Main text
Wiskott-Aldrich syndrome (WAS) is a complex primary immunodeficiency disorder caused by mutations in the WAS gene, which encodes the WAS protein (WASP). The WASP is expressed in nearly all hematopoietic cells and functions as a major effector of actin polymerization. Deficiency of the WASP in blood cells distresses actin cytoskeleton integrity, leading to defects in a broad range of cellular processes including cell movement, immune response, and blood clotting.1 Management of WAS often involves a comprehensive approach that addresses both the immune system deficiencies and potential autoimmune complications. In this issue of Molecular Therapy Methods and Clinical Development, Pille et al. describe the development of a gene correction strategy to target the integration of a therapeutic cassette containing a correct portion of the WAS gene sequence (WAS2–12) into intron 1 of the endogenous WAS gene.2 This study offers substantial evidence highlighting the feasibility of gene-editing-based targeted integration approaches in treating the disease, supporting previous findings.3
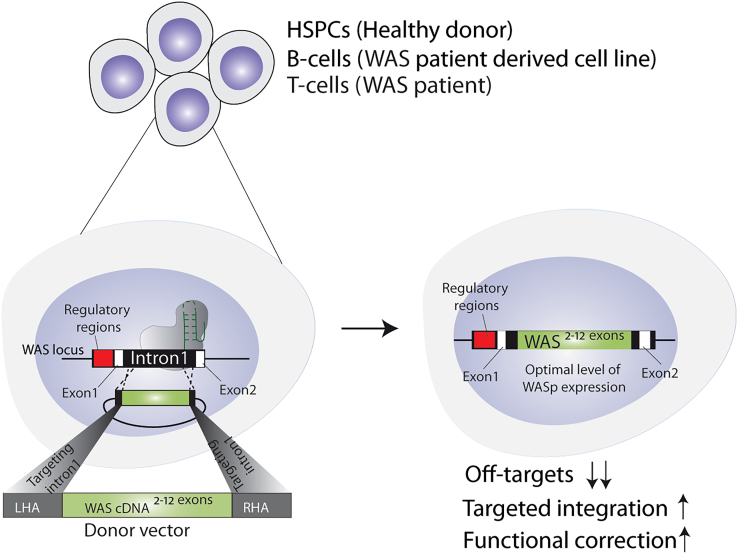
Although hematopoietic stem cell transplantation (HSCT) has shown success in treating WAS, its availability is limited to less than 20% of patients who have a suitable human leukocyte antigen-matched donor. Autologous gene therapy using a lentiviral vector (LV-GT) has been successfully attempted in patients with WAS; however, challenges remain such as ensuring the safety and efficiency of the gene delivery process and long-term monitoring of treated individuals.4,5 Indeed, long-term outcomes of LV-GT clinical trials have reported clear clinical benefits but have also highlighted the difficulties in obtaining a complete and sustained correction of the platelet compartment in patients with WAS.6 Another potential approach for the treatment of WAS is targeted gene insertion of a correct copy of the gene via CRISPR-Cas9 by harnessing the homology-directed repair (HDR) pathway. The hypothesis is rooted in the belief that a targeted and single-copy integration of a WAS transgene into the native locus will provide a more precise and physiologically relevant correction of the disease. To this aim, Pille et al. have developed a gene editing platform that integrates a cDNA encoding for WAS exons 2–12 into intron 1 of the endogenous WAS gene and showed its efficacy in healthy donor-derived hematopoietic stem and progenitor cells (HSPCs), as well as in a B cell line harboring a mutated WAS gene and in patient-derived T cells. The authors were able to reach >50% frequency of targeted integration in HSPCs, which retained their capability to differentiate into multiple hematopoietic lineages both in vitro and in vivo. Despite much lower rates of gene integration in WAS T cells (<10%), the platform also resolved the T cell-specific functional defects observed in WAS, suggesting the potential therapeutic value of this approach to patients with WAS (Figure 1).
Figure 1.
Schematic illustration of the gene editing approach used in this study in various cell types and the outcomes
This study provides further reassurance of the suitability of CRISPR-Cas-mediated targeted gene knockin for the treatment of WAS, as previously demonstrated using a similar platform introducing a cDNA encoding for WAS exons 1–12 into the WAS translational start site in WAS patient-derived HSPCs and T cells.3 The major difference of the strategy used by Pille et al. compared to the previous work is the targeting of WAS intron 1 for integration of the corrective WAS cassette. This strategy brings with it several advantages such as the maintenance of normal gene splicing favoring high transgene expression, as well as the lack of promoter regions in the homology arms of the adeno-associated virus donor cassette, which could eventually lead to unwanted gene expression dysregulation upon its random integration into the genome.7 At the same time, however, the strategy would not be amenable to patients with WAS bearing mutations in WAS exon 1, limiting its applicability to less than 85% of patients with WAS. Moreover, because integration into intron 1 does not induce disruption of the mutated WAS gene, this strategy would not be advantageous for a subgroup of WAS patients with congenital X-linked neutropenia, which carries gain-of-function mutations that result in a dominant and constitutively active mutated form of WASP.1 Another novelty of this study is the introduction of a reporter gene within the corrective cassette, which ideally would allow the selection and isolation of corrected cells for transplantation into patients. This is particularly important for WAS, where clinical evidence from HSCT and GT studies has highlighted the need for a high percentage of correction of myeloid precursors in patients (>50%) to neutralize the platelet defects. Current gene editing protocols employed to perform HDR in HSPCs suffer from poor engraftment and progressive decrease of corrected HSPCs in the bone marrow of transplanted animals, possibly due to an irreversible damage imposed to long-term HSCs (LT-HSCs) by the gene editing procedure.8 Although the study from Pille et al. did not assess the proportion of corrected LT-HSCs nor their persistence in secondary transplantation experiments, the inclusion of a selectable marker within the corrective cassette in a clinical setting could, in principle, assist in the improvement of the cell chimerism post-transplantation and thus in a more profound resolution of the WAS phenotype.
Given the presence of other competing strategies in the arena of therapeutic options for WAS, one important aspect of this study is the comparison between the level of WASP restoration achieved with targeted integration via CRISPR-Cas versus the LV-GT strategy. Although limited to only WAS patient-derived T-cells, the authors showed that knockin of the corrective cassette in the WAS locus allows the physiological expression of the WAS 2–12 cDNA from WAS endogenous regulatory regions and the restoration of protein expression at levels comparable to healthy T cells. This is in sharp contrast to the results seen in this and previous studies3 in WAS cells transduced with an LV carrying a WAS cDNA where reconstituted WASP levels looked seemingly lower than wild-type counterparts. Current clinical trials for WAS utilize an LV with a 1.6 kb fragment of the endogenous WAS promoter to drive human WASP expression, which may be insufficient to recapitulate full expression in certain hematopoietic lineages, thus explaining the reduced correction of platelet defects observed in treated patients. Therefore, the work from Pille et al. may represent a further confirmation of the increased suitability of targeted integration of WAS in its endogenous locus as a potential therapeutic option for patients with WAS. On the other hand, safety is of great concern for CRISPR-Cas9-based applications, where installing unwanted mutations at off-target sites could potentially lead to the development of cancerous lesions.
Using combined in silico and cell-based methods, the authors in this study identified 44 off-target sites, of which only one was confirmed through next-generation sequencing. While they suggest that this may pose relatively low risks for patients because of its location in intronic regions, performing further assessment to ensure the safety of the approach is essential. Indeed, regulatory elements located in introns, such as noncoding RNAs, have diverse functions and can significantly influence gene regulation; thus, monitoring their integrity after gene editing would be desirable from a therapeutic perspective.9
In conclusion, the work by Pille and colleagues represents a solid groundwork for the continued development of gene editing approaches for WAS, with the final aim being to provide the best line of treatment for patients with WAS lacking a suitable HSCT donor.
Acknowledgments
The authors of this commentary would like to thank funding by the Sparks-GOSH Children’s Charity Grant (V4522) and the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Asma Naseem, Email: a.naseem@ucl.ac.uk.
Alessia Cavazza, Email: a.cavazza@ucl.ac.uk.
References
- 1.Vieira R.C., Pinho L.G., Westerberg L.S. Understanding immunoactinopathies: A decade of research on WAS gene defects. Pediatr. Allergy Immunol. 2023;34 doi: 10.1111/pai.13951. [DOI] [PubMed] [Google Scholar]
- 2.Pille M., Avila J.M., Park S.H., Le C.Q., Xue H., Haerynck F., Saxena L., Lee C., Shpall E.J., Bao G., et al. Gene editing-based targeted integration for correction of Wiskott-Aldrich syndrome. Mol. Ther. Methods Clin. Dev. 2024;32 doi: 10.1016/j.omtm.2024.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai R., Romito M., Rivers E., Turchiano G., Blattner G., Vetharoy W., Ladon D., Andrieux G., Zhang F., Zinicola M., et al. Targeted gene correction of human hematopoietic stem cells for the treatment of wiskott - Aldrich syndrome. Nat. Commun. 2020;11:4034. doi: 10.1038/s41467-020-17626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C., et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341 doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K., et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnani A., Semeraro M., Adam F., Booth C., Dupré L., Morris E.C., Gabrion A., Roudaut C., Borgel D., Toubert A., et al. Long-term safety and efficacy of lentiviral hematopoietic stem/progenitor cell gene therapy for Wiskott-Aldrich syndrome. Nat. Med. 2022;28:71–80. doi: 10.1038/s41591-021-01641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiroli G., Ferrari S., Conway A., Jacob A., Capo V., Albano L., Plati T., Castiello M.C., Sanvito F., Gennery A.R., et al. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan0820. [DOI] [PubMed] [Google Scholar]
- 8.Lee B.C., Gin A., Wu C., Singh K., Grice M., Mortlock R., Abraham D., Fan X., Zhou Y., AlJanahi A., et al. Impact of CRISPR/HDR editing versus lentiviral transduction on long-term engraftment and clonal dynamics of HSPCs in rhesus macaques. Cell Stem Cell. 2024;31:455–466. doi: 10.1016/j.stem.2024.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]