Abstract
DNA-based molecular markers have great importance among other methods used for the authentication, detection, and identification of medicinal herbal species. Currently, it is more common to identify the medicinal herbal species (monoherbal or polyherbal forms) morphologically by using sensory, macroscopic, and microscopic methods. DNA-based markers made an easy for accurate detection of herbal species by using the polymerase chain reaction (PCR) which involves in vitro amplification of a particular region of DNA sequence.
In the current study, we used heterogenic parts for isolation of DNA from twelve important medicinal herbal species followed by purity determination, and yield calculation. We optimized a PCR reaction using universal primer sets to amplify the target DNA followed by DNA sequencing, and species identification. We also performed phylogenetic analysis for determining the evolutionary relationship between the herbal species, by using MEGAX32 software. Further, we prepared adulterated herbal species samples to validate the method.
The method was able to amplify the target gene through PCR in 11 out of 12 herbal species samples (sensitivity 91.66%).The DNA from cinnamon could not yield a truly amplified product. On DNA sequencing, all the amplified products were identified as true herbal species (specificity 100%). In the adulterated samples, non-specific DNA bands were observed after performing the PCR reaction, indicating the mixing of more than one herbal species.
To conclude, DNA sequencing-based molecular analysis is advantageous for the correct identification, and detection of adulterated herbal species.
Keywords: Adulteration, DNA sequencing, Herbal species, Polymerase chain reaction (PCR)
1. Introduction
Natural occurring herbal formulations play a pivotal role in agronomical products, nutritional supplements, botanical medicine, and cosmeceuticals. The use of medicinal important herbal species has been greatly raised as an alternative to conventional drugs all over the world. People’s interest in using medicinal herbs and medicinal products or folk medicines and cosmetics has grown since these items are thought to be safe and harmless as they include natural chemicals that are safe (Shimmer et al., 1994; Ahmed et al., 2006; Bown, 2002; herbal diversity, 2015).But this misleading concept may cause health risks including hypersensitivity in the users without calculating any risk and benefit ratio on the basis of data. In the developing counties, the condition is execrable due to lack of legislation.
Contamination and adulteration are very common malpractices in herbal raw material. Owing to a lack of proper quality checks by the regularity bodies, the adulterated herbal species are being sold in the herbal market and those too without a prescription. Usually, adulteration is done by using identical medicinal or nutritional herbal species and even in some instance a normal and technical person can’t identify the adulterant (De Smet, 1992; Schier et al., 1994; Keikin and Paloucek, 2008; Birdson and Forman, 2000). Currently, chemical methods are also used for the authentication of herbal species. These methods may also give impaired results due to the presence of some similar constituents in the herbal species. In this context, the DNA-based molecular marker technique provides a platform for the authentication of species produced from various parts of the plant such as fruit, seed, rhizome, flower, leaves, and root. DNA-based molecular markers are not only used in the fields of taxonomy, physiology, embryology, and genetics but also used in plant genetic science. This field is progressing and its application is increasing day by day in the commercially important medicinal herbs and nutritional food supplements such as food crops, and horticultural plants. Earlier it is being used in pharmacognostic characterization of herbal medicine and pharmacogenetics studies (Marmiroli et al., 2008; Joshi et al., 2004). In DNA-based assay, specifically the polymerase chain reaction (PCR) method is used in the identification and authentication of herbal species in foodstuffs, and for the authentication of meat products, seafood products, and dairy products (Focke et al., 2011). The conservation of plant species is easily made possible by DNA-based markers via phylogenetic analysis, and also applied in genotypes fingerprinting, and determination of purity as well (Gaine et al., 2015;). The innovation of PCR made the development of DNA-based markers easier with amplified efficiency and versatility. Correct identification of species is very important because some species having similar morphology that may contain toxic substances such as black cumin (Nigella sativa) can be adulterated with seeds of red creole onion (Allium cepa) or other species such as Clitoria guianensis; black pepper (Piper nigrum) is substituted with papaya seeds (Carica papaya); and the turmeric (Curcuma longa) is contaminated with (Curcuma zedoaria) which could be detected through the PCR. Even some toxic inorganic and synthetic organic pigment (i.e. yellow pigment lead chromate, PbcrO4) is also used for the pigmentation of adulterated species of Curcuma longa (Dhanya and Sasikumar, 2010; Hayakawa et al., 2010).
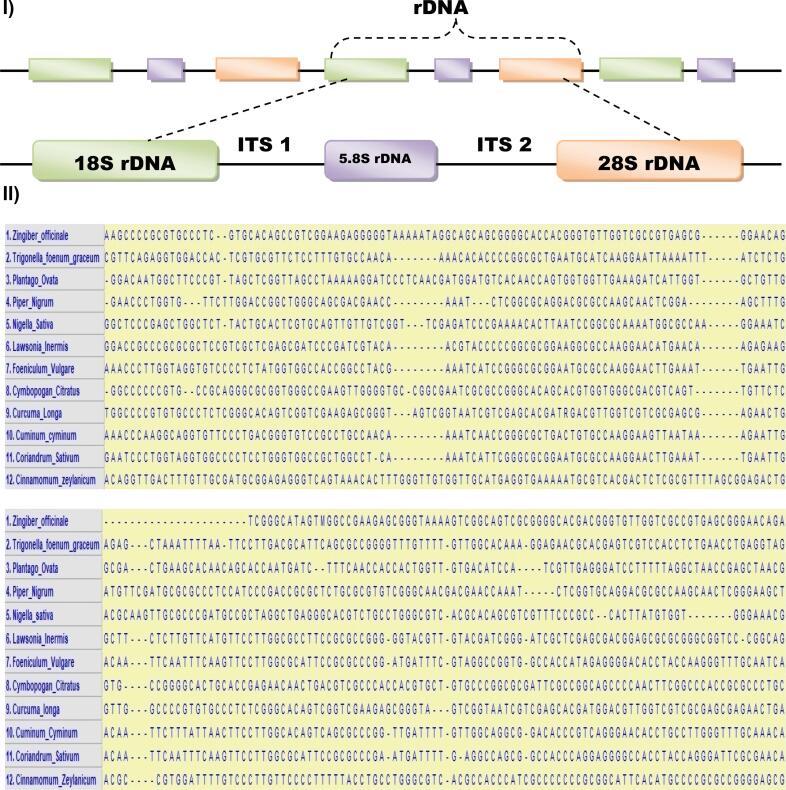
In this study, we have examined the heterogenic parts of herbal plant species as described in (Table 1). Isolation of the genomic DNA from the dried or fermented part of plant species is quite difficult due to its degradation (Mafra et al., 2008; John et al., 2012; Yu et al., 2020; John, 2017; Cardellina, 2020; Nybom et al., 2014; Dhanya et al., 2007). DNA extraction from the medicinal important herbal species is performed by cracking the cell wall, cell membrane and removing the proteins. For the PCR reaction, the selection of universal primer sets, and knowledge of the target sequencing region are very important to avoid false positive results. Herein, we targeted the Ribosomal DNA (rDNA) region which is present in the internal transcribed spacer (ITS) between the coding regions. The non-coding ITS regions ITS1 and ITS2 between 18S and 28S rDNA has proved to be a useful sequence for phylogenetic studies in many angiosperm families and for differentiation of families, genera, and species (Srivastava and Mishra, 2009; Liu et al., 2020; Gupta and Yunbi, 2008; Chen and Zhang, 2018; Kang and Long, 2017; Chiou et al., 2007; Rogers and Bendich, 1987). The level of sequence variation in both ITS regions suitable for phylogenetic analysis is found at various taxonomic levels within families, depending on the linkage, and spacer region of 5.8S rDNA as diagnostic tools for authentication (Larkin et al., 2007; Aliotta et al., 1996; Hall, 2020). The advantage of rDNA over the other coding regions is that the rDNA region is most abundant in the plant cells which provides the high number of replication in the genome (Pannecoucque and Hofte, 2017; Manokar et al., 2017). This study described the development of methods that are used for the isolation of genomic DNA from the important medicinal herbal species, and the PCR method for species-specific amplification using sequence differences in the rDNA region (Poczai and Hyvonen, 2010).
Table 1.
Medicinal herbal Species and condiments used for isolation of DNA.
| Spices | Family | Parts used | Medicinal properties |
|---|---|---|---|
|
Coriandrum sativum L. (Coriander) Cuminum cyminum L. (Cumin) Foeniculum vulgare M. (SweetFennel) |
Apiaceae |
Fruits Fruits Fruits |
Carminative* Diuretic, galactagauge* Antispasmodic* |
| Trigonella foenum-graceum L. (Fenugreek) | Leguminosae | Seeds | Hypoglycemic* |
| Lawsonia inermis L. (Henna) | Lythraceae | Leaves | Anti-mycotic* |
| Plantago Ovata Forsk. (Husk / Spogel) | Plantaginaceae | Seeds | Laxative, dysentery * |
| Piper nigrum L. (Black Piper) | Piperaceae | Fruits | Anti-cholerin* |
| Cymbopogan citrates L. (Lemon Grass) | Poaceae | Leaves | Anti-depressant* |
| Nigella stiva L. (Black Cumin) | Rununculaceae | Seeds | Anti puerperal fever* |
| Cinnamomum Zeylanicum(Cinnamon) | Lauraceae | Bark | Antimicrobial* |
|
Curcuma longa L. (Turmeric) Zingiber Officinale Boehm (Ginger) |
Zingiberaceae | Rhizome | Anti-inflammatory* Hypocholesterolaemic* |
(Ref.: Ross, 2005).
2. Material and methods
2.1. Sample collection of the herbal species
We have studied twelve different herbal species belonging to the nine different families (Table 1), some of which were in fresh form and some were commercially available in dried form. Initial identification of the herbs was made by organoleptic features. The herbs were carefully packed in separate bags to avoid any cross-contamination among the herbal species, and to minimize microbial contamination.
2.2. Methods for DNA extraction
2.2.1. Method-I: (Modified CTAB protocol for isolation of DNA)
It is a modified procedure of standard CTAB for DNA isolation (Ze-yu et al., 2017). In this method, a highly cationic surfactant lysis buffer CTAB (2 %) was prepared by using 2 % CTAB (cetyltrimethylammonium bromide), 1.4 M NaCl, 20 mM EDTA (ethylenediamine-tetraacetic acid salt), and 100 mM Tris-HCl (tris/ hydroxymethyl-aminomethan) at pH 8.0. The TE (Tris/EDTA) buffer was prepared by adding 10 mM Tris in 1 mM EDTA. An amount of 50 mg of a herbal sample was crushed into fine powder using liquid nitrogen in a cooled mortar and pestle. The powder was transferred into a micro-centrifuge tube, and 1 ml of preheated CTAB buffer was added into it, followed by adding 7μLof proteinase K (20 mg/ml) and 2 μL beta-mercaptoethanol. The mixture was incubated at 65 °C for 1 hr in a water bath. During incubation period, the mixture was gently mixed by inverting the tube at 5–10 min intervals. Then after 1 hr, the tube was kept at ambient temperature for 5 min.
An equal volume of the chloroform:isoamylalcohol solution in a ratio of (24:1) was added and mixed by gently inverting the tube several times, followed by centrifugation at 12000xgfor 15 min at 4 °C. The supernatant obtained was transferred carefully into a new micro-centrifuge tube while avoiding layers mixing, followed by the addition of 2/3rdvolume of isopropanol, and mixed well. Precipitated DNA was observed as thread-like structures. The micro-centrifuge tube was centrifuged at 12000xg for 5 min at 4 °C to pellet the DNA. The supernatant was carefully discarded without any disruption. Then DNA pellet was washed twice with 200 μL of freshly prepared ethanol (70 % v/v) and centrifugation at 12000xg for 3 min at 4 °C. After washing, DNA containing tube was kept for air-drying at ambient temperature for 30 min for complete evaporation of solvent from the tube. The pellet was suspended in 50 μL of the TE buffer (pH 8.0) and stored at −20 °C.
2.2.2. Method-II: Isolation of DNA through spin column
Isolation of DNA from the medicinal herbal species was carried out by an adsorption spin column (EZ-10 spin column plant genomic DNA Mini prep kit, BS425 Bio Basic Inc., Canada). For this, 50 mg herbal specie was grinded in liquid nitrogen and transferred into a micro-centrifuge tube. Then 150 μL PCL (Plant cell lysis solution) was added, the tube was shaken on a vortex and then incubated for 20 min at 65 °C, followed by adding 25 μL PPsolution and centrifuged at 8000xg for 5 min. Then 300 μL BP buffer was added to the column and incubated the mixture for 3 min at room temperature, followed by centrifugation at 8000xg for 2 min.The flow-through was discarded. Then, 500 μL of wash solution was added into the spin column followed by centrifugation at 8000xg for 2 min. The spin column was again centrifuged at 8000xg for 2 min to remove any remnant wash solution. The column was placed in a clean micro-centrifuge tube, and 30 μL of elution buffer was added into central part of the membrane in the column, followed by incubation of the column at 37 °C for 2 min. The column was centrifuged at 8000xg for 2 min in order to elute the DNA from the column. The aliquots of the purified genomic DNA were stored at −20 °C.
2.3. Qualitative and quantitative analysis of the isolated DNA through spectral categorization
The quality and purity of the DNA were further determined by using agarose gel electrophoresis and spectrophotometer. The quality of the isolated DNA and PCR amplified products was determined by loading the DNA onto a 2 % agarose gel electrophoresis which included a dye stain with ethidium bromide solution (0.01 %).The 1 Kbp ladder was loaded into the first slot of the gel to infer the size of the isolated DNA whereas the isolated DNA was added into the adjacent well of the gel. The electrophoresis was performed by applying 60 V for 1.5 h. The DNA bands were visualized with a UV-trans illuminator (Benchtop UV Transilluminator, UVP M−15 V, Thermo Fisher Scientific).
For quantification, DNA solution (2 μL) was measured at wavelengths 260 nm and 280 nm (Genova Nano, Micro-volume 737501, Genway). DNA was quantified by measuring the absorbance at 260 nm and multiplying by the dilution factor, using the relationship A260 of the Beer-Lambert equation.
2.4. PCR amplification
For the polymerase chain reaction, 1.0 μL of DNA template, 10 μL of PCR master mix (Thermo Fisher Scientific, USA) which contains Taq DNA polymerase, dNTPs, MgCl2, KCl and stabilizer, 1.0 μL of each of forward and reverse primers(10 pM), 7.0 μL of nuclease-free water, respectively were combined to form a total volume of 20 μL. After preparing the reaction mixture, samples were subjected to few seconds short spin and placed in a thermocycler (BIORAD, T1000) under specified conditions, as in the pre-denaturation step. The initial denaturation of the template DNA was performed at 94 °C for 3 min followed by amplification steps including denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and terminal elongation at 72 °C for 1 min (the amplification steps were repeated 35 times), and then 5 min for the final extension at 72 °C, followed by the hold at 4 °C (Focke et al., 2011).
The purity of the PCR products (5uL) was determined by 2 % agarose gel electrophoresis. The remaining PCR products were stored at −20 °C for sequence analysis.
2.5. DNA sequencing
The purified PCR products were sequenced with the help of DNA sequencer (AppliedBiosystem, SeqStudio Genetic Analyzer A46228, Thermofisher Scientific, USA).The obtained DNA sequences were submitted in NCBI GenBank with accession numbers, OL866137 (Coriandrum sativum L.), OL875149 (Cuminum cyminum L.), OL828773 (Foeniculum vulgare M.), OL889953 (Trigonella foenum-graceum L.), OL875245 (Lawsonia inermis L), OM279531 (Plantago Ovata Forsk.), OL765298 (Piper nigrum L.), OM279797 (Cymbopogan citrates L.), OL840850 (Nigella stiva L.), MZ673309 (Curcuma longa L.) and ON135430 (Zingiber Officinale Boehm). These sequences were subsequently used for phylogenetic analysis.
2.6. Sequencing alignment and phylogenetic tree analysis
The nucleotide sequences were aligned by Clustal-W technique using MEGA-X32 while the cladogram or phylogenetic tree was inferred by neighborhood- joining method with (1000 replicates) of 100 % bootstrap value. The maximum likelihood method was used to compute the tree distances (Hall, 2020; Larkin et al., 2007; Poczai et al., 2010).
3. Results
3.1. Qualitative and quantitative analysis of isolated DNA
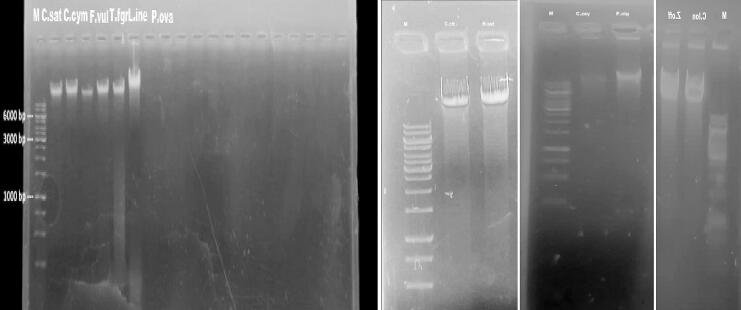
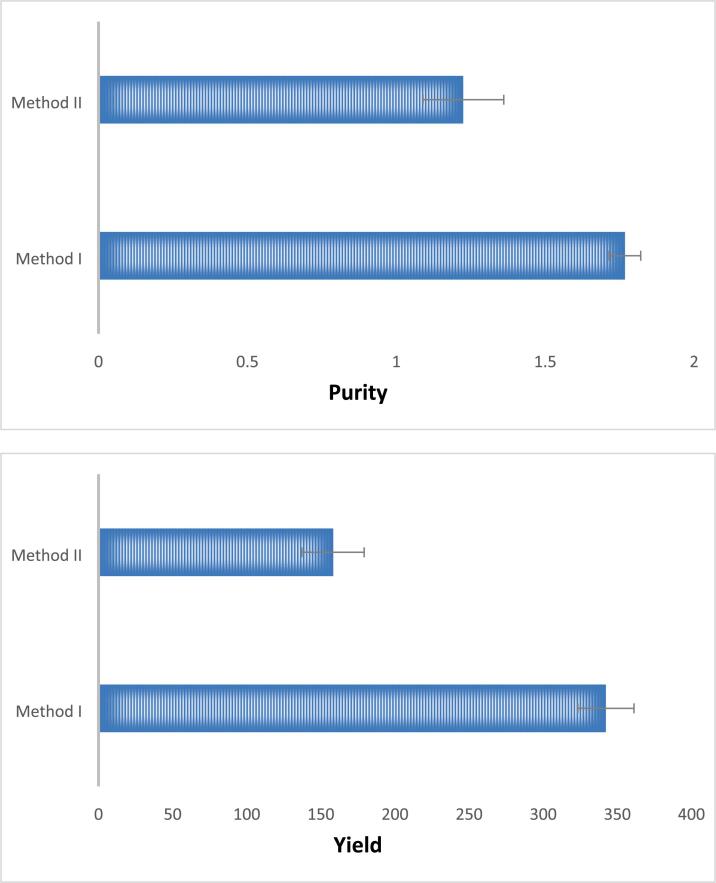
The purity of extracted DNA from 12 herbal species (Table 1) was determined by 2 % agarose gel electrophoresis. All the samples showed a uniform single band of above 6 Kb (Fig. 1).For quantification, the results showed that the DNA extracted from Method I exhibited a greater yield (342 ± 19 µg/ml) as compared to Method II (158 ± 21 µg/ml). Similarly, the purity of DNA (A260/A280) obtained from Method I revealed higher values (1.76 ± 0.05) in contrast with Method II (1.23 ± 0.14). However, method II was found to be quick and efficient (Fig. 2).
Fig. 1.
Isolated DNA analyzed on agarose gel. Where “M” is molecular weight marker (ladder genome 1 Kb) loaded into the gel.
Fig. 2.
Method I and Method II have different purity (A260/A280) and yield (ng/uL) of the genomic DNA extracted from the herbal species.
3.2. Amplification of the isolated DNA
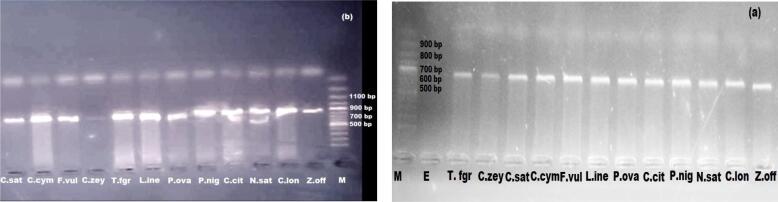
The amplification of targeted genes from the isolated DNA samples was performed using universal primer sets in a polymerase chain reaction (PCR) (Table 2). The isolates were amplified as distinct portions if one of the primer sets showed positive results. More than 93 % of the isolated DNA from Method I gave positive PCR reactions using primer set 1and primer set 2, while Method II gave (80 %) amplification results (Fig. 3).
Table 2.
Universal primer sets for the amplification of rDNA genes.
| Primer | Sequence Forward primer 5′→3′ Sequence Reverse primer 5′→3′ |
Length (bp) |
Target gene |
|---|---|---|---|
| Universal-1 | TGAACCTGCGGAAGGATCATTGT CGAGAGCCGAGATATCCGTTG |
320 | 18S rDNA gene 5.8S rDNA gene |
| Universal-2 | ACCATCGAGTCTTTGAACGCAAGT TATTGATATGCTTAAACTCAGCGGGT |
330 | 5.8S rDNA gene 28S rDNA gene |
Fig. 3.
Amplified product of PCR analyzed on gel after binding with primer set. (a) primer set 1 (b) primer set 2. “M” molecular weight marker (ladder genome 100 bp) and “E” is the empty, Amplicons with size less than 100 bp are considered as primer dimmers.
3.3. Verification and confirmation
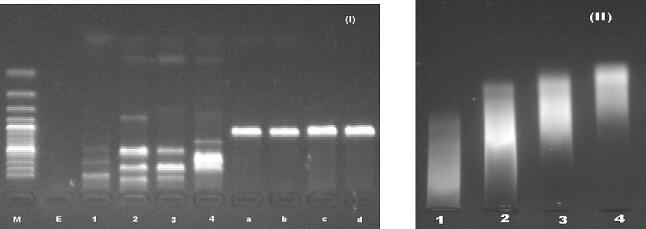
To further validate the suitability of the DNA authentication method for determining the purity and quality of herbal species self-made adulterations were made with a few experimental types. The species include the black piper, black cumin, lemon grass, and husk which were mixed with papaya seeds, red creole onion, ryegrass, and garden cress respectively in a ratio of 1:1 for PCR assessment (Table 3). The results showed that the DNA extracted from adulterated species with different primer sets gave incorrect amplification with messy bands while the individual amplified samples (without adulteration) revealed a single sharp band on agarose gel (Fig. 4).
Table 3.
Adulteration of herbal species performed for the method validation.
| Samples composition* | Results |
|---|---|
| Black Piper (Piper nigrum) with papaya seeds (Carica papaya) | Ir |
| Black Cumin (Nigella stiva) with red creole onion (Allium cepa) | Ir |
| Lemon Grass (Cymbopogan citrates) with perennial ryegrass (Lolium perenne) | Ir |
| Husk (Plantago Ovata) with garden cress (Lepidium sativum) | Ir |
Species mixed in equal amount, Ir: Incorrect result.
Fig. 4.
(I) PCR amplified product of mixed sample (1: Black piper with papaya seed, 2: Black cumin with red creole onion, 3: Lemon grass with perennial ryegrass, and 4: Husk with garden cress) revealed inappropriate bands with primer set 1 while amplified product of single sample (a: papaya seeds, b: red creole onion, c: perennial ryegrass, and d: garden cress) showed single sharp band of targeted region, similarly (II) exhibited impure band with primer set 2. Whereas “M” is the molecular weight marker (ladder genome) and “E” is the empty.
3.4. DNA sequencing and phylogenetic tree analysis
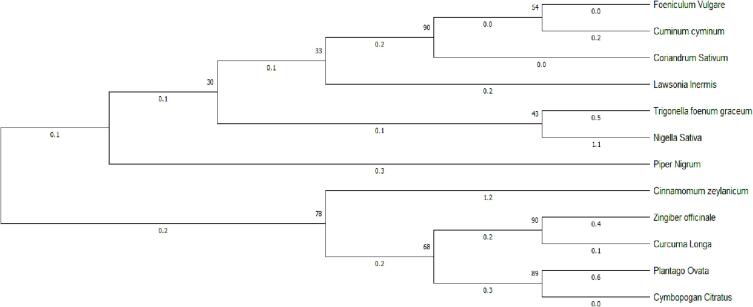
The amplified DNA was purified and the sequence was aligned by using the Clustal-W tool (Fig. 5). The evolutionary relationship among the 12 herbal species was determined by constructing a phylogeny with neighborhood-joining method using the MegaX32 tool (1000 bootstrap replicates). The results showed 30 to 90 % homology among the species (Fig. 6). The evolutionary detail was inferred by using the neighborhood-joining method, which showing the sum of the longitude of branch is 6.42574970 for its optimal tree. While the Maximum Composite Likelihood method explored the evolutionary distances was computed in which the units of the number of base substitutions each site. As a result of these 12 nucleotide sequences were analyzed. All those positions which found uncertain were removed from each sequence pair according to using pair wise deletion option. Ultimately total of 374 positions were constituted in the final dataset (Fig. 6).
Fig. 5.
rDNA sequence. (I) Colored blocks are the rDNA gene (18S, 5.8S, and 28S), a continuous line between the blocks are ITS 1 and ITS 2 region. (II) Sequence alignment of ITS region was formed by Clustal-W.
Fig. 6.
Evolutionary relationships of taxa. Phylogenetic tree obtained for twelve herbal species. Evolutionary analyses were conducted in MEGA X32 using neighborhood joining method.
4. Discussion
In the current study, the total DNA was isolated from 12 herbal species samples by using two different methods. The method I was a modified protocol of CTAB buffer in which the DNA was precipitated out and pelleted by centrifugation. This was in contrast with Method II which was based on DNA adsorption on a spin column as described earlier. The Isolated DNA was examined accordingly, as quantitative and qualitative analysis was done by photometric measurement; amplification was carried out by PCR; and the phylogenetic tree was constructed using the MEGAX32 software.
DNA-based molecular analysis has several advantages for the identification of herbal species over the traditional morphological methods (Focke et al., 2011; Joshi et al., 2004). It gives reliable and ambiguous free results on the other hand; an experienced person is required for species identification in traditional methods. In this study, Method II has been established for the isolation of DNA from all the herbal species. It is a reliable, efficient, and time-saving method consumes approximately 1 h for DNA extraction, compared with Method I which takes approximately 3 h (Ze-yu et al., 2017).
The amplification of the selected genes from the isolated DNA samples was performed using universal primer sets in a PCR. The designed primer sets (universal) are combined in the optimized PCR conditions which support the amplification of the specific genomic region for the detection and identification of 12 herbal species through subsequent DNA sequencing. The isolated DNA of all the herbal species showed a positive PCR reaction except cinnamon (Cinnamomum zeylanicum).In the case of cinnamon, the fermentation process occurred during storage condition, which might be one of the reasons for the degradation of DNA resulting in shorter fragments which was not appropriate for amplification by conventional PCR. In addition, the inhibitory nature and interfering nature of phenolic compounds might be the reason for DNA degradation (Khanuja et al., 1999; Aboul-Ftooh et al., 2019).
The PCR reaction resulted in various sizes of amplicons prior to optimization. Therefore, optimization of the PCR is very important to minimize the false positive results. Consequently, the reaction conditions were optimized by performing gradient PCR. In gradient PCR, we have selected various temperatures ranging from 55 °C to 65°Cfor obtaining the optimum primer annealing. The best annealing temperature was found to be at 60 °C by observing a sharp single band on the agarose gel. The deviation from this temperature would result in non-specific binding along with primer dimmers formation.
The process of validation and authentication was further verified and confirmed by performing self-made adulterations with a few experimental herbal species (Table 3) for PCR assessment during the study. The species usually used for adulteration has a similar morphology as the original herbal species (Focke et al., 2011; Gaine et al., 2015). The extracted DNA with different primer sets was examined and found inadequate amplification with disordered bands on the agarose gel with adulterated samples while uncontaminated samples were obtained as single pure band (Fig. 4). These false negative results of PCR with universal primers may be due to the inhibitory substances co-isolated with DNA from the other herbs. These findings further confirm that DNA-based characterization is the correct method for the authentication of herbs among the other analyses. In addition, it is a fast, reliable, and correct method for the authentication of medicinal herbs over conventional methods because the age, climate factor, and varying concentrations of the constituent do not affect the analysis of this method (Gaine et al., 2015). On the other hand, all herbal species gave the correct amplification results except cinnamon (Cinnamomum zeylanicum), which could not give a good quality band due to the too dried herbal sample or might be because of fermented bark the isolation of DNA results in DNA degradation. A good quality DNA can be obtained with fresh bark or leaves of cinnamon for DNA extraction. Also, larger amounts of secondary metabolites (such as polysaccharides and phenolic compounds) which have a negative impact on high quality DNA, result in a lower rate of amplification and DNA sequencing failures of the herbal species (Gaine et al., 2015; Sahu et al., 2012).
The amplified DNA was purified and the sequence was aligned by using the Clustal-W tool. The evolutionary relationship among the 12 species of herbs was determined by constructing a phylogeny (neighborhood-joining method) using the MegaX32 tool (1000 bootstrap replicates) (Tamura et al., 2004).In this analysis, the homology was ranging from 30 % to 90 %. Foeniculum vulgare (SweetFennel) and Cuminum cyminum (Cumin) having common ancestors which are closely related to each other and sharing the gene to Coriandrum sativum (Coriander) species with 90 % homology, similarly Trigonella foenum-graceum (Fenugreek) and Nigellasativa (Black Cumin)sharing 43 % homology, Zingiber officinale (Ginger)and Curcuma longa (Turmeric)sharing 90 % homology, Plantago ovata(Husk) and Cymbopogan citrates (Lemon Grass) sharing 89 % homology while Piper nigrum (BlackPepper), Lawsonia inermis (Henna), and Cinnamomum zeylanicum (cinnamon) are less closely related or distinctly related with them (Kumar et al., 2018).
5. Conclusion
To conclude, it was established that DNA-based molecular examination for determining the transparency and purity of herbal species has several benefits above the traditional old-styled procedures. This study will provide a new dimension in the advance research study as a tool for the authentication and standardization of the herbal species and help to promote the exploration of the rDNA of ITS region which remains undiscovered in most of the herbal species.
CRediT authorship contribution statement
Zeeshan Hyder: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. Ghazala Hafeez Rizwani: Conceptualization, Funding acquisition, Project administration, Supervision, Resources, Visualization, Writing – review & editing. Huma Shareef: Data curation, Investigation, Resources, Visualization, Writing – review & editing. Iqbal Azhar: Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – review & editing. Meraj Zehra: Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to Dr. Ishtiaq Ahmad Khan, In-charge of Jamil-ur-Rahman Center for Genome Research (PCMD, ICCBS, University of Karachi, Pakistan) for technical support and guidance.
Contributor Information
Zeeshan Hyder, Email: m.raffay@hamdard.edu.pk.
Ghazala Hafeez Rizwani, Email: director.hmi@hamdard.edu.pk.
Huma Shareef, Email: Huma.shareef@jsmu.edu.pk.
Iqbal Azhar, Email: Iazhar@uok.edu.pk.
Meraj Zehra, Email: Meraj.zehra@hamdard.edu.pk.
References
- Aboul-Maaty N.-F., Oraby H.-S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bulletin of the National Research Centre. 2019;43:25. doi: 10.1186/s42269-019-0066-1. [DOI] [Google Scholar]
- Ahmed I., Aqil F., Owais M., editors. Turning Medicinal Plants into Drugs. WILEY-VCH Verlag GmbH and Co.KGaA; 2006. pp. 30–37. [Google Scholar]
- Aliotta J.M., Pelletier J.J., Ware J.L., Moran L.S., Benner J.S., Kong H. Thermostable Bst DNA polymerase I lacks a 30 –> 50 proofreading exonuclease activity. Genet. Anal.: biomol. Eng. 1996;12:185–195. [PubMed] [Google Scholar]
- Birdson D., Forman L., editors. The Herbarium Handbook. Royal Botanical Garden; London, U.K.: 2000. [Google Scholar]
- Bown D. New Encyclopedia of Herbs and Their Uses. D.K; London: 2002. The Royal Horticultural Society. [Google Scholar]
- Cardellina J.H., II . ABC-AHP-NCNPR Botanical Adulterants Prevention Program; Austin, TX: 2020. Turmeric raw material and products laboratory guidance document. https://www.herbalgram.org/resources/botanical-adulterants-prevention-program/laboratory-guidance-documents/turmeric-raw-material-and-products-lab-guidance-document-february-2020/ [Google Scholar]
- Chen F., Zhang L. The sequenced angiosperm genomes and genome database. Frontier in Plant Science. 2018;9:418. doi: 10.3389/fpls.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou S.-J., Yen J.-H., Fang C.-L., Chen H.-L., Lin T.-Y. Authentication of medicinal herbs using PCR-amplified ITS2 with specific primers. Planta Med. 2007;73:1421–1426. doi: 10.1055/s-2007-990227. [DOI] [PubMed] [Google Scholar]
- De Smet P.A.G.M. In: Adverse Effects of Herbal Drugs. De Smet P.A.G.M., Keller K., Hänsel R., Chandler R.F., editors. Springer-Verlag; Heidelberg: 1992. Toxicological outlook on the quality assurance of herbal remedies; pp. 1–72. [Google Scholar]
- Dhanya K., Sasikumar B. Molecular marker based adulteration detection in traded food and agricultural commodities of plant origin with special reference to spices. FoodBiotechnol. Curr. Trends Biotechnol. Pharm. 2010;4:454–489. [Google Scholar]
- Dhanya K., Kizhakkayil J., Syamkumar S., Sasikumar B. Isolation and amplification of genomic DNA from recalcitrant dried berries of black pepper (Piper nigrum L.) A Medicinal Spice. Mol. Biotechnol. 2007;37:165–168. doi: 10.1007/s12033-007-0044-y. [DOI] [PubMed] [Google Scholar]
- Focke F., Haase I., Fischer M. DNA-Based identification of species: DNA isolation, whole geneome amplification and polymerase chain reaction. J. Agric. Food Chem. 2011;59:513–520. doi: 10.1021/jf103702s. [DOI] [PubMed] [Google Scholar]
- Gaine S.H., Upadhyay P., Das S., Sharma M.P. Authentication of medicinal plant by DNA markers. J. Plant Gene, Published by Elsevier b.v. 2015;4(83–99) doi: 10.1016/j.plgene.2015.10.002. https://creativecommons.org/licenses/by-nc-nd/4.0/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.K., Yunbi X. Genomics of major crops and model plant species. Int. J. Plant Genomics. 2008:2. doi: 10.1155/2008/171928. (Article ID 171928) [DOI] [Google Scholar]
- Hall B. G., 2020. Building phylogenetic tree from molecular data with MEGA, published by Oxford university press, Mol.Bio.Evol. 30(5): 1229-1235. [DOI] [PubMed]
- Hayakawa H., Kobayasm T., Minamiya Y., Yamamoto Y. Molecular identification of Turmeric (Curcuma longa, Zingiberacea) with high curcumin content. J. Jpn. Bot. 2010;85:263–269. [Google Scholar]
- Herbal Diversity, 2015. “AGTC Advance Gene Technology Crop”, Business development department Taiwan. https://www.agtc.com.tw.
- John K.W. Plant DNA barcodes: applications today and in the future. Institute of Botany, Chinese Academy of Sciences. 2017;55(4):291–307. [Google Scholar]
- John K. W., and Erickson D. L., (eds.), 2012. “DNA Barcodes: Methods and Protocols, Methods in Molecular Biology”, Springer Science, vol. 858:223-252.https://doi:10.1007/978-1-61779-591-6_11. [DOI] [PubMed]
- Joshi K., Chavan P., Warude D., Patwarhan B. Molecular marker in herbal drugs technology. Current Sci. 2004;87(2):159–165. https://www.jstor.org/stable/i24104699 [Google Scholar]
- Kang, Y., and Long, W., 2017. “DNA bar-coding analysis and phylogenetic relationships of tree species in tropical cloud forests”, 7(12564) 1-9. doi: 10.1038/s41598-017-13057-0. [DOI] [PMC free article] [PubMed]
- Keikin J.B., Paloucek F.P. 4th edn. Informa Healthcare; New York: 2008. Poisoning and Toxicology Handbook; pp. 577–582. [Google Scholar]
- Khanuja S.P.S., Shasany A.K., Darokar M.P., Kumar S. Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep. 1999;17:1. [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol.Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu Z., et al. Whole-genome sequencing and analysis of the Chinese herbal plant Gelsemium elegans. Acta Pharmaceutica Sinica B. 2020;10(2):374–382. doi: 10.1016/j.apsb.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra I., Ferreira I.M., Oliveira M.B.P. Food authentication by PCR-based methods. Eur. Food Res. Technol. 2008;227:649–665. [Google Scholar]
- Manokar J., Balasubramani S.P., Venkatasubramanian P. Nuclear ribosomal DNA e ITS region based molecular marker to distinguish Gmelina arborea Roxb. Ex Sm. from its substitutes and adulterants. J. of Ayurveda and Integrative Medicine. 2017;9(4):290–293. doi: 10.1016/j.jaim.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmiroli N., Maestri E., Gulli M., Malcevschi A., Peano C., Bordoni R., Bellis G. Methods for detection of GMOs in food and feed. Anal. Bioanal. Chem. 2008;392:369–384. doi: 10.1007/s00216-008-2303-6. [DOI] [PubMed] [Google Scholar]
- Nybom H., et al. DNA fingerprinting in botany: past, present, future. Invest. Genet. 2014;5(1):1–35. doi: 10.1186/2041-2223-5-1. https://www.investigativegenetics.com/content/5/1/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannecoucque J., Hofte M. Detection of rDNA ITS polymorphism in Rhizoctonia solani AG 2–1 isolates. Taylor & Francis Published. 2017;101:26–33. doi: 10.3852/08-084. [DOI] [PubMed] [Google Scholar]
- Poczai P., Hyvonen J. Nuclear ribosomal spacer regions in plant phylogenetics: problems and prospects. Mol Biol Rep. 2010;37:1897–1912. doi: 10.1007/s11033-009-9630-3. [DOI] [PubMed] [Google Scholar]
- Rogers S.O., Bendich A.J. Ribosomal RNA genes in plants: variability in copy number and in the intergenic spacer. Plant Mol. Biol. 1987;9:509–520. doi: 10.1007/BF00015882. [DOI] [PubMed] [Google Scholar]
- Ross I.A., 2005. “Medicinal plants of the world: Chemical Constituent, Traditional and Modern Medicinal Uses”. Humana press, Totowa, newjersy. ISBN: 1-59259-887-0.
- Sahu S.K., Thangaraj M., Kathiresan K. DNA extraction protocol for plants with high levels of secondary metabolites and polysaccharides without using liquid nitrogen and phenol. ISRN Molecular Biology. 2012;205049:6. doi: 10.5402/2012/205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier W., Sachsa B., Schultze W. Actual adulteration of herbal drugs. Dtsch A Poth Ztg. 1994;134:4569–4576. [Google Scholar]
- Shimmer, O., Kruger, A., Paulini, H., Haefele, 1994. “ Pharmazie”, 49: 448- 451. [PubMed]
- Srivastava S., Mishra N. Genetic markers - a cutting-edge technology in herbal drug research. J. Chem. Pharm. Res. 2009;1(1):1–18. [Google Scholar]
- Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wu, X., Liu C., Newmaster S., Ragupathy S., Kress W. J., 2020. “Progress in the use of DNA barcodes in the identification and classification of medicinal plants”, J. Ecotoxicology and Environmental Safety Published by Elsevier Inc., 208:1-7. doi: 10.1016/j.ecoenv.2020.111691. [DOI] [PubMed]
- Ze-yu F., Jian-cheng S., Jameson P.E. A rapid and cost-effective protocol for plant genomic DNA isolation using regenerated silica columns in combination with CTAB extraction. J. Integr. Agric. 2017;16(8):1682–1688. doi: 10.1016/S2095-3119(16)61534-4. [DOI] [Google Scholar]