Abstract
Simple Summary
Studies have revealed a rapid global and continental loss of genetic resources for native sheep breeds that is more critical in Europe and the Caucasus region. Therefore, an urgent step is needed to halt this negative trend. Viable and functional epididymal spermatozoa could be retrieved from castrated, slaughtered, or accidentally dead animals with good pregnancy outcomes; however, many factors reported to affect the quality and cryo-tolerance of artificial vagina-collected, as well as electro-ejaculated, ram spermatozoa have not been extensively studied in ram epididymal spermatozoa despite being a cheap alternative for gene conservation. Given the context mentioned above, we assessed the effects of three different commercial soy lecithin-based semen extenders (AndroMed®, BioXcell®, and OviXcell®) and two spermatozoa concentrations (200 × 106/mL vs. 400 × 106/mL) on the freezability of ram epididymal spermatozoa. BioXcell® and OviXcell® produced significantly higher post-thaw specific kinematics and better protected the ram epididymal spermatozoa head membrane compared to AndroMed®. In contrast, the normal tail morphology is better maintained in AndroMed®. The 400 × 106 spermatozoa/mL concentration better preserved the ram epididymal spermatozoa head membrane integrity. The ideal concentration for cryopreserving ram epididymal spermatozoa is 400 × 106 spermatozoa/mL. However, the extenders must be optimized for more effective ram epididymal spermatozoa freezing.
Abstract
There are limited studies on the factors affecting the success of ram epididymal spermatozoa (REPS) cryopreservation. On this note, the current study assessed the influence of three commercial soy lecithin-based semen extenders, AndroMed® (AND), BioXcell® (BIO), and OviXcell® (OVI), and two concentrations (400 × 106 vs. 200 × 106 spermatozoa/mL) on the pre-freeze and post-thaw quality of REPS. The REPS were retrieved from nine adult rams’ testes and diluted with each of the three extenders to both concentrations. Straws were frozen manually. Standard motility (SMP) and kinematic parameters (KPs) were assessed via a CASA, while spermatozoa viability, morphology, and acrosomal integrity were assessed via the Kovács–Foote staining technique. The concentration did not significantly affect the pre-freeze and post-thaw SMP and KPs of REPS. BIO and OVI had significantly higher pre-freeze and post-thaw BCFs, post-thaw VAP, and the percentage of all intact heads than AND. In contrast, AND had a significantly lower percentage of REPS with tail defects than BIO and OVI. The 400 × 106 spermatozoa/mL concentration resulted in a significantly higher percentage of all intact heads than the 200 × 106 spermatozoa/mL concentration. Freezing significantly increased tail defects and decreased the percentage of REPS with distal cytoplasmic droplets. The cryopreservation of REPS at the 400 × 106 spermatozoa/mL concentration is recommended. All three extenders must be optimized to preserve the viability, membrane integrity, and better normal morphology of REPS; the reason for increased tail abnormality after the freezing/thawing process needs to be studied.
Keywords: AndroMed®, BioXcell®, OviXcell®, soy lecithin, ram epididymal spermatozoa, cryopreservation, membrane integrity
1. Introduction
Semen cryopreservation is an important technique that facilitates modern assisted reproductive technologies and ensures the conservation of valuable animal genetic resources (AGRs) in the form of frozen semen for several years or decades, while maintaining its viability and fertilizing ability. Sperm collection in animals with erectile dysfunction is compromised or not feasible via artificial vaginas or electro-ejaculators [1]. Therefore, epididymal spermatozoa (EPS) collection and cryopreservation provides the cheapest and easiest alternative means of collecting spermatozoa to conserve AGRs of a valuable sire in the case of sudden death or castration [2]. Moreover, studies in different species proved that EPS resulted in good-to-excellent pregnancy rates following AI in boar (92.0%) [3], cattle (58.8%) [4], sheep (87.5%, 58.5%, and 55.0%) [5,6,7], goats (61.2%) [8], stallions (27.8% and 64.0%) [9,10], and red deer (75.0%) [11]. Therefore, it is considered the most viable, cheapest, and easiest way to conserve the genetic resources of endangered, threatened, or valuable animals that die accidentally.
Semen extenders are solutions that provide nourishment and protect sperm cells from injury during the cooling and freezing process. They are one factor that affects the fertility of cervical insemination [12]. Some researchers have reported that they greatly influence the quality of frozen–thawed EPS in sheep [12,13] and alpacas [2]. Extenders can be conventional or commercially prepared. The most used ram semen extender is tris-citric egg yolk. The commercially available ones are classified based on their origin/composition. They include those that are soy lecithin-based (AndroMed®, BioXcell®, Biociphos Plus®, Botu-Bov®–soy lecithin, and OviXcell®), egg-yolk-based (Biladyl®, Botu-Bov® Triladyl®, and BullXcell®), milk-based (INRA96®), and protein-free (OptiXcell®) [14,15,16,17,18]. Different studies were conducted on the effects of commercially prepared semen extenders on the freezability of the spermatozoa of bulls [15,16,19,20], buffalo [21], goat bucks [22], and rams [23,24,25]. In recent years, there has been a call by researchers against the use of egg-yolk-based extenders due to the wide variability of their components and microbial contamination risk, leading to endotoxin production, reducing spermatozoa post-thaw viability and acrosomal integrity [25,26]. An alternative cold-shock protector for egg yolk is plant-based lecithin. Several studies have been conducted on the effects of different semen extenders on the freezability of artificial vagina (AV)-collected ram spermatozoa [27,28,29]; however, there have been fewer studies on ram epididymal spermatozoa (REPS), particularly on the effects of soy lecithin-based commercially available semen extenders [12,13]. Most of the studies on the effects of soy lecithin-based semen extenders primarily focused on AV-collected ram spermatozoa [17,25,26,30,31,32]. Moreover, the studies conducted on REPS were mostly on the effects of collection methods [13], handling/storage conditions or transportation temperature [33,34,35], washing [36], egg yolk-based extenders [12,13], and the effects of buffers and sugar combinations [37] on their post-thaw quality characteristics. This being the case, there is a need to explore other factors affecting REPS’s post-thaw quality.
The dilution rate or sperm-freezing concentration effect is another exciting factor worth investigating regarding REPS freezability. Some researchers have reported it to affect the quality/success of AV-collected cryopreserved spermatozoa in sheep [26,38,39]. The lower concentration (200 × 106 spermatozoa/mL) was reported to result in better post-thaw quality parameters than the higher doses (400 × 106 or 800 × 106 spermatozoa/mL) [26,40], however, extreme dilution was found to negatively affect the membrane integrity of ram spermatozoa and cause capacitation-like changes [41], and cryopreservation has an additive effect that damages the cells [38]. Moreover, for a successful artificial insemination program, the technique employed in depositing spermatozoa into the receptive female reproductive tract determines the dilution rate [39]. Hence, it is important to identify the most ideal dilution rate/sperm concentration with which to freeze REPS. Moreover, there are fewer or no studies on the ideal spermatozoa concentration of REPS that leads to less detrimental effects on its post-thaw quality. On this note, the current study attempted to investigate the effects of three different commercially available soy lecithin-based semen extenders (AndroMed® (AND), BioXcell® (BIO), and OviXcell® (OVI), with compositions detailed in Table 1) and two different spermatozoa concentrations (400 × 106 vs. 200 × 106 spermatozoa/mL), or their most suitable interactions, on the freezability of REPS. The current study did not consider the breed effect because the sole aim was to identify the ideal concentration, extender, or their most suitable interactions for freezing REPS, regardless of the breed, to enhance the gene conservation of local sheep breeds.
Table 1.
Compositions of the three commercial soy lecithin-based semen extenders.
| AndroMed® (100 mL) | BioXcell® (1000 mL) | OviXcell® (100 mL) |
|---|---|---|
| Phospholipids | Glycine (0.2 g/L) | Amino acid |
| TRIS | TRIS (2.3 g/L) | Buffers |
| Citric acid | Monohydrate citric acid (2.5 g/L) | |
| Sodium citrate (6.2 g/L) Potassium chloride (0.8 g/L) Hydrate of calcium lactate (0.7 g/L) |
Salts | |
| Sugars | Fructose (1.2 g/L) Monohydrate lactose (0.8 g/L) Anhydrous glucose (0.5 g/L) |
Sugars |
| Antioxidants | Taurine (0.005 g/L) | Taurine |
| Glycerol (6.7%) | Glycerol (7.0%/40.2 g/L) | Glycerol |
| Tylosin (5.7 mg) | Tylosin tartrate (0.33 g/L) | Tylosin tartrate |
| Gentamicin (28.6 mg) | Gentamycin sulphate (0.24 g/L) | Gentamicin |
| Spectinomycin (34.3 mg) | Spectinomycin | Spectinomycin sulfate (<0.2%) |
| Lincomycin (17.2 mg) | Licospectin 100 (0.385 g/L) | Lincomycin hydrochloride |
| Soy lecithin | Soy lecithin (1.5 g/L) | Soy lecithin |
| Ultrapure water | Ultrapure water (ad 1000 mL) | Ultrapure water |
Sources: Extenders’ leaflets; [42].
2. Materials and Methods
2.1. Media, Reagents, and Materials
Three different commercial semen extenders, AndroMed® (AND) (13503/1200 CSS One-step, 200 mL), BioXcell® (BIO) (016218 Easy to use, 250 mL), and OviXcell® (OVI) (020997 Ready-to-use extender, 100 mL), were purchased from Minitube Ltd. (Tiefenbach, Germany) and IMV technologies (L’Aigle, France). The AND and BIO extenders were reconstituted according to the manufacturers’ guidelines, filled into sterilized 10 mL centrifuge tubes, and stored at frozen conditions until required. All other plastic wares were purchased from Falcons® (Corning Inc., Corning, NY, USA), and 0.25 mL transparent semen straws were purchased from IMV Technologies (L’Aigle, France).
2.2. Study Location and Testicle Collection
The study was conducted at the Hungarian University of Agriculture and Life Sciences, spermatology laboratory, Herceghalom, Hungary. Nine pairs of intact testes were collected from 9 adult healthy rams (with health status according to the relevant EU regulations) of different breeds, Merino (4), Racka (3), and Dorper (2), from slaughterhouses in Hungary between November 2022 and March 2023. They were transported to the laboratory in a cold box within 2 h and processed individually within 24 h to simulate field conditions, as described by Egerszegi et al. [43].
2.3. Epididymal Sperm Collection
The testes were weighed using a digital weighing scale after removing the scrotal sac and lamina parietalis of the tunica vaginalis. Each cauda epididymis (CE) was carefully separated and weighed, and the spermatozoa were retrieved through slicing. The visceral layer of the tunica vaginalis covering the CE was carefully removed to avoid blood contamination. The stripped CE was washed with a PBS solution and then sliced with a scalpel in a Petri dish containing 3 mL of a tris-citric acid fructose buffer solution (Tris (Hydroxyl methylamino methane), 3.028 g; citric acid monohydrate, 1.70 g; fructose, 1.25 g; and distilled water up to 100 mL), as described by Ahmed et al. [36]. The sliced CE was placed in the tris buffer solution for 10 min to enhance spermatozoa collection, rinsed with 2 mL of the tris buffer, and filtered with gauze sheets; the final volume was then recorded. The tris buffer solution was added to each sample from each CE, making an equal volume of 10 mL, and centrifuged at 880 g for 10 min, according to Ahmed et al. [36]; see Figure 1. Finally, the supernatant was removed, and the pellets that were retrieved from both CEs of the same ram with a good mass motility score of 4–5 were mixed.
Figure 1.
Epididymal sperm collection via the slicing method. (a) Stripping the CE for slicing. (b) Stripped CE ready for slicing. (c) Slicing the stripped CE. (d) Rinsing the sliced CE. (e) Filtering the retrieved epididymal spermatozoa. (f) Centrifuging the retrieved epididymal spermatozoa.
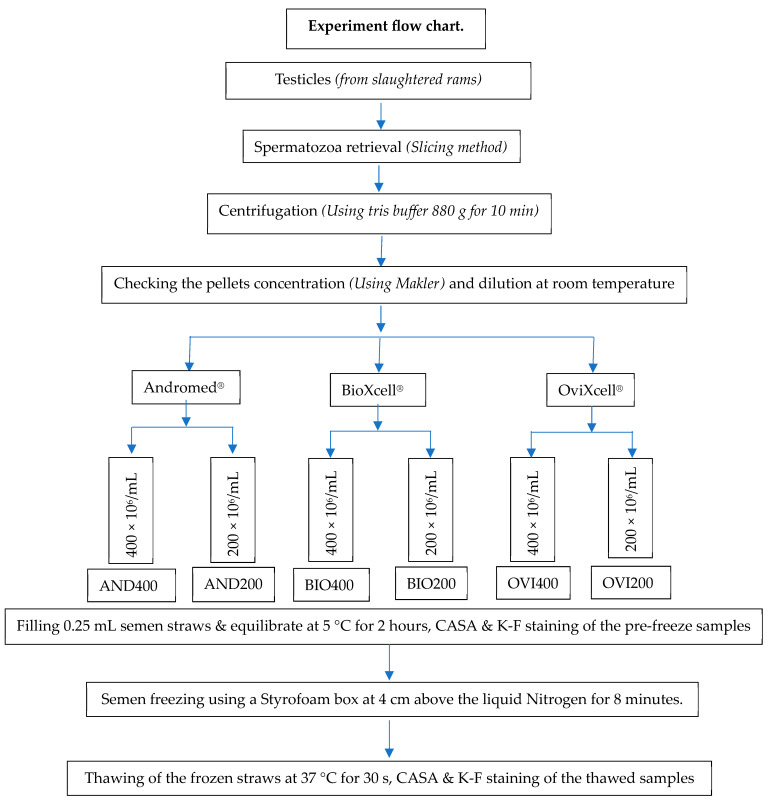
2.4. Sample Dilution, Equilibration and Freezing
Samples were checked for concentration with a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel), using a phase-contrast microscope at ×200 magnification. Part of the sample was taken and divided into three aliquots, and each of the aliquots was diluted with one of the commercial semen extenders to a concentration of 400 × 106 spermatozoa/mL at room temperature to give AND 400, BIO 400, and OVI 400. Part of each extended sample was aliquoted again and further diluted with the corresponding extender to a final concentration of 200 × 106 spermatozoa/mL, giving AND 200, BIO 200, and OVI 200. The extended samples were manually filled and sealed using polyvinyl alcohol (PVA) into well-labelled and color-coded French Mini straws.
The filled and sealed straws were equilibrated in a refrigerator (5 °C for 2 h). The freezing of REPS was conducted in a similar way as conventional AV-collected spermatozoa freezing. It was carried out manually in a Styrofoam box at 4 cm above the liquid nitrogen (LN2) for 8 min. Finally, the frozen straws were plunged into the LN2 for permanent storage. After about 2 weeks, the frozen samples were thawed (37 °C for 30 s) and assessed for standard motility and kinematic parameters. Smears were prepared for membrane integrity and morphology evaluation (Figure 2).
Figure 2.
Experiment flowchart.
2.5. Sample Quality Assessment
2.5.1. Standard Motility and Kinematic Parameters
Pre-freeze and post-thawed spermatozoa’s motility and kinematic parameters were assessed using a computer-assisted sperm analyzer (CASA) (Sperm VisionTM Version 3.8 software, Minitübe Ltd., Tiefenbach, Germany). The samples were diluted to a 50–60 × 106 spermatozoa/mL concentration using the same extender. At least 10 random fields per sample or a total of 500 spermatozoa were analyzed for standard motility (total motility (TM, %) and progressive motility (PM, %)) and kinematic parameters: curvilinear velocity (VCL, μm/s), average path velocity (VAP, μm/s), straight line velocity (VSL μm/s), linearity (LIN = VSL/VCL × 100, %), straightness (STR = VSL/VAP × 100, %), beat cross frequency (BCF, Hz), wobble (WOB = VAP/VCL × 100, %), and amplitude of lateral head displacement (ALH, μm), as described by Goovaerts et al. [44], Kang et al. [4], and Bergstein-Galan et al. [45].
2.5.2. Viability and Morphology Assessment
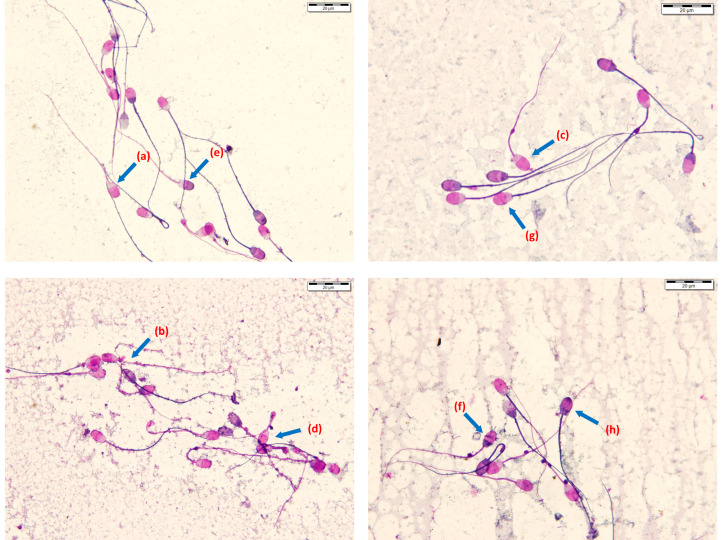
The acrosome, head, and tail membrane integrity, as well as the morphology, of spermatozoa were evaluated via a modified Kovács–Foote staining method, using a 0.16% Chicago sky blue 6B (Sigma-Aldrich, St. Louis, MO, USA, C-8679) viability stain, neutral red (Sigma N 2880), formaldehyde fixation, and 7.5% Giemsa solution (Sigma GS-500) in distilled water prepared freshly before use for acrosome staining [46,47]. The procedure involved the viability staining of the diluted samples and the air-drying of the slides, fixation for 4 min, followed by rinsing with tap and distilled water, and finally staining with a Giemsa solution for 3.5–4 h. After this, rinsing with tap water and the differentiation of the stained slides in distilled water for 2 min were carried out for better categorization of the spermatozoa. Slides were evaluated using an oil-immersion objective with bright-field microscopy at ×1000 magnification with a yellow filter for better live/dead differentiation [46]. A total of three hundred cells were counted on each slide and classified into eight categories: intact head, intact tail, and acrosome membrane (Intact); normal morphology (IHITIA); intact with a proximal cytoplasmic droplet (IPD); intact with a distal cytoplasmic droplet (IDD); intact with a tail defect (bent, broken, hairpin curved, or coiled tail) (IBT); intact head and tail, damaged acrosome (IHITDA); damaged head with intact tail (DHIT); intact head with damaged tail (IHDT); and damaged head, damaged tail, and damaged acrosome (DHDTDA), as described by Kútvölgyi et al. [46]. Different spermatozoa categories are shown in Figure 3. In addition, all distal cytoplasmic droplets and all bent, hairpin-curved tails were counted regardless of intact or damaged membranes, and per cent, all intact spermatozoa (IHITIA + IPD + IDD + IBT), all intact heads (IHITIA + IPD + IDD + IBT + IHITDA + IHDT), and all intact tails (IHITIA + IPD + IDD + IBT + IHITDA + DHIT) were also calculated. The values obtained for each category were presented in percentages.
Figure 3.
The different post-thaw ram epididymal spermatozoa categories stained with the modified Kovács–Foote staining technique (magnification × 1000, using a light microscope with an oil immersion objective). (a) Intact head, intact tail, and acrosome membrane (Intact: IHITIA). (b) Intact with a proximal droplet (IPD). (c) Intact with distal droplet (IDD). (d) Intact with a bent tail (IBT). (e) Intact head, tail, damaged acrosome (IHITDA). (f) Damaged head with intact tail (DHIT). (g) Intact head with damaged tail (IHDT). (h) Damaged head, damaged tail, and damaged acrosome (DHDTDA).
2.6. Data Analysis
Data from pre-freeze, post-thaw, and Kovács–Foote-stained REPS were collected, recorded, and analyzed for descriptive statistics, using IBM® SPSS® statistical software version 29. Normality was checked using a Shapiro–Wilk test, and transformations were achieved using a two-step transformation. A general linear model using two-way analysis of variance was used to analyze the effects of extender and sperm concentration (400 × 106 vs. 200 × 106 spermatozoa/mL), as well as their interaction, on standard motility, kinematic parameters, viability, and morphological parameters, with the level of the significance set at p < 0.05. Means were separated using the Tukey post hoc test. The effects of freezing using different commercial soy lecithin-based semen extenders and the overall effects of freezing and thawing on the percentage of distal droplets and tail defects were analyzed using Student’s paired-sample t-test, and the significance difference was checked using a two-tailed test. The results are presented as means ± standard errors of means (SEs).
3. Results
3.1. General Parameters of Ram Epididymal Spermatozoa
In the current study, we determined certain parameters related to the ram testicles and cauda epididymal (CE) weight, in addition to the concentration of the spermatozoa retrieved from rams of different breeds (Table 2). The mean testicular weight, epididymal weight, and spermatozoa concentration obtained were 157.78 ± 22.15 g, 14.25 ± 1.38 g, and 9061.44 ± 845.53 × 106/mL, respectively.
Table 2.
General parameters of the ram epididymal spermatozoa retrieved from different ram breeds.
| Parameters | Range | Mean ± SE |
|---|---|---|
| Testicular weight (g) | 113.07–308.09 | 157.78 ± 22.15 |
| Cauda epididymal weight (g) | 7.89–20.39 | 14.25 ± 1.38 |
| Spermatozoa concentration (106/mL) | 5800–14,240 | 9061.44 ± 845.53 |
SE: standard error of means, n = 9.
3.2. Effects of Three Different Commercial Soy Lecithin-Based Semen Extenders and Two Spermatozoa Concentrations on the Standard Motility and Kinematic Parameters of Pre-Freeze Ram Epididymal Spermatozoa
The effects of the three different commercial soy lecithin-based semen extenders and two spermatozoa concentrations on pre-freeze REPS are presented in Table 3. There was no significant (p > 0.05) interaction between the extender and the spermatozoa concentrations for all the parameters studied, so we present the main treatment effect. Similarly, the standard motility and all kinematic parameters showed no significant (p > 0.05) difference among the extenders and between the two spermatozoa concentrations, except for BCF. The BIO and OVI extenders had significantly (p < 0.05) higher BCFs (30.18 ± 1.1 and 29.99 ± 1.0 Hz) than the AND extender (26.80 ± 0.8 Hz).
Table 3.
Effects of three different commercial soy lecithin-based semen extenders and two spermatozoa concentrations on the standard motility and kinematic parameters of pre-freeze ram epididymal spermatozoa.
| Extenders | Standard Motility and Kinematic Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM (%) | PM (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | LIN (%) | STR (%) | BCF (Hz) | WOB (%) | ALH (μm) | |
| AndroMed® | 72.22 ± 3.2 | 64.89 ± 3.4 | 163.94 ± 5.8 | 76.85 ± 2.3 | 54.05 ± 2.4 | 32.83 ± 1.3 | 70.00 ± 2.2 | 26.80 ± 0.8 a | 46.61 ± 0.5 | 5.55 ± 0.2 |
| BioXcell® | 69.00 ± 3.8 | 62.44 ± 4.0 | 168.11 ± 3.9 | 82.21 ± 2.3 | 60.64 ± 3.1 | 35.50 ± 1.6 | 72.83 ± 2.4 | 30.18 ± 1.1 b | 48.44 ± 0.6 | 5.21 ± 0.2 |
| OviXcell® | 67.61 ± 3.7 | 60.78 ± 3.9 | 169.06 ± 3.2 | 83.16 ± 2.2 | 62.00 ± 3.4 | 35.94 ± 1.5 | 73.22 ± 2.2 | 29.99 ± 1.0 b | 48.56 ± 0.7 | 5.27 ± 0.1 |
| p-value | 0.633 | 0.727 | 0.863 | 0.336 | 0.215 | 0.267 | 0.463 | 0.020 | 0.080 | 0.695 |
| Conc. (106/mL) | ||||||||||
| 200 | 67.85 ± 3.3 | 61.26 ± 3.4 | 167.48 ± 3.7 | 81.14 ± 1.9 | 59.80 ± 2.5 | 35.26 ± 1.2 | 72.85 ± 1.9 | 29.24 ± 0.9 | 48.04 ± 0.6 | 5.25 ± 0.2 |
| 400 | 71.37 ± 2.5 | 64.15 ± 2.8 | 166.59 ± 3.6 | 80.34 ± 1.9 | 58.00 ± 2.5 | 34.56 ± 1.2 | 71.19 ± 1.9 | 28.71 ± 0.8 | 47.70 ± 0.5 | 5.42 ± 0.2 |
| p-value | 0.170 | 0.231 | 0.556 | 0.379 | 0.302 | 0.808 | 0.584 | 0.834 | 0.985 | 0.181 |
| p-value Ext. * Conc. | 0.619 | 0.643 | 0.852 | 0.744 | 0.659 | 0.887 | 0.840 | 0.854 | 0.946 | 0.712 |
Conc.: concentration. TM: total motility. PM: progressive motility. VCL: curvilinear velocity. VAP: average pathway velocity. VSL: straight line velocity. LIN: linearity of movement. STR: straightness. BCF: beat cross frequency. WOB: wobble. ALH: amplitude of the lateral head displacement. Ext. * Conc.: extender * concentration interaction effects. SE: standard error of means, n = 9. Means in the same column with different superscripts a,b differ significantly.
3.3. Effects of Three Different Commercial Soy Lecithin-Based Extenders and Two Spermatozoa Concentrations on Standard Motility and Kinematic Parameters of Post-Thaw Ram Epididymal Spermatozoa
Table 4 presents the effects of the three commercial soy lecithin-based semen extenders and two spermatozoa concentrations on the REPS’s post-thaw standard motility and kinematic parameters. There was no significant (p > 0.05) interaction between the extender and spermatozoa concentrations for all of the studied parameters, so we present the main effect of the extenders and the spermatozoa concentrations. The standard motility parameters of the post-thaw REPS were also not significantly (p > 0.05) different among the extenders and between the spermatozoa concentrations. The BIO and OVI extenders had statistically the same post-thaw VAPs (77.78 ± 3.2 vs. 80.48 ± 3.1 μm/s) and BCFs (32.81 ± 1.1 vs. 32.46 ± 1.0 Hz) and were significantly (p < 0.05) higher than the AND extender (67.72 ± 3.5 μm/s and 28.72 ± 0.9 Hz). Moreover, OVI had significantly higher (p < 0.05) per cent WOB than the AND extender (50.56 ± 0.8 vs. 47.67 ± 0.7 %), while BIO and OVI were statistically the same (49.56 ± 0.9 vs. 50.56 ± 0.8 %). All other kinematic parameters were statistically the same (p > 0.05) among the extenders and between the spermatozoa concentrations.
Table 4.
Effects of the three different commercial soy lecithin-based semen extenders and two spermatozoa concentrations on the standard motility and kinematic parameters of post-thaw ram epididymal spermatozoa.
| Extenders | Standard Motility and Kinematic Parameters (Mean ± SE) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TM (%) | PM (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | LIN (%) | STR (%) | BCF (Hz) | WOB (%) | ALH (μm) | |
| AndroMed® | 34.89 ± 3.9 | 27.11 ± 3.4 | 139.55 ± 6.3 | 67.72 ± 3.5 a | 50.58 ± 3.3 | 35.72 ± 1.4 | 74.06 ± 2.3 | 28.72 ± 0.9 a | 47.67 ± 0.7 a | 4.41 ± 0.2 |
| BioXcell® | 38.83 ± 3.5 | 31.50 ± 3.1 | 156.72 ± 5.0 | 77.78 ± 3.2 b | 58.96 ± 3.9 | 37.11 ± 1.8 | 74.28 ± 2.5 | 32.81 ± 1.1 b | 49.56 ± 0.9 ab | 4.42 ± 0.2 |
| OviXcell® | 37.61 ± 3.7 | 31.56 ± 3.5 | 157.39 ± 5.4 | 80.48 ± 3.1 b | 61.46 ± 3.9 | 38.33 ± 1.7 | 75.00 ± 2.4 | 32.46 ± 1.0 b | 50.56 ± 0.8 b | 4.55 ± 0.2 |
| p-value | 0.893 | 0.509 | 0.191 | 0.024 | 0.154 | 0.554 | 0.816 | 0.012 | 0.044 | 0.849 |
| Concentrations (106/mL) | ||||||||||
| 200 | 34.33 ± 2.3 | 27.33 ± 2.2 | 150.40 ± 5.3 | 75.43 ± 3.2 | 57.92 ± 3.4 | 37.74 ± 1.4 | 75.41 ± 1.9 | 31.83 ± 0.9 | 49.37 ± 0.8 | 4.33 ± 0.1 |
| 400 | 39.89 ± 3.5 | 32.78 ± 3.1 | 152.04 ± 4.3 | 75.22 ± 2.4 | 56.07 ± 2.8 | 36.37 ± 1.3 | 73.48 ± 1.9 | 30.83 ± 0.8 | 49.15 ± 0.6 | 4.58 ± 0.2 |
| p-value | 0.170 | 0.249 | 0.878 | 0.957 | 0.534 | 0.486 | 0.566 | 0.400 | 0.815 | 0.250 |
| p-value Ext. * Conc. | 0.723 | 0.946 | 0.648 | 0.855 | 0.913 | 0.976 | 0.959 | 0.827 | 0.882 | 0.927 |
TM: total motility. PM: progressive motility. VCL: curvilinear velocity. VAP: average pathway velocity. VSL: straight line velocity. LIN: linearity of movement. STR: straightness. BCF: beat cross frequency. WOB: wobble. ALH: amplitude of the lateral head displacement. Ext. * Conc.: extender * concentration interaction effects. SE: Standard error of means, n = 9. Means in the same column with different superscripts a,b differ significantly.
3.4. Effects of Different Soy Lecithin-Based Commercial Semen Extenders and the Two Spermatozoa Concentrations on the Post-Thaw Viability and Morphological Characteristics of Ram Epididymal Spermatozoa
The effects of different soy lecithin-based commercial semen extenders and the two spermatozoa concentrations on the post-thaw viability and morphological characteristics of the REPS are presented in Table 5. There was no significant (p > 0.05) interaction between the extenders and the spermatozoa concentrations. Similarly, neither the extender nor the spermatozoa concentration significantly affects the percentage of the post-thaw REPS with IHITIA. The AND extender had a significantly (p < 0.05) lower percentage of the intact REPS with bent tails (IBT), all intact heads, and all bent tails categories (2.56 ± 0.6, 34.64 ± 3.2, and 9.74 ± 1.4%) than the BIO (8.14 ± 1.5, 45.33 ± 3.3, and 18.33 ± 2.4%) and OVI (7.19 ± 1.3, 44.68 ± 2.9, and 17.39 ± 1.7%) extenders. In contrast, the BIO and OVI extenders were statistically the same and had a lower percentage of categories of REPS with DHIT than the AND extender: 2.91 ± 0.7 and 2.53 ± 0.4 vs. 6.31 ± 1.1, respectively. The 400 × 106 spermatozoa/mL concentration resulted in a significantly (p < 0.05) higher percentage of all intact head categories than the 200 × 106 spermatozoa/mL (45.15 ± 5.1 vs. 37.95 ± 3.4%) concentration. The extenders and the spermatozoa concentrations did not affect all of the other parameters.
Table 5.
Effects of the three different commercial soy lecithin-based semen extenders and two spermatozoa concentrations on the viability and morphological parameters of post-thaw ram epididymal spermatozoa.
| Extenders | Viability and Morphological Parameters (Mean ± SE) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHITIA (%) | IPD (%) | IDD (%) |
IBT (%) |
IHITDA (%) |
DHIT (%) |
IHDT (%) |
DHDTDA (%) |
All Intact (%) | All Intact Head (%) | All Intact Tail (%) | All Distal Droplets (%) |
All Bent Tails (%) |
|
| AndroMed® | 5.92 ± 1.2 | 0.87 ± 0.3 | 9.72 ± 1.4 | 2.56 ± 0.6 a | 0.04 ± 0.0 | 6.31 ± 1.1 | 15.52 ± 1.8 | 59.06 ± 3.4 | 19.08 ± 2.2 | 34.64 ± 3.2 a | 25.42 ± 2.9 | 28.44 ± 2.9 | 9.74 ± 1.4 a |
| BioXcell® | 6.55 ± 1.1 | 0.91 ± 0.2 | 9.44 ± 1.4 | 8.14 ± 1.5 b | 0.03 ± 0.0 | 2.91 ± 0.7 | 20.27 ± 2.5 | 51.73 ± 3.3 | 25.03 ± 1.5 | 45.33 ± 3.3 b | 27.97 ± 1.9 | 21.89 ± 2.7 | 18.33 ± 2.4 b |
| OviXcell® | 7.46 ± 1.3 | 0.68 ± 0.2 | 9.33 ± 1.5 | 7.19 ± 1.3 b | 0.02 ± 0.0 | 2.53 ± 0.4 | 20.00 ± 1.8 | 52.79 ± 3.0 | 24.66 ± 2.4 | 44.68 ± 2.9 b | 27.21 ± 2.6 | 20.33 ± 2.4 | 17.39 ± 1.7 b |
| p-value | 0.717 | 0.613 | 0.981 | 0.003 | 0.866 | 0.155 | 0.143 | 0.242 | 0.094 | 0.030 | 0.771 | 0.100 | 0.0001 |
| Con. (106/mL) | |||||||||||||
| 200 | 5.43 ± 1.3 | 0.70 ± 0.3 | 9.03 ± 2.1 | 6.39 ± 1.9 | 0.04 ± 0.0 | 4.83 ± 0.8 | 16.36 ± 2.2 | 57.23 ± 4.1 | 21.55 ± 2.8 | 37.95 ± 3.4 A | 26.41 ± 3.7 | 24.15 ± 4.1 | 16.81 ± 2.9 |
| 400 | 7.86 ± 1.9 | 0.94 ± 0.3 | 9.96 ± 2.1 | 5.53 ± 1.1 | 0.02 ± 0.0 | 3.00 ± 0.9 | 20.83 ± 4.0 | 51.83 ± 5.1 | 24.29 ± 2.9 | 45.15 ± 5.1 B | 27.32 ± 3.4 | 22.96 ± 3.7 | 13.49 ± 2.1 |
| p-value | 0.186 | 0.079 | 0.587 | 0.820 | 0.703 | 0.165 | 0.151 | 0.160 | 0.267 | 0.049 | 0.760 | 0.713 | 0.118 |
| p-value Ext. * Conc. | 0.337 | 0.692 | 0.946 | 0.819 | 0.374 | 0.876 | 0.783 | 0.918 | 0.750 | 0.724 | 0.984 | 0.833 | 0.277 |
IHITIA: intact head, intact tail, and acrosome membrane (Intact). IPD: intact with proximal droplet. IDD: intact with distal droplets. IBT: intact with a bent tail. IHITDA: intact head and tail, damaged acrosome. DHIT: damaged head with intact tail. IHDT: intact head with damaged tail. DHDTDA: damaged head, damaged tail, and damaged acrosome. Ext. * Conc.: extender * concentration interaction effect. SE: standard error of the means. Stained with a modified Kovács–Foote staining technique, three hundred cells were evaluated and categorized per slide, using a bright-field microscope with an oil-immersion objective at ×1000 magnification, n = 9. Means in the same column with different superscripts among the extenders a,b and between the spermatozoa concentrations A,B differ significantly.
3.5. Effect of Freezing with Different Commercial Soy Lecithin-Based Semen Extenders on All Distal Droplets and Tail Defects of Ram Epididymal Spermatozoa
Table 6 presents the effects of freezing REPS with different commercial semen extenders on all distal droplets and tail defects of REPS. Considering that the 200 and 400 million spermatozoa/mL concentrations were statistically the same (Table 5), the data were pooled to assess the effects of freezing REPS on all distal droplets and all bent tails, in addition to the overall effects of freezing. Significant (p < 0.05) differences existed between the pre-freeze and the post-thaw distal droplets, as well as the bent tails, in all of the extenders and the overall means: AND, 38.51 ± 4.8 vs. 28.17 ± 2.9% and 5.52 ± 1.3 vs. 9.74 ± 1.4%; BIO, 32.92 ± 5.5 vs. 21.72 ± 2.8 and 11.24 ± 2.7 vs. 18.33 ± 2.4%; and OVI, 26.62 ± 3.6 vs. 20.33 ± 2.5% and 11.31 ± 2.4 vs. 17.39 ± 1.7%. And the overall means were 32.69 ± 2.7 vs. 23.41 ± 1.6 % and 9.29 ± 1.3 vs. 15.15 ± 1.2% for all distal droplets and all bent tails, respectively.
Table 6.
Effects of freezing and thawing with different commercial soy lecithin-based semen extenders on distal droplets and tail defects of ram epididymal spermatozoa.
| All Distal Droplets | p-Values | All Bent Tails | p-Values | |||
|---|---|---|---|---|---|---|
| Extender | Pre-Freeze | Post-Thaw | Pre-Freeze | Post-Thaw | ||
| AND | 38.51 ± 4.8 a | 28.17 ± 2.9 b | 0.002 | 5.52 ± 1.3 a | 9.74 ± 1.4 b | 0.003 |
| BIO | 32.92 ± 5.5 a | 21.72 ± 2.8 b | 0.009 | 11.24 ± 2.7 a | 18.33 ± 2.4 b | 0.003 |
| OVI | 26.62 ± 3.6 a | 20.33 ± 2.5 b | 0.032 | 11.31 ± 2.4 a | 17.39 ± 1.7 b | 0.002 |
| Overall | 32.69 ± 2.7 a | 23.41 ± 1.6 b | 0.0001 | 9.29 ± 1.3 a | 15.15 ± 1.2 b | 0.0001 |
AND: AndroMed® extender. BIO: BioXcell® extender. OVI: OviXcell® extender. SE: standard error of the means, n = 9. Stained with a modified Kovács–Foote staining technique, three hundred cells were evaluated and categorized per slide using a bright-field microscope with an oil-immersion objective at ×1000 magnification. Means in the same row with different superscripts a,b differ significantly.
4. Discussion
It is well established that cryopreservation decreases spermatozoa viability, functionality, and fertilizing ability [19,39,48]. Furthermore, many factors affecting the success of REPS’s cryopreservation have not been extensively studied, as in AV- and EE-collected ram spermatozoa [26,49]. Among these are the spermatozoa concentration and the diluents used, in particular the readily available commercial soy lecithin-based extenders. On this note, the current study attempted to investigate the earlier-mentioned factors of the pre-freeze quality and freezability of postmortem REPS. Furthermore, the diluents/extenders were reported to affect the freezability of EPS in different species [2,12,13]. Moreover, the animal-based semen extenders were reported to contain variable compositions with a high microbial contamination risk, reducing spermatozoa’s post-thaw viability and acrosome integrity compared to the plant-based extenders [27]. Hence, it is important to identify the ideal commercially available soy lecithin-based diluent and spermatozoa concentration for freezing REPS.
The average weight of the testes and the CE processed in this study (157.78 ± 22.15 and 14.25 ± 1.38 g) were slightly lower than what was reported by Kaabi et al. [34]: 191.11 ± 4.9 and 18.14 ± 0.4 (g), respectively. However, our results presented higher values of standard error, which might be attributed to individual animal differences due to age, season, and breed effects.
The kinematics are important in determining spermatozoa functionality and freezing/thawing success, and spermatozoa with higher BCFs and lower ALH result in a high PM [50]. Similarly, the VAP parameter is preferred over the PM in predicting fresh and post-thaw bull spermatozoa fertilizing potentials [51]. Moreover, the kinematic parameters show relatively high breed similarities in sheep; however, specific kinematic parameters, like the VCL, might vary even between individual sperm from 50 to 320 μm/s in a single field of analysis, and the spermatozoa sub-population with the highest velocity has higher cervical mucus penetration and fertilization rates [50], with the VCL and VAP being the only kinematic parameters that showed a significant positive correlation with cervical mucus penetration in sheep [52] and litter size in pigs [53]. The pregnancy rate in sheep has a strong and significant positive correlation with the spermatozoa PM and VAP (r = 0.62), LIN (r = 0.86), and STR (r = 0.55), but it is negatively correlated with the VCL (r = −0.65), while the average litter size is positively correlated with LIN (r = 0.87) and STR (r = 0.77) [54].
The current study demonstrates that there was no significant (p > 0.05) difference among the three commercial soy lecithin-based semen extenders, AND, BIO, and OVI, and between the spermatozoa concentration, 200 × 106/mL or 400 × 106/mL, on standard motility parameters of pre-freeze and post-thaw REPS. These findings contrast with the findings of D’Alessandro et al. [39], who reported that the freezing concentration effect on the freezability of AV-collected ram spermatozoa was due to extender differences (milk-based vs. egg yolk-based); however, in the current study, all of the extenders compared were soy lecithin-based, and this might be why we could not observe any significant difference among them. Similarly, Abdussamad et al. [15] reported no significant (p > 0.05) difference in the post-thaw TM between two different egg yolk-based extenders in bull cryopreserved spermatozoa and between two soy lecithin-based semen extenders. Our result for the post-thaw motility parameters agrees with Braga et al. [19] and Ondřej et al. [55], who reported no significant difference in motility between the AND and BIO extenders in bull AV-collected post-thaw spermatozoa. It also tallies with that of Akçay et al. [26] in rams. Similarly, Fernandes et al. [27] reported no significant difference (p > 0.05) in the post-thaw TM (33.7 vs. 41.7%) and PM (4.6 vs. 5.0%) between the AND and OVI extenders in Portuguese Merino breed AV-collected spermatozoa.
For the pre-freeze kinematics, the BCF was the only parameter that was significantly (p < 0.05) different among the extenders. The REPS diluted with the BIO and OVI extenders had significantly (p < 0.05) higher BCFs than those in the AND extender. A higher BCF value was reported to be associated with increased fertilization rates [44].
The REPS frozen in the BIO and OVI extenders had statistically the same post-thaw VAP and BCF and were significantly higher (p < 0.05) than the AND extender. Therefore, freezing REPS in the BIO and OVI extenders might lead to a higher fertilization rate than that when using the AND extender. This is because the higher BCFs and lower ALH of sperm heads could facilitate zona pellucida penetration [44], and a higher VAP might lead to higher cervical mucus penetration and fertilization rates [52]. The WOB parameter depicts the degree of oscillation of the sperm head/balancing [56]. The spermatozoa concentration did not affect the parameter but differed significantly between the AND and OVI extenders. Moreover, spermatozoa with higher progression tend to have higher cryo-survival and fertilization potentials [57]. Our results of the effects of semen extenders on the WOB parameter contradict the findings of Dorado et al. [58] in goat bucks and Domingo et al. [59] in rabbits, that being that semen extenders have no significant effect on the WOB parameter. With regard to the spermatozoa concentrations, our result was not in agreement with that of Akçay et al. [26] and Nascimento et al. [40], who reported better post-thaw quality parameters in AV-collected ram spermatozoa frozen at 200 × 106/mL than at 400 × 106/mL. This might be due to the differences in spermatozoa source, as well as the extenders’ compositions. Moreover, D’Alessandro et al. [39] and Akçay et al. [26] reported that increasing the freezing concentration to 800 × 106/mL has a more significant negative influence on the post-thaw quality of ram spermatozoa.
In the current study, we used the Kovács–Foote viability staining technique to evaluate the REPS’s head, tail, and acrosome membrane integrity, as well as morphology. Although the technique is a subjective evaluation, it is economical, as it does not require a costly device and permits the investigator to see damage/abnormalities in the spermatozoa. Using this method, the acrosome, head, and tail membranes of the sperm can be assessed separately, ensuring the precise determination of lesions’ locations. The retained cytoplasmic droplets are caused by incomplete maturation in the epididymis, leading to abnormal spermatozoa morphology and, thus, impairing viability and capacitation in boars [60,61]. The percentage of distal droplets was also reported to increase significantly with bulls’ age [62], and it is positively correlated with ROS production in men [63]. Additionally, the presence of distal droplets has been associated with a higher percentage of ubiquitinated protein and morphological abnormality, and they also harbored 15-lipoxygenases, which are responsible for mitochondria degradation in ejaculated boar spermatozoa [64,65]; however, they are a normal organelle in EPS, and the complete absence of them indicates spermatogenesis abnormality [66]. Similarly, maintaining tail/flagella integrity is very important because it aids spermatozoa’s heads in achieving fertilization [50]. AND preserved the REPS’s normal tail morphology better than the BIO and OVI extenders did. The 400 × 106 spermatozoa/mL concentration was superior in preserving the REPS’s head membrane integrity compared to the 200 × 106 spermatozoa/mL (45.15 ± 5.1 vs. 37.95 ± 3.4%) concentration. Freezing REPS with the BIO or OVI extender better maintained the REPS’s head membrane compared to the AND extender, as indicated by their significantly higher percentage of all intact head values. The highest value of “all intact heads” that we observed in the current study was in BIO, 45.33 ± 3.3%, and was slightly below what was reported by [36], 51.38 ± 4.44%, using the eosin–nigrosin staining technique. The percentage of spermatozoa with IHDT recorded in the current study (15.52 ± 1.8 to 20.27 ± 2.5%) was similar to that reported in bulls, 20%; boars and rams, 5 to 25% [67]; deer, 20% [68]; and stallions, 19.0% [69].
We supposed that the percentage of “all intact” cells corresponds to the percentage of “live” spermatozoa with intact cell membranes and presumably actively moving spermatozoa, while cells with damaged tails and intact heads are supposed to not move and, hence, be non-fertile in vivo [67]. The percentage of all intact spermatozoa observed in the current study ranges between 19.08 ± 2.2 and 25.03 ± 1.5%. It agrees with Salamon and Maxwell’s report [49] that only about 20–30% of post-thawed ram spermatozoa remain biologically intact.
The highest post-thaw percentage of all distal cytoplasmic droplets observed in the current study (AND: 28.17 ± 2.9%) was comparable to that reported in goat bucks (27.8%) [70], but it was lower than that of Kaabi et al. [34] (55.1 ± 5.3%) in rams under similar conditions; however, in the latter experiment, both proximal and distal cytoplasmic droplets were counted. They retrieved spermatozoa in different ways: no extender was used in the slicing procedure to allow sperm to swim out, and there was no centrifugation step in the protocol. These steps probably enhanced the drifting of the distal droplets in some of the cells. Centrifugation was reported to reduce the number of distal cytoplasmic droplets in collared peccaries (Pecari tajacu Linnaeus) [71] and in cat epididymal spermatozoa [72]. Therefore, more studies are needed to confirm this speculation in rams. Freezing REPS with all of the extenders showed a significant difference in the percentage of all distal droplets and all tail defects. We observed that the bent tails increased by about the same percentage as the distal droplets decreased in the frozen samples compared to the pre-freeze condition. The reason for this may be that spermatozoa’s moving tails suddenly get stuck and enclose the droplet and become a spermatozoon with a distal midpiece reflex (also called a hairpin-curved tail), or the osmotic changes during the freezing/thawing process can cause the bending of the tail for some of the distal droplet-bearing sperm. This phenomenon seems to have occurred more in the BIO and OVI extenders than the AND extender, which resulted in a higher percentage of spermatozoa with bent tails in the former extenders than in the latter. Similarly, the overall mean proved that freezing in general significantly (p < 0.05) increases the percentage of REPS with tail defects (9.29 ± 1.3 vs. 15.15 ± 1.2%), with a significant decrease in the percentage of all distal droplets (32.69 ± 2.7 vs. 23.41 ± 1.6%). Our result of the percentage of distal droplets was consistent with the findings of Kaabi et al. [34].
5. Conclusions
Ram epididymal spermatozoa can behave differently than ejaculated spermatozoa during the freezing/thawing process; the membrane structure could be more unstable, so improving and optimizing the freezing technique of REPS is needed. The BIO and OVI extenders showed significantly higher post-thaw VAP and BCFs and were superior to the AND extender in preserving the ram epididymal spermatozoa head membrane integrity. In contrast, the AND extender was superior in maintaining the normal tail morphology of ram epididymal spermatozoa compared to the BIO and OVI extenders. Freezing significantly decreased the percentage of spermatozoa with distal cytoplasmic droplets and increased the percentage of ram epididymal spermatozoa with tail defects; these phenomena could be connected. All three commercial soy lecithin-based extenders must be optimized to better preserve the viability, membrane integrity, and normal morphology of REPS. Future studies should investigate the effect of extenders and spermatozoa concentration on REPS’s mitochondrial membrane potentials, ATP content, and in vivo fertility. Ram epididymal spermatozoa are suggested to freeze in the 400 × 106 spermatozoa/mL concentration, as it better preserves the head membrane integrity of REPS compared to cryopreserving in the 200 × 106 spermatozoa/mL concentration. The effect of centrifugation on REPS distal cytoplasmic droplets and the reason for increased tail abnormalities after the freezing/thawing process need to be studied.
For postmortem gamete extraction and cryopreservation, selecting the best cryopreservation procedure for ex situ in vitro gene conservation is essential. Using the appropriate extender allows the samples to be stored more successfully; therefore, our research is a valuable step in this regard.
Acknowledgments
The authors would like to thank István Egerszegi for the Dorper testicles and Orosz és Tsa Ltd., Hungary, for the Racka and Merino ram testicles. We also thank James Kachingwa Lugata and Hamza Yusuf Adam of ATBU, Bauchi, for their guidance in the data analysis.
Author Contributions
All authors contributed to the manuscript. M.A.M., funding acquisition, investigation, data curation, formal analysis, visualization, writing—original draft preparation, and writing—review and editing; G.K., conceptualization, supervision, methodology, resources, investigation, project administration, and writing—review and editing; J.R.S., investigation and data acquisition; V.J.D., investigation and data acquisition; A.T., investigation; N.V., supervision, resources, and writing—review and editing; S.B., conceptualization, supervision, methodology, investigation, resources, validation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable, because the study does not involve live animals, only organs received from slaughterhouses.
Informed Consent Statement
Not applicable. Only organs from slaughterhouse (testicles and epididymes) were used for the study. We received the organs directly from the slaughterhouse.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding Statement
This study was supported by the Stipendium Hungaricum Scholarship Program (SH ID: 254555).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kang S.-S., Kim U.-H., Jeon M.-H., Lee M.-S., Cho S.-R. Comparison of Spermatozoa Recovery Methods on Cauda Epididymal Sperm of Hanwoo Bulls. J. Anim. Reprod. Biotechnol. 2018;33:321–326. doi: 10.12750/JET.2018.33.4.321. [DOI] [Google Scholar]
- 2.Mamani-Mango G., Moina Gonzales M., Ramos Hidalgo M., Mendoza Mallma J., Ruiz Béjar J., Rivas Palma V., Mellisho Salas E. Effect of Extender and Freezing Rate on Quality Parameters and In Vitro Fertilization Capacity of Alpaca Spermatozoa Recovered from Cauda Epididymis. Biopreserv. Biobank. 2019;17:39–45. doi: 10.1089/bio.2018.0021. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T., Akiyoshi T., Kan M., Mori M., Teshima H., Shimada M. Artificial Insemination with Seminal Plasma Improves the Reproductive Performance of Frozen-Thawed Boar Epididymal Spermatozoa. J. Androl. 2012;33:990–998. doi: 10.2164/jandrol.111.015115. [DOI] [PubMed] [Google Scholar]
- 4.Kang S.-S., Kim U.-H., Ahn J.S., Won J.I., Cho S.-R. Improvement of Pregnancy Rate after Deep Uterine Artificial Insemination with Frozen-Thawed Cauda Epididymal Spermatozoa in Hanwoo Cattle. J. Anim. Reprod. Biotechnol. 2021;36:82–90. doi: 10.12750/JARB.36.2.82. [DOI] [Google Scholar]
- 5.Ehling C., Rath D., Struckmann C., Frenzel A., Schindler L., Niemann H. Utilization of Frozen–Thawed Epididymal Ram Semen to Preserve Genetic Diversity in Scrapie Susceptible Sheep Breeds. Theriogenology. 2006;66:2160–2164. doi: 10.1016/j.theriogenology.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Rickard J.P., Pini T., Soleilhavoup C., Cognie J., Bathgate R., Lynch G.W., Evans G., Maxwell W.M.C., Druart X., de Graaf S.P. Seminal Plasma Aids the Survival and Cervical Transit of Epididymal Ram Spermatozoa. Reproduction. 2014;148:469–478. doi: 10.1530/REP-14-0285. [DOI] [PubMed] [Google Scholar]
- 7.Fernández Abella D., Da Costa M., Guérin Y., Dacheux J.L. Fertility of Undiluted Ram Epididymal Spermatozoa Stored for Several Days at 4 °C. Animal. 2015;9:313–319. doi: 10.1017/S1751731114002109. [DOI] [PubMed] [Google Scholar]
- 8.Ocampo L.C., DelaRosa I.C.J., Lofranco J.O.C., Ortiz J.G.M., Ocampo M.B. Live Birth after Artificial Insemination Using Cryopreserved Epididymal Sperm Recovered from the Cauda Epididymis of Slaughtered Non-Descript Bucks in the Philippines. Int. J. Agric. Technol. 2021;17:2183–2196. [Google Scholar]
- 9.Miró J., Morató R., Vilagran I., Taberner E., Bonet S., Yeste M. Preservation of Epididymal Stallion Sperm in Liquid and Frozen States: Effects of Seminal Plasma on Sperm Function and Fertility. J. Equine Vet. Sci. 2020;88:102940. doi: 10.1016/j.jevs.2020.102940. [DOI] [PubMed] [Google Scholar]
- 10.Heise A., Kähn W., Volkmann D.H., Thompson P.N., Gerber D. Influence of Seminal Plasma on Fertility of Fresh and Frozen-Thawed Stallion Epididymal Spermatozoa. Anim. Reprod. Sci. 2010;118:48–53. doi: 10.1016/j.anireprosci.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Soler A.J., García A.J., Fernández-Santos M.R., Esteso M.C., Garde J.J. Effects of Thawing Procedure on Postthawed In Vitro Viability and In Vivo Fertility of Red Deer Epididymal Spermatozoa Cryopreserved at −196 °C. J. Androl. 2003;24:746–756. doi: 10.1002/j.1939-4640.2003.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 12.Álvarez M., Tamayo-Canul J., Martínez-Rodríguez C., López-Urueña E., Gomes-Alves S., Anel L., Martínez-Pastor F., de Paz P. Specificity of the Extender Used for Freezing Ram Sperm Depends of the Spermatozoa Source (Ejaculate, Electroejaculate or Epididymis) Anim. Reprod. Sci. 2012;132:145–154. doi: 10.1016/j.anireprosci.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Lone F., Islam R., Khan M., Sofi K. Effect of Different Egg Yolk-Based Extenders on the Quality of Ovine Cauda Epididymal Spermatozoa during Storage at 4 °C: Effect of Extenders on Ovine Epididymal Sperm. Reprod. Domest. Anim. 2012;47:257–262. doi: 10.1111/j.1439-0531.2011.01847.x. [DOI] [PubMed] [Google Scholar]
- 14.Rastegarnia A., Shahverdi A., topraggaleah T.R., Shafiepour V. In Vitro Comparison of Soybean Lecithin-Based Extenders for Cryopreservation of Buffalo (Bubalus Bubalis) Semen. Comp. Clin. Pathol. 2014;23:893–900. doi: 10.1007/s00580-013-1708-6. [DOI] [Google Scholar]
- 15.Abdussamad A.M., Detterer J., Gauly M., Holtz W. Comparison of Various Semen Extenders and Addition of Prostaglandin F2 on Pregnancy Rate in Cows. Animal. 2016;10:655–659. doi: 10.1017/S1751731115002256. [DOI] [PubMed] [Google Scholar]
- 16.Aires V.A., Hinsch K.-D., Mueller-Schloesser F., Bogner K., Mueller-Schloesser S., Hinsch E. In Vitro and in Vivo Comparison of Egg Yolk-Based and Soybean Lecithin-Based Extenders for Cryopreservation of Bovine Semen. Theriogenology. 2003;60:269–279. doi: 10.1016/S0093-691X(02)01369-9. [DOI] [PubMed] [Google Scholar]
- 17.Khatun A., Fazili M.R., Malik A.A., Shah R.A., Khan H.M., Choudhury A.R., Malik A. In Vitro Assessment of Tris Egg Yolk and Soybean Lecithin Based Extenders for Cryopreservation of Crossbred Ram Semen. Cryoletters. 2021;42:73–80. [PubMed] [Google Scholar]
- 18.Murphy E.M., Murphy C., O’Meara C., Dunne G., Eivers B., Lonergan P., Fair S. A Comparison of Semen Diluents on the in Vitro and in Vivo Fertility of Liquid Bull Semen. J. Dairy. Sci. 2017;100:1541–1554. doi: 10.3168/jds.2016-11646. [DOI] [PubMed] [Google Scholar]
- 19.Braga M.Q., Franco R.V.R., Rodrigues L.F., Galeli G., Oliveira K.M., Reis F.A.C., Nishikawa M.F.A., Moura E.P. 110 Comparison Of Andromed®, Bioxcell®, And Botu-Bov® Extenders For Cryopreservation Of Bull Sexed Semen. Reprod. Fertil. Dev. 2007;19:172. doi: 10.1071/RDv19n1Ab110. [DOI] [Google Scholar]
- 20.Crespilho A.M., Sá Filho M.F., Dell’Aqua J.A., Nichi M., Monteiro G.A., Avanzi B.R., Martins A., Papa F.O. Comparison of in Vitro and in Vivo Fertilizing Potential of Bovine Semen Frozen in Egg Yolk or New Lecithin Based Extenders. Livest. Sci. 2012;149:1–6. doi: 10.1016/j.livsci.2012.05.011. [DOI] [Google Scholar]
- 21.Meena G.S., Raina V.S., Gupta T.K., Mohanty, Bishist R. Comparative Performance of Biociphos and Egg Yolk Based Extenders for Buffalo Semen Cryopreservation. Indian. J. Anim. Sci. 2010;80:414–417. [Google Scholar]
- 22.Jiménez-Rabadán P., Ramón M., García-Álvarez O., Maroto-Morales A., del Olmo E., Pérez-Guzmán M.D., Bisbal A., Fernández-Santos M.R., Garde J.J., Soler A.J. Effect of Semen Collection Method (Artificial Vagina vs. Electroejaculation), Extender and Centrifugation on Post-Thaw Sperm Quality of Blanca-Celtibérica Buck Ejaculates. Anim. Reprod. Sci. 2012;132:88–95. doi: 10.1016/j.anireprosci.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Gil J., Lundeheim N., Söderquist L., Rodríguez-Martínez H. Influence of Extender, Temperature, and Addition of Glycerol on Post-Thaw Sperm Parameters in Ram Semen. Theriogenology. 2003;59:1241–1255. doi: 10.1016/S0093-691X(02)01177-9. [DOI] [PubMed] [Google Scholar]
- 24.Fukui Y., Kohno H., Togari T., Hiwasa M., Okabe K. Fertility after Artificial Insemination Using a Soybean-Based Semen Extender in Sheep. J. Reprod. Dev. 2008;54:286–289. doi: 10.1262/jrd.20004. [DOI] [PubMed] [Google Scholar]
- 25.Kulaksiz R., Çebi Ç., Akçay E. The Effect of Different Extenders on the Motility and Morphology of Ram Sperm Frozen or Stored at 4 °C. Turk. J. Vet. Anim. Sci. 2012 doi: 10.3906/vet-1103-11. [DOI] [Google Scholar]
- 26.Akçay E., Kulaksiz R., Daşkin A., Çebi C., Tekin K. The Effect of Different Dilution Rates on Post-Thaw Quality of Ram Semen Frozen in Two Different Egg-Yolk Free Extenders. Slov. Vet. Res. 2012;42:97–102. [Google Scholar]
- 27.Fernandes M., Hernández P.R., Simões J., Barbas J.P. Effects of Three Semen Extenders, Breeding Season Month and Freezing–Thawing Cycle on Spermatozoa Preservation of Portuguese Merino Sheep. Animals. 2021;11:2619. doi: 10.3390/ani11092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ari U., Kulaksiz R., Öztürkler Y. Freezability of Tushin Ram Semen Extended with Goat or Cow Milk Based Extenders: Freezability of Tushin Ram Semen Extended with Goat or Cow Milk. Reprod. Domest. Anim. 2011;46:975–979. doi: 10.1111/j.1439-0531.2011.01769.x. [DOI] [PubMed] [Google Scholar]
- 29.Bohlooli S., Cedden F., PishJang J., Razzaghzadeh S., Bozoğlu S. The Effectof Different Extenders on Post-Thaw Sperm Viability, Motility and Membrane Integrity in Cryopreserved Semen of ZandiRam. J. Basic. Appl. Sci. Res. 2012;2:1120–1123. [Google Scholar]
- 30.Khalifa T., Lymberopoulos A., Theodosiadou E. Association of Soybean-Based Extenders with Field Fertility of Stored Ram (Ovis Aries) Semen: A Randomized Double-Blind Parallel Group Design. Theriogenology. 2013;79:517–527. doi: 10.1016/j.theriogenology.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Sharafi M., Forouzanfar M., Hosseini S.M., Hajian M., Ostadhosseini S., Hosseini L., Abedi P., Nili N., Rahmani H.R., Javaheri A.R., et al. In Vitro Comparison of Soybean Lecithin Based-Extender with Commercially Available Extender for Ram Semen Cryopreservation. Int. J. Fertil. Steril. 2009;3:149–152. doi: 10.22074/IJFS.2009.45788. [DOI] [Google Scholar]
- 32.Emamverdi M., Zhandi M., Shahneh A.Z., Sharafi M., Akhlaghi A., Motlagh M.K., Dadkhah F., Davachi N.D. Flow Cytometric and Microscopic Evaluation of Post-Thawed Ram Semen Cryopreserved in Chemically Defined Home-Made or Commercial Extenders. Anim. Prod. Sci. 2015;55:551. doi: 10.1071/AN13215. [DOI] [Google Scholar]
- 33.Lone F.A., Islam R., Khan M.Z., Sofi K.A. Effect of Transportation Temperature on the Quality of Cauda Epididymal Spermatozoa of Ram. Anim. Reprod. Sci. 2011;123:54–59. doi: 10.1016/j.anireprosci.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Kaabi M., Paz P., Alvarez M., Anel E., Boixo J.C., Rouissi H., Herraez P., Anel L. Effect of Epididymis Handling Conditions on the Quality of Ram Spermatozoa Recovered Post-Mortem. Theriogenology. 2003;60:1249–1259. doi: 10.1016/S0093-691X(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 35.Tamayo-Canul J., Alvarez M., López-Urueña E., Nicolas M., Martinez-Pastor F., Anel E., Anel L., de Paz P. Undiluted or Extended Storage of Ram Epididymal Spermatozoa as Alternatives to Refrigerating the Whole Epididymes. Anim. Reprod. Sci. 2011;126:76–82. doi: 10.1016/j.anireprosci.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed T., Islam R., Lone F.A., Malik A.A. Effect of Washing on the Post-Thaw Quality of Cryopreserved Ram Epididymal Spermatozoa. Vet. World. 2016;9:519–523. doi: 10.14202/vetworld.2016.519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed T. Cryopreservation of Ram Cauda Epididymal Spermatozoa Using Different Buffers and Sugar Combinations. JAR. 2019;9:927–933. doi: 10.30954/2277-940X.06.2019.22. [DOI] [Google Scholar]
- 38.Leahy T., Marti J.I., Mendoza N., Pérez-Pé R., Muiño-Blanco T., Cebrián-Pérez J.A., Evans G., Maxwell W.M.C. High Pre-Freezing Dilution Improves Post-Thaw Function of Ram Spermatozoa. Anim. Reprod. Sci. 2010;119:137–146. doi: 10.1016/j.anireprosci.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 39.D’Alessandro A.G., Martemucci G., Colonna M.A., Bellitti A. Post-Thaw Survival of Ram Spermatozoa and Fertility after Insemination as Affected by Prefreezing Sperm Concentration and Extender Composition. Theriogenology. 2001;55:1159–1170. doi: 10.1016/S0093-691X(01)00474-5. [DOI] [PubMed] [Google Scholar]
- 40.Nascimento J., Raphael C.F., Andrade A.F.C., Alonso M.A., Celeghini E.C.C., Arruda R.P. Effects of Sperm Concentration and Straw Volume on Motion Characteristics and Plasma, Acrosomal, and Mitochondrial Membranes of Equine Cryopreserved Spermatozoa. J. Equine Vet. Sci. 2008;28:351–358. doi: 10.1016/j.jevs.2008.04.010. [DOI] [Google Scholar]
- 41.Maxwell W.M., Johnson L.A. Physiology of Spermatozoa at High Dilution Rates: The Influence of Seminal Plasma. Theriogenology. 1999;52:1353–1362. doi: 10.1016/S0093-691X(99)00222-8. [DOI] [PubMed] [Google Scholar]
- 42.Penitente-Filho J.M., Dias J.C., Oliveira F.A., Silveira C.O., Torres C.A. Correlation between Sperm Motility and Hypoosmotic Swelling Test on Cryopreserved Goat Semen. Magistra Cruz Das Almas. 2017;27:468–472. [Google Scholar]
- 43.Egerszegi I., Sarlos P., Ratky J. Cryopreservation of Epididymal Spermatozoa of Black Racka Rams from Hortobágy-a Possible Means of Gene Preservation. Magyar Állatorvosok Lapja. 2012;134:524–528. [Google Scholar]
- 44.Goovaerts I.G.F., Hoflack G.G., Van Soom A., Dewulf J., Nichi M., de Kruif A., Bols P.E.J. Evaluation of Epididymal Semen Quality Using the Hamilton–Thorne Analyser Indicates Variation between the Two Caudae Epididymides of the Same Bull. Theriogenology. 2006;66:323–330. doi: 10.1016/j.theriogenology.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Bergstein-Galan T.G., Weiss R.R., Kozicki L.E., Bicudo S.D. Sperm Subpopulations in Ejaculated Sperm and Spermatozoa Recovered from Ovine Epididymides up to 48 h after Death. Anim. Reprod. Sci. 2017;187:20–27. doi: 10.1016/j.anireprosci.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Kútvölgyi G., Stefler J., Kovács A. Viability and Acrosome Staining of Stallion Spermatozoa by Chicago Sky Blue and Giemsa. Biotech. Histochem. 2006;81:109–117. doi: 10.1080/10520290600931007. [DOI] [PubMed] [Google Scholar]
- 47.Kovács A., Foote R.H. Viability and Acrosome Staining of Bull, Boar and Rabbit Spermatozoa. Biotech. Histochem. 1992;67:119–124. doi: 10.3109/10520299209110020. [DOI] [PubMed] [Google Scholar]
- 48.Watson P.F. The Causes of Reduced Fertility with Cryopreserved Semen. Anim. Reprod. Sci. 2000;60–61:481–492. doi: 10.1016/S0378-4320(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 49.Salamon S., Maxwell W.M.C. Storage of Ram Semen. Anim. Reprod. Sci. 2000;62:77–111. doi: 10.1016/S0378-4320(00)00155-X. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Horst G. Computer Aided Sperm Analysis (CASA) in Domestic Animals: Current Status, Three D Tracking and Flagellar Analysis. Anim. Reprod. Sci. 2020;220:106350. doi: 10.1016/j.anireprosci.2020.106350. [DOI] [PubMed] [Google Scholar]
- 51.Nagy Á., Polichronopoulos T., Gáspárdy A., Solti L., Cseh S. Correlation between Bull Fertility and Sperm Cell Velocity Parameters Generated by Computer-Assisted Semen Analysis. Acta Vet. Hung. 2015;63:370–381. doi: 10.1556/004.2015.035. [DOI] [PubMed] [Google Scholar]
- 52.Robayo I., Montenegro V., Valdés C., Cox J. CASA Assessment of Kinematic Parameters of Ram Spermatozoa and Their Relationship to Migration Efficiency in Ruminant Cervical Mucus. Reprod. Domest. Anim. 2008;43:393–399. doi: 10.1111/j.1439-0531.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 53.Holt W.V. Basic Aspects of Frozen Storage of Semen. Anim. Reprod. Sci. 2000;62:3–22. doi: 10.1016/S0378-4320(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 54.Sinapov B., Yotov S. Relationship between Some Kinematic Parameters of Ram Semen and Reproductive Performance of Dairy Sheep after Application of Assisted Reproductive Technologies. Int. J. Curr. Microbiol. App. Sci. 2023;12:307–317. doi: 10.20546/ijcmas.2023.1209.030. [DOI] [Google Scholar]
- 55.Ondřej Š., Jiří Š., Jan B., Pavla M., Lucie T., Dole~alová M., Petra F., Ludk S., Radko R. Low Density Lipoprotein - Important Player in Increasing Cryoprotective Efficiency of Soybean Lecithin-Based Bull Semen Extenders. Anim. Reprod. 2019;16:267–276. doi: 10.21451/1984-3143-AR2018-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanga B.M., Qamar A.Y., Raza S., Bang S., Fang X., Yoon K., Cho J. Semen Evaluation: Methodological Advancements in Sperm Quality-Specific Fertility Assessment - A Review. Anim. Biosci. 2021;34:1253–1270. doi: 10.5713/ab.21.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo J.A., Montanero J., Calero R., Roy T.J. Identification of Sperm Subpopulations with Defined Motility Characteristics in Ejaculates from Ile de France Rams. Anim. Reprod. Sci. 2011;129:22–29. doi: 10.1016/j.anireprosci.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Dorado J., Rodríguez I., Hidalgo M. Cryopreservation of Goat Spermatozoa: Comparison of Two Freezing Extenders Based on Post-Thaw Sperm Quality and Fertility Rates after Artificial Insemination. Theriogenology. 2007;68:168–177. doi: 10.1016/j.theriogenology.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 59.Domingo P., Olaciregui M., González N., De Blas I., Gil L. Comparison of Different Semen Extenders and Cryoprotectant Agents to Enhance Cryopreservation of Rabbit Spermatozoa. Czech J. Anim. Sci. 2019;64:59–66. doi: 10.17221/53/2018-CJAS. [DOI] [Google Scholar]
- 60.Henning H., Luther A.-M., Höfner-Schmiing L., Waberski D. Compensability of an Enhanced Incidence of Spermatozoa with Cytoplasmic Droplets in Boar Semen for Use in Artificial Insemination: A Single Cell Approach. Sci. Rep. 2022;12:21833. doi: 10.1038/s41598-022-26020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henning H., Luther A.-M., Waberski D. A High Incidence of Sperm with Cytoplasmic Droplets Affects the Response to Bicarbonate in Preserved Boar Semen. Animals. 2021;11:2570. doi: 10.3390/ani11092570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandal D.K., Kumar M., Tyagi S. Effect of Age on Spermiogram of Holstein Friesian × Sahiwal Crossbred Bulls. Animal. 2010;4:595–603. doi: 10.1017/S1751731109991273. [DOI] [PubMed] [Google Scholar]
- 63.Aziz N., Saleh R.A., Sharma R.K., Lewis-Jones I., Esfandiari N., Thomas A.J., Agarwal A. Novel Association between Sperm Reactive Oxygen Species Production, Sperm Morphological Defects, and the Sperm Deformity Index. Fertil. Steril. 2004;81:349–354. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 64.Fischer K.A., Van Leyen K., Lovercamp K.W., Manandhar G., Sutovsky M., Feng D., Safranski T., Sutovsky P. 15-Lipoxygenase Is a Component of the Mammalian Sperm Cytoplasmic Droplet. Reproduction. 2005;130:213–222. doi: 10.1530/rep.1.00646. [DOI] [PubMed] [Google Scholar]
- 65.Kuster C.E., Hess R.A., Althouse G.C. Immunofluorescence Reveals Ubiquitination of Retained Distal Cytoplasmic Droplets on Ejaculated Porcine Spermatozoa. J. Androl. 2004;25:340–347. doi: 10.1002/j.1939-4640.2004.tb02798.x. [DOI] [PubMed] [Google Scholar]
- 66.Xu H., Yuan S.-Q., Zheng Z.-H., Yan W. The Cytoplasmic Droplet May Be Indicative of Sperm Motility and Normal Spermiogenesis. Asian J. Androl. 2013;15:799–805. doi: 10.1038/aja.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagy S., Házas G., Bali Papp A., Iváncsics J., Szász F., Szász F., Kovács A., Foote R.H. Evaluation of Sperm Tail Membrane Integrity by Light Microscopy. Theriogenology. 1999;52:1153–1159. doi: 10.1016/S0093-691X(99)00207-1. [DOI] [PubMed] [Google Scholar]
- 68.Nagy S., Kovács A., Zubor T., Zomborszky Z., Tóth J., Horn P. Evaluation of Membrane Integrity of Frozen/Thawed Deer Spermatozoa: Short Communication. Acta Vet. Hung. 2001;49:223–227. doi: 10.1556/004.49.2001.2.12. [DOI] [PubMed] [Google Scholar]
- 69.Kútvölgyi G. Ph. D. Thesis. Kaposvári Egyetem; Kaposvár, Hungary: 2013. Development of Qualification of Fresh and Frozen Stallion Semen, Investigation of Factors Affecting Sperm Quality Using a New Evaluation Method [A Friss És Mélyhűtött Ménsperma Minősítésének Fejlesztése, a Sperma Minőségét Befolyásoló Tényezők Vizsgálata Egy Új Bírálati Módszer Alkalmazásával] [DOI] [Google Scholar]
- 70.Turri F., Madeddu M., Gliozzi T.M., Gandini G., Pizzi F. Effect of Testicle Postmortem Storage on Goat Frozen-Thawed Epididymal Sperm Quality as a Tool to Improve Genebanking in Local Breeds. Animal. 2014;8:440–447. doi: 10.1017/S1751731113002279. [DOI] [PubMed] [Google Scholar]
- 71.Bezerra J.A.B., da Silva A.M., Peixoto G.C.X., da Silva M.d.A., Franco de Oliveira M., Silva A.R. Influence of Recovery Method and Centrifugation on Epididymal Sperm from Collared Peccaries (Pecari Tajacu Linnaeus, 1758) Zool. Sci. 2014;31:338–342. doi: 10.2108/zs130149. [DOI] [PubMed] [Google Scholar]
- 72.Tebet J.M., Martins M.I.M., Chirinea V.H., Souza F.F., Campagnol D., Lopes M.D. Cryopreservation Effects on Domestic Cat Epididymal versus Electroejaculated Spermatozoa. Theriogenology. 2006;66:1629–1632. doi: 10.1016/j.theriogenology.2006.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.