Abstract
Utilization of internal ribosome entry segment (IRES) structures in the 5′ noncoding region (5′NCR) of picornavirus RNAs for initiation of translation requires a number of host cell factors whose distribution may vary in different cells and whose requirement may vary for different picornaviruses. We have examined the requirement of the cellular protein poly(rC) binding protein 2 (PCBP2) for hepatitis A virus (HAV) RNA translation. PCBP2 has recently been identified as a factor required for translation and replication of poliovirus (PV) RNA. PCBP2 was shown to be present in FRhK-4 cells, which are permissive for growth of HAV, as it is in HeLa cells, which support translation of HAV RNA but which have not been reported to host replication of the virus. Competition RNA mobility shift assays showed that the 5′NCR of HAV RNA competed for binding of PCBP2 with a probe representing stem-loop IV of the PV 5′NCR. The binding site on HAV RNA was mapped to nucleotides 1 to 157, which includes a pyrimidine-rich sequence. HeLa cell extracts that had been depleted of PCBP2 by passage over a PV stem-loop IV RNA affinity column supported only low levels of HAV RNA translation. Translation activity was restored upon addition of recombinant PCBP2 to the depleted extract. Removal of the 5′-terminal 138 nucleotides of the HAV RNA, or removal of the entire IRES, eliminated the dependence of HAV RNA translation on PCBP2.
Members of the family Picornaviridae employ a unique initiation mechanism for translation of their uncapped RNA genomes (for reviews, see references 13 and 40). In contrast to ribosomes scanning from the capped 5′ ends of the majority of cellular mRNAs, the long (600- to 1,000-nucleotide [nt]) and highly structured 5′ noncoding region (5′NCR) of picornavirus RNAs mediates the binding of ribosomal subunits internally at an internal ribosome entry segment (IRES), a region consisting of 400 to 500 nt. Despite little similarity in primary nucleotide sequences and marked variations in predicted secondary structures of the 5′NCRs among the different genera of picornaviruses, the IRES elements are functionally equivalent. Analysis of potential higher-order folds in the 5′NCRs of both viral and cellular RNAs suggest the presence of a common core structure at the 3′ end of the IRES that may represent the actual site of ribosome entry (27). Binding of the ribosomal initiation complex to the IRES element is presumably facilitated by one or more cellular trans-acting protein factors distinct from the canonical initiation factors. These factors may vary with respect to distribution in different cells and requirement by different picornaviruses. Rabbit reticulocyte lysates (RRL) support accurate and efficient translation in vitro of cardiovirus and aphthovirus IRESes. In a reconstituted in vitro system, a set of purified canonical initiation factors alone were able to mediate internal ribosomal entry of encephalomyocarditis virus (EMCV) RNA, although some enhancement of initiation complex formation was observed upon addition of the cellular polypyrimidine tract binding protein (PTB) (34). In contrast, poliovirus (PV) and rhinovirus RNAs require supplementation of RRL with HeLa cell factors for correct and efficient translation (7, 12, 21). Function of the hepatitis A virus (HAV) IRES in RRL may require additional factors present in mammalian liver at higher concentrations than present in RRL (17). Addition of ribosomal salt wash (RSW) from other mouse tissues or cell lines did not stimulate HAV translation in RRL (17, 22). However, recently we showed that HeLa cells support the translation of HAV RNA in vivo and that translation of HAV RNA is efficient in HeLa cell extract (18). None of the picornavirus IRESes has been reported to function in a wheat germ translation system. Several proteins, most of which were isolated from HeLa cells, interact with distinct regions in picornavirus 5′NCRs and have functional specificity regarding IRES utilization. These include the La autoantigen (p52), specific for PV RNA translation (29, 30); PTB (p57), which shows interaction with several picornavirus IRESes (9, 21, 24, 31, 32); and poly(rC) binding protein 2 (PCBP2; p39), shown to be required for PV translation (5, 6, 14).
The human cellular protein PCBP2 was identified in RSW of HeLa cells by RNA affinity column chromatography using PV stem-loop IV RNA (5). PCBP2 is present in most mammalian tissues, and it is related to a class of RNA binding proteins which contain three internal peptide repeats corresponding to K-homologous (KH) domains, originally identified in heterogeneous nuclear ribonucleoprotein K (28, 39). PCBP2 does not contain other known nucleic acid binding motifs, and therefore the KH domain is believed to determine its RNA binding activity. Previous demonstration of binding to homopolymeric RNA sequences showed strong binding to poly(rC) in vitro and lesser binding to poly(rG) and poly(U) (28). The longest homopolymeric sequence in the PV stem-loop IV binding site is four C residues. It is likely that PCBP2 has other RNA recognition specificities as well. Preliminary data from competition assays showed that the IRES elements of different picornaviruses such as coxsackievirus B3, human rhinovirus 14, EMCV, and HAV were able to compete with PV RNA for binding of PCBP2 (4a). Dildine and Semler (11) showed the competition of 5′NCRs of coxsackievirus B3, human rhinovirus 14, and Theiler’s murine encephalomyelitis virus for formation of a complex between PV stem-loop IV sequences and protein extract from HeLa cells, later shown to represent PCBP2 (5).
HAV, a distant relative of PV, was chosen to examine a possible broader role of PCBP2 in IRES-mediated translation of other picornaviruses. The genome of HAV is composed of a single-stranded positive-sense RNA approximately 7,500 nt in length with a structural organization and gene order characteristic of picornaviruses. It encodes a predicted polyprotein of 2,227 amino acids, cleaved to functional polypeptides, preceded by a 734-nt-long 5′NCR. Computer-assisted folding predictions and biochemical probing showed that the HAV 5′NCR forms extensive higher-order structures, typical for the long 5′NCRs of picornaviruses, which usually contain about six predicted stem-loop domains (8, 27). Also as found for the other picornaviruses, the HAV 5′NCR contains multiple AUG codons upstream of the translation initiation codon, which are not used for translation initiation (41). However, as the sole member of the genus Hepatovirus within the family Picornaviridae, HAV shows many unique features. The nucleotide and amino acid sequences of the HAV genome are only distantly related to those of other picornaviruses. HAV grows inefficiently in cell cultures, usually establishing a persistent, nonlytic infection in the cultured cells and failing to inhibit host cell protein synthesis. Only one HAV-encoded proteinase, 3C, has been identified (16, 20, 23, 35, 36), and details of the polyprotein processing scheme in vivo have not been demonstrated. At the level of RNA translation, there is evidence that capsid coding sequences downstream of the initiation codon are involved in translation of the RNA in vitro, unlike the case for other picornaviruses (18, 45). No specific function of any trans-acting factor has been identified to date for translation or RNA replication. Proteins with molecular masses of 30, 39, and 110 kDa, isolated from RSW of BS-C-1 cells and FRhK-4 cells, as well as PTB, present in RRL and HeLa cell extract, were found to bind to different regions within the 5′NCR of HAV (9), although the biological significance of these interactions has not been tested. A 39-kDa protein which interacted with stem-loop IIIa of the HAV 5′NCR was identified as glyceraldehyde-3-phosphate dehydrogenase (37); a functional role in translation of HAV of this protein also remains to be determined. The HAV proteinase 3C was found to bind to the 5′ terminus of the HAV genome (26).
In this study, we investigated the possibility of PCBP2 binding within the HAV 5′NCR. Competition RNA mobility assays conducted with recombinant PCBP2 (rPCBP2), 32P-labeled PV stem-loop IV RNA, and the HAV 5′NCR as competitor RNA clearly demonstrate the interaction of PCBP2 with the 5′NCR of HAV. Binding appears to be restricted to the 5′-terminal region of the 5′NCR. Furthermore, the depletion of PCBP2 from HeLa cell extract, used to support cell-free translation of HAV RNA, resulted in reduced translational capacity of the 5′NCR, which was restored by the addition of rPCBP2. The data support a functional role for PCBP2 in HAV RNA translation.
MATERIALS AND METHODS
Plasmid construction.
Plasmids pPsP* (18), pT220-460 (11), pH139-4977 (18), and pT7-HAV1 (19) were described previously. Plasmids constructed in this study were derived from pHAV/7(735) (41), containing the complete cDNA sequence of the cell culture-adapted HAV, HM175p35, under SP6 promoter control. It contains substitutions at nt 735 and 736, changing the AUG codon at this position to GCG in order to direct translation initiation to the following start codon at nt 741, which is the predominant one used in infected cells (41).
The numbering used to describe nucleotide positions in the HAV genome in the following plasmids is adjusted to the sequence of the wild-type HAV strain HM175 (10).
The HAV subclone pH749 contains the entire 5′NCR. It was derived from pHAV/7(735) by digestion with XbaI downstream of the 5′NCR (nt 744) and in the polylinker sequence. Separation of the fragments by agarose gel electrophoresis and gel purification (QIAquick; Qiagen Inc.) eliminated the coding sequences and the 3′NCR. Ligation of the remaining 3.5-kb fragment yielded the subclone pH749, harboring nt 1 to 749 of the HAV genome under SP6 promoter control. From this parental subclone we generated a series of 5′-end and internal deletions to construct pH148-749, pH292-749, pH533-749, pH667-749, and pH749Δ158-292, using the following strategies.
To delete nt 1 to 147, 1 to 291, 1 to 532, or 1 to 666, respectively, from the 5′ end of the HAV 5′NCR, plasmid pH749 was digested with HindIII, which cuts 7 nt downstream the SP6 promoter start site, and with an appropriate second restriction endonuclease within the 5′NCR (SspI, XcmI, NsiI, or EcoO109I, respectively). The protruding ends were treated with the Klenow fragment of DNA polymerase I, and the cDNAs lacking the particular 5′-end sequences were used for blunt-end ligation after gel purification. If SspI and EcoO109I, restriction endonucleases which cut pH749 twice, were used, a two-point ligation was performed. Each resulting plasmid, pH148-749, pH292-749, pH533-749, and pH667-749, harbors the nucleotide sequences of the HAV genome indicated by numbers in the name.
The internal deletion from nt 158 to 292 within the HAV 5′NCR was created by digestion of pH749 with PstI and XcmI, which cut in the HAV 5′NCR at nt 157 and 284, respectively. The large 3.4-kb fragment was isolated by agarose gel electrophoresis and gel purification following incubation with Klenow enzyme to produce blunt ends. Ligation with T4 DNA ligase generated plasmid pH749Δ158-292.
The construct pH667-4977, lacking most of the 5′NCR, was generated by digestion of pH667-749 with XbaI and EcoRI, followed by insertion of the 4.2-kb XbaI-EcoRI fragment comprising nt 744 to 4977 of pT7-HAV1. The resulting plasmid contained nt 667 to 4977 of the HAV genome under SP6 promoter control.
All plasmids were propagated by standard procedures in Escherichia coli DH5α cells, using ampicillin selection. The appropriate nucleotide sequences throughout the junctions of the ligated fragments were verified by analysis using Sequenase version 2.0 (U.S. Biochemical) and [35S]dATP according to the manufacturer’s instructions.
RNA synthesis in vitro.
Circular plasmids were linearized with the appropriate restriction enzyme. The linear DNAs were then used to direct transcription in a 20-μl reaction mixture in the presence of [3H]UTP with a MEGAscript kit (Ambion) according to the supplier’s instructions to produce transcripts up to 750 nt in length. For production of transcripts longer than 750 nt, a 50-μl reaction mixture was prepared by using a MAXIscript kit (Ambion) in the presence of [3H]UTP. Following transcription, the reaction mixtures were treated with RNase-free DNase I at 37°C for 15 min. Transcripts were phenol-chloroform extracted prior to analysis of the integrity of the RNA by agarose gel electrophoresis and quantification by measuring the incorporated 3H. T7 or SP6 RNA polymerase was used, depending on the promoter sequence present in the plasmid to allow the production of sense transcripts. Although all RNAs were 3H labeled, they are referred to as unlabeled RNA in competition experiments. Plasmid pT220-460 was linearized with HindIII and used for synthesis of 32P-labeled PV stem-loop IV RNA by using a MAXIscript T7 kit (Ambion). Twenty microcuries of [32P]CTP (3,000 Ci/mmol) was added to 20 μl of transcription reaction mixture. Unincorporated nucleotides were removed by passage over a G-50 Sephadex Quick Spin column (Boehringer Mannheim), and measurement of incorporated 32P was used for quantification of the transcripts.
Preparation of cell extracts.
RSW from HeLa S3 cells and FRhK-4 cell monolayers were prepared as described by Brown and Ehrenfeld (7). The RSW was concentrated by precipitation with ammonium sulfate, and the 0 to 40% saturation precipitate (A-cut) was resuspended and dialyzed against 20 mM Tris (pH 7.4)–100 mM KCl–0.2 mM EDTA–7 mM β-mercaptoethanol–5% glycerol overnight at 4°C. Total protein concentration in the A-cut preparations was determined by the Bradford method, using a commercial kit (Pierce).
HeLa S3 cell extract (S10) and HeLa S3 cell translation initiation factors (RSW) for cell-free translation reactions were prepared as described previously (3, 42) and combined (HeLa S10-RSW extract) in a relative volume-to-volume ratio (74:26) used for translation assays in vitro prior to translation and depletion.
Western blot analysis.
Protein extracts mixed with Laemmli sample buffer were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Schleicher & Schuell). The blot was incubated for 60 min with Tris-buffered saline containing 3% milk and then for 60 min with the primary antibody raised against rPCBP2 (6). After a wash with Tris-buffered saline containing 0.05% Tween 20, the membrane was incubated for 30 min with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G Fc (Promega) as the secondary antibody, followed by color detection as recommended by the manufacturer.
Depletion of HeLa cell extract.
PCBP2-depleted extract was prepared by RNA affinity column chromatography using PV stem-loop IV RNA as described previously (6). Briefly, 2 ml of combined HeLa S10-RSW extract was depleted of PCBP2 by passage over immobilized PV stem-loop IV RNA, derived from pT220-460, at 4°C. Approximately 1 mg of PV stem-loop IV RNA was covalently coupled to preswollen CNBr-activated Sepharose 4B (Pharmacia) in 0.1 M NaCH3CO2 for 24 h at 4°C (1-ml packed-bed volume). The column was equilibrated with translation buffer containing 20 mM HEPES-KOH (pH 7.4), 91 mM KCH3CO2, 2.6 mM KCl, 3.4 mM Mg(CH3CO2)2, and 4 mM dithiothreitol. The flowthrough was passed four additional times over the column. To reuse the column, proteins bound to PV stem-loop IV RNA were eluted with 1 M KCl, and the column was washed with 2 M KCl and stored in translation buffer. Control extract (mock-depleted HeLa S10-RSW extract) was passaged five times over a column without coupled RNA.
Translation assay in vitro.
Translation assays of various viral transcripts were performed in HeLa S10-RSW extracts (2, 42) that were either mock depleted or depleted of PCBP2 (6). The cell-free translation assays were performed in a 10-μl reaction mixture containing 500 ng of transcript in the presence of a mixture of [35S]methionine and [35S]cysteine. The final concentration of potassium in the translation mixture was reduced to 100 mM instead of 138 mM, adjusted for optimal translation of HAV RNA. Reactions performed with PCBP2-depleted extracts were supplemented with 200 nM rPCBP2 for restoration assays. The control assays were supplemented with the appropriate buffer. After incubation for 90 min at 30°C, samples were diluted in Laemmli sample buffer and analyzed by SDS-PAGE (11% gel). Following electrophoresis, the gel was stained with Coomassie blue, dried and subsequently subjected to autoradiography.
RNA mobility shift assay and competition assay.
RNA mobility shift assays were performed as previously described (4) except that rPCBP2 was used instead of protein extract from HeLa cells. Briefly, purified rPCBP2 (2 pmol), generously provided by Todd B. Parsley (6, 33), was preincubated with tRNA (1 μg/μl)–heparin (0.25 μg/μl)–40 mM KCl–5 mM HEPES (pH 7.4)–2 mM MgCl2–0.1 mM EDTA–2 mM dithiothreitol for 10 min at 30°C before addition of 0.05 pmol of 32P-labeled RNA or labeled RNA mixed with competitor RNA and further incubation for 15 min at 30°C. Glycerol was added to a final concentration of 10%, and the RNA-protein complex was separated from free RNA on a 4% nondenaturing polyacrylamide gel in Tris-borate-EDTA buffer at 50 V and 4°C overnight. The gel had been preequilibrated for 30 min at 280 V and 4°C. Following electrophoresis, the gel was dried and exposed to X-ray film (Kodak BioMax, Kodak Corp.).
RESULTS
Presence of PCBP2 in FRhK-4 cells.
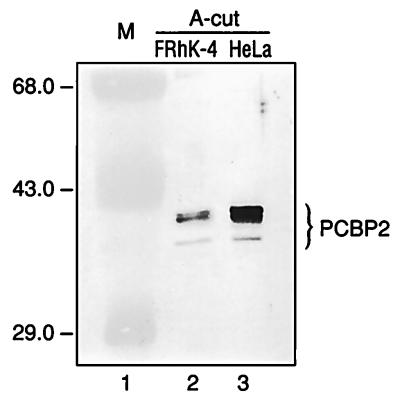
The cellular factor PCBP2, which has been shown to play a role in translation of PV RNA, was isolated from RSW of HeLa cells, a cell line which has not been reported to be permissive for HAV replication. We wanted to determine whether PCBP2 was required for translation of HAV RNA. Although we have recently shown that HAV RNA translated efficiently in HeLa cells (18), it was important to determine whether PCBP2 was expressed in cells known to support growth of HAV, such as the fetal rhesus monkey cell line FRhK-4. The A-cut of FRhK-4 cell RSW was prepared and compared with the HeLa cell A-cut for detection of PCBP2. Equal amounts of protein from both A-cut preparations were separated by SDS-PAGE and subjected to analysis by Western blot using antiserum raised against rPCBP2. As shown in Fig. 1, the anti-rPCBP2 serum recognized the same protein pattern, thought to represent phosphorylated variants of PCBP2 (28) in both HeLa and FRhK-4 cells. The immunoreactive proteins appeared to be present at a lower concentration in FRhK-4 cell RSW A-cut than in the corresponding HeLa cell fraction. There may be less PCBP2 present in FRhK-4 cells than in HeLa cells; however, there may be weaker recognition of FRhK-4 PCBP2 by this antiserum, since it was raised against the human PCBP2 and FRhK-4 cells are of rhesus monkey origin. In addition, the immunoreactive proteins could be a combination of PCBP2 and PCBP1, since the antiserum used is cross-reactive (6). PCBP1 and PCBP2 differ in amino acid sequence by only about 10% and are closely related members of the same gene family (1, 25).
FIG. 1.
Western blot analysis of HeLa and FRhK-4 cell fractions for PCBP2. Thirty micrograms of protein from the A-cut preparations derived from FRhK-4 (lane 2) and HeLa S3 (lane 3) cell RSW were resolved by SDS-PAGE and transferred to a nitrocellulose membrane for immunoblot analysis using antiserum raised against rPCBP2. Immunoreactive proteins recognized by anti-rPCBP2 antiserum are indicated to the right; molecular masses of marker proteins (M; lane 1) are indicated in kilodaltons to the left.
PCBP2 binding to the HAV 5′NCR.
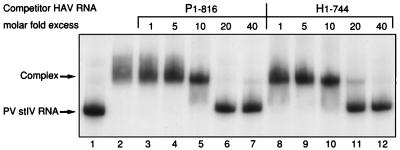
To determine whether PCBP2 binds to the 5′NCR of HAV RNA and to assess its binding affinity to HAV sequences compared to PV sequences, competition RNA mobility shift assays were performed. As shown previously (5), PV stem-loop IV RNA, representing nt 220 to 460 from the PV 5′NCR, forms a stable complex with purified rPCBP2 (Fig. 2, lanes 1 and 2). Addition of increasing amounts of unlabeled HAV RNA containing nt 1 to 744 (H1-744), representing the entire 5′NCR of HAV, to the binding reaction resulted in competition for binding of rPCBP2 with PV stem-loop IV RNA (Fig. 2, lanes 8 to 12). Effective competition for rPCBP2 binding was achieved in this assay with a 20-fold molar excess of the unlabeled HAV RNA over 32P-labeled PV stem-loop IV RNA. The same ratio of unlabeled RNA of the PV 5′NCR (P1-816) to 32P-labeled PV stem-loop IV RNA was necessary for competition for rPCBP2 (Fig. 2, lanes 3 to 7), indicating that the HAV 5′NCR interacts with PCBP2 and that the affinity for binding of PCBP2 to the HAV 5′NCR is similar to that for binding of PCBP2 to the PV 5′NCR.
FIG. 2.
Mobility and competition assay of RNAs from PV and HAV 5′NCR. 32P-labeled PV stem-loop IV RNA (nt 220 to 460) was incubated without (lane 1) and with rPCBP2 (lane 2) and separated on a 4% native polyacrylamide gel. Competition for complex formation between 32P-labeled PV stem-loop IV RNA and rPCBP2 was tested in the presence of 1- to 40-fold molar excesses of transcripts representing the PV 5′NCR (P1-816; lanes 3 to 7) or transcripts representing the HAV 5′NCR (H1-744; lanes 8 to 12). Free labeled PV stem-loop IV (stIV) RNA and the RNA-protein complex are indicated to the left.
Mapping of PCBP2 binding site within the HAV 5′NCR.
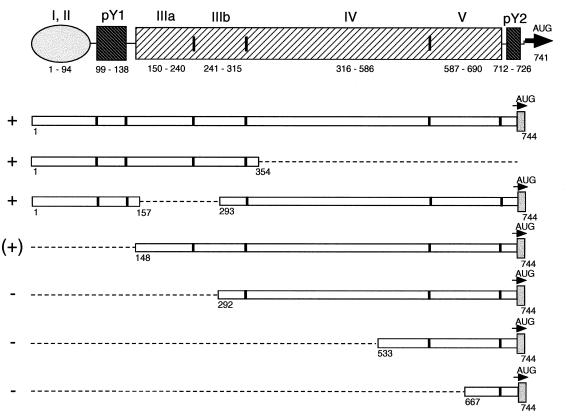
PCBP2 has been shown to bind preferentially to homopolymeric poly(rC) sequences (28), and specifically to the PV stem-loop IV RNA (5) and the 5′-terminal 90 nt of the PV 5′NCR (stem-loop I) (14, 33), that form a cloverleaf-like structure. Lesser binding activity was demonstrated to poly(rG) and poly(U) (28). The 5′NCR of HAV contains two pyrimidine-rich tracts located at the 5′ end between nt 99 to 138 (pY1 [38]) and at the 3′ end between nt 712 to 726 (pY2 [38]), in addition to several stable stem-loop structures, which could serve as potential binding sites for PCBP2. To identify the region(s) of the HAV 5′NCR that interacts with the cellular protein PCBP2, a series of RNA fragments of the HAV 5′NCR, depicted in Fig. 3, were used as unlabeled competitor RNAs during RNA mobility shift assays with 32P-labeled PV stem-loop IV RNA and rPCBP2.
FIG. 3.
Schematic representation of RNA fragments of the HAV 5′NCR used as competitor RNAs in mobility shift assays with 32P-labeled PV stem-loop IV RNA and rPCBP2. Open bars represent sequences of the 5′NCR comprising nucleotides indicated below each fragment. The dotted lines indicate the extent of deletions in the 5′NCR. The vertical lines indicate the borders of the predicted stem-loop structures I to V and the pyrimidine-rich tracts at the 5′ (pY1) and 3′ (pY2) ends of the 5′NCR (8, 38) as depicted above the diagram of the RNA fragments. +, (+), or − at the left indicates whether the particular RNA fragment competes, competes weakly, or does not compete with PV stem-loop IV RNA for binding of rPCBP2.
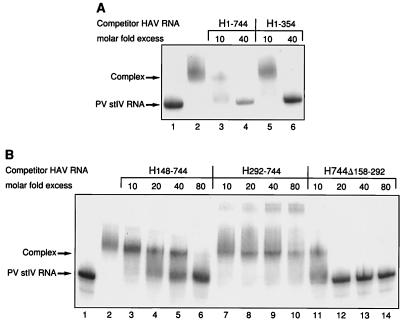
In the first analysis, an RNA segment comprising the 5′ half of the 5′NCR (nt 1 to 354) was used as competitor RNA at 10- and 40-fold molar excesses over the radioactive labeled probe. As shown in Fig. 4A, lanes 5 and 6, the first 354 nt of the HAV 5′NCR (H1-354) showed competition for rPCBP2 binding to PV stem-loop IV RNA similar to that seen with the entire 5′NCR (H1-744) (Fig. 4A, lanes 3 and 4). The RNA representing the entire HAV 5′NCR competes slightly better than the RNA of the fragment H1-354, likely due to an increase in stability of the relevant RNA structure when presented in the context of the larger RNA. A similar effect was observed for competition between the intact PV 5′NCR and an RNA representing only the stem-loop IV domain (4). The region H1-354 includes several predicted stem-loop structures thought to be upstream of the IRES (nt 1 to 94), the pY1 pyrimidine-rich tract, and stem-loop IIIa,b structure (nt 150 to 315), considered an essential part of the IRES (8). Further fragmentation (nt 1 to 160 or 140 to 248) of the 5′ half resulted in poor competition (data not shown), possibly due to disruption of the overall structure.
FIG. 4.
RNA mobility shift and competition assay with 32P-labeled PV stem-loop IV RNA (nt 220 to 460), rPCBP2, and various HAV 5′NCR fragments as competitor RNA analyzed on a 4% native polyacrylamide gel. (A) Labeled RNA (PV nt 220 to 460) was incubated with rPCBP2 in the absence (lane 2) or in the presence of unlabeled competitor HAV RNA comprising nt 1 to 744 (lanes 3 and 4) or comprising the 5′ half of the HAV 5′NCR from nt 1 to 354 (lanes 5 and 6) at the molar excess indicated. Lane 1 was loaded with 32P-labeled PV stem-loop IV (stIV) RNA only. (B) Labeled RNA (PV nt 220 to 460) was incubated with rPCBP2 in the presence of competitor RNA comprising nt 148 to 744 (lanes 3 to 6), nt 292 to 744 (lanes 7 to 10), and nt 1 to 744 with the internal deletion from nt 158 to 292 (lanes 11 to 14). Labeled PV stem-loop IV RNA alone was loaded in lane 1 and as complex with rPCBP2 in lane 2. Free RNA and the RNA-protein complex are indicated to the left.
Another set of RNAs used for competition represented increasing lengths of 3′-end sequences of the HAV 5′NCR. Transcripts representing small RNA fragments from the 3′ third, nt 533 to 744 derived from pH533-749 or nt 677 to 744 derived from pH667-749, which include the pY2 pyrimidine-rich tract of the HAV 5′NCR, failed to compete for binding of rPCBP2 (data not shown), as did transcripts of the 3′ half of the HAV 5′NCR derived from pH292-749 (Fig. 4B, lanes 7 to 10). Although, H292-744 failed to displace the PV probe from the PCBP2 complex, some interaction between these macromolecules appears to occur at high HAV RNA concentrations, causing the appearance of new bands with an increased shift in mobility. Finally, transcripts comprising nt 148 to 744 of the HAV 5′NCR (H148-744) showed a weak competition for binding of rPCBP2, causing complete displacement of the PV stem-loop IV RNA only at a molar ratio of 80:1 (Fig. 4B, lanes 3 to 6). Nonspecific binding of rPCBP2 was tested by using RNA transcribed from pGEM digested with RsaI as the competitor. There was no competition for rPCBP2 binding detectable even at a molar ratio of competitor RNA to specific probe of 80:1 (data not shown and shown previously [4]).
The results of the competition assays indicated that the binding site for PCBP2 within the 5′NCR of HAV is in the 5′ half of the 5′NCR and that the 3′ half of the HAV 5′NCR does not compete for PCBP2 binding. To localize the binding region more precisely, an RNA fragment of the HAV 5′NCR with an internal deletion from nt 158 to 292 (H744Δ158-292) was prepared. This construct removes the stem-loop IIIa,b structure. Figure 4B, lanes 11 to 14, shows the results obtained in assays using the internally deleted HAV 5′NCR as competitor RNA. H744Δ158-292 competed efficiently for binding of rPCBP2 with PV stem-loop IV RNA at a molar ratio of 10:1, indicating that the first 157 nt, including the 5′-terminal pyrimidine-rich tract of the HAV 5′NCR, are involved in binding of PCBP2. An RNA fragment comprising only the 5′-terminal 160 nt demonstrated direct binding to rPCBP2 by mobility shift (data not shown), confirming the interaction of this region of the HAV 5′NCR with PCBP2.
Effect of PCBP2 on translation in vitro of HAV RNA.
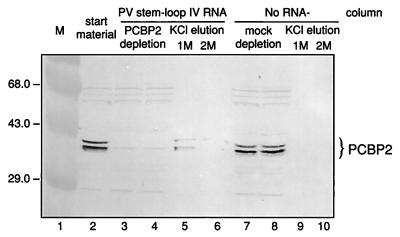
To determine whether the presence of PCBP2 affected translation directed by the 5′NCR of HAV RNA, translation assays were performed with cell extracts which had been depleted of PCBP2. Successful depletion of PCBP2 from combined HeLa S10-RSW extracts by PV stem-loop IV RNA affinity column chromatography was verified by Western blot analysis with anti-rPCBP2 serum (Fig. 5). The RNA affinity column removed PCBP2 from the cell extract almost completely (Fig. 5, lanes 3 and 4). Minor residual amounts of PCBP2 were variably detectable after depletion in different preparations. Elution of the column with 1 M KCl recovered PCBP2 from the PV stem-loop IV RNA column. The high-salt eluate showed the typical multiple forms of PCBP2 (Fig. 5, lane 5). PCBP2 was not removed when cell extract was passed over a column without coupled RNA (mock-depleted HeLa S10-RSW extract) (Fig. 5, lanes 7 and 8), and subsequently no PCBP2 was recovered in the 1 or 2 M KCl eluate from the control column (Fig. 5, lanes 9, 10).
FIG. 5.
Western blot analysis of combined HeLa S10-RSW extract, using antiserum raised against rPCBP2. The extract was depleted of PCBP2 by RNA affinity column chromatography with immobilized PV stem-loop IV RNA (nt 220 to 460). Lane 2, untreated extract; lanes 3 and 4, extract passaged five times over the RNA affinity column; lanes 5 and 6, 1 and 2 M KCl eluates; lanes 7 to 10, control extract passaged over a column without any immobilized RNA (mock-depleted extract) and its high-salt eluates. Molecular masses of marker proteins (M; lane 1) are indicated in kilodaltons to the left; immunoreactive proteins recognized by anti-rPCBP2 antiserum are indicated to the right.
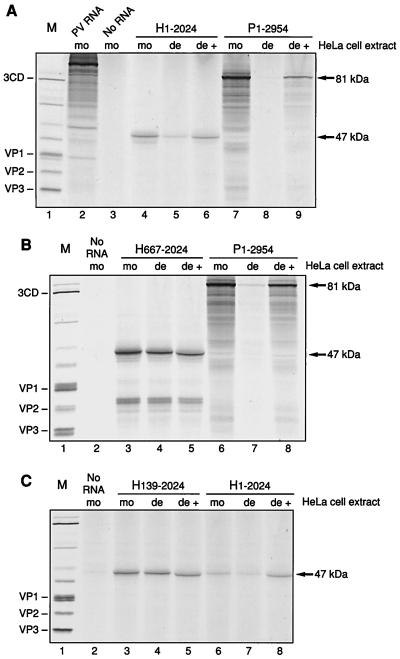
HAV RNA was translated in the PCBP2-depleted and the mock-depleted HeLa S10-RSW extracts. Truncated HAV RNA representing nt 1 to 2024 derived from pT7-HAV1 was used to program PCBP2-depleted HeLa S10-RSW extracts or mock-depleted control extract; for comparison, truncated PV RNA representing nt 1 to 2954 derived from pPsP* was translated. To simplify the pattern of translation products, transcripts were prepared from plasmids linearized so as to encode only capsid protein sequences. Figure 6A shows the translation products obtained in this assay derived from HAV RNA H1-2024 (lanes 4 to 6) and from PV RNA P1-2954 (lanes 7 to 9) with predicted molecular masses of 47 and 81 kDa, respectively. Translation of HAV RNA was decreased in PCBP2-depleted extract (lane 5) compared to mock-depleted extract (lane 4). Restoration of this decreased translation efficiency was achieved by addition of rPCBP2 (Fig. 6A, lane 6), indicating a specific effect of PCBP2 for translation of HAV. However, depletion of PCBP2 did not cause as complete a reduction in translation as that seen using PV RNA (Fig. 6A, lanes 7 to 9). Thus, HAV RNA requires PCBP2 for efficient translation, although it can still be translated at a low level when the majority of the cellular factor PCBP2 has been depleted from the extract. The incomplete restoration of translation with rPCBP2 in this assay for PV RNA may be due to addition of nonsaturating amounts of rPCBP2. In the case of HAV RNA, saturating amounts of rPCBP2 were used, as determined in a dose-response assay (data not shown).
FIG. 6.
Translation of truncated HAV and PV RNAs driven by different 5′NCR regions in HeLa S10-RSW extracts. The H1-2024 (lanes 4 to 6) and P1-2954 (lanes 7 to 9) (A), H667-2024 (lanes 3 to 5) and P1-2954 (lanes 6 to 8) (B), and H139-2024 (lanes 3 to 5) and H1-2024 (lanes 6 to 8) (C) transcripts were used to program translation extracts in the presence of [35S]methionine and [35S]cysteine. Translation assays were carried out in mock-depleted extract (mo), PCBP2-depleted extract (de), and PCBP2-depleted extract supplemented with rPCBP2 (de +). PV-encoded polypeptides obtained from PV-infected HeLa cells labeled with [35S]methionine served as protein marker (M; lane 1) and are identified on the left. HeLa S10-RSW extract programmed with 100 ng of PV RNA isolated from purified PV virions was used as internal translation control (panel A, lane 2). Due to the short incubation time used in this assay, the PV polyprotein is mainly uncleaved. HeLa cell extract programmed with no RNA is analyzed in lane 3 of panel A and lanes 2 of panels B and C. The predicted protein sizes of the translation products are indicated to the right.
The specificity of PCBP2 stimulation of HAV IRES-mediated translation was tested by construction of an HAV RNA with a short, relatively unstructured 5′NCR, designed to be translated by scanning from the 5′ end. HAV RNA containing nt 667 to 2024, lacking almost the entire 5′NCR, was then translated in PCBP2-depleted or mock-depleted HeLa S10-RSW extract. Figure 6B shows that translation of HAV RNA comprising nt 667 to 2024 occurred with the same efficiency in the two extracts (lanes 3 and 4), and the addition of rPCBP2 to the depleted extract showed no stimulation (lane 5), verifying that the effect of PCBP2 on HAV RNA is a function of IRES-mediated translation. Supplementation of PCBP2-depleted extract with rPCBP2 yielded a translation product which appeared to migrate slightly faster than the products obtained with mock- or PCBP2-depleted extract alone. This effect was caused by the high protein concentration of added rPCBP2, which migrates at about 43 kDa, and could be reproduced by adding increasing amounts of rPCBP2 to mock-depleted or PCBP2-depleted extract (data not shown). Translation products (about 30 kDa) of the H667-2024 RNA smaller than the expected 47-kDa protein were detectable, most probably due to aberrant internal initiations on this RNA.
The mobility shift competition assays described above indicated that the first 157 nt of the HAV 5′NCR mediated binding of PCBP2. Transcripts containing nt 139 to 2024 with a disrupted potential binding site for rPCBP2 were prepared and used to program HeLa cell extract which was mock depleted or depleted of PCBP2. As predicted, depletion of PCBP2 had no effect on translation of this RNA (Fig. 6C; compare lanes 3 and 4). Similarly, addition of exogenous rPCBP2 to the depleted extract had no influence on the efficiency of translation (lane 5). Consistent with the assignment of sequences or structures formed by the 5′-terminal 138 nt in the binding of PCBP2, HAV RNA comprising nt 139 to 744, used as competitor RNA in the mobility shift assay with PV stem-loop IV RNA and rPCBP2, also failed to compete for rPCBP2 binding at a molar ratio of competitor RNA to specific probe of 20:1 (data not shown). RNA of the entire HAV 5′NCR competes efficiently at this molar concentration. Comparison of the efficiencies of translation of HAV RNAs H1-2024 and H139-2024 in the control, mock-depleted extract showed that deletion of the 5′-terminal 138 nt of the HAV 5′NCR resulted in more efficient translation under these conditions than the HAV RNA containing the entire 5′NCR (Fig. 6C, lanes 3 and 6). This effect has been observed previously (18, 44). Interestingly, addition of rPCBP2 to the depleted extract restored translation of H1-2024 to levels similar to those seen when the PCBP2 binding sequences were not present (lane 8 and 5).
DISCUSSION
Internal initiation of translation mediated by picornavirus IRES structures may be facilitated by multiple host cell proteins in addition to the canonical initiation factors (for a review, see reference 40). Addition of PTB to a set of purified initiation factors enhanced the formation of the translation initiation complex of a variant of EMCV IRES-driven translation (24, 34). The 5′NCR of HAV RNA has been shown to bind specifically to several proteins derived from the RSW of BS-C-1, FRhK-4, and HeLa cells (9), although most of these proteins were not identified. A 39-kDa protein that was present in BS-C-1 and FRhK-4, but not in HeLa, cells and that cross-linked to several regions of the HAV 5′NCR and bound specifically to stem-loop IIIa of the HAV 5′NCR was subsequently identified as glyceraldehyde-3-phosphate dehydrogenase (37). It was not determined whether these interactions provide a functional role in HAV translation.
A cellular RNA binding protein, PCBP2, has been shown previously to bind to stem-loop IV in the 5′NCR of PV RNA, and this binding appears essential for IRES-mediated translation of PV RNA (5, 6). PCBP2 binds also to sequences in the 5′-terminal cloverleaf-like structure (stem-loop I) formed by the first 90 nt of PV RNA and has been implicated in replication of PV RNA (14, 33). To determine whether PCBP2 had a potential role in HAV RNA translation, we investigated the binding of rPCBP2 to the 5′NCR of HAV RNA and examined the translation of this RNA in an extract depleted of PCBP2. We made use of the specific binding between PV stem-loop IV RNA and rPCBP2 in a competition RNA mobility shift assay with competitor RNAs representing the 5′NCR of HAV or segments of this 5′NCR.
The results showed that the HAV 5′NCR competes for binding of PCBP2 to PV stem-loop IV and that the binding site is located within the 5′ third of the HAV 5′NCR. Maintenance of the overall structure of the 5′NCR proved to be important for PCBP2 interaction, since most fragments of this RNA region tested for direct binding to PCBP2 resulted in only weak and nonspecific retarded migration of the RNA (data not shown). Additional cellular or viral proteins could also affect the binding of PCBP2 to HAV RNA, a possibility not tested in this study. The 5′-terminal 157-nt sequence of the HAV 5′NCR was able to specifically compete for binding of PCBP2 if presented in the context of the HAV 5′NCR harboring an internal deletion from nt 158 to 292. The 5′-terminal sequences of the HAV 5′NCR contain a pyrimidine-rich tract from nt 99 to 138 which may provide a favorable binding site for PCBP2, since PCBP2 was originally characterized as demonstrating strong binding to poly(rC) (28, 43). The 3′ half of the 5′NCR is predicted to form several stem-loop structures and also contains a pyrimidine-rich tract from nt 712 to 726. These sequences did not compete for binding of PCBP2. Even the very small RNA probe from nt 667 to 744, designed to expose the pyrimidine-rich tract, did not bind PCBP2. Thus, the cellular protein PCBP2 seems to interact with HAV RNA containing the pyrimidine-rich tract from nt 99 to 138 yet not with the pyrimidine-rich tract located at the 3′ end between nt 712 to 726. If the pyrimidine-rich tract is the site of PCBP2 binding, the secondary structure and the primary sequence context surrounding the tract may be responsible for the differential binding of the two pyrimidine-rich tracts. Additional investigations are necessary to specify the exact binding site.
The affinities of binding to PCBP2 appear quite comparable between the PV and the HAV 5′NCRs, as indicated by the equal molar concentrations of the two 5′NCRs required to compete with the stem-loop IV probe (Fig. 2). However, the binding sites on the two picornaviral RNAs appear to be quite different. The PV 5′NCR binds PCBP2 at stem-loop IV, a large and complex internal domain (nt 235 to 440) within the IRES element (5), as well as at a subloop of the cloverleaf-like structure (nt 1 to 90) at the 5′ terminus of the 5′NCR upstream of the IRES (33). The HAV RNA binding site could be localized only to within the 5′ one-third of the 5′NCR (nt 1 to 157). Neither a terminal cloverleaf-like structure nor anything resembling the downstream PV stem-loop IV domain is predicted to form in this region of the HAV RNA, and the pyrimidine-rich tract including the 5′-terminal region in HAV RNA that is likely responsible for its PCBP2 binding is not present in PV RNA. Despite this apparent difference in binding sites, translation of both PV and HAV RNAs is significantly reduced in HeLa cell extracts depleted of PCBP2. These observations suggest that the role of PCBP2 in facilitating IRES-mediated initiation of translation is to stabilize the complex secondary and tertiary structure of the IRES, rather than to identify similar structural determinants in the RNA. It is, of course, also possible that PCBP2 needs only to bind somewhere within or near the IRES in order to direct other factors to the initiation complex. Binding of PCBP2 to the 5′ end of the HAV RNA may also have a role in RNA replication, as is the case for PV RNA (14, 15, 33); however, no obvious similarity between the two viruses exists in this region.
Previous results showed that a 39-kDa protein identified as glyceraldehyde-3-phosphate dehydrogenase bound to the predicted stem-loop IIIa (nt 155 to 235) of the HAV 5′NCR (37). This 39-kDa protein could also be cross-linked to nt 1 to 148 and nt 597 to 740 of the HAV 5′NCR (9). The cross-linking activity was detected in RSW of BS-C-1 and FRhK-4, but not HeLa, cells. We have shown that PCBP2, also an approximately 39-kDa protein, binds to HAV RNA by a competition RNA mobility shift assay. PCBP2 is present in both HeLa and FRhK-4 cells. It is not known whether PCBP2 cross-links to HAV RNA. It appears, therefore, that the observed binding of these two similar-sized proteins may not be related.
Translation assays with PCBP2-depleted HeLa cell extract demonstrated a requirement for the binding of PCBP2 for efficient translation of HAV RNA, when the translation is directed by the complete HAV 5′NCR. Addition of rPCBP2 to the depleted extracts specifically restored the reduced translation activity of the PCBP2-depleted extract. Residual translation activity of HAV RNA was still detectable in the PCBP2-depleted extract and was greater than that detected with PV RNA in the same extract preparation. It is possible that HAV RNA needs a lower concentration of PCBP2 for functional translation than PV RNA, and since the depletion procedure fails to remove all of the PCBP2, there may be enough PCBP2 remaining in the extract to support a low level of translation of HAV RNA. Alternatively, removal of PCBP2 from the translation extract may allow another trans-acting factor present only in minor amounts to interact with the HAV 5′NCR in a manner to support viral RNA translation on a low level.
The finding that translation promoted by the HAV 5′NCR lacking the 5′-terminal 138 nt, a sequence which is upstream of the HAV IRES element, was independent of PCBP2 and more efficient was rather surprising. Considering that the possible role of PCBP2 binding is to promote the correct three-dimensional organization of the 5′NCR of HAV RNA necessary to facilitate internal ribosome entry, it may be that the HAV 5′NCR sequences lacking the 5′-terminal 138 nt adopt the correct structure spontaneously without the need for PCBP2 binding to promote the appropriate folding. This may suggest another reason for the finding that HAV RNA containing the entire 5′NCR translates, albeit poorly, in PCBP2-depleted extracts. This activity might reflect a minor population of HAV RNA molecules already in a conformation folded in the appropriate secondary structure without the help of PCBP2. Recently, Gamarnik and Andino (15) proposed that the RNA element at the 5′ end of the PV genome contains overlapping signals for translation and replication of RNA. Their model suggests that the binding of the cellular protein PCBP to the RNA enhances translation and stimulates production of viral proteins. When sufficient concentrations of the viral protein 3CD become available, both 3CD and PCBP are bound, resulting in repression of translation and formation of a ribonucleoprotein complex that initiates viral RNA replication. The data presented here suggest a similar model for regulation of the utilization of the HAV 5′NCR RNA as a template for translation and transcription. In the absence of other viral or cellular proteins, PCBP binding facilitates translation, perhaps by stabilizing the IRES conformation. Binding of other proteins to the 5′-terminal RNA sequence might displace or alter PCBP binding so as to favor a replication structure over a translation structure. The presence of the 5′-terminal RNA sequences were observed to inherently downregulate translation, perhaps because of their potential to form the replication-specific structure.
ACKNOWLEDGMENTS
We thank Todd B. Parsley for generously providing purified rPCBP2, and we are grateful to Ollie Richards and Yolanda Bell for helpful discussions.
This work was supported by Public Health Service grants AI26350 and AI12387 from the National Institutes of Health.
REFERENCES
- 1.Aasheim H-C, Loukianova T, Deggerdal A, Smeland E B. Tissue specific expression and cDNA structure of a human transcript encoding a nucleic acid binding [oligo(dC)] protein related to the pre-mRNA binding protein K. Nucleic Acids Res. 1994;22:956–964. doi: 10.1093/nar/22.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blyn L B, Chen R, Semler B L, Ehrenfeld E. Host cell proteins binding to domain IV of the 5′ noncoding region of poliovirus RNA. J Virol. 1995;69:4381–4389. doi: 10.1128/jvi.69.7.4381-4389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Blyn, L. B., and J. Graff. Unpublished data.
- 5.Blyn L B, Swiderek K M, Richards O, Stahl D C, Semler B L, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blyn L B, Towner J, Semler B L, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown B A, Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979;97:396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- 8.Brown E A, Day S P, Jansen R W, Lemon S M. The 5′ nontranslated region of hepatitis A virus RNA: secondary structure and elements required for translation in vitro. J Virol. 1991;65:5828–5838. doi: 10.1128/jvi.65.11.5828-5838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K H, Brown E A, Lemon S M. Cell type-specific proteins which interact with the 5′ nontranslated region of hepatitis A virus RNA. J Virol. 1993;67:6716–6725. doi: 10.1128/jvi.67.11.6716-6725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen J I, Ticehurst J R, Purcell R H, Buckler-White A, Baroudy B M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987;61:50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dildine S L, Semler B L. Conservation of RNA-protein interactions among picornaviruses. J Virol. 1992;66:4364–4376. doi: 10.1128/jvi.66.7.4364-4376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorner A J, Semler B L, Jackson R J, Hanecak R, Duprey E, Wimmer E. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J Virol. 1984;50:507–514. doi: 10.1128/jvi.50.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrenfeld E. Initiation of translation by picornavirus RNAs. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 549–573. [Google Scholar]
- 14.Gamarnik A V, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 15.Gamarnik A V, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauss-Müller V, Jürgensen D, Deutzmann R. Autoproteolytic cleavage of recombinant 3C proteinase of hepatitis A virus. Virology. 1991;182:861–864. doi: 10.1016/0042-6822(91)90630-t. [DOI] [PubMed] [Google Scholar]
- 17.Glass M J, Summers D F. Identification of a trans-acting activity from liver that stimulates HAV translation in vitro. Virology. 1993;193:1047–1050. doi: 10.1006/viro.1993.1225. [DOI] [PubMed] [Google Scholar]
- 18.Graff J, Ehrenfeld E. Coding sequences enhance internal initiation of translation by hepatitis A virus RNA in vitro. J Virol. 1998;72:3571–3577. doi: 10.1128/jvi.72.5.3571-3577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmon S A, Richards O C, Summers D F, Ehrenfeld E. The 5′-terminal nucleotides of hepatitis A virus RNA, but not poliovirus RNA, are required for infectivity. J Virol. 1991;65:2757–2760. doi: 10.1128/jvi.65.5.2757-2760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmon S A, Updike W, Jia X-Y, Summers D F, Ehrenfeld E. Polyprotein processing in cis and in trans by hepatitis A virus 3C protease cloned and expressed in Escherichia coli. J Virol. 1992;66:5242–5247. doi: 10.1128/jvi.66.9.5242-5247.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson R J, Hunt S L, Gibbs C L, Kaminski A. Internal initiation of translation of picornavirus RNAs. Mol Biol Rep. 1994;19:147–159. doi: 10.1007/BF00986957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia X-Y, Schepper G, Brown D, Updike W, Harmon S, Richards O, Summers D, Ehrenfeld E. Translation of hepatitis A virus RNA in vitro: aberrant internal initiations influenced by 5′ noncoding region. Virology. 1991;182:712–722. doi: 10.1016/0042-6822(91)90612-f. [DOI] [PubMed] [Google Scholar]
- 23.Jia X-Y, Ehrenfeld E, Summers D F. Proteolytic activity of hepatitis A virus 3C protein. J Virol. 1991;65:2595–2600. doi: 10.1128/jvi.65.5.2595-2600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaminski A, Jackson R J. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA. 1998;4:626–638. doi: 10.1017/s1355838298971898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiledjian M, Wang X, Liebhaber S A. Identification of two KH domain proteins in the α-globin mRNP stability complex. EMBO J. 1995;14:4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusov Y, Gauss-Müller V. In vitro RNA binding of the hepatitis A virus proteinase 3C (HAV 3Cpro) to secondary structure elements within the 5′ terminus of the HAV genome. RNA. 1997;3:291–302. [PMC free article] [PubMed] [Google Scholar]
- 27.Le S Y, Sidiqui A, Maizel J V. A common structural core in the internal ribosome entry site of picornavirus, hepatitis c virus, and pestivirus. Virus Genes. 1996;12:135–147. doi: 10.1007/BF00572952. [DOI] [PubMed] [Google Scholar]
- 28.Leffers H, Delgaard K, Celis J E. Characterization of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–453. [PubMed] [Google Scholar]
- 29.Meerovitch K, Pelletier J, Sonenberg N. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989;3:1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- 30.Meerovitch K, Svitkin Y V, Lee H S, Lejbkowicz F, Kenan D J, Chan E K L, Agol V I, Keene J D, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niepmann M. Porcine polypyrimidine tract-binding protein stimulates translation initiation at the internal ribosome entry site of foot-and-mouth-disease virus. FEBS Lett. 1996;388:39–42. doi: 10.1016/0014-5793(96)00509-1. [DOI] [PubMed] [Google Scholar]
- 32.Niepmann M, Petersen A, Meyer K, Beck E. Functional involvement of polypyrimidine tract-binding protein in translation initiation complexes with the internal ribosome entry site of foot-and-mouth disease virus. J Virol. 1997;71:8330–8339. doi: 10.1128/jvi.71.11.8330-8339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsley T B, Towner J S, Blyn L B, Ehrenfeld E, Semler B L. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 34.Pestova T, Hellen C U T, Shatsky I N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultheiss T, Kusov Y Y, Gauss-Müller V. Proteinase 3C of hepatitis A virus (HAV) cleaves the HAV polyprotein P2-P3 at all sites including VP1/2A and 2A/2B. Virology. 1994;198:275–281. doi: 10.1006/viro.1994.1030. [DOI] [PubMed] [Google Scholar]
- 36.Schultheiss T, Sommergruber W, Kusov Y, Gauss-Müller V. Cleavage specificity of purified recombinant hepatitis A virus 3C proteinase on natural substrates. J Virol. 1995;69:1727–1733. doi: 10.1128/jvi.69.3.1727-1733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz D E, Hardin C C, Lemon S M. Specific interaction of glyceraldehyde 3-phosphate dehydrogenase with the 5′-nontranslated RNA of hepatitis A virus. J Biol Chem. 1996;271:14134–14142. doi: 10.1074/jbc.271.24.14134. [DOI] [PubMed] [Google Scholar]
- 38.Shaffer D R, Brown E A, Lemon S M. Large deletion mutations involving the first pyrimidine-rich tract of the 5′ nontranslated RNA of human hepatitis A virus define two adjacent domains associated with distinct replication phenotypes. J Virol. 1994;68:5568–5578. doi: 10.1128/jvi.68.9.5568-5578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siomi H, Matunis M J, Michael W M, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionary conserved motif. Nucleic Acids Res. 1993;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart S R, Semler B L. RNA determinants of picornavirus cap-independent translation initiation. Semin Virol. 1998;8:242–255. [Google Scholar]
- 41.Tesar M, Harmon S A, Summers D F, Ehrenfeld E. Hepatitis A virus polyprotein synthesis initiates from two alternative AUG codons. Virology. 1992;186:609–618. doi: 10.1016/0042-6822(92)90027-m. [DOI] [PubMed] [Google Scholar]
- 42.Todd S, Towner J S, Semler B L. Translation and replication properties of the human rhinovirus genome in vivo and in vitro. Virology. 1997;229:90–97. doi: 10.1006/viro.1996.8416. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Kiledjian M, Weiss I M, Liebhaber S A. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human α-globin mRNA stability. Mol Cell Biol. 1995;15:1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whetter L E, Day S P, Elroy-Stein O, Brown E A, Lemon S M. Low efficiency of the 5′ nontranslated region of hepatitis A virus RNA in directing cap-independent translation in permissive monkey kidney cells. J Virol. 1994;68:5253–5263. doi: 10.1128/jvi.68.8.5253-5263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Kaplan G G. Characterization of replication-competent hepatitis A virus constructs containing insertions at the N terminus of the polyprotein. J Virol. 1998;72:349–357. doi: 10.1128/jvi.72.1.349-357.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]