in quality, stabilized the overall certainty of reported effect size estimates (see Appendix A, Table A4).
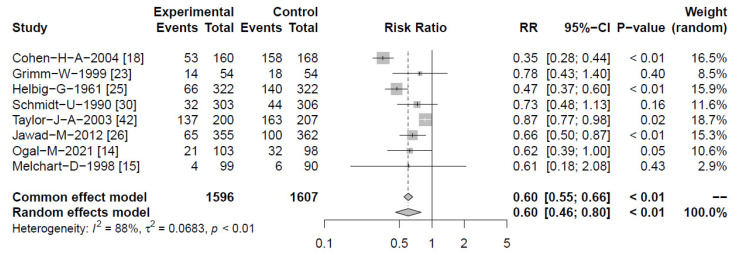
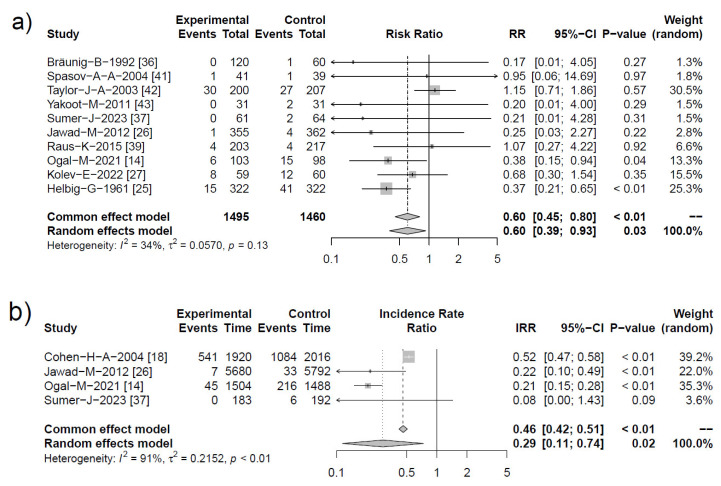
The risk for overall RTIs was lower than the risk for at least one episode. This was assumed to be a result of a diminished risk for recurrent infections and relapses, and was further explored. The risk for participants experiencing recurrent RTIs was calculated by pooling results from eight clinical studies comprising 3203 subjects, comparing Echinacea with control (mostly within a preventive scenario) [14,15,18,23,25,26,30,42]. A significant reduction of recurrences and relapses was found in the Echinacea group indicating a RR = 0.60 [95% CI 0.46–0.80; p < 0.01], but at significant heterogeneity of I2 = 88% (Figure 4).
Figure 4.
Forest plot showing meta-analysis of proportion of Echinacea-treated subjects experiencing recurrent RTIs/relapses compared with control. Shown are “events” (pts with recurrences), “total” (participants) for Echinacea (“experimental”) and control, risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies.
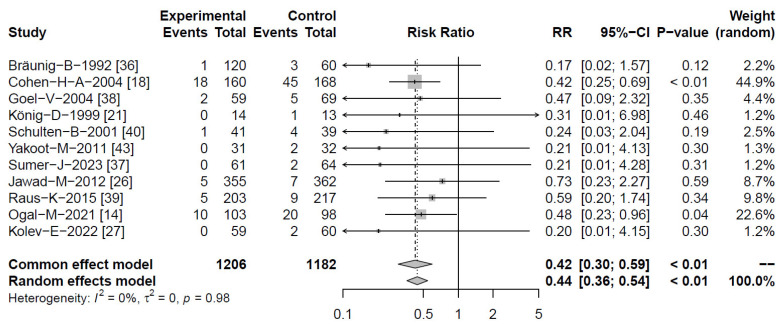
2.4.2. Reduction of RTI Complications
We pooled results pertaining to numbers of participants experiencing RTI complications, as well as the total numbers of complications occurring. Results were available from 11 [14,18,21,26,27,36,37,38,39,40,43] and 13 clinical studies [14,18,21,23,26,27,29,36,37,38,39,40,43], including 2388 and 2695 subjects, respectively. For both analyses, heterogeneity was either absent or moderate, pointing to a highly robust estimated effect size. A pronounced reduction of risk of complications was observed (RR = 0.44 [95% CI 0.36–0.54; p < 0.01]) which was highly comparable with the overall number of complications occurring. The two largest studies (both of good methodological quality with Jadad scores ≥ 4) provided results that were consistent with the estimated effect size upon meta-analysis. Consequently, the results from a sub-analysis including only high-quality studies provided a highly similar RR = 0.47 [95% CI 0.37–0.58] for participants with complications, again at the absence of heterogeneity. The estimated effect size was thus considered to be reliable (see Figure 5 and Appendix A Table A4).
Figure 5.
Forest plots showing meta-analysis of proportion of Echinacea-treated subjects experiencing complications compared with control. Shown are “events” (complications), “total” (participants) for Echinacea (“experimental”) and placebo risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies.
2.4.3. Antibiotic Prescriptions
Finally, we tested whether the use of Echinacea would also affect the need for antibiotics, as assessed by the number of participants treated with antibiotics, number of antibiotic treatment days and pooled mean differences between reported antibiotic treatment durations per individual. See Figure 6a,b for more information.
Figure 6.
Forest plots showing meta-analysis of: (a) number of Echinacea-treated subjects receiving antibiotic therapy compared with control. Shown are “events” (pts or days with antibiotics), “total” (participants) for Echinacea (“experimental”) and placebo, risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies. (b) Number of overall antibiotic treatment days, showing individual risk ratio (IRR). Most studies reported the number of patients receiving antibiotic therapy.
Results referring to antibiotic use were retrieved from ten clinical studies, of which seven trials were assigned a high methodological quality (Jadad score 5) [14,25,26,27,37,42,43] and three trials a poor methodological quality (Jadad score 1 and 2) [36,39,41]. Heterogeneity was calculated to be low with I2 = 34% and insignificant (p > 0.1). For the number of participants treated with antibiotics, both common and random effect models provided similar risks that were significant, with RR = 0.60 [95% CI 0.39–0.93; p = 0.03] for the random effects model (Figure 6a). Both Helbig and Taylor et al. [25,42] provided considerable cumulative weight of more than 50%, with the former study being of low quality and the latter describing a treatment study. Interestingly, upon exclusion of therapeutic studies [36,37,41,42,43], pure prevention studies provided an even more pronounced effect, with RR = 0.46 [95% CI 0.27–0.76; p = 0.01].
Figure 6b illustrates effects of Echinacea treatment on the overall duration of antibiotic therapy that was significantly reduced showing an individual risk ratio (IRR) = 0.29 [95% CI 0.11–0.74; p < 0.02]. Maximal benefits were achieved using a combined therapeutic approach with basic prevention dosing and dose-increase during acute illness, as shown by Cohen et al. [18]. The latter reported 541 versus 1084 days with antibiotic therapy for Echinacea and placebo, respectively, IRR 0.52 [95% CI 0.47–0.58; p < 0.01]. This result was only surpassed by Ogal et al. who showed an impressive 80% reduction from 216 to 45 antibiotic treatment days, IRR 0.21 [95% CI 0.15–0.28; p < 0.01] (Figure 6b) [14].
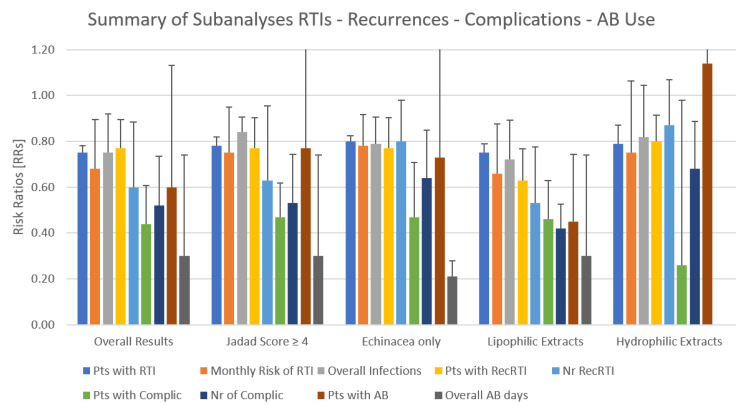
2.4.4. Subanalyses
As per registration of this meta-analysis, it was the clear intention to include all randomized controlled clinical trials investigating any Echinacea species, regardless of study design, quality, manufacturing method or addition of further supplements to the Echinacea product. It was also declared necessary to conduct sub-analysis on more discrete study selection criteria, yielding results which are discussed in the following section and provided in Appendix A Table A4 and Figure A1. Pooling of high-quality studies overall did not increase consistency (I2) but the magnitude of treatment effect and its statistical significance remained consistent with the overall meta-analyses throughout. The monthly risk for RTIs marginally increased to RR = 0.75 (p < 0.01), the effects on recurrent RTI, complications and most importantly, the 70% reduction of antibiotic treatment days remained stable and significant (Appendix A, Figure A1).
The separation of lipophilic from hydrophilic extracts revealed a clear distinction not only in terms of the monthly risk for RTIs (lipophilic vs hydrophilic RR = 0.66 [p < 0.01] vs. 0.75 [n.s.]), but also for recurrent RTIs (RR = 0.53 [p < 0.01] vs. 0.87 [n.s.]) and complications (RR = 0.42 [p < 0.01] vs. 0.68 [n.s.]), highlighting important differences between Echinacea preparations.
There remains the question as to whether the addition of supplements would further enhance the benefits of Echinacea. As shown in Appendix A Table A4, results are inconsistent, where a trend towards higher monthly RTI RR values was balanced by an opposite trend for antibiotic use with a lower RR value and tighter CIs for Echinacea-only preparations. However, it is important to note that this analysis may be fundamentally influenced by the wide variety of Echinacea formulations, introducing more variance than additives, as shown previously.
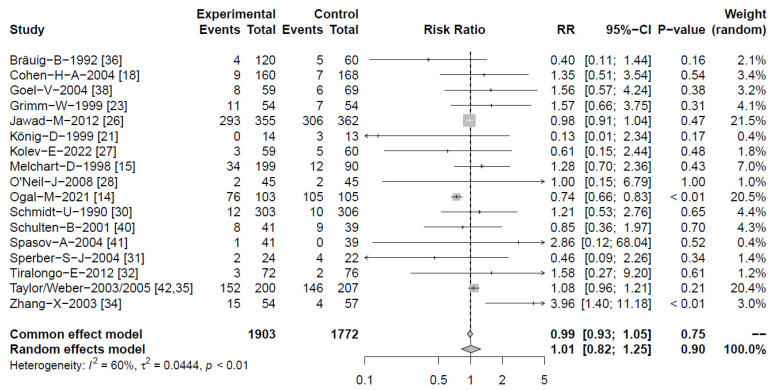
2.4.5. Adverse Events
Information regarding the occurrence of AEs was retrieved from 17 clinical studies (Figure 7) [14,15,18,21,23,26,27,28,30,31,32,34,36,38,40,41,42]. For both Echinacea and control, an overall number of 633 events were reported from 1903 and 1772 participants, respectively. The resulting risk and odds ratio for Echinacea versus control yielded highly similar values of OR = 0.99 [95% CI 0.64–1.47] and RR = 1.01 [0.85–1.20]; p = 0.90. The largest study by Jawad et al. [26] assessed the occurrence of AEs over four months long-term use, with similar figures for Echinacea and placebo [RR = 0.98 [95% CI 0.91–1.04]]. Ogal et al. investigated the same Echinacea preparation (Echinaforce®) to find significantly lower AEs in comparison with control (3 × 50 mg vitamin C), due to reduced RTI complications including otitis media or bronchitis [14]. Overall, AEs most often concerned mild gastro-intestinal complaints, which were self-limiting without medicinal intervention.
Figure 7.
Information regarding occurrence of AEs from 17 clinical studies. Forest plots showing meta-analysis of proportion of Echinacea-treated subjects experiencing AEs compared with control. Shown are “events” (AEs), “total” (participants) for Echinacea (“experimental”) and placebo risk ratios (RR) employing a common and random effect model, heterogeneity (I2), confidence intervals (95%-CI), p-value and individual weight of respective studies.
3. Discussion
Global antibiotic use continues to rise despite governmental education programs (i.e., antibiotic stewardship) promoting their judicious use [49]. Every day of oral beta-lactam administration is estimated to increase the risk of carrying penicillin resistant pneumococci by 4% [50]. RTIs represent the most common reason for antibiotic use in not only the ambulatory but also inpatient and self-medication settings [51]. Reducing the antibiotic use for RTIs thus represents a unique opportunity to control the overuse of antibiotics in the future.
We investigated the potential of Echinacea species to prevent initial viral RTIs, thereby reducing secondary (likely bacterial) RTI complications and the need for antibiotics. Positive associations between Echinacea and the three levels of prevention could be demonstrated, showing a reduction of overall RTIs by ~32% at a RR = 0.68 [95% CI 0.61–0.77], of recurrences by approximately 40% at RR = 0.60 [95% CI 0.46–0.80] and of complications by approximately 56% at RR = 0.44 [95% CI 0.36–0.54]. These reductions resulted in approximately 40% fewer participants requiring antibiotics (RR = 0.60 [95% CI 0.39–0.93]) and a 70% reduction of antibiotic treatment days (RR = 0.30 [0.12–0.73]), both results on antibiotic use being statistically significant (p < 0.05). The former result included two trials [39,42] on the acute use of Echinacea and their exclusion aligned to figures on overall antibiotic treatment days. This supports the beneficial effects of long-term, preventative Echinacea supplementation. The difference between the two outcomes (antibiotic prescriptions vs. treatment duration) might also originate from using heterogeneous Echinacea preparations. Upon exclusion of hydrophilic extracts (pressed juices) as used in Taylor and Spasov [39,41], the RR = 0.45 [95% CI 0.30–0.66] for patients requiring antibiotics corresponded well with the value for antibiotic treatment days.
Heterogeneity in meta-analysis is a crucial, yet common factor increasing variance to the estimated effect: varying manufacturing techniques (lipophilic versus hydrophilic extracts or further supplements), study designs (prevention versus acute treatment) or the methodological quality of included studies. We addressed this potential weakness by applying distinct selection criteria in function of the respective research question to attribute benefits to the various characteristics of heterogeneity in a sub-analysis. The differentiation between preparations used in trials more clearly revealed the correlation between RTIs, secondary complication and antibiotic use that was most convincingly demonstrated for lipophilic extracts. Those consisted mostly of alcoholic extracts from freshly harvested Echinacea purpurea herbs and roots (Echinaforce extract). This finding is consistent with data from Schapowal (2015) or Karsch–Voelk (2014), who also revealed important differences between Echinacea preparations [13,52]. An interesting observation was the fact that the two largest studies providing strongest effect sizes both investigated children preventively treated for three–four months using lipophilic preparations [14,18]. The RTI risks were very low with RRs = 0.47 and 0.67, recurrence risk RRs = 0.35 and 0.62, complication risk RRs = 0.42 and 0.48, leading to overall antibiotic treatment day IRR = 0.52 and 0.21 and fewer patients requiring antibiotics for the latter study, RR = 0.38 [0.15–0.94] (all p-values < 0.05).
It is reasonable to assume that reported broad-spectrum antiviral effects of alcoholic fresh-plant Echinacea extracts contribute to the preventative benefit of such products [10]. This alone however may not fully explain the observed strong decrease on the level of antibiotic prescriptions. Immuno-modulation or tertiary antibacterial effects may support the recovery process of acute illness rendering antibiotic use unnecessary, however more research is warranted to further elucidate the accumulating trend from RTI prevention to antibiotic reduction [11,12].
Our results compare to previous meta-analyses from David and Cunningham (2019) [44], Karsch–Voelk et al. (2014) [52] and Shah et al. (2006) [53], each drawing conclusion on nine or ten prevention studies including less than 2000 participants [45,52,53]. Ten years ago, Karsch–Voelk et al. conferred, despite significant heterogeneity, a pooled RR for RTIs prevention of 0.83 [95% CI 0.75–0.92]. In a more recent analysis, David and Cunningham found a RR for RTI prevention by Echinacea of 0.78 [95% CI 0.68–0.88], whereas Shah expressed preventive effects in a pooled odds ratio of OR = 0.42 for the same parameter [95% CI 0.25–0.71]. The above analyses did not cover more recent literature or studies written in the German language, which our study did include.
Our results are based on data from 5652 study subjects included in 30 studies, yielding a comparable overall RR for RTIs of 0.68 [0.61–0.77, p < 0.01]. Similar to Shah, odds ratios found in our study were notably lower than risk ratios and, in our study, OR = 0.53 [0.42–0.67, p < 0.01] approached the value found by Shah, i.e., a reduction by over 50% in the absence of heterogeneity.
This work demonstrates for the first time how Echinacea reduces antibiotic prescriptions and overall therapy duration on the level of a meta-analysis referring to randomized controlled clinical studies. Along with results on RTI incidence and duration, no previous meta-analyses investigated the sequence of incident RTI, RTI recurrences, RTI complications and the use of antibiotics, therefore no comparative effects are available. The strategy to reduce antibiotic use through RTI prevention is very promising and has been described similarly for influenza and pneumococcal vaccines, which are associated with a 10–40% reduction of antibiotic prescriptions or antibiotic days [54]. This effect has now been demonstrated to be applicable to Echinacea as well, while results shown for alcoholic fresh-plant Echinacea extracts (55–70%, Appendix A, Table A4) seem to exceed the effectiveness of the aforementioned vaccinations. A combined approach of vaccination plus Echinacea supplementation may provide even superior effects, however this would require confirmation in a confirmatory clinical study.
The safety profile of Echinacea was evaluated by previous meta-analyses along with the present research. David deduced a relative risk of RR = 1.09 [0.95–1.25] for participants reporting at least one adverse event [44] and Karsch–Voelk found a 2.4% dropout rate due to adverse events with Echinacea compared to 0.8% with placebo [52]. The latter, however, wrongly referenced the data from the largest trial by Jawad, therefore the result has to be questioned. We looked at overall occurring adverse events as safety parameter to find the very same number of adverse events occurring with Echinacea therapy and control, i.e., 633 AEs in a sample of 1903 and 1772 participants, OR = 0.99 [95% CI 0.636–1.47] and RR = 1.01 [0.82–1.25]; p = 0.90. These figures indicate a highly positive safety profile. In comparison with David and Karsch–Voelk, we looked at the total occurring adverse events rather than patients experiencing events [45,52]. However, both analyses underscore the very good safety profile of Echinacea extracts used for prevention and acute therapy. Taylor found an increase in allergic reactions for a pressed-juice formulation used in children, however this was not confirmed by Ogal or Cohen for lipophilic extracts, even upon long-term use over three–four months [14,18,42]. In most cases, adverse events were mild, self-limiting, gastrointestinal in nature and did not require medical intervention.
Our analysis has limitations. First, we did not restrict publication date and also regarded early scientific studies prior to 2000, when reporting guidelines were not as strict. Hence, some publications received lower quality ratings. This however does not necessary indicate a low quality of the study per se. They may still provide valid results, a conclusion supported by the fact that analysis of only high-quality studies did not significantly change the overall result or decrease heterogeneity overall.
Secondly, we carried out a series of sub-analysis accounting for the variability of included studies mentioned above. More extensive diversification would have been interesting but would have exceeded the scope of this work. Previous research focused on RTI prevention in immunologically susceptible individuals, finding better results in comparison to more robust subjects [13]. We did not explicitly investigate this population in more detail.
In conclusion, Echinacea could provide an effective and safe means to prevent RTIs and secondary complications to thereby significantly reduce the need for antibiotic prescriptions. However, due caution is implicated in the selection of the particular Echinacea product as differences may exist.
4. Methods
The purpose of this systematic review and meta-analysis was to investigate the potential of Echinacea species to prevent and treat RTI under the conditions of a RCT (randomized controlled trial). As an outcome, a trial had to compare at least one of the following between groups over the study period: RTIs, recurrent RTIs, complications of RTIs or use of antibiotics. Further, we collected reports on AEs for the assessment of Echinacea’s safety profile.
The protocol for this systematic review was registered on INPLASY under protocol ID: 4969-1. We carried out a comprehensive search of literature on EMBASE, PubMed, Google Scholar and Cochrane DARE from the respective databases’ day of inception until 30 June 2023 without restriction for language, publication status or particular patient groups and according to guidance [55,56]. An example literature search strategy is given in Table 1.
Table 1.
Example search strategy.
| Step | Search |
|---|---|
| 1 | Echinacea.mp. or exp Echinacea/ |
| 2 | coneflower.mp. |
| 3 | Black Sampson.mp. |
| 4 | 1 or 2 or 3 |
| 5 | (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (exp animals/ not humans.sh.) |
| 6 | 4 and 5 |
| 7 | Remove duplicates from 6 |
| 8 | limit to controlled, randomized human RTI studies |
In addition, we screened the clinical trials register clinicaltrials.gov for completed studies with results on Echinacea. Some articles were available in German and thus literature was sought by any language and via screening bibliographies of identified trials and review articles. We did not include articles in Arabic [48]. Identified hits from the above searches were checked for duplicates using EndNote. Resulting hits were then displayed with abstract and title. Two review authors (GG, MS) were involved in the final selection of clinical articles studying Echinacea for treatment or prevention of RTIs in humans using a controlled setting. Random allocation to verum and control group was a prerequisite for inclusion in order to yield homogenous and comparable collectives. Articles were further regarded if information on (recurrent) RTIs, their complications and/or usage of antibiotics were reported. Two authors independently carried out the study selection process (GG, MS), whereas native speaking authors reviewed the German literature (GH, RS).
The resulting list of referenced literature was checked for consistency and completeness, and discrepancies were solved mutually. Study details were retrieved and data were extracted using a standard extraction form capturing authors, reference, study registration number, Echinacea species and manufacturing method, dosage, details on comparator, studied indication, methodology, patient sample, RTI occurrence, complications, antibiotic use and adverse events. We contacted investigators and sponsors of registered clinical studies in case of missing data. Results on recurring RTIs were deduced from the number of relapses/recurrences from the first dose of Echinacea until the end of treatment phase including any follow-up period, as defined by Schapowal (2015) [13]. Patients with and incidences of complications and/or bacterial superinfections were deduced from the same observation period retrieving reports for tonsillitis/pharyngitis, tracheitis, lymphadenitis, bronchitis, pneumonia, sinusitis, conjunctivitis, otitis media (acuta) or adverse events on respiratory system disorders. Regarding the use of antibiotics, we searched for the number of patients requiring antibiotics as well as treatment duration where available.
According to pre-published protocol, our primary parameters were the odds for (recurrent) RTIs, of complications, respectively, the need for antibiotics during the time of Echinacea intervention and follow-up period in comparison with the control. Additionally, we evaluated results on the patient level, i.e., the number of patients reporting ≥1 RTI, recurrent RTIs, complications or those with antibiotic therapy. Accounting for the varying therapy/observation durations of included studies, we expressed results in terms of monthly occurrence of RTIs as well.
Our risk of bias assessment largely referred to the work by David et al. that used the Cochrane collaboration’s risk of bias tool [44,48]. Additional literature not included by their work was assessed independently. We also applied the Jadad et al. scoring method to estimate the studies’ methodological qualities [45]. Risk of publication bias across selected studies was scrutinized using funnel plots in order to detect asymmetries within trials referred to in the meta-analysis.
We quantitatively estimated effect sizes using meta-analysis and forest plots displaying odds rations (OR) and risk ratios (RR) with their respective 95% confidence intervals (CI) for binary data. For continuous parameters (e.g., duration of antibiotic therapy) we synthesized the incidence risk ratio (IRR) between groups.
Where quantitative data was available, we synthesized the results of the included studies by meta-analysis with the R language for statistical programming version R-4.3.1 using the “meta” package. Due to heterogeneity between studies, we conservatively applied a random effect model but compared results to the fixed effect model as well. For the binary outcomes we used the “metabin” function, which uses the Mantel–Haenszel method for pooling and the DerSimonian–Laird estimator for tau². For measures of event counts, the “metafor” function was used. Between study heterogeneity was assessed using the I² statistic [57,58].
Analogous to David et al. [44], we deduced the number of participants with ≥1 infection from the total number of infections occurring and the number of subjects with recurring infections/relapses. Information pertaining to the occurrence of episodes was principally retrieved from David et al. after confirmation regarding where data was available. In contrast to a Cochrane review by Karsch–Voelk et al. [52], we included Spasov et al. [41] in our analysis, who compared to standard treatment instead of placebo, as well as trials published thereafter. Melchart, Bräuning, Turner (2005), Sumer and Forth et al. used multiple Echinacea species, extraction methods or dosage strengths [15,17,33,36,37]. We pooled effects from the treatment groups into one comparison each. Sumer et al. [37] used 4 arms comparing increased dosing during acute RTI episodes with a low, preventative dosage [36]. The latter was conservatively considered as the control treatment. Vonau and Coegniet et al. [59,60] studied preventive applications of Echinacea for urinary tract infections and were therefore excluded, as were studies investigating anything other than RTIs. Cohen et al. [18] reported a number of subjects experiencing otitis media, tonsillopharyngitis or pneumonia individually, and we calculated the mean of subjects experiencing any of the three complications.
This work intentionally aimed to survey a wide range of studies in the primary analysis to obtain a general overview on preparations containing Echinacea at first. Consequently, we included non-treatment controlled or actively controlled studies only if appropriately randomized. We did not restrict the study to a single Echinacea species or manufacturing technique, and preparations that contained further ingredients like zinc, other herbs or vitamins were included. We collected information on RTIs, recurrent RTIs/relapses, RTI complications and antibiotic therapies reported from the time of the onset of Echinacea intake until the end of follow up during the studies. Our investigation was in alignment with the latest recommendations by the PRISMA working group for reporting meta-analyses [61].
Finally, we decided ad hoc to investigate the safety profile of Echinacea while comparing the occurrence of adverse events during intervention. For this parameter, we solely referred to comparisons of Echinacea versus placebo in healthy subjects. Trials in patients with underlying illness like cancer, with concomitant antibiotic therapy or comparisons to oseltamivir, were excluded as they were expected to skew the basis for establishing the net effect of Echinacea with respect to safety.
Acknowledgments
We kindly thank Roland Schoop for giving input into the conceptualization of this work and the manuscript.
Appendix A
Table A1.
Description of included studies and assessment of methodological quality according to Jadad scoring [45].
| Study/ Registry |
Echinacea Species | Control | Extraction Method | Supplement | Duration of Treatment/Observation | Daily Dose/Amount of Echinacea [mg] | Participant Number (N, ITT) | Age [Years] | Cold Definition | Jadad Score [18] |
|---|---|---|---|---|---|---|---|---|---|---|
| Bendel R et al., 1988 [20] | EPAr + EPUr Esberitox | NT | Ethanolic Extract | Thuiae occid, Baptisia | 50 days in addition to Chemotherapy Prevention |
3 × 50 drops | 50 | >18 | Respiratory Infection induced Stop of Chemotherapy medically confirmed | 2 |
| Bendel R et al., 1989 [19] | EPAr + EPUr Esberitox | NT | Ethanolic Extract | Thuiae occid, Baptisia | 12 Chemotherapy Cycles à 14 days Prevention |
3 × 25 drops | 67 | >18 | Respiratory Infection induced Stop of Chemotherapy medically confirmed | 1 |
| Bräuning B et al., 1992 [36] | EPUr | Placebo | Ethanolic Extract | None | Therapy 8–10 days | Dosis 1 = 90 drops/450 mg Dosis 2 = 2 × 90 drops/900 mg |
180 | 18–60 | Flu-like Infections, clinically confirmed (virally vs. bacterial) | 1 |
| Cohen HA et al., 2004 [18] | EPU + EAN | Placebo | Glycerol extract | Propolis + Vitamin C | 3 mts Prevention |
2–4 × 5–7.5 mL 500–1500 mg | 328 | 1–5 | Patient reported- medically confirmed |
4 |
| Forth H, Beuscher N, 1981 [17] | EPAr + EPUr (Esberitox) | Placebo (20 mg Vit C) | Ethanolic extract | Thuiae occid, Baptisia | 3 × 14d cycles for up to 17 weeks Prevention |
3 × 25 drops or 3 × 1 tablet | 95 | >18 | Patient reported Rhinitis | 1 |
| Freyer HU, 1974 [22] | EPAr + EPUr | NT | Ethanolic extract | Thujae occid, Baptisia | 6 weeks Prevention |
3 × 20 drops | 284 | 6–17 | “infections” not further described | 1 |
| Goel V et al., 2004 [38] | EPU | Placebo | Ethanol | None | 7 days Therapy |
1st day: 10 × 4 mL 6 days: 4 × 4 mL |
128 ≥2 colds/y |
18–65 | Patient reported Confirmed by study nurse/physician | 5 |
| Grimm (1999)/Schoeneberger (1996) [23,62] | EPUh | Placebo | Pressed-juice | None | 2 mts Prevention |
2 × 4 mL 6200 mg 2) |
108 ≥3 colds/y |
>11 | Patient reported- Confirmed by physician |
5 |
| Hall, H et al., 2007 [24] | EPUh | Placebo | Pressed Juice | none | 28 days Prevention |
4 × 2 capsules/8000 mg | 32 | >17 | Incidence of URTI Patient-reported outcome | 4 |
| Helbig (1961) [25] | EPUr + EANr (Esberitox) | NT | Ethanolic extract | Thujae occid/Baptisia | 1 mt Prevention |
3 × 20 drops | 644 | 1–3 | Infections of Upper Respiratory Tract | 0 |
| Jawad et al., 2012 [26] | EPU h + r (Echinaforce) | Placebo | Ethanolic extract | None | 4 mts Prevention |
3–5 × 0.9 mL 2.7–4.5 mL 2400–4000 mg |
717 | >17 | Patient reported–confirmed by Jackson method Virally confirmed infections |
5 |
| König D, 1999 or Berg A (1998) [21] | EPUh | Placebo/Magnesium | Pressed Juice i.c. placebo and Biomagnesin |
None | 28 days Prevention |
3 × 40 drops/8000 mg | 42 (Athletes) | >17 | Incidence of URTI Infection, Training failures | 3 |
| Kolev E et al., 2022 [27] | EPU h + r (Echinaforce) | NT | Ethanolic extract | None | 5 months Prevention |
3–5 × 2 tablets (400 mg)/2400–4000 mg | 119 | 18–75 | Patient reported, physician and virally-confirmed infections | 2 |
| Melchart (1998) 3-arm study [15] |
EPUr | Placebo | Ethanolic extract | None | 3 mts Prevention |
2 × 50 drops 1800 mg 3) |
99 (90 placebo) =/>3 colds/y |
18–65 | Patient reported- Confirmed by physician |
4 |
| Melchart (1998) 3-arm study [15] |
EANr | Ethanolic extract | None | 3 mts Prevention |
2 × 50 drops 1800 mg 3) |
100 (90 placebo) |
18–65 | Patient reported- Confirmed by physician |
||
| O’Neil J et al., 2008 [28] | EPU | Placebo | Dried Echinacea, not specified | None | 8 weeks Prevention |
3 × 2 capsules/1800 mg | 90 | 18–65 | Patient reported- Study staff confirmed |
4 |
| Ogal M et al., 2021 NCT02971384 [14] |
EPU h + r (Echinaforce) | Placebo (VitC) | Ethanolic extract | None | 4 months Prevention |
3–5 × 1 tablet (400 mg)/1200–2000 mg | 203 | 4–12 | Patient reported, physician and virally-confirmed infections | 5 |
| Awad OG, 2020 2015NBA5732814 [29] |
EPU root | Azithromycin (AZT) vs. NTC | Powder | None | 6 × 10 days over 6 months Prevention |
3 × 5 mL (250 mg)/1500 mg + AZT I.c. no prevention/ATZ prevention |
300 | 5–16 | Recurrent tonsillitis, reported by parents | 1 |
| Schmidt U et al., 1990 [30] | EAN | Placebo | Ethanolic extract | Eupatorium/Baptisia | 2 month Prevention |
1 × 12 mL/1440 mg 1) | 609 | >17 | Patient reported- Confirmed by physician |
4 |
| Schulten B et al., 2001 [40] | EPUh (Echinacin®) |
Placebo | Pressed Juice | None | 10 days Therapy |
2 × 5 mL (7750 mg) | 80 | >17 | Patient reported confirmed by Jackson method (full picture of cold) | 3 |
| Spasov AA et al., 2004 [41] | EPUh | NT (standard therapy) | Pressed Juice | None (i.a. std treatment) | 10 days Therapy |
3 × 10 drops | 80 | 4–11 | Patient reported, Physician confirmed uncomplicated RTIs | 2 |
| Sperber SJ et al., 2004 [31] | EPUh Echinaguard |
Placebo | Pressed juice | None | 14d Prevention |
3 × 2.5 mL | 46 | 18–65 | Artificially Rhinovirus Infection, Jackson definition | 3 |
| Sumer J et al., 2023 [37] | EPU (h + r) (Echinaforce) |
Ethanolic extract | None | 10 days Therapy |
1–5 tablets (3360 mg) or 2–7 sprays (1120 mg) 3360–16,800 mg |
246 | >17 | Patient reported, physician and virally-confirmed flu-like infections | 4 | |
| Tiralongo E et al., 2012 [32] | EPUr + EANr (MediHerb) | Placebo | Ethanolic extract | None | 5–9 weeks Prevention |
Priming dose 2 × 1 tabs followed by exposition dose 2 × 2 tabs sick dose 3 × 2 tabs/3825 mg and 7650 mg | 148 | 18–65 | Natural exposition (air travel) | 5 |
| Turner RB et al., 2005 [16] 4-arm study |
EANr | Placebo | 20% Ethanolic extract | None | 7 days Prevention 5 days Therapy |
3 × 1.5 mL (300 mg)/900 mg | 206 | >17 | Artificially Rhinovirus Infection, Jackson definition Patient reported, physician and virally-confirmed flu-like infections |
4 |
| Turner RB et al., 2005 [16] 4-arm study |
EANr | Placebo | 60% Ethanolic extract | None | 7 days Prevention 5 days Therapy |
3 × 1.5 mL (300 mg)/900 mg | 203 | >17 | 4 | |
| Turner RB et al., 2005 [16] 4-arm study |
EANr | Placebo | CO2 extract | None | 7 days Prevention 5 days Therapy |
3 × 1.5 mL (300 mg)/900 mg | 196 | >17 | 4 | |
| Turner RB et al., 2000 [33] | EPU | Placebo | Powder Almost no alkylamides |
None | 19 days (14 days prevention + 5 days therapy) Prevention |
3 × 1 capsule/900 mg | 92 | >17 | Artificial Rhinovirus Infection, | 3 |
| Taylor (2003)/Weber (2005) [35,42] | EPUh (Echinacin®) | Placebo | Pressed juice | None- | 4/1 week Therapy |
2 × 3.75–5 mL 7.5–10 mL 7500–10,000 mg |
407/401 | 2–11 | Study staff confirmed | 5 |
| Yakoot M et al., 2011 [43] | E (Immumax) |
Placebo | Extract | Garlic, Nigella sativa, Panax ginseng, Vitamin C, Zinc | 14 days Therapy |
2 × 1 capsule (120 mg)/240 mg | 63 | 38 (Mean) | Patient reported- Confirmed by physician |
5 |
| Zhang X et al., 2003 [34] | EPUr | Placebo | Powdered root | None | 8 weeks Prevention |
2 × 1 capsule (294 mg)/588 mg | 111 | 18–65 | Patient reported- Confirmed by physician |
3 |
E…Echinacea (not further specified) EAN…Echinacea angustifolia. EPU…Echinacea purpurea. h…herb. r…root. NT…No-treatment control. ITT…Intention-to-treat. 1) with 120 mg/mL EA extract; 2) product contains 22% Ethanol for stabilisation; 3) at 20 drops / ml and δ = 0.9 gr/mL.
Table A2.
Methodological Quality Assessment according to Cochrane Risk of Bias Tool and Jadad Scoring [46,55].
| Study | Random Sequence Generation | Allocation Concealment | Blinding Patients/Personel | Blinding Outcome Assess | Incomplete Outcome | Select Reporting | Other Bias | Overall Jadad [0–5] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Bendel, 1988 [20] | + | + | - | - | + | + | + | 2 | - | =High Risk |
| Bendel, 1989 [19] | ? | + | - | - | + | + | + | 1 | ||
| Bräunig, 1992 [36] | ? | ? | ? | ? | + | - | + | 1 | + | =Low Risk |
| Cohen, 2004 [18] | + | + | + | + | ? | + | + | 4 | ||
| Forth, 1981 [17] | + | - | - | ? | ? | - | ? | 1 | ? | =Unclear |
| Freyer, 1974 [22] | + | - | - | - | + | + | + | 1 | ||
| Goel, 2004 [38] | + | + | + | + | + | + | + | 5 | ||
| Grimm, 1996 [23] | + | ? | + | + | + | + | + | 5 | ||
| Hall, 2007 [24] | + | + | + | + | ? | ? | + | 4 | ||
| Helbig, 1961 [25] | ? | - | - | - | + | + | + | 1 | ||
| Jawad, 2012 [26] | + | + | + | + | ? | ? | + | 5 | ||
| Kolev, 2022 [27] | + | - | - | + | + | + | + | 2 | ||
| Berg, 1998 [21] | ? | + | + | ? | + | + | + | 3 | ||
| Melchart, 1998 [15] | + | + | - | ? | + | + | + | 4 | ||
| Ogal, 2021 [14] | + | + | ? | + | + | + | + | 5 | ||
| O’Neil, 2008 [28] | + | + | + | + | - | + | + | 4 | ||
| Osama 2020 [29] | + | + | - | - | - | + | ? | 1 | ||
| Raus, 2015 [39] | + | + | + | + | + | + | + | 5 | ||
| Schmidt 1990 [30] | ? | + | ? | ? | + | + | + | 4 | ||
| Schulten, 2001 [40] | + | - | + | + | + | ? | + | 3 | ||
| Spasov, 2004 [41] | + | - | - | - | ? | ? | ? | 2 | ||
| Sperber, 2004 [31] | ? | ? | + | + | + | + | + | 3 | ||
| Sumer, 2023 [37] | + | + | ? | ? | + | + | + | 4 | ||
| Taylor03-Weber05 [35,42] | + | + | + | + | + | + | + | 5 | ||
| Tiralongo, 2012 [32] | + | + | + | + | ? | + | + | 5 | ||
| Turner, 2000 [33] | ? | ? | ? | + | + | + | + | 3 | ||
| Turner, 2005 [16] | + | ? | + | + | + | + | + | 4 | ||
| Yakoot, 2011 [43] | + | + | + | + | + | ? | + | 5 | ||
| Zhang, 2003 [34] | + | + | - | - | ? | + | + | 3 |
Table A3.
Results overall.
| RTIs/Pts with RTIs | Recurrent RTIs/Pts with Recurrent RTIs | Complications/Pts with Complications | Pts with AB/AB Treatment Days/Mean Difference [Days] | Adverse Events (Number of Events) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Registry |
Echinacea (N) | Control (N) | Echinacea | Control | Echinacea | Control | Echinacea | Control | Echinacea | Control |
| Bendel R et al., 1988 [20] | 24/12 (33) | 30/17 (34) | - | - | - | - | - | - | Safety of Echinacea during Chemotherapy not assessed | |
| Bendel R et al., 1989 [19] | 0/0 (25) | 1/1 (25) | - | - | - | - | - | - | Safety of Echinacea during Chemotherapy not assessed | |
| Bräunig B et al., 1992 [36] | - | - | - | - | 1/2 (120) | 4/3 (60) | 0/0 | 1/- | 4 | 5 |
| Cohen HA et al., 2004 [18] | 138/85 (160) | 308/150 (168) | 53 | 158 | 54/18 (160) | 136/45 (168) | -/541/3.40 (160) | -/1084/6.50 (168) | 9 | 7 |
| Forth H, Beuscher N, 1981 [17] | 22/22 (66) | 19/19 (29) | None reported | None reported | ||||||
| Freyer HU, 1974 [22] | 43/43 (140) | 74/74 (144) | 0 | 0 | ||||||
| Goel V et al., 2004 [38] | - | - | - | - | 2/2 (59) | 5/5 (69) | - | - | 8 | 6 |
| Grimm (1999)/Schoeneberger (1996) [23,62] | 35/42 (54) | 40/50 (54) | 14/7 (54) | 18/8 (54) | 37 (54) | 54 (54) | - | - | 11 | 7 |
| Hall, H et al., 2007 [24] | 7/7 (18) | 7/7 (14) | - | - | - | - | - | - | Not reported | Not reported |
| Helbig 1961 [25] | 66/- (322) | 140/- (322) | 66 | 140 | - | - | 15 (322) | 41 (322) | 0 | 0 |
| Jawad (2012) [26] | 149/112 (355) | 188/131 (362) | 65/28 (355) | 100/43 (362) | 5/5 (355) | 7/7 (362) | 1/7/0.02 (355) | 4/33/0.09 (362) | 293 | 306 |
| König D, 1999 or Berg A (1998) [21] | 0/0 (14) | 4/4 (13) | - | - | 0/0 (14) | 1/1 (13) | - | - | 0 | 3 |
| Kolev E et al., 2022 [27] | 21/21 (59) | 29/29 (60) | - | - | 0/0 (59) | 2/2 (60) | 8 (59) | 12 (60) | 3 | 5 |
| Melchart (1998) 3-arm study [15] |
- | - | 4/4 (EPUr) (99) | 6/6 (90) | - | - | - | - | 13 | 12 |
| Melchart (1998) 3-arm study [15] |
- | - | 7/7 (EAN) (100) | - | - | - | - | 21 | ||
| O’Neil J et al., 2008 [28] | 9/9 (45) | 14/14 (45) | - | - | - | - | - | - | (8%) 2 | (7%) 2 |
| Ogal M et al., 2021 NCT02971384 [14] |
61/40 (103) | 86/54 (98) | 21/16 (103) | 32/22 (98) | 11/10 (103) | 30/20 (98) | 6/45/0.44 (103) | 15/216/2.20 (98) | 76 | 105 |
| Awad OG, 2020 2015NBA5732814 [29] |
2/- (100) | 4/- (100) | - | - | 2 (100) | 4 (100) | - | - | Not assessed as in combination with AZT | |
| Raus K et al., 2015 EUDRA-CT 2010-021571-88 [39] |
- | - | - | - | 5/5 (203) | 9/9 (217) | 4 (203) | 4 (217) | Not assessed as in comparison with Oseltamivir | |
| Schmidt U et al., 1990 [30] | 164/132 (303) | 199/155 (306) | 32 | 44 | 12 | 10 | ||||
| Schulten B et al., 2001 [40] | - | - | 1/1 (41) | 4/4(39) | 8 | 9 | ||||
| Spasov AA et al., 2004 [41] | - | - | 1 (41) | 1 (39) | 1 | 0 | ||||
| Sperber SJ et al., 2004 [31] | 14/14 (24) | 20/20 (22) | 2 | 4 | ||||||
| Sumer J et al., 2023 [37] | - | - | 0/0 (61) | 2/2 (64) | 0/0/0 (61) | 2/6/0.09 (64) | Comparison of different Echinacea galenic forms, no non-Echinacea reference. | |||
| Tiralongo E et al., 2012 PHM0608HREC [32] |
31/31 (72) | 43//43 (76) | 3 | 2 | ||||||
| Turner RB et al., 2000 [33] | 11/11 (50) | 14/14 (42) | 0 No significant side effect seen |
0 | ||||||
| Turner RB et al., 2005 [16] | 73/73 (149) | 58/58 (103) | 2% (prevention phase) | 2% (prevention phase) | ||||||
| Taylor (2003) [42] Weber (2005) [35] |
- - |
- - |
137/110 (200) | 163/142 (207) | 30 (200) | 27 (207) | 152 | 146 | ||
| Yakoot M et al., 2011 [43] | - | - | 0/0 (31) | 2/2 (32) | 0 (31) | 2 (31) | No significant difference between groups but no listing of AEs | |||
| Zhang X et al., 2003 [34] | 25/44 (54) | 33/57 (57) | 15 | 4 | ||||||
Pts…Participants. RTI…Respiratory Tract Infections. AB…Antibiotics.
Table A4.
Results from sub-analysis with resulting risk ratios per analysis section.
| Subanalysis | Subjects with RTI | Monthly Risk of RTI | Overall Infections | Subjects with Recurrent RTI | Number of Recurrent RTI | Subjects with Complication | Number of Complications | Subjects with AB | Overall AB Days |
|---|---|---|---|---|---|---|---|---|---|
| Overall Result | 0.75 [0.69–0.81] I2 = 27% |
0.68 [0.61–0.77] I2 = 29% |
0.75 [0.69–0.82] I2 = 55% |
0.77 [0.68–0.88] I2 = 0% |
0.60 [0.46–0.80] I2 = 88% |
0.44 [0.36–0.54] I2 = 0% |
0.52 [0.43–0.64] I2 = 32% |
0.60 [0.39–0.93] I2 = 34% |
0.30 [0.12–0.73] I2 = 91% |
| Jadad Score ≥ 4 | 0.78 [0.71–0.86] I2 = 40% |
0.75 [0.64–0.87] I2 = 32% |
0.84 [0.80–0.88] I2 = 0% |
0.77 [0.68–0.88] I2 = 0% |
0.63 [0.46–0.87] I2 = 88% |
0.47 [0.37–0.58] I2 = 0% |
0.53 [0.41–0.68] I2 = 52% |
0.77 [0.34–1.45] I2 = 34% |
0.30 [0.12–0.73] I2 = 91% |
| Lipophilic Extracts | 0.75 [0.66–0.83] I2 = 47% |
0.66 [0.56–0.78] I2 = 50% |
0.72 [0.64–0.81] I2 = 68% |
0.63 [0.51–0.78] I2 = 0% |
0.53 [0.39–0.73] I2 = 72% |
0.46 [0.36–0.58] I2 = 0% |
0.42 [0.35–0.50] I2 = 0% |
0.45 [0.30–0.66] I2 = 0% |
0.30 [0.12–0.73] I2 = 91% |
| Hydrophilic Extracts | 0.79 [0.67–0.94] I2 = 0% |
0.75 [0.56–1.02] ns, 0% |
0.82 [0.66–1.02] ns, I2 = 15% |
0.80 [0.67–0.96] I2 = 0% |
0.87 [0.66–1.14] Ns, I2 = 0% |
0.26 [0.05–1.26] Ns, I2 = 0% |
0.68 [0.50–0.92] I2 = 0% |
1.14 [0.76–1.73] I2 = 0% |
No study providing data |
| Echinacea only | 0.80 [0.75–0.85] I2 = 0% |
0.78 [0.71–0.85] I2 = 0% |
0.79 [0.74–0.85] I2 = 7% |
0.77 [0.68–0.88] I2 = 0% |
0.80 [0.67–0.96] I2 = 16% |
0.47 [0.34–0.65] I2 = 0% |
0.64 [0.54–0.77] I2 = 0% |
0.73 [0.41–1.33] Ns, I2 = 26% |
0.21 [0.15–0.29] I2 = 0% |
Green 0–40% low heterogeneity. Rose 41–60% moderate heterogeneity. Light Red 61–75% substantial heterogeneity. Dark Red > 75% Considerable heterogeneity or non-significant result.
Figure A1.
Graphical illustration of sub-analysis results.
Appendix B
Figure A2.
Funnel plot in detail referred to Figure 2 of main text.
Figure A3.
Abbey plot in detail referred to Figure 2 of main text.
Figure A4.
Funnel plot in detail referred to Figure 3 of main text.
Figure A5.
Abbey plot in detail referred to Figure 3 of main text.
Author Contributions
Conceptualization, G.G., R.S., M.O., A.B., S.K., G.H. and W.V.B.; methodology, G.G., R.S., M.O., A.B., W.C.A., S.K., G.H. and S.L.J.; software, G.H.; validation, G.G., G.H., S.V., M.S., B.H.-C. and A.B.; formal analysis, G.G., G.H., R.S., S.V., M.S., B.H.-C. and A.B.; investigation, G.G., G.H., R.S., M.S., B.H.-C., A.B. and W.V.B.; resources, G.G., A.B. and G.H.; data curation, G.H., A.B. and B.H.-C.; writing—original draft preparation, G.G., G.H., M.O. and M.S.; writing—review and editing, all authors; visualization, G.H.; supervision, S.K., S.L.J. and A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All articles reviewed have been included in the reference list.
Conflicts of Interest
R.S., W.C.A. and S.L.J. received honoraria from A.Vogel AG Switzerland for consulting work. G.H. received honoraria for statistical evaluation of this meta-analysis. The other authors declare no conflicts of interest.
Funding Statement
This research was financially supported in part by A.Vogel AG, Switzerland.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nunes-Silva C., Vilares A.T., Schweitzer V., Castanhinha S., Martins A., Lopes M.J., Ascoli-Bartoli T., Canelas G., Keir H.R., Cunha F., et al. Non-COVID-19 respiratory viral infection. Breathe. 2022;18:210151. doi: 10.1183/20734735.0151-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X., Ren J., Li R., Gao Y., Zhang H., Li J., Zhang J., Wang X., Wang G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. eClinicalMedicine. 2021;37:100986. doi: 10.1016/j.eclinm.2021.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/s0140-6736(16)31678-6. Erratum in Lancet 2017, 389, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2019. [(accessed on 1 February 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 5.Lang K. What do we know about covid in immunocompromised people? BMJ. 2023;383:1612. doi: 10.1136/bmj.p1612. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins J. 2023. [(accessed on 1 February 2024)]. Available online: https://coronavirus.jhu.edu/map.html.
- 7.Brueggemann A.B., Jansen van Rensburg M.J., Shaw D., McCarthy N.D., Jolley K.A., Maiden M.C.J., van der Linden M.P.G., Amin-Chowdhury Z., Bennett D.E., Borrow R., et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health. 2021;3:e360–e370. doi: 10.1016/s2589-7500(21)00077-7. Erratum in Lancet Digit. Health 2021, 3, e360–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandi A., Pecetta S., Bloom D.E. Global antibiotic use during the COVID-19 pandemic: Analysis of pharmaceutical sales data from 71 countries, 2020–2022. eClinicalMedicine. 2023;57:101848. doi: 10.1016/j.eclinm.2023.101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.P.R., Westwood D., Daneman N., MacFadden D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleschka S., Stein M., Schoop R., Hudson J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian Influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol. J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Declerck K., Novo C.P., Grielens L., Van Camp G., Suter A., Vanden Berghe W. Echinacea purpurea (L.) Moench treatment of monocytes promotes tonic interferon signaling, increased innate immunity gene expression and DNA repeat hypermethyl-ated silencing of endogenous retroviral sequences. BMC Complement. Med. Ther. 2021;21:141. doi: 10.1186/s12906-021-03310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma M., Schoop R., Hudson J.B. Echinacea as an antiinflammatory agent: The influence of physiologically relevant pa-rameters. Phytother. Res. 2009;23:863–867. doi: 10.1002/ptr.2714. [DOI] [PubMed] [Google Scholar]
- 13.Schapowal A., Klein P., Johnston S.L. Echinacea Reduces the Risk of Recurrent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv. Ther. 2015;32:197–200. doi: 10.1007/s12325-015-0194-4. [DOI] [PubMed] [Google Scholar]
- 14.Ogal M., Johnston S.L., Klein P., Schoop R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: A randomized, blinded, controlled clinical trial. Eur. J. Med. Res. 2021;26:33. doi: 10.1186/s40001-021-00499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melchart D., Walter E., Linde K., Brandmaier R., Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections. Arch. Fam. Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 16.Turner R.B., Bauer R., Woelkart K., Hulsey T.C., Gangemi J.D. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N. Engl. J. Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 17.Forth H., Beuscher N. Beeinflussung der Häufigkeit banaler Erkältungsinfekte durhc Esberitox. Z. Für Allg. 1981;57:2272–2275. [PubMed] [Google Scholar]
- 18.Cohen H.A., Varsano I., Kahan E., Sarrell M., Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: A randomized. Arch. Pediatr. Adolesc. Med. 2004;158:217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 19.Bendel R., Bendel V., Renner K., Carstens V., Stolze K. Zusatzbehandlung mit Esbertox N bei Patientinnen mit chemostrahlentherapeutischer Behandlung eines fortgeschrittenen Mammakarzinoms. Onkologie. 1989;12((Suppl. S3)):32–38. doi: 10.1159/000216701. [DOI] [PubMed] [Google Scholar]
- 20.Bendel R., Renner K., Stolze K. Zusatzbehandlung mit Esberitox bei Patientinnen mit kurativer adjuvanter Bestrahlung nach Mammakarzinom. Strahlenther. Onkol. 1988;164:278–283. [PubMed] [Google Scholar]
- 21.Berg A., Northoff H., Konig D., Weinstock C., Grathwohl D., Parnham M.J., Stuhlfauth I., Keul J. Influence of Echinacin (EC31) treatment on the exercise-induced immune response in athletes. J. Clin. Res. 1998;1:367–380. [Google Scholar]
- 22.Freyer H.U. Häufigkeit banaler Infekte im Kindeslater und Möglichkeiten der Prophylaxe. Forschritte Med. 1974;92:165–168. [PubMed] [Google Scholar]
- 23.Grimm W., Müller H.-H. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am. J. Med. 1999;106:138–143. doi: 10.1016/S0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 24.Hall H., Fahlman M., Engels H. Echinacea Purpurea and Mucosal Immunity. Int. J. Sports Med. 2007;28:792–797. doi: 10.1055/s-2007-964895. [DOI] [PubMed] [Google Scholar]
- 25.Helbig G. Unspezifische Reizkörpertherapie zur Infektionsprophylaxe. Med. Klin. 1961;56:1512–1514. [PubMed] [Google Scholar]
- 26.Jawad M., Schoop R., Suter A., Klein P., Eccles R. Safety and efficacy profile of Echinacea purpurea to prevent common cold episodes: A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2012;2012:841315. doi: 10.1155/2012/841315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolev E., Mircheva L., Edwards M.R., Johnston S.L., Kalinov K., Stange R., Gancitano G., Berghe W.V., Kreft S. Echinacea Purpurea For the Long-Term Prevention of Viral Respiratory Tract Infections during COVID-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. Front. Pharmacol. 2022;13:856410. doi: 10.3389/fphar.2022.856410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’neil J., Hughes S., Lourie A., Zweifler J. Effects of echinacea on the frequency of upper respiratory tract symptoms: A randomized, double-blind, placebo controlled trial. Ann. Allergy Asthma Immunol. 2008;100:384–388. doi: 10.1016/S1081-1206(10)60603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awad O.G.A.-N. Echinacea can help with Azithromycin in prevention of recurrent tonsillitis in children. Am. J. Otolaryngol. 2020;41:102344. doi: 10.1016/j.amjoto.2019.102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt U., Albrecht M., Schenk N. Pflanzliches Immunstimulans senkt Häufigkeit grippaler Infekt. Nat. Und Gan-Zheitsmedizin. 1990;3:277–281. [Google Scholar]
- 31.Sperber Steven J., Shah Leena P., Gilbert Richard D., Ritchey Thomas W., Monto A.S. Echinacea purpurea for prevention of ex-perimental rhinovirus colds. Clin. Infect. Dis. 2004;38:1367–1371. doi: 10.1086/386324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiralongo E., Lea R.A., Wee S.S., Hanna M.M., Griffiths L.R. Randomised, double blind, placebo-controlled trial of Echinacea sup-plementation in air travellers. Evid Based Complement Altern. Med. 2012;2012:417267. doi: 10.1155/2012/417267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner Ronald B., Riker Donald K., Gangemi J.D. Ineffectiveness of echinacea for prevention of experimental rhinovirus colds. Antimicrob. Agents Chemother. 2000;44:1708–1709. doi: 10.1128/AAC.44.6.1708-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Lowe D., Badesha G., Ishaq S., Rai H., Williams P. A Double-Blinded Placebo Controlled Trial Evaluating the Effectiveness of Echinacea in Countering Upper Respiratory Tract Infections. 2003 Unpublished Report . [Google Scholar]
- 35.Weber W., Taylor J.A., Stoep A.V., Weiss N.S., Standish L.J., Calabrese C. Echinacea purpureafor prevention of upper respiratory tract infections in children. J. Altern. Complement. Med. 2005;11:1021–1026. doi: 10.1089/acm.2005.11.1021. [DOI] [PubMed] [Google Scholar]
- 36.Bräuning B., Dorn M., Knick E. Echinacea purpurea radix zur Stärkung der körpereigenen Abwehr bei grippalen Infekten. Z. Für Phytother. 1992;13:7–13. [Google Scholar]
- 37.Sumer J., Keckeis K., Scanferla G., Frischknecht M., Notter J., Steffen A., Kohler P., Schmid P., Roth B., Wissel K., et al. Novel Echinacea formulations for the treatment of acute respiratory tract infections in adults—A randomized blinded controlled trial. Front. Med. 2023;10:948787. doi: 10.3389/fmed.2023.948787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel V., Lovlin R., Barton R., Lyon M.R., Bauer R., Lee T.D.G., Basu T.K. Efficacy of a standardized echinacea preparation (EchinilinTM) for the treatment of the common cold: A randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2004;29:75–83. doi: 10.1111/j.1365-2710.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- 39.Rauš K., Pleschka S., Klein P., Schoop R., Fisher P. Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial. Curr. Ther. Res. 2015;77:66–72. doi: 10.1016/j.curtheres.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulten B., Bulitta M., Ballering-Brühl B., Köster U., Schäfer M. Efficacy of echinacea purpurea in patients with a common cold. A placebo-controlled, randomised, doubleblind clinical trial. Arzneimittelforschung. 2001;51:563–568. doi: 10.1055/s-0031-1300080. [DOI] [PubMed] [Google Scholar]
- 41.Spasov A.A., Ostrovskij O.V., Chernikov M.V., Wikman G. Comparative controlled study of Andrographis paniculate fixed com-bination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004;18:47–53. doi: 10.1002/ptr.1359. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J.A., Weber W., Standish L., Quinn H., Jama J.G. Efficacy and safety of Echinacea in treating upper respiratory tract infec-tions in children: A randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 43.Yakoot M., Salem A. Efficacy and safety of a multiherbal formula with vitamin C and zinc (Immumax) in the management of the common cold. Int. J. Gen. Med. 2011;4:45–51. doi: 10.2147/IJGM.S16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David S., Cunningham R. Echinacea for the prevention and treatment of upper respiratory tract infections: A systematic review and meta-analysis. Complement. Ther. Med. 2019;44:18–26. doi: 10.1016/j.ctim.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Hróbjartsson A., Boutron I., Turner L., Altman D.G., Moher D., Cochrane Bias Methods Group Assessing risk of bias in ran-domised clinical trials included in Cochrane Reviews: The why is easy, the how is a challenge. Cochrane Database Syst. Rev. 2013;4:ED000058. doi: 10.1002/14651858.ED000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials:is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 47.A Wahl R., Aldous M.B., A Worden K., Grant K.L. Echinacea purpurea and osteopathic manipulative treatment in children with recurrent otitis media: A randomized controlled trial. BMC Complement. Altern. Med. 2008;8:56. doi: 10.1186/1472-6882-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahmati M.B., Safdarian F., Hamedi Y., Khadem A.A., Rezai M.S. Efficacy and Safety of Echinacea Root Extracts in the Treatment of Pediatric Common Cold: A Randomized Clinical Trial. J. Maz. Univ. Med. Sci. 2012;22:12–18. [Google Scholar]
- 49.Cars T., Eriksson I., Granath A., Wettermark B., Hellman J., Norman C., Ternhag A. Antibiotic use and bacterial complications following upper respiratory tract infections: A population-based study. BMJ Open. 2017;7:e016221. doi: 10.1136/bmjopen-2017-016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasrin D., Collignon P.J., Roberts L., Wilson E.J., Pilotto L.S., Douglas R.M. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: Prospective cohort study. BMJ. 2002;324:28. doi: 10.1136/bmj.324.7328.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lebanova H.V., Stoev S.N., Veleva N.R., Belcheva S.P., Madzharov V.G., Gueorguiev S.R. Prevalence of Self-Medication with Antibiotics in Europe: A Scoping Review. J. Biomed. Clin. Res. 2023;16:5–16. doi: 10.2478/jbcr-2023-0001. [DOI] [Google Scholar]
- 52.Karsch-Völk M., Barrett B., Kiefer D., Bauer R., Ardjomand-Woelkart K., Linde K. Echinacea for preventing and treating the common cold. Emergencias. 2014;2014:CD000530. doi: 10.1002/14651858.CD000530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah S., Sander S., White C.M., Rinaldi M., Coleman C. Evaluation of echinacea for the prevention and treatment of the common cold: A meta-analysis. Lancet Infect. Dis. 2007;7:473–480. doi: 10.1016/S1473-3099(07)70160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Heuvel L., Paget J., Dückers M., Caini S. The impact of influenza and pneumococcal vaccination on antibiotic use: An updated systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2023;12:70. doi: 10.1186/s13756-023-01272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lefebvre C., Glanville J., Briscoe S., Littlewood A., Marshall C., Metzendorf M.-I., Noel-Storr A., Rader T., Shokraneh F., Thomas J., et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M.S., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2020. [(accessed on 1 February 2024)]. version 6.1; Updated September 2020. Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 56.Tjosvold L. Randomized Controlled Trials/Controlled Clinical Trials: A Cut and Paste Search Strategy adapted from the Cochrane ENT Group for Web of Science. Adapted from “RCT Filters used by Cochrane ENT. Oxford (UK): Cochrane ENT Group; 2018.” John W. Scott Health Sciences Library, University of Alberta. Rev. 14 January 2021. [(accessed on 1 February 2024)]. Available online: https://docs.google.com/document/d/1aEVsIaeXVZY8_BND-sUB_19sTTMccgoxNUdznH54ilQ/edit.
- 57.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 59.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonau B., Chard S., Mandalia S., Wilkinson D., Barton S.E. Does the extract of the plant Echinacea purpurea influence the clinical course of recurrent genital herpes? Int. J. STD AIDS. 2001;12:154–158. doi: 10.1258/0956462011916947. [DOI] [PubMed] [Google Scholar]
- 61.PRISMA-P Group. Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoeneberger C. The influence of the immuno stimulating effects of pressed juice from Echinacea purpurea on the course and severity of cold infections. Forum Immunol. 1992;8:18–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All articles reviewed have been included in the reference list.