Abstract
The antioxidant and anti-inflammatory effects of hormetic nutrition for enhancing stress resilience and overall human health have received much attention. Recently, the gut–brain axis has attracted prominent interest for preventing and therapeutically impacting neuropathologies and gastrointestinal diseases. Polyphenols and polyphenol-combined nanoparticles in synergy with probiotics have shown to improve gut bioavailability and blood–brain barrier (BBB) permeability, thus inhibiting the oxidative stress, metabolic dysfunction and inflammation linked to gut dysbiosis and ultimately the onset and progression of central nervous system (CNS) disorders. In accordance with hormesis, polyphenols display biphasic dose–response effects by activating at a low dose the Nrf2 pathway resulting in the upregulation of antioxidant vitagenes, as in the case of heme oxygenase-1 upregulated by hidrox® or curcumin and sirtuin-1 activated by resveratrol to inhibit reactive oxygen species (ROS) overproduction, microbiota dysfunction and neurotoxic damage. Importantly, modulation of the composition and function of the gut microbiota through polyphenols and/or probiotics enhances the abundance of beneficial bacteria and can prevent and treat Alzheimer’s disease and other neurological disorders. Interestingly, dysregulation of the Nrf2 pathway in the gut and the brain can exacerbate selective susceptibility under neuroinflammatory conditions to CNS disorders due to the high vulnerability of vagal sensory neurons to oxidative stress. Herein, we aimed to discuss hormetic nutrients, including polyphenols and/or probiotics, targeting the Nrf2 pathway and vitagenes for the development of promising neuroprotective and therapeutic strategies to suppress oxidative stress, inflammation and microbiota deregulation, and consequently improve cognitive performance and brain health. In this review, we also explore interactions of the gut–brain axis based on sophisticated and cutting-edge technologies for novel anti-neuroinflammatory approaches and personalized nutritional therapies.
Keywords: Gut–brain axis, inflammation, Nrf2 pathway, hormesis, probiotics, polyphenols, neurological disorders, organoids
1. Introduction
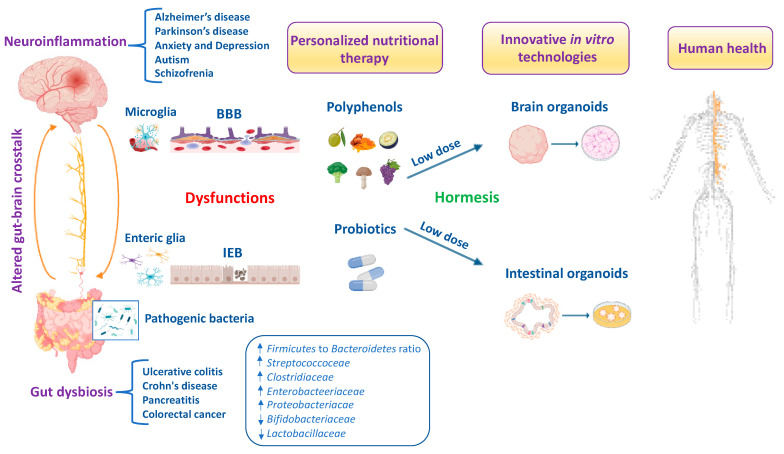
Growing evidence has highlighted the interaction of the gut–brain axis as a critical determinant of human health and/or disease and a key regulator of host physiology, inflammatory responses and redox homeostasis [1]. The high prevalence of gut and brain disorders poses serious public health challenges worldwide [2,3]. Perturbations in gut microbiota composition are characterized by increased oxidative stress, neuroinflammation and progressive neuronal death, leading to the development of brain disorders, particularly Alzheimer’s disease (AD), Parkinson’s disease (PD), schizophrenia, autism spectrum disorder (ASD), depression and anxiety [4,5,6,7,8]. For this reason, the gut microbiota, the complex ecosystem of microorganisms in the gastrointestinal tract, is also called “the second brain” or “the forgotten endocrine organ” [9]. Indeed, an imbalance of the gut microbiota can lead to increased permeability of the intestinal epithelial barrier (IEB) with the release of proinflammatory cytokines and promotion of a neuroinflammatory response [10]. Recently, preclinical evidence suggests that gut dysbiosis is closely associated with increased circulating neurotoxic mediators, such as TNF-α, IL-1β, IL-6 and C-reactive protein, which can directly cause low-grade inflammation predisposing to progressive gastrointestinal and nervous system disorders [11,12]. Furthermore, patients with brain damage and amyloidosis exhibit a perturbated gut microbiome with the abundance of Escherichia/Shigella significantly associated with an increase in proinflammatory cytokine gene profiles and a reduction in the abundance in the anti-inflammatory Eubacterium rectale [13]. It is well established that oxidative stress induces progressive degeneration and neuronal death [14]. Specifically, the imbalance between free-radical production and the efficiency of antioxidant defense pathways triggers neuronal dysfunction and degeneration, being vagal sensory neurons particularly vulnerable to reactive byproducts species of oxidative stress including hydrogen peroxide, 4-hydroxynonenal (4-HNE), malondialdehyde (MDA) and acrolein [15]. It is interesting to note that moderate stress is protective for the organism because it activates adaptive resilience pathways of crucial importance for maintaining cell survival and an optimal quality of life. However, when stress exceeds the intracellular defense capacity, sensory neurons to counteract ROS overproduction activate neuroprotective pathways involving the translocation of antioxidant nuclear factor erythroid 2–related factor 2 (Nrf2) from cytosol into the nucleus and the upregulation of detoxification phase II enzymes, termed vitagenes, particularly heat shock protein 70 (Hsp70), heme oxygenase-1 (HO-1), sirtuin-1 (Sirt1), thioredoxin (Trx)/thioredoxin reductase system, NADPH quinone oxidoreductase 1 (NQO1) and γ-glutamylcysteine synthetase (γ-GCs), which is part of the glutathione redox system to protect against the initiation and progression of neurodegenerative disorders [16,17,18,19,20,21,22]. Likewise, the disequilibrium in the function and composition of the gut microbiota promotes the production of toxic metabolites and proinflammatory cytokines that destroy the IEB with the activation of local and distant immune cells and deregulation of enteric neurons, astrocytes and microglial cells, which ultimately reduces the amount of beneficial substances such as short-chain fatty acids (SCFAs), neurotransmitters such as gamma amino butyric acid (GABA), acetylcholine, serotonin, norepinephrine, histamine and anti-inflammatory lipid mediators such as lipoxin A4 [23,24]. This culminates in a BBB dysfunction that triggers a vicious circle converging in neuroinflammation, and predisposing neuronal and glial cells to apoptotic death, primarily in the cerebral cortex and hippocampus, underlying the development of dementia [25]. Of note, gut dysbiosis associated to the onset and progression of neurodegeneration is characterized by a significative reduction in specific microbial species belonging to the Firmicutes and Bifidobacterium phyla, and an increase in pathogenic species, mainly pro-inflammatory bacteria of the Proteobacteria and Bacteroidetes phyla [26]. Similarly, individuals with neuropsychiatric disorders (i.e., depression, anxiety, schizophrenia and ASD) showed higher levels of Enterobacteriaceae, Alistipes and Clostridium, and lower levels of Faecalibacterium [27,28,29,30]. Therefore, the gut–brain axis is emerging as an attractive target for the discovery of new natural dietary supplements to restore intestinal inflammation due to alterations in gut microbiota bacteria for brain health and cognitive function [31,32]. The human gut metabolome is the result of a complex chemical interplay between commensal microbes and their hosts. Indeed, the gut microbiota produces thousands of metabolites which specifically interact with various bacterial species and strains, dietary interventions (e.g., probiotics and polyphenols) and host molecules, including amyloids and dopamine, which may reach the brain to regulate neurological function. In particular, the gut microbiota produces SCFAs that strengthen the intestinal barrier, stimulate the intestinal L-cell to secrete glucagon-like peptide 1 (GLP-1) and gastrointestinal peptide (GIP) to directly or indirectly inhibit NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation, insulin resistance and ROS [33]. Furthermore, SCFAs stimulate intestinal gluconeogenesis (IGN) in intestinal epithelial cells and, together with GLP-1/GIP, activate the vagus nerve from the enteric nervous system (ENS) and brain-derived neurotrophic factor (BDNF) signaling in the brain, reinforcing the importance of gut and neural signaling networks to promote brain health [33]. Importantly, a meta-analysis study observed that dietary anthocyanins effectively reduced the Firmicutes/Bacteroidetes ratio and increased the content of SCFAs including acetic acid, propionic acid and butyric acid to improve gut health depending on the duration and dosage of treatment [34]. Recent clinical evidence reported that SCFAs levels are altered in the fecal and urine samples of patients with ASD [35], as well as in gut and feces among patients with AD and PD [36]. The interplay between polyphenols and gut–brain axis signaling provides an important tool to modulate gut microbiota composition and the abundance of specific beneficial bacteria for maintaining intestinal barrier integrity and CNS function [37]. Furthermore, the dietary intake of probiotics in adequate doses interferes with potential pathogens alleviating the symptoms not only of inflammatory bowel diseases (IBD) but also of neuropsychiatric and degenerative disorders via the gut–brain axis in experimental models [38] and in patients [39,40]. This review integrates the emerging literature focused on the molecular mechanisms and cellular pathways linking gut microbiota disorders to IEB and BBB dysfunctions, underlying the development of nervous system disorders. Therefore, identifying the mechanisms by which specific vital gut microorganisms and/or dietary natural compounds influence intestinal immune cells or potentially cross the BBB to affect central nervous system cells will help us achieve a deeper comprehension of gut–brain axis crosstalk for human health. Finally, antioxidant and anti-inflammatory signaling pathways activated by hormetic nutrients including polyphenols alone and/or in synergy with probiotics using the innovative organoid technology for microbiota-based neuroprotective and anti-neuroinflammatory therapeutic options are also discussed.
2. Hormetic Nutrition and Redox Resilience Signaling in Gut–Brain Axis
Hormetic nutrition is a novel idea that emerged from the extensive research over the past two decades on the occurrence of hormesis in the biological and biomedical sciences, especially in the areas of food science and nutrition. Hormesis represents the confluence of a wide range of adaptive strategies that mediate the upregulation of several highly co-ordinated series of processes at the cell, organ and organismic levels to protect against endogenous and exogenous stress insults and/or events. The hormesis adaptions are expressed via the occurrence of dose–time responses that are biphasic with a low or moderate dose stimulation and a higher dose inhibition. Interestingly, the low-dosage stimulating responses result in enhanced stress resilience/adaptive capacity via anti-inflammatory and antioxidant molecular networks that promote healthy aging and longevity, prevent the onset or slow the progression of neurodegenerative and chronic gastrointestinal diseases. Thus, the hormetic dose response integrates the responses of both single natural compounds and complex mixtures in their biological responses, showing consistency with the quantitative features of the hormetic pattern in the gut–brain axis. It is important to remember that toxic compounds including ROS, inflammatory cytokines and microbial metabolites are also essential at low levels in the initial phases, as they create minimal stress which is fundamental for the activation of antioxidant pathways and resilience mechanisms promoting gut barrier integrity and neuroprotection. Of relevance, the cellular stress resilience response activated by hormetic nutrients is emerging as a promising preventive and therapeutic strategy to counteract oxidative stress, inflammatory states and gut microbiota dysregulation occurring both in gastrointestinal and brain disorders [41,42]. Specifically, numerous polyphenols, including resveratrol, hidrox®, curcumin, Crocus sativus L., mushrooms and blueberry modulate and upregulate the Nrf2 signaling pathway and stress resilience vitagenes to preserve gut and brain homeostasis during pathological processes [43,44,45,46,47,48]. Several preclinical and clinical studies highlighted that hormetic nutrition with appropriate dosages of antioxidant polyphenols alone and/or in synergistic action with probiotics shows powerful neuroprotective and therapeutic effects by activating the Nrf2 pathway and related vitagenes to reduce gut dysbiosis and attenuate mitochondrial dysfunction, neuroinflammation and cognitive impairment, as well as symptoms of schizophrenia and depression (Figure 1) [49,50,51,52,53,54]. The neuroprotective potential of these natural substances is thought to be mediated via the ENS, neuroendocrine system and vagus nerve, as well as the spinal nerves of the gut [55,56,57,58,59,60,61]. In this scenario, probiotics also refer to “psychobiotics”, candidate species of live symbiotic bacteria that, when ingested in adequate amounts, confer brain health effects to the host for an innovative nutritional approach targeting gut microbiota to treat various neurological and psychiatric disorders [62,63]. Notably, probiotics, such as Lactobacillus paracasei, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum, Lactobacillus helveticus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium breve and Bifidobacterium infantis, improve the central expression of BDNF, N-methyl-D-aspartatic acid (NMDA) receptor and other neuroactive peptides involved in synaptic and neural plasticity to enhance memory, cognition and behavior, and reduce microglial activation in a wide range of neurological illness including anxiety and depression [64,65,66,67].
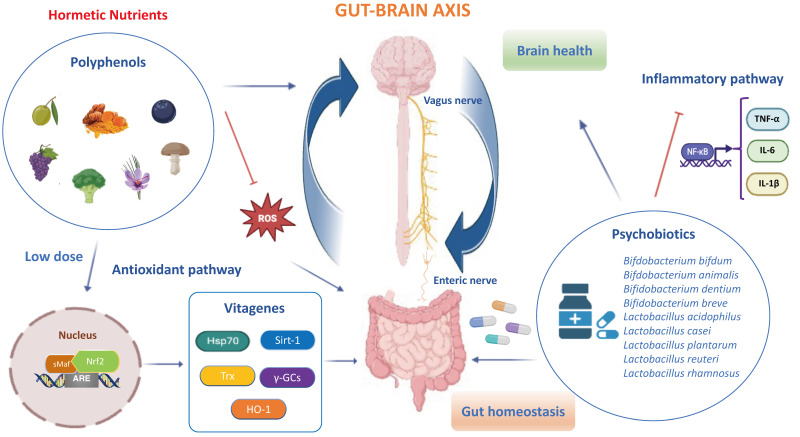
Figure 1.
Hormetic nutrition modulates redox stress resilience vitagenes via gut–brain axis.
A moderate intake of hormetic nutrients including polyphenols and psychobiotics promotes gut and brain healthy effects through the activation of Nrf2 signaling and stress resilience vitagenes [62,68,69]. In particular, polyphenols (i.e., hidrox®, curcumin, sulforaphane, resveratrol, blueberry and mushrooms), in synergy with psychobiotics, particularly the Bifidobacterium and lactobacillus phyla, upregulate antioxidant vitagenes including Hsp70, HO-1, γ-GCs, Sirt1 and Trx to inhibit oxidative stress and inflammatory NF-κB dependent pathways (i.e., TNF-α, IL-6 and IL-1β), leading to gut dysbiosis and the onset of CNS disorders via microbiota–vagus nerve–brain interactions. Therefore, hormetic nutrients can be considered promising therapeutic agents capable of restoring gut homeostasis and brain health during pathological conditions.
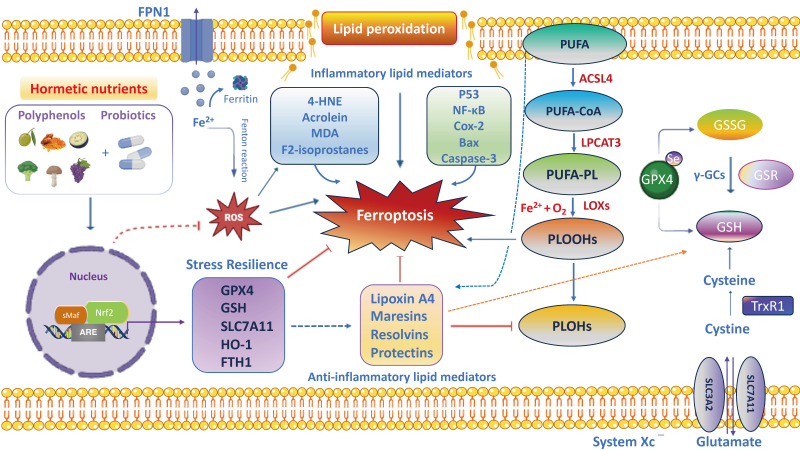
The possibility of adopting natural antioxidant therapies in the prevention and initial phases of intestinal and neuronal disorders has been documented as being able to restore or delay the subsequent phases or to achieve remission [61,63]. Therefore, hormetic nutrition is a new evolving field in its application to gut and brain health, in both preventative and restorative study models. Accordingly, hormesis establishes that a low dose of polyphenols and/or polyphenol-combined nanoparticles, in synergy with probiotics, may modulate multiple antioxidant avenues, including Nrf2 and cellular phase II antioxidant enzymes (e.g., superoxide dismutase, catalase, glutathione peroxidases, thioredoxins/thioredoxin reductases, peroxiredoxins) and non-enzymatic antioxidant molecules (e.g., glutathione, hydrogen sulfide, carnosine, coenzyme Q and bilirubin), ultimately acting as neuroprotective and therapeutic agents [55]. In fact, the moderate consumption of natural compounds exerts gut and brain healthy effects by promoting the activation of stress resilience vitagenes and the inhibition of NF-κB-dependent pro-inflammatory pathways [53,56]. This postulates antioxidant vitagenes as a promising target for anti-neurodegenerative approaches based on the use of polyphenols in order to induce powerful neuroprotective and anti-neuroinflammatory effects in synergy with probiotics of clinical relevance, as a potential option to drugs, or the enhancement of classical neuro- and gastrointestinal therapies [58]. However, in the brain, a high dose of polyphenols can produce neurotoxicity related to the increased production of ROS and inflammatory markers with subsequent neuronal apoptosis or depletion of cellular resilience pathways [60]. The bidirectional crosstalk between the intestinal microflora and the brain occurs through a variety of pathways, including the vagus nerve, immune system, metabolic and neuroendocrine pathways and bacteria-derived metabolites. Compelling evidence suggests a critical role of gut dysbiosis-derived neuroinflammation in exacerbating the onset of neurodegenerative and neuropsychiatric disorders, opening up novel directions to explore gut–brain crosstalk and its underlying molecular mechanisms. It is noteworthy that systemic inflammation triggered by bacterial products in the blood stream may stimulate a strong production of pro-inflammatory cytokines by cells from the innate and the adaptive immune system, which can spread through the blood, promote the permeabilization of the BBB and reach the brain. Accordingly, CD4+ T-cells, when stimulated, release high levels of NF-κB, chemokines and inflammatory cytokines (e.g., TNF-α, IL-1β, IFN-γ and IL-6) and microglial activation markers (e.g., glial fibrillary acidic protein (GFAP), Sox-10 and TLRs), which in turn produce increased levels of glutamate, TNF-α and reactive oxygen and nitrogen species (ROS/RNS), inducing neuronal death and the further generation of oxidized and nitrated proteins, thereby indicating a tight association between neurodegenerative disorders and gut inflammation [70]. In this scenario, the nutritional therapeutic approach using hormetic nutrients, including polyphenols, in synergy with probiotics is attracting considerable interest in the scientific community to prevent and treat inflammation associated to pathophysiological changes in the diversity of the gut microbiota leading to nervous system disorders along the gut–brain axis [71,72]. Of particular interest, probiotics in combination with polyphenols induce a pharmacokinetic resilience to peripheral inflammation that ultimately protects against brain neuroinflammation by modifying immune cell recruitment to potentially preserve BBB integrity and prevent cognitive dysfunction under chronic stress conditions [73]. Importantly, microbial-derived metabolites modulate the intestinal immune response via the activation of aryl hydrocarbon receptor (AHR) to promote resilience to stress-induced psychiatric disorders by evoking adaptive changes [74]. Notably, it has been documented that a grape-derived prebiotic, in synergistic combination with the probiotics Lactobacillus plantarum and Bifidobacterium longum, acts as a ligand to the AHR on antigen-presenting cells or directly on the naïve CD4+ T cells, impacting their response to stress and ultimately leading to a reduction in the proportions of the Treg and Th17 lymphocytes in the periphery, in particular in the ileum and liver. The resilience response to inflammation in the periphery ultimately protects against neuroinflammation in the brain (i.e., pre-frontal cortex and hippocampus) by altering the recruitment of immune cells and potentially protecting BBB integrity in gut–brain axis disorders [74]. In addition, magnolol, a lignan found in Magnolia officinalis at a dose of 10 mg/kg, effectively increased the serum levels of tryptophan metabolites including kynurenic acid, 5-hydroxyindoleacetic acid, indole-3-acetic acid, indolelactic acid and indoxylsulfuric acid, which are endogenous AHR ligands to reduce pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and attenuate dextran-induced colitis in rodents [75]. Recent preclinical research described curcumin as capable of suppressing the activation of the TLR4/NF-κB/STAT pathway that regulates the expression of pro-inflammatory genes [71]. In addition, a treatment with curcumin nanoparticles significantly decreased the mucosal mRNA expression of TNF-α, IL-1β, IL-6, CXCL1 and CXCL2 in colonic epithelial tissues [76]. Overall, according to hormesis, the dose is a crucial determinant in promoting gastro- and neuroprotective or harmful effects of nutritional therapies, in particular with polyphenols alone and/or in synergistic combination with probiotics; therefore, this must be carefully assessed.
2.1. Resveratrol
The 3,5,4-trihydroxy-trans-stilbene also known as resveratrol is a natural polyphenolic compound produced by various plants in response to injury or pathogens such as bacteria or fungi. Food sources of resveratrol include the skin of grapes, berries and red wine [77]. It promotes numerous pharmacological properties including antioxidant, anti-inflammatory, anti-aging and anti-carcinogenic effects, gut microbiota homeostasis, neuroprotection, xenohormetic effects and resilience to stress. In congruence with this, pre-clinical evidence has shown that resveratrol is neuroprotective via modulating the intestinal flora and gut–brain axis dose-dependently [78]. Interestingly, gut bacteria and, in particular, Bifidobacterium infantis and Lactobacillus acidophilus, convert the glycosidic form, resveratrol-3-O-β-d glucoside, also called piceid, into the bioactive form, trans-resveratrol [79,80]. Resveratrol and its derivative pterostilbene have been shown to protect against intestinal morphological alterations, increased gut permeability, redox imbalance and dysbiosis. In particular, the molecular mechanism of action of resveratrol on the gut microbiota is its ability to act on duodenal-mucosal Sirt1 and, thus, improve insulin sensitivity and lower hepatic glucose levels [81]. In addition to its action on duodenal mucosa, resveratrol improves hypothalamic insulin sensitivity [81] and mitochondrial biogenesis [82], upregulating Sirt1 via the gut–brain axis. Furthermore, a study conducted by Chen et al. showed that a resveratrol-supplemented diet (300 mg/kg) modulates the microbiota by inhibiting the abundance of Escherichia and Actinobacillus and increasing the growth of Bacteroides, Lactobacillus and Bifidobacterium after 2 weeks in piglets [83]. Furthermore, resveratrol regulates the gut microbiota by activating anti-inflammatory lipid mediators including lipoxin A4, resolvins, protectins and maresins [84]. Intriguingly, resveratrol confers neuroprotection via modulating intestinal immune dysfunction. Specifically, the health effects induced by resveratrol in the brain are attributed to promoting the Th1/Th2 balance towards Th2 polarization and skewing the Treg/Th17 balance towards Treg in the SI-LP and subsequently reducing small intestinal pro-inflammatory cytokines (IL-17A, IL-23, IL-10, IFN-γ and IL-4) and intestinal vascular permeability via attenuating the circulating levels of inflammatory cytokines from the small intestine and alleviating cytokines-mediated BBB disruption and neuroinflammation in mice with a focal cerebral ischemia/reperfusion injury [85]. Of equal importance, resveratrol regulates the balance of neurotransmitters such as BDNF and serotonin 5-hydroxytryptamine (5-HT) via the GLP-1 pathway and the activation of Sirt1 and Foxo genes in the intestine and CNS [86]. Indeed, several authors demonstrated that resveratrol exhibits anxiolytic and antidepressant effects similar to drugs in a range from 10 mg/kg to 80 mg/kg per day in a dose-dependent manner, upregulating both ERK and BDNF, as well as antioxidant markers (i.e., SOD and catalase), and downregulating inflammatory markers in animal models [87,88,89]. Unfortunately, few clinical trials have evaluated the health-promoting effects of resveratrol in gut–brain axis disorders. A recent study of ten participants with a mild decline in cognition demonstrated that 72 g of grape consumption for a 6-month period was related to a marked protection from longitudinal changes in brain metabolism and cognitive function, with an amelioration in attention and working memory performance [90]. In addition, another human study of 12 healthy volunteers observed interindividual differences in trans-resveratrol metabolism closely related to the microbial diversity in feces samples. In particular, the study showed that an oral dose of 0.5 mg of trans-resveratrol induced lower abundances of Firmicutes and higher abundances of Bacteroidetes, Actinobacteria (Bifidobacteriaceae and Coriobacteriaceae), Verrucomicrobia and Cyanobacteria. The authors identified two new resveratrol metabolites, 3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxybibenzyl (lunularin), produced by intestinal bacteria [91]. Furthermore, a recent double-blind, placebo-controlled randomized clinical study on sixty-two participants demonstrated that resveratrol at a dosage of 250 mg twice per day in synergy with risperidone reduced hyperactivity and secondary deficits such as social withdrawal, stereotypic behavior and inappropriate speech in patients with ASD compared to a placebo group [92]. Lastly, a randomized, placebo-controlled, double-blind, multicenter 52-week phase 2 study demonstrated that resveratrol at an oral dose of 500 mg once daily was able to permeate the BBB, acting on the CNS and increasing brain volume loss in patients with mild to moderate AD [93].
2.2. Curcumin
Curcumin is a polyphenolic compound present in Curcuma longa Linn rhizome (Zingiberaceae). The recent interest in its therapeutic potential derives from numerous biological effects including antioxidant [94], anti-inflammatory [95], gut microbiota homeostasis [96] and neuroprotection [97] in a dose-dependent manner. Emerging preclinical and clinical literature reported the health-promoting effects of curcumin in gut and brain disorders [98,99]. In the gut microbiota, a treatment of curcumin significantly altered the ratio between beneficial/pathogenic bacterial strains by increasing the growth of Bifidobacteria and Lactobacilli and reducing the loads of Prevotellaceae, Coriobacterales, Enterobacteria and Enterococci [100]. Indeed, a dose of 500 mg of curcumin and boswellia extracts significantly improved bloating and abdominal pain symptoms in patients suffering from irritable bowel syndrome (IBS) and small bowel dysbiosis after 30 days of supplementation [101]. In the CNS, curcumin provided neuroprotection, ameliorating motor deficits, the aggregation of α-synuclein and microglial activation with a remarkable abundance of Lactobacillacea and Lachnospiraceae, increasing the serum levels of methionine, tyrosine, sarcosine and creatine, and depleting the growth of Aerococcaceae and Staphylococcaceae in a mouse model of PD [97]. In particular, the neuroprotection induced by curcumin resulted in the upregulation of redox sensory genes such as HO-1 and NQO1 via the Nrf2-ARE pathway [102]. Moreover, low doses (20 and 40 mg/kg) of curcumin decreased the sensitivity of the intestinal tract-produced antidepressant- and anti-anxiety-like effects by elevating serotonin, BDNF and p-CREB levels in the hippocampus in a rat model of IBS [103]. In humans, a randomized controlled study conducted by Wynn et al. demonstrated the effectiveness of curcumin capsules at a dose of 360 mg/day (twice daily as an oral dose) in ameliorating BDNF levels and enhancing cognitive performance in patients with schizophrenia after 8 weeks [104]. In addition, a randomized, double-blind trial of 28 participants proved that oral supplementation of curcumin (2.5 g) for three months was able to decrease uremic toxins such as p-cresyl sulfate plasma levels in hemodialysis patients, modulating the gut microbiota [105]. Collectively, curcumin could represent a potential therapeutic option against brain disorders through the regulation of intestinal dysbiosis, thus improving the abundance of beneficial bacteria and maintaining a proper gut–brain axis without any apparent toxicity.
2.3. Blueberry
Blueberries are polyphenolic compounds rich in anthocyanins, fiber and sugars with powerful antioxidant and anti-inflammatory properties capable of modulating the gut microbiota and brain function in a biphasic dose response manner. It is noteworthy that blueberry positively influences gut microbiota composition, exhibiting antimicrobial and antiadhesion properties against pathogenic bacteria and ultimately contributing to brain health [106,107]. Accordingly, recent preclinical evidence reported that the anthocyanin-rich extract obtained from blueberries at a dose of 30 mg/day (i) reduced neuroinflammation and gut dysbiosis by modulating gut microbiota composition, especially increasing the abundance of Lactobacillales and decreasing the Clostridiales population; (ii) enhanced serotonin levels in the cerebral prefrontal cortex and intestine; and (iii) reversed the synaptic dysfunction in rodent models of autism-like behaviors [106]. In addition, a dose of 300 mg/kg per day of polyphenol-rich blueberry–mulberry extract significantly changed the gut microbiota by enhancing the abundance of Lactobacillus, Streptococcus and Lactococcus, and decreasing the abundance of Blautia and Allobaculum. This was positively correlated with an increase in beneficial metabolites, such as α-linolenic acid, vanillic acid and N-acetylserotonin, to alleviate inflammation and cognitive impairment along the gut–brain axis in aged mice after 6 weeks of treatment [107]. Furthermore, low doses (6%) of blueberry anthocyanin-rich extracts attenuate high-fat diet-induced oxidative stress and improve hippocampal status, as well as neuronal function, through an increase in antioxidant enzymes (i.e., SOD and GSH-Px). Notably, the modulation of the antioxidant pathway stimulated probiotics (Bifidobacterium and Lactobacillus) and SCFAs producers (Roseburia, Faecalibaculum and Parabacteroides), improving the colon environment in C57BL/6 mice [108,109]. Human studies showed that the consumption of a wild blueberry powder beverage in men increased live Bifidobacterium activity after six weeks [110]. Moreover, a pilot study conducted on 17 women reported that a low dose of 38 g of polyphenol-rich fractions purified from blueberry (i.e., anthocyanins/flavonol glycosides, sugar/acid fraction, proanthocyanidins and total polyphenols) and/or prebiotic mix modulated the fecal microbiota composition of healthy adults and was correlated with an increase in the antioxidant activity in blood. The study observed that the fecal microbiota fermented with total polyphenols and sugar/acid fraction supplementation exhibited a higher abundance of Enterobacteriaceae and Bifidobacteriaceae, while anthocyanins/flavonol glycosides supplementation and prebiotic mix produced a significant reduction in Escherichia/Shigella (Enterobacteriaceae) and an increase in the Lachnospiraceae and Bacteroidaceae families [111]. In addition, a daily supplementation with blueberry powder (0.5 c) whole-fruit equivalent improved cognitive performance in middle-aged patients with insulin resistance after 12 weeks [112]. Similarly, supplementation with blueberry powder at a dosage of 12.5 g per packet enhanced the brain response and memory function in older adults with MCI and a risk of dementia [113]. Furthermore, a double-blind, parallel randomized controlled trial conducted in 61 healthy older adults supplemented with 26 g of freeze-dried wild blueberry showed significant improvements in vascular and cognitive function, as well as in the gut microbiota composition, by increasing beneficial bacteria such as Ruminiclostridium and Christensellenacea [114]. Another randomized, placebo-controlled clinical study reported that blueberry intake (25 g/day) during a period of 18 days markedly decreased the plasma concentrations of pro-inflammatory 9,10-, 12,13-dihydroxy-9Z-octadecenoic acids (diHOMEs), increased anti-inflammatory docosahexaenoic acid (DHA)- and eicosapentaenoic acid (EPA)-generated hydroxydocosahexaenoic acids and specialized pro-resolving oxylipins after intense exercise in untrained adults compared to a placebo [115]. Finally, a recent double-blind, randomized, cross-over study reported that 30 g of highbush freeze-dried blueberry powder produced from equal proportions of Tifblue® and Rubel® varieties (equivalent to 180 g fresh blueberries) significantly attenuated abdominal symptoms and ameliorated general markers of well-being, quality of life and life functioning compared to placebo in patients with gastrointestinal disorders after six weeks of treatment [116].
2.4. Hidrox®
Hidrox® (HD) is a freeze–dried powder extract from the aqueous fraction of olives obtained from defatted olive pulps, during the processing of Olea europaea L. after olive oil extraction [117]. An amount of 12% HD extract contains polyphenols with high antioxidant potential. The most abundant polyphenol in HD is hydroxytyrosol (40–50%), while 5–10% contains oleuropein, approximately 20% oleuropein aglycone and gallic acid and 0.3% tyrosol [118]. Recent preclinical evidence demonstrated that HD possesses significant antioxidant and anti-neuroinflammatory effects by upregulating the Nrf2 pathway and vitagenes and downregulating NF-κB signaling to prevent or delay the neurodegenerative process characteristic of AD and PD [119,120]. Furthermore, other recent evidence showed that low doses (10 mg/kg) of HD modulate oxidative stress and neuroinflammation through a significant reduction in proinflammatory mediators, such as IL-1β, IL-6 and TNF-α, and a significant induction of the Nrf2/HO-1 pathway by restoring GSH, SOD and catalase levels in the bladder and spinal cord of rodent models [121]. In addition, studies on Caenorhabditis elegans used as model of PD have reported that low doses of 250 μg of HD display healthy effects by improving lifespan and stress resistance, mainly exerting a neuroprotective action by reducing neurotoxic misfolded α-synuclein aggregates in dopaminergic neurons [122,123]. Clinical research documented the health effects of hydroxytyrosol on cognitive function. Notably, a randomized, double-blind, placebo-controlled, parallel-group study on 72 subjects showed that 3 g twice daily of desert olive tree pearls (DOTPs) containing 162 times more polyphenol hydroxytyrosol than olive oil, improved cognitive function (memory, attention, reaction time and executive function) in middle-aged and older adults [124]. Furthermore, in the intestine, hydroxytyrosol supplementation exerts anti-inflammatory effects in dextran sodium sulfate-induced ulcerative colitis by augmenting the colonic Nrf2 pathway, inhibiting NLRP3 inflammasome activation (interleukin-18 and interleukin-1β levels) and modulating gut microbiota in rodents [125,126]. It is interesting to note that an oral administration of 100 mg/kg body weight HT modulated colonic gut microbiota by increasing the number of Firmicutes and Lactobacillus and reducing the relative amount of Bacteroidetes. The gut beneficial effects were correlated with the activities of serum Nrf2 antioxidant enzymes (i.e., SOD, GSH-Px, catalase and NQO1) in the small intestine of mice [127]. Finally, another randomized, controlled, double-blind, crossover human study of 12 participants showed that the ingestion of virgin olive oil (25 mL/day) containing a mixture of olive oil and thyme phenolic compounds (500 mg) determined changes in the gut microbiota by increasing Bifidobacteria populations, decreasing blood-LDL in hypercholesterolemic patients after 3 weeks [128]. Overall, hidrox® can be taken into consideration as a novel approach of therapeutic intervention for intestinal and brain disorders as it leads to changes in gut beneficial bacteria and ultimately enhances the cognitive function and quality of life in humans.
2.5. Crocus sativus L.
Crocus sativus L., widely known as saffron, is a perennial plant belonging to the Iridaceae family. Commonly, it is cultivated in Iran, Spain, Morocco, Turkey, India, Greece and Italy [129]. The flowers comprise six purple tepals where both petals and sepals are fused; moreover, they present a white style surrounded by three yellow stamens and a red stigma separated into three threads. Saffron performs several pharmacological activities such as antioxidant [130], anti-inflammatory [131], hepatoprotective [132], neuroprotective [133], antidepressant [134] and anti-carcinogenic activity [135], and maintains gut microbiota balance [136]. Recent literature documented the heathy effects of saffron in gut–brain axis disorders. Notably, it has been documented that there are significant antibacterial dose-response effects of saffron polyphenols in the reduction of Lactobacillus and Clostridium species in the cecal microbiome in vivo [136]. The major bioactive molecules of saffron include crocin (C44H64O24), crocetin (C20H24O4), picrocrocin (C16H26O7) and safranal (C10H14O). Interestingly, a study conducted by Khodir et al. showed the anti-inflammatory and anti-oxidant activity of crocin at a low dose of 20 mg/kg orally via the upregulation of Nrf2/HO-1 signaling and the downregulation of caspase-3 activity in the colon of rats [137]. Furthermore, safranal inhibited gut tissue damage induced by dextran sodium sulfate, ROS and intestinal epithelial cell death, showing significant protective effects in maintaining intestinal homeostasis in drosophila [138]. Furthermore, a recent study demonstrated that a new herbal formulation containing Edgeworthia gardneri (Wall.) Meisn., Sibiraea angustata and Crocus sativus L., at a total dose of 26 g, improved hyperglycaemia and modulated gut dysbiosis by reducing lipopolysaccharide (LPS) levels; the same study also reported a dose-dependent decrease in circulating levels of IL-6 and TNF-α along with an increase in the amount of Proteobacteria and Actinobacteria and a reduction in the Firmicutes/Bacteroidetes ratio in diabetic fatty rats after 6 weeks [139]. In the brain, saffron constituents have been shown to be neuroprotective by upregulating Nrf2 signaling and stress resilience vitagenes in numerous brain disorders, including neurodegenerative and neuropsychiatric pathologies, in a hormetic dose response manner [133]. In line with this, saffron (40 mg/kg) was synergistically associated with endurance exercise, increased BDNF, serotonin and muscular neurotrophin-3 mRNA levels in the hippocampus of rats [140]. Furthermore, the neuroprotective efficacy of saffron tea infusion (90 mg styles/200 mL) against aflatoxin B1-induced neurotoxic damage in terms of enhancing learning and memory performance and cholinergic activity, as well as reducing monoaminergic and oxidative markers in the brain of rodent models after 2 weeks, has also been demonstrated [141]. Further evidence revealed that a low dose of 30 mg/kg via oral gavage of crocin improved unpredictable chronic mild stress-induced anxiety and depression through a downregulation of brain oxidative stress, cortical malondialdehyde, inflammatory mediators (i.e., TNF-α and IL-6) and corticosterone serum levels after 4 weeks in rats [142]. Finally, a clinical trial conducted by Kell and coworkers on 128 participants with low mood showed that affron® at a dosage of 28 mg per day for 4 weeks improved mood, anxiety and stress management without side effects compared to a placebo group [143]. Overall, the data suggest that saffron and its active compounds show an excellent safety profile and could represent potential candidates for the prevention and treatment of gut and brain disorders.
2.6. Polyphenols of Nutritional Mushrooms
The recent literature has shown great attention to the beneficial effects of nutritional mushroom polyphenols contained in Hericium erinaceus and Coriolus versicolor for treating gut microbiota dysbiosis and cognitive damage [144,145]. Notably, extensive evidence documented that the polyphenols of HE and CV inhibit neuroinflammation and oxidative stress to prevent and/or slow the onset of major neurodegenerative diseases, including AD and PD [55,146,147], and psychiatric disorders such as ASD [148].
2.6.1. Hericium erinaceus
Hericium erinaceus (HE) is a medicinal mushroom containing several bioactive constituents with anti-cancer, anti-inflammatory, anti-oxidative and neuroprotective properties [55,148,149]. In particular, HE contains erinacines and hericerin that induce a neurotrophic effect by stimulating the nerve growth factor (NGF) biosynthesis responsible for the survival, growth and differentiation of neurons [150]. Interestingly, HE also contains a great number of polyphenols acting as strong antioxidants for the inhibition of tyrosinase and free radical scavenging [151]. Preclinical evidence showed that HE can be used as a prebiotic to regulate the gut microbial community in animal models [152]. In particular, it has been demonstrated that an intake with 0.8 g of HE for 16 weeks can regulate the gut microbial community including the pathogenic intestinal genera Campylobacter, Streptococcus and Tyzzerella implicated in inflammation and obesity in aged dogs [152]. Moreover, the supplementation of HE improved gut microbiota composition, producing SCFAs both in rats with ulcerative colitis [153] and in healthy adults [154] to enhance the gut barrier function. In the brain, HE attenuated p-tau and amyloid-β deposition while in the intestine it improved gut microbiota diversity by promoting the growth of SCFAs-producing bacteria and by suppressing the abundance of Helicobacter. In the same study, HE was also shown to enhance the activation of superoxide dismutase, catalase and glutathione peroxidase, and inhibit the secretion of malondialdehyde and 4-hydroxynonenal, confirming that in APP/PS1 mice this mushroom promotes neuroprotective effects via upregulation of the Nrf2 pathway [155]. Furthermore, HE influenced the composition of the intestinal microbiota, particularly increasing the relative abundance of beneficial bacteria such as Lachnospiraceae, Ruminococcaceae and Akkermansiaceae, and reducing the microbial population of Muribaculaceae, Rikenellaceae, Lactobacillaceae and Bacteroidaceae, promoting the immunomodulatory effects via the NF-κB, MAPK and PI3K/Akt pathways in vitro and in experimental animal models after 28 days [156]. In addition, HE mycelium-derived polysaccharide supplementation at a dose of 500 mg/kg per day markedly improved the nutritional status and decreased the incidence of diarrhea and intestinal inflammation through an increase in Lactobacillus reuteri and a reduction in Streptococcus lutetiensis in cynomolgus monkey used as a model of ulcerative colitis after 4 weeks [157]. In addition, other authors also demonstrated that a new polysaccharide isolated from HE (HEP10) suppressed the LPS/DSS-induced production of iNOS and COX-2 and proinflammatory mediators such as TNF-α, IL-6 and IL-1β, as well as NLRP3 inflammasome, in rodent models of colitis and in macrophages. Furthermore, HEP10 reversed the dextran sulfate sodium (DSS)-induced changes in both composition and structure of intestinal bacteria by reducing the Proteobacteria phylum and increasing Akkermansia muciniphila dose-dependently [158]. Finally, a recent pilot trial conducted by Xie et al. on 13 healthy individuals observed that 3 g/day of HE powder taken as a food supplement modulated gut microbiota composition and serum biochemical parameters. Notably, the study found that HE significantly upregulated beneficial bacteria, such as Bifidobacterium and Bacteroidetes, as well as SCFAs-producing bacteria, such as Roseburia and Faecalibacterium, and downregulated pathobionts, such as Escherichia_Shigella, Streptococcus thermophilus, Bacteroides caccae and Anaerostipes hadrus, in blood and fecal samples [159].
2.6.2. Coriolus versicolor
Coriolus versicolor (CV) is another nutritional mushroom that acts as a prebiotic to modulate the intestinal microbiome composition [160] and brain function [60]. Recent evidence reported the anti-inflammatory and antioxidant properties of CV in experimental models [161,162,163]. In line with this, it has been shown that CV at a dose of 200 mg/kg induced antioxidant and anti-inflammatory effects, upregulating the Nrf2/HO1 pathway and downregulating the Toll-like receptors 4 (TLR4)/NF-kB cascade in a murine model of colitis [161]. Interestingly, the synergistic combination of HE and CV at a dose of 200 mg/kg orally for 28 days upregulated antioxidant Nrf2/HO-1 redox signaling and increased anti-inflammatory lipoxin A4 to inhibit the neuroinflammation (NF-κB pathway) and apoptosis of dopaminergic neurons in a rodent model of PD [162] and TBI [147]. In addition, a very recent clinical study by our research group demonstrated that a CV treatment for 6 months at a moderate dosage promoted neuroprotective effects by downregulating α-synuclein and NF-κB-mediated pro-inflammatory cytokines and upregulating the Nrf2 pathway and stress resilience of vitagenes to counteract oxidative damage, particularly of protein carbonyls and 4-hydroxynonenal (4-HNE), in the lymphocytes of patients with Meniere’s disease and at increased risk of developing neurodegenerative disorders [163]. Finally, more recent evidence has shown that CV improves gut dysbiosis, predominantly suppressing Clostridium (belonging to Firmicutes) and increasing the Bacteroides phylum both in the serum and cecal contents [164].
3. Allostasis and Resilience in Gut and Brain
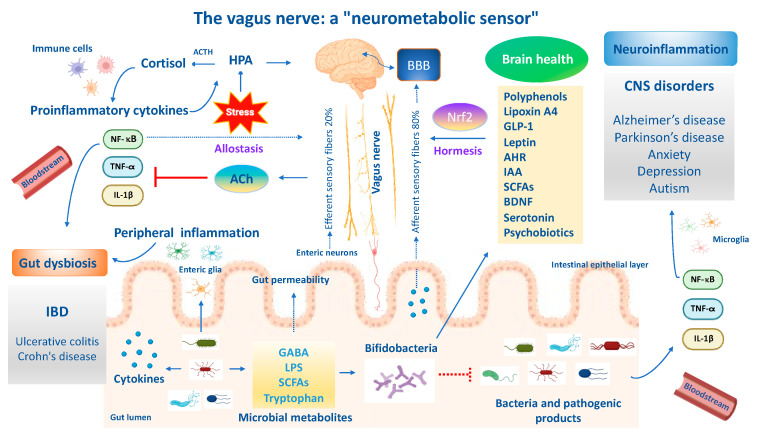
Stress adaption to environmental challenges is also defined as “allostasis”. The concept of allostasis emerges as a new view of stress and resilience to it, specifically referring to the process that maintains cellular homeostasis and drives other physiological changes in response to environmental perturbations [165]. Allostasis responses are not considered negative but reflect the fact that stress adaptive responses are crucial for survival, healthspan and lifespan extension [165]. The gut and brain are central organs of oxidant stress and resilience/adaptation to stress (allostasis), but they also contribute to pathophysiology (‘‘allostatic load/overload’’) when they are overused or perturbated. Brain resilience denotes the capacity of an organism to cope to neuronal insults (e.g., neurotoxicity, neuroinflammation and oxidative stress) or psychological perturbations through the activation of allostasis mechanisms and neuronal mediators to promote stress adaptation and mental health. In line with this, the neuronal mediators of stress adaptation implicated in gut and brain homeostasis—such as HPA; cortisol; the autonomic nervous system and vagus nerve; metabolic hormones and immune system mediators; neurotrophins, such as BDNF; endogenous neurotransmitters such as GABA, glutamate, acetylcholine and serotonin; cellular stress response mediators such as Nrf2 and vitagenes; and anti-inflammatory lipid mediators (lipoxin A4)—promote adaptation to several stressors (e.g., oxidative stress, inflammation and mitochondrial disfunction), but the same mediators display biphasic effects and can also participate in pathophysiology when they are dysregulated or overused with respect to their physiological balanced network (allostasis load and overload) [166]. Therefore, acute stress, also defined as “eustress”, is beneficial in the short term since it enhances the adaptive capabilities of an organism and increases the antioxidant resilience responses, hormesis and cross-tolerance mechanisms, and is considered an example of allostasis; however, in the long term it turns into chronic stress, also called “distress”, and is maladaptive, leading to a dysregulated Nrf2 pathway and predisposing one to the onset and progression of central nervous system disorders [167]. For instance, proinflammatory cytokines can modulate the production of corticosteroids, which in turn can suppress inflammatory cytokine production. Likewise, the sympathetic and parasympathetic systems exert different effects on inflammatory cytokines, with the former stimulating their production and the latter inhibiting them. It is important to emphasize that allostasis and allostatic load and overload apply to the gut–brain axis [168]. An optimal gut equilibrium due to the amounts of beneficial bacteria and their metabolites promotes functional neural activity that drives synaptic plasticity, mediated in part by the vagus nerve and systemic hormones, but also by endogenous excitatory and inhibitory neurotransmitters, amino acids, neurotrophic factors and other mediators. Changes in how such adaptive mediators respond likely explain the changes in the vulnerability of the gut microbiome to environmental stressors such as proinflammatory cytokines produced by pathogenic bacteria, leading to intestinal dysbiosis or favoring mental illnesses including AD, PD, depression and autism. Indeed, the gut and brain are constantly adapting to a changing environment. This is the very essence of allostatic load/overload applied to the gut and brain [168]. Therefore, allostatic overload results in dysregulated HPA axis regulation, elevated chemokines and inflammatory cytokines, reduced synaptic plasticity and persistently activated enteric microglia, culminating in a dysbiotic gut microbiota. The functional crosstalk between the gut–vagal sensory neurons–brain axis is also implicated in allostasis for stress adaptation and resilience [168]. Several independent pathways and bioactive molecules contribute to the gut–brain axis bidirectional signaling. These pathways include proinflammatory mediators, metabolic signaling, oxidative markers, stress modulators, dietary nutrients, neuroendocrine factors and a direct neuronal crosstalk via the vagus nerve [169,170]. Inflammatory processes protecting the body from injury or invasion also impose an allostatic load and the vagus nerve mediates the inflammatory reflex. Notably, afferent vagus sensory fibers sense peripheral inflammatory cytokines produced by gut bacteria and convey signals to the brain to generate an adaptive or maladaptive response. The latter triggers a neuroinflammatory cascade and dysregulated BBB, culminating in impaired cognitive function. On the other hand, inflammatory signals activate the hypothalamus via vagal afferents, triggering cholinergic vagal efferent fibers to suppress the release of local and serum proinflammatory cytokines and macrophages, restoring the control mechanisms of the adaptive anti-inflammatory reflex [171]. Importantly, afferents and efferent vagal sensory fibers in allostasis are implicated in modulating the potentially detrimental effects of allostatic load/overload for gut and brain health. Importantly, allostatic load is a cumulative measure of physiological dysregulation and is influenced by multiple factors including nutrition. Emerging evidence reported that berries intake and their bioactive polyphenols alleviate stress by modulating the BDNF and HPA axis in the hippocampus, which in turn reduces neuroinflammation and attenuates allostasis load scores, ultimately improving resilience and potentially reducing the severity of stress-related disorders [172]. Similarly, probiotics are known to affect the composition and function of microbiota for the maintenance of allostasis adaptations, ultimately promoting brain resilience in the context of environmental stress challenges related to perturbations of gut microbial diversity (dysbiosis), leading to systemic inflammation/neuroinflammation [170]. In the same way, the ingestion of a strain of Lactobacillus in mice has been shown to attenuate stress-induced neurogenic skin inflammation (allostasis) and the inhibition of hair growth along the skin–gut–brain axis [173]. Overall, the antioxidant nutritional approach in synergy with psychobiotics targeting crucial pathways and neuronal mediators including the Nrf2 pathway to modify the composition of the gut microbiota and stimulate vagal sensory neurons could provide an effective alternative treatment paradigm to promote allostasis and resilience during stressful challenges in gut–brain axis disorders.
4. The Role of Epigenomics and Nutrition in Gut–Brain Axis
Epigenomics has grown rapidly over the past two decades due to its importance in numerous physiological and pathophysiological processes, including gut–brain axis disorders [174]. Epigenetics consists of DNA methylations, histone modifications and the dysregulation of micro-RNAs in order to regulate the accessibility of specific DNA regions to transcription factors and allow them to adapt the genomic expression to the environmental changes [175]. Emerging evidence demonstrated that epigenetics mediates gene–environment interactions and contributes to the vulnerability of neurodegenerative and psychiatric disorders such as depression and anxiety, schizophrenia and autism [176]. Accordingly, studies documented epigenetic aberrations such as DNA methylations, histone modifications or miRNAs dysregulations found in the postmortem brain or blood cells of patients with psychotic or autism-spectrum disorders tightly linked to the pathogenesis [177]. Epigenetics plays a crucial role in regulating host physiology by altering the metabolic activity of the intestinal microbiome, which depends on the environment and nutrition. Interestingly, gut microbiota bacteria synthesize and modulate their hosts to produce metabolites, such as SCFAs [178], tryptophan and indole derivatives [179] and neurotransmitters including GABA, glutamate, acetylcholine, dopamine, norepinephrine [180], Nrf2 and polyphenols [181]. The epigenetic interactions between the gut–brain axis via several bacterial-produced metabolites and cytokines and their ability to cross the BBB allow them to explicate their neuroactive properties that are potentially implicated in mental health and/or disease. Importantly, SCFAs are important mediators by which bacteria affect the host epigenome and can inhibit the activity of histone deacetylases (HDACs), resulting in chromatin changes generally associated with increased histone acetylation of non-coding target genes [182]. Among SCFAs, butyrate and propionate produced by commensal microbials induced the differentiation of Treg cells and ameliorated intestinal inflammation and the development of colitis via an enhancement of histone H3 acetylation in the promoter of naïve T cells and expression of the Foxp3 gene in vitro and in vivo [183]. Of relevance, changes in DNA methylation have been ascribed to an increase in pro-inflammatory cells, in particular promoters of IL-6 and IL-1β, as well as promotors of chemokine and chemokine receptors, such as CXCL13 and CXCR3, with significantly hypomethylated and elevated levels of neutrophils found in stools of children affected by ASD specifically associated with dysbiosis, an inflammatory state and gut permeability features [184]. Nowadays, nutritional therapy through polyphenols and/or probiotics that interact with DNA, also referred to as “nutriepigenomics”, represents a complementary and alternative approach in gut and nervous system disorders, demonstrating positive effects in subjects with epigenetic variations and drug resistance [185].
4.1. Tryptophan, Kynurenine and Indole Pathways
Tryptophan metabolism along the serotonin, kynurenine and indole pathways can be directly influenced by gut microbials. Some bacteria strains (i.e., Escherichia coli, Clostridium sp. and Bacteroides sp.) harbor a tryptophanase enzyme responsible for the conversion of tryptophan into indole and its derivatives, such as indole-3-aldehyde (IAld), indole-3-acetic-acid (IAA) and indole-3-propionic acid, which can give rise to a wide range of neuroactive signaling molecules. Additionally, tryptophan can be metabolized into 5-HT via aromatic amino acid decarboxylase (AAAD) activity, or kynurenine by the enzymes tryptophan-2,3-dioxygenase (TDO) or the ubiquitous indoleamine-2,3-dioxygenase (IDO). Lipopolysaccharides (LPS), an inflammatory cell wall component from Gram-negative bacteria, can induce the expression of IDO, increasing the conversion of tryptophan to kynurenine. The latter, produced from the periphery, is also a potent agonist of AHR capable of crossing the BBB to regulate the intestinal immune system [186]. On the other hand, circulating SCFAs, such as butyrate, can directly modulate central kynurenine pathways. Specifically, butyrate has been demonstrated to inhibit IDO activity and to modulate the kynurenine pathway in a STAT1/HDAC-dependent manner [187]. In addition, in stressed mice, the production of H2O2 by Lactobacillus strains, such as Lactobacillus reuteri, can be protective against the development of despair behaviors by directly inhibiting intestinal indolamine 2,3-dioxygenase 1 (IDO1) expression and decreasing the circulating kynurenine levels [187]. Conversely, the reduction in Firmicutes and the subsequent decrease in SCFAs synthesis observed in MDD patients has been linked to increased inflammation, and cytokines are also known to promote tryptophan utilization for kynurenine synthesis via IDO activity [188]. This pathway gives rise to the neurotoxic metabolite quinolinic acid that crosses the BBB to reach the CNS and reduces central serotonergic availability [189]. Clinical research showed that children with ASD exhibit an altered tryptophan metabolism, with reduced levels of tryptophan and an increased kynurenine to tryptophan ratio in the plasma [190], suggesting a shift in the tryptophan metabolism from serotonin synthesis to the kynurenine pathway. Furthermore, in these ASD children, a correlation was observed between altered concentrations of tryptophan and serotonin with gut dysbiosis thorough a significant decrease in Dorea and Blautia, as well as Sutterella, and an increase in Clostridiales associated with the severity of the symptoms [190]. Probiotic species belonging to Lactobacillus and Bifidobacterium may shift the host tryptophan metabolism by suppressing the kynurenine pathway. Furthermore, Lactobacillus are reported to be able to degrade tryptophan into indole compounds, such as IAld, ILA and IAA [191]. In addition, the administration of Lactobacillus rhamnosus leads to the remodeling of the DNA methylation code at the BDNF and Tph1A promoter genes in the gut and brain of zebrafish after 28 days [192]. Recent evidence reported that indole-3-lactic acid can regulate gut homeostasis. Notably, an Escherichia coli strain isolated from the feces of tinidazole-treated individuals showed that indole-3-lactic acid reduced the susceptibility of mice to dextran sulfate sodium-induced colitis by inhibiting the production of epithelial CCL2/7, thereby reducing the accumulation of inflammatory macrophages in vitro and in vivo [193]. Similarly, the supplementation of polyphenols extracted from Fu brick tea (post-fermented tea) promoted the transformation by gut microbiota bacteria of tryptophan into indole-3-acetic acid attenuating colitis, immune cells infiltration and inflammatory cytokines release through a direct enhancement of AHR-mediated protection in rodents, dose-dependently [194]. The dietary enrichment of fibers from oat and rye brans enhanced the production of SCFAs, leading to improved gut integrity, reduced liver inflammation and the expression of genes related to tryptophan metabolism, in particular, tryptophan hydroxylase 1 (TPH-1) mRNA activity and enhanced indole propionic acid production in mice [195] and in humans [196].
4.2. γ-Aminobutyric Acid
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter of the CNS. It is produced by various bacteria, including Bacteroides, Bifidobacterium, Lactobacillus and Escherichia spp., and this involves ENS homeostasis and disturbance, such as acid secretion, bowel motion, gastric emptying and abdominal pain [197]. Alterations in GABAergic gene promotors, such as GAD2, NPY and SST, involves H3K4 methylation as well as histone modifications leading to prefrontal dysfunction and the development of schizophrenia and autism [198]. Probiotic therapy with L. rhamnosus (JB-1) reduced GABA(Aα2) mRNA expression in the prefrontal cortex and amygdala, but increased GABA(Aα2) in the hippocampus, inhibiting stress-induced corticosterone and anxiety- and depression-related behavior in a rodent model via the vagus nerve [199]. Translational studies showed that L. reuteri PBS072 and B. breve BB077 are potential probiotic candidates for improving stress resilience, cognitive functions and sleep quality through the inhibition of the epigenetic enzyme LSD1, promotion of GABA and expression of serotonin [200]. Furthermore, probiotic-fermented buckwheat significantly increased the contents of GABA, rutin, total polyphenols and total flavonoids by improving oxidative stress and chronic inflammation, reversing the high-fat diet-induced intestinal dysbiosis through a reduction in the ratio of Firmicutes and Bacteroidetes and improving the abundance of SCFA-producing bacteria, such as Bacteroides, Lactobacillus and Blautia, in murine models of dyslipidemia [201]. Finally, a prenatal treatment with resveratrol-activating histone deacetylase Sirt1 prevented epigenetic alterations caused by valproic acid in the GABA receptor and synaptic proteins and the expansion of initial damage resulting in the maintenance of the neuronal composition in the medial prefrontal cortex and hippocampus in a rat model of autism [202].
4.3. Hypothalamic–Pituitary–Adrenal Axis
Epigenetic modifications are involved in neurophysiological changes, the inflammatory response, hypothalamic–pituitary–adrenal (HPA) axis activity and glucocorticoid receptor resistance, and they increase the risk for the development of metabolic and mental disorders [203]. Preclinical genetic evidence demonstrated that prenatal stress induces manic behavior in female offspring rats accompanied by hyperactivity of the HPA axis, epigenetic adaptations in the activity of HDAC and DNMT, and acetylation in the histones H3K9 and H3K14, as well as increased levels of adrenocorticotropic hormone (ACTH) [204]. Glucocorticoid receptor (NR3C1) is an important target for epigenetic regulation in the HPA axis. Importantly, decreased expression of the glucocorticoid receptor (NR3C1) gene, which is also susceptible to epigenetic modulation, is a strong indicator of impaired HPA axis control and function. Accordingly, recent evidence demonstrated that epigenetic changes driving the reprogramming of NR3C1 expression and regulation contribute to the aberrant expression profile of genes, particularly variations in FKBP5 underlying the HPA axis impairment which likely decreases resilience to the adaptive stress response and increases susceptibility to psychiatric disorders and suicide in teenagers [205]. In addition, epigenetic variations of the NR3C1 gene have also been associated with early-life experiences in both animals and humans. Studies of early experiences in rats showed that DNA methylation at CpG islands on the NR3C1 promoter in specific regions of the brain was altered by maternal care, resulting in NR3C1 expression and HPA responses to stress [206]. In humans, stressful life events (e.g., trauma and abuse) have been correlated with higher DNA methylation at the NR3C1 promoter region and biological markers of HPA axis activity, such as salivary cortisol [207]. The gut microbial balance and/or a modified diet through probiotics supplementation and polyphenols may prevent or restore epigenetic alterations (e.g., proinflammatory genes in autism) [208]. Notably, children with autism and LPS-exposed rat model of autism exhibited lower SCFA concentrations and an overactivation of the HPA axis. Notably, SCFA-producing bacteria, such as Lactobacillus, might be the key differential microbiota between the control and LPS-exposed offspring [208]. Interestingly, a sodium butyrate treatment contributed to regulate the HPA axis, in particular corticosterone and corticotropin-releasing hormone receptor 2 expressions, ultimately improving anxiety and social deficit behaviors in autism-like rats [209]. Finally, a recent study demonstrated that a polyphenols-enriched diet, such as with quercetin (20 mg/day), xanthohumol (10 mg/day) and phlorotannins (20 mg/day), ameliorated the dysregulation of the HPA axis and monoamine neurotransmitters (i.e., dopamine and 5-hydroxyindoloacetic acid) and this was correlated with marked changes in bacterial composition and diversity, with a significant increase in Enterorhabdus, Asteroplasma, Lachnospiraceae and Coprococcus, ultimately reversing depression in animal models of early-life stress caused by maternal deprivation [210].
5. Polyphenol Nanoparticle Delivery Systems in Gut and Brain Disorders
Recently, several scientists have highlighted the importance of novel polyphenol nanoparticle delivery systems to enhance the bioavailability and stability of circulating polyphenols and ultimately preserve gut and brain health [211,212].
5.1. Polyphenol Nanoparticles for Brain Health
Nowadays, nanotechnology increasingly represents a promising approach for the delivery of therapeutic agents into the CNS, in particular polyphenol nanoparticles capable of improving the overall bioavailability and therefore diffusing more efficiently across the BBB to enhance brain delivery [213]. Preclinical evidence demonstrated that an intravenous injection of curcumin loaded with T807-modified nanoparticles at a dosage of 5 mg/kg was capable of effectively crossing the BBB by enhancing its permeation into the brain. Notably, curcumin loaded with T807-modified nanoparticles displayed a high binding affinity to the hyperphosphorylated form of tau protein in neurons by reducing its levels and inhibiting neuronal cell death to attenuate AD progression both in vitro and in vivo [214]. Moreover, other authors observed the neuroprotective potential of curcumin-loaded lipid-core nanocapsules in a murine model of AD. The results of this study showed that a low dosage of 10 or 1 mg/kg p.o. of curcumin-loaded lipid-core nanocapsules for 14 days provided higher neuroprotection than the high dose of 50 mg/kg p.o. of free curcumin by reducing levels of Aβ1-42-induced proinflammatory circulating cytokines, such as TNF-α, IL-6, IL-1β and IFN-γ, in the prefrontal cortex, hippocampus and serum of aged mice [215]. In addition, Sadegh et al. documented the low doses (4 mg/kg) of curcumin-nanostructured lipid carriers passing through the BBB and targeting oxidative stress markers (ADP/ATP ratio, lipid peroxidation and ROS formation) in the hippocampal tissue, resulting in enhanced learning and memory performance in vitro and in AD rats [216,217]. Moreover, curcumin loaded with chitosan and bovine serum albumin nanoparticles at a dosage of 100 μg/mL effectively increased the drug penetration across the BBB, promoted the phagocytosis of the Aβ peptide and further supported the activation of microglia by repressing TLR4-MAPK/NF-κB signaling and M1 macrophage polarization in vitro [218]. In particular, selenium nanoparticles raised the number of beneficial bacteria including Bifidobacterium, Dubosiella, Desulfovibrio and Gordonibacter to inhibit the Aβ aggregate-induced neurotoxicity, downregulating the expression of NLRP3 inflammasome and the inflammatory cytokine secretion nitrite (NO), interleukin-6 (IL-6), IL-1β and TNF-α, leading to neuroinflammation and death in vitro and in vivo models of AD [211]. A recent clinical study evaluated the effects of daily oral use of curcumin dispersed with colloidal nanoparticles (named Theracurmin®), a highly bioavailable form of curcumin, on memory performance. This study demonstrated that Theracurmin® containing 90 mg of curcumin taken twice daily improved both the memory and attention in non-demented adults, and this was strongly associated with reductions in amyloid plaque and tau accumulation in the hypothalamus and amygdala after 18 months of treatment without any toxic effects [219].
5.2. Polyphenol-Nanoparticles for Gut Health
In the intestine, oral treatment (0.2%) with curcumin nanoparticles through the inhibition of NF-κB activation repressed mucosal inflammation and increased butyrate-producing bacteria, Clostridium cluster IV and XIVa, with an elevation of fecal butyrate levels and an expansion of regulatory T cells (Tregs) in the colonic mucosa in experimental animal models of IBD [220]. Notably, the prebiotic effects of oral polyphenol nanoparticles in modulating gut microbiota and brain function relieving anxiety- and depression-like behaviors and cognitive impairment in a mouse model of acute colitis have been also reported [218]. Specifically, an oral treatment (dose of 1 mg mL−1, 200 μL) of polyphenol armored with TNFα–small interfering RNA and gallic acid-mediated graphene quantum dot (GAGQD)-encapsulated bovine serum albumin nanoparticle, with a chitosan and tannin acid multilayer, prolonged the residence time in the colon and regulated gut microbial homeostasis by increasing Lactobacillus and inhibiting the expression of GABA receptors via gut–brain axis crosstalk, consequently alleviating neuroinflammation and improving the mood and cognitive recovery of IBD model mice [221]. Furthermore, it has been shown by Li et al. that an oral treatment of TGN-Res@SeNPs nanocomposites effectively improved AD by inhibiting Aβ aggregation and deposition in the hippocampus, decreasing ROS and enhancing antioxidant enzyme activities, leading to a downregulation of the neuroinflammatory cascade, particularly the NFκB/MAPK/Akt signaling pathway, and ultimately alleviating gut microbiota disorders by decreasing proinflammatory gut pathogens such as Alistipes, Helicobacter, Rikenella, Desulfovibrio and Faecalibaculum in cells and rodents [222]. Interestingly, the health effects of selenium nanoparticles coated with dihydromyricetin (DMY), a natural polyphenol extracted from grapevine tea (Tg-CS/DMY@SeNPs), in modulating gut microbiota diversity have been proven. Despite numerous preclinical studies, unfortunately, clinical trials investigating the potential effects of polyphenol nanoparticle delivery systems in the prevention and treatment of gut disorders are lacking [223,224]. A recent clinical study demonstrated that a treatment with Theracurmin® (360 mg/day) for 3 months was effective and safe in patients with Crohn’s disease, and is capable of ameliorating symptoms [225]. Finally, a placebo-controlled trial revealed that an oral dose of 500 mg/tablet of curcumin–phospholipid supplementation (Meriva®) taken twice daily decreased plasma pro-inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1), IFN-γ and IL-4, and lipid peroxidation by modulating gut microbiota composition through a reduction in the bacterial community of Enterobacter and Escherichia-Shigella, and a significant increase in the relative abundance of Lachnoclostridium, Lactobacillaceae and Prevotellaceae after 6 months in subjects with chronic kidney disease [224]. Taken together, the data indicate that polyphenol-based nanoparticles could represent natural candidates for innovative therapeutic purposes in gastrointestinal and brain disorders as they are more bioavailable and stable in the intestine, and are capable of modulating the gut microbiota and crossing the BBB with potential pharmacological effects at lower doses than polyphenols alone.
6. The Probiotics for Gut and Brain Health
Gut–brain crosstalk influences neural, endocrine and immune pathways. Probiotics activating signaling molecules involved in the cellular stress response could represent an innovative strategy to counteract oxidative stress in gut–brain axis disorders. Recent evidence has documented that changes in the microbiota due to probiotic consumption can modify resilience to stress in vitro, in vivo and in humans [71,226,227,228].
6.1. Neuropsychiatric Disorders
Emerging evidence suggests the potential therapeutic benefits of psychobiotics acting through the gut–brain axis to affect neuronal development, function and behavior, representing a novel approach for mental health in neuropsychiatric disorders including anxiety and depression, ASD and schizophrenia [229]. In line with this, Hao et al. demonstrated that Faecalibacterium prausnitzii (ATCC 27766) exhibited psychobiotic effects by increasing cecal SCFAs and plasma IL-10 levels, and decreasing corticosterone and IL-6 levels in rats with anxiety and depression-like behaviors caused by chronic unpredictable mild stress [230]. Furthermore, Bifidobacterium breve CCFM1025 significantly inhibited depression- and anxiety-like behaviors by mitigating HPA axis hyperactivity and inflammation, upregulating BDNF levels and downregulating c-Fos levels, raising the serotonergic system in the gut and brain of chronic stressed mice after 5 weeks [231].
6.1.1. Depression and Anxiety
Recent evidence reported that Lactobacillus gasseri NK109 alleviated colitis and gut dysbiosis, leading to psychiatric disorders such as depression and memory deficits induced by exposure to Escherichia coli K1 by inhibiting neuroinflammatory NF-κB signaling and IL-1β expression and increasing BDNF levels in the hippocampus of mice via the gut–vagus nerve–brain axis [232]. Similarly, the oral administration of Lactobacillus reuteri NK33 in synergistic combination with Bifidobacterium adolescentis NK98 at a dosage of 1 × 109 colony forming unit (CFU)/day significantly inhibited NF-κB activation, IL-6 expression and LPS levels, and induced hippocampal BDNF expression and CREB phosphorylation, alleviating anxiety and depression symptoms by suppressing gut dysbiosis through the inhibition of Proteobacteria in cells and in rodents [233]. In addition, the oral administration of Lactococcus lactis (1 × 109 CFU mL−1) attenuated anxiety and depressive-like behaviors in response to chronic unpredictable mild stress with the improvement of 5-hydroxytryptamine (5-HT) metabolism in serum and colon via the modulation of gut microbiome composition after 5 weeks in vivo [234]. Numerous clinical studies highlighted the protective and therapeutic role of neuroactive probiotics in reducing the inflammatory cascade and kynurenine levels and increasing BDNF expression via a functional crosstalk between the gut–brain axis in major depressive disorders [235,236]. Indeed, the SANGUT study conducted in 120 patients with depression for 12 weeks demonstrated that a gluten-free diet and the synergistic combination of psychobiotics, particularly Lactobacillus helveticus and Bifidobacterium longum at a dosage of 3 × 109 CFU, inhibited the immune–inflammatory cascade and improved both psychiatric symptoms and gut barrier integrity [237]. In addition, a recent randomized, double-blind, placebo-controlled study demonstrated in 63 participants with chronic stress that the intake of Lactiplantibacillus plantarum HEAL9 significantly reduced the plasma concentrations of two pro-inflammatory markers (soluble fractalkine and CD163) in subjects exposed to acute stress compared to placebo after four weeks [238]. Interestingly, gut inflammation induced by pathogenic bacteria and their products can directly reach the brain via the systemic circulation, triggering neuroinflammation with the activation of a cytokine cascade and the release of TNF-α, IL-6 and IL-1β, which in turn influenced various neuronal processes and promote depressive-like behaviors along the gut–vagus nerve–brain axis. Notably, gut microbiota inflammation triggers an alteration of tryptophan–kynurenine metabolism and the formation of neurotoxic quinolinic acid in the brain, resulting in mental health problems including depressive and schizophrenia symptoms [186]. Accordingly, a double-blind, randomized, placebo-controlled study conducted on 60 patients with depressive and anxiety symptoms showed that the probiotic bacteria Lactobacillus Plantarum 299v decreased plasma kynurenine levels, improving cognitive performance and function compared to the placebo [235]. Moreover, depressed patients had an altered fecal microbiota composition, with increased abundance of Enterobacteriaceae and Alistipes and reduced levels of Faecalibacterium compared to controls [27]. In addition, a randomized, double-blind, placebo-controlled clinical trial of 40 participants with a diagnosis of major depressive disorder (MDD) showed that probiotic capsules containing strains of Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g) and Bifidobacterium bifidum (2 × 109 CFU/g) increased antioxidant biomarkers including plasma GSH levels and decreased serum high-sensitivity C-reactive protein (hs-CRP) and insulin levels compared to the placebo after 8 weeks [239]. Lastly, a recent randomized placebo-controlled trial of 82 patients with depression documented that the probiotic OMNi-BiOTiC® Stress Repair plus biotin with at least 7.5 billion organisms per 1 portion (3 g) increased beneficial bacteria including Ruminococcus gauvreauii and Coprococcus in the gut and upregulated vitamin B6/B7 metabolism, improving psychiatric symptoms compared to the placebo after 28 days of treatment [240].
6.1.2. Autism
Importantly, a randomized controlled study demonstrated in children with ASD, anxiety and gastrointestinal symptoms that a probiotic formulation (Vivomixx®) containing 450 billion lyophilized bacterial cells belonging to Lactobacillus and Bifidobacterium taken twice a day exerted health effects not only in reducing gut dysbiosis but also in improving language and cognitive functions, as well as neurotransmission and connectivity attenuating inflammatory markers (TNF-α, IL-6) plasminogen activator inhibitor-1 (PAI-1) and chemical pollutants (phthalates) in plasma and urine after 6 months [241]. Another randomized, double-blinded, placebo-controlled pilot trial revealed that a daily intake of a Lactobacillus plantarum PS128 probiotic (6 × 1010 CFU) in combination with oxytocin reduced serum inflammatory markers such as IL-1 β with positive socio-behavioral symptoms related to the increase of Eubacterium hallii in ASD patients after 28 weeks [242].
6.2. Neurodegenerative Disorders
Recently, probiotic supplements have been considered to improve cognitive function via the gut–brain axis in major neurodegenerative disorders including AD and PD [39,243]. Preclinical evidence demonstrated that a new formulation of lactic acid bacteria and bifidobacteria (SLAB51) at a dosage of 200 bn bacteria/Kg/day improved the cognitive function and plasma concentration of gut hormones such as ghrelin, leptin, GLP1 and GIP, as well as reduced pro-inflammatory cytokines, by modulating the gut microbiota composition with an increase in Bifidobacterium spp. and a reduction in Campylobacterales in 3xTg-AD mice after 24 weeks [244]. The same authors also observed that probiotic SLAB51 showed antioxidant effects, upregulating the Sirt1 pathway, which in turn reduced ROS production and preserved brain redox homeostasis in 3xTg-AD mice after 16 weeks [245]. Furthermore, Ma et al. demonstrated that a synergistic treatment with Lactobacillus mucosae NK41 and Bifidobacterium longum NK46 promoted anti-inflammatory effects by inhibiting LPS levels, NF-κB activation and TNF-α expression, resulting in an increase in BDNF expression, modulating the gut microbiota composition by enhancing the population of Bacteroides including Odoribactericeae and reducing Firmicutes and Proteobacteria phyla, as well as suppressing Aβ amyloid accumulation in the hippocampus of 5xFAD mice [246]. Numerous clinical trials reported that a daily intake of probiotics reduces oxidative stress, gut dysbiosis, neuroinflammatory cytokines and cognitive impairment in patients with AD and PD [247,248,249]. In congruence with this, a recent study observed that probiotic supplementation with kefir at the minimum dose of 2 ml/kg for 90 days influenced CNS function via the gut–brain axis, impacting positively on brain function (ameliorating memory, language, visual-spatial function, executive functions, conceptualization and abstraction abilities), systemic pro-inflammatory cytokines (downregulating IL-1, IL-6, IL-8, IL-12 and TNF-α), systemic antioxidant enzymes (upregulating GSH, GPx and SOD) and DNA repair/apoptosis (enhancing PARP-1 and p53 expression) in elderly patients with AD [247]. Recent randomized clinical controlled studies have demonstrated that the probiotic formulation containing Lactobacillus acidophilus, Bifidobacterium bifidum and Bifidobacterium longum (2 × 109 CFU/day each) co-supplemented with selenium (200 μg/day) significantly improved cognitive function in AD patients [248] and the movement in PD patients [249] via a reduction in C-reactive protein and malondialdehyde, and an enhancement of antioxidant GSH after 12 weeks. In addition, a randomized study performed by Aljumaah et al. demonstrated that Lactobacillus rhamnosus GG reduced the relative abundances of Prevotella ruminicola and Bacteroides thetaiotaomicron, improving cognitive dysfunction in patients with MCI and promoting healthy brain aging in an elderly population [250]. Another recent randomized, double-blind placebo-controlled study conducted on 130 patients with MCI observed that a daily intake of Bifidobacterium breve MCC1274 (2 × 1010 CFU) for 24 weeks changed the gut microbiota composition by preventing the brain atrophy progression that ultimately improved the cognitive function in older patients with MCI [251]. Taken together, data from the emerging literature highlight that the gut microbiota and brain communicate with each other via the gut–brain axis, and this bidirectional crosstalk implicates that bacteria-derived metabolites can have a positive or negative impact on the CNS, leading to brain health and/or the onset of neurodegenerative disorders. Therefore, a proper dose of probiotics could represent great promise for the treatment of dementia in humans, as they interfere with a range of metabolic and antioxidant signaling pathways involved in the maintenance of cellular resilience homeostasis, which is of crucial importance for neural cells’ survival and the quality complex of human life.
7. The Vagus Nerve a “Neurometabolic Sensor” of Gut–Brain Axis
The gut–brain axis involves the autonomic nervous system (ANS), including the parasympathetic nervous system, that is, the vagus nerve (VN), originating from the cranial parasympathetic nucleus and innervates numerous structures and organs such as the heart and gastrointestinal tract. The VN is the longest nerve of the organism and a key neural pathway between the gut and the brain, containing 80% and 20% of afferent and efferent fibers, respectively [252]. It plays an essential role in interoceptive awareness. Notably, the VN acts as a “neurometabolic sensor” because it is able to sense the microbiota metabolites through its afferent fibers to transmit this information from the gut to the brain, where it is then integrated in the central autonomic network to generate an adequate or maladaptive response. The latter could perpetuate a pathological state of the digestive tract or could favor psychiatric and neurodegenerative disorders, as well as gastrointestinal diseases [253,254]. Under physiological conditions, the VN is involved in gut and brain homeostasis because it senses the “milieu intérieur” of the gut through the interaction of nutrients and/or gut peptides (i.e., cholecystokinin, GLP-1, leptin and serotonin), with vagal afferents boosting the responses to maintain neurological and gastrointestinal health status [255]. The VN possesses anti-inflammatory properties by regulating gut dysbiosis due to peripheral cytokines release through its vagal afferent fibers and the activation of the HPA axis, which in turn stimulates the secretion of cortisol by the adrenal glands, whereas through vagal efferent fibers it induces a vagovagal reflex called the cholinergic anti-inflammatory pathway [256]. The cholinergic anti-inflammatory pathway is mediated by enteric neurons that innervate intestinal lamina propria to stimulate the release of acetylcholine (ACh) at the synaptic junction, which, via binding to the α-7-nicotinic ACh receptors (α7nAChR) of macrophages, ultimately inhibits the release of TNFα, IL-1β and IL-6 [256] (Figure 2). Perturbations of vagal homeostasis due to oxidative stress trigger a neuropathological cascade with the release of pro-inflammatory cytokines and glucocorticoids that act on vagal receptors and the microbiota, leading to dysbiosis and the onset of gastrointestinal disorders, ultimately promoting brain damage [257].
Figure 2.
Schematic overview of the vagus nerve as “neurometabolic sensor” of the gut–brain axis. The vagus nerve is a “neurometabolic sensor” of the gut–brain axis, since it senses microbiota metabolites, including SCFAs, LPS, tryptophan and GABA, generated by pathogenic bacteria, as well as those produced by beneficial bacteria such as Bifidobacteria through its afferent fibers, to transfer this gut information to the brain, where it is integrated into the central autonomic network and then generates an adapted or inappropriate response. The abundance of pathogenic bacteria implicates a shift towards a pro-inflammatory state in the gut, yielding increased gut permeability and the subsequent triggering of a peripheral inflammatory response, which in turn impacts vagal sensory neurons through its afferent fibers, culminating in neuroinflammation and impaired cognitive function. Elevated inflammatory cytokines in the bloodstream and stress activate the HPA axis through ACTH, leading to cortisol secretion which affects the immune cells and contributes to cytokines release. On the other hand, the efferent sensory fibers activate the cholinergic anti-inflammatory pathway that stimulates the release of acetylcholine (ACh) to inhibit the secretion of proinflammatory cytokines such as TNFα, Il-1β and Il-6. At the appropriate dose, hormetic nutrients, especially polyphenols in synergy with psychobiotics, as well as Bifidobacteria produced-metabolites such as lipoxin A4, GLP-1, leptin, SCFAs, BDNF, IAA, AHR and serotonin targeting the Nrf2 pathway, can directly stimulate the vagus nerve through its afferent sensory fibers to attenuate or reverse the pathophysiological process, converging in the onset of IBD and central nervous system disorders in order to restore gut and brain homeostasis to environmental challenges (hormesis/allostasis response). IBD: inflammatory bowel diseases, CNS: central nervous system, AHR: aryl hydrocarbon receptor, IAA: indole-3-acetic acid, LPS: lipopolysaccharide, HPA axis: hypotalamic pituitary-adrenal axis, ACTH: adrenocorticotropic hormone, SCFA: short-chain fatty acids, GABA: γ-aminobutyric acid.
The stimulation of vagal afferent fibers in the gut increases the transmission of the monoaminergic brain system, especially norepinephrine, serotonin and dopamine, and recently it has emerged as an interesting nondrug therapy not only for the treatment of gastrointestinal and neurodegenerative disorders, such as IBD and PD, but also of major psychiatric conditions, such as depression and anxiety disorders, in which gut–brain crosstalk is dysfunctional [257,258]. More recently, effective natural therapeutic options, in particular, dietary supplementation with polyphenols and/or probiotics targeting antioxidant pathways to enhance the vagal tone and inhibit cytokine production in both neurological and gut disorders via VN modulation, have also been documented [259,260]. For this reason, polyphenols alone and/or in synergy with probiotics are important nondrug therapies to promote gut and brain resilience to pathological challenges (i.e., stress, inflammation and gut dysbiosis). In line with this, preclinical and clinical studies documented the healthy effects of probiotics in repressing neurological and gut disorders via VN stimulation [199,261,262,263]. Accordingly, Bravo et al. reported that Lactobacillus rhamnosus (JB-1) represses stress-induced corticosterone and anxiety- and depression-related behavior in mice through VN stimulation [199]. In addition, it has been also reported that Lactobacillus reuteri (~1 × 108 organisms/day) reversed the social behavioral deficits in the gut in a VN-dependent manner via increasing the oxytocin levels in the brain and specifically in the ventral tegmental area of dopaminergic neurons, ultimately promoting synaptic plasticity between enteroendocrine cells and vagal afferents in mouse models of ASD [262]. Human studies, especially a double-blind randomized clinical trial on 60 women affected by normal weight obese and obesity, demonstrated that an intake for 3 weeks of a psychobiotics oral suspension containing strains of Streptococcus thermophilus, Streptococcus thermophiles, Bifdobacterium animalis subsp. Lactis, Bifdobacterium bifdum, Lactococcus lactis subsp. Lactis, Lactobacillus delbrueckii spp. Bulgaricus, Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus reuteri at a dose of 3 g/day modulated the body composition, microbial contamination, psychopathological scores and eating behavior via the VN in pre-obese–obese women [263]. Intriguingly, Takada and colleagues demonstrated that a daily administration of milk fermented with Lactobacillus casei strain Shirota (1.0 × 109 CFU/mL) for 8 weeks markedly repressed salivary cortisol levels, preventing the onset of physical symptoms in academically stressed students compared to the placebo group [261]. In support of this, the same authors also observed that an intragastric administration of Lactobacillus casei strain Shirota (2 × 1010 CFU/mL) stimulated gastric vagal afferent activity and sent sensory signals through the VN to the nucleus tractus solitarius (NTS) of the brain, thereby modulating HPA axis reactivity and subsequently suppressing the stress-initiated cortisol response dose-dependently [261]. Likewise, compelling evidence documented that a daily intake of polyphenols, such as resveratrol and polyunsaturated fatty acids (PUFAs), modulates the gut microbiota via the endocannabinoid pathway and light, odor and taste receptors, which, in turn, transfer their messages to the other organs, in particular to the brain via the VN [264]. The protective mechanism of resveratrol on the intestinal microbiota is mediated by the upregulation of the Sirt1 pathway, which increases insulin sensitivity and reduces hepatic glucose production through the activation of the hypothalamic KATP channel and the innervation of the hepatic VN [265]. Importantly, polyphenols, in particular flavones and flavone metabolites, are able to modulate neural transmission. In this regard, Ishii et al. observed that a single oral dose of 50 mg/kL of flavan-3-ols, a catechin and procyanidin fraction, crosses nerves and affects adipose tissue through sympathetic nerve activation and by upregulating thermogenic transcription factors, such as eroxisome proliferator-activated receptor γ coactivator (PGC)-1α, PR domain-containing PRDM16 and mitochondrial uncoupling protein 1 (UCP-1), in mouse adipose tissues [266]. The active flavonoid isoliquiritigenin (10 μM) contained in Glycyrrhiza glabra L. root, Kaempferol-3-rhamnoside and rosmarinic acid exerted neuroprotection, activating GABA receptors and decreasing intracellular Ca2+ and glutamate release into cerebrocortical terminals from synaptic vesicles in murine models [267,268,269]. In addition, a dose of 25 or 50 mg/kg of epigallocatechin gallate remarkably attenuated the neuronal expression of NADPH-d/nNOS and cell death in the motor neurons following peripheral nerve injury [270]. Equally importantly, genistein isoflavone, a tyrosine kinase inhibitor, reduced the Ca2+ influx through T-type CaV3.3 voltage-gated ion channels, affecting nerve activation in a concentration-dependent manner in vitro and in silico [271]. Furthermore, it has been reported that a daily supplementation with matured hop-derived bitter acids (35 mg/day) found in beer enhanced the hippocampal memory and prefrontal cortex-related to cognitive function via VN stimulation in rodent models of neurodegeneration [272]. Recently, Skiba et al. evaluated the pharmacometabolic effects of pteryxin (20 μM), a natural coumarin compound, demonstrating that this substance purposely augmented and restored whole-body acetylcholine, choline and serotonin levels in zebrafish larvae mediated by VN stimulation. Therefore, pteryxin could be considered an adjunctive therapeutic approach to treat refractory epilepsy [273]. Interestingly, it has been also demonstrated that ayurvedic medicine, particularly centella asiatica extract (200 mg), alleviated colitis by inhibiting inflammatory cell infiltration with reduced myeloperoxidase activity in the colon and upregulated the expression of tight junction protein (ZO-1, claudin-1 and E-cadherin) to enhance the intestinal mucosa permeability and the abundance of beneficial bacteria, such as the Firmicutes phylum, and reduce the abundance of the Proteobacteria phylum in order to restore intestinal motility and promote c-Kit expression in the colon and 5-HT in the brain [274]. Finally, a very recent randomized, double-blind, placebo-controlled cross-over trial performed on 11 healthy subjects observed that the consumption of 500 mg of UP360 containing Aloe vera, Poria cocos mushroom and Rosmarinus officinalis extract increased chemokine levels through the activation of innate immune cell markers (i.e., NKT cells, monocytes, CD8+ T cells and γδT cells), which was followed by an increase in the antioxidant pathways (i.e., superoxide dismutase and catalase), leading to a significant reduction in TNF-α levels via vagal communication [275]. Overall, the therapeutic effects of psychobiotics and/or natural compounds on intestinal inflammation and brain damage are likely due to the activation of the Nrf2-dependent antioxidant pathways that inhibit gut-mediated cytokine release via VN stimulation.
8. Ferroptosis and Nrf2 Signaling in Gut–Brain Axis: Relevance to Natural Therapy
Ferroptosis is a newly iron- and lipid peroxidation-dependent cell death cascade first described by Dixon and colleagues in 2012 [276]. It is caused by the redox state depletion of the intracellular antioxidant microenvironment, which is tightly controlled by glutathione (GSH) and glutathione peroxidase 4 (GPX4) and is responsible for regulating ROS levels via the Nrf2 pathway. Therefore, the depletion and inhibition of GSH antioxidant levels inactivates and represses the decomposition of toxic lipid hydroperoxides into lipid alcohols to initiate ferroptosis. Ferroptosis is thus promoted by the suppression of cysteine uptake, reduced GSH levels or inactivation of the lipid repair enzyme GPX4. Emerging evidence indicates that lipid peroxidation and GSH metabolism dysfunction trigger ferroptosis by promoting neuronal loss and CNS-associated damage in a range of neurodegenerative disorders, as well as affecting gut microbiota homeostasis, leading to the pathogenesis or progression of gastrointestinal diseases [277]. Nrf2 is known to regulate ferroptosis in multiple pathways and the mechanism of action in which natural compounds target Nrf2 to inhibit ferroptosis is attracting the attention of many researchers. It is equally noteworthy that the upregulation of Nrf2 and its target vitagenes (HO-1, NQO-1 and Trx) activated by dietary polyphenols, such as flavonoids, contributes not only to neuroprotection, delaying brain degeneration [278], but is also highly linked to gut microbiota homeostasis during ferroptosis [279,280]. Consistent with this, recent preclinical evidence elucidated the underlying molecular mechanism of quercetin, a natural flavonoid, on gastrointestinal inflammation and revealed that at a concentration of 50 mg/kg it reduced dietary mycotoxins-induced intestinal ferroptosis by attenuating the inflammatory TLR4/NF-κB signaling pathway in mice [281]. In addition, quercetin reduced lipid peroxidation (e.g., downregulating 4-HNE and upregulating the GSH/GSSG ratio) and lipid accumulation, inhibiting hepatic ferroptosis in vitro and in vivo [282]. In the brain, low doses of quercetin have shown significant neuroprotective effects by attenuating cognitive impairment and ameliorating motor behavioral disorders via the inhibition of ferroptosis and the activation of the Nrf2 pathway in vitro and in vivo models of AD and PD [283,284]. In addition, curcumin has been reported as a novel ferroptosis inhibitor due to its protective effects as an iron chelator, thus preventing GSH depletion, GPX4 inactivation and lipid peroxidation in pancreatic cells [285], as well as 6-hydroxydopamine-induced nigral dopaminergic neuronal degeneration in PD rats [286] in a dose-dependent manner. Sulforaphane is known as an Nrf2 activator, a mechanism of action for anti-oxidative and anti-inflammatory activities and a key negative regulator of ferroptosis [287]. Notably, evidence indicated that sulforaphane inhibited ferroptosis, downregulating lactate dehydrogenase, Fe2+, malondialdehyde and acyl-CoA synthetase long-chain family member 4 (ACSL4), and upregulating Nrf2, GSH, glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11) in vitro and in vivo [288] (Figure 3).
Figure 3.
Potential mechanism of action of nutrients in preventing ferroptosis via Nrf2 pathway. Hormetic nutrients, such as polyphenols, in synergy with probiotics upregulate the Nrf2 antioxidant pathway and intracellular resilience scavengers including GPX4, GSH, SLC7A11, HO-1, ferritin heavy chain 1 (FTH1), which in turn activates anti-inflammatory lipid mediators such as lipoxin A4, maresins, resolvins and protectins generated by polyunsaturated-fatty-acid (PUFA) to block ferroptosis and lipid peroxidation triggered from pro-inflammatory lipid mediators such as 4-HNE, acrolein, COX-2 and MDA; F2-isoprostanes; inflammatory cytokines including NF-κB; and apoptotic mediators such as P53, Bax and Caspase-3 [277,280,289]. In particular, GPX4 is able to oxidize glutathione (GSH) to oxidized glutathione (GSSG) to reduce lipid peroxides and restore cellular redox homeostasis. GSH-reductase (GSR) reproduced GSH from GSSG, which was also synthesized by γ-GCs. Cystine enters cells through a system Xc- transporter and is reduced to cysteine via the thioredoxin reductase 1 (TrxR1) to synthesize GSH. The inhibition of cystine input blocks the synthesis of GSH in cells and promotes ferroptosis. PUFA activated by acyl-CoA synthetase long-chain family member 4 (ACSL4) starts lipid peroxidation and converts to PUFA acyl-CoA to generate phospholipid (PUFA-PL) by lysopho-sphatidylcholine acyltransferase 3 (LPCAT3) in the lipid membrane, which reacts with molecular oxygen through the Fenton reaction to generate peroxide radicals that promote the dehydrogenation of PUFA to toxic phospholipid hydroperoxide (PLOOHs), which is mediated by lipoxygenases (LPXs) to drive lipid peroxidation and form the corresponding alcohol (PLOH) and lipid radicals. The storage of iron is mediated by ferroportin 1 (FPN1) and ferritin is responsible for the regulation of iron homeostasis. Lipoxin A4 and other anti-inflammatory lipid mediators activated by nutrients block the toxic lipid cascade via activation of the Nrf2 pathway and antioxidant systems of crucial importance for maintaining functional intracellular repair mechanisms such as GPX4 and GSH, thus preventing ferroptosis in both the gut and the brain.
Similarly, epigallocatechin-3-gallate (EGCG) protected against radiation-induced intestinal damage by scavenging ROS and inactivating ferroptosis via the upregulation of the Nrf2 pathway and its antioxidant proteins SLC7A11, HO-1 and GPX4 [289]. A recent study showed that EGCG exhibits therapeutic effects against the nonalcoholic steatohepatitis induced by a deficient diet in methionine and choline through shifting gut dysbiosis (i.e., Oxalobacter, Oscillibacter, Coprococcus_1 and Desulfovibrio versus Bacteroides, Bifidobacteria and Lactobucillus), improving the hepatic injury, lipid accumulation, fibrosis and ferroptosis pathway after 4 weeks in animals [280]. Ginkgolide B, a terpenoid found in Ginkgo biloba, by targeting the Nrf2/GPX4 signaling pathway, has been shown to ameliorate AD-related cognitive impairment in senescence mice by decreasing the iron content in the brain, transferrin receptor 1 (TFR1) and nuclear receptor coactivator 4 (NCOA4) expression, and by increasing ferritin heavy chain (FTH1) expression [290]. Interestingly, salidroside, a polyphenol contained in Rhodiola Rosea L., effectively reduced intracellular Fe2+ levels, attenuating lipid peroxidation and mitochondrial damage and enhancing the expression of GPX4 and SLC7A11 by targeting Nrf2/HO1 signaling to block neuronal ferroptosis in hippocampal cells and Aβ1-42-induced AD mice [291]. In the same way, the probiotic formulation containing three strains of Lactobacilli (Lacticaseibacillus rhamnosus LR04 (DSM16605), Lactiplantibacillus plantarum LP14 (DSM33401) and Lacticaseibacillus paracasei (LPC09)) and two strains of Bifidobacteria (Bifidobacterium breve BR03 (DSM16604) and B632 (DSM24706)) completely restored the harmful effects of ferroptosis and gut inflammation due to dinitrobenzene sulfonic acid exposure in colon tissues of ex vivo models (organ-on-chip) [292]. Taken together, the abovementioned recent findings elucidate the crucial role of the Nrf2 pathway and vitagenes activated by natural mitigators (i.e., polyphenols or probiotics) as a rational line of therapy for ferroptosis-induced neurodegeneration and intestinal dysfunction.
9. Nutritional Therapeutic Interventions Using Innovative In Vitro Modeling
Recent research in stem cell biology has led to the successful three-dimensional culture of tissue in vitro, also known as “organoids”, or three-dimensional organ-like structures composed of functional, live cells that can self-renew and spatially organize. The ENS strongly influences mucosal immunity and epithelial function, and is currently suggested as an important contributor to IBD development and progression [293]. On the other hand, ENS dysfunction may lead to bidirectional consequences for the gut and the nervous system, as various neurotransmitters are released from ENS neurons and can in turn act on the intestinal epithelium (gut–brain axis), exacerbating peripheral inflammation and the development of neuropathologies [294]. Notably, the direct crosstalk between the gut epithelium and specific primary afferent fibers is conducted by enterochromaffin cells (EC), which are proposed as chemosensors of the intestinal epithelial that modulate vagal sensory neurons, bacteria products and metabolites, chemical irritants, inflammatory mediators and neurotransmitters from the gut directly to the nervous system [295]. In this light, intestinal and brain organoids provide an innovative in vitro platform to explore cellular communication and molecular mechanisms of host–microbe interaction and inter-organ crosstalk between the intestine and CNS in health and/or disease. To note, the discovery of novel natural therapeutic candidates, including polyphenols and/or probiotics tested on organoids, could be relevant for personalized nutritional medicine of gut and brain disorders, ultimately revolutionizing research in the fields of neuroscience and gastroenterology (Figure 4) [296]. Recent findings have found that ferulic acid, a polyphenolic compound, promotes the survival and differentiation of mouse intestine organoids, prevents inflammation and ensures gut health by protecting the intestinal epithelial barrier; thus, it could be considered as a potential preventive or alleviating component in the diet of IBD patients [297]. Furthermore, a study revealed that a low dose (0.1%) of mixture composed of functional amino acids (L-arginine, L-leucine, L-valine, L-isoleucine and L-cystine) and 100 ppm of a polyphenol-rich extract from grape seeds and skins regulates epithelial homeostasis and modulates the gut microbiota in vivo and in intestinal organoids [298]. In addition, another study by Tveter et al. demonstrated that the administration of proanthocyanidin-rich extract of grape polyphenols improves glucose metabolism, decreases pathogenic gut bacteria associated with nuclear transcription factor farnesoid X receptor (FXR) inhibition and downregulates ceramide synthesis genes (e.g., Smpd3, Cers4 and Sptlc2) in the intestine of db/db mice after 4 weeks [299]. Interestingly, a recent research conducted by Elbadawi et al. showed that a low dose of 10 g/mL of a new herbal preparation termed STW 5-II and consisting of six synergistic medicinal extracts, i.e., Iberis amara L., Glycyrrhiza glabra L., Chamomilla recutita R., Menthae piperitae L., Melissa officinalis L. and Carum carvi L., reduced levels of corticotropin-releasing factor (CRF), mediating IL-6, IL-1β and TNFα expression and serotonin release, using mouse intestinal organoids as a model of IBS disorder dose-dependently [300]. Likewise, the application of intestinal organoids and the effectiveness of probiotics in maintaining gut epithelial regeneration and homeostasis against inflammation have been extensively documented [301,302,303]. In particular, Hou et al. showed the efficacy of Lactobacillus reuteri D8 (106 CFU/g) in regenerating the intestinal barrier and activating the intestinal epithelial proliferation of organoids subjected to TNF-α damage. The authors observed that Lactobacillus reuteri D8 reduced TNF-α and stimulated intestinal organoids with lamina propria lymphocytes (LPLs) to secrete IL-22 via STAT3 pathway ex vivo and in vivo [301]. Furthermore, Wu and coworkers confirmed that Lactobacillus reuteri D8 protected the intestinal mucosal barrier’s integrity, repaired intestinal damage and reduced inflammation induced by TNF in intestinal organoids or C. rodentium infection in mice via the activation of the Wnt/β-catenin signaling pathway [302]. Intriguingly, other recent evidence showed postbiotic effects of a new probiotic strain of Lactobacillus reuteri DS0384 isolated from the feces of a healthy newborn, and found that it promotes intestinal epithelial maturation and protects the intestinal epithelium from IFNγ/TNFα-induced injury in human intestinal organoids and infant mice [303]. Furthermore, Engevik et al. demonstrated that Lactobacillus reuteri ATCC PTA 6475 metabolites, including ethanol, upregulate the serotonin transporter (SERT), restoring normal serotonin levels for maintaining intestinal homeostasis in mouse colonic organoids [304]. The same authors also investigated another probiotic, Bifidobacterium dentium, and showed that its metabolites, particularly acetate, stimulated enterochromaffin cells to secrete 5-hydroxytryptamine (5-TH) within the intestinal epithelium of human enteroid cells and adult mice via the gut–brain axis. Notably, the study revealed that Bifidobacterium dentium changed the expression of key intestinal serotonin receptors, particularly isoforms 2a (Htr2a) and 4 (Htr4), and the 5-HT transporter, a serotonin transporter (Sert), upregulating the expression of Htr2a in the hippocampus and normalizing anxiety like-behaviors in mice [305]. Most recently, an intriguing study showed the beneficial effects of the intestinal bacterium Lactobacillus plantarum in modulating the intestinal microbiota and improving gut barrier integrity by reducing inflammation-related pathways (TNF-alpha and NF-κB) and increasing levels of L-arginine in both hepatic and intestinal organoids and in mouse models of nonalcoholic steatohepatitis [306]. Concerning neurodegenerative disorders, recent advances using the human midbrain organoids documented that mutation in the DNAJC6 gene encoding HSP40 auxilin caused PD pathogenesis including autophagy defects, α-syn aggregation and dopaminergic neuron degeneration, contributing to juvenile-onset PD [307]. Lastly, a recent study conducted by Ji and collogues reported that exosomes from brain organoids relieved H2O2-induced oxidative stress and apoptosis in rat midbrain astrocytes by suppressing ROS overproduction, lipid peroxidation, mitochondrial dysfunction and the expression of pro-apoptotic genes, and they promoted the differentiation of human-induced pluripotent stem cells (iPSCs) into dopaminergic neurons via homeobox transcription factor 1 alpha (LMX1A) upregulation due to increased levels of neurotrophic factors including neurotrophin-4 (NT-4) and glial-cell-derived neurotrophic factor (GDNF) [308]. Taking together, to date, there are few studies in the literature that have investigated the therapeutic potential of a nutritional approach with polyphenols and/or probiotics using organoid models to prevent or inhibit gut and brain inflammation and related disorders in order to improve human health. It is hoped that in the future personalized nutritional medicine in this interesting field will become more promising and will be taken into consideration.
Figure 4.
Personalized nutritional therapy using organoid models in gut–brain axis disorders.
An altered gut–brain crosstalk due to an increase in pathogenic bacteria, such as Streptococcoceae, Clostridiaceae, Enterobacteriaceae and Proteobacteriaceae, as well as a reduction in beneficial bacteria, such as Bifidobacteriaceae and Lactobacillaceae, triggers gut dysbiosis and neuroinflammation, which in turn leads to enteric glia and microglia activation and, consequently, BBB and IEB dysfunctions, ultimately culminating in the development of gastrointestinal diseases including inflammatory bowel diseases (i.e., ulcerative colitis and Crohn’s disease), pancreatitis and colorectal cancer and CNS disorders (i.e., AD, PD, depression and anxiety, autism and schizophrenia). Personalized nutritional therapy through polyphenols and probiotics at low doses (hormesis) tested in innovative in vitro models, especially the brain and intestinal organoids, can prevent or inhibit an inflammatory cascade and restore the physiological composition of the gut microbiota and subsequent brain function in order to promote human health. increase, decrease.
10. Conclusions and Future Perspectives
In conclusion, both indirect and direct crosstalk between intestinal microbiota and the CNS along the gut–brain axis provides a rationale for non-invasive and affordable therapeutic innovations in brain disorders including neurodegenerative and psychiatric diseases. In this new way, hormetic nutrition through polyphenols and/or probiotics targeting the antioxidant Nrf2 pathway and stress resilient vitagenes to inhibit oxidative stress and inflammatory pathways, as well as ferroptosis, could represent an effective therapy to manipulate alterations in the gut microbiome leading to brain dysfunction in order to prevent or slow the onset of major cognitive disorders. Notably, hormetic nutrients can stimulate the vagus nerve as a means of directly modulating microbiota–brain interactions for therapeutic purposes to mitigate or reverse the pathophysiological process, restoring gut and brain homeostasis, as reported by extensive preclinical and clinical studies. Interestingly, emerging research highlighted that gut microbiome composition and function can predict healthy aging and longevity in humans. Indeed, a positive correlation was attributed to the phylum of Firmicutes and a negative association was attributed to the abundance of Bacteroides [309]. Notably, the abundance of pathogenic bacteria involves a shift towards a pro-inflammatory state in the gut, yielding increased gut permeability and subsequent triggering of a peripheral inflammatory response, which in turn impacts vagal sensory neurons, culminating in neuroinflammation and impaired cognitive function. Therefore, a deeper understanding of the composition, function and expression of gut microbiota bacteria, as well as their modulation through low doses (hormesis) of probiotics in synergistic combination with polyphenols, and especially with the more bioavailable polyphenol nanoparticles, can restore intestinal homeostasis and promote brain health (allostasis/adaptive response) in both young and elderly populations. Lastly, novel and sophisticated in vitro modeling through the use of gut and brain organoids to explore the relationship between the intestinal microenvironment, host–microbes interaction and inter-organ crosstalk, as well as the underlying mechanism of action of polyphenols/probiotics targeting the antioxidant Nrf2 pathway, could shed more light on personalized nutritional therapies to prevent or attenuate intestinal dysbiosis and peripheral inflammation/neuroinflammation leading to the degeneration of sensory neurons and cognitive impairment, and could ultimately predict positive outcomes in gut–brain axis disorders.
Acknowledgments
This original review is part of the special issue “The Interplay of Oxidative Stress, the Gut–Brain Axis and Neurodegenerative Diseases” proposed by Maria Scuto; The authors extend their appreciation to the technical support provided by Miljana Nikolic.
Author Contributions
Conceptualization, M.S.; investigation, M.S.; resources, A.T.S., V.C., F.R., S.M.S. and G.M.R.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and A.T.S.; visualization, M.S., V.C., S.M.S., F.R., G.M.R. and A.T.S.; supervision, M.S., A.T.S. and V.C.; Figures, M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cryan J.F., O‘Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 2.Bäuerl C., Collado M.C., Diaz Cuevas A., Viña J., Pérez Martínez G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018;66:464–471. doi: 10.1111/lam.12882. [DOI] [PubMed] [Google Scholar]
- 3.Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Jaafari H., Houghton L.A., West R.M., Agrawal A., Aziz I., Black C.J., Corsetti M., Shuweihdi F., Eugenicos M., Paine P.A., et al. The national prevalence of disorders of gut brain interaction in the United Kingdom in comparison to their worldwide prevalence: Results from the Rome foundation global epidemiology study. Neurogastroenterol. Motil. 2023;35:e14574. doi: 10.1111/nmo.14574. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q., Luo Y., Ray Chaudhuri K., Reynolds R., Tan E.K., Pettersson S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 6.Sanctuary M.R., Kain J.N., Chen S.Y., Kalanetra K., Lemay D.G., Rose D.R., Yang H.T., Tancredi D.J., German J.B., Slupsky C.M., et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE. 2019;14:e0210064. doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan X., Chen B., Duan Z., Xia Z., Ding Y., Chen T., Liu H., Wang B., Yang B., Wang X., et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 2021;13:1987779. doi: 10.1080/19490976.2021.1987779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y., Liu Y., Cheng K., Zhou C., Wang H., et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019;5:eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donati Zeppa S., Agostini D., Ferrini F., Gervasi M., Barbieri E., Bartolacci A., Piccoli G., Saltarelli R., Sestili P., Stocchi V. Interventions on Gut Microbiota for Healthy Aging. Cells. 2022;12:34. doi: 10.3390/cells12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 11.Agirman G., Yu K.B., Hsiao E.Y. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 12.Parker A., Romano S., Ansorge R., Aboelnour A., Le Gall G., Savva G.M., Pontifex M.G., Telatin A., Baker D., Jones E., et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10:68. doi: 10.1186/s40168-022-01243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Dasuri K., Zhang L., Keller J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013;62:170–185. doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Musgrove R.E., Helwig M., Bae E.J., Aboutalebi H., Lee S.J., Ulusoy A., Di Monte D.A. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular α-synuclein transfer. J. Clin. Investig. 2019;129:3738–3753. doi: 10.1172/JCI127330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scuto M., Trovato Salinaro A., Caligiuri I., Ontario M.L., Greco V., Sciuto N., Crea R., Calabrese E.J., Rizzolio F., Canzonieri V., et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021;199:111551. doi: 10.1016/j.mad.2021.111551. [DOI] [PubMed] [Google Scholar]
- 17.Cornelius C., Trovato Salinaro A., Scuto M., Fronte V., Cambria M.T., Pennisi M., Bella R., Milone P., Graziano A., Crupi R., et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: Role of vitagenes. Immun. Ageing. 2013;10:41. doi: 10.1186/1742-4933-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trovato Salinaro A., Cornelius C., Koverech G., Koverech A., Scuto M., Lodato F., Fronte V., Muccilli V., Reibaldi M., Longo A., et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder linked to Alzheimer’s disease. Front. Pharmacol. 2014;5:129. doi: 10.3389/fphar.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese V., Scapagnini G., Davinelli S., Koverech G., Koverech A., De Pasquale C., Trovato Salinaro A., Scuto M., Calabrese E.J., Genazzani A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell Commun. Signal. 2014;8:369–384. doi: 10.1007/s12079-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelius C., Koverech G., Crupi R., Di Paola R., Koverech A., Lodato F., Scuto M., Trovato Salinaro A., Cuzzocrea S., Calabrese E.J., et al. Osteoporosis and Alzheimer pathology: Role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front. Pharmacol. 2014;5:120. doi: 10.3389/fphar.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese V., Scuto M., Trovato Salinaro A., Dionisio G., Modafferi S., Ontario M.L., Greco V., Sciuto S., Schmitt C.P., Calabrese E.J., et al. Hydrogen Sulfide and Carnosine: Modulation of Oxidative Stress and Inflammation in Kidney and Brain Axis. Antioxidants. 2020;9:1303. doi: 10.3390/antiox9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilipenko V., Narbute K., Amara I., Trovato A., Scuto M., Pupure J., Jansone B., Poikans J., Bisenieks E., Klusa V., et al. GABA-containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J. Neurosci. Res. 2019;97:708–726. doi: 10.1002/jnr.24396. [DOI] [PubMed] [Google Scholar]
- 23.Welcome M.O. Gut Microbiota Disorder, Gut Epithelial and Blood-Brain Barrier Dysfunctions in Etiopathogenesis of Dementia: Molecular Mechanisms and Signaling Pathways. NeuroMol. Med. 2019;21:205–226. doi: 10.1007/s12017-019-08547-5. [DOI] [PubMed] [Google Scholar]
- 24.Mangino M.J., Brounts L., Harms B., Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marizzoni M., Cattaneo A., Mirabelli P., Festari C., Lopizzo N., Nicolosi V., Mombelli E., Mazzelli M., Luongo D., Naviglio D., et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimers Dis. 2020;78:683–697. doi: 10.3233/JAD-200306. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang Z., Yang R., Wang W., Qi L., Huang T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020;17:288. doi: 10.1186/s12974-020-01961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alshammari M.K., AlKhulaifi M.M., Al Farraj D.A., Somily A.M., Albarrag A.M. Incidence of Clostridium perfringens and its toxin genes in the gut of children with autism spectrum disorder. Anaerobe. 2019;61:102114. doi: 10.1016/j.anaerobe.2019.102114. [DOI] [PubMed] [Google Scholar]
- 30.Eicher T.P., Mohajeri M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients. 2022;14:2661. doi: 10.3390/nu14132661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017;7:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimidi E., Rossi M., Whelan K. Irritable bowel syndrome and diet: Where are we in 2018? Curr. Opin Clin. Nutr. Metab. Care. 2017;20:456–463. doi: 10.1097/MCO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 33.Jackson A., Forsyth C.B., Shaikh M., Voigt R.M., Engen P.A., Ramirez V., Keshavarzian A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019;10:1245. doi: 10.3389/fneur.2019.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapoor P., Tiwari A., Sharma S., Tiwari V., Sheoran B., Ali U., Garg M. Effect of anthocyanins on gut health markers, Firmicutes-Bacteroidetes ratio and short-chain fatty acids: A systematic review via meta-analysis. Sci. Rep. 2023;13:1729. doi: 10.1038/s41598-023-28764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 36.Obrenovich M.E.M. Leaky gut, Leaky brain? Microorganisms. 2018;6:107. doi: 10.3390/microorganisms6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng H., Zhang D., Wu J., Liu J., Zhou Y., Tan Y., Feng W., Peng C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine. 2023;119:154979. doi: 10.1016/j.phymed.2023.154979. [DOI] [PubMed] [Google Scholar]
- 38.Emge J.R., Huynh K., Miller E.N., Kaur M., Reardon C., Barrett K.E., Gareau M.G. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G989–G998. doi: 10.1152/ajpgi.00086.2016. [DOI] [PubMed] [Google Scholar]
- 39.Du Y., Li Y., Xu X., Li R., Zhang M., Cui Y., Zhang L., Wei Z., Wang S., Tuo H. Probiotics for constipation and gut microbiota in Parkinson’s disease. Parkinsonism Relat. Disord. 2022;103:92–97. doi: 10.1016/j.parkreldis.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Li N., Yang J.J., Zhao D.M., Chen B., Zhang G.Q., Chen S., Cao R.F., Yu H., Zhao C.Y., et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020;157:104784. doi: 10.1016/j.phrs.2020.104784. [DOI] [PubMed] [Google Scholar]
- 41.Bjørklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Bucciantini M., Leri M., Scuto M., Ontario M., Trovato Salinaro A., Calabrese E.J., Calabrese V., Stefani M. Xenohormesis underlyes the anti-aging and healthy properties of olive polyphenols. Mech. Ageing Dev. 2022;202:111620. doi: 10.1016/j.mad.2022.111620. [DOI] [PubMed] [Google Scholar]
- 43.Cordaro M., Siracusa R., Fusco R., D‘Amico R., Peritore A.F., Gugliandolo E., Genovese T., Scuto M., Crupi R., Mandalari G., et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants. 2020;9:660. doi: 10.3390/antiox9080660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osakabe N., Shimizu T., Fujii Y., Fushimi T., Calabrese V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules. 2024;14:234. doi: 10.3390/biom14020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leri M., Scuto M., Ontario M.L., Calabrese V., Calabrese E.J., Bucciantini M., Stefani M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020;21:1250. doi: 10.3390/ijms21041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calabrese E.J., Dhawan G., Kapoor R., Agathokleous E., Calabrese V. Rhodiola rosea and salidroside commonly induce hormesis, with particular focus on longevity and neuroprotection. Chem. Biol. Interact. 2023;380:110540. doi: 10.1016/j.cbi.2023.110540. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese E.J., Hayes A.W., Pressman P., Dhawan G., Kapoor R., Agathokleous E., Calabrese V. Flavonoids commonly induce hormetic responses. Arch. Toxicol. 2024;98:1237–1240. doi: 10.1007/s00204-024-03684-8. [DOI] [PubMed] [Google Scholar]
- 48.Amara I., Ontario M.L., Scuto M., Lo Dico G.M., Sciuto S., Greco V., Abid-Essefi S., Signorile A., Trovato Salinaro A., Calabrese V. Moringa oleifera Protects SH-SY5YCells from DEHP-Induced Endoplasmic Reticulum Stress and Apoptosis. Antioxidants. 2021;10:532. doi: 10.3390/antiox10040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amara I., Salah A., Timoumi R., Annabi E., Scuto M., Trovato A., Neffati F., Calabrese V., Abid-Essefi S. Effect of di(2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney. Cell Stress Chaperones. 2020;25:919–928. doi: 10.1007/s12192-020-01127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amara I., Scuto M., Zappalà A., Ontario M.L., Petralia A., Abid-Essefi S., Maiolino L., Signorile A., Trovato Salinaro A., Calabrese V. Hericium erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020;21:2138. doi: 10.3390/ijms21062138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calabrese V., Santoro A., Trovato Salinaro A., Modafferi S., Scuto M., Albouchi F., Monti D., Giordano J., Zappia M., Franceschi C., et al. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018;96:1641–1662. doi: 10.1002/jnr.24244. [DOI] [PubMed] [Google Scholar]
- 52.Trovato Salinaro A., Pennisi M., Di Paola R., Scuto M., Crupi R., Cambria M.T., Ontario M.L., Tomasello M., Uva M., Maiolino L., et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing. 2018;15:8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ontario M.L., Siracusa R., Modafferi S., Scuto M., Sciuto S., Greco V., Bertuccio M.P., Trovato Salinaro A., Crea R., Calabrese E.J., et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and hidrox. Mech. Ageing Dev. 2022;203:111637. doi: 10.1016/j.mad.2022.111637. [DOI] [PubMed] [Google Scholar]
- 54.Fusco R., Gugliandolo E., Siracusa R., Scuto M., Cordaro M., D‘Amico R., Evangelista M., Peli A., Peritore A.F., Impellizzeri D., et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology. 2020;9:238. doi: 10.3390/biology9090238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cordaro M., Trovato Salinaro A., Siracusa R., D‘Amico R., Impellizzeri D., Scuto M., Ontario M.L., Cuzzocrea S., Di Paola R., Fusco R., et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants. 2021;10:1664. doi: 10.3390/antiox10111664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordaro M., Scuto M., Siracusa R., D‘amico R., Peritore A.F., Gugliandolo E., Fusco R., Crupi R., Impellizzeri D., Pozzebon M., et al. Effect of N-palmitoylethanolamine-oxazoline on comorbid neuropsychiatric disturbance associated with inflammatory bowel disease. FASEB J. 2020;34:4085–4106. doi: 10.1096/fj.201901584RR. [DOI] [PubMed] [Google Scholar]
- 57.Peritore A.F., Crupi R., Scuto M., Gugliandolo E., Siracusa R., Impellizzeri D., Cordaro M., D‘amico R., Fusco R., Di Paola R., et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord. Drug Targets. 2020;19:27–43. doi: 10.2174/1871527319666200107094732. [DOI] [PubMed] [Google Scholar]
- 58.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fusco R., Scuto M., Cordaro M., D‘Amico R., Gugliandolo E., Siracusa R., Peritore A.F., Crupi R., Impellizzeri D., Cuzzocrea S., et al. N-Palmitoylethanolamide-Oxazoline Protects against Middle Cerebral Artery Occlusion Injury in Diabetic Rats by Regulating the SIRT1 Pathway. Int. J. Mol. Sci. 2019;20:4845. doi: 10.3390/ijms20194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cosentino A., Agafonova A., Modafferi S., Trovato Salinaro A., Scuto M., Maiolino L., Fritsch T., Calabrese E.J., Lupo G., Anfuso C.D., et al. Blood-Labyrinth Barrier in Health and Diseases: Effect of Hormetic Nutrients. Antioxid. Redox Signal. 2024;40:542–563. doi: 10.1089/ars.2023.0251. [DOI] [PubMed] [Google Scholar]
- 61.Guglielmetti S., Bernardi S., Del Bo’ C., Cherubini A., Porrini M., Gargari G., Hidalgo-Liberona N., Gonzalez-Dominguez R., Peron G., Zamora-Ros R., et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: Study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020;20:77. doi: 10.1186/s12877-020-1472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R., Hamidi G.A., Salami M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bambury A., Sandhu K., Cryan J.F., Dinan T.G. Finding the needle in the haystack: Systematic identification of psychobiotics. Br. J. Pharmacol. 2018;175:4430–4438. doi: 10.1111/bph.14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Ceccarelli G., Brenchley J.M., Cavallari E.N., Scheri G.C., Fratino M., Pinacchio C., Schietroma I., Fard S.N., Scagnolari C., Mezzaroma I., et al. Impact of High-Dose Multi-Strain Probiotic Supplementation on Neurocognitive Performance and Central Nervous System Immune Activation of HIV-1 Infected Individuals. Nutrients. 2017;9:1269. doi: 10.3390/nu9111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chunchai T., Thunapong W., Yasom S., Wanchai K., Eaimworawuthikul S., Metzler G., Lungkaphin A., Pongchaidecha A., Sirilun S., Chaiyasut C., et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018;15:11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mörkl S., Butler M.I., Holl A., Cryan J.F., Dinan T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020;9:171–182. doi: 10.1007/s13668-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lackner S., Sconocchia T., Ziegler T., Passegger C., Meier-Allard N., Schwarzenberger E., Wonisch W., Lahousen T., Kohlhammer-Dohr A., Mörkl S., et al. Immunomodulatory Effects of Aronia Juice Polyphenols-Results of a Randomized Placebo-Controlled Human Intervention Study and Cell Culture Experiments. Antioxidants. 2022;11:1283. doi: 10.3390/antiox11071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Interdonato L., Marino Y., Impellizzeri D., D‘Amico R., Siracusa R., Fusco R., Cammilleri G., Pantano L., Modafferi S., Abdelhameed A.S., et al. Autophagy machinery plays an essential role in traumatic brain injury-induced apoptosis and its related behavioral abnormalities in mice: Focus on Boswellia sacra gum resin. Front. Physiol. 2024;14:1320960. doi: 10.3389/fphys.2023.1320960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devos D., Lebouvier T., Lardeux B., Biraud M., Rouaud T., Pouclet H., Coron E., Bruley des Varannes S., Naveilhan P., Nguyen J.-M., et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Qu Y., Li X., Xu F., Zhao S., Wu X., Wang Y., Xie J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-κB Axis. Front. Immunol. 2021;12:679897. doi: 10.3389/fimmu.2021.679897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurowska A., Ziemichód W., Herbet M., Piątkowska-Chmiel I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients. 2023;15:1436. doi: 10.3390/nu15061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westfall S., Caracci F., Estill M., Frolinger T., Shen L., Pasinetti G.M. Chronic Stress-Induced Depression and Anxiety Priming Modulated by Gut-Brain-Axis Immunity. Front. Immunol. 2021;12:670500. doi: 10.3389/fimmu.2021.670500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westfall S., Caracci F., Zhao D., Wu Q.L., Frolinger T., Simon J., Pasinetti G.M. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav. Immun. 2021;91:350–368. doi: 10.1016/j.bbi.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao L., Xiao H.T., Mu H.X., Huang T., Lin Z.S., Zhong L.L.D., Zeng G.Z., Fan B.M., Lin C.Y., Bian Z.X. Magnolol, a Natural Polyphenol, Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice. Molecules. 2017;22:1218. doi: 10.3390/molecules22071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Islam T., Koboziev I., Albracht-Schulte K., Mistretta B., Scoggin S., Yosofvand M., Moussa H., Zabet-Moghaddam M., Ramalingam L., Gunaratne P.H., et al. Curcumin Reduces Adipose Tissue Inflammation and Alters Gut Microbiota in Diet-Induced Obese Male Mice. Mol. Nutr. Food Res. 2021;65:e2100274. doi: 10.1002/mnfr.202100274. [DOI] [PubMed] [Google Scholar]
- 77.Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Y.C., Li J., Zhang M., Pan J.C., Yu Y., Zhang J.B., Zheng L., Si J.M., Xu Y. Resveratrol Improves Brain-Gut Axis by Regulation of 5-HT-Dependent Signaling in the Rat Model of Irritable Bowel Syndrome. Front. Cell. Neurosci. 2019;8:13–30. doi: 10.3389/fncel.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Theilmann M.C., Goh Y.J., Nielsen K.F., Klaenhammer T.R., Barrangou R., Abou Hachem M. Lactobacillus acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals. mBio. 2017;8:e01421-17. doi: 10.1128/mBio.01421-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basholli-Salihu M., Schuster R., Mulla D., Praznik W., Viernstein H., Mueller M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst. Eng. 2016;39:1879–1885. doi: 10.1007/s00449-016-1662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Côté C.D., Rasmussen B.A., Duca F.A., Zadeh-Tahmasebi M., A Baur J., Daljeet M., Breen D.M., Filippi B.M., Lam T.K.T. Resveratrol activates duodenal SIRT1 to reverse insulin resistance in rats through a neuronal network. Nat. Med. 2015;21:498–505. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]
- 82.Koh Y.C., Lin S.J., Hsu K.Y., Nagabhushanam K., Ho C.T., Pan M.H. Pterostilbene Enhances Thermogenesis and Mitochondrial Biogenesis by Activating the SIRT1/PGC-1α/SIRT3 Pathway to Prevent Western Diet-Induced Obesity. Mol. Nutr. Food Res. 2023;67:e2300370. doi: 10.1002/mnfr.202300370. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Zhang H., Chen Y., Jia P., Ji S., Zhang Y., Wang T. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J. Anim. Sci. Biotechnol. 2021;12:70. doi: 10.1186/s40104-021-00589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diaz-Gerevini G.T., Repossi G., Dain A., Tarres M.C., Das U.N., Eynard A.R. Beneficial action of resveratrol: How and why? Nutrition. 2016;32:174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 85.Dou Z., Rong X., Zhao E., Zhang L., Lv Y. Neuroprotection of Resveratrol Against Focal Cerebral Ischemia/Reperfusion Injury in Mice through a Mechanism Targeting Gut-Brain Axis. Cell. Mol. Neurobiol. 2019;39:883–898. doi: 10.1007/s10571-019-00687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung J.Y., Jeong J.H., Song J. Resveratrol Modulates the Gut-Brain Axis: Focus on Glucagon-Like Peptide-1, 5-HT, and Gut Microbiota. Front. Aging Neurosci. 2020;12:588044. doi: 10.3389/fnagi.2020.588044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baghaei Naeini F., Hassanpour S., Asghari A. Resveratrol exerts anxiolytic-like effects through anti-inflammatory and antioxidant activities in rats exposed to chronic social isolation. Behav. Brain Res. 2023;438:114201. doi: 10.1016/j.bbr.2022.114201. [DOI] [PubMed] [Google Scholar]
- 88.Chen J.J., Shen J.X., Yu Z.H., Pan C., Han F., Zhu X.L., Xu H., Xu R.T., Wei T.Y., Lu Y.P. The Antidepressant Effects of Resveratrol are Accompanied by the Attenuation of Dendrite/Dendritic Spine Loss and the Upregulation of BDNF/p-cofilin1 Levels in Chronic Restraint Mice. Neurochem. Res. 2021;46:660–674. doi: 10.1007/s11064-020-03200-1. [DOI] [PubMed] [Google Scholar]
- 89.Shen J., Qu C., Xu L., Sun H., Zhang J. Resveratrol exerts a protective effect in chronic unpredictable mild stress-induced depressive-like behavior: Involvement of the AKT/GSK3β signaling pathway in hippocampus. Psychopharmacology. 2019;236:591–602. doi: 10.1007/s00213-018-5087-1. [DOI] [PubMed] [Google Scholar]
- 90.Lee J., Torosyan N., Silverman D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017;87:121–128. doi: 10.1016/j.exger.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Bode L.M., Bunzel D., Huch M., Cho G.S., Ruhland D., Bunzel M., Bub A., Franz C.M., Kulling S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 92.Hendouei F., Sanjari Moghaddam H., Mohammadi M.R., Taslimi N., Rezaei F., Akhondzadeh S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2020;45:324–334. doi: 10.1111/jcpt.13076. [DOI] [PubMed] [Google Scholar]
- 93.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A., Brewer J.B., Rissman R.A., Raman R., Aisen P.S. Alzheimer’s Disease Cooperative Study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abrahams S., Haylett W.L., Johnson G., Carr J.A., Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 95.White C.M., Pasupuleti V., Roman Y.M., Li Y., Hernandez A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;146:104280. doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
- 96.Di Meo F., Margarucci S., Galderisi U., Crispi S., Peluso G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients. 2019;11:2426. doi: 10.3390/nu11102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scuto M.C., Mancuso C., Tomasello B., Ontario M.L., Cavallaro A., Frasca F., Maiolino L., Trovato Salinaro A., Calabrese E.J., Calabrese V. Curcumin, Hormesis and the Nervous System. Nutrients. 2019;11:2417. doi: 10.3390/nu11102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopresti A.L., Smith S.J., Rea A., Michel S. Efficacy of a curcumin extract (Curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: A randomised, double-blind, placebo-controlled study. BMC Complement. Med. Ther. 2021;21:40. doi: 10.1186/s12906-021-03220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thota R.N., Rosato J.I., Dias C.B., Burrows T.L., Martins R.N., Garg M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients. 2020;12:1032. doi: 10.3390/nu12041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zam W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018;2018:1367984. doi: 10.1155/2018/1367984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giacosa A., Riva A., Petrangolini G., Allegrini P., Fazia T., Bernardinelli L., Peroni G., Rondanelli M. Beneficial Effects on Abdominal Bloating with an Innovative Food-Grade Formulation of Curcuma longa and Boswellia serrata Extracts in Subjects with Irritable Bowel Syndrome and Small Bowel Dysbiosis. Nutrients. 2022;14:416. doi: 10.3390/nu14030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dai W., Wang H., Fang J., Zhu Y., Zhou J., Wang X., Zhou Y., Zhou M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018;140:65–71. doi: 10.1016/j.brainresbull.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 103.Yu Y., Wu S., Li J., Wang R., Xie X., Yu X., Pan J., Xu Y., Zheng L. The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: Involvement of 5-HT-dependent signaling. Metab. Brain Dis. 2015;30:47–55. doi: 10.1007/s11011-014-9554-z. [DOI] [PubMed] [Google Scholar]
- 104.Wynn J.K., Green M.F., Hellemann G., Karunaratne K., Davis M.C., Marder S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018;195:572–573. doi: 10.1016/j.schres.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 105.Salarolli R.T., Alvarenga L., Cardozo L.F.M.F., Teixeira K.T.R., de S.G. Moreira L., Lima J.D., Rodrigues S.D., Nakao L.S., Fouque D., Mafra D. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int. Urol. Nephrol. 2021;53:1231–1238. doi: 10.1007/s11255-020-02760-z. [DOI] [PubMed] [Google Scholar]
- 106.Serra D., Henriques J.F., Sousa F.J., Laranjo M., Resende R., Ferreira-Marques M., de Freitas V., Silva G., Peça J., Dinis T.C.P., et al. Attenuation of Autism-like Behaviors by an Anthocyanin-Rich Extract from Portuguese Blueberries via Microbiota-Gut-Brain Axis Modulation in a Valproic Acid Mouse Model. Int. J. Mol. Sci. 2022;23:9259. doi: 10.3390/ijms23169259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H., Xiao C., Wang F., Guo X., Zhou Z., Jiang Y. Blueberry-Mulberry Extract Alleviates Cognitive Impairment, Regulates Gut Metabolites, and Inhibits Inflammation in Aged Mice. Foods. 2023;12:860. doi: 10.3390/foods12040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Si X., Bi J., Chen Q., Cui H., Bao Y., Tian J., Shu C., Wang Y., Tan H., Zhang W., et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021;69:3658–3666. doi: 10.1021/acs.jafc.0c07637. [DOI] [PubMed] [Google Scholar]
- 109.Huang F., Marungruang N., Kostiuchenko O., Kravchenko N., Burleigh S., Prykhodko O., Hållenius F.F., Heyman-Lindén L. Identification of Nordic Berries with Beneficial Effects on Cognitive Outcomes and Gut Microbiota in High-Fat-Fed Middle-Aged C57BL/6J Mice. Nutrients. 2022;14:2734. doi: 10.3390/nu14132734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vendrame S., Guglielmetti S., Riso P., Arioli S., Klimis-Zacas D., Porrini M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011;59:12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 111.Ntemiri A., Ghosh T.S., Gheller M.E., Tran T.T.T., Blum J.E., Pellanda P., Vlckova K., Neto M.C., Howell A., Thalacker-Mercer A., et al. Whole Blueberry and Isolated Polyphenol-Rich Fractions Modulate Specific Gut Microbes in an In Vitro Colon Model and in a Pilot Study in Human Consumers. Nutrients. 2020;12:2800. doi: 10.3390/nu12092800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krikorian R., Skelton M.R., Summer S.S., Shidler M.D., Sullivan P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients. 2022;14:1619. doi: 10.3390/nu14081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boespflug E.L., Eliassen J.C., Dudley J.A., Shidler M.D., Kalt W., Summer S.S., Stein A.L., Stover A.N., Krikorian R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018;21:297–305. doi: 10.1080/1028415X.2017.1287833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wood E., Hein S., Mesnage R., Fernandes F., Abhayaratne N., Xu Y., Zhang Z., Bell L., Williams C., Rodriguez-Mateos A. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2023;117:1306–1319. doi: 10.1016/j.ajcnut.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nieman D.C., Sakaguchi C.A., Omar A.M., Davis K.L., Shaffner C.E., Strauch R.C., Lila M.A., Zhang Q. Blueberry intake elevates post-exercise anti-inflammatory oxylipins: A randomized trial. Sci. Rep. 2023;13:11976. doi: 10.1038/s41598-023-39269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilder-Smith C.H., Materna A., Olesen S.S. Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders. Nutrients. 2023;15:2396. doi: 10.3390/nu15102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soni M.G., Burdock G.A., Christian M.S., Bitler C.M., Crea R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006;44:903–915. doi: 10.1016/j.fct.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Miraglia N., Bianchi D., Trentin A., Volpi N., Soni M.G. Safety assessment of non-animal chondroitin sulfate sodium: Subchronic study in rats, genotoxicity tests and human bioavailability. Food Chem. Toxicol. 2016;93:89–101. doi: 10.1016/j.fct.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 119.Cordaro M., Trovato Salinaro A., Siracusa R., D‘Amico R., Impellizzeri D., Scuto M., Ontario M.L., Crea R., Cuzzocrea S., Di Paola R., et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants. 2021;10:818. doi: 10.3390/antiox10050818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siracusa R., Scuto M., Fusco R., Trovato A., Ontario M.L., Crea R., Di Paola R., Cuzzocrea S., Calabrese V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants. 2020;9:824. doi: 10.3390/antiox9090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D‘Amico R., Trovato Salinaro A., Cordaro M., Fusco R., Impellizzeri D., Interdonato L., Scuto M., Ontario M.L., Crea R., Siracusa R., et al. Hidrox® and Chronic Cystitis: Biochemical Evaluation of Inflammation, Oxidative Stress, and Pain. Antioxidants. 2021;10:1046. doi: 10.3390/antiox10071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Rosa G., Brunetti G., Scuto M., Trovato Salinaro A., Calabrese E.J., Crea R., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan Enhancement by Olive Polyphenols in C. elegans Wild Type and Parkinson’s Models. Int. J. Mol. Sci. 2020;21:3893. doi: 10.3390/ijms21113893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brunetti G., Di Rosa G., Scuto M., Leri M., Stefani M., Schmitz-Linneweber C., Calabrese V., Saul N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020;21:2588. doi: 10.3390/ijms21072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoon J., Sasaki K., Nishimura I., Hashimoto H., Okura T., Isoda H. Effects of Desert Olive Tree Pearls Containing High Hydroxytyrosol Concentrations on the Cognitive Functions of Middle-Aged and Older Adults. Nutrients. 2023;15:3234. doi: 10.3390/nu15143234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miao F. Hydroxytyrosol alleviates dextran sodium sulfate-induced colitis by inhibiting NLRP3 inflammasome activation and modulating gut microbiota in vivo. Nutrition. 2022;97:111579. doi: 10.1016/j.nut.2021.111579. [DOI] [PubMed] [Google Scholar]
- 126.Wang Q., Wang C., Abdullah, Tian W., Qiu Z., Song M., Cao Y., Xiao J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022;70:2241–2252. doi: 10.1021/acs.jafc.1c07568. [DOI] [PubMed] [Google Scholar]
- 127.Han H., Zhong R., Zhang S., Wang M., Wen X., Yi B., Zhao Y., Chen L., Zhang H. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J. Nutr. Biochem. 2023;113:109256. doi: 10.1016/j.jnutbio.2022.109256. [DOI] [PubMed] [Google Scholar]
- 128.Martín-Peláez S., Mosele J.I., Pizarro N., Farràs M., de la Torre R., Subirana I., Pérez-Cano F.J., Castañer O., Solà R., Fernandez-Castillejo S., et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017;56:119–131. doi: 10.1007/s00394-015-1063-2. [DOI] [PubMed] [Google Scholar]
- 129.Caser M., Demasi S., Stelluti S., Donno D., Scariot V. Crocus sativus L. Cultivation in Alpine Environments: Stigmas and Tepals as Source of Bioactive Compounds. Agronomy. 2020;10:1473. doi: 10.3390/agronomy10101473. [DOI] [Google Scholar]
- 130.Hatziagapiou K., Kakouri E., Lambrou G.I., Bethanis K., Tarantilis P.A. Antioxidant Properties of Crocus sativus L. and Its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s and Parkinson’s Disease. Curr. Neuropharmacol. 2019;17:377–402. doi: 10.2174/1570159X16666180321095705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeinali M., Zirak M.R., Rezaee S.A., Karimi G., Hosseinzadeh H. Immunoregulatory and Anti-Inflammatory Properties of Crocus sativus (Saffron) and Its Main Active Constituents: A Review. Iran. J. Basic Med. Sci. 2019;22:334–344. doi: 10.22038/ijbms.2019.34365.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saleem N., Ahmad M., Kamran S., Riaz H., Mehmood Y., Raza S. Hepatoprotective Effect of Crocus sativus on Amiodarone-Induced Liver Toxicity. Br. J. Pharm. Res. 2016;12:1–11. doi: 10.9734/BJPR/2016/27219. [DOI] [Google Scholar]
- 133.Scuto M., Modafferi S., Rampulla F., Zimbone V., Tomasello M., Spano’ S., Ontario M.L., Palmeri A., Trovato Salinaro A., Siracusa R., et al. Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: From molecular basis to therapy. Mech. Ageing Dev. 2022;205:111686. doi: 10.1016/j.mad.2022.111686. [DOI] [PubMed] [Google Scholar]
- 134.Siddiqui M., Saleh M.M., Basharuddin S.B.B., Zamri S.B., Mohd Najib M.B., Che Ibrahim M., Binti Mohd Noor N., Binti Mazha H., Mohd Hassan N., Khatib A. Saffron (Crocus sativus L.): As an Antidepressant. J. Pharm. Bioallied Sci. 2018;10:173. doi: 10.4103/JPBS.JPBS_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samarghandian S., Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Agarwal N., Kolba N., Jung Y., Cheng J., Tako E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology In Vivo (Gallus gallus) Nutrients. 2022;14:220. doi: 10.3390/nu14010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khodir A.E., Said E., Atif H., ElKashef H.A., Salem H.A. Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats. Biomed. Pharmacother. 2019;110:389–399. doi: 10.1016/j.biopha.2018.11.133. [DOI] [PubMed] [Google Scholar]
- 138.Lei X., Zhou Z., Wang S., Jin L.H. The protective effect of safranal against intestinal tissue damage in Drosophila. Toxicol. Appl. Pharmacol. 2022;439:115939. doi: 10.1016/j.taap.2022.115939. [DOI] [PubMed] [Google Scholar]
- 139.Li M., Ding L., Hu Y.L., Qin L.L., Wu Y., Liu W., Wu L.L., Liu T.H. Herbal formula LLKL ameliorates hyperglycaemia, modulates the gut microbiota and regulates the gut-liver axis in Zucker diabetic fatty rats. J. Cell. Mol. Med. 2021;25:367–382. doi: 10.1111/jcmm.16084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akbari-Fakhrabadi M., Najafi M., Mortazavian S., Memari A.H., Shidfar F., Shahbazi A., Heshmati J. Saffron (Crocus sativus L.), Combined with Endurance Exercise, Synergistically Enhances BDNF, Serotonin, and NT-3 in Wistar Rats. Rep. Biochem. Mol. Biol. 2021;9:426–434. doi: 10.52547/rbmb.9.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Linardaki Z.I., Lamari F.N., Margarity M. Saffron (Crocus sativus L.) Tea Intake Prevents Learning/Memory Defects and Neurobiochemical Alterations Induced by Aflatoxin B1 Exposure in Adult Mice. Neurochem. Res. 2017;42:2743–2754. doi: 10.1007/s11064-017-2283-z. [DOI] [PubMed] [Google Scholar]
- 142.Abbaszade-Cheragheali A., Beheshti F., Kakhki S., Khatibi S.R., Dehnokhalaji F., Akbari E., Fathi H., Safari Farimani S. Crocin, the main active saffron (Crocus sativus L.) constituent, as a potential candidate to prevent anxiety and depressive-like behaviors induced by unpredictable chronic mild stress. Neurosci. Lett. 2022;791:136912. doi: 10.1016/j.neulet.2022.136912. [DOI] [PubMed] [Google Scholar]
- 143.Kell G., Rao A., Beccaria G., Clayton P., Inarejos-García A.M., Prodanov M. Saffron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017;33:58–64. doi: 10.1016/j.ctim.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Ren Y., Geng Y., Du Y., Li W., Lu Z.M., Xu H.Y., Xu G.H., Shi J.S., Xu Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018;57:67–76. doi: 10.1016/j.jnutbio.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Trovato A., Siracusa R., Di Paola R., Scuto M., Fronte V., Koverech G., Luca M., Serra A., Toscano M.A., Petralia A., et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology. 2016;53:350–358. doi: 10.1016/j.neuro.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 146.Scuto M., Di Mauro P., Ontario M.L., Amato C., Modafferi S., Ciavardelli D., Trovato Salinaro A., Maiolino L., Calabrese V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019;21:284. doi: 10.3390/ijms21010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.D‘Amico R., Trovato Salinaro A., Fusco R., Cordaro M., Impellizzeri D., Scuto M., Ontario M.L., Lo Dico G., Cuzzocrea S., Di Paola R., et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants. 2021;10:898. doi: 10.3390/antiox10060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Modafferi S., Lupo G., Tomasello M., Rampulla F., Ontario M., Scuto M., Salinaro A.T., Arcidiacono A., Anfuso C.D., Legmouz M., et al. Antioxidants, Hormetic Nutrition, and Autism. Curr. Neuropharmacol. 2023;22:1156–1168. doi: 10.2174/1570159X21666230817085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hou X.-X., Liu J.-Y., Li Z.-Y., Chang M.-C., Guo M., Feng C.-P., Shi J.-Y. Fruiting body polysaccharides of Hericium erinaceus induce apoptosis in human colorectal cancer cells via ROS generation mediating caspase-9-dependent signaling pathways. Food Funct. 2020;11:6128–6138. doi: 10.1039/D0FO00916D. [DOI] [PubMed] [Google Scholar]
- 150.Lai P.L., Naidu M., Sabaratnam V., Wong K.H., David R.P., Kuppusamy U.R., Abdullah N., Malek S.N. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms. 2013;15:539–554. doi: 10.1615/IntJMedMushr.v15.i6.30. [DOI] [PubMed] [Google Scholar]
- 151.Kim S. Antioxidant Compounds for the Inhibition of Enzymatic Browning by Polyphenol Oxidases in the Fruiting Body Extract of the Edible Mushroom Hericium erinaceus. Foods. 2020;9:51. doi: 10.3390/foods9070951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cho H.W., Choi S., Seo K., Kim K.H., Jeon J.H., Kim C.H., Lim S., Jeong S., Chun J.L. Gutmicrobiota profiling in aged dogs after feeding pet food contained Hericium erinaceus. J. Anim. Sci. Technol. 2022;64:937–949. doi: 10.5187/jast.2022.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shao S., Wang D., Zheng W., Li X., Zhang H., Zhao D., Wang M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019;71:411–422. doi: 10.1016/j.intimp.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 154.Pérez-Reytor D., Puebla C., Karahanian E., García K. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front. Physiol. 2021;12:650313. doi: 10.3389/fphys.2021.650313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guan Y., Shi D., Wang S., Sun Y., Song W., Liu S., Wang C. Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients. 2023;15:3799. doi: 10.3390/nu15173799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang Y., Ye H., Zhao C., Ren L., Wang C., Georgiev M.I., Xiao J., Zhang T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021;262:117668. doi: 10.1016/j.carbpol.2021.117668. [DOI] [PubMed] [Google Scholar]
- 157.Ren Z., Xu Z., Amakye W.K., Liu W., Zhao Z., Gao L., Wang M., Ren J. Hericium erinaceus mycelium-Derived Polysaccharide Alleviates Ulcerative Colitis and Modulates Gut Microbiota in Cynomolgus Monkeys. Mol. Nutr. Food Res. 2023;67:e2200450. doi: 10.1002/mnfr.202200450. [DOI] [PubMed] [Google Scholar]
- 158.Ren Y., Sun Q., Gao R., Sheng Y., Guan T., Li W., Zhou L., Liu C., Li H., Lu Z., et al. Low Weight Polysaccharide of Hericium erinaceus Ameliorates Colitis via Inhibiting the NLRP3 Inflammasome Activation in Association with Gut Microbiota Modulation. Nutrients. 2023;15:739. doi: 10.3390/nu15030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xie X.Q., Geng Y., Guan Q., Ren Y., Guo L., Lv Q., Lu Z.M., Shi J.S., Xu Z.H. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients. 2021;13:1008. doi: 10.3390/nu13031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pallav K., Dowd S.E., Villafuerte J., Yang X., Kabbani T., Hansen J., Dennis M., Leffler D.A., Newburg D.S., Kelly C.P. Effects of polysaccharopeptide from Trametes versicolor and amoxicillin on the gut microbiome of healthy volunteers: A randomized clinical trial. Gut Microbes. 2014;5:458–467. doi: 10.4161/gmic.29558. [DOI] [PubMed] [Google Scholar]
- 161.Impellizzeri D., Fusco R., Genovese T., Cordaro M., D‘Amico R., Trovato Salinaro A., Ontario M.L., Modafferi S., Cuzzocrea S., Di Paola R., et al. Coriolus versicolor Downregulates TLR4/NF-κB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants. 2022;11:406. doi: 10.3390/antiox11020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cordaro M., Modafferi S., D‘Amico R., Fusco R., Genovese T., Peritore A.F., Gugliandolo E., Crupi R., Interdonato L., Di Paola D., et al. Natural Compounds Such as Hericium erinaceus and Coriolus versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines. 2022;10:2505. doi: 10.3390/biomedicines10102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Di Paola R., Siracusa R., Fusco R., Ontario M., Cammilleri G., Pantano L., Scuto M., Tomasello M., Spanò S., Trovato Salinaro A., et al. Redox Modulation of Meniere Disease by Coriolus versicolor Treatment, a Nutritional Mushroom Approach with Neuroprotective Potential. Curr. Neuropharmacol. 2023 doi: 10.2174/1570159X22666231206153936. Online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tang H., Zha Z., Tan Y., Li Y., Jiao Y., Yang B., Xiong Q., Yin H., Wang H. Extraction and characterization of polysaccharide from fermented mycelia of Coriolus versicolor and its efficacy for treating nonalcoholic fatty liver disease. Int. J. Biol. Macromol. 2023;248:125951. doi: 10.1016/j.ijbiomac.2023.125951. [DOI] [PubMed] [Google Scholar]
- 165.Karatsoreos I.N., McEwen B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 166.Mc Ewen B.S., Gianaros P.J. Stress and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kupriyanov R., Zhdanov R. The eustress concept: Problems and outlooks. World J. Med. Sci. 2014;11:179–185. [Google Scholar]
- 168.Teckentrup V., Kroemer N.B. Mechanisms for survival: Vagal control of goal-directed behavior. Trends Cogn. Sci. 2024;28:237–251. doi: 10.1016/j.tics.2023.11.001. [DOI] [PubMed] [Google Scholar]
- 169.Köhler C.A., Maes M., Slyepchenko A., Berk M., Solmi M., Lanctôt K.L., Carvalho A.F. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: Mechanisms and pathophysiological role in Alzheimer’s disease. Curr. Pharm. Des. 2016;22:6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 170.Westfall S., Iqbal U., Sebastian M., Pasinetti G.M. Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2019;168:147–181. doi: 10.1016/bs.pmbts.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 171.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang L., Muscat J.E., Chinchilli V.M., Kris-Etherton P.M., Al-Shaar L., Richie J.P. Berry Consumption in Relation to Allostatic Load in US Adults: The National Health and Nutrition Examination Survey, 2003–2010. Nutrients. 2024;16:403. doi: 10.3390/nu16030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arck P., Handjiski B., Hagen E., Pincus M., Bruenahl C., Bienenstock J., Paus R. Is there a g‘ut-brain-skin axis’? Exp. Dermatol. 2010;19:401–405. doi: 10.1111/j.1600-0625.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 174.Louwies T., Johnson A.C., Orock A., Yan T., van Meerveld B.G. The microbiota-gut-brain axis: An emerging role for the epigenome. Exp. Biol. Med. 2020;245:138–145. doi: 10.1177/1535370219891690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu L., Li Y., Tollefsbol T.O. Gene-environment interactions and epigenetic basis of human diseases. Curr. Issues Mol. Biol. 2008;10:25–36. doi: 10.21775/cimb.010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Begum N., Mandhare A., Tryphena K.P., Srivastava S., Shaikh M.F., Singh S.B., Khatri D.K. Epigenetics in depression and gut-brain axis: A molecular crosstalk. Front. Aging Neurosci. 2022;14:1048333. doi: 10.3389/fnagi.2022.1048333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abdolmaleky H.M., Zhou J.R., Thiagalingam S. An update on the epigenetics of psychotic diseases and autism. Epigenomics. 2015;7:427–449. doi: 10.2217/epi.14.85. [DOI] [PubMed] [Google Scholar]
- 178.Koh A., de Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 179.Caspani G., Kennedy S., Foster J.A., Swann J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell. 2019;6:454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohórquez D.V. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;61:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Woo V., Alenghat T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022;14:2022407. doi: 10.1080/19490976.2021.2022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 184.Cuomo M., Coretti L., Costabile D., Della Monica R., De Riso G., Buonaiuto M., Trio F., Bravaccio C., Visconti R., Berni Canani R., et al. Host fecal DNA specific methylation signatures mark gut dysbiosis and inflammation in children affected by autism spectrum disorder. Sci. Rep. 2023;13:18197. doi: 10.1038/s41598-023-45132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blum K., Thanos P.K., Wang G.J., Febo M., Demetrovics Z., Modestino E.J., Braverman E.R., Baron D., Badgaiyan R.D., Gold M.S. The Food and Drug Addiction Epidemic: Targeting Dopamine Homeostasis. Curr. Pharm. Des. 2018;23:6050–6061. doi: 10.2174/1381612823666170823101713. [DOI] [PubMed] [Google Scholar]
- 186.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 187.Martin-Gallausiaux C., Larraufie P., Jarry A., Beguet-Crespel F., Marinelli L., Ledue F., Reimann F., Blottiere H.M., Lapaque N. Butyrate produced by commensal bacteria down-regulates indolamine 2,3-dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front. Immunol. 2018;9:2838. doi: 10.3389/fimmu.2018.02838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maes M., Leonard B.E., Myint A.M., Kubera M., Verkerk R. The new 5‘-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 189.Kaluzna-Czaplinska J., Gatarek P., Chirumbolo S., Chartrand M.S., Bjorklund G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019;59:72–88. doi: 10.1080/10408398.2017.1357534. [DOI] [PubMed] [Google Scholar]
- 190.Luna R.A., Oezguen N., Balderas M., Venkatachalam A., Runge J.K., Versalovic J., Veenstra-VanderWeele J., Anderson G.M., Savidge T., Williams K.C. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell. Mol. Gastroenterol. Hepatol. 2017;3:218–230. doi: 10.1016/j.jcmgh.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cervantes-Barragan L., Chai J.N., Tianero M.D., Di Luccia B., Ahern P.P., Merriman J., Cortez V.S., Caparon M.G., Donia M.S., Gilfillan S., et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cuomo M., Borrelli L., Della Monica R., Coretti L., De Riso G., D‘Angelo Lancellotti di Durazzo L., Fioretti A., Lembo F., Dinan T.G., Cryan J.F., et al. DNA Methylation Profiles of Tph1A and BDNF in Gut and Brain of L. rhamnosus-Treated Zebrafish. Biomolecules. 2021;11:142. doi: 10.3390/biom11020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yu K., Li Q., Sun X., Peng X., Tang Q., Chu H., Zhou L., Wang B., Zhou Z., Deng X., et al. Bacterial indole-3-lactic acid affects epithelium-macrophage crosstalk to regulate intestinal homeostasis. Proc. Natl. Acad. Sci. USA. 2023;120:e2309032120. doi: 10.1073/pnas.2309032120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang X., Shi L., Wang N., Li Q., Zhang L., Han N., Yan T., Ren D., Zhang B., Zhao Y., et al. Gut Bacterial Indole-3-acetic Acid Induced Immune Promotion Mediates Preventive Effects of Fu Brick Tea Polyphenols on Experimental Colitis. J. Agric. Food Chem. 2023;71:1201–1213. doi: 10.1021/acs.jafc.2c06517. [DOI] [PubMed] [Google Scholar]
- 195.Kundi Z.M., Lee J.C., Pihlajamäki J., Chan C.B., Leung K.S., So S.S.Y., Nordlund E., Kolehmainen M., El-Nezami H. Dietary Fiber from Oat and Rye Brans AmeliorateWestern Diet-Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021;65:e1900580. doi: 10.1002/mnfr.201900580. [DOI] [PubMed] [Google Scholar]
- 196.Tuomainen M., Lindström J., Lehtonen M., Auriola S., Pihlajamäki J., Peltonen M., Tuomilehto J., Uusitupa M., de Mello V.D., Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes. 2018;8:35. doi: 10.1038/s41387-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Strandwitz P., Kim K.H., Terekhova D., Liu J.K., Sharma A., Levering J., McDonald D., Dietrich D., Ramadhar T.R., Lekbua A., et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang H.S., Matevossian A., Whittle C., Kim S.Y., Schumacher A., Baker S.P., Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J. Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nobile V., Giardina S., Puoci F. The Effect of a Probiotic Complex on the Gut-Brain Axis: A Translational Study. Neuropsychobiology. 2022;81:116–126. doi: 10.1159/000518385. [DOI] [PubMed] [Google Scholar]
- 201.Yan J., Xue Q., Chen W., Wang K., Peng D., Jiang J., Li P., Du B. Probiotic-fermented rice buckwheat alleviates high-fat diet-induced hyperlipidemia in mice by suppressing lipid accumulation and modulating gut microbiota. Food Res. Int. 2022;155:111125. doi: 10.1016/j.foodres.2022.111125. [DOI] [PubMed] [Google Scholar]
- 202.Santos-Terra J., Deckmann I., Carello-Collar G., Nunes G.D., Bauer-Negrini G., Schwingel G.B., Fontes-Dutra M., Riesgo R., Gottfried C. Resveratrol Prevents Cytoarchitectural and Interneuronal Alterations in the Valproic Acid Rat Model of Autism. Int. J. Mol. Sci. 2022;23:4075. doi: 10.3390/ijms23084075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dubois T., Reynaert C., Jacques D., Lepiece B., Zdanowicz N. From Family Surroundings to Intestinal Flora, A Literature Review Concerning Epigenetic Processes in Psychiatric Disorders. Psychiatr. Danub. 2020;32:158–163. [PubMed] [Google Scholar]
- 204.Possamai-Della T., Cararo J.H., Aguiar-Geraldo J.M., Peper-Nascimento J., Zugno A.I., Fries G.R., Quevedo J., Valvassori S.S. Prenatal Stress Induces Long-Term Behavioral Sex-Dependent Changes in Rats Offspring: The Role of the HPA Axis and Epigenetics. Mol. Neurobiol. 2023;60:5013–5033. doi: 10.1007/s12035-023-03348-1. [DOI] [PubMed] [Google Scholar]
- 205.Rizavi H.S., Khan O.S., Zhang H., Bhaumik R., Grayson D.R., Pandey G.N. Methylation and expression of glucocorticoid receptor exon-1 variants and FKBP5 in teenage suicide-completers. Transl. Psychiatry. 2023;13:53. doi: 10.1038/s41398-023-02345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weaver I.C., Champagne F.A., Brown S.E., Dymov S., Sharma S., Meaney M.J., Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J. Neurosci. 2005;5:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Perroud N., Paoloni-Giacobino A., Prada P., Olié E., Salzmann A., Nicastro R., Guillaume S., Mouthon D., Stouder C., Dieben K., et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Transl. Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Amadi C.N., Orish C.N., Frazzoli C., Orisakwe O.E. Dietary interventions for autism spectrum disorder: An updated systematic review of human studies. Psychiatriki. 2022;33:228–242. doi: 10.22365/jpsych.2022.073. [DOI] [PubMed] [Google Scholar]
- 209.Wang X., Sun Z., Yang T., Lin F., Ye S., Yan J., Li T., Chen J. Sodium butyrate facilitates CRHR2 expression to alleviate HPA axis hyperactivity in autism-like rats induced by prenatal lipopolysaccharides through histone deacetylase inhibition. mSystems. 2023;8:e0041523. doi: 10.1128/msystems.00415-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Donoso F., Egerton S., Bastiaanssen T.F.S., Fitzgerald P., Gite S., Fouhy F., Ross R.P., Stanton C., Dinan T.G., Cryan J.F. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology. 2020;116:104673. doi: 10.1016/j.psyneuen.2020.104673. [DOI] [PubMed] [Google Scholar]
- 211.Reddy V.P., Aryal P., Robinson S., Rafiu R., Obrenovich M., Perry G. Polyphenols in Alzheimer’s Disease and in the Gut–Brain Axis. Microorganisms. 2020;8:199. doi: 10.3390/microorganisms8020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang L., Cui Y., Liang H., Li Z., Wang N., Wang Y., Zheng G. Multifunctional Selenium Nanoparticles with Different Surface Modifications Ameliorate Neuroinflammation through the Gut Microbiota-NLRP3 Inflammasome-Brain Axis in APP/PS1 Mice. ACS Appl. Mater. Interfaces. 2022;14:30557–30570. doi: 10.1021/acsami.2c06283. [DOI] [PubMed] [Google Scholar]
- 213.Shen Y., Cao B., Snyder N.R., Woeppel K.M., Eles J.R., Cui X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018;16:13. doi: 10.1186/s12951-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gao C., Wang Y., Sun J., Han Y., Gong W., Li Y., Feng Y., Wang H., Yang M., Li Z., et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020;108:285–299. doi: 10.1016/j.actbio.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 215.Giacomeli R., Izoton J.C., Dos Santos R.B., Boeira S.P., Jesse C.R., Haas S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019;1721:146325. doi: 10.1016/j.brainres.2019.146325. [DOI] [PubMed] [Google Scholar]
- 216.Sadegh Malvajerd S., Izadi Z., Azadi A., Kurd M., Derakhshankhah H., Sharifzadeh M., Akbari Javar H., Hamidi M. Neuroprotective potential of curcumin-loaded nanostructured lipid carrier in an animal model of Alzheimer’s disease: Behavioral and biochemical evidence. J. Alzheimer’s Dis. 2019;69:671–686. doi: 10.3233/JAD-190083. [DOI] [PubMed] [Google Scholar]
- 217.Sadegh Malvajerd S., Azadi A., Izadi Z., Kurd M., Dara T., Dibaei M., Sharif Zadeh M., Akbari Javar H., Hamidi M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 2018;10:728–739. doi: 10.1021/acschemneuro.8b00510. [DOI] [PubMed] [Google Scholar]
- 218.Yang R., Zheng Y., Wang Q., Zhao L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced A_ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018;13:330. doi: 10.1186/s11671-018-2759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Small G.W., Siddarth P., Li Z., Miller K.J., Ercoli L., Emerson N.D., Martinez J., Wong K.P., Liu J., Merrill D.A., et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry. 2018;26:266–277. doi: 10.1016/j.jagp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 220.Ohno M., Nishida A., Sugitani Y., Nishino K., Inatomi O., Sugimoto M., Kawahara M., Andoh A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE. 2017;12:e0185999. doi: 10.1371/journal.pone.0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.He H., Qin Q., Xu F., Chen Y., Rao S., Wang C., Jiang X., Lu X., Xie C. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci. Adv. 2023;9:eadf3887. doi: 10.1126/sciadv.adf3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li C., Wang N., Zheng G., Yang L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces. 2021;13:46406–46420. doi: 10.1021/acsami.1c14818. [DOI] [PubMed] [Google Scholar]
- 223.Yang L., Wang Y., Li Z., Wu X., Mei J., Zheng G. Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery: Impact on insulin resistance and gut microbiota in obesity-related Alzheimer’s disease. Carbohydr. Polym. 2023;310:120714. doi: 10.1016/j.carbpol.2023.120714. [DOI] [PubMed] [Google Scholar]
- 224.Pivari F., Mingione A., Piazzini G., Ceccarani C., Ottaviano E., Brasacchio C., Dei Cas M., Vischi M., Cozzolino M.G., Fogagnolo P., et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients. 2022;14:231. doi: 10.3390/nu14010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sugimoto K., Ikeya K., Bamba S., Andoh A., Yamasaki H., Mitsuyama K., Nasuno M., Tanaka H., Matsuura A., Kato M., et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn’s Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns Colitis. 2020;14:1693–1701. doi: 10.1093/ecco-jcc/jjaa097. [DOI] [PubMed] [Google Scholar]
- 226.Kobayashi Y., Kinoshita T., Matsumoto A., Yoshino K., Saito I., Xiao J.Z. Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study. J. Prev. Alzheimers Dis. 2019;6:70–75. doi: 10.14283/jpad.2018.32. [DOI] [PubMed] [Google Scholar]
- 227.Lee Y., Nguyen T.L., Roh H., Kim A., Park J., Lee J.Y., Kang Y.R., Kang H., Sohn M.Y., Park C.I., et al. Mechanisms underlying probiotic effects on neurotransmission and stress resilience in fish via transcriptomic profiling. Fish Shellfish Immunol. 2023;141:109063. doi: 10.1016/j.fsi.2023.109063. [DOI] [PubMed] [Google Scholar]
- 228.Kim C.S., Jung S., Hwang G.S., Shin D.M. Gut microbiota indole-3-propionic acid mediates neuroprotective effect of probiotic consumption in healthy elderly: A randomized, double-blind, placebo-controlled, multicenter trial and in vitro study. Clin. Nutr. 2023;42:1025–1033. doi: 10.1016/j.clnu.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 229.Oroojzadeh P., Bostanabad S.Y., Lotfi H. Psychobiotics: The Influence of Gut Microbiota on the Gut-Brain Axis in Neurological Disorders. J. Mol. Neurosci. 2022;72:1952–1964. doi: 10.1007/s12031-022-02053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hao Z., Wang W., Guo R., Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019;104:132–142. doi: 10.1016/j.psyneuen.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 231.Tian P., O‘Riordan K.J., Lee Y.K., Wang G., Zhao J., Zhang H., Cryan J.F., Chen W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress. 2020;12:100216. doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yun S.W., Kim J.K., Lee K.E., Oh Y.J., Choi H.J., Han M.J., Kim D.H. A Probiotic Lactobacillus gasseri Alleviates Escherichia coli-Induced Cognitive Impairment and Depression in Mice by Regulating IL-1β Expression and Gut Microbiota. Nutrients. 2020;12:3441. doi: 10.3390/nu12113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jang H.M., Lee K.E., Kim D.H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients. 2019;11:819. doi: 10.3390/nu11040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gao K., Farzi A., Ke X., Yu Y., Chen C., Chen S., Yu T., Wang H., Li Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022;13:957–969. doi: 10.1039/D1FO03723D. [DOI] [PubMed] [Google Scholar]
- 235.Rudzki L., Ostrowska L., Pawlak D., Malus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 236.Schneider E., Doll J.P.K., Schweinfurth N., Kettelhack C., Schaub A.C., Yamanbaeva G., Varghese N., Mählmann L., Brand S., Eckert A., et al. Effect of short-term, high-dose probiotic supplementation on cognition, related brain functions and BDNF in patients with depression: A secondary analysis of a randomized controlled trial. J. Psychiatry Neurosci. 2023;48:E23–E33. doi: 10.1503/jpn.220117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Karakula-Juchnowicz H., Rog J., Juchnowicz D., Łoniewski I., Skonieczna-Żydecka K., Krukow P., Futyma-Jedrzejewska M., Kaczmarczyk M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): A 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 2019;18:50. doi: 10.1186/s12937-019-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Önning G., Hillman M., Hedin M., Montelius C., Eriksson J., Ahrné S., Jönsson P. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double blind, placebo-controlled study. Physiol. Behav. 2020;221:113083. doi: 10.1016/j.physbeh.2020.113083. [DOI] [PubMed] [Google Scholar]
- 239.Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M., Jafari P., Akbari H., Taghizadeh M., Memarzadeh M.R., Asemi Z., Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 240.Reininghaus E.Z., Platzer M., Kohlhammer-Dohr A., Hamm C., Mörkl S., Bengesser S.A., Fellendorf F.T., Lahousen-Luxenberger T., Leitner-Afschar B., Schöggl H., et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients. 2020;12:3422. doi: 10.3390/nu12113422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Santocchi E., Guiducci L., Fulceri F., Billeci L., Buzzigoli E., Apicella F., Calderoni S., Grossi E., Morales M.A., Muratori F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry. 2016;16:183. doi: 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kong X.J., Liu J., Liu K., Koh M., Sherman H., Liu S., Tian R., Sukijthamapan P., Wang J., Fong M., et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients. 2021;13:1552. doi: 10.3390/nu13051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hsu Y.C., Huang Y.Y., Tsai S.Y., Kuo Y.W., Lin J.H., Ho H.H., Chen J.F., Hsia K.C., Sun Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients. 2023;16:16. doi: 10.3390/nu16010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J.S., Nasuti C., Fiorini D., Boarelli M.C., Rossi G., Eleuteri A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bonfili L., Cecarini V., Cuccioloni M., Angeletti M., Berardi S., Scarpona S., Rossi G., Eleuteri A.M. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD Mouse Model. Mol. Neurobiol. 2018;55:7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ma X., Kim J.K., Shin Y.J., Son Y.H., Lee D.Y., Park H.S., Kim D.H. Alleviation of Cognitive Impairment-like Behaviors, Neuroinflammation, Colitis, and Gut Dysbiosis in 5xFAD Transgenic and Aged Mice by Lactobacillus mucosae and Bifidobacterium longum. Nutrients. 2023;15:3381. doi: 10.3390/nu15153381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ton A.M.M., Campagnaro B.P., Alves G.A., Aires R., Côco L.Z., Arpini C.M., Guerra E., Oliveira T., Campos-Toimil M., Meyrelles S.S., et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxidative Med. Cell. Longev. 2020;13:2020–2638703. doi: 10.1155/2020/2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tamtaji O.R., Heidari-Soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E., Tajabadi-Ebrahimi M., Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019;38:2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 249.Tamtaji O.R., Taghizadeh M., Daneshvar Kakhaki R., Kouchaki E., Bahmani F., Borzabadi S., Oryan S., Mafi A., Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 250.Aljumaah M.R., Bhatia U., Roach J., Gunstad J., Azcarate Peril M.A. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin. Nutr. 2022;41:2565–2576. doi: 10.1016/j.clnu.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 251.Asaoka D., Xiao J., Takeda T., Yanagisawa N., Yamazaki T., Matsubara Y., Sugiyama H., Endo N., Higa M., Kasanuki K., et al. Effect of Probiotic Bifidobacterium breve in Improving Cognitive Function and Preventing Brain Atrophy in Older Patients with Suspected Mild Cognitive Impairment: Results of a 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2022;88:75–95. doi: 10.3233/JAD-220148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bonaz B., Bazin T., Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Breit S., Kupferberg A., Rogler G., Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry. 2018;9:9–44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bonaz B., Sinniger V., Pellissier S. Vagus Nerve Stimulation at the Interface of Brain-Gut Interactions. Cold Spring Harb. Perspect. Med. 2019;9:a034199. doi: 10.1101/cshperspect.a034199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bonaz B., Sinniger V., Pellissier S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bonaz B., Sinniger V., Pellissier S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front. Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hosomoto K., Sasaki T., Yasuhara T., Kameda M., Sasada S., Kin I., Kuwahara K., Kawauchi S., Okazaki Y., Yabuno S., et al. Continuous vagus nerve stimulation exerts beneficial effects on rats with experimentally induced Parkinson’s disease: Evidence suggesting involvement of a vagal afferent pathway. Brain Stimul. 2023;16:594–603. doi: 10.1016/j.brs.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 258.George M., Nahas Z., Borckardt J.J., Anderson B., Burns C., Kose S., Short E.B. Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Expert Rev. Neurother. 2007;7:63–74. doi: 10.1586/14737175.7.1.63. [DOI] [PubMed] [Google Scholar]
- 259.Melrose J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants. 2023;12:663. doi: 10.3390/antiox12030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu J., Dai Q., Qu T., Ma J., Lv C., Wang H., Yu Y. Ameliorating effects of transcutaneous auricular vagus nerve stimulation on a mouse model of constipation-predominant irritable bowel syndrome. Neurobiol. Dis. 2024;193:106440. doi: 10.1016/j.nbd.2024.106440. [DOI] [PubMed] [Google Scholar]
- 261.Takada M., Nishida K., Kataoka-Kato A., Gondo Y., Ishikawa H., Suda K., Kawai M., Hoshi R., Watanabe O., Igarashi T., et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016;28:1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- 262.Sgritta M., Dooling S.W., Buffington S.A., Momin E.N., Francis M.B., Britton R.A., Costa-Mattioli M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101:246–259.e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.De Lorenzo A., Costacurta M., Merra G., Gualtieri P., Cioccoloni G., Marchetti M., Varvaras D., Docimo R., Di Renzo L. Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. J. Transl. Med. 2017;15:135. doi: 10.1186/s12967-017-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Repossi G., Das U.N., Eynard A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020;51:105–114. doi: 10.1016/j.arcmed.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 265.Knight C.M., Gutierrez-Juarez R., Lam T.K., Arrieta-Cruz I., Huang L., Schwartz G., Barzilai N., Rossetti L. Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes. 2011;11:2691–2700. doi: 10.2337/db10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ishii Y., Muta O., Teshima T., Hirasima N., Odaka M., Fushimi T., Fujii Y., Osakabe N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients. 2021;13:4214. doi: 10.3390/nu13124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lin T., Lu C.W., Hsieh P.W., Chiu K.M., Lee M.Y., Wang S.J. Natural Product Isoliquiritigenin Activates GABAB Receptors to Decrease Voltage-Gate Ca2+ Channels and Glutamate Release in Rat Cerebrocortical Nerve Terminals. Biomolecules. 2021;11:1537. doi: 10.3390/biom11101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lin T., Hung C.F., Weng J.R., Hsieh T.Y., Wang S.J. Kaempferol 3-Rhamnoside on Glutamate Release from Rat Cerebrocortical Nerve Terminals Involves P/Q-Type Ca2+ Channel and Ca2+/Calmodulin-Dependent Protein Kinase II-Dependent Pathway Suppression. Molecules. 2022;27:1342. doi: 10.3390/molecules27041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang C., Hsieh P.W., Kuo J.R., Wang S.J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules. 2021;11:1029. doi: 10.3390/biom11071029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wei I.H., Tu H.C., Huang C.C., Tsai M.H., Tseng C.Y., Shieh J.Y. (-)-Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. BMC Neurosci. 2011;12:52. doi: 10.1186/1471-2202-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rangel-Galván M., Rangel A., Romero-Méndez C., Dávila E.M., Castro M.E., Caballero N.A., Meléndez Bustamante F.J., Sanchez-Gaytan B.L., Meza U., Perez-Aguilar J.M. Inhibitory Mechanism of the Isoflavone Derivative Genistein in the Human CaV3.3 Channel. ACS Chem. Neurosci. 2021;12:651–659. doi: 10.1021/acschemneuro.0c00684. [DOI] [PubMed] [Google Scholar]
- 272.Ayabe T., Fukuda T., Ano Y. Improving Effects of Hop-Derived Bitter Acids in Beer on Cognitive Functions: A New Strategy for Vagus Nerve Stimulation. Biomolecules. 2020;10:131. doi: 10.3390/biom10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Skiba A., Pellegata D., Morozova V., Kozioł E., Budzyńska B., Lee S.M., Gertsch J., Skalicka-Woźniak K. Pharmacometabolic Effects of Pteryxin and Valproate on Pentylenetetrazole-Induced Seizures in Zebrafish Reveal Vagus Nerve Stimulation. Cells. 2023;12:1540. doi: 10.3390/cells12111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li H., Chen X., Liu J., Chen M., Huang M., Huang G., Chen X., Du Q., Su J., Lin R. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 2021;267:113445. doi: 10.1016/j.jep.2020.113445. [DOI] [PubMed] [Google Scholar]
- 275.Yu L., McGarry S., Cruickshank D., Jensen G.S. Rapid increase in immune surveillance and expression of NKT and γδT cell activation markers after consuming a nutraceutical supplement containing Aloe vera gel, extracts of Poria cocos and rosemary. A randomized placebo-controlled cross-over trial. PLoS ONE. 2023;18:e0291254. doi: 10.1371/journal.pone.0291254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gao W., Zhang T., Wu H. Emerging Pathological Engagement of Ferroptosis in Gut Diseases. Oxidative Med. Cell. Longev. 2021;2021:4246255. doi: 10.1155/2021/4246255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yuan Y., Zhai Y., Chen J., Xu X., Wang H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules. 2021;11:923. doi: 10.3390/biom11070923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Xu Z., Sun X., Ding B., Zi M., Ma Y. Resveratrol attenuated high intensity exercise training-induced inflammation and ferroptosis via Nrf2/FTH1/GPX4 pathway in intestine of mice. Turk. J. Med. Sci. 2023;53:446–454. doi: 10.55730/1300-0144.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ning K., Lu K., Chen Q., Guo Z., Du X., Riaz F., Feng L., Fu Y., Yin C., Zhang F., et al. Epigallocatechin Gallate Protects Mice against Methionine-Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota To Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega. 2020;5:20800–20809. doi: 10.1021/acsomega.0c01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ye Y., Jiang M., Hong X., Fu Y., Chen Y., Wu H., Sun Y., Wang X., Zhou E., Wang J., et al. Quercetin Alleviates Deoxynivalenol-Induced Intestinal Damage by Suppressing Inflammation and Ferroptosis in Mice. J. Agric. Food Chem. 2023;71:10761–10772. doi: 10.1021/acs.jafc.3c02027. [DOI] [PubMed] [Google Scholar]
- 282.Jiang J.J., Zhang G.F., Zheng J.Y., Sun J.H., Ding S.B. Targeting Mitochondrial ROS-Mediated Ferroptosis by Quercetin Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity. Front. Pharmacol. 2022;13:876550. doi: 10.3389/fphar.2022.876550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Liu Y., Xue N.J., Zheng R., Yan Y.Q., Wang Z.X., Li Y.L., Ying C.Z., Song Z., Tian J., Pu J.L., et al. Quercetin Protects against MPP/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis. Oxidative Med. Cell. Longev. 2022;2022:7769355. doi: 10.1155/2022/7769355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hofmann J., Spatz P., Walther R., Gutmann M., Maurice T., Decker M. Synthesis and Biological Evaluation of Flavonoid-Cinnamic Acid Amide Hybrids with Distinct Activity against Neurodegeneration in Vitro and in Vivo. Chemistry. 2022;39:e202200786. doi: 10.1002/chem.202200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kose T., Vera-Aviles M., Sharp P.A., Latunde-Dada G.O. Curcumin and (-)-Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals. 2019;12:26. doi: 10.3390/ph12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Du X.X., Xu H.M., Jiang H., Song N., Wang J., Xie J.X. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci. Bull. 2012;23:253–258. doi: 10.1007/s12264-012-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang X., Chen X., Zhou W., Men H., Bao T., Sun Y., Wang Q., Tan Y., Keller B.B., Tong Q., et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B. 2022;12:708–722. doi: 10.1016/j.apsb.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang Y.Q., Shi C.X., Zhang D.M., Zhang L.Y., Wang L.W., Gong Z.J. Sulforaphane, an NRF2 agonist, alleviates ferroptosis in acute liver failure by regulating HDAC6 activity. J. Integr. Med. 2023;21:464–473. doi: 10.1016/j.joim.2023.08.002. [DOI] [PubMed] [Google Scholar]
- 289.Xie L.W., Cai S., Zhao T.S., Li M., Tian Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic. Biol. Med. 2020;161:175–186. doi: 10.1016/j.freeradbiomed.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 290.Shao L., Dong C., Geng D., He Q., Shi Y. Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem. Biophys. Res. Commun. 2021;572:7–14. doi: 10.1016/j.bbrc.2021.07.081. [DOI] [PubMed] [Google Scholar]
- 291.Yang S., Xie Z., Pei T., Zeng Y., Xiong Q., Wei H., Wang Y., Cheng W. Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Abeta1-42-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin. Med. 2022;17:82. doi: 10.1186/s13020-022-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Monzani R., Gagliardi M., Clemente N., Saverio V., Pańczyszyn E., Santoro C., Yissachar N., Visciglia A., Pane M., Amoruso A., et al. The Gut-Ex-Vivo System (GEVS) Is a Dynamic and Versatile Tool for the Study of DNBS-Induced IBD in BALB/C and C57BL/6 Mice, Highlighting the Protective Role of Probiotics. Biology. 2022;11:1574. doi: 10.3390/biology11111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jarret A., Jackson R., Duizer C., Healy M.E., Zhao J., Rone J.M., Bielecki P., Sefik E., Roulis M., Rice T., et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell. 2020;180:813–814. doi: 10.1016/j.cell.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 294.Drokhlyansky E., Smillie C.S., Van Wittenberghe N., Ericsson M., Griffin G.K., Eraslan G., Dionne D., Cuoco M.S., Goder-Reiser M.N., Sharova T., et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182:1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O‘Donnell T.A., Brierley S.M., Ingraham H.A., Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Scuto M., Ontario M.L., Salinaro A.T., Caligiuri I., Rampulla F., Zimbone V., Modafferi S., Rizzolio F., Canzonieri V., Calabrese E.J., et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radic. Biol. Med. 2022;179:59–75. doi: 10.1016/j.freeradbiomed.2021.12.267. [DOI] [PubMed] [Google Scholar]
- 297.Hwang H.J., Lee S.R., Yoon J.G., Moon H.R., Zhang J., Park E., Yoon S.I., Cho J.A. Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells. Antioxidants. 2022;11:1448. doi: 10.3390/antiox11081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Beaumont M., Lencina C., Painteaux L., Viémon-Desplanque J., Phornlaphat O., Lambert W., Chalvon-Demersay T. A mix of functional amino acids and grape polyphenols promotes the growth of piglets, modulates the gut microbiota in vivo and regulates epithelial homeostasis in intestinal organoids. Amino Acids. 2022;54:1357–1369. doi: 10.1007/s00726-021-03082-9. [DOI] [PubMed] [Google Scholar]
- 299.Tveter K.M., Villa-Rodriguez J.A., Cabales A.J., Zhang L., Bawagan F.G., Duran R.M., Roopchand D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care. 2020;8:e001386. doi: 10.1136/bmjdrc-2020-001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Elbadawi M., Ammar R.M., Rabini S., Klauck S.M., Efferth T. Modulation of Intestinal Corticotropin-Releasing Hormone Signaling by the Herbal Preparation STW 5-II: Possible Mechanisms for Irritable Bowel Syndrome Management. Pharmaceuticals. 2022;15:1121. doi: 10.3390/ph15091121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wu H., Xie S., Miao J., Li Y., Wang Z., Wang M., Yu Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11:997–1014. doi: 10.1080/19490976.2020.1734423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lee H., Jung K.B., Kwon O., Son Y.S., Choi E., Yu W.D., Son N., Jeon J.H., Jo H., Yang H., et al. Limosilactobacillus reuteri DS0384 promotes intestinal epithelial maturation via the postbiotic effect in human intestinal organoids and infant mice. Gut Microbes. 2022;14:2121580. doi: 10.1080/19490976.2022.2121580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Engevik M., Ruan W., Visuthranukul C., Shi Z., Engevik K.A., Engevik A.C., Fultz R., Schady D.A., Spinler J.K., Versalovic J. Limosilactobacillus reuteri ATCC 6475 metabolites upregulate the serotonin transporter in the intestinal epithelium. Benef. Microbes. 2021;12:583–599. doi: 10.3920/BM2020.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Engevik M.A., Luck B., Visuthranukul C., Ihekweazu F.D., Engevik A.C., Shi Z., Danhof H.A., Chang-Graham A.L., Hall A., Endres B.T., et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol. Gastroenterol. Hepatol. 2021;11:221–248. doi: 10.1016/j.jcmgh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kim D.Y., Park J.Y., Gee H.Y. Lactobacillus plantarum ameliorates NASH-related inflammation by upregulating L-arginine production. Exp. Mol. Med. 2023;55:2332–2345. doi: 10.1038/s12276-023-01102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wulansari N., Darsono W.H.W., Woo H.J., Chang M.Y., Kim J., Bae E.J., Sun W., Lee J.H., Cho I.J., Shin H., et al. Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease-linked DNAJC6 mutations. Sci. Adv. 2021;7:eabb1540. doi: 10.1126/sciadv.abb1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ji X., Zhou S., Wang N., Wang J., Wu Y., Duan Y., Ni P., Zhang J., Yu S. Cerebral-Organoid-Derived Exosomes Alleviate Oxidative Stress and Promote LMX1A-Dependent Dopaminergic Differentiation. Int. J. Mol. Sci. 2023;24:11048. doi: 10.3390/ijms241311048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wilmanski T., Diener C., Rappaport N., Patwardhan S., Wiedrick J., Lapidus J., Earls J.C., Zimmer A., Glusman G., Robinson M., et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021;3:274–286. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]