Abstract
This work aims at characterizing the interplay between human immunodeficiency virus type 1 (HIV-1) and the antiapoptotic cellular protein Bcl-2 responsible for a persistent infection in lymphoblastoid T (J.Jhan) or monocytic (U937) cells. We report that the kinetics of Bcl-2 protein level during the establishment of a chronic infection is biphasic, characterized by a transient decrease followed by restoration to the initial level. The extent and duration of this transient decrease were inversely correlated with the basal level of Bcl-2 as shown by kinetics of Bcl-2 levels in J.Jhan or U937 clones exhibiting different levels of Bcl-2. Using these clones, we also showed that Bcl-2 downregulates HIV-1 replication. Therefore, the cells overexpressing Bcl-2 are characterized by a low viral burden which, in turn, has little effect on the level of this protein. The observed bipasic kinetics is the result of a dual regulation of Bcl-2 induced by HIV-1 infection itself: an upregulation at the transcriptional level of the bcl-2 gene concomitant with a downregulation at the protein level. Convergent data suggest that this downregulation is caused by the oxidative stress induced by the infection itself as shown by the associated modulations of glutathione and thioredoxin levels and by the prevention of these dysregulations by N-acetylcysteine. Altogether, these data indicate that infection first results in a decrease of Bcl-2, permitting an initial boost of replication. Then, as the synthesis at the transcriptional level proceeds, the replication is negatively controlled by Bcl-2 to reach a balance characterized by low virus production and a level of Bcl-2 compatible with cell survival. We suggest that the basal level of Bcl-2, together with infection-inducible transcription factors able to activate bcl-2 gene transcription, is a critical cellular determinant in the tendency toward an acute or a persistent infection.
The main feature of human immunodeficiency virus (HIV) infection is persistent replication. This chronic replication correlates with the permanent viral burden in lymphoid organs (37). One of the causes of this viral burden is the spreading of the virus from persistently infected macrophages (9) or from certain subsets of dendritic cells (5). Macrophages, particularly in pathologically affected tissues such as the brain, actively replicate the virus and are laden with virus particles (49). In contrast to these reservoir cells, infected but also bystander lymphocytes (11) die from various causes including apoptosis. Indeed, ex vivo studies clearly show that T lymphocytes from HIV-infected individuals exhibit a higher rate of apoptosis than lymphocytes from normal subjects (2, 11, 13). The apoptosis of infected peripheral blood T lymphocytes raised the question of the ability of other target cells such as macrophages to survive HIV infection and to sustain a persistent infection. This might relate to a particular host-virus interaction which prevents programmed cell death despite virus expression.
Several cellular gene products have been shown to induce or inhibit apoptosis. Among them, the cellular protein Bcl-2 was clearly demonstrated to inhibit apoptosis (19) induced by a variety of signals (34, 35) including infection by cytolytic viruses (18, 28, 29, 47) and oxidative stress (20, 52). Convergent data indicate that one of the major roles of Bcl-2 is to maintain the integrity of mitochondrial membrane function (27) and therefore to inhibit the release of apoptogenic factors such as cytochrome c (24, 30, 51) from mitochondria to cytosol. A downregulation of Bcl-2 expression was demonstrated in T lymphocytes from HIV-infected individuals and might ultimately be a possible cause of their increased rate of apoptosis ex vivo (3).
Furthermore, the role of oxidative stress as a cause of apoptosis of T lymphocytes has been proposed in HIV infection. HIV type 1 (HIV-1) infection causes a chronic ongoing inflammation as shown by high plasmatic levels of inflammatory cytokines (10) and production of reactive oxygen intermediates in HIV-1-seropositive individuals (8). This oxidative stress was demonstrated by a decrease of the concentration of the main antioxidant molecules such as plasmatic and lymphocyte glutathione (4, 7, 45) or lymphocyte thioredoxin (31). This oxidative stress is the result of the constitutive production of H2O2 by neutrophils at all stages of the disease (8). In addition, HIV-1-infected cells undergo an endogenous oxidative stress related to the inhibitory effect of the viral protein Tat on the activity of the manganese superoxide dismutase (12, 50), leading to an increase in endogenous reactive oxygen intermediates. In vivo studies with animal models showed that oxidative stress induces an immunodeficiency with T-lymphocyte depletion (14–16, 26). This conclusion fits with the observation that the high rate of apoptosis of CD4+ T cells from HIV-infected individuals can be decreased ex vivo by the addition of antioxidant compounds such as N-acetylcysteine (NAC) (36).
The aim of this work was to determine whether there is a particular interplay between HIV expression and Bcl-2 in cells which tolerate a chronic and active infection and to define the mechanisms governing such an interplay. The main question is whether a downregulation of Bcl-2 occurs in these cells as a consequence of HIV infection and whether it is deleterious to the cells, or whether there is any mechanism preventing this potentially deleterious regulation. We addressed this question in two cellular models of viral persistence: the lymphoblastoid T-cell (J.Jhan) and monocytic cell (U937) lines infected by HIV-1B-LAI.
We report here that the expression of viral proteins in these cells is followed as in T lymphocytes by a decrease of Bcl-2 level, but the main difference is that this decrease is only transient. Using J.Jhan or U937 cellular clones exhibiting different levels of Bcl-2, we demonstrate that the extent and duration of this decrease (accompanied by a proportional rate of apoptosis) are dependent upon the initial basal level of Bcl-2 and that a restoration of this initial level is required for long-term survival of the cells. Reciprocally, using these clones, we demonstrate that Bcl-2 negatively controls HIV expression. This regulation of Bcl-2 expression by the virus was further analyzed. We show that infection by itself induces an increase of Bcl-2 at the transcriptional level which compensates for the downregulation at the protein level. We propose that this downregulation is caused by the oxidative stress associated with infection.
MATERIALS AND METHODS
Plasmids.
The pNL4-3 vector (1), a kind gift of M. A. Martin, was used as an infectious HIV-1 (LAI strain) provirus. The plasmids P1-luc and P2-luc, carrying the luciferase reporter gene under the control of the P1 or P2 promoter of the bcl-2 gene (44), were derived from p18-21H (32). The P1-luc and P2-luc constructs contain a 2,400-bp BamHI-SacI (−3700 to −1289) and a 1,289-bp SacI-HindIII (−1289 to +1) fragment, respectively, fused to the luciferase gene in pGL3 (Promega). To construct CMV-s bcl-2 and CMV-as bcl-2 vectors, the 1,900-bp EcoRI fragment encompassing the human bcl-2 cDNA (44) was inserted in the sense or antisense orientation downstream of the cytomegalovirus early promoter in the pcDNA3 expression vector (Invitrogen). pSV2-TK-neo (22) carries the neomycin resistance gene under the control of the early simian virus 40 promoter.
Cells and culture conditions.
The J.Jhan human lymphoblastoid CD4+ T-cell line (derived from the Jurkat cell line; a gift from J. D. Fox, London, England) and the human myelomonocytic U937 cell line as well as transfectants (see below) derived from these cell lines were grown in RPMI 1640 (GIBCO-BRL) supplemented with 5% fetal calf serum, glutamine, and antibiotics.
J.Jhan or U937 transfectants expressing distinct levels of Bcl-2.
J.Jhan or U937 cells were transfected with CMV-s bcl-2, CMV-as bcl-2, or the control vector pcDNA3 by an electroporation procedure, with a single pulse of 520 V/cm and 1,500 μF for J.Jhan cells and a pulse of 600 V/cm and 2,100 μF for U937 cells. Stable transfectants were selected on the basis of their resistance to 1 mg of G418 per ml for J.Jhan cells and 0.8 mg/ml for U937 cells. G418-resistant cells were cloned at 0.3 cells per well and maintained in G418-containing medium. Selection of clones expressing distinct levels of Bcl-2 was performed on the basis of PCR analysis of sequences of the transfected plasmids and of protein content by Western immunoblot analysis. Since the clones originated from a population of cells heterogeneous for CD4 expression, we made the selection of these clones also on the basis of a high level of CD4 determined by flow cytometry analysis.
J.Jhan or U937 transfectants expressing the luciferase gene under the control of the P1 or P2 promoter of the bcl-2 gene.
J.Jhan or U937 cells were cotransfected with P1-luc or P2-luc and pSV2-TK-neo by electroporation as described above. Neomycin-resistant cells were not cloned but maintained in G418 as pools of transfectants. Under this selective pressure, the two mock-infected populations (J.Jhan and U937) were characterized by a stable constitutive luciferase expression at least during the time of the experiment (see Fig. 5). Luciferase activity was measured according to the standard procedure (43).
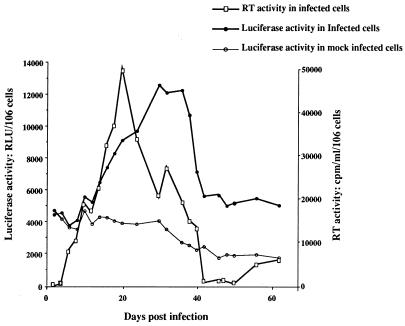
FIG. 5.
Modulations of the activity of the P2 promoter of the bcl-2 gene over the course of HIV-1 infection in J.Jhan cells. Luciferase activity was monitored in J.Jhan cells stably cotransfected with P2-luc and pSV2-TK-neo vectors, at various times postinfection. Luciferase activity was determined in cell lysates of infected or mock-infected cells as relative light units (RLU) per 106 cells. In parallel, virus replication was monitored in infected cells by measuring the RT activity in the cell supernatant, expressed in counts per minute per 106 living cells. The data shown here are representative of two independent experiments.
Infection with HIV-1.
To standardize all infections of T (J.Jhan) and monocytic (U937) cells, we prepared HIV-1 infectious supernatants from Cos-7 cells: pNL4-3 vector was transfected in subconfluent Cos-7 cells by a standard calcium phosphate coprecipitation procedure. The viral supernatant was harvested 48 h after transfection. p24gag protein was determined in cell-free supernatants, by an enzyme-linked immunoadsorbent assay (Sanofi/Pasteur), and all infections were standardized to the p24gag levels: 150 ng of p24gag was used to infect 107 J.Jhan or U937 cells. J.Jhan or U937 cells were exposed to HIV-1B-LAI for 1 h at 37°C. Cells were then washed to remove residual free virus, and cultures were established at 3 × 105 cells/ml. At various times postinfection, aliquots of viral supernatants were collected for an analysis of reverse transcriptase (RT) activity, by a previously described technique (42). In the experiment presented in Fig. 7, NAC was added at 10 mM from day 2 postinfection and cells were incubated in fresh medium containing this concentration of NAC every other day from day 3.
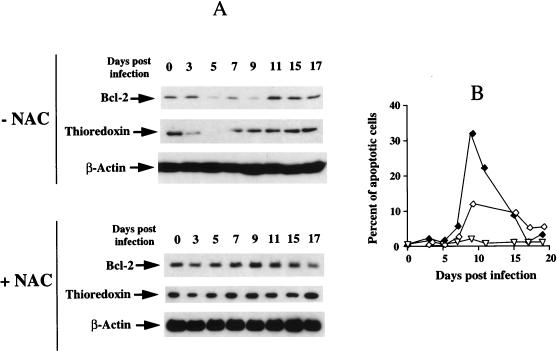
FIG. 7.
NAC counteracts both the decrease of Bcl-2 and thioredoxin and the apoptosis associated with HIV infection. (A) Levels of Bcl-2, thioredoxin, and β-actin were determined by Western immunoblot analysis in the J.Jhan control clone infected and cultured either in the absence (−NAC) or in the presence of 10 mM NAC (+NAC) The level of β-actin was also determined to standardize the amount of proteins used. (B) In parallel, the rate of apoptosis was determined by the YOPRO technique as described in Materials and Methods. Percentages of apoptosis were determined in cells infected in the absence (⧫) or in the presence (◊) of NAC and in mock-infected cells cultured in the presence of NAC (▿).
Determination of viability and apoptosis rates.
At various times postinfection, the percentages of viable and apoptotic cells were determined. Cell viability was assessed by the trypan blue exclusion technique. Apoptosis was evaluated by two distinct techniques: the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique (Oncor) and the use of a DNA intercalating agent, YOPRO 1 (Interchim), which does not label living cells and permits accurate detection of apoptotic cells (21). Apoptosis rates were determined by fluorescence microscopy or flow cytometry.
Western blot analysis.
At various times postinfection, whole-cell extracts were prepared for Western immunoblot analysis; 106 cells were incubated in 10 μl of lysis buffer: 0.2% Triton X-100, 500 mM NaCl, 500 mM sucrose, 1 mM EDTA, 0.15 mM spermine, 0.5 mM spermidine, 10 mM HEPES (pH 8), 200 μM phenylmethylsulfonyl fluoride, 2 μg of leupeptin per ml, 2 μg of pepstatin per ml, 24 IU of aprotinin per ml, and 7 mM β-mercaptoethanol. Twenty micrograms of proteins was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunodetection. Mouse monoclonal antibodies were raised against amino acids 41 to 54 of human Bcl-2 (clone 124; DAKO, Glostrup, Denmark), amino acids 85 to 104 of human thioredoxin (clone 11) (23) (provided by Fujirebio Inc., Tokyo, Japan), amino acids 285 to 304 of HIV-1 p24gag (39), and a slightly modified synthetic β-actin N-terminal peptide (AC-74; Sigma Immunochemicals, St. Louis, Mo.). Horseradish peroxidase-conjugated sheep anti-mouse antibody was used as a secondary reagent (Amersham). The antigen-antibody complexes were revealed by enhanced chemiluminescence (ECL; Amersham).
RNA extraction and Northern blot analysis.
Total cellular RNA preparations were obtained by the guanidinium isothiocyanate method as described by Chomczynski and Sacchi (6). For Northern blot analysis, total RNA was electrophoresed through a 1.5% agarose-formaldehyde gel, then blotted onto a nylon filter by capillarity with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer, and fixed by UV exposition for 5 min. Filters were initially prehybridized for 24 h at 42°C in 50% (vol/vol) formamide–5× Denhardt solution–5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]–0.5% (wt/vol) sodium dodecyl sulfate–20 μg of sonicated and denatured salmon sperm DNA per ml. Hybridization was then carried out overnight at 50°C, in the presence of the radiolabeled probe, and autoradiography was performed after the washing procedure.
The probe used for bcl-2 mRNA detection was a 1.9-kb fragment encompassing the complete cDNA of bcl-2. A β-actin cDNA probe (1) was used as an internal control.
Determination of intracellular glutathione.
Glutathione analysis was carried out by a modification of the method described by Tietze (48). Briefly, at various times postinfection, 3 × 106 cells were pelleted and resuspended in 50 μl of phosphate-buffered saline–200 μl of 0.25% Triton X-100 and deproteinized by the addition of 10% trichloroacetic acid in 0.01 N HCl. After centrifugation, trichloroacetic acid was extracted from the supernatant with diethyl ether. Aliquots were assayed for glutathione content with 0.2 mM NADPH, 0.3 mM dithionitrobenzoic acid, and 2 U of glutathione reductase per ml. Absorbance was recorded at 412 nm.
RESULTS
A chronic and productive HIV-1 infection of lymphoblastoid T (J.Jhan) or monocytic (U937) cells is accompanied by a transient decrease of Bcl-2 associated with a decrease of cell viability.
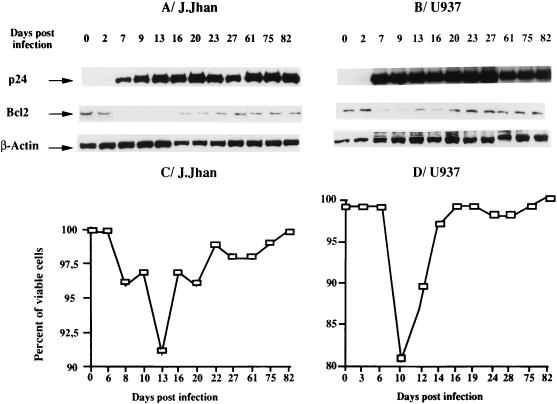
At various times postinfection, the levels of Bcl-2 as well as those of the viral protein p24gag and the β-actin were determined by Western blot analysis (Fig. 1). Expression of viral proteins as indicated by p24gag expression (a very faint band was observed at day 2 but was clearly visible at day 7) was associated with a marked decrease, although transient, of Bcl-2 in the two cell populations whereas the β-actin level was unchanged over the course of infection. This decrease of Bcl-2 level (Fig. 1A, days 7 to 20, and B, days 7 to 16) was accompanied by a moderate loss of viability as shown in Fig. 1C (9% at most) and D (17% at most), and the restoration of viability correlated with the restoration of Bcl-2 level.
FIG. 1.
Kinetics of the level of Bcl-2 over the course of HIV-1 infection in the lymphoblastoid T-cell (J.Jhan) (A) and monocytic cell (U937) (B) lines. Levels of Bcl-2 were analyzed by Western immunoblot analysis at various times postinfection. In parallel, p24gag antigen expression was also evaluated as a criterion of viral protein expression. The level of β-actin was also determined to standardize the amount of proteins used. In parallel, the percentage of viable cells was determined by trypan blue exclusion (C and D). The data shown here are representative of two independent experiments.
The extent and duration of the transient decrease of Bcl-2 level during HIV-1 infection are dependent upon the constitutive cellular level of Bcl-2.
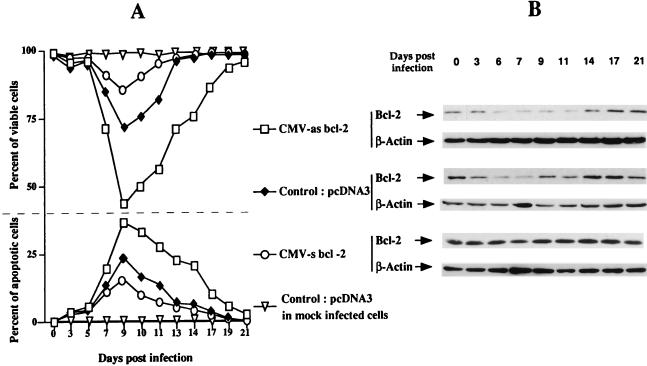
The experiments presented in Fig. 1 were performed with populations of cells relatively heterogeneous for their levels of Bcl-2. Thus, to determine the precise relation between the extent and duration of this transient decrease and the basal level of Bcl-2, we studied the kinetics of Bcl-2 (and the potential association with cell viability) in J.Jhan cell clones exhibiting different levels of this protein. Three of these clones were obtained by transfecting J.Jhan cells with either CMV-s bcl-2 (leading to a high-Bcl-2-expressing clone), CMV-as bcl-2 (leading to a low-Bcl-2-expressing clone), or pcDNA3, the cloning vector (leading to the control clone). As shown in Fig. 2B, we determined Bcl-2 levels at various times postinfection in these three clones by Western blot analysis. In parallel, we determined both the percentage of viable cells (by the trypan blue exclusion technique) and the percentage of apoptotic cells (by the TUNEL and YOPRO techniques) over the course of infection. Figure 2A shows that in noninfected cells (day 0) or in infected cells prior to the expression of viral protein (p24gag was clearly detectable at day 5 in this experiment), the rate of viability is about 98 to 100% in all clones irrespective of their basal Bcl-2 level. In contrast, expression of viral proteins triggered a decrease in Bcl-2 level (Fig. 2A) accompanied by a loss of viability which was mainly due to apoptosis (Fig. 2B), as shown by comparing the curves of viability and apoptosis. Nevertheless, the decrease of Bcl-2 was not a consequence of the decrease in cell viability since the level of β-actin determined in the same experiment was unchanged irrespective of the clone tested. The rate and duration of Bcl-2 decrease inversely correlated with the basal level of Bcl-2. Indeed, the decrease of Bcl-2 as well as the apoptosis rate were marked (about 40% at day 9) in cells expressing the lowest basal level of Bcl-2, whereas the decrease of Bcl-2 was hardly visible and the apoptosis much more moderate (about 10% at day 9) in cells expressing a high basal level of this protein. The clone expressing an average amount of Bcl-2 showed intermediate rates of decrease of Bcl-2 and apoptosis (about 25% at day 9). Similar observations were made for U937 clones exhibiting different levels of Bcl-2 (data not shown). We conclude that apoptosis is associated with the decrease of Bcl-2 level (rather than with a low basal level) induced by expression of viral proteins, but the extent and duration of this decrease depend on the basal level of Bcl-2.
FIG. 2.
Percentages of viable and apoptotic cells (A) in J.Jhan clones exhibiting distinct basal levels of Bcl-2 (B) over the course of HIV-1 infection. Levels of Bcl-2 were determined by Western immunoblot analysis in J.Jhan cells stably transfected with either CMV-as bcl-2, control pcDNA3, or CMV-s bcl-2 vectors at different times postinfection. The level of β-actin, as a nonantioxidant protein, was also determined to standardize the amount of proteins used. In parallel, the rate of apoptosis was determined by the TUNEL technique as described in Materials and Methods. The percentage of viable cells was determined by trypan blue exclusion. Percentages of apoptosis and viability were also determined in mock-infected J.Jhan cells transfected with pcDNA3.
Bcl-2 negatively regulates HIV replication.
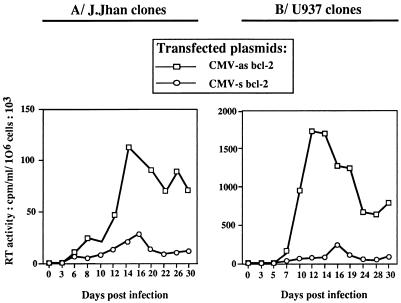
We then tried to understand why in cells expressing a high level of Bcl-2 the transient decrease of Bcl-2 was hardly visible (with almost no impairment in cell viability). We hypothesized that high levels of Bcl-2 might negatively control the level of HIV replication. Consequently, the low rate of viral protein expression in the cells would not affect Bcl-2 level. To verify this hypothesis, we measured the RT activities in the culture supernatants of J.Jhan (Fig. 3A) and U937 (Fig. 3B) clones at different times postinfection. We showed that RT levels were significantly lower in J.Jhan or U937 clones overexpressing Bcl-2 (stable transfectants containing the vector CMV-s bcl-2), than in low-Bcl-2-expressing clones (stable transfectant containing the vector CMV-as bcl-2). Together, these data clearly indicate that the cellular Bcl-2 protein negatively regulates HIV replication.
FIG. 3.
Comparative kinetics of HIV-1 replication in J.Jhan or U937 clones exhibiting distinct basal levels of Bcl-2. HIV-1 replication was determined in J.Jhan (A) or U937 (B) cells stably transfected with CMV-as bcl-2 or CMV-s bcl-2 at various times postinfection. HIV-1 replication was evaluated by determination of the RT activity (counts per minute per 106 living cells). The data shown here are representative of two independent experiments.
HIV-1 infection increases Bcl-2 synthesis at the transcriptional level.
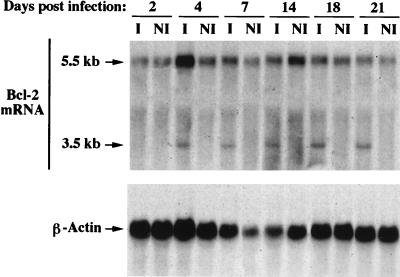
We then addressed the question of the possible mechanism(s) involved in the transient decrease and in the replenishment of Bcl-2 associated with infection. We tested the possibility that this process might occur at the transcriptional level. Using Northern blot analysis (Fig. 4), we tested the expression of bcl-2 gene transcripts at different times postinfection in J.Jhan cells. A major transcript of 5.5 kb was present in control cells, and its level was unchanged (considering β-actin levels) in infected cells. A second transcript of 3.5 kb was detectable only in infected cells when HIV-1 p24 expression started being detectable on day 4 postinfection (data not shown). This indicates that HIV infection induces an increase in the steady-state level of the 3.5-kb mRNA (see Discussion).
FIG. 4.
bcl-2 mRNA expression in J.Jhan cells infected or not by HIV-1. Northern blot analysis was performed on J.Jhan cell extracts at different times postinfection (I) or post-mock infection (NI) as described in Materials and Methods. β-Actin probe was used as a control for loading.
HIV infection increases the initiation rate of transcription from the P2 promoter of the bcl-2 gene.
We then determined whether this increased expression of the 3.5-kb transcript was associated with an increase in transcription initiation from the two known promoters of the bcl-2 gene, termed P1 and P2 (44). Therefore, we tested the activity of the P1 and P2 promoters driving the synthesis of luciferase in stably transformed J.Jhan cells over the course of HIV-1 infection. We determined luciferase activity in cell lysates as well as RT activity in the cell supernatants, at various times postinfection. Figure 5 shows that viral protein expression, as assessed by RT activity, was accompanied by an increase of P2 promoter activity (starting from day 5 and culminating between days 30 and 36), followed by a progressive decrease. It is worth noting that as bcl-2 gene transcription increased, virus replication decreased, confirming again the negative control of Bcl-2 over virus replication. An equilibrium chacterized by a low virus production and a P2 promoter activity sustained at a higher level than in mock-infected cells was then reached. In contrast, the P1 promoter activity was not modified over the course of HIV-1 infection (data not shown). These results indicate that infection itself increases the synthesis of Bcl-2 by increasing the initiation rate of transcription from the P2 promoter.
The upregulation of transcription level of the bcl-2 gene starts concomitantly with the decrease observed at the protein level (Fig. 1A, days 7 to 20), indicating that this decrease is not due to a downregulation at the transcriptional level but is most probably related to an increased degradation of Bcl-2.
HIV infection induces a decrease of Bcl-2, associated with oxidative stress.
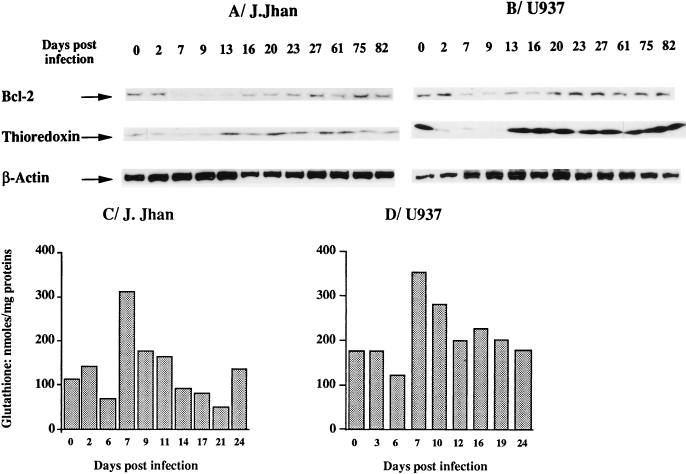
HIV infection is known to induce oxidative stress, as demonstrated by the decrease in cellular concentration of the main antioxidant molecules such as plasmatic and lymphocyte glutathione (4, 7, 45) or lymphocyte thioredoxin (32). Since Bcl-2 was demonstrated to have antioxidant functions, we determined whether the downregulation of Bcl-2 at the protein level was related to the oxidative stress induced by HIV infection. In the experiment partially presented in Fig. 1, we also monitored the level of thioredoxin over the course of HIV-1 infection in lymphoblastoid T (J.Jhan) or monocytic (U937) cells. The comparison of kinetics of Bcl-2 and that of thioredoxin is shown in Fig. 6. The thioredoxin level showed the same decrease as Bcl-2 (Fig. 6A and B; between days 2 and 9). However, the restoration of the level of thioredoxin occurred more rapidly than that of Bcl-2.
FIG. 6.
Relationship between the dysregulation of Bcl-2 and that of thioredoxin and glutathione over the course of HIV infection. Comparison of the kinetics of thioredoxin and Bcl-2 levels over the course of HIV-1 infection in the lymphoblastoid T-cell (J.Jhan) (A) and monocytic cell (U937) (B) lines. The infection experiment is that represented in Fig. 1. Consequently, data concerning Bcl-2 and β-actin levels were derived from Fig. 1, whereas the thioredoxin level is shown in comparison. Kinetics of glutathione concentrations was determined over the course of HIV-1 infection in the lymphoblastoid T-cell (J.Jhan) (C) and monocytic cell (U937) (D) lines. Glutathione concentrations were determined as described in Materials and Methods and were expressed as nanomoles of reduced glutathione per 106 cells. Each value is the mean of duplicates.
The variations in glutathione concentration were also determined by an enzymatic technique. A dysregulation of glutathione level was similarly observed in both cell lines (Fig. 6C and D) with a brief but reproducible decrease at day 6. From day 7, a compensatory upregulation was observed followed by a progressive shift towards the basal level. The restoration of glutathione level preceded those of thioredoxin and Bcl-2. These data suggest that the mechanism underlying the downregulation of Bcl-2 at the protein level is associated with infection-induced oxidative stress revealed by the concomitant decrease of glutathione and thioredoxin with Bcl-2. In the experiment presented in Fig. 7, we performed an infection of the J.Jhan control clone in the presence or absence of the antioxidant NAC. We showed that NAC prevented the associated decreases of Bcl-2 and thioredoxin during HIV infection (Fig. 7A). These data correlated with a reduced rate (12%) of apoptosis in the presence of NAC during infection, whereas this rate of apoptosis peaked at 32% in the absence of NAC (Fig. 7B). Altogether, these findings support a direct link between HIV-induced oxidative stress and the downregulation of Bcl-2.
DISCUSSION
In this report, we provide evidence that the persistence of HIV-1 infection in two cell lines was the result of a virus-host interaction leading to a regulation of the level of Bcl-2 by the virus and reciprocally of the virus level by Bcl-2. We showed that the establishment of a chronic and active HIV infection of lymphoblastoid T (J.Jhan) (Fig. 1A) or monocytic (U937) (Fig. 1B) cells was accompanied by a decrease of Bcl-2, such as that observed in peripheral blood lymphocytes from infected patients (3). The main difference was that this decrease was only transient and was associated with a moderate loss of viability. In these cells, used as a model of virus persistence, the restoration of Bcl-2 level required for the restoration of cell viability readily occurs. Using J.Jhan cell clones exhibiting different levels of Bcl-2, we demonstrated that the extent and duration of the transient decrease of Bcl-2 level during HIV-1 infection were dependent on the constitutive Bcl-2 level of the cells (Fig. 2). More precisely, the extent and duration of the decrease in Bcl-2 level were more important when the clone expressed a lower basal level of Bcl-2, whereas this decrease was hardly detectable in the high-Bcl-2-expressing clone. This strong difference could explain some discrepancies in the literature concerning the effect of HIV-1 infection on the level of Bcl-2 (38). The consequences of infection for Bcl-2 level should be interpreted on the basis of a given initial Bcl-2 level and also of the time of infection.
This strong difference in Bcl-2 kinetics in the different Bcl-2 clones was due to the control of Bcl-2 over virus replication and reciprocally of the virus over Bcl-2 level. We showed that Bcl-2 negatively regulates HIV-1 replication (Fig. 3), implying that the cells expressing a high level of Bcl-2 are characterized by a low virus production which, in turn, does not much modify Bcl-2 level. Other reports mentioned that overexpression of Bcl-2 in cells infected with viruses such as alphavirus (28) or Semliki Forest virus (41) leads to a decrease in the replication levels of such viruses and to a prolonged survival of the infected cells.
We then analyzed the mechanisms involved in the regulation of Bcl-2 expression in our cell system. The bcl-2 gene has two distinct promoters and displays a complex structure and strategy for expression (44). Considerable heterogeneity in the expression of bcl-2 transcripts was observed in different cell lines. In J.Jhan cells, we showed two major transcripts of 5.5 and 3.5 kb, which are often described and have been shown to contain the open reading frame coding for the Bcl-2 protein (239 amino acids). The 3.5-kb transcript was detectable only in infected cells. Unexpectedly, in contrast with the decrease observed at the protein level, an increased synthesis of Bcl-2 was shown at the transcriptional level as demonstrated by the accumulation of the 3.5-kb transcript in infected cells (Fig. 4). This increase in the steady-state level of the 3.5-kb mRNA correlated with an increase in the initiation rate of transcription from the P2 promoter (Fig. 5). Furthermore, the progressive increase of bcl-2 gene transcription correlated with a progressive decrease of viral replication (RT), confirming again the negative control of Bcl-2 over virus replication. An equilibrium is thus created between HIV replication and Bcl-2 level, which permits the establishment of a chronic infection with no damage to the cells. We propose that this mechanism of induction of bcl-2 transcription compensates for the initial decrease induced concomitantly by infection and permits the establishment of virus persistence.
We showed that this phenomenon of dysregulation of Bcl-2 at the protein level is in keeping with the general pattern of dysregulation of the main cellular antioxidant molecules glutathione and thioredoxin (Fig. 6), suggesting a role for infection-induced oxidative stress in apoptosis. The transient disruption of the glutathione and thioredoxin levels might also contribute to the observed loss of viability (although coincidence of their kinetics with cell viability was less obvious than that of Bcl-2). The fact that NAC counteracts these effects of HIV infection provides further evidence for the role of an infection-induced oxidative stress in the dysregulation of Bcl-2 and in the subsequent induction of apoptosis (Fig. 7).
Work is in progress in our laboratory to study more extensively the relation between this oxidative stress and the degradation of Bcl-2 and thioredoxin. The cellular proteases that can induce degradation of Bcl-2 and thioredoxin will be investigated. Bcl-2 has been suggested to be cleaved by HIV protease, thus explaining the death of HIV-infected lymphocytes (46). However, no evidence of discrete cleavage products could be observed in infected T lymphocytes in this report, nor in the cell lines we used in our experiments (data not shown). Other proteases suspected of cleaving Bcl-2, such as caspases, were shown to be induced by infection with Sindbis virus (33). The potential role of these proteases in HIV infection remains to be tested. The absence of intermediate degradation products might also suggest a role for the proteasome, which has been involved in various apoptosis situations (17, 40).
Bcl-2 is a member of a family of proteins (34) that includes Bax, a conserved homolog that heterodimerizes in vivo with Bcl-2 and promotes cell death. The proportion of family members with antiapoptotic properties such as Bcl-2 and Bax determines the survival or death of cells following an apoptotic stimulus. It is not excluded that the levels of other antiapoptotic proteins are similarly influenced by the redox status of the cells over the course of infection (25). In this case, the pattern of Bcl-2 expression might reflect that of the other members of the family.
Altogether, our data suggest that HIV infection induces oxidative stress which initiates apoptosis by degradation of all the antioxidant systems including Bcl-2, except when this degradation is compensated for by an induction of Bcl-2 synthesis. As Bcl-2 upregulation proceeds, a negative control of virus replication occurs, limiting the oxidative stress and Bcl-2 degradation. We propose that cells are predisposed to undergo a persistent or an acute HIV-1 infection, according to their basal level of Bcl-2 and their ability to sustain bcl-2 gene transcription. This last point relates to the existence of (redox-dependent?) transcription factors induced by infection itself. To support these conclusions, work is in progress in our laboratory to investigate whether macrophages, which were shown to be one of the major reservoirs of the virus, fulfill the requirements for sustaining a chronic infection as defined in cell lines. This work on macrophages will also address the question of whether macrophage-tropic isolates of HIV-1 induce the same type of kinetics of antioxidant molecules including Bcl-2 as does the T-lymphocyte-tropic strain (HIV-1LAI) that we used in our experiments.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale pour la Recherche sur le SIDA. H. Masutani was supported by a fellowship of the CNRS and was a recipient of a travel award from the Naito Medical Research Foundation.
We thank J. Yodoi for providing the antibody against thioredoxin.
REFERENCES
- 1.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 3.Boudet F, Lecoeur H, Gougeon M L. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol. 1996;156:2282–2293. [PubMed] [Google Scholar]
- 4.Buhl R, Jaffe H A, Holroyd K J, Wells F B, Mastrangeli A, Saltini C, Cantin A M, Crystal R G. Glutathione deficiency and HIV. Lancet. 1990;335:546. doi: 10.1016/0140-6736(90)90783-2. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 5.Cameron P, Pope M, Granelli P A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukoc Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Eck H P, Gmunder H, Hartmann M, Petzoldt D, Daniel V, Droge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe-Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 8.Elbim C, Prevot M H, Bouscarat F, Franzini E, Chollet M S, Hakim J, Gougerot-Pocidalo M-A. Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming. Blood. 1994;84:2759–2766. [PubMed] [Google Scholar]
- 9.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner R K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 10.Emilie D, Fior R, Jarrousse B, Marfaing K A, Merrien D, Devergne O, Crevon M C, Maillot M C, Galanaud P. Cytokines in HIV infection. Int J Immunopharmacol. 1994;16:391–396. doi: 10.1016/0192-0561(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 11.Finkel T H, Tudor W G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 12.Flores S C, Marecki J C, Harper K P, Bose S K, Nelson S K, McCord J M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc Natl Acad Sci USA. 1993;90:7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gougeon M L, Lecoeur H, Heeney J, Boudet F. Comparative analysis of apoptosis in HIV-infected humans and chimpanzees—relation with lymphocyte activation. Immunol Lett. 1996;51:75–81. doi: 10.1016/0165-2478(96)02558-8. [DOI] [PubMed] [Google Scholar]
- 14.Gougerot-Pocidalo M-A, Fay M, Roche Y, Chollet M S. Mechanisms by which oxidative injury inhibits the proliferative response of human lymphocytes to PHA. Effect of the thiol compound 2-mercaptoethanol. Immunology. 1988;64:281–288. [PMC free article] [PubMed] [Google Scholar]
- 15.Gougerot-Pocidalo M-A, Fay M, Roche Y, Lacombe P, Marquetty C. Immune oxidative injury induced in mice exposed to normobaric O2: effects of thiol compounds on the splenic cell sulfhydryl content and Con A proliferative response. J Immunol. 1985;135:2045–2051. [PubMed] [Google Scholar]
- 16.Grever M R, Thompson V N, Balcerzak S P, Sagone A J. The effect of oxidant stress on human lymphocyte cytotoxicity. Blood. 1980;56:284–288. [PubMed] [Google Scholar]
- 17.Grimm L M, Goldberg A L, Poirier G G, Schwartz L M, Osborne B A. Proteasomes play an essential role in thymocyte apoptosis. EMBO J. 1996;15:3835–3844. [PMC free article] [PubMed] [Google Scholar]
- 18.Hinshaw V S, Olsen C W, Dybdahl S N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hockenbery D, Nunez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 20.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 21.Idziorek T, Estaquier J, De B F, Ameisen J C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 22.Israël N, Gougerot-Pocidalo M-A, Aillet F, Virelizier J L. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992;149:3386–3393. [PubMed] [Google Scholar]
- 23.Kitaoka Y, Sorachi K, Nakamura H, Masutani H, Mitsui A, Kobayashi F, Mori T, Yodoi J. Detection of adult T-cell leukemia-derived factor/human thioredoxin in human serum. Immunol Lett. 1994;41:155–161. doi: 10.1016/0165-2478(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 24.Kluck R M, Bossy W E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 25.Korsmeyer S J, Yin X M, Oltvai Z N, Veis N D, Linette G P. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- 26.Kraut E H, Segal M, Sagone A J. Evaluation of the role of oxygen radicals in polymorphonuclear leukocyte aggregation. Inflammation. 1982;6:161–167. doi: 10.1007/BF00916240. [DOI] [PubMed] [Google Scholar]
- 27.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 28.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the Bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 31.Masutani H, Naito M, Takahashi K, Hattori T, Koito A, Takatsuki K, Go T, Nakamura H, Fujii S, Yoshida Y, Okuma M, Yodoi J. Dysregulation of adult T-cell leukemia-derived factor (ADF)/thioredoxin in HIV infection: loss of ADF high-producer cells in lymphoid tissues of AIDS patients. AIDS Res Hum Retroviruses. 1992;8:1707–1715. doi: 10.1089/aid.1992.8.1707. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita T, Harigai M, Hanada M, Reed J C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 33.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunez G, Clarke M F. The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol. 1994;4:399–403. doi: 10.1016/0962-8924(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 35.Nunez G, Merino R, Grillot D, Gonzalez G M. Bcl-2 and Bcl-x: regulatory switches for lymphoid death and survival. Immunol Today. 1994;15:582–588. doi: 10.1016/0167-5699(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 36.Olivier R, Lopez O, Mollerau M, Dragic T, Guetard D, Montagnier L. Prevention of early cell death in peripheral blood lymphocytes of HIV infected individuals by an antioxidant, N-acetyl-cysteine. In: Pasquier C, Olivier R, editors. Oxidative stress, cell activation and viral infection. Vol. 1. Basel, Switzerland: Birkhauser; 1994. pp. 323–332. [Google Scholar]
- 37.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 38.Park I W, Kondo E, Bergeron L, Park J, Sodroski J. Effects of human immunodeficiency virus type 1 infection on programmed cell death in the presence or absence of bcl-2. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;12:321–328. doi: 10.1097/00042560-199608010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Robert-Hebmann V, Emiliani S, Jean F, Resnicoff M, Traincard F, Devaux C. Clonal analysis of murine B cell response to the human immunodeficiency virus type 1 (HIV1)-gag p17 and p25 antigens. Mol Immunol. 1992;29:729–738. doi: 10.1016/0161-5890(92)90183-x. [DOI] [PubMed] [Google Scholar]
- 40.Sadoul R, Fernandez P A, Quiquerez A L, Martinou I, Maki M, Schroter M, Becherer J D, Irmler M, Tschopp J, Martinou J C. Involvement of the proteasome in the programmed cell death of NGF-deprived sympathetic neurons. EMBO J. 1996;15:3845–3852. [PMC free article] [PubMed] [Google Scholar]
- 41.Scallan M F, Allsopp T E, Fazakerley J K. bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz O, Henin Y, Marechal V, Montagnier L. A rapid and simple colorimetric test for the study of anti-HIV agents. AIDS Res Hum Retroviruses. 1988;4:441–448. doi: 10.1089/aid.1988.4.441. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz O, Virelizier J L, Montagnier L, Hazan U. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene. 1990;88:197–205. doi: 10.1016/0378-1119(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 44.Seto M, Jaeger U, Hockett R D, Graninger W, Bennett S, Goldman P, Korsmeyer S J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staal F J, Roederer M, Israelski D M, Bubp J, Mole L A, McShane D, Deresinski S C, Ross W, Sussman H, Raju P A. Intracellular glutathione levels in T cell subsets decrease in HIV-infected individuals. AIDS Res Hum Retroviruses. 1992;8:305–311. doi: 10.1089/aid.1992.8.305. [DOI] [PubMed] [Google Scholar]
- 46.Strack P R, Frey M W, Rizzo C J, Cordova B, George H J, Meade R, Ho S P, Corman J, Tritch R, Korant B D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci USA. 1996;93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 49.Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, Bureau J-F, Montagnier L, Brahic M. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- 50.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 52.Zhong L T, Sarafian T, Kane D J, Charles A C, Mah S P, Edwards R H, Bredesen D E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci USA. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]