Abstract
Objectives:
Previously reported data regarding growth parameters in individuals with fragile X syndrome (FXS) are inconsistent. We conducted longitudinal analysis of height and body mass index (BMI) in large number of individuals with FXS.
Methods:
Age- and sex-specific z-scores for height and BMI of 1,223 individuals with FXS were calculated based on published normative data. Mixed-effect linear regression models were fit separately for males and females, z-scores for height and weight were regressed against age and adjusted for intellectual disability (ID) and psychotropic medication use.
Results:
Mean height z-score for both sexes decreased with age and was lower than normative data. Mean BMI z-score was greater than normative data in both sexes and this disparity increased with age. BMI z-score in females was greater for those with moderate or severe ID than those with no or mild ID. Individuals taking antipsychotics had higher BMI z-scores than those taking no or other medications; those taking anticonvulsants or stimulants had lower BMI z-scores.
Conclusions:
Individuals with FXS are at elevated risk for overweight and obesity. The risk is higher in individuals taking antipsychotics and among females with severe ID. These findings warrant increased attention to obesity prevention for all individuals with FXS.
Introduction
Worldwide the prevalence of obesity between 1980 and 2013 has increased by 27.5% in adults and 47.1% in children1. Individuals with intellectual disability (ID) are considered a high-risk group for obesity, as studies have shown an increased obesity rates in children and adults with ID when compared to typically functioning controls2,3,4,5. Fragile X syndrome (FXS), caused by the silencing of Fragile X mental Retardation gene (FMR1) is the most common monogenic cause of inherited ID and autism spectrum disorder (ASD)6. Since the gene defect is located on chromosome X, the condition is typically more severe in males.
Recently concern have increased regarding obesity in childhood onset neurodevelopmental disordersand ASD. Because such neurodevelopmental disorders start early in life, an association with obesity starting in childhood could predispose them to long-term cardiovascular disease and reduce long term quantity of life7. It is most importantto understand the modifiable factors that might lead to obesity in children with neurodevelopmental disorders. For example, converging evidence suggest that the use of antipsychotic drugs can lead to obesity, lipid and blood glucose dysregulation and increased risk for premature cardiometabolic morbidity and mortality8. Thus, understanding if there is an association between obesity and FXS would be important for better management of this major neurodevelopmental disorder.
Several studies have examined obesity in FXS but have provided inconsistent results. A published survey of 885 families with FXS found that the prevalence of obesity in adults with FXS was similar to the prevalence in the general population; however, the obesity prevalence in boys with FXS was higher (31%) than in typically developing age matched controls (18%)9. A study conducted by the Fragile X Clinical and Research Consortium, which included 260 patients with FXS (198 males and 62 females), concluded that the weight of children and adolescents with FXS could be higher than in the general population10, but this conclusion could have been influenced by an outlier subgroup of obese patients. A more recent study analyzed longitudinal data of 147 males and females with FXS followed during a 7-to-9-year period concluded that BMI progressively increases with age11.
To better understand the association between FXS and obesity, we utilized a unique longitudinal data source. The Fragile X Online Registry with Accessible Research Database (FORWARD) is a longitudinal, multi-site collaborative database created through a cooperative agreement with the Centers for Disease Control (CDC). The FORWARD database is the largest such database in the US12. The FORWARD database includes data on cognitive functioning, problem behavior and current treatments as well as anthropometric measures. We analyzed FORWARD data to establish the prevalence of obesity in FXS in relation to normative data. We assessed group differences related to age, gender, severity of ID, and treatment with psychotropic medications.
Methods
Design of the FORWARD study
The FORWARD data collected from 33 participating clinical sites was obtained from 2012 to 2018. All participants have a full mutation confirmed by genetic testing. Individuals with FXS may enter the study at any point in the lifespan, and an attempt is made to evaluate each participant annually. At baseline, data are collected on a set of three forms designed by the project, Registration, Clinician Report, Parent Report, and three standardized assessment measures, the Aberrant Behavior Checklist (ABC), the Social Responsiveness Scale (SRS) and the Social Communication Questionnaire (SCQ). De-identified data were entered by site-based staff into a central REDCap database maintained at Columbia University. At annual follow-ups, data were collected on the Clinician, Parent Report and ABC forms. Given the clinic-based nature of the cohort, it is not possible to follow all FORWARD participants longitudinally. The anthropometric, medication and cognitive assessment data are contained in the clinician form and demographic information in the Registration form. All FORWARD subjects are consented for participation at the clinic of recruitment, and the study protocol has been approved by the IRB at each clinic site. The FORWARD study has been described in detail previously12.
Study Sample and Statistical Analysis:
Height and weight data collected in 2,492 FORWARD baseline and follow-up evaluations were performed on 1,306 individuals with a confirmed full mutation This collected data was based on measurements by the clinic health professionals during the in person visits at the participating clinics.728 (55.7%) of the individuals had only a baseline evaluation, 265 (20.3%) had a total of two evaluations, 153 (11.7%) had three evaluations, 64 (4.9%) had four evaluations, 59 (4.5%) had five evaluations, 35 (2.7%) had 6 evaluations, and 2 (0.2%) had seven evaluations. 183 evaluations (6%) were excluded from the analyses due to inconsistencies in change over time or improbable age-specific values that could not be resolved. Specifically, observations were removed for subjects in whom height measurements decreased by ≥3cm at any time; height measurements that increased by ≥3 cm per month; height measurements that increased by more than 20cm after age 20; BMI z-scores that changed by ≥2 SD from one observation to the next; and BMI values ≥50. The final sample size for analysis was 1,223. Sensitivity analyses including the excluded observations yielded results consistent with those reported below.
To compare BMI and height in the study sample with corresponding parameters in the general population (normative data) z-scores for height and BMI were calculated using WHO formulations and data for participants aged 0-2 years13 and CDC formulations and data for participants older than 2 years14. Mixed-effect linear regression models of BMI and height z-scores were developed; the initial models were fit with age as a continuous predictor, and indicators for use of eight classes of medications were included as covariates: alpha-agonists, antipsychotics, anxiolytics, mood stabilizers, non-SSRI anti-depressants, SSRIs, anticonvulsants, stimulants. An age-sex interaction was included in the models so that sex-specific estimates of the relationship of age and BMI could be generated. These models featured random intercepts for subject and an AR(1) correlation structure for repeated observations within subject. Contrasts were derived from the model parameters to estimate mean z-scores by age group (0-5, 6-10, 11-18, 19+), and to determine whether these means were significantly different from the published normative data. To assess the influence of disease severity, separate models were also fit including ID (dichotomized as mild disability or less vs moderate or greater disability) as a predictor. Contrasts were derived from this model to estimate mean z-scores for participants of different ID levels. Some younger participants were reported to be developmentally delayed but did not have an assessment of ID severity. These participants were excluded from these analyses. The initial analyses included all evaluations in which height and weight data was obtained, including those from participants with only one evaluation. Separate analyses were also conducted in which only those with longitudinal data (2 or more evaluations) were included. All analyses were performed using SAS version 9.4. Significance level was set at 5%.
Results:
As shown in Table 1, the sample of 1,223 consisted of 954 males and 269 females who were on average ~12 years of age at the time of registration in FORWARD; 23.1% were 0-5 years of age, 31.3% were 6-10, 27.6% were 11-18 and 18.1% were 19 or older. The sample was predominantly white, non-Hispanic (75.7%), 7.3% Black non-Hispanic, 12.8% Hispanic and 2.9% Asian. For 513 individuals who had 2 or more assessments, the mean number of months between first and last assessment was 38.1 months.
Table 1.
Individuals with fragile X syndrome included in the study
| Total Sample (n=1223) |
Males (n=954) |
Females (n=269) |
||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Race/ethnicity | ||||||
| White non-Hispanic | 926 | 75.7% | 733 | 76.8% | 193 | 71.7% |
| Black non-Hisp | 89 | 7.3% | 69 | 7.2% | 20 | 7.4% |
| Asian | 36 | 2.9% | 28 | 2.9% | 8 | 3.0% |
| Hispanic | 156 | 12.8% | 110 | 11.5% | 46 | 17.1% |
| Other | 16 | 1.3% | 14 | 1.5% | 2 | 0.7% |
| Age at Baseline [Mean (SD)] | 1223 | 12.3 (9.3) | 954 | 12.2 (9.0) | 269 | 12.5 (10.3) |
| Age at Baseline -- categorized | ||||||
| 0-5 | 282 | 23.1% | 217 | 22.7% | 65 | 24.2% |
| 6-10 | 383 | 31.3% | 298 | 31.2% | 85 | 31.6% |
| 11-18 | 337 | 27.6% | 264 | 27.7% | 73 | 27.1% |
| 19+ | 221 | 18.1% | 175 | 18.3% | 46 | 17.1% |
| Number of Follow up (FU) Assessments | ||||||
| 1 | 710 | 58.1% | 549 | 57.6% | 161 | 59.9% |
| 2 | 247 | 20.2% | 197 | 20.7% | 50 | 18.6% |
| 3+ | 266 | 21.7% | 208 | 21.7% | 58 | 21.5% |
| Time Followed in months (Only those with >1 FU Assessments) [Mean (SD)] | 513 | 38.1 (20.4) | 405 | 38.4 (20.6) | 108 | 36.9 (19.7) |
| Height, cm [Mean (SD)] | 1223 | 140.2 (27.0) | 954 | 141.0 (28.1) | 269 | 137.2 (22.5) |
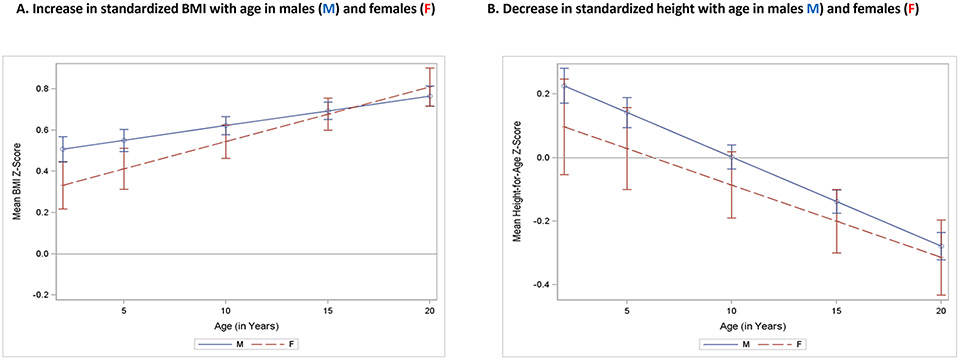
Model-based estimates of height and BMI z-scores by age, adjusted for the effects of medications and ID are shown in Table 2. Among 2-year-old males, the mean BMI z-score of individuals with FXS was 0.54 SD greater than normative data (p<.0001) and increased steadily with age to .77 SD greater than the normative reference at age 20 (p<.0001). Similarly, among females, BMI z-score was 0.38 SD greater than normative data among females (p=0.0135) at age 2 and increased to .83 SD greater at age 20. The trend of increase in BMI z-score with age for both males and females is shown graphically in Figure 1a. While the slope of increase for females is somewhat steeper than for males, this difference is not statistically significant (p=0.1284).
Table 2.
BMI and Height Z-Scores Relative to the General Population among Males and Females with FXS
| BMI Z-Score | Height Z-Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex and Age | Estimate | SE | DF | t Value | Pr > |t| | Estimate | SE | DF | t Value | Pr > |t| |
| Males | ||||||||||
| 2 y.o | 0.5419 | 0.1147 | 1011 | 4.73 | <.0001 | 0.1115 | 0.1066 | 1011 | 1.05 | 0.2956 |
| 5 y.o. | 0.5793 | 0.1088 | 1011 | 5.32 | <.0001 | 0.0322 | 0.1012 | 1011 | 0.32 | 0.7504 |
| 10 y.o. | 0.6416 | 0.1016 | 1011 | 6.31 | <.0001 | −0.1000 | 0.0947 | 1011 | −1.06 | 0.2912 |
| 15 y.o. | 0.7039 | 0.0982 | 1011 | 7.17 | <.0001 | −0.2322 | 0.0915 | 1011 | −2.54 | 0.0113 |
| 20 y.o. | 0.7662 | 0.0991 | 1011 | 7.73 | <.0001 | −0.3643 | 0.0920 | 1011 | −3.96 | <.0001 |
| Females | ||||||||||
| 2 y.o | 0.3772 | 0.1524 | 1011 | 2.47 | 0.0135 | −0.0052 | 0.1732 | 1011 | −0.03 | 0.9761 |
| 5 y.o. | 0.4531 | 0.1399 | 1011 | 3.24 | 0.0012 | −0.0678 | 0.1543 | 1011 | −0.44 | 0.6614 |
| 10 y.o. | 0.5797 | 0.1254 | 1011 | 4.62 | <.0001 | −0.1722 | 0.1334 | 1011 | −1.29 | 0.1973 |
| 15 y.o. | 0.7062 | 0.1211 | 1011 | 5.83 | <.0001 | −0.2765 | 0.1288 | 1011 | −2.15 | 0.0320 |
| 20 y.o. | 0.8328 | 0.1281 | 1011 | 6.50 | <.0001 | −0.3808 | 0.1425 | 1011 | −2.67 | 0.0076 |
Figure 1.
BMI-z scores and Height z scores for age
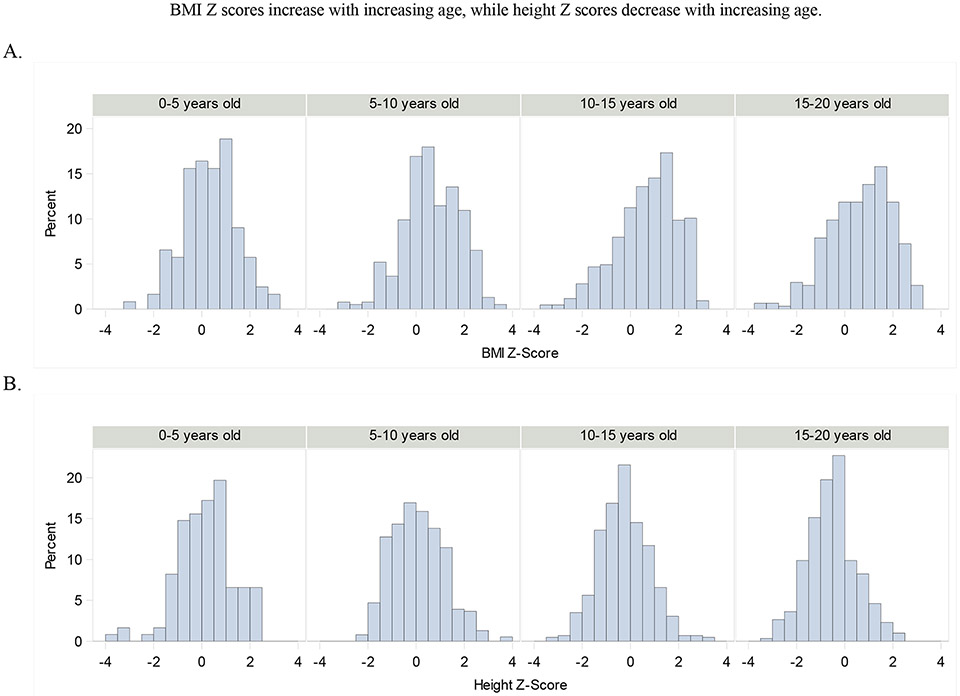
Among 2-year-old males, the mean height z-score of individuals with FXS was 0.11 SD greater than in normative data, a non-significant difference (p<0.2956) and decreased steadily with age to −0.36 SD slightly greater than the normative data at age 20 (p<.0001). Similarly, among females, height z-score at age 2 was −0.01 SD less than the normative data among females, a non-significant difference (p=.9761) and decreased to −0.38 SD less than the normative data at age 20 (p<0.0076). The trend of decrease in height z-score with age for both males and females is shown graphically in Figure 1b. The difference in decline of height z-score with age between the sexes is not statistically significant (p=0.4661). Figure 2 shows distributions of FXS individuals BMI-Z scores (A) and height Z scores (B) at different ages.
Figure 2.
Distributions of FXS individuals BMI-z scores (A) and height z scores (B) at different ages.
In summary, among individuals with FXS, BMI is significantly greater compared to the normative reference at all ages and among both males and females, and this disparity increases with age. Height decreases with age and is significantly lower than in the normative reference by adulthood.
There was no significant difference between males and females in BMI z-scores (p=0.1797) or height z-scores (p = 0.3159), controlling for age, and the gender-by-age interaction was also not significant (BMI p= 0.1284, height p =0.4661), indicating no significant sex difference in the association between age and BMI or height z-scores.
In terms of medications, as shown in Table 3 those using antipsychotics had higher BMI z-scores (p <.0001) while people taking anticonvulsants or stimulants had lower BMI z-scores (p=0.0266; p<.0001, respectively) than those not taking medications, adjusting for age and the effects of other medications. No medication was found to have significant influence on height z-scores.
Table 3.
Effects of Medications on BMI
| Effect | DF | Den DF |
F Value |
Pr > F | |
|---|---|---|---|---|---|
| Age,mos | 1 | 1012 | 17.86 | <.0001 | |
| AlphaAgonists | 1 | 1012 | 1.46 | 0.2270 | |
| Antipsychotics | 1 | 1012 | 17.24 | <.0001 |
|
| Anxiolytic | 1 | 1012 | 0.02 | 0.9019 | |
| Mood Stabilizers | 1 | 1012 | 2.90 | 0.0887 | |
| Non-SSRI AntiDepressants | 1 | 1012 | 0.00 | 0.9495 | |
| SSRIs | 1 | 1012 | 0.87 | 0.3512 | |
| Seizure Medications | 1 | 1012 | 4.93 | 0.0266 | |
| Stimulants | 1 | 1012 | 23.10 | <.0001 |
Those using Antipsychotics had higher BMI Z-scores (Diff = 0.1977, p <.0001) while people taking Seizure medications (Diff = −0.2087, p = 0.0266) or Stimulants had lower BMI Z-scores (Diff = −0.2168, p<.0001) than those not taking medications, adjusting for age and the effects of other medications
The results shown in Table 4 indicate that ID severity was significantly associated with BMI z-score only among females (female p = 0.001, male p = 0.125). Females with moderate ID or greater, had higher mean BMI z-scores compared with those with mild ID or less, adjusted for medications and age (score difference=0.5021; p=0001). ID severity had no significant relationship with height z-score for either males (p = 0.3262) or females (p= 0.9205).
Table 4.
BMI-Z score is greater among those with intellectual disability (ID) among females, but not among males
| Females | BMI z-score Estimate (age 10) |
Standard Error |
DF | t Value | Pr > |t| |
|---|---|---|---|---|---|
| Moderate, Severe or Profound ID VersusNo ID/Borderline ID/Developmental Delay | 0.4075 | 0.1484 | 193 | 2.75 | .0066 |
| Males | |||||
| Moderate, Severe or Profound ID VersusNo ID/Borderline ID/Developmental Delay | 0.078 | 0.0552 | 757 | 1.40 | .1605 |
To determine that the significant effects reported above hold up when the analysis is limited to individuals with longitudinal data, the models were repeated, limited with participants with two or more annual visits (n=513). Demographic characteristics of those in this subset are similar to those shown in Table 1.
Among individuals with more than one visit, as with the analyses of the entire cohort, age was significantly associated with BMI z-score (p=0.0053) and older individuals had higher average BMI z-scores than younger patients in each age group (Table 5). Similarly, as in the full sample, height z-score decreased significantly with age (p<.0001).
Table 5.
Individuals with two or more visits included in the longitudinal analyses
| Total Sample (n=513) |
Males (n=405) |
Females (n=108) |
|||||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | Prob |
| Race/ethnicity based on f1q8x and f1q6 | . | . | . | 0.3640 | |||
| White non-Hispanic | 412 | 80.3% | 332 | 82.0% | 80 | 74.1% | |
| Black non-Hisp | 34 | 6.6% | 26 | 6.4% | 8 | 7.4% | |
| Asian | 14 | 2.7% | 9 | 2.2% | 5 | 4.6% | |
| Hispanic | 50 | 9.7% | 36 | 8.9% | 14 | 13.0% | |
| Other | 3 | 0.6% | 2 | 0.5% | 1 | 0.9% | |
| Age at the time of registration, based on f1q4 [Mean (SD)] | 513 | 11.8 (8.4) | 405 | 11.9 (8.4) | 108 | 11.6 (8.3) | 0.8074 |
| Age at Registration--categorized | . | . | . | 0.6705 | |||
| 0-5 | 101 | 19.7% | 79 | 19.5% | 22 | 20.4% | |
| 6-10 | 184 | 35.9% | 148 | 36.5% | 36 | 33.3% | |
| 11-18 | 145 | 28.3% | 110 | 27.2% | 35 | 32.4% | |
| 19+ | 83 | 16.2% | 68 | 16.8% | 15 | 13.9% | |
The BMI z score slope among those with multiple visits was similar to that of the entire study cohort, with a progressive increase in BMI z-score discrepancies with increasing age. (longitudinal sample: b=0.018, p<.0001; full sample: b=0.013, p=.0053).
Discussion
This is the largest study of height and BMI in patients with FXS reported to-date (see Table 6). Unlike most previous studies, we report data in all age groups and assess the association of BMI and height z-scores to the ID severity and the use of psychotropic medications. Unlike some of the previous studies that showed increased BMI only in children or adolescents with FXS, our study found significantly elevated BMI z-scores into adulthood and increasing BMI with age compared to the normative population. These findings should motivate increased attention in FXS clinics to weight control and surveillance for potential obesity associated disorders.
Table 6.
Previous studies of obesity among individuals with fragile X and the FORWARD study
| Reference number |
Source of FXS Participants | Age Range |
Number of Patients |
Males | Females | Related Variables | Type of Study |
|---|---|---|---|---|---|---|---|
| 6 | National Fragile X Foundation, FRAXA Research Foundation, Conquer Fragile X Foundation | all ages | 963 | 839 | 236 | gender, age, overall health, learning, food habits | cross sectional |
| 7 | FXCRC | all ages | 260 | 198 | 62 | age, gender | cross sectional |
| 8 | University of Wisconsin study | >19 | 134 | age, gender, ASD | longitudinal−9 years | ||
| Current study | FORWARD Database, version 4 | all ages | 1223 | 954 | 269 | age, gender, cognition psychoactive medications | Longitudinal, median follow up =38.9 months |
BMI z-scores were significantly higher among individuals who used anti-psychotic medications. These findings are in concordance with previous studies that showed increased weight gain in young individuals with mental disorders, who were treated with antipsychotic medications15. It has also been shown that weight loss programs are less efficient for individuals on antipsychotic medications16. This medication-associated weight gain may involve interference of the medication with neurohormone receptor signaling, mitochondrial function or the constitution of the patients’ microbiome17 These findings suggest that weight awareness and weight control programs should be initiated for patients with FXS at the onset of antipsychotic medication therapy. While BMI z-scores were elevated among those with antipsychotic medications and severe ID, the increase in BMI z-score with age and the disparity in BMI z-score at all ages with respect to the general population remain statistically significant even after adjusting for the effects of medication and ID.
When evaluating obesity in FXS, it is important to consider the presence of a distinct group of FXS individuals with severe obesity, food seeking behavior and hypogonadism, who resemble patients with Prader-Willi syndrome referred to as Prader-Willi phenotype in Fragile X18,19,20,21. Individuals with apparent characteristics of this phenotype could not be identified based on our sample data. While the results of the analyses limited to those with longitudinal data were consistent with those that include participants with only one visit, it is important to note that participants may be recruited into FORWARD at any age, and that the span of longitudinal data for most individuals is only 2-3 years. If age at entry into FORWARD is associated with disease severity or medication use, this creates a potential for bias. However, our models incorporated adjustment for the effects of psychotropic medication and severity of ID. Moreover, our results show that BMI is significantly greater at each age than it is in the general population; this finding is not subject to bias with respect to age of study entry.
Only FXS females showed an association between the degree of ID and BMI z-scores (Table 4). A possible explanation of this finding may be related to a narrower range of ID in males that would decrease the statistical power to detect differences between the ID severity groups.
Metformin, a drug used to treat type 2 diabetes, was recently found to decrease obesity, improve language capability, and reduce aberrant behavior in patients with FXS’22,23. Expanded clinical trials with metformin are ongoing’22,23. Metformin is also effective in treating antipsychotic induced weight gain and may be considered even in FXS individuals without obesity. In addition to elevated obesity in the FORWARD sample, we found a significant reduction in mean height z-score with increasing age. In both males and females, at age 15, mean adult height became significantly lower among those with FXS than in the general population and further decreased by adulthood. Greater height of younger FXS individuals is consistent with results of previous studies in which height was measured over time10,24. However, we believe our study is the first to report that mean height in FXS individuals at an older age is substantially lower than in the general population.
In conclusion, 1) BMI z-scores in FXS were higher than in the published normative data at all ages; 2) this disparity increased with age among both sexes and 3) height z-scores in FXS decrease with age and by adulthood are well below the mean height of the general population. These findings call for increased attention to weight control programs in FXS clinics. This is especially relevant for patients with more severe ID and those with prescribed antipsychotic medications.
Study limitations
FXS individuals with severe manifestations may be underrepresented in this study because of the difficulties and, possibly, the reluctance of their caregivers to travel to the clinic for a research visit. Female FXS patients with mild or absent symptoms are also likely underrepresented. Finally, individuals from some minority groups may be underrepresented in the clinic population due to underdiagnosis and limitations in access.
Acknowledgements
We would like to thank the members of the Steering Committee of FORWARD for their review of the manuscript and their important and helpful comments.
Contributor Information
Tse-Hwei Choo, Columbia University, Department of Psychiatry.
Qing Xu, Columbia University, Department of Psychiatry.
Dejan Budimirovic, Kennedy Krieger Institute, Department of Psychiatry.
Reymundo Lozano, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences.
Richard E. Frye, Phoenix Children's Hospital, Child Health
Howard Andrews, Columbia University, Data Coordinating Center.
Milen Velinov, Rutgers University New Brunswick, Pediatrics.
References
- 1.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–s185. [PubMed] [Google Scholar]
- 2.Emerson E, Robertson J, Baines S et al. Obesity in British children with and without intellectual disability: cohort study. BMC Public Health. 2016; 16:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De S, Small J, Baur LA. Overweight and obesity among children with developmental disabilities. J Intellect Dev Disabil. 2008;33(1):43–47. [DOI] [PubMed] [Google Scholar]
- 4.Bégarie J, Maiíano C, Leconte P et al. The prevalence and determinants of overweight and obesity among French youths and adults with intellectual disabilities attending special education schools. Res Dev Disabil. 2013;34(5):1417–1425. [DOI] [PubMed] [Google Scholar]
- 5.Bandini L, Danielson M, Esposito LE, et al. Obesity in children with developmental and/or physical disabilities. Disabil Health J. 2015;8(3):309–316. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann WE, Kidd SA, Andrews HF et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics. 2017. (Suppl 3):S194–S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahathuduwa CN, West BD, Blume J, Dharavath N, Moustaid-Moussa N, Mastergeorge A. The risk of overweight and obesity in children with autism spectrum disorders: A systematic review and meta-analysis. Obes Rev. 2019. Dec;20(12):1667–1679 [DOI] [PubMed] [Google Scholar]
- 8.Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, Newcomer JW. Metabolic Effects of Antipsychotics on Adiposity and Insulin Sensitivity in Youths: A Randomized Clinical Trial. JAMA Psychiatry. 2018. 75(8):788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raspa M, Bailey DB, Bishop E et al. Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. Am J Intellect Dev Disabil. 2010;115(6):482–495. [DOI] [PubMed] [Google Scholar]
- 10.Kidd SA, Lachiewicz A, Barbouth D, et al. Fragile X syndrome: a review of associated medical problems. Pediatrics. 2014;134(5):995–1005. [DOI] [PubMed] [Google Scholar]
- 11.Usher LV, DaWalt LS, Hong J et al. Trajectories of Change in the Behavioral and Health Phenotype of Adolescents and Adults with Fragile X Syndrome and Intellectual Disability: Longitudinal Trends Over a Decade [published online ahead of print, 2020 Feb 10], J Autism Dev Disord. 2020; 10.1007/s10803-020-04367-w. doi: 10.1007/s10803-020-04367-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman SL, Kidd SA, Riley C, et al. FORWARD: A Registry and Longitudinal Clinical Database to Study Fragile X Syndrome. Pediatrics. 2017;139(Suppl 3):S183–S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006; 450:76–85. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 15.Shymko G, Grace T, Jolly N, et al. Weight gain and metabolic screening in young people with early psychosis on long-acting injectable antipsychotic medication (aripiprazole vs paliperidone). Early Interv Psychiatry. 2020. Jul 26. doi: 10.1111/eip.13013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Shukla AP, Mandel LS, Tchang BG et al. . Medical Weight-Loss Outcomes in Patients Receiving Concomitant Psychotropic Medication: A Retrospective Cohort Study. Obesity (Silver Spring). 2020. Sep;28(9): 1671–1677. [DOI] [PubMed] [Google Scholar]
- 17.Bretler T, Weisberg H, Koren O et al. The effects of antipsychotic medications on microbiome and weight gain in children and adolescents. BMC Med. 2019;17(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fryns JP, Haspeslagh M, Dereymaeker AM et al. A peculiar subphenotype in the fra(X) syndrome: extreme obesity-short stature-stubby hands and feet-diffuse hyperpigmentation. Further evidence of disturbed hypothalamic function in the fra(X) syndrome? Clin Genet. 1987;32(6):388–392. [DOI] [PubMed] [Google Scholar]
- 19.De Vries BB, Niermeijer MF. The Prader-Willi-like phenotype in fragile X patients: a designation facilitating clinical (and molecular) differential diagnosis. J Med Genet. 1994;31(10):820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowicki ST, Tassone F, Ono MY, et al. The Prader-Willi phenotype of fragile X syndrome. J Dev Behav Pediatr. 2007;28(2):133–138. [DOI] [PubMed] [Google Scholar]
- 21.Muzar z, Lozano R, Kolevzon A et al. The neurobiology of the Prader-Willi phenotype of fragile X syndrome. Intractable Rare Dis Res. 2016;5(4):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biag HMB, Potter LA, Wilkins V et al. Metformin treatment in young children with fragile X syndrome. Mol Genet Genomic Med. 2019. Nov;7(11):e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dy ABC, Tassone F, Eldeeb M et al. Metformin as targeted treatment in fragile X syndrome. Clin Genet. 2018. Feb;93(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loesch DZ, Huggins RM, Hoang NH. Growth in stature in fragile X families: a mixed longitudinal study. Am J Med Genet. 1995;58(3):249–56. [DOI] [PubMed] [Google Scholar]