Abstract
The GPRC6A receptor is a recently “deorphanized” class C G protein–coupled receptor. We and others have shown that this receptor is coactivated by basic l-α-amino acids and divalent cations, whereas other groups have also suggested osteocalcin and testosterone to be agonists. Likewise, the GPRC6A receptor has been suggested to couple to multiple G protein classes albeit via indirect methods. Thus, the exact ligand preferences and signaling pathways are yet to be elucidated. In the present study, we generated a Chinese hamster ovary (CHO) cell line that stably expresses mouse GPRC6A. In an effort to establish fully the signaling properties of the receptor, we tested representatives of four previously reported GPRC6A agonist classes for activity in the Gq, Gs, Gi, and extracellular-signal regulated kinase signaling pathways. Our results confirm that GPRC6A is activated by basic l-α-amino acids and divalent cations, and for the first time, we conclusively show that these responses are mediated through the Gq pathway. We were not able to confirm previously published data demonstrating Gi- and Gs-mediated signaling; neither could we detect agonistic activity of testosterone and osteocalcin. Generation of the stable CHO cell line with robust receptor responsiveness and optimization of the highly sensitive homogeneous time resolved fluorescence technology allow fast assessment of Gq activation without previous manipulations like cotransfection of mutated G proteins. This cell-based assay system for GPRC6A is thus useful in high-throughput screening for novel pharmacological tool compounds, which are necessary to unravel the physiologic function of the receptor.
Introduction
The G protein–coupled receptor family C, group 6, member A (GPRC6A) receptor is a recently identified class C G protein-coupled receptor (GPCR) (Wellendorph and Bräuner-Osborne, 2004). A promiscuous range of nonselective ligands and a broad but low-level expression profile have complicated elucidation of the physiologic function of this receptor. Based on studies in GPRC6A knockout mice, the receptor is now suggested to be involved in inflammatory, metabolic, and endocrine regulation; however, the specific role of GPRC6A is still unknown (Pi et al., 2008, 2011, 2012; Wellendorph et al., 2009; Rossol et al., 2012; Smajilovic et al., 2013; Clemmensen et al., 2013a,b).
In 2004–2005, we were the first to clone and “deorphanize” GPRC6A, which proved to be activated by l-α-amino acids and in particular basic amino acids (Wellendorph and Bräuner-Osborne, 2004; Wellendorph et al., 2005). Divalent cations such as calcium have also been shown to activate the receptor directly (Pi et al., 2005; Pi and Quarles, 2012) or to serve as coagonists, which positively modulate the l-α-amino acid response (Kuang et al., 2005; Christiansen et al., 2007; Wellendorph et al., 2007). Furthermore, others have demonstrated receptor activation on stimulation with testosterone and osteocalcin in the presence of extracellular calcium (Pi et al., 2010, 2011; Oury et al., 2011; Pi and Quarles, 2012). The closest related GPCR is the odorant goldfish 5.24 receptor, which signals through the Gq pathway (Speca et al., 1999; Christiansen et al., 2006). Likewise, we and the group of Hampson have used the oocyte expression system to show that mouse GPRC6A (mGPRC6A) activates a calcium-sensitive chloride channel on stimulation with l-α-amino acids and cations indicative of Gq coupling (Kuang et al., 2005; Wellendorph et al., 2005; Christiansen et al., 2007). Using mGPRC6A-transfected human embryonic kidney (HEK293) cells and pathway selective inhibitors, the Quarles group has shown that downstream serum-response element (SRE) and extracellular signal-regulated kinase (ERK) are activated by divalent cations (Gq and Gi pathways) (Pi et al., 2005, 2010; Pi and Quarles, 2012), l-arginine (pathway not investigated) (Pi et al., 2011), the steroid testosterone (Gi pathway, Gq not investigated (Pi et al., 2010), and the peptide osteocalcin (Gq pathway, Gi not investigated) (Pi et al., 2011). In addition, this group has shown that all four agonist classes lead to cAMP accumulation in the GPRC6A-HEK293 cell line and thus is likely also to be Gs coupled (Dreaden et al., 2012; Pi et al., 2012). Finally, the Karsenty group has shown that osteocalcin leads to a bell-shaped, concentration-dependent increase in cAMP, indicating Gs coupling, but no osteocalcin-mediated activation of the Gq or ERK pathways in TM3 Leydig cells. These osteocalcin responses, however, were not shown specifically to be mediated by GPCR6A (Oury et al., 2011). Thus, conflicting findings regarding GPRC6A signaling have been reported, and physiologic relevant ligands and signaling pathways have yet to be identified.
Although GPCRs are known to be activated by a broad range of ligands (Pierce et al., 2002), it is unprecedented that the same receptor subtype should have evolved to be endogenously activated by four highly different structural classes of ligands: amino acids, cations, a steroid, and a 49-amino-acid peptide (Fig. 1). From a phylogenetic point of view, class C GPCRs are perceived as nutrient receptors derived from amino acid and nutrient transporters from bacteria (Conklin and Bourne, 1994; Kuang et al., 2006). Thus, all other human class C GPCRs with a “Venus-flytrap domain” like GPRC6A sense amino acids, cations, or sugars, and no other subtype in class C has previously been suggested to be activated by steroids or peptides of the size of osteocalcin (Bräuner-Osborne et al., 2007). The reports of GPCR6A being activated by testosterone or osteocalcin have thus generated considerable curiosity.
Fig. 1.
Structures of previously reported GPRC6A ligands. (A) The amino acid l-ornithine; (B) the steroid testosterone; (C) the peptid osteocalcin (porcine) (adapted from Protein Data Bank code 1Q8H); (D) the GPRC6A-selective antagonist compound 1; and (E) the amino acid sequence of bovine osteocalcin (bOC) and the differences compared with porcine osteocalcin (pOC).
A major limitation when testing the GPRC6A receptor is the presence of l-α-amino acids and divalent cations in the cell culture media. These ligands might be responsible for activation and subsequent desensitization of the receptor; hence, it has been difficult to obtain robust responses when characterizing mGPRC6A recombinantly expressed in mammalian cell lines (Wellendorph et al., 2005). Accordingly, previous studies of mGPRC6A signaling have suffered from low-throughput techniques, manipulations such as cotransfection with chimeric or mutated G proteins biasing the signaling pathway, or measurement of downstream effects such as SRE or ERK activation, which is difficult to assign to specific signaling pathways.
To circumvent these limitations and for the first time enable direct measurement of GPRC6A signaling, we generated a Chinese hamster ovary (CHO) cell line that stably expresses mGPRC6A (mGPRC6A-CHO) using the Flp-In system (Invitrogen, Paisley, UK). To elucidate fully the signaling of GPRC6A, pharmacological characterization was carried out using this cell line by testing representatives of the four previously reported GPRC6A agonist classes using the highly sensitive high-throughput homogeneous time-resolved fluorescence (HTRF) technology for detection of the Gq, Gs , and Gi pathways and Western blotting for detection of ERK activation.
Materials and Methods
Ham’s F-12 GlutaMAX medium, DMEM GlutaMAX medium, dialyzed fetal bovine serum, penicillin (104 U/ml)-streptomycin (104 µg/ml) mixture, Hygromycin B (50 mg/ml), Zeocin (100 mg/ml), Dulbecco’s phosphate-buffered saline (DPBS), 0.05% trypsin-EDTA, Hanks’ balanced salt solution (HBSS), the Fluo-4 NW calcium assay kit, TAE buffer (10×), NuPAGE LDS sample buffer (4×), NuPAGE sample-reducing agent (10×), NuPAGE Novex 4%–12% Bis-Tris 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol) gels (1.5-mm-thick, 15-well), NuPAGE MOPS SDS running buffer (20×), NuPAGE antioxidant, XCell SureLock Mini-Cell, NuPAGE transfer buffer (20×), polyvinylidene difluoride/filter paper sandwiches and XCell II blot module, c-myc mouse monoclonal antibody, and goat anti-mouse IgG HRP conjugate were all purchased from Invitrogen. l-Ornithine (l-Orn), calcium chloride (CaCl2), magnesium chloride (MgCl2), lithium chloride (LiCl), ATP, paraformaldehyde, Trizma hydrochloride solution (pH 7.4 and 7.6), dimethyl sulfoxide (DMSO), probenecid, 3-isobutyl-1-methylxanthine (IBMX), forskolin, poly-d-lysine, HEPES, bovine serum albumin, cell dissociation solution nonenzymatic, Tween-20, sodium azide, skim milk powder, radioimmunoprecipitation assay buffer, Tris-buffered saline (TBS), U73122, and testosterone were all purchased from Sigma-Aldrich (St. Louis, MO). Testosterone was dissolved in DMSO to 100 mM. University of Bonn-Gq inhibitor compound (UBO-QIC) was purchased from Prof. Evi Kostenis (University of Bonn, Bonn, Germany) and dissolved to 10 mM in DMSO. SuperSignal enzyme-linked immunosorbent assay (ELISA) Femto stable peroxidase solution and SuperSignal ELISA Femto Luminol enhancer solution were purchased from Thermo Fischer Scientific (Slangerup, Denmark). Prestained protein molecular weight marker, donkey anti-rabbit IgG HRP-link whole antibody, mouse IgG HRP-link whole antibody, and Amersham ECL prime Western blotting detection reagents were purchased from VWR (Radnor, PA). Uncarboxylated mouse osteocalcin was purchased from Bachem (Bubendorf, Switzerland). Osteocalcin was dissolved in HBSS buffer containing 20 mM HEPES, 1 mM Ca2+, 1 mM Mg2+ (pH 7.4) to 100 µM. Myo-[2-3H]inositol and polylysine YSi SPA beads were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Green GoTaq Reaction buffer (5×); magnesium chloride solution (25 mM); GoTaq DNA polymerase (5 U/µl); and a set of dATP, dCTP, dGTP, dTTP (100 mM each) were all purchased from Promega (Madison, WI), and primers were purchased from TAG Copenhagen A/S (Copenhagen, Denmark). The IP-One kit and the cAMP dynamic 2 kit were purchased from Cisbio (Codolet, France). Complete protease inhibitor cocktail tablets were purchased from Roche (Basel, Switzerland), and phospho-p44/42 MAPK (ERK1/2) rabbit antibody and p44/42 MAPK (ERK1/2) mouse antibody were purchased from Cell Signaling Technology (Danvers, MA). Compound 1 (2-[N-methyl(2′-morpholino-2′-oxoethyl)amino]-1-(2′′-phenyl-1H-indol-3′′-yl)-ethanone) was synthesized by Henrik Johansson and Daniel S. Pedersen (University of Copenhagen, Denmark) as previously described (Gloriam et al., 2011).
Generation of CHO and HEK 293 Cell Lines Stably Expressing Mouse and Human GPRC6A.
Mouse GPRC6A was previously tagged in the N terminus with the signal peptide of mGluR5 to promote cell surface expression and c-myc to enable detection of cell surface expression by ELISA (Wellendorph et al., 2005). This construct was transferred from the previously published pEGFP-N1 vector (Wellendorph et al., 2005) to the pcDNA5/FRT/V5-His-Topo vector (Invitrogen) by PCR using the primers m6A_cloning_forward, 5′-ACCATGGTCCTTCTGTTGATC-3′ and m6A_cloning_reverse, 5′-TCATATACTTGAACTTCTTTTCTG-3′). Likewise, c-myc-tagged human GPRC6A (hGPRC6A) was transferred from the pEGFPN1 vector to the pcDNA5/FRT/V5-His-Topo vector by using the primers hC6A_cloning_forward, 5′-AGTGCCACCATGGTCCTTCTGT-3′ and hC6A_cloning_reverse, 5′-GCCGCCATCTCCTAAGGCTTATCAT-3′. The absence of mutations in all constructs was verified by DNA sequencing (Eurofins MWG Operon, Ebersberg, Germany). Flp-In-CHO and Flp-In-HEK293 cells were maintained in Ham’s F12 and DMEM media, respectively, supplemented with 10% (v/v) dialyzed fetal bovine serum, 2 mM l-glutamine, and 1% penicillin-streptomycin mixture in a humidified atmosphere (95% air and 5% CO2). To generate Flp-In-CHO or Flp-In-HEK293 cells stably expressing mGPRC6A (mGPRC6A-CHO or mGPRC6A-HEK293), cells were transfected with a 1:9 ratio mixture of pcDNA5/FRT/V5-His/Topo construct and pOG44 using Polyfect (Qiagen, West Sussex, UK) according to the manufacturer’s instructions. Twenty-four hours after the transfection, fresh medium was applied, and 48 hours after the transfection, the medium was changed again to fresh medium containing 600 or 200 µg/ml Hygromycin B to initiate the selection of stably expressing mGPRC6A-CHO or mGPRC6A-HEK293 cells, respectively. Likewise, Flp-In-CHO and Flp-In-HEK293 cells stably expressing hGPRC6A were generated.
Reverse-Transcription Polymerase Chain Reaction.
RNA was extracted from mGPRC6A-CHO and Flp-In-CHO cells using the RNeasy kit (Qiagen, Hilden, Germany), and subsequently 1 µg of RNA was transcribed into cDNA using the QuantiTect reverse transcription (RT) kit (Qiagen, Hilden, Germany). mGPRC6A cDNA was amplified by using intron-spanning primer sequences previously reported (Wellendorph et al., 2009): m6A_RT-polymerase chain reaction (PCR)_forward 5′-GCCCTGGTCAAATGAAGAAA-3′ and m6A_ RT-PCR_reverse 5′-TGATGTAGCCCAGCATGGTA-3′. Amplification of the ubiquitously expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as control, in which the following primers were used: GAPDH_forward 5′-TGAAGGTCGGTGTGAACGGATTTGG-3′ and GAPDH_reverse 5-CATGTAGGCCATGAGGTCCACCAC-3′. GoTaq DNA polymerase (5 U/µl) was used for the PCR reaction, which was run as follows: 95°C for 5 minutes, 95°C for 30 seconds, 55°C (for mGPRC6A primers), and 64°C (for GAPDH primers) for 30 seconds for annealing, 72°C for 2 minutes, and 72°C for 10 minutes for 30 cycles. Amplified PCR products were verified on a 1% agarose gel containing ethidium bromide, and cDNA bands were detected using the PhotoDoc-It imaging system (UVP, Upland, CA).
Transient Expression in tsA201 Cells.
The tsA201 cells were transfected using Polyfect according to the manufacturer’s protocol (Qiagen). Cells were cotransfected either with constructs encoding mGPRC6A and empty vector (transfection ratio 1:1) or constructs encoding mGPRC6A and Gq(G66D) (transfection ratio 1:1) as previously described (Christiansen et al., 2007). tsA201 cells transfected with empty vector were used as negative control.
ELISA.
One day before the assay, 105 cells/well were cultured in a poly-d-lysine–treated white with clear bottom CulturPlate-96 (PerkinElmer Life and Analytical Sciences) at 37°C and 5% CO2 for 24 hours. On the day of the assay, cells were fixed to the plate using 50 µl/well fixing solution (DPBS + 4% paraformaldehyde) for 5 minutes. The 96-well plate was washed twice with DPBS containing 1 mM Ca2+ (DPBS-Ca), followed by the addition of 100 µl/well blocking solution (0.3 g dry milk, 10 µl of 1 M CaCl2, 250 µl of Trizma hydrochloride solution pH 7.4, 250 µl of Trizma hydrochloride solution pH 7.6, add up to total volume of 10 ml with dH20). The plate was incubated at room temperature for at least 30 minutes. After blocking, the plate was incubated with 75 µl/well primary antibody (c-myc mouse monoclonal antibody diluted 1:1000 in blocking solution) at room temperature for 45 minutes. Subsequently, the plate was washed with 100 µl/well blocking solution and twice with 100 µl/well DPBS-Ca; 75 µl/well secondary antibody (goat anti-mouse IgG HRP conjugate diluted 1:1500 in blocking solution) was added, and the plate was incubated at room temperature for 45 minutes. The plate was then washed four times with 100 µl/well blocking solution and four times with 100 µl/well DPBS-Ca; 60 µl/well DPBS-Ca was added, and the detection solution was prepared (SuperSignal ELISA Femto stable peroxidase solution and SuperSignal ELISA Femto luminol enhancer solution (Thermo Fisher Scientific, Waltham, MA), 1:1); 20 µl/well detection solution was added to the plate, and chemiluminescence was measured immediately on an EnSpire reader (PerkinElmer Life and Analytical Sciences).
IP-One Assay.
Ligands were prepared in 2× final concentration in ligand buffer (HBSS buffer, 40 mM LiCl, 1 mM Ca2+, 1 mM Mg2+), and 5 µl/well ligand solution in triplicates was added to a 384-well OptiPlate (PerkinElmer). Subconfluent cells were detached from the cell culture dish by using cell dissociation solution at 37°C. The cells were centrifuged at 1100 rpm for 5–7 minutes, after which the cell pellet was resuspended in the appropriate volume of 37°C assay buffer (HBSS buffer, 20 mM HEPES, 1 mM Ca2+, 1 mM Mg2+ pH 7.4) to achieve a concentration of 107 cells/ml (for the stably expressing mGPRC6A-CHO cells and the control Flp-In-CHO cells) and 6 × 106 cells/ml (for the transiently expressing tsA201 cells). Cell suspension of 5 µl/well then was added to the plate, which was then sealed and incubated at 37°C for 1 hour, followed by 15 minutes’ incubation at room temperature. The detection solution was prepared as follows: IP-One conjugate and lysis buffer + 2.5% anti-IP1 cryptate Tb conjugate + 2.5% d-myo-inositol monophosphate (IP1)-d2 conjugate; 10 µl/well detection solution was added to the plate, which was then incubated away from light for 1 hour at room temperature. The plate was read on EnVision multilabel reader (PerkinElmer Life and Analytical Sciences); excitation at 340 nm and measurements of emission at 615 nm and 665 nm. The fluorescence resonance energy transfer ratios (665 nm/615 nm) were converted to IP1 concentrations by interpolating values from an IP1 standard curve generated from an IP1 calibrator, provided by the manufacturer (Cisbio).
To test for Gq pathway signaling, cells were pretreated for 1 hour before the assay in normal media containing 1 µM UBO-QIC. Ligands to be tested were prepared in ligand buffer containing the same amount of UBO-QIC. For inhibition using U73122, the cell suspensions were made in assay buffer supplemented with 20 µM U73122 and incubated for 10 minutes before addition of the cells.
IP Turnover Assay.
The assay was carried out as previously described (Christiansen et al., 2007). Transiently expressing tsA201 cells were stimulated with an EC25 concentration of l-Orn (25 µM) for 30 minutes at 37°C in the absence or presence of 0.1 and 1 µM concentrations of osteocalcin and in the presence of 1 mM Ca2+ and Mg2+. A concentration of 1 mM, l-Orn was used to assess the maximum response. Three different forms of osteocalcin were tested: osteocalcin form 1, human mature carboxylated osteocalcin (Sigma O5761); and form 2, mouse mature uncarboxylated osteocalcin (Bachem H-6552); form 3, mouse mature carboxylated osteocalcin (Bachem 4063515). Stock solutions were 1 mM prepared in either DPBS (forms 1 and 2) or 0.1% trifluoracetic acid in H2O (form 3).
Fluo-4 Calcium Assay.
One day before the assay, 105 cells/well were cultured in a 96-well black with clear-bottom plate at 37°C and 5% CO2. On the day of the assay, the plate was washed with 100 µl/well DPBS, followed by the addition of 50 µl/well dye-loading solution. The plate was incubated for 1 hour at 37°C and 5% CO2. The dye loading solution had been prepared by adding 10 ml of probenecid-buffer (HBSS buffer, 20 mM HEPES, 1 mM Ca2+, 1 mM Mg2+, pH 7.4, supplemented with 2.5 mM probenecid) to component A in the Fluo-4 NW calcium assay kit (Invitrogen). Ligands were prepared in 4× concentrations and distributed in a clear 96-well ligand plate. After incubation with the dye loading solution, the plate was washed once with 100 µl/well probenecid-buffer, followed by addition of 100 µl/well probenecid buffer. The plate was read using a FlexStation Benchtop multi-mode microplate reader (Molecular Devices, Sunnyvale, CA) with an excitation filter of 485 nm and emission at 525 nm. The ligand (33 µl/well) was added to the plate.
cAMP Dynamic 2 Assay.
Ligands were prepared in 2× final concentration in ligand buffer (HBSS buffer, 20 mM HEPES, 100 µM IBMX, 1 mM Ca2+, 1 mM Mg2+, pH 7.4); 40 µM forskolin was added to the ligand buffer when measuring Gi signaling, and 5 µl/well ligand solution in triplicate was added to a small-volume 384-well white Greiner plate (Bio-One, Frickenhausen, Germany). Cell suspensions were prepared as described for the IP-One assay to achieve a concentration of 2 × 106 or 1.2 × 106 cells/ml for measurements of Gs and Gi signaling, respectively. Cell suspension (5 µl/well) then was added to the plate, which was then covered with a lid and incubated at room temperature on a plate shaker at 450 rpm for 30 minutes. Two detection solutions were prepared as follows; cAMP conjugate and lysis buffer + 5% anti-cAMP cryptate Tb conjugate and cAMP conjugate and lysis buffer + 5% cAMP d2 conjugate; 5 µl/well of each conjugate solution was added to the plate, which was then incubated away from light for 1 hour at room temperature. The plate was read on EnVision multilabel reader (PerkinElmer Life and Analytical Sciences); excitation was at 340 nm and measurements of emission at 615 and 665 nm. The fluorescence resonance energy transfer ratio (665 nm/615 nm) was converted to cAMP concentrations by interpolating values from a cAMP standard curve generated from a cAMP calibrator provided by the manufacturer (Cisbio).
ERK Assay.
Forty-eight hours before the assay, 3 × 105 cells/well (for mGPRC6A-CHO) and 4.5 × 105 cells/well (for Flp-In-CHO) were cultured in a six-well clear culture plate. On the day of the assay, cells were washed with 2 ml/well washing buffer (HBSS, 20 mM HEPES, 1 mg/ml bovine serum albumin, 1 mM Ca2+, 1 mM Mg2+, pH 7.4, although without Ca2+ and Mg2+ when testing Ca2+ as a ligand) for 4 hours at 37°C. Ligands were prepared in final concentrations in ligand buffer (HBSS, 20 mM HEPES, 1 mM Ca2+, 1 mM Mg2+, pH 7.4, although without Ca2+ and Mg2+ when testing Ca2+ as a ligand), and 900 µl/well was added. The plates were incubated for 20 minutes at 37°C, as pilot studies had shown maximum ERK activation 20 minutes after the addition of l-Orn or Ca2+. Subsequently, the cells were washed twice with ice-cold DPBS; 70 µl/well ice-cold lysis buffer (7 ml of RIPA buffer + 1 protease inhibitor cocktail tablet) was added, and the plates were incubated on ice for 10–15 minutes. The protein concentration in each sample was determined by using the Bio-Rad protein determination kit (Bio-Rad Laboratories, Hercules, CA). SDS-PAGE was run by using the XCell SureLock Mini-Cell kit (Invitrogen), and samples were loaded on a NuPAGE 4–12% Bis-Tris gel. The gel was run at 175 V and 125 mA for 1 hour, and the proteins were subsequently transferred from the gel to a polyvinylidene difluoride membrane by using the XCell SureLock Mini-Cell kit and the XCell II Blot module. At first, the membranes were prepared by 2-minute incubation in 96% ethanol, and the transfer was then run at 30 V and 110–170 mM for 1 hour. Subsequently, the membranes were incubated in blocking solution [5% dry milk in TBST (TBS 1×) with 0.2% Tween-20] for 1 hour at room temperature on a tilting table, followed by overnight incubation at 4°C. Membranes were subjected to 1-hour incubation in primary antibody (rabbit anti-P-ERK1/2 antibody diluted 1:1000 in 1% dry milk in TBST with 0.02% sodium azide), followed by 1-hour incubation in secondary antibody (anti-rabbit antibody HRP conjugate diluted 1:5000 in 1% dry milk in TBST) at room temperature on a tilting table. The Amersham ECL Prime Western blotting-detection reagents were mixed 1:1, and 1 ml of the detection mix was then added to the protein side of the membranes. Imaging of the chemiluminescence was carried out by using the FluorChem HD2 system from Alpha Innotech (San Leandro, CA). Then the membranes were stripped of the first antibodies by 0.2 M NaOH, followed by incubation with total ERK (T-ERK) antibodies (mouse anti-T-ERK1/2 antibody and anti-mouse antibody HRP conjugate) and imaging as described already. Relative quantification of the Western blots was carried out by measuring the intensity of the bands using the publicly available ImageJ Software program (http://rsbweb.nih.gov/ij/download.html) and by normalizing the P-ERK response to the corresponding T-ERK response.
Data Analysis.
All data analysis was carried out using Prism GraphPad version 5.0a for Mac OS X (GraphPad Software, San Diego, CA). Concentration-response curves were fitted by nonlinear regression using eq. 1 for sigmoidal concentration-response function with variable slope:
| (1) |
in which X is the logarithm of the concentration, R is the response, Rmax is the maximal response, Rmin is the minimal response, EC50 is the concentration giving half-maximum response, and nH is the Hill coefficient, which describes the steepness of the curve. Statistical analysis [unpaired Student’s t test or one-way analysis of variance (ANOVA) followed by Dunnett’s test] was performed where appropriate and as indicated in the figure captions. Statistical significance has been determined at the following levels: *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Expression of Mouse GPRC6A in the CHO Cell Line.
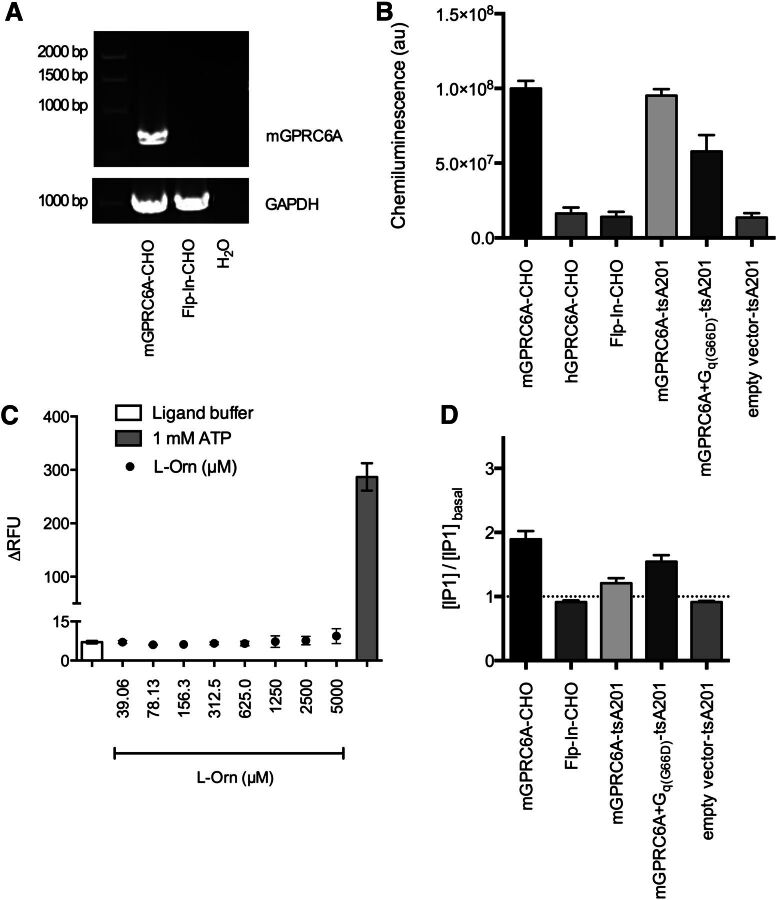
A stable mGPRC6A-CHO cell line was generated, and mRNA expression of the receptor was verified by RT-PCR (Fig. 2A). Furthermore, the cell surface expression of the receptor was determined by ELISA, as surface expression of GPCRs is a prerequisite for functional activity. Before insertion into the Flp-In-CHO genome, the receptor was tagged with a c-myc epitope at the N terminus, thereby allowing receptor expression to be detected using anti-c-myc antibody. The surface expression of the receptor in mGPRC6A-CHO was about 7-fold higher than the nonspecific background level detected in Flp-In-CHO (Fig. 2B), confirming that the receptor is present in the cell membrane when stably expressed in the CHO cell line.
Fig. 2.
Evaluation of cell lines stably and transiently expressing GPRC6A. (A) RT-PCR performed on total RNA extracted from the mGPRC6A-CHO and Flp-In-CHO cell lines. Water was used as a negative control. Bands were detected by using intron-spanning primers specific for (top) mGPRC6A and (bottom) the ubiquitously, endogenously expressed GAPDH. Only the shown bands were detected. (B) ELISA to detect cell-surface expression of c-myc tagged GPRC6A in the mGPRC6A-CHO, hGPRC6A-CHO, and Flp-In-CHO cell lines, and in tsA201 cells transiently expressing mGPRC6A alone, mGPRC6A and Gq(G66D), or empty vector alone. Surface-expressed receptors were detected using c-myc mouse monoclonal antibody and goat anti-mouse IgG HRP conjugate. Luminescence was measured after addition of a chemiluminescent HRP substrate. Data are shown as means ± S.E.M. of at least three independent experiments performed in triplicate. (C) Measurements of intracellular Ca2+ release. Responses to l-Orn and ATP were measured in mGPRC6A-CHO using the Fluo-4 NW calcium assay kit. Data are ΔRFU (peak fluorescence units after agonist addition subtracted fluorescence before agonist addition) and are shown as means ± S.E.M. of two independent experiments performed in triplicate. (D) Measurements of IP1 accumulation in response to 1 mM l-Orn as assessed by the HTRF IP-One assay. Responses were measured in the mGPRC6A-CHO, hGPRC6A-CHO, and Flp-In-CHO cell lines and in tsA201 cells transiently expressing mGPRC6A alone, mGPRC6A and Gq(G66D), or empty vector alone. Data are means ± S.E.M. of at least three independent experiments performed in triplicate.
In parallel, a CHO cell line stably expressing c-myc-tagged human GPRC6A (hGPRC6A) was generated. mRNA expression was verified by RT-PCR (data not shown); however, no surface expression of the receptor was detected when using anti-c-myc antibody in ELISA (Fig. 2B). Accordingly, l-Orn was inactive in the IP-One and P-ERK assays in this cell line (data not shown). This finding is consistent with previously published data (Wellendorph and Bräuner-Osborne, 2004; Wellendorph et al., 2005), in which no surface expression or functional responses of hGPRC6A was obtained in any of the applied expression systems.
Activation of the Gq Signaling Pathway.
The Gq protein directly activates the phosphatidylinositol-specific phospholipase C (PI-PLC), which is responsible for hydrolysis of phosphatidylinositol 4,5-bisphosphate into the second-messenger molecules diacylglycerol and inositol 1,4,5-trisphosphate (IP3). IP3 is degraded into d-myo-inositol bisphosphate and d-myo-inositol monophosphate (IP1) (Luttrell, 2008; Trinquet et al., 2011). Further degradation of IP1 is inhibited by lithium chloride (Parthasarathy et al., 1994), which can be added to the ligand buffer, and thus allows assessment of Gq activity by measuring IP1 accumulation (Trinquet et al., 2006).
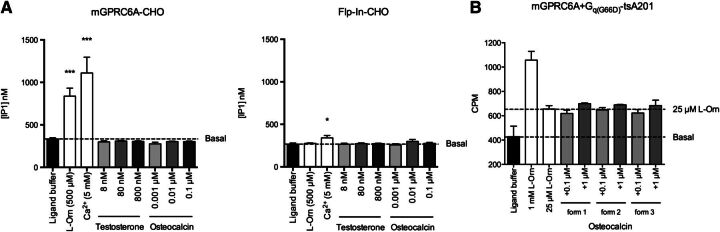
Four ligands (l-Orn, Ca2+, testosterone, and osteocalcin) were tested for their ability to activate the Gq pathway in the mGPRC6A-CHO and Flp-In-CHO cell lines using the HTRF IP-One assay. l-Orn was tested at 500 µM because pilot studies showed a maximum IP-One response in mGPRC6A-CHO at this concentration, and Ca2+ was tested in 5 mM concentrations because of assay interference greater than this concentration (data not shown). Testosterone and osteocalcin were previously reported to induce maximum response in the ERK pathway at 80 nM and 60 ng/ml, respectively (Pi and Quarles, 2012); thus, the ligands were tested in corresponding concentrations in the IP-One assay. In addition, they were tested in both 10-fold higher and 10-fold lower concentrations.
A significant increase in IP1 concentration was seen in mGPRC6A-CHO when testing both l-Orn and Ca2+, and Ca2+ also triggered a minor, but statistically significant, response in Flp-In-CHO cells. Neither testosterone nor osteocalcin induced IP1 accumulation in any of the concentrations tested (Fig. 3A). Given that two other groups previously reported osteocalcin to be a GPRC6A agonist, we also tested this ligand in our previously published assay in which mGPRC6A is cotransfected with the mutated Gq(G66D) protein and receptor activation is measured as generation of IP1–3 by using scintillation proximity assay beads (Christiansen et al., 2007). In addition, osteocalcin was tested in the presence of ∼EC20 of l-Orn, which would allow detection of both agonism and positive allosteric modulation. However, osteocalcin was also inactive in these settings (Fig. 3B).
Fig. 3.
Activity testing in the Gq signaling pathway. (A) Measurements of IP1 accumulation as a result of Gq activation. Responses to the ligands l-Orn, Ca2+, testosterone, and osteocalcin were measured in mGPRC6A-CHO and Flp-In-CHO cells, respectively, by means of the HTRF IP-One assay. Data are means ± S.E.M. of four independent experiments performed in triplicate. Significant differences from basal (ligand buffer) were calculated by performing a one-way ANOVA followed by Dunnett’s post-test (*P < 0.05, ***P < 0.001). (B) Activity testing of three different commercially available recombinant forms of osteocalcin using the IP turnover assay. Osteocalcin were tested in the presence of EC25 of l-Orn in tsA201 cells cotransfected with mGPRC6A and Gq(G66D). Results are shown as CPM and are means ± S.D. of a single representative experiment performed in duplicate. An additional experiment gave similar results. Significant differences from basal (ligand buffer) were calculated by performing a one-way ANOVA followed by Dunnett’s post-test (not significant, P > 0.05).
The mGPRC6A-CHO cell line was shown to retain a stable, functional l-Orn response in the IP-One assay for at least 30 passages. In parallel, we also generated a stably expressing mGPRC6A-HEK293 cell line using the Flp-In system. However, this cell line was proven to lose the l-Orn-mediated IP-One response after only a few passages and thus was not characterized further (data not shown).
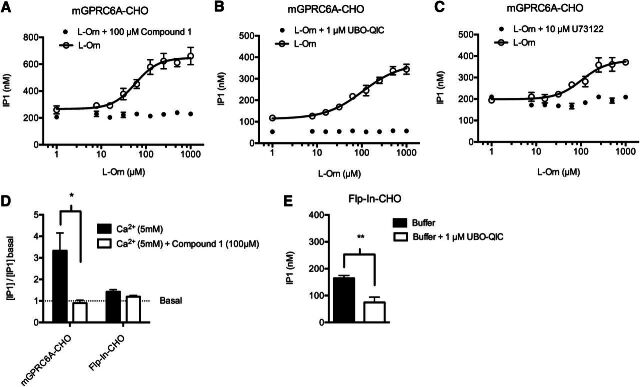
Concentration-response curves of l-Orn established a potency in mGPRC6A-CHO (Fig. 4A) of EC50 = 41.0 µM (pEC50 ± S.E.M. = 4.40 ± 0.02, n = 32). This potency is comparable to a previously published EC50 value of l-Orn at mGPRC6A transiently coexpressed with Gq(G66D) in tsA201 cells; EC50 = 63.6 µM (Christiansen et al., 2007). The lack of l-Orn response in the host cell line Flp-In-CHO strongly indicates that the response in mGPRC6A-CHO is in fact mediated by the GPRC6A receptor. This was further confirmed by the complete inhibition of l-Orn–induced IP1 accumulation by a GPRC6A-selective antagonist, compound 1 (Gloriam et al., 2011) (Fig. 4A). This antagonist has proven to be selective toward GPRC6A when tested against a panel of receptors, including the homologous calcium-sensing receptor (Gloriam et al., 2011).
Fig. 4.
Validation of mGPRC6A pharmacology as mediated by the Gq signaling pathway. (A, B, and C), concentration-response curve of l-Orn using mGPRC6A-CHO cells in the presence and absence of (A) the GPRC6A-selective antagonist compound 1, (B) the specific Gq inhibitor UBO-QIC, or (C) the specific PI-PLC inhibitor U73122. Responses are shown as IP1 accumulation measured by the HTRF IP-One assay. Data are means ± S.D. of a single representative experiment performed in triplicate. Two additional experiments gave similar results. (D) Ca2+-induced IP1 production in mGPRC6A-CHO and Flp-In-CHO cells in the presence and absence of compound 1. Data are normalized to the basal level of IP1 in ligand buffer and are shown as means ± S.E.M. of three independent experiments performed in triplicate. Statistical comparison was performed within each group (each cell line) by using an unpaired student’s t test (* P < 0.05). (E) The basal level of IP1 in ligand buffer in the Flp-In-CHO cell line in the presence and absence of 1 µM UBO-QIC. Data are means ± S.E.M. of three independent experiments performed in triplicate. Statistical comparison was performed by using an unpaired student’s t test (**P < 0.01).
Ca2+-mediated IP1 accumulation was also measured with and without the antagonist present (Fig. 4D), which showed that compound 1 completely inhibited the Ca2+-induced IP1 production in mGPRC6A-CHO, whereas the minor Ca2+ response in Flp-In-CHO was not significantly changed when compound 1 was added, thereby demonstrating that the Ca2+ response in mGPRC6A-CHO is mediated by GPRC6A.
Additionally, we used the specific Gq inhibitor UBO-QIC (Fujioka et al., 1988) to demonstrate that the observed IP1 response of l-Orn was indeed mediated by Gq activation (Fig. 4B). However, we noted that the basal level of IP1 was decreased on incubation with UBO-QIC. A similar decrease in basal IP1 was also observed in the Flp-In-CHO cell line on stimulation with UBO-QIC (Fig. 4E), thus indicating constitutive activity of the Gq pathway in CHO cells. The Ca2+-mediated IP1 production in mGPRC6A-CHO cells was likewise inhibited by UBO-QIC (data not shown). To demonstrate further the involvement of the Gq signaling pathway, we used the specific PI-PLC inhibitor U73122 (1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione) (Smallridge et al., 1992), which indeed inhibited the l-Orn-induced IP1 response (Fig. 4C).
The stably expressing mGPRC6A-CHO cell line was tested in the Fluo-4 NW calcium assay, which measures release of intracellular calcium in response to ligand stimulation. Concentrations of l-Orn ranging from 30 µM to 5 mM were tested for their ability to increase the level of intracellular calcium; however, no responses were seen at any of the concentrations tested; 1 mM ATP was included as a positive control (Fig. 2C). These data indicate that the Fluo-4 calcium assay is not a sufficiently sensitive method for detection of mGPRC6A signaling in the mGPRC6A-CHO cell line. Thus, the IP-One assay seems to be more sensitive than previously used methods, as it enables measurements of robust responses by direct Gq coupling.
To elucidate further the sensitivity of the IP-One assay, we tested our previously published transient expression system, in which tsA201 cells were cotransfected with mGPRC6A and a mutated Gq(G66D) protein. First, the receptor surface expression was determined in the different cellular expression systems by ELISA. Comparable levels of mGPRC6A surface expression were seen between the stable CHO expression system and tsA201 cells transiently expressing mGPRC6A alone. However, when coexpressing Gq(G66D), the surface expression of mGPRC6A was decreased by approximately 40% (Fig. 2B). In parallel, the functional response of mGPRC6A was tested in the stable and transient expression systems using the IP-One assay; 1 mM l-Orn induced a 1.9-fold increase in IP1 levels in the stably expressing mGPRC6A-CHO cell line. Despite a comparable level of surface expression in mGPRC6A-tsA201, 1 mM l-Orn induced only a minor increase in IP1. However, with Gq(G66D) coexpressed, it was possible to achieve a 1.5-fold increase in IP1 on stimulation with l-Orn (Fig. 2D). Thus, the signal-enhancing Gq(G66D) protein seems necessary to achieve a markedly functional response in the IP-One assay when using a transient expression system. The functional response is lower than in the stable mGPRC6A-CHO cell line, but the level of receptor surface expression is accordingly lower in the transiently expressing tsA201 cells. Collectively, the results indicate that the IP-One assay is not sufficiently sensitive to enable direct measurements of functional responses in the transiently expression system without having Gq(G66D) present.
Gs and Gi Signaling.
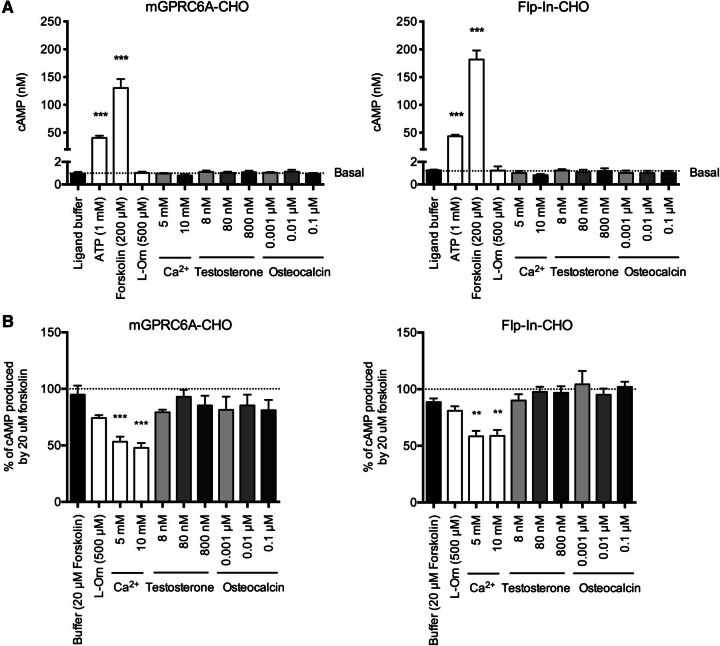
Both Gs and Gi proteins affect the enzymatic activity of adenylate cyclase, which is responsible for the conversion of ATP into the second-messenger cAMP. Gs proteins stimulate adenylate cyclase, whereas the Gi protein inhibits the enzyme, resulting in increased and decreased levels of cAMP, respectively (Kristiansen, 2004; Luttrell, 2008). In the HTRF cAMP dynamic 2 assay, activation of the Gs and Gi pathways is assessed by measuring the level of cAMP (Degorce et al., 2009). The four ligands (l-Orn, Ca2+, testosterone, and osteocalcin) were tested in the same concentration as in the IP-One assay, except Ca2+ was tested at both 5 mM and 10 mM concentrations.
Activation of the Gs signaling pathway was accounted for by measuring whether any of the applied ligands induced cAMP production. Forskolin and ATP were used as positive controls, as they triggered significant increases in cAMP in both mGPRC6A-CHO and Flp-In-CHO. None of the other ligands induced any cAMP production, indicating no Gs coupling of GPRC6A when stably expressed in CHO cells (Fig. 5A).
Fig. 5.
Measurements of (A) cAMP accumulation as a result of Gs activation and (B) cAMP inhibition as a result of Gi activation. Responses to ATP, forskolin, l-Orn, Ca2+, testosterone, and osteocalcin were measured in mGPRC6A-CHO and Flp-In-CHO cells, respectively, by using the HTRF cAMP assay. (A) Data are means ± S.E.M. of three independent experiments performed in triplicate. Significant differences from basal (ligand buffer) were determined by performing a one-way ANOVA followed by Dunnett’s post-test (***P < 0.001). (B) Data are normalized to the cAMP production in response to 20 µM forskolin and are shown as means ± S.E.M. of five independent experiments performed in triplicate. Significant differences from basal (20 µM forskolin) were determined by performing a one-way ANOVA followed by Dunnett’s post-test (**P < 0.01, ***P < 0.001).
When testing for Gi activity, adenylate cyclase is preactivated with forskolin to generate basal levels of cAMP, which allows inhibition to be measured on receptor activation (Degorce et al., 2009). Both 5 mM and 10 mM Ca2+ significantly inhibited the cAMP level in both cell lines (Fig. 5B), and other Ca2+ concentrations also triggered equal responses in both mGPRC6A-CHO and Flp-In-CHO (data not shown). Thus, the Ca2+-induced inhibition of cAMP is a nonspecific effect. None of the other ligands triggered decreased levels of cAMP, indicating that GPRC6A is not a Gi-coupled receptor in the CHO cell line.
As l-Orn induces a significant concentration-dependent response in the Gq pathway, different concentrations ranging from 100 µM to 10 mM were tested in the cAMP assay. However, none of the tested l-Orn concentrations induced any response in the Gs or in the Gi signaling pathway (data not shown).
Activation of the ERK Pathway.
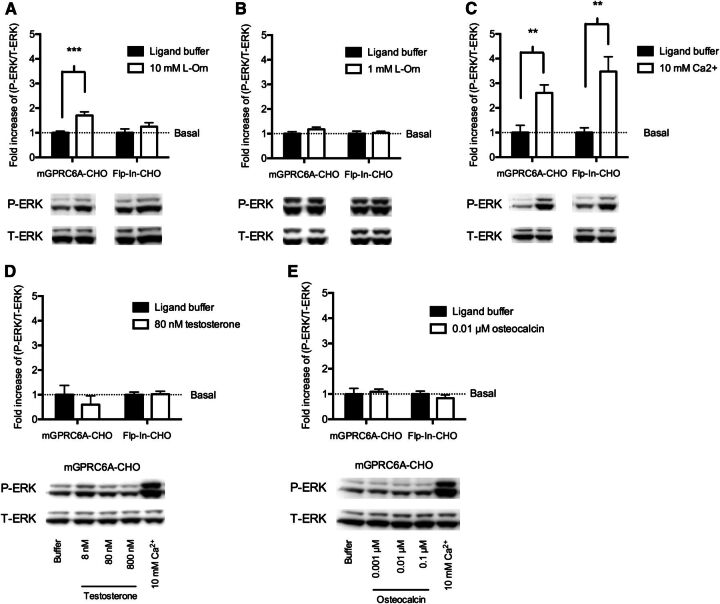
Many GPCRs are capable of initiating the MAPK/extracellular signal-regulated kinase (ERK) pathway, which is involved in regulation of cell proliferation through modulation of gene transcription. Two of the most important kinases are ERK1 and ERK2, which are activated by phosphorylation of specific serine and threonine residues (P-ERK1/2). Both G protein-dependent and -independent pathways, such as the β-arrestin pathway, can trigger ERK activation (Luttrell, 2008). The four ligands (l-Orn, Ca2+, testosterone, and osteocalcin) were tested for their ability to activate ERK in the mGPRC6A-CHO and Flp-In-CHO cell lines by using the Western blot technique.
In the mGPRC6A-CHO cells, but not in the Flp-In-CHO cells, 10 mM l-Orn was shown to induce increased levels of P-ERK. The level of T-ERK was similar in all samples. Quantification of the Western blots proved the l-Orn response to be significant only in mGPRC6A-CHO and not in Flp-In-CHO (Fig. 6A). At 1 mM l-Orn, no activation of ERK was seen in mGPRC6A-CHO (Fig. 6B). Collectively, these results show that l-Orn triggers GPRC6A-mediated ERK activation, which is consistent with the Gq activation seen in mGPRC6A-CHO on l-Orn stimulation, although higher concentrations of l-Orn seem to be needed to achieve a detectable response in the ERK pathway.
Fig. 6.
Measurements of ERK activation in mGPRC6A-CHO and Flp-In-CHO cells on stimulation with (A) 10 mM l-Orn, (B) 1 mM l-Orn, (C) 10 mM Ca2+, (D) 80 nM testosterone, and (E) 0.01 µM osteocalcin. ERK activation was assessed by using antibodies against phosphorylated ERK (P-ERK) and total ERK (T-ERK) after Western blotting. Quantification of the western blots has been performed by using the ImageJ program (http://rsbweb.nih.gov/ij/download.html) and by normalizing the P-ERK response to the corresponding T-ERK response. Furthermore, the data have been normalized to the basal level of P-ERK/T-ERK in ligand buffer and are shown as means ± S.E.M. of (A, B, C) three or (D, E) two independent experiments. Statistical comparison between basal (ligand buffer) and the ligand was performed within each group (each cell line) by using an unpaired student’s t test (**P < 0.01, ***P < 0.001).
In both mGPRC6A-CHO and Flp-In-CHO cells, 10 mM Ca2+ triggered increased P-ERK levels, and quantification of the responses showed significant activation of ERK in both cell lines (Fig. 6C). In addition, 3 mM and 5 mM Ca2+ induced equal ERK activation in mGPRC6A-CHO and Flp-In-CHO cells (data not shown); thus, the Ca2+-mediated ERK phosphorylation at these three concentrations is unrelated to GPRC6A. Different concentrations of both testosterone and osteocalcin were also tested for their ability to phosphorylate ERK; however, neither of the concentrations triggered any increase in the level of P-ERK in mGPRC6A-CHO. We included 10 mM Ca2+ as a positive control. Quantification of the 80 nM testosterone and 0.01 µM osteocalcin bands further confirmed that no increase in P-ERK was seen (Fig. 6, D and E). Thus, testosterone and osteocalcin do not activate the ERK pathway in the mGPRC6A-CHO cell line.
Discussion
Conflicting data regarding the signaling of GPRC6A have been reported; thus, the physiologic relevant ligands and signaling pathways of the receptor remain elusive. In the present study, we generated a CHO cell line that stably expresses the mouse GPRC6A receptor. This cell line was used for systematically testing different intracellular signaling pathways on stimulation with previously reported GPRC6A-agonists (l-Orn, Ca2+, testosterone, and osteocalcin) to delineate the signaling properties of the GPRC6A receptor.
We showed that l-Orn triggered a GPRC6A-mediated response in mGPRC6A-CHO cells when testing the Gq signaling pathway, as evidenced by IP1 generation. This finding is in accordance with previously published data showing that GPRC6A is a promiscuous l-α-amino acid–sensing receptor with a preference for basic amino acids activating an endogenous calcium-sensitive chloride channel in Xenopus laevis oocytes (Kuang et al., 2005; Wellendorph et al., 2005). Previously, a 2×2-hour washing protocol and a signal-enhancing mutated Gq(G66D) protein construct were requisites for achieving responses through the Gq pathway in mammalian cell lines (Christiansen et al., 2007; Wellendorph et al., 2007), but this is not a necessity in the current setup. To our knowledge, this is the first time that robust Gq protein activation has been demonstrated for the GPRC6A receptor without using techniques for improving signaling or assays that do not measure G protein pathways directly.
When testing the mGPRC6A-CHO cell line in the Fluo-4 NW calcium assay, no functional response could be detected, contrary to the IP-One assay, which therefore seems to be a more sensitive method. In our previously published GPRC6A setup, it was possible to measure responses only when coexpressing the signal-enhancing Gq(G66D) protein. When applying this expression system in our current IP-One setup, only a minor response could be detected when expressing mGPRC6A alone. Thus, despite using the more sensitive IP-One assay, this method is still not sufficiently effective to enable robust functional measurements in transient expression systems without using the signal-enhancing Gq(G66D) protein. Conclusively, our ability to characterize mGPRC6A signaling through coupling to endogenous G proteins arises from a combination of using the highly sensitive HTRF technology and our stably expressing CHO cell line.
Divalent cations have been claimed to work as either direct agonists at the GPRC6A receptor (Pi et al., 2005) or coagonists with amino acids (Christiansen et al., 2007). In the present study, Ca2+ behaves as an agonist at mGPRC6A when measuring activation of the Gq pathway. In the HTRF IP-One assay, cells in high-suspension density are subjected to ligand stimulation for 1 hour, during which the level of IP1 accumulates. It is well known that l-amino acids can be released from cells during such an incubation, which can lead to indirect activation of amino acid receptors (Thomsen et al., 1994; Desai et al., 1995). It is thus plausible that the observed Ca2+ response is caused by positive modulation of low-level amino acid levels that build up during the ligand incubation. However, we cannot rule out that Ca2+ is directly activating the mGPRC6A receptor, as it is not possible to confirm the presence or absence of amino acid buildup in the buffer with the present assay setup.
l-Orn cannot necessarily be classified as an agonist either, as the IP-One experiments were conducted in a buffer containing 1 mM Ca2+ and 1 mM Mg2+. It seems possible that both basic amino acids and divalent cations are equally required to obtain robust GPRC6A-responses, and in that regard, the ligands may instead be classified as coagonists. This is consistent with the fact that both amino acids and cations will be present in concentrations capable of activating GPRC6A under most physiologic situations. Thus, our results confirm previous results that basic l-α-amino acids and divalent cations are ligands for the mGPRC6A receptor (Kuang et al., 2005; Pi et al., 2005; Wellendorph et al., 2005; Christiansen et al., 2007).
Through use of the specific Gq inhibitor UBO-QIC and the PI-PLC inhibitor U73122, we unequivocally confirm that the l-Orn and Ca2+-induced IP1 production is specifically mediated by the Gq signaling pathway in this system.
GPRC6A has previously been reported to activate the downstream signaling molecules SRE and ERK via Gi (Pi et al., 2005, 2010) and to increase cAMP levels via Gs in HEK293 cells (Dreaden et al., 2012). Activation of the Gs pathway by osteocalcin in Leydig TM3 cells has also been suggested, albeit not directly demonstrated to be mediated by GPRC6A (Oury et al., 2011). In the present study, we show that mGPRC6A couples to neither Gs nor Gi when stably expressed in the CHO cell line. CHO cells have been used extensively to study Gs and Gi-coupled GPCRs for two decades (Jones et al., 1991; Horie et al., 1995; Schroder et al., 2010), and it is thus not due to lack of components of these signaling pathways that we fail to measure Gs- or Gi-mediated responses by any of the four ligand classes.
The Quarles group previously demonstrated activation of the P-ERK pathway on stimulation with basic amino acids, divalent cations, testosterone, and osteocalcin (Pi et al., 2005, 2010, 2011), albeit the latter ligand activity was not confirmed in Leydig TM3 cells by the Karsenty group (Oury et al., 2011). Here we have shown that l-Orn triggers mGPRC6A-specific ERK phosphorylation. These results were expected, since ERK phosphorylation is a downstream effect of several signaling pathways including the Gq pathway (Luttrell, 2008), which is activated by l-Orn in mGPRC6A-CHO cells. In analogy, activation of Gq by the homologous calcium-sensing receptor also leads to ERK1/2 phosphorylation (Kifor et al., 2001; Thomsen et al., 2012). However, it is noteworthy that high l-Orn concentrations were needed to detect responses in Western blotting compared with the amount of l-Orn needed to give maximum effect in the IP-One assay. Thus, the potency of l-Orn seems to be lower in the ERK pathway than in the Gq pathway, and consequently it has not been possible to determine an exact EC50 value of l-Orn in the ERK signaling pathway.
Ca2+-induced ligand activation of mGPRC6A has also been shown in the Gq signaling pathway; thus, it was expected that Ca2+ likewise would activate ERK in mGPRC6A-CHO. However, the response in the ERK pathway turned out to be nonspecific. These nonspecific effects may be triggered by unknown mechanisms, for example, G protein–independent effects in the CHO cell lines, which potentially mask the Gq-mediated Ca2+ response in the ERK pathway.
Contrary to previous reports, neither testosterone nor osteocalcin elicited any response in the ERK pathway or any of the other tested G protein–signaling pathways. Thus, the role of testosterone and osteocalcin as GPRC6A agonists, as stated previously by others (Pi et al., 2005, 2010, 2011, 2021; Oury et al., 2011), cannot be supported by the present study. Because of the strong interest in osteocalcin as a GPRC6A agonist, we also tested this ligand in a combined agonist-positive allosteric modulator mode in tsA cells, a transformed HEK293 cell line (Chahine et al., 1994), where we also failed to detect receptor activity.
Our results have not provided us with an explanation for the contradictory data regarding GPRC6A signaling. It is likely that inconsistencies between reports on GPRC6A pharmacology are accounted for by the choice of cell expressing system, cell growth conditions, or the applied methods. Different cell lines might display different levels of receptor density, G proteins, and other signaling molecules, which can lead to cell-type specific agonist signaling profiles (Kenakin, 2011). The results can also be influenced by the messenger molecule being measured, which may limit signal amplification and sensitivity. Others have measured ERK phosphorylation and SRE activation (Pi et al., 2005), both of which are downstream to many signaling pathways and might consequently give rise to increased interference from nonspecific pathways. In comparison, more upstream signaling pathway components have been measured in the current work, which, along with the application of a GPRC6A selective antagonist and the specific inhibitors UBO-QIC and U73122, convincingly validate the specificity of the observed signaling.
In conclusion, we provide clear evidence that the mGPRC6A receptor is Gq-coupled in response to l-α-amino acids and divalent cations. In addition, we report the successful development of a stable mGPRC6A-CHO cell line and its usefulness for measuring Gq-mediated responses in the robust and sensitive HTRF IP-One assay. Notably, this precludes the previously used “tricks” of using chimeric receptors, mutated or chimeric G proteins, or extensive prewashing of the cells, which allows direct and unbiased measurement of receptor activation. The assay enables screening for novel and more selective GPRC6A ligands that may serve as pharmacological tool compounds, which are highly required to study the physiologic function and therapeutic potential of the receptor. We were unable to confirm previously reported activation of Gi and Gs pathways by GPRC6A and the agonistic activity of testosterone and osteocalcin. Further studies are thus warranted to elucidate completely which ligand classes and G protein pathways are used by the GPRC6A receptor.
Acknowledgments
The authors thank Henrik Johansson and Dr. Daniel Sejer Pedersen for synthesizing compound 1, Maria Brinck Hansen and Kasper Lind Hansen for generating the pcDNA5/FRT/V5-His/TOPO-mGPRC6A plasmid and Dr. Christoffer Clemmensen for fruitful discussions and help with quantification of the Western blots.
Abbreviations
- ANOVA
analysis of variance
- bOC
bovine osteocalcin
- CHO
Chinese hamster ovary
- compound 1
2-[N-methyl(2′-morpholino-2′-oxoethyl)amino]-1-(2′′-phenyl-1H-indol-3′′-yl)-ethanone
- DMSO
dimethylsulfoxide
- DPBS
Dulbecco’s phosphate-buffered saline
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPCR
G protein–coupled receptor
- GPRC6A
G protein–coupled receptor family C, group 6, member A
- HBSS
Hanks’ balanced salt solution
- HEK
human embryonic kidney (cell line)
- hGPRC6A
human GPRC6A
- HTRF
homogeneous time-resolved fluorescence
- IBMX
3-isobutyl-1-methylxanthine
- IP1
d-myo-inositol monophosphate
- IP3
inositol 1,4,5 trisphosphate
- L-Orn
L-ornithine
- MAPK
mitogen-activated protein kinase
- mGPRC6A
mouse GPRC6A
- P-ERK
phosphorylated ERK
- PI-PLC
phosphatidylinositol-specific phospholipase C
- pOC
porcine osteocalcin
- RT-PCR
reverse-transcription polymerase chain reaction
- SRE
serum-response element
- TBS
Tris-buffered saline
- T-ERK
total ERK
- U73122
1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione
- UBO-QIC
University of Bonn-Gq inhibitor compound
Authorship Contributions
Participated in research design: Jacobsen, Nørskov-Lauritsen, Thomsen, Smajilovic, Wellendorph, Larsson, Lehmann, Bhatia, Bräuner-Osborne.
Conducted experiments: Jacobsen, Nørskov-Lauritsen, Thomsen, Smajilovic, Wellendorph.
Contributed new reagents or analytic tools: Thomsen, Bhatia.
Performed data analysis: Jacobsen, Nørskov-Lauritsen, Thomsen, Smajilovic, Wellendorph.
Wrote or contributed to the writing of the manuscript: Jacobsen, Nørskov-Lauritsen, Thomsen, Smajilovic, Wellendorph, Larsson, Lehmann, Bhatia, Bräuner-Osborne.
Footnotes
This work was supported by grants from the Danish Council for Independent Research Medical Sciences (H.B.-O., V.K.B., P.W.); the Investment Capital for University Research (UNIK) Food, Fitness and Pharma for Health and Disease program (H.B.-O.); the Carlsberg Foundation (S.S.); and the Drug Research Academy (A.R.B.T.).
References
- Bräuner-Osborne H, Wellendorph P, Jensen AA. (2007) Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8:169–184. [DOI] [PubMed] [Google Scholar]
- Chahine M, Bennett PB, George AL, Jr, Horn R. (1994) Functional expression and properties of the human skeletal muscle sodium channel. Pflugers Arch 427:136–142. [DOI] [PubMed] [Google Scholar]
- Christiansen B, Hansen KB, Wellendorph P, Bräuner-Osborne H. (2007) Pharmacological characterization of mouse GPRC6A, an L-alpha-amino-acid receptor modulated by divalent cations. Br J Pharmacol 150:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen B, Wellendorph P, Bräuner-Osborne H. (2006) Activity of L-alpha-amino acids at the promiscuous goldfish odorant receptor 5.24. Eur J Pharmacol 536:98–101. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Pehmøller C, Klein AB, Ratner C, Wojtaszewski JFP, Bräuner-Osborne H. (2013a) Enhanced voluntary wheel running in GPRC6A receptor knockout mice. Physiol Behav 118:144–151. [DOI] [PubMed] [Google Scholar]
- Clemmensen C, Smajilovic S, Madsen AN, Klein AB, Holst B, Bräuner-Osborne H. (2013b) Increased susceptibility to diet-induced obesity in GPRC6A receptor knockout mice. J Endocrinol 217:151–160. [DOI] [PubMed] [Google Scholar]
- Conklin BR, Bourne HR. (1994) Homeostatic signals: marriage of the flytrap and the serpent. Nature 367:22. [DOI] [PubMed] [Google Scholar]
- Degorce F, Card A, Soh S, Trinquet E, Knapik GP, Xie B. (2009) HTRF: a technology tailored for drug discovery—a review of theoretical aspects and recent applications. Curr Chem Genomics 3:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MA, Burnett JP, Mayne NG, Schoepp DD. (1995) Cloning and expression of a human metabotropic glutamate receptor 1 alpha: enhanced coupling on co-transfection with a glutamate transporter. Mol Pharmacol 48:648–657. [PubMed] [Google Scholar]
- Dreaden EC, Gryder BE, Austin LA, Tene Defo BA, Hayden SC, Pi M, Quarles LD, Oyelere AK, El-Sayed MA. (2012) Antiandrogen gold nanoparticles dual-target and overcome treatment resistance in hormone-insensitive prostate cancer cells. Bioconjug Chem 23:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Koda S, Morimoto Y, Biemann K. (1988) Structure of FR900359, a cyclic depsipeptide from Ardisia crenata sims. J Org Chem 53:2820–2825. [Google Scholar]
- Gloriam DE, Wellendorph P, Johansen LD, Thomsen AR, Phonekeo K, Pedersen DS, Bräuner-Osborne H. (2011) Chemogenomic discovery of allosteric antagonists at the GPRC6A receptor. Chem Biol 18:1489–1498. [DOI] [PubMed] [Google Scholar]
- Horie K, Itoh H, Tsujimoto G. (1995) Hamster alpha 1B-adrenergic receptor directly activates Gs in the transfected Chinese hamster ovary cells. Mol Pharmacol 48:392–400. [PubMed] [Google Scholar]
- Jones SV, Heilman CJ, Brann MR. (1991) Functional responses of cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol 40:242–247. [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302. [DOI] [PubMed] [Google Scholar]
- Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. (2001) Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol 280:F291–F302. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. (2004) Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103:21–80. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. (2005) Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem 93:383–391. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Maclean D, Wang M, Hampson DR, Chang BS. (2006) Ancestral reconstruction of the ligand-binding pocket of Family C G protein-coupled receptors. Proc Natl Acad Sci USA 103:14050–14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM. (2008) Reviews in molecular biology and biotechnology: transmembrane signaling by G protein-coupled receptors. Mol Biotechnol 39:239–264. [DOI] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, et al. (2011) Endocrine regulation of male fertility by the skeleton. Cell 144:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy L, Vadnal RE, Parthasarathy R, Devi CS. (1994) Biochemical and molecular properties of lithium-sensitive myo-inositol monophosphatase. Life Sci 54:1127–1142. [DOI] [PubMed] [Google Scholar]
- Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, et al. (2008) GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3:e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, et al. (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Parrill AL, Quarles LD. (2010) GPRC6A mediates the non-genomic effects of steroids. J Biol Chem 285:39953–39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Quarles LD. (2012) GPRC6A regulates prostate cancer progression. Prostate 72:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. (2012) GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology 153:4608–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Wu Y, Quarles LD. (2011) GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 26:1680–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650. [DOI] [PubMed] [Google Scholar]
- Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schöneberg T, Schaefer M, Krügel U, et al. (2012) Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun 3:1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, Müller A, Blättermann S, Mohr-Andrä M, Zahn S, et al. (2010) Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol 28:943–949. [DOI] [PubMed] [Google Scholar]
- Smajilovic S, Clemmensen C, Johansen LD, Wellendorph P, Holst JJ, Thams PG, Ogo E, Bräuner-Osborne H. (2013) The L-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in L-arginine-induced insulin release. Amino Acids 44:383–390. [DOI] [PubMed] [Google Scholar]
- Smallridge RC, Kiang JG, Gist ID, Fein HG, Galloway RJ. (1992) U-73122, an aminosteroid phospholipase C antagonist, noncompetitively inhibits thyrotropin-releasing hormone effects in GH3 rat pituitary cells. Endocrinology 131:1883–1888. [DOI] [PubMed] [Google Scholar]
- Speca DJ, Lin DM, Sorensen PW, Isacoff EY, Ngai J, Dittman AH. (1999) Functional identification of a goldfish odorant receptor. Neuron 23:487–498. [DOI] [PubMed] [Google Scholar]
- Thomsen AR, Hvidtfeldt M, Bräuner-Osborne H. (2012) Biased agonism of the calcium-sensing receptor. Cell Calcium 51:107–116. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Hansen L, Suzdak PD. (1994) L-glutamate uptake inhibitors may stimulate phosphoinositide hydrolysis in baby hamster kidney cells expressing mGluR1a via heteroexchange with L-glutamate without direct activation of mGluR1a. J Neurochem 63:2038–2047. [DOI] [PubMed] [Google Scholar]
- Trinquet E, Bouhelal R, Dietz M. (2011) Monitoring Gq-coupled receptor response through inositol phosphate quantification with the IP-One assay. Expert Opin Drug Discov 6:981–994. [DOI] [PubMed] [Google Scholar]
- Trinquet E, Fink M, Bazin H, Grillet F, Maurin F, Bourrier E, Ansanay H, Leroy C, Michaud A, Durroux T, et al. (2006) d-Myo-inositol 1-phosphate as a surrogate of d-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation. Anal Biochem 358:126–135. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Bräuner-Osborne H. (2004) Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335:37–46. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Burhenne N, Christiansen B, Walter B, Schmale H, Bräuner-Osborne H. (2007) The rat GPRC6A: cloning and characterization. Gene 396:257–267. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. (2005) Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol 67:589–597. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P, Clément-Lacroix P, Bettler B, Bräuner-Osborne H. (2009) No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42:215–223. [DOI] [PubMed] [Google Scholar]