Abstract
Antibodies that neutralize primary isolates of human immunodeficiency virus type 1 (HIV-1) appear during HIV-1 infection but are difficult to elicit by immunization with current vaccine products comprised of monomeric forms of HIV-1 envelope glycoprotein gp120. The limited neutralizing antibody response generated by gp120 vaccine products could be due to the absence or inaccessibility of the relevant epitopes. To determine whether neutralizing antibodies from HIV-1-infected patients bind to epitopes accessible on monomeric gp120 and/or oligomeric gp140 (ogp140), purified total immunoglobulin from the sera of two HIV-1-infected patients as well as pooled HIV immune globulin were selectively depleted of antibodies which bound to immobilized gp120 or ogp140. After passage of each immunoglobulin preparation through the respective columns, antibody titers against gp120 and ogp140 were specifically reduced at least 128-fold. The gp120- and gp140-depleted antibody fraction from each serum displayed reduced neutralization activity against three primary and two T-cell line-adapted (TCLA) HIV-1 isolates. Significant residual neutralizing activity, however, persisted in the depleted sera, indicating additional neutralizing antibody specificities. gp120- and ogp140-specific antibodies eluted from each column neutralized both primary and TCLA viruses. These data demonstrate the presence and accessibility of epitopes on both monomeric gp120 and ogp140 that are specific for antibodies that are capable of neutralizing primary isolates of HIV-1. Thus, the difficulties associated with eliciting neutralizing antibodies by using current monomeric gp120 subunit vaccines may be related less to improper protein structure and more to ineffective immunogen formulation and/or presentation.
Neutralizing antibodies (NAbs) are an important component of protective immunity against numerous viruses (7, 12, 23, 27–29, 64, 68), and most effective vaccines elicit pathogen-specific NAbs (reviewed in references 11 and 56). Since protection of humans against human immunodeficiency virus type 1 (HIV-1) infection has not been achieved, the role of NAbs in protective immunity against HIV-1 is not known. However, based on the experience with other viruses, it is reasonable to assume that NAbs play a role in protection against infection by HIV-1. Abs capable of neutralizing HIV-1 in vitro develop naturally, over several years, in HIV-infected patients (10, 31, 41, 44, 57, 60, 77, 78). However, immunization with monomeric forms of the HIV-1 envelope glycoprotein gp120 results in production of Abs which neutralize T-cell line-adapted (TCLA) viruses (4, 62, 65) but have only marginal activity against primary isolates of HIV-1 (41, 42, 66, 79). Possible explanations for this weak neutralizing activity against primary viral isolates, in contrast to the potent NAbs that can develop during natural infection, include the inaccessibility or absence of relevant epitopes on the immunogen. Monoclonal Abs (MAbs) which potently neutralize primary isolates can bind to gp120, but it has been suggested that the neutralizing capacity of these Abs correlates more closely with the efficiency of binding to epitopes exposed on the oligomeric form of gp120 (22, 45, 49, 59), as the oligomeric protein may more closely resemble the native structure of HIV-1 gp120/gp41 (21, 51, 61, 70). In support of these studies, recent work in our laboratory has shown that immunization of rabbits with oligomeric gp140 (ogp140) can elicit moderate levels of NAbs against some primary isolates (74).
Several experimental approaches have been used to identify the epitope specificity of NAbs from the sera of HIV-infected patients (3, 6, 37, 43, 53, 67, 75). In antibody depletion studies, Abs which bound to both linear and conformation-dependent epitopes of gp120 or to the V3 loop peptide of gp120 were found to have a role in the neutralization of TCLA viruses (3, 37, 43, 53, 67, 75). Work from our laboratory extended these results to show that V3-specific Abs had a marginal role in neutralizing primary viral isolates (75). In this study, we depleted sera of Abs which bind to monomeric gp120 or to ogp140 and evaluated their role in the neutralization of three primary isolates and two TCLA strains of HIV-1. We show that HIV-1 serum Abs directed to either monomeric gp120 or ogp140 can neutralize primary isolates of HIV-1. These data suggest that the epitopes important in mediating HIV-1 serum neutralization of primary isolates are present on subunit HIV-1 envelope (Env) glycoproteins and that further optimization of the presentation of these epitopes on vaccine products could improve their immunogenicity.
MATERIALS AND METHODS
Cells and viruses.
Peripheral blood mononuclear cells (PBMCs) were isolated by leukophoresis of blood from HIV-1- and hepatitis B virus-seronegative donors and then subjected to centrifugation over lymphocyte separation medium. PBMCs were stored in liquid nitrogen at 3 × 107 cells/ml in RPMI 1640 medium (Quality Biological Inc., Gaithersburg, Md.) containing 20% heat-inactivated fetal calf serum (FCS; PAA Laboratories Inc., Newport Beach, Calif.) and 10% dimethyl sulfoxide. For treatment with phytohemagglutinin (PHA; Difco Laboratories, Detroit, Mich.), cells were thawed and cultured for 24 h at 106 cells/ml in RPMI 1640 medium containing 15% FCS and 20 U of recombinant human interleukin-2 (IL-2; Boehringer, Mannheim, Germany) per ml (complete medium) in the presence of PHA at 1 μg/ml. Cells were washed free of the PHA-containing medium after 24 h and were incubated for an additional 48 to 72 h in complete medium. TCLA HIV-1IIIB and HIV-1MN and the primary isolate HIV-1US056 were obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health. HIV-1US1 and HIV-1CM237 are primary clade B isolates obtained from infected subjects from the National Naval Medical Center, Bethesda, Md., and Chiang Mai, Thailand, respectively. Virus titers were determined in PHA/IL-2-stimulated PBMCs.
Ab and protein reagents.
Sera from patients US20 and US22, selected based on their broad and strong neutralization of primary HIV-1 isolates, were obtained with informed consent from clade B HIV-infected subjects. All HIV-1 sera and normal human sera (NHS; Sigma Chemical Co., St. Louis, Mo.) were heat inactivated at 56°C for 30 min prior to use. The purified polyclonal immunoglobulin (purified Ig) fraction was purified from sera US20 and US22 by using an EZ-SEP kit (Pharmacia Biotech, Piscataway, N.J.) prior to affinity column depletion and purification. To ensure that equivalent amounts of purified Ig and serum were used in subsequent binding and virus neutralization assays, the volume of purified Ig was adjusted to return the titers of Abs to gp120, ogp140, and p24 to the levels of unfractionated HIV-1 sera. Samples were concentrated in Centricon 30 spin concentrators (Amicon, Danvers, Mass.) with multiple spins at 5,000 × g for 25 min. HIV-1 immune globulin (HIVIG; 50 mg/ml) is a preparation of purified polyclonal Ig derived from the plasma of multiple HIV-infected donors and was kindly provided by Chris Saban (NABI, Boca Raton, Fla.). The donors contributing to HIVIG were clinically asymptomatic and had CD4 lymphocyte counts greater than or equal to 400 cells/ml, high anti-p24 antibody titers, and undetectable p24 antigen (16). MAb T4 was kindly provided by Pat Earl (National Institutes of Health) (20). Monomeric gp120451 and ogp140451 were affinity purified from the culture medium of a cell line (6D5) chronically infected with HIV-1451 as described previously (35, 36, 76). The ogp140451 preparation is comprised mostly of trimers/tetramers and dimers, with some monomers (76), and is a truncated version of full-length gp160, with the truncation occurring just prior to the transmembrane domain. Recombinant p24 was obtained from MicroGeneSys Inc. (Meriden, Conn.); V3MN peptide (YNKRKRIHIGPGRAFYTTKNIIGC), corresponding to the V3 region of gp120, and gp41 peptide gp41582 (QARILAVERYLKDQQLLGIWGCSGKLIC), corresponding to the immunodominant domain of gp41 and contained within ogp140451, were synthesized by Synthecell (Gaithersburg, Md.). Reduced, carboxymethylated gp120MN was generously provided by Genentech Inc. (South San Francisco, Calif.).
Column depletion.
gp120451 and ogp140451 were coupled to separate CNBr-activated Sepharose 4B beads as described by the manufacturer (Pharmacia Biotech). Prior to incubation with purified Ig derived from sera of HIV-1-infected patients or with NHS, 0.4 ml of packed gp120 or ogp140-coupled Sepharose 4B beads was treated in a 2-ml polystyrene column (Pierce, Rockford, Ill.) with 0.4 ml of NHS to reduce nonspecific binding of Abs. The beads were then washed with 0.8 ml of phosphate-buffered saline (PBS) and incubated with 0.2 ml of purified Ig and 0.4 ml of PBS in a 1.5-ml tube for 4 h at room temperature on a rotating wheel. The mixture was transferred to a polystyrene column, and the eluate (depleted fraction) was collected. The beads in the column were washed twice with 0.4 ml of PBS, and each wash was added to the main eluate. The combined eluate was then reincubated with a fresh 0.4 ml of packed, coupled Sepharose beads for an additional 4 h at room temperature on a rotating wheel. This material was transferred to a new column, and the eluate was collected. These beads were washed three times with 0.4 ml of PBS, and the washes were added to the main eluate. The eluate combined with the three washes was considered the final depleted fraction. gp120- or ogp140-depleted fractions were concentrated in Centricon spin concentrators to a volume at which the enzyme-linked immunosorbent assay (ELISA) titer of anti-p24 Abs was equal to that of the undepleted Ig. In the case of depleted NHS, the sample was concentrated to the original total IgG concentration.
The gp120- or ogp140-coupled Sepharose beads with bound serum Abs were washed five times with 1 ml of PBS, followed by two washes with 1 ml of 500 mM NaCl to remove weakly or nonspecifically associated Abs. For US20 and US22, Ab was eluted with 2.5 ml of 100 mM Na2CO3, pH 11. For HIVIG, Ab was eluted with 100 mM Na2CO3 as described above, followed by addition of 1.8 ml of 100 mM H3PO4, pH 2. The efficiency of Ab recovery was improved twofold by this sequence of high- and low-pH elution. The high- and low-pH solutions containing eluted Abs were neutralized with 1 N HCl and 1 M NaPO4, respectively. All data for affinity-purified Abs from HIVIG refer to Abs combined from the high- and low-pH elutions, except for the HIVIG data shown in Table 2, which are for Na2CO3-eluted Abs alone. Columns were prepared for reuse by serial washes with 100 mM Na2CO3 and 100 mM H3PO4 followed by extensive washing with PBS. Eluted Abs were concentrated to the original volume in Centricon 30 spin concentrators as described above. Where indicated, V3MN peptide was immobilized to Sepharose, and Ab depletion and affinity purification were done as described above and previously (76).
TABLE 2.
gp120 and ogp140 columns selectively deplete and affinity purify Abs to conformational epitopes of gp120
| Samplea | Ab binding to indicated antigen by SPR (RU)
|
Ratio, native gp120/ denatured gp120 | ||
|---|---|---|---|---|
| ogp140451 | Native gp120MN | Denatured gp120MN | ||
| Purified Ig | 3,499 | 3,545 | 492 | 7.2 |
| gp120451-depleted fraction | 2,331 | 323 | 217 | 1.5 |
| ogp140451-depleted fraction | 143 | 372 | 256 | 1.5 |
| V3MN-depleted fraction | 2,645 | 2,569 | 53 | 48.3 |
| gp120451 Abs | 1,324 | 3,523 | 571 | 6.2 |
| ogp140451 Abs | 3,671 | 4,088 | 576 | 7.1 |
| V3MN Abs | 81 | 283 | 244 | 1.2 |
All derived from HIVIG. For details of preparation, see Table 1, footnote a; the dilutions of affinity-purified (eluted) Abs were adjusted to yield gp120 or ogp140 binding levels equivalent to those of polyclonal Ig as described in Materials and Methods.
Ab binding levels in depleted and affinity-purified Ig samples.
Unfractionated serum, purified Ig, and column-depleted and affinity-purified Ab fractions were assayed for amount of Ab reactive to specific HIV-1 peptides and to subunit HIV-1 Env glycoproteins, or for the amount of total Ig, by ELISA as described previously (1, 72, 74). Briefly, gp120451, ogp140451, p24, or synthetic peptides V3MN and gp41582 (1 μg/ml) in PBS (pH 7.4, with 0.01% thimerosal) were coated overnight at 4°C onto Immulon 2 microtiter plates (Dynatech, Chantilly, Va.). Plates were washed twice with wash buffer (PBS with 0.1% Tween 20, pH 7.4) prior to incubation with twofold dilutions of Ab-containing samples diluted in serum diluent (wash buffer with 5% skim milk, pH 7.4) for 1 h at 37°C. Plates were washed three times with wash buffer and incubated with horseradish peroxidase-conjugated goat anti-human IgG (diluted 1:8,000 in serum diluent) (Kirkegaard & Perry, Gaithersburg, Md.). After a 1-h incubation at 37°C, plates were washed three times, after which substrate [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS); Kirkegaard & Perry] was added. The reaction was stopped with 0.5% sodium dodecyl sulfate after 30 min at 37°C. Alternatively, total serum IgG concentrations were determined by coating plates with unlabeled anti-human IgG and detecting captured human IgG by using horseradish peroxidase-conjugated goat anti-human IgG as described elsewhere (1). Concentrations were determined by using a human IgG standard (Sigma). Relative levels of Ab specific for native and denatured forms of HIV-1 gp120 were determined by surface plasmon resonance (SPR) as described previously (72–74).
Virus neutralization assays.
Virus neutralization assays were performed with PHA/IL-2-stimulated PBMCs by methods similar to those previously described (40, 41). Isolates of HIV-1 (100 50% tissue culture infective doses) were preincubated, in quadruplicate wells, with 5 to 8 serial 2- to 10-fold dilutions of HIV-1 sera, purified Ig, column-depleted Ab, or affinity-purified Ab in 0.05-ml 96-well culture plates (PGC, Frederick, Md.) for 45 min at 37°C. Controls included virus preincubated with NHS and PBS. Dilutions were made in RPMI 1640 containing 15% FCS. HIV-1 sera, purified Ig, and gp120- or ogp140-depleted Ab, previously normalized by ELISA reactivity to p24 antigen as described above, were prepared at the same initial sample dilutions. The initial dilutions of eluted gp120451- and ogp140451-specific purified Abs were adjusted so that the ELISA reactivity of each sample to gp120 or ogp140 was equivalent to that of the undepleted purified Ig. Then 1.5 × 105 PHA-activated PBMCs in 0.05 ml were added to the Ab-virus mixture. After incubation for 18 to 24 h at 37°C, the infected cells were washed five times with 0.45 ml of RPMI 1640 containing 15% FCS to remove unabsorbed virus and residual antibody to p24 (39). Cells were resuspended in 0.25 ml of complete medium, and 0.22 ml was distributed into wells of a 96-well tissue culture plate (Costar, Cambridge, Mass.). Culture supernatants from infected cells were tested on day 4, 5, or 6, depending on virus growth kinetics (40), and viral growth was determined by measuring p24 antigen in the culture medium by ELISA (Coulter, Miami, Fla.). Virus neutralization was determined by measurement of the fraction of remaining infectious virus after exposure to Ab. This value was obtained by dividing the amount of p24 antigen produced at each dilution of HIV-1 Abs by the amount produced in the absence of HIV-specific Abs. Fifty percent, 90%, and 99% neutralization titers were determined by linear regression analysis.
RESULTS
Efficient depletion of monomeric gp120- and ogp140-binding Abs from purified Ig of HIV-infected patients.
To determine the relative importance of Abs specific for monomeric gp120 and ogp140 in neutralization of primary and TCLA isolates of HIV-1, purified Ig from three sources (US20, US22, and HIVIG) was selectively depleted of Abs which bound to gp120451 or ogp140451. To test the efficiency of the affinity column depletion and purification procedures, the titers of Abs in the depleted and affinity-purified fractions reactive with ogp140451, gp120451, p24, and two peptides, V3MN and gp41582, were determined by ELISA. Screening against p24 antigen was included as a control to test and correct for nonspecific loss of Ig during the depletion procedure, thus permitting direct comparison of Ab titers in the various fractions against ogp140451, gp120451, V3MN, and gp41582. Peptide gp41582, present only on ogp140451, was included as an additional control for the specificity of the gp120451 column. One of the samples, HIVIG, was also depleted of V3MN-binding Abs by using a V3MN-Sepharose column to confirm results of a previous study (75) and to compare directly the roles of V3- versus gp120- and ogp140-specific Abs in the neutralization of primary and TCLA HIV-1 isolates.
ELISA titers of column-depleted and affinity-purified fractions against various antigens are shown in Table 1 for US20 and HIVIG. The data for US22 were similar and are not shown. As mentioned previously, the volumes of the gp120- and ogp140-depleted samples were adjusted such that the Ab responses against the control antigen p24 were equivalent to those of the purified Ig samples, as shown in Table 1. This required an approximately twofold concentration, indicating some nonspecific loss of antibody during the depletion procedure. While the p24-binding Ab titers for purified Ig and depleted samples were comparable, the gp120451- and/or ogp140451-binding Ab titers were substantially lower, indicating a selective depletion of HIV-1 envelope-specific Abs. The gp120451-binding Ab titer of each purified Ig preparation was reduced at least 128-fold (greater than 99% reduction) after passage through the Sepharose-gp120451 column (gp120-depleted fractions [Table 1]). Similarly, the ogp140451 binding Ab titer of each purified Ig preparation after passage through the Sepharose-ogp140451 column was reduced at least 256-fold (ogp140451-depleted fractions [Table 1]). Abs reactive with gp120451 were efficiently removed after passage through either the gp120451 or ogp140451 columns, while the majority of Abs reactive with ogp140451 were efficiently removed by immobilized ogp140451 but not by gp120451, indicating the presence of a substantial proportion of ogp140451-specific Abs reactive with epitopes either within gp41 or within gp120 but unique to its oligomeric configuration.
TABLE 1.
Efficient removal of HIV-1 envelope-binding Abs from purified Ig from HIV-infected patients
| Samplea | ELISA endpoint antibody titer against:
|
||||
|---|---|---|---|---|---|
| ogp140451 | gp120451 | V3MN | gp41582 | p24 | |
| US20 | |||||
| Purified Ig | 409,600 | 51,200 | 12,800 | 6,400 | 204,800 |
| gp120-depleted fraction | 204,800 (2b) | 400 (128) | 3,200 (4) | 6,400 (1) | 204,800 |
| ogp140-depleted fraction | 1,600 (256) | 400 (128) | 1,600 (8) | <400 (>16) | 204,800 |
| gp120 Abs | 25,600 | 12,800 | 400 | <100 | <100 |
| ogp140 Abs | 51,200 | 12,800 | 400 | 400 | 800 |
| HIVIG | |||||
| Purified Ig | 1,638,400 | 409,600 | 25,600 | 102,400 | 1,600,000 |
| gp120-depleted fraction | 819,200 (2) | 200 (2,048) | 3,200 (8) | 102,400 (1) | 1,600,000 |
| ogp140-depleted fraction | 200 (8,192) | 400 (1,024) | 6,400 (4) | 12,800 (8) | 1,600,000 |
| V3MN-depleted fraction | 819,200 (2) | 409,600 (1) | <100 (>256) | NDc | 1,600,000 |
| gp120 Abs | 102,400 | 102,400 | 3,200 | <800 | 1,600 |
| ogp140 Abs | 409,600 | 102,400 | 3,200 | 25,600 | 3,200 |
Purified Ig, purified Ig from HIV-1 serum; gp120-, ogp140-, and V3MN-depleted fractions, HIV-1 sera depleted over gp120, ogp140, and V3MN columns, respectively; gp120 Abs and ogp140 Abs, Abs purified from HIV-1 serum by using gp120 and ogp140 affinity columns, respectively. Values for depleted fractions were normalized to the unfractionated Ig by correcting for the amount of Ab to p24, and affinity-purified (eluted) Abs were concentrated to the original volume of the Ig applied to each column, as described in Materials and Methods.
Fold reduction compared to Ig.
ND, not determined.
To characterize more precisely the accessibility of specific epitopes on the column-immobilized gp120 and ogp140 used in these experiments, the extent of depletion of V3MN- and gp41582-specific Abs on each column was determined. The gp120451-depleted fraction retained the full reactivity by ELISA against the gp41 peptide (gp41582), while reactivity was diminished in the gp140-depleted fraction. Interestingly, while reactivity to gp41582 was reduced >16-fold in US20, only an 8-fold reduction was obtained for HIVIG despite the 8,192-fold reduction in reactivity against the entire ogp140451 protein. There was also only a modest four- to eightfold reduction in Ab titer to V3MN in both the gp120- and gp140-depleted material from each purified Ig preparation, in contrast to the more than 256-fold reduction in V3-specific Abs after HIVIG was passed through the Sepharose V3MN column. Thus, depletion of Abs to the whole gp120451 or ogp140451 proteins by using the gp120 or ogp140 affinity columns was more efficient than depletion of Abs against specific immunodominant epitopes. However, while some Abs specific for immunodominant linear epitopes were less efficiently depleted, total gp120- and ogp140-specific Abs were reduced >99%.
Affinity-purified gp120- and ogp140-specific Abs retain binding capacity.
Abs which had bound column-immobilized gp120451 or ogp140451 were eluted and evaluated for efficiency of recovery and specificity of reactivity by ELISA against the panel of proteins and peptides used in the assays described above for the depleted fractions. As shown in Table 1, there was minimal nonspecific binding of Abs to each column, as demonstrated by the small amount of affinity-purified Abs reactive with p24 (representing <0.5% of the initial p24 reactivity). Elution of Abs from either column and concentration of each fraction to its original volume resulted in recovery of approximately 25% of the initial gp120- or ogp140-specific binding activity (compare data for gp120 and ogp140 Abs with data for purified Ig). Similar percentages of gp120451 reactivity were recovered from both the gp120451 and ogp140451 columns for all three purified Ig preparations (data shown for only US20 and HIVIG in Table 1). In contrast, Abs recovered from the ogp140 column displayed greater reactivity to ogp140 than did Abs recovered from the gp120 column, probably because of the presence of Abs to gp41 in the ogp140-purified Abs. These data demonstrate that gp120- and ogp140-specific Abs were selectively enriched by binding to the affinity columns and that the eluted affinity-purified Abs retained binding capacity.
Affinity-purified gp120- and ogp140-specific Abs bind preferentially to native gp120.
To confirm that Sepharose-bound gp120451 and ogp140451 were capable of binding Abs specific for conformational epitopes within gp120, the gp120451- and ogp140451-depleted and affinity-purified Abs from HIVIG were analyzed by SPR for binding to conformationally intact gp120 and denatured (reduced, carboxymethylated) gp120. HIVIG bound preferentially to native gp120, with a native/denatured gp120 binding ratio of 7.2 (Table 2), consistent with previous results for sera from HIV-1 infected individuals (46, 72). Both gp120451- and ogp140451-depleted fractions were preferentially depleted of Abs to native gp120. In both cases, there was a reduction in the native/denatured gp120 binding ratio from 7.2 (found in the unfractionated HIVIG) to 1.5 in the depleted samples. This was calculated by dividing antibody binding (in RU) to native, monomeric gp120 (3,542 RU) by binding to denatured gp120 (492 RU). In contrast, Abs to linear epitopes were selectively removed when HIVIG was depleted by using the V3MN-coupled Sepharose beads. The native/denatured gp120 binding ratio rose to 48.3, consistent with the relative accessibility of the V3 region on native and denatured gp120. These data also indicate that the majority of denatured gp120-specific Abs within HIVIG are specific for V3. The affinity-purified gp120451 and ogp140451 Abs had reactivities to native and denatured gp120 similar to those of unfractionated HIVIG, with ratios of 6.2 and 7.1, respectively. These results demonstrate that both immobilized gp120451 and ogp140451 were able to bind Abs to conformational epitopes, suggesting that the tertiary structure of gp120 was retained in the column matrix. In addition, the presence of oligomeric gp140-specific epitopes within the immobilized gp140 column, but not the gp120 column, was demonstrated by using an oligomer-specific MAb (T4 [20]) that bound only to the former (data not shown). In summary, both gp120451 and ogp140451 columns depleted HIV-1 sera preferentially of Abs specific for conformational gp120 epitopes, and affinity-purified Abs from both columns preferentially bound native gp120.
Purified Ig from HIV-1 sera retains neutralizing activity against primary and TCLA HIV-1.
To confirm that the viral inhibitory activities of the sera from HIV-infected patients US20 and US22 were Ig mediated and not due to other factors such as chemokines (13), the viral neutralization titers of purified Ig from these two sera against three primary isolates and two TCLA viruses were compared to the viral neutralization titers of the respective sera. Samples from the third source of purified Ig, HIVIG, were assayed similarly, but the original serum pool was not available for comparison. Of the three purified Ig preparations, US20 had the lowest titer of Abs to gp120451 and ogp140451, as measured by ELISA, yet demonstrated the strongest neutralization of the three HIV-1 primary isolates US1, CM237, and 056 (Table 3). The serum and purified Ig of US20 had approximately equal neutralization titers (ID50 and ID90) against these viruses. US22 serum and purified Ig also had similar ID50 values against the primary isolates, although the ID90 of the purified Ig was two- to threefold less than that of the serum (Table 3). HIVIG had a high neutralization titer against HIV-1CM237 but relatively low neutralization titers against HIV-1US1 and HIV-1056. All three purified Ig preparations had comparably strong neutralization activity against TCLA HIV-1MN. Their neutralization titers against HIV-1IIIB were lower and more comparable to those observed against the primary isolates. These data demonstrate that the purified Ig from US20 and US22 sera retained most of the ability of the sera to neutralize primary and TCLA HIV-1. Neutralizing activity for these sera did not correlate with the total amount of Abs to monomeric gp120451 or ogp140451. This finding is consistent with other studies showing no correlation between HIV-1 Env-specific MAb binding titers to gp120 and neutralization (22, 45, 50, 59).
TABLE 3.
Ab binding and viral neutralization titers of sera and purified Ig from HIV-1-infected patients
| Samplea | ELISA titer of Abs reactive with:
|
50%/90% viral neutralization titer against:
|
|||||
|---|---|---|---|---|---|---|---|
| gp120451 | ogp140451 | Primary isolate
|
TCLA isolate
|
||||
| US1 | CM237 | 056 | IIIB | MN | |||
| Patient US20 | |||||||
| Serum | 51,200 | 409,600 | 240/138 | 302/161 | 187/111 | 1,967/734 | 3,409/973 |
| Purified-Ig | 51,200 | 409,600 | 204/121 | 298/152 | 209/121 | 1,191/452 | 2,527/954 |
| Patient US22 | |||||||
| Serum | 409,600 | 3,276,800 | 70/15 | 67/29 | 38/11 | 120/40 | 4,502/1,943 |
| Purified-Ig | 409,600 | 3,276,800 | 53/<5 | 56/10 | 45/<5 | 114/21 | 3,783/1,658 |
| HIVIG | 409,600 | 1,638,400 | 22/13 | 281/36 | 15/5 | 97/65 | 3,692/907 |
The Ig fractions of US20 and US22 were normalized to the respective sera for Ab reactivity to gp120451, ogp140451, and p24 as described in Materials and Methods.
Depletion of gp120- and ogp140-specific Abs from HIV-1 sera diminishes neutralizing activity against TCLA and primary HIV-1.
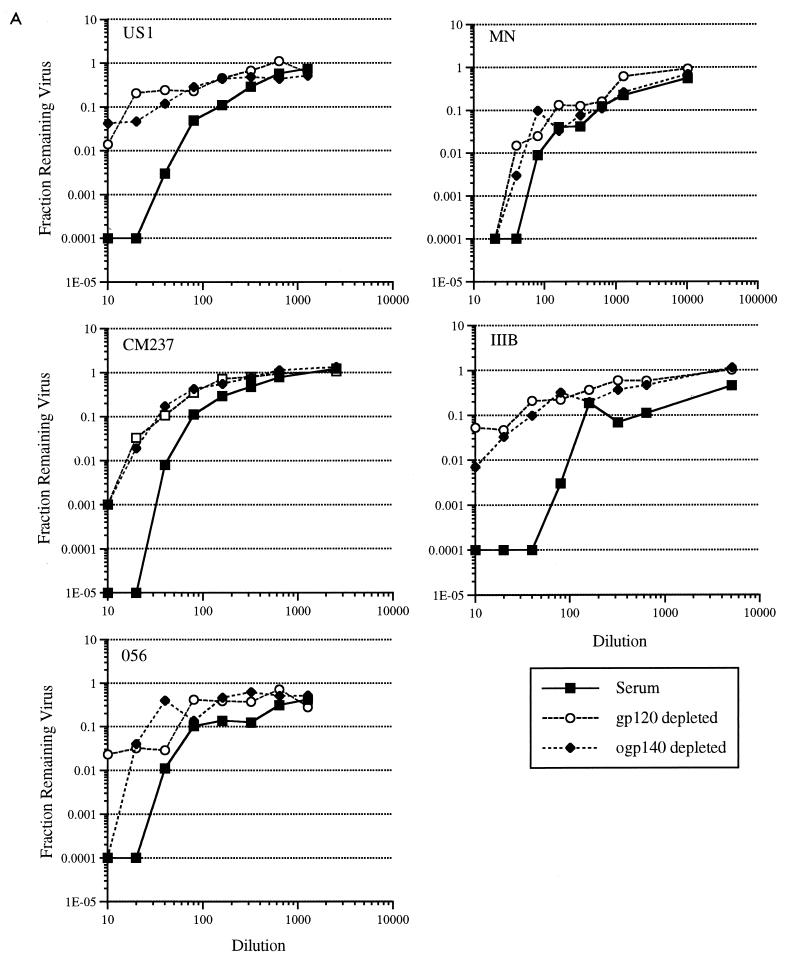
To determine the importance of gp120- and ogp140-specific Abs from individual sera in the neutralization of HIV-1, gp120451- and ogp140451-depleted fractions and affinity-purified Abs from US20 and US22 were evaluated for neutralizing capacity against TCLA and primary HIV-1 isolates. After standardization of the depleted fractions to the purified Ig by the amount of Abs to p24, the gp120- and ogp140-depleted fractions from US20 and US22 had reduced neutralizing activity against all viruses evaluated (Fig. 1A; Table 4). The level of reduction in neutralization was similar for the gp120451- and ogp140451-depleted fractions. The increase in virus growth in the presence of the gp120- and gp140-depleted fractions was most striking against HIV-1IIIB, with a 2- to 3-log10 difference between the depleted and unfractionated material (Fig. 1A). In the presence of lower dilutions of depleted US20 fractions, a greater than 100-fold increase in virus growth of HIV-1US1, HIV-1CM237, and HIV-1056 primary isolates was obtained (Fig. 1A). The corresponding ID90 and ID99 (neutralizing titers) of the depleted US20 material against the primary isolates of HIV-1 were reduced 2- to 10-fold (Table 4). The more weakly neutralizing US22 serum had a 2- to 10-fold reduction in ID50 against these viruses (Table 4). Despite the reduction of neutralizing capacity against primary and TCLA HIV-1 isolates in both the gp120- and gp140-depleted US20 fractions, the remaining Abs in these depleted fractions retained a substantial (i.e., greater than 90% neutralization at the higher Ig concentration) level of neutralizing activity (Fig. 1A; Table 4).
FIG. 1.
Preparation of purified Ig and depleted samples, design of viral neutralization assays, and evaluation of p24 antigen were as described in Materials and Methods. (A) Results for gp120- and ogp140-depleted material from US20; (B) results for HIVIG. Each point represents the average from triplicate wells from one experiment of four that gave similar results. Depleted fractions were standardized to the purified Ig and serum by equating reactivity to p24 such that equivalent amounts of each were added. Diluted samples were evaluated by ELISA for titers of Abs to p24 after the neutralization assay to confirm that equivalent amounts were added. The amount of viral growth in comparable dilutions of NHS was used as the standard for 100% virus growth. Purified Ig and depleted fractions were assessed for neutralizing capacity against primary (US1, CM237, and 056) and TCLA (MN and IIIB) HIV-1 isolates. gp120-depleted and ogp140-depleted fractions correspond to HIV-1 serum US20 or HIVIG depleted over the gp120 or ogp140 affinity columns, respectively.
TABLE 4.
Viral neutralization titers of depleted and affinity-purified US20, US22, and HIVIG
| Serum | Neutralization titera
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampleb | Primary isolate
|
TLCA isolate
|
||||||||||||||
| US1
|
CM237
|
056
|
IIIB
|
MN
|
||||||||||||
| 50% | 90% | 99% | 50% | 90% | 99% | 50% | 90% | 99% | 50% | 90% | 99% | 50% | 90% | 99% | ||
| US20 | Expt A | |||||||||||||||
| Serum | 240 | 138 | 62 | 302 | 161 | 66 | 187 | 111 | 52 | 1,967 | 734 | 179 | 3,409 | 973 | 162 | |
| Purified Ig | 204 | 121 | 58 | 298 | 152 | 58 | 209 | 121 | 55 | 1,191 | 452 | 113 | 2,527 | 954 | 237 | |
| gp120 depleted | 132 | 27 | <10 | 100 | 51 | 20 | 188 | 48 | <10 | 218 | 28 | <10 | 362 | 181 | 68 | |
| ogp140 depleted | 178 | 32 | <10 | 102 | 50 | 18 | 106 | 57 | 23 | 89 | 39 | 12 | 475 | 230 | 82 | |
| Expt B | ||||||||||||||||
| Purified Ig | 256 | 97 | 24 | 280 | 158 | 69 | 209 | 117 | 51 | 2,633 | 179 | <20 | 2,399 | 904 | 224 | |
| gp120 Abs | 335 | 149 | 47 | 224 | 73 | <20 | 229 | 33 | <20 | NDc | ND | ND | 5,217 | 1,073 | 112 | |
| ogp140 Abs | 405 | 239 | 113 | 398 | 180 | 58 | 242 | 132 | 56 | 2,907 | 873 | 156 | 3,497 | 1,240 | 281 | |
| US22 | Expt A | |||||||||||||||
| Serum | 70 | 15 | <5 | 67 | 29 | 9 | 38 | 11 | <5 | 120 | 40 | 8 | 4,502 | 1,943 | 584 | |
| Purified Ig | 53 | <5 | <5 | 56 | 10 | <5 | 45 | <5 | <5 | 114 | 21 | <5 | 3,783 | 1,658 | 509 | |
| gp120 depleted | <5 | <5 | <5 | 23 | <5 | <5 | 9 | <5 | <5 | <5 | <5 | <5 | 1,401 | 460 | <100 | |
| ogp140 depleted | <5 | <5 | <5 | 12 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 4,799 | <100 | <100 | |
| Expt B | ||||||||||||||||
| Purified Ig | 36 | <10 | <10 | 44 | 11 | <10 | 32 | <10 | <10 | 264 | <25 | <25 | 3,783 | 1,658 | 509 | |
| gp120 Abs | 34 | <10 | <10 | 18 | <10 | <10 | 67 | <10 | <10 | 156 | <25 | <25 | 9,320 | 3,370 | 786 | |
| ogp140 Abs | 32 | <10 | <10 | 21 | <10 | <10 | 35 | <10 | <10 | 153 | <25 | <25 | 13,963 | 7,401 | 2,985 | |
| HIVIG | Expt A | |||||||||||||||
| Purified Ig | 22 | 13 | 6 | 281 | 36 | 4 | 15 | 5 | <4 | 97 | 65 | 37 | 3,692 | 907 | 122 | |
| gp120 depleted | <4 | <4 | <4 | 62 | 11 | <4 | <4 | <4 | <4 | 24 | 16 | 9 | 140 | 69 | 25 | |
| ogp140 depleted | <4 | <4 | <4 | 83 | 4 | <4 | <4 | <4 | <4 | 23 | 10 | <4 | 192 | 95 | 35 | |
| V3MN depleted | 40 | 10 | <4 | 165 | 21 | <4 | 11 | <4 | <4 | 21 | 13 | 7 | 129 | 78 | 38 | |
| Expt B | ||||||||||||||||
| Purified Ig | 22 | 13 | 6 | 281 | 36 | 4 | 15 | 5 | <4 | 97 | 65 | 37 | 3,692 | 907 | 122 | |
| gp120 Abs | 43 | 4 | <4 | 84 | <4 | <4 | ND | ND | ND | ND | ND | ND | 2,889 | 639 | 74 | |
| ogp140 Abs | 65 | <4 | <4 | 86 | 6 | <4 | ND | ND | ND | ND | ND | ND | 2,227 | 757 | 161 | |
Neutralization titers for unfractionated, depleted, and affinity-purified Ig were determined by linear regression analysis of data such as those shown in Fig. 1 and 2, using an Excel computer program.
Data for experiments A and B are from separate neutralization assays, evaluating depleted and affinity-purified material, respectively. The same polyclonal Ig samples were included for both experiments.
ND, not done.
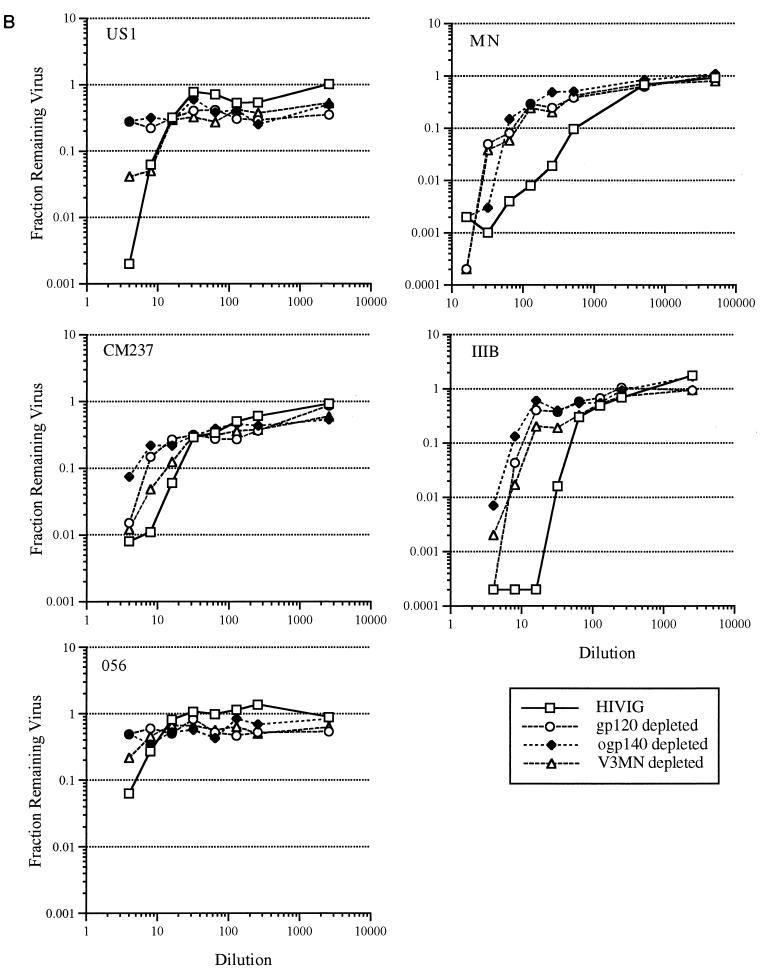
Previous data showed that V3-specific Abs were important in mediating neutralization of TCLA (37, 53, 75) but not primary (75) HIV-1 isolates. To confirm these previous studies using HIVIG and to provide a basis for comparison with gp120- and ogp140-depleted samples, HIVIG was passed through a V3MN-coupled Sepharose column, resulting in a >256-fold reduction in V3MN-specific Abs (Table 1). In agreement with previous findings, neutralization of TCLA HIV-1MN by V3MN-depleted HIVIG was substantially reduced (≥20-fold against MN), with minimal corresponding reduction in neutralizing capacity against the primary isolates US1, CM237, and 056 (Table 4; Fig. 1B). In contrast, both gp120- and ogp140-depleted HIVIG had reduced primary isolate neutralization capacity. For example, while V3MN-specific depletion failed to achieve a twofold reduction in ID50 and ID90 against primary HIV-1 isolates, gp120- and ogp140-specific depletion reduced the ID50 and ID90 approximately fourfold or more. The reduction in neutralizing titer of gp120- and ogp140-depleted HIVIG against TCLA isolate IIIB was comparable to the reduction against the primary HIV-1 isolates (Table 4), while a greater reduction in MN neutralizing titer was obtained. As obtained previously with US20, despite the reduction in primary isolate neutralizing capacity, in the case of CM237, significant neutralizing Abs remained in the gp120- and ogp140-depleted HIVIG.
To confirm that the procedures for purified Ig purification and Ab depletion, elution, and concentration did not introduce factors which nonspecifically inhibited viral growth, each experiment included negative controls in which the purified Ig from NHS was processed on the gp120 and ogp140 columns identically to the HIV-1 samples. Neither the purified Ig, the mock-depleted gp120 and ogp140 fractions, nor the mock-purified Abs from NHS had detectable neutralization against the viruses used in the study (data not shown). As an additional control, all HIVIG samples evaluated above were subjected to an identical experiment substituting a clade E HIV-1 isolate (9461 [74]), against which the clade B HIVIG had marginal neutralizing activity. None of these samples inhibited or enhanced viral growth of the clade E isolate.
gp120 and ogp140 affinity-purified antibodies neutralize both TCLA and primary HIV-1 isolates.
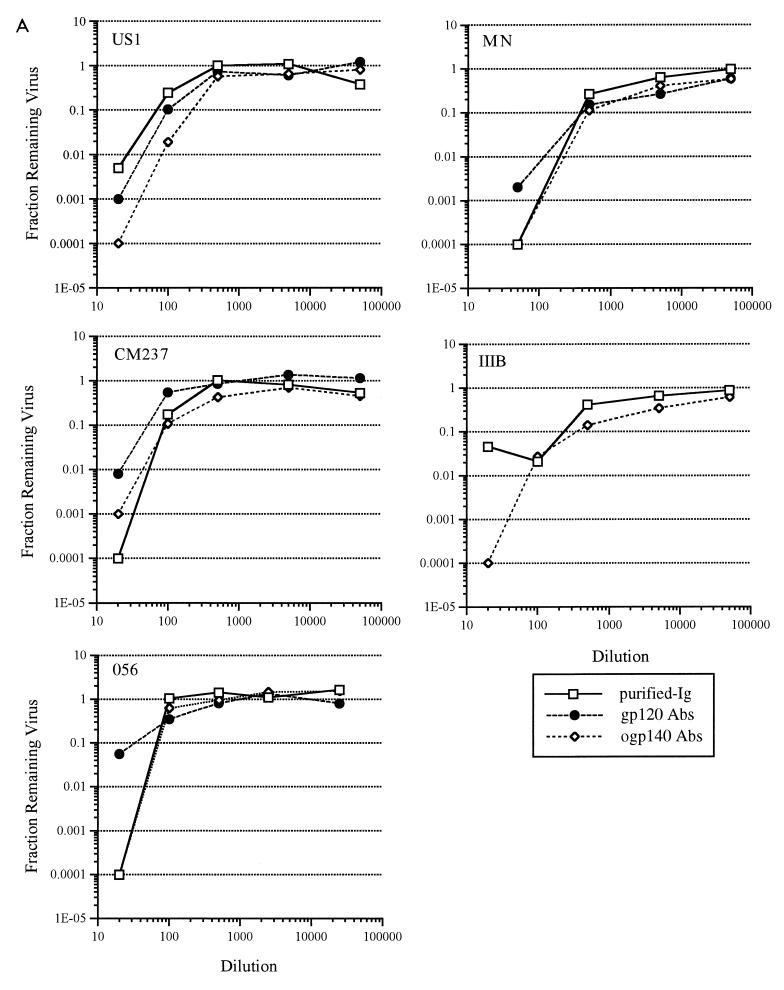
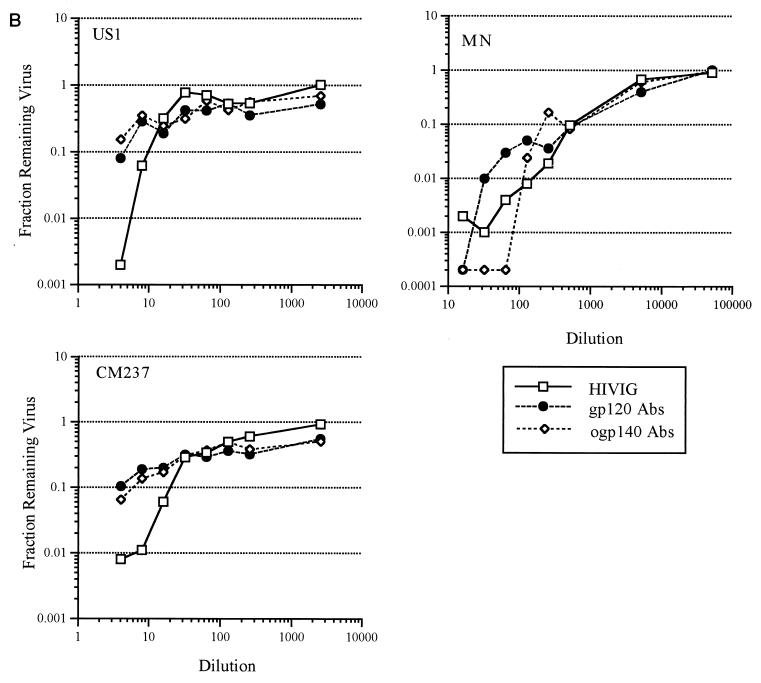
US20, US22, and HIVIG affinity-purified Abs from the gp120 and ogp140 columns were tested for neutralizing activity against both TCLA and primary HIV-1 isolates. Prior to evaluation for neutralization activity, the concentrations of gp120- and ogp140-affinity purified Abs in the Ab fractions were adjusted so that the ELISA titer against gp120 or ogp140 was equivalent to that of unfractionated purified Ig. Abs recovered from both gp120 and ogp140 columns from US20 had substantial neutralization activity against the two TCLA and three primary isolates of HIV-1 (Fig. 2A; Table 4). This activity was quantitatively similar to that of the unfractionated purified Ig. Furthermore, to compare the potencies of the gp120 and ogp140 Abs, the concentration of IgG in the initial dilution of each sample from US20 was determined and found to be as follows: serum, 645.5 μg/ml; purified Ig, 648.7 μg/ml; gp120 Abs, 10.1 μg/ml; and ogp140 Abs, 29.7 μg/ml. Based on IgG concentration, the 99% inhibitory concentrations against HIV-1US1 of gp120- and ogp140-specific Abs were similar at 4 and 5 μg/ml, respectively (data not shown), suggesting similar neutralizing potencies of the gp120 and ogp140 affinity-purified Abs. gp120- and ogp140-specific Abs from US22 and HIVIG also neutralized both TCLA and primary HIV-1 isolates, but to a more limited degree than US20 (data shown for HIVIG in Fig. 2B and Table 4). Thus, both gp120- and ogp140-specific, affinity-purified Abs from HIV-infected patients had potent neutralizing activity (90 to 99.9% at the lowest dilutions studied) against primary isolates and TCLA HIV-1.
FIG. 2.
Affinity-purified antibodies from the gp120451 and ogp140451 affinity columns for US20 (A) and from HIVIG (B) were compared with the respective unfractionated Ig for neutralization activity against primary (US1, CM237, and 056) and TCLA (MN and IIIB) HIV-1 isolates. Abs affinity purified from the monomeric gp120 column (gp120 Abs) or ogp140 column (ogp140 Abs) were standardized to the unfractionated serum and/or purified Ig by adding equivalent amounts of reactivity to gp120 or ogp140, respectively. Dilutions were evaluated by ELISA for titers to gp120 and/or ogp140 after the assay to confirm that equivalent amounts of reactivity had been added.
DISCUSSION
We have demonstrated that HIV-1 serum Abs which neutralize primary HIV-1 isolates bind to both soluble monomeric gp120451 and ogp140451. Monomeric gp120 and ogp140 coupled to Sepharose beads efficiently removed >99% of serum Abs specific for the homologous protein. Elution of bound Abs from both gp120 and ogp140 columns yielded gp120- and ogp140-specific Abs which were normalized to original anti-Env reactivity and evaluated for HIV-1 neutralizing activity. The affinity-purified antibodies specific for gp120 were directed predominantly to epitopes exposed on native gp120. Less efficient depletion by gp120 or ogp140 was obtained against specific immunodominant epitopes such as V3 (V3MN) and the immunodominant domain in gp41 (gp41582), suggesting either reduced accessibility of these epitopes on immobilized gp120 or ogp140, differences in amino acid sequence of the epitope in the protein used to deplete (i.e., 451) and the peptide used for the antibody binding assay (MN or LAI), or insufficient concentration of the epitope within the protein immobilized on the column. For example, column immobilized peptide V3MN was capable of complete depletion of HIV-1 serum V3 antibody, but the concentration of the V3 region in the V3 affinity column material was approximately 100-fold higher than the concentration of V3 in the gp120 or ogp140 affinity columns. Therefore, while the total gp120- and ogp140-binding Ab titers were reduced 100-fold, some epitope-specific Ab populations may have been less efficiently depleted.
Removal of more than 99% of gp120- or ogp140-binding Abs from the sera of HIV-infected patients resulted in a significant decrease in neutralization titers against three primary and two TCLA isolates of HIV-1. This was in contrast to V3-depletion of HIV-1 sera in this study as well as in a previous study (74), where removal of V3 antibodies reduced neutralization against TCLA but not primary HIV-1 isolates. These data suggest the presence of antibodies with specificities outside of linear V3 epitopes and present on both gp120 and ogp140 with neutralizing activity against multiple primary HIV-1 isolates. In addition, affinity-purified gp120451- and ogp140451-specific Abs neutralized the infectivities of both TCLA and primary HIV-1 isolates. The gp120-specific Abs purified from both the gp120451 and ogp140451 affinity columns were predominantly directed to epitopes present on native but not denatured gp120, consistent with observations that HIV-1 serum gp120-specific Abs are directed predominantly to native gp120 (46, 72) and that many broadly neutralizing MAbs are specific for discontinuous epitopes within gp120 (9, 26, 52, 71). MAbs specific for HIV-1 gp41 which potently neutralize HIV-1 have been identified (14, 15, 47); however, the comparable HIV-1-neutralizing activities of gp120 and ogp140 affinity-purified Abs from HIVIG, US20, and US22 suggest that a significant portion of the primary HIV-1 isolate-neutralizing activity of polyclonal serum Ig is present in Abs with gp120 epitope specificities. For example, Abs from US20 recovered from the gp120 and ogp140 columns had comparable neutralizing potencies against HIV-1US1 as well as HIV-1CM237 and HIV-1056. Therefore, the presence of gp41-specific antibodies in the ogp140 affinity-purified fraction did not contribute to enhanced neutralizing activity. It remains to be determined whether fine epitope specificities of the gp120-specific Abs from the gp120 and ogp140 affinity-purified sera are similar or whether the two fractions have distinct antibody populations with comparable neutralizing activities.
The oligomeric protein used in this study is comprised of a truncated gp160 (gp140) which is secreted from a chronically infected cell line derived from HUT78 cells. Although it naturally assembles and is secreted in culture media as monomers, dimers, and trimers/tetramers (76), the extent of resemblance, after being immobilized onto Sepharose, to native gp120/gp41 expressed on the surface of virions and infected cells is not clearly defined. This may help explain why despite extensive depletion of Abs to conformational epitopes on ogp140 (a 256-fold reduction in titer against ogp140451), substantial neutralizing activity (greater than 90% of total neutralizing activity of US20 Ig) remained in the depleted fraction. NAbs specific for the C terminus of gp41 (truncated in ogp140) or to conformational epitopes of gp120/gp41 requiring proper quaternary structure dependent on either membrane expression or the presence of an entire intact gp41 may not have been absorbed from the sera analyzed. The depletion studies, by selectively removing gp120 conformational antibody, demonstrated the presence of some properly folded gp120 within the gp120- and gp140-coupled matrices. Binding of MAb T4, which maps to an oligomer-specific epitope within gp41 (19, 20), indicated the presence of some oligomeric gp140 after coupling to the column.
Another explanation may be the presence of NAbs type specific for the various primary isolates evaluated which were not efficiently depleted with the 451 strain of gp120 and ogp140 used for this study. These data indicate a significant proportion (up to 50%) of type-specific primary isolate NAb. Additionally, critical gp160 epitopes may be absent on the column-immobilized gp120 or ogp140, possibly secondary to immobilizing the proteins onto the Sepharose beads. For example, gp120 has been shown to undergo conformational changes after binding CD4 that result in enhanced accessibility or exposure of previously cryptic epitopes (17, 18, 24, 58, 69). Recent HIV-1 envelope structural data have also identified conserved regions of gp120 involved with interactions with CD4 and/or coreceptors which are hidden on the native gp120 protein by glycosylation and the hypervariable loop domains (38, 55, 80). Abs directed to these regions may be induced during natural infection but would not be expected to be efficiently depleted by using soluble gp120 and ogp140. Finally, NAbs directed against nonenvelope (8, 32, 48, 63) or nonvirus-encoded proteins (5, 25, 34, 54) which also would not have been depleted by using the HIV-1 Env-specific reagents have been identified. It should be noted that the inability to remove completely serum neutralizing activity by binding to immobilized gp120, even when evaluated against the homologous TCLA virus (43, 67), has been observed previously.
The presence of epitopes accessible on both monomeric gp120 and ogp140 (antigenicity) capable of depleting HIV-1 serum neutralizing activity against primary HIV-1 isolates as well as affinity purifying broadly neutralizing Ab activity is inconsistent with their role as immunogens. Monomeric forms of gp120 have proven effective in eliciting NAb against TCLA HIV-1 isolates but with limited neutralizing activity against primary HIV-1 isolates (2, 30, 33, 41, 42, 62, 78). ogp140 has been shown to elicit Abs capable of neutralizing some primary HIV-1 isolates, but these responses have been restricted to date to those which are particularly susceptible to Ab-mediated neutralization (74). Broadly neutralizing Ab responses against primary isolates as observed with the gp120 and ogp140 affinity-purified sera have not been achieved in vaccine studies. Therefore, there appears to be a significant dislinkage between antigenicity and immunogenicity of soluble HIV-1 subunit vaccines. This may be related to structural instability of critical conformational epitopes within gp120 as expressed in gp120 or ogp140 and a more stringent requirement for eliciting an immune response. Potent NAbs may be capable of binding with lower affinity to these soluble HIV-1 envelope preparations but not effectively elicited by using these same proteins. Dose, route, timing of immunizations, and adjuvant formulations have been shown to qualitatively alter the quality of the Ab immune response in rabbits after immunization with ogp140 (74). This finding raises the possibility that further stabilization of immunogen, by oligomerization or adjuvant selection, may preserve neutralizing epitopes. Alternatively, in natural infection, potent NAbs against heterologous primary viral isolates develop over several years, and therefore more optimal maturation of the immune response may be required to elicit NAbs by immunization.
Our laboratory is continuing to identify epitopes on gp120/gp41 which are recognized by NAbs, with the goal of applying this knowledge toward design of an immunogenic vaccine. Comparison of the epitope specificities of gp120- and ogp140-specific Abs from a potently neutralizing HIV-1 serum with similarly purified Abs from vaccinee sera should help identify gaps in the latter. In addition, we are determining whether the remaining neutralizing activity in the gp120451- and ogp140451-depleted fractions, similar to what was seen in other studies (43, 67), is related to heterologous NAbs which did not bind to gp120451 and ogp140451 or to Abs with epitope specificities not optimally presented by gp120 or ogp140. Finally, extensions of these type of studies to non-clade B HIV-1 isolates will be important to determine the relative contribution of various HIV-1 Env-specific antibodies to HIV-1 isolates circulating in areas where vaccine efficacy trials may be performed.
ACKNOWLEDGMENTS
We thank patients and staff of the Infectious Diseases Clinic at the National Naval Medical Center, Bethesda, Md. for sera used in these studies. We also thank I. Kalisz for technical assistance, C. Drew for graphic support, C. Sapan for HIVIG, P. Earl for MAbs, L. Loomis-Price for helpful discussions, and P. Gomatos for review of the manuscript.
This work was supported in part by cooperative agreement DAMD17-93-V-3004, between the U.S. Army Medical Research and Material Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
REFERENCES
- 1.Artenstein A W, VanCott T C, Sitz K V, Robb M L, Wagner K F, Veit S C D, Rogers A B, Garner R P, Byron J W, Burnett P R, Birx D L. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: a comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–271. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Belshe R B, Graham B S, Keefer M C, Gorse G J, Wright P, Dolin R, Matthews T, Weinhold K, Bolognesi D P, Sposto R, Stablein D M, Twadell T, Berman P W, Gregory T, Izu A E, Walker M C, Fast P. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS Vaccine Clinical Trials Network. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 3.Berkower I, Murphy D, Smith C C, Smith G E. A predominant group-specific neutralizing epitope of human immunodeficiency virus type 1 maps to residues 342 to 511 of the envelope glycoprotein gp120. J Virol. 1991;65:5983–5990. doi: 10.1128/jvi.65.11.5983-5990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 5.Briant L, Benkirane M, Girard M, Hirn M, Iosef C, Devaux C. Inhibition of human immunodeficiency virus type 1 production in infected peripheral blood mononuclear cells by human leukocyte antigen class I-specific antibodies: evidence for a novel antiviral mechanism. J Virol. 1996;70:5213–5220. doi: 10.1128/jvi.70.8.5213-5220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broliden P A, Clapham G A von, P, Rosen J, Fenyo E M, Wahren B, Broliden K. Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci USA. 1992;89:461–465. doi: 10.1073/pnas.89.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruguera M, Bayas J M, Vilella A, Tural C, Gonzalez A, Vidal J, Dal-Re R, Salleras L. Immunogenicity and reactogenicity of a combined hepatitis A and B vaccine in young adults. Vaccine. 1996;14:1407–1411. doi: 10.1016/s0264-410x(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 8.Buratti E, Tisminetzky S G, D’Agaro P, Baralle F E. A neutralizing monoclonal antibody previously mapped exclusively on human immunodeficiency virus type 1 gp41 recognizes an epitope in p17 sharing the core sequence IEEE. J Virol. 1997;71:2457–2462. doi: 10.1128/jvi.71.3.2457-2462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall A, Scharff M D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R T, Markowitz L E, Albrecht P, Stewart J A, Mofenson L M, Preblud S R, Orenstein W A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F, De Vico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 14.Conley A J, Kessler J N, Boots L J, Tung J S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, and anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotropia J, Ugen K E, Kliks S, Broliden K, Broliden P A, Hoxie J A, Srikantan V, Williams W V, Weiner D B. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against diverse laboratory isolates. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;12:221–232. doi: 10.1097/00042560-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Cummins L M, Weinhold K J, Matthews T J, Langlois A J, Perno C F, Condie R M, Allain J P. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood. 1991;77:1111–1117. [PubMed] [Google Scholar]
- 17.De Vico A, Silver A, Thronton A M, Sarngadharan M G, Pal R. Covalently crosslinked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology. 1996;218:258–263. doi: 10.1006/viro.1996.0188. [DOI] [PubMed] [Google Scholar]
- 18.De Vico A L, Rahman R, Welch J, Crowley R, Lusso P, Sarngadharan M G, Pal R. Monoclonal antibodies raised against covalently crosslinked complexes of human immunodeficiency virus type 1 gp120 and CD4 receptor identify a novel complex-dependent epitope on gp 120. Virology. 1995;211:583–588. doi: 10.1006/viro.1995.1441. [DOI] [PubMed] [Google Scholar]
- 19.Earl P L, Broder C C, Doms R W, Moss B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J Virol. 1997;71:2674–2684. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank A L, Taber L H, Glezen W P, Geyer E A, McIlwain S, Paredes A. Influenza B virus infections in the community and the family. The epidemics of 1976–1977 and 1979–1980 in Houston, Texas. Am J Epidemiol. 1983;118:313–325. doi: 10.1093/oxfordjournals.aje.a113638. [DOI] [PubMed] [Google Scholar]
- 24.Gershoni J, Denisova G, Raviv D, Smorodinsky N, Buyaner D. HIV binding to its receptor creates specific epitopes for the CD4/gp120 complex. FASEB J. 1993;7:1185–1187. doi: 10.1096/fasebj.7.12.7690724. [DOI] [PubMed] [Google Scholar]
- 25.Gomez M B, Hildreth J E. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorny M K, Moore J P, Conley A J, Karwowska S, Sodroski J, Williams C, Burda S, Boots L J, Zolla P S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groothuis J R, Simoes E A, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 28.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 29.Hammon W M, Coriel L L, Wehrle P F. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. JAMA. 1953;151:1272–1285. [PubMed] [Google Scholar]
- 30.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 31.Ho D D, Sarngadharan M G, Hirsch M S, Schooley R T, Rota T R, Kennedy R C, Chanh T C, Sato V L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987;61:2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kageyama S, Katsumoto T, Taniguchi K, Ismail S I, Shimmen T, Sasao F, Gao M, Owatari S, Wakamiya N, Tsuchie H, Ueda S, Shiraki K, Kurimura T. Neutralization of human immunodeficiency virus type 1 (HIV-1) with antibody from carriers’ plasma against HIV-1 protein p17. Acta Virol. 1996;40:195–200. [PubMed] [Google Scholar]
- 33.Kahn J O, Sinangil F, Baenziger J, Murcar N, Wynne D, Coleman R L, Steimer K S, Dekker C L, Chernoff D. Clinical and immunologic responses to human immunodeficiency virus (HIV) type 1SF2 gp120 subunit vaccine combined with MF59 adjuvant with or without muramyl tripeptide dipalmitoyl phosphatidylethanolamine in non-HIV-infected human volunteers. J Infect Dis. 1994;170:1288–1291. doi: 10.1093/infdis/170.5.1288. [DOI] [PubMed] [Google Scholar]
- 34.Kalter D C, Gendelman H E, Meltzer M S. Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules. Immunol Lett. 1991;30:219–227. doi: 10.1016/0165-2478(91)90029-a. [DOI] [PubMed] [Google Scholar]
- 35.Kalyanaraman V S, Pal R, Gallo R C, Sarngadharan G. A unique human immunodeficiency virus culture secreting soluble gp160. AIDS Res Hum Retroviruses. 1988;4:319–329. doi: 10.1089/aid.1988.4.319. [DOI] [PubMed] [Google Scholar]
- 36.Kalyanaraman V S, Rodriguez V, Veronese F, Rahman R, Lusso P, DeVico A L, Copeland T, Oroszlan S, Gallo R C, Sarngadharan M G. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1990;6:371–380. doi: 10.1089/aid.1990.6.371. [DOI] [PubMed] [Google Scholar]
- 37.Kang C Y, Nara P, Chamat S, Caralli V, Ryskamp T, Haigwood N, Newman R, Kohler H. Evidence for non-V3-specific neutralizing antibodies that interfere with gp120/CD4 binding in human immunodeficiency virus 1-infected humans. Proc Natl Acad Sci USA. 1991;88:6171–6175. doi: 10.1073/pnas.88.14.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola J R, Burke D S. Antigen detection in neutralization assays: high levels of interfering anti-p24 antibodies in some plasma. AIDS Res Hum Retroviruses. 1993;9:1173–1174. doi: 10.1089/aid.1993.9.1173. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 40.Mascola J R, Louder M K, Van Cott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Matthews T, McDanal C, Greenwell T, Streilein A, Davison D, Ruland M, Bolognesi D. Serological reactivity from HIV-1 vaccine recipients participating in the AIDS vaccine clinical trials network. AIDS Res Hum Retroviruses. 1994;10:S55. [Google Scholar]
- 43.Matthews T J, Langlois A J, Robey W G, Chang N T, Gallo R C, Fischinger P J, Bolognesi D P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci USA. 1986;83:9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 45.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas C F R, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niedrig M, Harthus H P, Broker M, Meloen R, Gelderblom H, Pauli G. Characterization of murine monoclonal antibodies directed against the submembrane protein p17 of HIV-1. Hybridoma. 1993;12:431–439. doi: 10.1089/hyb.1993.12.431. [DOI] [PubMed] [Google Scholar]
- 49.Parren P W, Fisicaro P, Labrijn A F, Binley J M, Yang W P, Ditzel H J, Barbas C F R, Burton D R. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type 1 envelope. J Virol. 1996;70:9046–9050. doi: 10.1128/jvi.70.12.9046-9050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner M R, Hideshima T, Cannon T, Mukherjee M, Mayer K H, Byrn R A. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991;146:4325–4332. [PubMed] [Google Scholar]
- 53.Profy A T, Salinas P A, Eckler L I, Dunlop N M, Nara P L, Putney S D. Epitopes recognized by the neutralizing antibodies of an HIV-1-infected individual. J Immunol. 1990;144:4641–4647. [PubMed] [Google Scholar]
- 54.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 56.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 57.Robert G M, Brown M, Gallo R C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985;316:72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- 58.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawyer L S, Wrin M T, Crawford M L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schawaller M, Smith G E, Skehel J J, Wiley D C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz D H, Gorse G, Clements M L, Belshe R, Izu A, Duliege A M, Berman P, Twaddell T, Stablein D, Sposto R, et al. Induction of HIV-1-neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1IIIB rgp120 subunit vaccine. Lancet. 1993;342:69–73. doi: 10.1016/0140-6736(93)91283-r. [DOI] [PubMed] [Google Scholar]
- 63.Shang F, Huang H, Revesz K, Chen H C, Herz R, Pinter A. Characterization of monoclonal antibodies against the human immunodeficiency virus matrix protein, p17gag: identification of epitopes exposed at the surfaces of infected cells. J Virol. 1991;65:4798–4804. doi: 10.1128/jvi.65.9.4798-4804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smithburn K C, Mahaffy A F. Immunization against yellow fever. Studies on the time of development and the duration of induced immunity. Am J Trop Med Hyg. 1945;25:217–223. [Google Scholar]
- 65.Steimer K S, Haigwood N L. Immunization of primates with native, recombinant HIV-SF2 gp120 generates broadly effective neutralizing antibodies directed to conformational epitopes. AIDS Res Hum Retroviruses. 1992;8:1391. doi: 10.1089/aid.1992.8.1391. [DOI] [PubMed] [Google Scholar]
- 66.Steimer K S, Sakamoto D, Yi D S, West D, Baenziger J, Sinangil F. Primary isolate neutralizing activity of human antibodies directed to recombinant, native HIV-1 SF2 gp120 (rgp120SF2) J Cell Biochem. 1994;18B:114. [Google Scholar]
- 67.Steimer K S, Scandella C J, Skiles P V, Haigwood N L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991;254:105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- 68.Suss J, Sinnecker H. Immune reactions against rabies viruses—infection and vaccination. Exp Pathol. 1991;42:1–9. doi: 10.1016/s0232-1513(11)80028-9. [DOI] [PubMed] [Google Scholar]
- 69.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas D J, Wall J S, Hainfeld J F, Kaczorek M, Booy F P, Trus B L, Eiserling F A, Steven A C. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991;65:3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.VanCott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 73.VanCott T C, Bethke F R, Kalyanaraman V, Burke D S, Redfield R R, Birx D L. Preferential antibody recognition of structurally distinct HIV-1 gp120 molecules. J Acquired Immune Defic Syndr. 1994;7:1103–1115. [PubMed] [Google Scholar]
- 74.VanCott T C, Mascola J R, Kaminski R W, Kalyanaraman V, Hallberg P L, Burnett P R, Ulrich J T, Rechtman D J, Birx D L. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 76.VanCott T C, Veit S C D, Kalyanaraman V, Earl P, Birx D L. Characterization of a soluble, oligomeric HIV-1 gp160 protein as a potential immunogen. J Immunol Methods. 1995;183:103–117. doi: 10.1016/0022-1759(95)00038-c. [DOI] [PubMed] [Google Scholar]
- 77.Weiss R A, Clapham P R, Cheingsong P R, Dalgleish A G, Carne C A, Weller I V, Tedder R S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985;316:69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- 78.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquired Immune Defic Syndr. 1994;7:211–219. [PubMed] [Google Scholar]
- 79.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]