Abstract
Background: Many epigenetic factors, including microRNAs, are involved in the process of changing gene expressions. Small non-coding RNA molecules, called miRNAs, are responsible for regulating gene translation by silencing or degrading target mRNAs. It is acknowledged that for many diseases, they may be novel diagnostic and prognostic biomarkers. Patients with autoimmune thyroid diseases are more likely to develop nodules in the thyroid tissue, and Hashimoto’s thyroiditis and Graves’ disease predispose patients to thyroid cancer. We evaluated the concentrations of microRNA molecules (miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p, miR-150-5p) in the blood of children with thyroid disorders. In addition, we wished to identify molecules whose change in concentration predisposes to the development of thyroid cancer. Aim: The aim of this study is to evaluate selected epigenetic elements by analyzing the levels of miR-15a-5p, miR-126-3p, miR-142-5p, miR-150-5p and miR-21-5p in the blood of pediatric patients with Graves’ disease (n = 25), Hashimoto’s thyroiditis (n = 26) and thyroid nodular disease (n = 20) compared to a control group of healthy children (n = 17). Materials and Methods: The study consists of groups of children and adolescents aged 10–18 years with autoimmune thyroid disease, with thyroid nodular disease compared to a control group. The miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p molecules were determined through an immunoenzymatic assay using BioVendor reagents. Results: There is a statistically significant decrease in the expression of the miR-15a-5p in children with Graves’ disease (21.61 vs. 50.22 amol/μL, p = 0.03) and in patients with thyroid nodular disease compared to controls (20.23 vs. 50.22 amol/μL, p = 0.04). Higher levels of the miR-142-5p molecule are found in patients with thyroid disease (with GD-3.8 vs. 3.14 amol/μL, p = 0.01; with HT-3.7 vs. 3.14 amol/μL, p = NS, with thyroid nodular disease-4.16 vs. 3.14 amol/μL, p = 0.04). Lower levels of miR-126-3p were noted in the GD group compared to the control group (7.09 vs. 7.24 amol/μL, p = 0.02). No statistically significant changes in the expressions of miR-150-5p and miR-21-5p molecules were observed in the study groups. Conclusions: 1. The overexpression of the miR-142-5p molecule occurs in children and adolescents with thyroid diseases. 2. Decreased blood levels of miR-15a-5p predispose patients to the formation of focal lesions in the thyroid gland. 3. Identifying a lower expression of the miR-126-3p molecule in the blood of children with GD requires careful follow-up for the development of focal lesions in the thyroid gland and evaluation for their potential malignancy.
Keywords: miRNA, Hashimoto’s thyroiditis, Graves’ disease, thyroid nodular disease
1. Introduction
Among the multifactorial pathogenesis of autoimmune thyroid diseases (AITDs) are genetic predisposition and environmental factors. Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) are associated with a dysfunction in the immune system. The impaired immune tolerance of thyroid antigen molecules results in a defective immune response. There is an incorrect identification of the body’s own cells, which impairs the safety of tissues and organs. Abnormal immunotolerance results from impaired suppression involving regulatory T cells in peripheral immunocompetent tissues (Tregs) [1]. It is known that patients with autoimmune thyroid diseases are more likely to develop focal lesions in the thyroid tissue, and Hashimoto’s thyroiditis and Graves’ disease predispose patients to the development of thyroid cancer [2,3,4,5]. To date, many mutations in oncogenes and tumor suppressor genes have been found in patients with malignant tumors. It is known that most of the human genome does not have the ability to encode proteins. Only 2% have this capability. RNA encodes most of the non-coding genome. Mutations in RNA can cause many human diseases, including autoimmunity, or cancer [6].
MicroRNAs (miRNAs) are small 22-nucleotide non-coding molecule RNAs that regulate gene translation by silencing or degrading target mRNAs. By binding to the 3′ non-translational regions (3′ UTR) of the RNAs of protein-coding genes, they signal the translation or degradation of the messenger molecule. MiRNAs are involved in many biological processes, such as proliferation, metabolism, hemostasis, apoptosis and inflammation, the dysfunction of which may play an important role in the pathogenesis of many diseases. Thus, they may be considered as new diagnostic and prognostic biomarkers for a variety of defects.
Studies have shown that miR-15a affects pancreatic β-cells, thereby playing a role in insulin synthesis. Increased levels of miR-15a are observed in patients with diabetes. The more metabolically unstable the diabetes, the higher the amount in serum [7]. Downregulated levels of miR-15a are observed in many cancers, including colorectal cancer [8], liver cancer [9], prostate cancer [10], chronic lymphocytic leukaemia [11], nasopharyngeal carcinoma [12], malignant melanoma [13], human brain glioma [14] and breast cancer [15].
In contrast, another miRNA that regulates the expressions of many cancer-related genes is miR-126-3p. It is also endothelium-specific because it also regulates angiogenesis and blood vessel integrity [16]. It acts either as a tumor suppressor or as an oncogene in various types of cancer. As has been reported in published studies, the miR-126-3p expression is found to be downregulated in malignant thyroid cancer samples compared to benign thyroid nodules. A decreased miR-126-3p expression is associated with clinically more aggressive papillary thyroid cancer [17].
The miR-142-5p molecule also presents high specificity for hematopoietic cells and increased levels are observed in many pathological conditions such as inflammation [18], immune disorders [19] and cancers, for example, retinoblastoma [20] and breast cancer [21]. Moreover, the downregulation of miR-142-5p may serve as an additional indicator of metastasis in thyroid cancer [22].
The miR-21-5p is a typical onco-miRNA. It is one of the first identified miRNA molecules. It modulates the expressions of many tumor-related target genes. In addition to tissues, it is found in various types of extracellular fluids, such as plasma, serum, cerebrospinal fluid, saliva, gastric fluid, pancreatic juice, sputum and pancreatic cyst fluid. A high level of the miR-21-5p expression is a negative predictor of survival in various cancers [23,24,25]. It plays an important role in vascular smooth muscle cell proliferation and apoptosis, cardiac cell growth and death, aortic valve calcification and cardiac fibroblast function [26]. Moreover, the expression of miR-21-5p is significantly upregulated in patients with Hashimoto’s thyroiditis [27].
miR-150-5p is another microRNA with an expression that is an important regulator of immune cell differentiation and activation. A particular selective expression of miR-150-5p occurs from lymph nodes, the spleen and in mature B and T lymphocytes. The finding of lower levels of miR-150-5p in cancer patients predicted worse responses to chemotherapy and shorter survival. In contrast, a high expression of miR-150 in patient tumor samples indicates a better prognosis and better response to chemotherapy. Moreover, the overexpression of miR-150-5p leads to a reduced migration and invasion of colorectal cancer cells. MiR-150-5p also acts as a suppressor of metastasis in colorectal cancer [28].
There are very few studies evaluating the concentrations of these molecules in children. We would like to assess the concentrations of microRNA molecules (miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p, miR-150-5p) in children with thyroid disorders, the predisposition to the development of focal lesions in thyroid gland and the risk of developing malignancy, considering predisposition to cancer [29,30].
2. Materials and Methods
This study was performed with 25 patients with newly diagnosed GD (mean age 14.36 ± 3.6), 26 with newly diagnosed HT (mean age, 14.18 ± 3.9), 20 with a nodular goiter in euthyroid (mean age, 13.7 ± 2.9) and 17 children without any autoimmune diseases (mean age 12.58 ± 2.6) recruited from the Department of Pediatrics, Endocrinology, Diabetology, with Cardiology Divisions and from the Pediatric Endocrinology Outpatient Clinic of Medical University in Bialystok. The diagnoses of autoimmune thyroid diseases were based on medical history, physical examination, laboratory and ultrasound investigations [31,32]. Patients with thyroid diseases had no other autoimmune comorbidities. We excluded other endocrinopathies. A clinical diagnosis of hyperthyroidism in GD was confirmed by elevated thyroid hormones in serum and the suppression of TSH (thyroid- stimulating hormone) to values close to zero with positive antibodies against the receptor for thyroid-stimulating hormone (TRAb = anti-TSH), positive anti-thyroid peroxidase antibodies (aTPO) and anti-thyroglobulin antibodies (aTG). In clinically evident hypothyroidism in HT, serum TSH levels were elevated at reduced concentrations of thyroid hormones (fT4—free thyroxine, fT3—triiodothyronine), usually accompanied by elevated thyroid antibodies (aTPO and/or aTG, rarely blocking anti-TSH). Children and adolescents with GD and HT exhibited a typical thyroid picture for AITD on ultrasound. Patients with nodular goiters underwent fine-needle aspiration biopsies (FNABs) to exclude thyroid cancer. All patients started appropriate therapy for autoimmune thyroid pathology. Patients with newly diagnosed GD started therapy with methimazole and b-blockers, administered orally. Patients with newly diagnosed HT received l-thyroxine orally in appropriate doses as part of their therapy. The control group consisted of 17 healthy children with no personal or family history of any AITDs. They were euthyroid and negative for thyroid antibodies. All controls had normal thyroid glands on ultrasonography. Before enrollment, all patients’ and controls’ parents and all children over 16 years old signed informed consent forms. The protocol for the study was approved by the Local Bioethical Committee at the Medical University of Bialystok.
2.1. Assessment of the Thyroid Hormone Concentration and Anti-Thyroid Antibody Titers
Blood for analysis was collected in the morning from the basilic vein. The serum levels of free thyroxine (fT4), free triiodothyronine (fT3) and TSH were determined based on electrochemiluminescence ‘ECLIA’ with a Cobas E411 analyzer (Roche Diagnostics, Rotkreuz, Switzerland). Normal values for fT4 ranged between 1.1 and 1.7 ng/dL; for fT3, between 2.3 and 5.0 pg/mL; and for TSH, between 0.28 and 4.3 (µIU/L). TR-Ab, aTPO and aTG antibodies were measured in all samples using ECLIA with a Modular Analytics E170 analyzer (Roche Diagnostics). The positive values for antithyroid antibodies were >1.75 U/L for TRAb, >34 IU/mL for aTPO-Abs and >115 IU/mL for aTG-Abs. The blood samples for microRNAs and for thyroid hormones and antithyroid antibodies analyses were harvested at the same time points.
2.2. Assessment of miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p Levels
Whole blood for miRNA analysis was collected in PAX-Gene Blood RNA Tubes (IVD) (Qiagen, Germantown, MD, USA) and treated according to the manufacturer’s protocol. Blood for miRNA determination was provided before the drug treatment was started. Subsequently, the blood underwent centrifugation at 2000× g for 20 min at zero acceleration and deceleration within a refrigerated centrifuge. The resulting supernatant was transferred into 2 mL Eppendorf tubes. To obtain purified plasma, the supernatant was subjected to a secondary centrifugation step at 20,000× g for 15 min in a refrigerated centrifuge. Hemolysis in the plasma samples was assessed through visual examination and spectrophotometric analysis at a wavelength of 414 nm. The plasma was then divided into 0.5 mL aliquots and promptly stored at −80 °C until the subsequent RNA extraction process. The miRNA from the plasma was extracted using a miRNA Isolation Kit Serum/Plasma (BioVendor R&D, Brno, Czech Republic) according to the manufacturer’s protocol. The isolated miRNAs were quantified via hybridization to complementary biotinylated DNA oligonucleotide probes. The DNA/RNA hybrids were then captured using a microtiter plate-immobilized monoclonal antibody specific to perfectly match the DNA/miRNA hybrids using miREIA—a novel, immunoassay-based method of miRNA quantification—following the manufacturer’s instructions (BioVendor R&D, Brno, Czech Republic). The next steps followed standard ELISA protocols. The results were reported as concentrations of miR-15a-5p (amol/μL), miR-126-3p (amol/μL), miR-142-5p (amol/μL), miR-21-5p (amol/μL) and miR-150-5p (amol/μL) in the samples.
2.3. Statistical Analysis
The p-value obtained using Fisher’s exact test [33] was used to assess the association between miRNA prevalence and the median unbiased estimator (mid-p) of the odds ratio (as well as its 95% exact confidence interval). Parametric or non-parametric methods were used to determine statistically significant differences between disease-defined groups, depending on whether the assumptions of normality and homogeneity of variance were met. Due to the issue of multiple tests during the post hoc analysis, the p-value correction method of the false discovery rate was used [34,35]. A p-value < 0.05 was considered significant for all calculations. The R software (version 4.2.3.) environment was used for all calculations [36].
3. Results
Age and anthropometric parameters in the study groups of children with GD, HT and nodular goiter compared to the control group did not have statistically significant differences (Table 1).
Table 1.
Clinical characteristics of patients with Graves’ disease (GD), with Hashimoto’s thyroiditis (HT) and with nodular goiter and of control group.
| GD (Mean ± SD) |
p^ | HT (Mean ± SD) |
p^^ | Nodular Goiter (Mean ± SD) | p^^^ | Control Group | |
|---|---|---|---|---|---|---|---|
| children (female/male) | 25 (15/10) | 26 (20/6) | 20 (15/5) | 17 (8/9) | |||
| age (years) | 14.36 ± 3.6 | NS | 14.18 ± 3.9 | NS | 13.7 ± 2.9 | NS | 12.58 ± 2.6 |
| fT4 (ng/dL) | 12.68 ± 33.5 | p < 0.001 | 1.18 ± 0.24 | p < 0.01 | 2.31 ± 4.2 | NS | 1.55 ± 0.62 |
| fT3 (pg/mL) | 17.99 ± 34.39 | p < 0.001 | 2.54 ± 1.1 | NS | 4.93 ± 0.1 | NS | 3.61 ± 2.12 |
| TSH (mIU/L) | 0.96 ± 0.22 | p < 0.001 | 8.58 ± 20.45 | NS | 2.27 ± 1.54 | NS | 2.16 ± 0.95 |
| anti-TSH (U/L) | 17.84 ± 31.86 | ||||||
| aTG (IU/mL) | 248.3 ± 348.71 | p < 0.02 | 441.96 ± 442.89 | p < 0.001 | 201.78 ± 433.6 | NS | 25.25 ± 22.95 |
| aTPO (IU/mL) |
238.94 ± 182.76 | p < 0.001 | 172.31 ± 121.99 | p < 0.001 | 43.34 ± 33.9 | NS | 23.92 ± 33.9 |
| treatment | methamizole/ b-blocker |
l-thyroxine | none |
SD—Standard Deviation; p—p-value; NS—statistically non-significant—p-value > 0.05. p^—correlation between group of patients with GD compared to control group. p^^—correlation between group of patients with HT compared to control group. p^^^—correlation between group of patients with nodular goiter compared to control group.
3.1. Results for miR-15a-5p
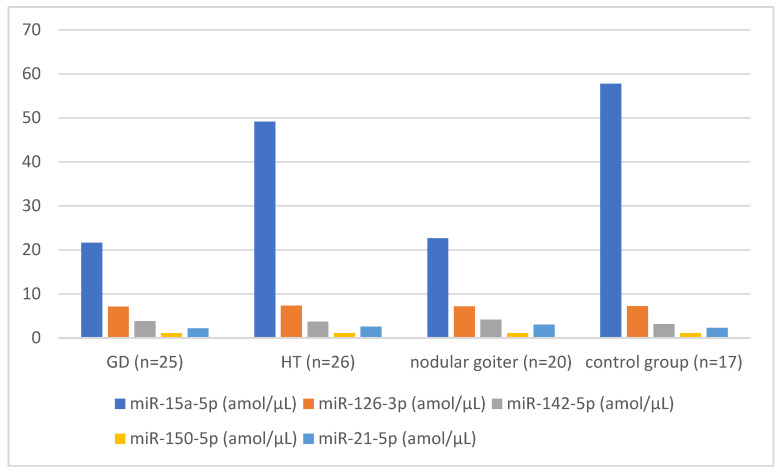
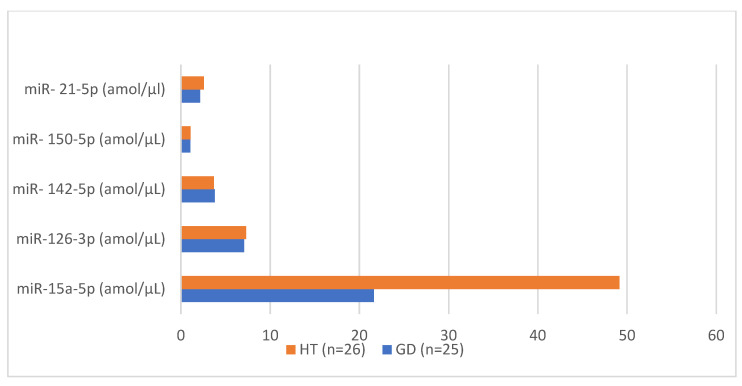
Our study shows that miR-15a-5p levels were lower in patients with GD and with nodular goiter compared to those in healthy children (21.61 amol/µL in GD vs. 50.2 amol/µL in control group, p = 0.03; 20.3 amol/µL in nodular goiter vs. 50.22 amol/µL in control group, p = 0.049, Figure 1). There were lower levels in patients with HT compared to the control group, but not statistically significant (42.99 amol/µL vs. 50.22 amol/µL; p = NS) (Table 2). In comparing both groups with autoimmune diseases, HT and GD, miRNA-15a-5p was higher in patients with HT (49.15 amol/µL vs. 21.61 amol/µL; p = NS) (Table 3). A negative correlation between aTPO levels and miRNA 15-a-5p levels was identified in the group of patients with GD. Moreover, a positive correlation between miRNA15a-5p levels and TRAb titers was observed in GD patients (Table 4 and Table 5).
Figure 1.
Management of particular miRNA molecules (miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p) in groups with Graves’ disease (GD), with Hashimoto’s thyroiditis (HT) and with nodular goiter and in control group.
Table 2.
MiR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p in groups with Graves’ disease (GD), with Hashimoto’s thyroiditis (HT) and with nodular goiter compared to control group.
| Patients with GD (n = 25) | Patients with HT (n = 26) |
Patients with Nodular Goiter (n = 20) | Control Group (n = 17) |
|
|---|---|---|---|---|
| miR-15a-5p amol/µL |
21.61 (p = 0.03) p^ | 49.15 (p = NS) p^^ |
22.63 (p = 0.04)
p^^^ |
57.74 |
| miR-126-3p amol/µL |
7.09 (p = 0.02)
p^ |
7.31 (p = NS) p^^ |
7.14 (p = NS) p^^^ |
7.24 |
| miR-142-5p amol/µL |
3.80 (p = 0.01)
p^ |
3.70 (p = NS) p^^ |
4.16 (p = 0.04)
p^^^ |
3.14 |
| miR-150-5p amol/µL |
1.07 (p = NS) p^ |
1.08 (p = NS) p^^ |
1.07 (p = NS) p^^^ |
1.06 |
| miR-21-5p amol/µL |
2.16 (p = NS) p^ |
2.58 (p = NS) p^^ |
3.04 (p = NS) p^^^ |
2.28 |
p—p-value; NS—statistically non-significant—p-value > 0.05; p^—correlation between group of patients with GD compared to control group. p^^—correlation between group of patients with HT compared to control group. p^^^—correlation between group of patients with nodular goiter compared to control group.
Table 3.
MiR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p in group with Graves’ disease (GD) compared to group with Hashimoto’s thyroiditis (HT).
| Patients with GD (n = 25) | Patients with HT (n = 26) | |
|---|---|---|
| miR-15a-5p | 21.61 amol/µL | 49.15 amol/µL (p = NS) |
| miR-126-3p | 7.09 amol/µL | 7.31 amol/µL (p = NS) |
| miR-142-5p | 3.8 amol/µL | 3.7 amol/µL (p = NS) |
| miR-150-5p | 1.07 amol/µL | 1.08 amol/µL (p = NS) |
| miR-21-5p | 2.16 amol/µL | 2.58 amol/µL (p = NS) |
p—p-value; NS—statistically non-significant—p-value > 0.05; p-correlation between group of patients with GD compared to group with HT.
Table 4.
The correlation between miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p levels and thyroid hormones levels and anti-thyroid antibodies in the group with Graves’ disease (GD).
| miR-150-5p | miR-21-5p | miR-15a-5p | miR-126-3p | miR-142-5p | |
|---|---|---|---|---|---|
| TSH | r = −0.099 p = NS |
r = −0.304 p = NS |
r = −0.011 p = NS |
r = 0.132 p = NS |
r = −0.447 p = NS |
| fT4 | r = 0.143 p = NS |
r = 0.357 p = NS |
r = 0.078 p = NS |
r = 0.135 p = NS |
r = 0.416 p = NS |
| fT3 | r = 0.188 p = NS |
r = 0.1343 p = NS |
r = 0.109 p = NS |
r = 0.119 p = NS |
r = 0.293 p = NS |
| aTG | r = −0.342 p = NS |
r = −0.555 p = NS |
r = −0.445 p = NS |
r = −0.422 p = NS |
r = 0.7118 p = NS |
| aTPO | r = 0.429 p = NS |
r = 0.081 p = NS |
r = −0.892
p = 0.007 |
r = −0.648 p = NS |
r = −0.188 p = NS |
| TRAb | r = −0.37 p = NS |
r = 0.015 p = NS |
r = 0.765
p = 0.045 |
r = 0.729 p = NS |
r = 0.148 p = NS |
p—p-value; NS—statistically non-significant—p-value > 0.05; r—correlation coefficient.
Table 5.
The correlation between miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p levels and thyroid hormones levels and anti-thyroid antibodies in the group with Hashimoto’ thyroiditis (HT).
| miR-150-5p | miR-21-5p | miR-15a-5p | miR-126-3p | miR-142-5p | |
|---|---|---|---|---|---|
| TSH | r = −0.067 p = NS |
r = −0.76 p = NS |
r = −0.065 p = NS |
r = 0.132 p = NS |
r = −0.065 p = NS |
| fT4 | r = 0.037 p = NS |
r = 0.029 p = NS |
r = 0.212 p = NS |
r = 0.035 p = NS |
r = 0.011 p = NS |
| aTG | r = 0.098 p = NS |
r = 0.087 p = NS |
r = 0.261 p = NS |
r = 0.100 p = NS |
r = 0.077 p = NS |
| aTPO |
r = 0.485
p = 0.019 |
r = 0.489
p = 0.018 |
r = 0.152 p = NS |
r = 0.478
p = 0.021 |
r = 0.491
p = 0.017 |
p—p-value; NS—statistically non-significant—p-value > 0.05; r—correlation coefficient.
3.2. Results for miR-126-3p
miR-126-3p levels were lower in patients with GD compared to healthy children (7.09 amol/µL in GD group vs. 7.24 amol/µL in control group, p = 0.02). There were higher levels in patients with HT compared to control group, but not statistically significant (7.31 amol/µL vs. 7.24 amol/µL; p = NS). In children with nodular goiter, miRNA-126-3p levels were slightly lower than in the control group (7.14 amol/µL vs. 7.24 amol/µL; p = NS) (Table 2, Figure 1). In comparing both groups with autoimmune diseases, HT and GD, miRNA-126-3p was higher in patients with HT (7.31 amol/µL vs. 7.09 amol/µL; p = NS) (Table 3, Figure 2). A positive correlation between aTPO levels and miRNA-126-3p was found in the group of patients with HT (r = 0.478; p < 0.05; Table 5). A negative correlation between fT3 levels and miRNA 126-3p levels was identified in the group of patients with nodular goiter (Table 6).
Figure 2.
Management of particular miRNA molecules (miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p) in group with Graves’ disease (GD) compared to group with Hashimoto’s thyroiditis (HT).
Table 6.
The correlation between miR-15a-5p, miR-126-3p, miR-142-5p, miR-21-5p and miR-150-5p levels and thyroid hormones levels and anti-thyroid antibodies in the group with nodular goiter.
| miR-150-5p | miR-21-5p | miR-15a-5p | miR-126-3p | miR-142-5p | |
|---|---|---|---|---|---|
| TSH | r = 0.443 p = NS |
r = 0.091 p = NS |
r = −0.963 p = NS |
r = 0.509 p = NS |
r = 0.712 p = NS |
| fT4 | r = 0.859 p = NS |
r = −0.086 p = NS |
r = −0.662 p = NS |
r = 0.895 p = NS |
r = 0.976 p = NS |
| fT3 | r = −0.992 p = NS |
r = −0.073 p = NS |
r = 0.302 p = NS |
r = −0.999
p = 0.029 |
r = −0.977 p = NS |
| aTG | r = −0.568 p = NS |
r = −0.212 p = NS |
r = 0.914 p = NS |
r = −0.628 p = NS |
r = −0.806 p = NS |
| aTPO | r = 0.681 p = NS |
r = −0.061 p = NS |
r = 0.592
p = 0.007 |
r = 0.624 p = NS |
r = 0.403 p = NS |
p—p-value; NS—statistically non-significant—p-value > 0.05; r—correlation coefficient.
3.3. Results for miR-142-5p
Our study shows that miR-142-5p levels were significance higher in patients with GD and with nodular goiter compared to those in healthy children (3.8 amol/µL in GD vs. 3.14 amol/µL in control group, p = 0.01; 4.16 amol/µL in nodular goiter vs. 3.14 amol/µL in control group, p = 0.04, Figure 1). Also, there were higher levels in patients with HT compared to the control group, but not statistically significant (3.7 amol/µL vs. 3.14 amol/µL; p = NS) (Table 2). In comparing both groups with autoimmune diseases, HT and GD, miR-142-5p levels were similar in both patient groups (3.7 amol/µL in HT vs. 3.8 amol/µL in GD; p = NS) (Table 3, Figure 2). A positive correlation between aTPO levels and miRNA-142-5p was identified in the group of patients with HT (r = 0.491; p = 0.917; Table 5). There was no statistically significant correlation between miR-142-5p, thyroid hormones levels and anti-thyroid antibodies in the other studied groups (Table 4 and Table 6).
3.4. Results for miR-150-5p
miR-150-5p levels were similar in patients with GD, HT and nodular goiter compared to those in healthy children (1.07 amol/µL in GD vs. 1.06 amol/µL in control group, p = NS; 1.08 amol/µL in HT vs. 1.06 amol/µL in control group, p = NS; 1.07 amol/µL in nodular goiter vs. 1.06 amol/µL in control group, p = NS) (Table 2, Figure 1). In comparing both groups with autoimmune diseases, HT and GD, miR-150-5p levels were similar in both patient groups (1.06 amol/µL in HT vs. 1.07 amol/µL in GD; p = NS) (Table 3, Figure 2). A positive correlation between aTPO levels and miRNA-150-5p was identified in the group of patients with HT (r = 0.485; p = 0.019; Table 5). There was no statistically significant correlation between miRNA-150-5p thyroid hormones levels and anti-thyroid antibodies in the groups of patients with GD and with nodular goiter (Table 4 and Table 6). In the group of patients with GD, a positive correlation was found between miR-150-5p and miR-21-5p (r = 0.495; Table 7), and also between miR-150-5p and miRNA-142-5p (r = 0.486; Table 7).
Table 7.
The correlations between miRNAs in the group with GD.
| miR-15a-5p | miR-150-5p | miR-21-5p | miR-126-3p | miR-142-5p | |
|---|---|---|---|---|---|
| miR-15a-5p | r = 1 | r = 0.175 | r = −0.133 | r = 0.018 | r = −0.024 |
| miR-150-5p | r = 0.175 | r = 1 | r = 0.495 | r = −0.133 | r = 0.486 |
| miR-21-5p | r = −0.133 | r = 0.495 | r = 1 | r = 0.28 | r = 0.533 |
| miR-126-3p | r = 0.018 | r = −0.133 | r = 20.8 | r = 1 | r = 0.3 |
| miR-142-5p | r = −0.24 | r = 0.486 | r = 0.533 | r = 0.03 | r = 1 |
r—correlation coefficient.
3.5. Results for miR-21-5p
Our study shows that miR-21-5p levels were not significance higher in patients with HT or with nodular goiter compared to those in healthy children (2.58 amol/µL in HT vs. 2.28 amol/µL in control group, p = NS; 3.04 amol/µL in nodular goiter vs. 2.28 amol/µL in control group, p = NS). MiR-21-5p levels were lower in patients with GD compared to healthy children (2.16 amol/µL in GD vs. 2.28 amol/µL in control group, p = NS) (Table 2, Figure 1). In comparing both groups with autoimmune diseases, HT and GD, miR-21-5p levels were higher in the group with HT than the group with GD (2.58 amol/µL in HT vs. 2.16 amol/µL in GD; p = NS) (Table 3). A positive correlation between aTPO levels and miRNA-21-5p was identified in the group of patients with HT (r = 0.489; p = 0.018; Table 5). There was no statistically significant correlation between miR-21-5p thyroid hormones levels and anti-thyroid antibodies in the patients with GD or nodular goiter (Table 4 and Table 6). In the group of patients with nodular goiter, a positive correlation was found between miR-21-5p and miR-150-5p (r = 0.555; Table 8), and also between miR-21-5p and miR-142-5p (r = 0.487; Table 8).
Table 8.
The correlations between miRNAs in the group with nodular goiter.
| miR-15a-5p | miR-150-5p | miR-21-5p | miR-126-3p | miR-142-5p | |
|---|---|---|---|---|---|
| miR-15a-5p | r = 1 | r = 0.247 | r = −0.023 | r = −0.269 | r = 0.171 |
| miR-150-5p | r = 0.247 | r = 1 | r = 0.555 | r = −0.020 | r = 0.528 |
| miR-21-5p | r = −0.023 | r = 0.555 | r = 1 | r = 0.043 | r = 0.487 |
| miR-126-3p | r = −0.269 | r = −0.02 | r = 0.043 | r = 1 | r = −0.075 |
| miR-142-5p | r = 0.171 | r = 0.528 | r = 0.487 | r = −0.075 | r = 1 |
r—correlation coefficient
4. Discussion
The different levels of miRNAs were associated with GD and HT, which may play a role in the pathogenesis of these diseases. It is known that autoimmune thyroid diseases predispose patients to the development of thyroid cancer. In recent years, several reports have been published confirming different serum or tissue miRNA levels in autoimmune thyroid diseases and thyroid cancers in adults. There are still no reports on children evaluating miRNA levels as a risk for autoimmune endocrine disorders.
In the present study, none of the observed miRNAs were differentially expressed in the sera of HT patients compared to healthy controls. Our study showed that miR-15a-5p levels in serum were lower in GD patients and with nodular goiter compared to healthy children. Moreover, miR-142-5p levels were higher in the same groups. Only miR-126-3p serum levels were lower in patients with GD.
As a regulator of the expression of many cancer-related genes, downregulated miR-126-3p may impact the development of malignant thyroid cancer. Xiong Y. et al. [17] reported that a reduced miR-126-3p expression is associated with papillary thyroid cancer. The risk of developing thyroid cancer in nodules in patients with autoimmune diseases is higher than in patients with nodular goiter without autoimmune diseases. So, GD patients with lower levels of miR-126-3p require careful follow-up for the development of focal lesions in the thyroid gland. This still requires further studies. There are no studies in the literature evaluating the molecule in the sera of GD patients.
In our study, we noticed that miR-142-5p levels were higher in patients with AITDs and with nodular goiter compared to healthy children, but not statistically significant higher levels in patients with HT compared to the control group. Trummer O. et al. showed significantly higher expression levels for miR-142-3p in patients with HT [27]. Zhu et al. reported positive associations of TgAb and miR-142-5p levels in HT serum [37]. In our subgroup analysis, we found no evidence of a significant correlation between antithyroid antibodies and miR-142-5p. It is possible that the detectable overexpression of miR-142-5p may be due to both active secretion as a result of inflammation in patients with AITD and the origin of autoimmune cell death or the proliferation of abnormal cells. We suspect that miR-142-5p may be a useful marker of abnormal thyrocytes.
In addition, Yao et al. found that miR-21-5p may be related to atherosclerosis in patients with subclinical hypothyroidism, and miR-150-5p seems to be a sensitive risk marker in predicting endothelial dysfunction in patients with arteriosclerosis [38]. We did not report any significant differences between miR-21-5p serum levels and thyroid hormones levels in the studied groups. To explore this topic further, it would be necessary to compare miRNA levels with lipid concentrations and other markers of atherosclerosis.
In reviewing the relevant literature, there are no studies evaluating these miRNAs in children’ serum compared to thyroid tissue. Jin et al. found that miR-15a was down-expressed in human papillary thyroid cancer (PTC) tissues [39]. They showed that the upregulation of miR-15a mimics could inhibit tumor growth. Hu J. et al. found that the miR-15a expression in the PTC tissues was lower compared with in nodular goiter tissues and perineoplastic thyroid tissues [40], so it might be a useful biomarker and promising target in the diagnosis of papillary thyroid carcinoma. The findings of Jiang et al. [41] and Zhang et al. [42] indicated that miR-15a and miR-21-5p may exert tumor-suppressive effects on PTC. On the other hand, Jianugo W. et al. demonstrated that the expression levels of these miRNAs were increased in PTC compared with those noted in normal tissues [43]. Further, Wu L. et al. confirmed that miR-21-5p was secreted by hypoxic thyroid cancer cells and transferred to endothelial cells and was upregulated in the sera of patients with PTC [44]. In our study, although miR-21-5p levels were not significantly higher in patients with HT or with nodular goiter and were lower in patients with GD compared to healthy children, in the future, it would be worthwhile to assess serum miR-21-5p levels in children with thyroid cancer.
The miRNAs can be differentially expressed in AITD patients in thyroid tissue, the orbit and/or serum or plasma. The ideal study would evaluate miRNAs in different tissues in the same patient. There are no studies in the literature evaluating serum miRNAs in children and adolescents with autoimmune thyroid diseases and nodular goiter. Despite the rather small number of study groups, we view these results as a prelude to further research. In the future, we plan to expand the size of the study group and add a group of thyroid cancer patients. To sum up, the analysis of different miRNAs in clinical practice could allow us to differentiate thyroid diseases, asses the risk of developing thyroid cancer and use appropriate treatments. These miRNAs can be useful in monitoring nodules in thyroid glands in patients with AITDs. The assessment of the serum levels of miRNAs may be a practical noninvasive method for the follow-up of patients’ therapy including thyroidectomy, but it still requires a lot of thorough research to improve our knowledge on the subject.
5. Conclusions
This study reveals the following conclusions:
The overexpression of the miR-142-5p molecule occurs in children and adolescents with thyroid diseases.
Decreased blood levels of miR-15a-5p predispose patients to the formation of focal lesions in the thyroid gland.
Identifying a lower expression of the miR-126-3p molecule in the blood of children with GD requires careful follow-up for the development of focal lesions in the thyroid gland and evaluation for their potential malignancy.
Author Contributions
Conceptualization, B.S. and A.B.; methodology, B.S., A.S., A.K.-P., M.D., H.B.-S., J.N., B.M. and A.B.; software, B.S., F.B. and A.B.; validation, F.B. and A.B.; formal analysis, B.S. and A.B.; investigation, B.S. and A.B.; resources, B.S.; data curation, B.S.; writing—original draft preparation, B.S.; writing—review and editing, B.S. and A.S.; visualization, B.S.; supervision, B.S. and A.B.; project administration, B.S.; funding acquisition, B.S. and A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The search on humans was approved by Ethics Committee at Medical University in Bialystok, Poland. Number of approval code—APK.002.483.2020 (date 28 January 2021) and code—APK.002.102.2023 (date 16 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Medical University of Bialystok (SUB/1/NN/21/001/1142).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Weetman A.P. An update on the pathogenesis of Hashimoto’s thyroiditis. Endocrinol. Investig. 2021;44:883–890. doi: 10.1007/s40618-020-01477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keefe G., Culbreath K., Cherella C., Smith J.R., Zendejas B., Shamberger R.C., Richman D.M., Hollowell M.L., Modi B.P., Wassner A.J. Autoimmune Thyroiditis and Risk of Malignacy in Children with Thyroid Nodules. Thyroid. 2022;32:1109–1117. doi: 10.1089/thy.2022.0241. [DOI] [PubMed] [Google Scholar]
- 3.Borysewicz-Sańczyk H., Pasławska M., Zdrodowska M., Sawicka B., Pomaski J., Handkiewicz-Junak D., Krajewska J., Czarniecka A., Jarząb B., Dzięcioł J., et al. Childhood thyroid carcinoma- single- centre experience. Endokrynol. Pol. 2021;72:676–677. doi: 10.5603/EP.a2021.0086. [DOI] [PubMed] [Google Scholar]
- 4.Borysewicz-Sańczyk H., Sawicka B., Bossowski F., Dzięcioł J., Bossowski A. Elastographic Evaluation of Thyrodi Nodules in Children and Adolescents with Hashimoto’s Thyroiditis and Nodular Goiter with Reference to Cytological and/or Histopathological Diagnosis. J. Clin. Med. 2022;11:6339. doi: 10.3390/jcm11216339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borysewicz-Sańczyk H., Sawicka B., Karny A., Bossowski F., Marcinkiewicz K., Rusak A., Dzięcioł J., Bossowski A. Suspected Malignant Thyroid Nodules in Children and Adolescents According to Ultrasound Elastography and Ultrasound-Based Risk Stratification Systems-Experience from One Center. J. Clin. Med. 2022;11:1768. doi: 10.3390/jcm11071768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Martino M., Esposito F., Capone M., Pallante P., Fusco A. Noncoding RNAs in Thyroid- Follicular-Cell-Derived Carcinomas. Cancers. 2022;14:3079. doi: 10.3390/cancers14133079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Z.Q. MicroRNA targets and biomarker validation for diabetes-associated cardiac fibrosis. Pharmacol. Res. 2021;174:105941. doi: 10.1016/j.phrs.2021.105941. [DOI] [PubMed] [Google Scholar]
- 8.Liu L., Yao H., Zhou X., Chen J., Chen G., Shi X., Wu G., Zhou G., He S. MiR-15a-3p regulates ferroptosis via targeting glutathione peroxidase GPX4 in colorectal cancer. Mol. Carcinog. 2022;61:301–310. doi: 10.1002/mc.23367. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Li D., Yang Y., Wang J. MiR-15a-5p regulates Liver Cancer Cell Migration, Apoptosis and Cell Cycle Progression by Targeting Transcription Factor E2F3. Crit. Rev. Eukaryot. Gene Expr. 2022;32:1–10. doi: 10.1615/CritRevEukaryotGeneExpr.2022042503. [DOI] [PubMed] [Google Scholar]
- 10.Xu P., Wang Y., Deng Z., Tan Z., Pei X. MicroRNA-15a promotes prostate cancer cell ferroptosis by inhibiting GPX4 expression. Oncol. Lett. 2022;23:67. doi: 10.3892/ol.2022.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutter K., Rulicke T., Szabo T.G., Andersen L., Villunger A., Herzog S. The miR-15a/16-1 and miR-15b/16-2 clusters regulate early B cell development by limiting Il-7 receptor expression. Front. Immunol. 2022;13:967914. doi: 10.3389/fimmu.2022.967914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Cheng C., Luo X., Xia Q., Zhang Y., Long X., Jiang Q., Fang W. Retraction Note: CDK4 and miR-15a comprise an abnormal automodulatory feedback loop stimulating the pathogenesis and inducing chemotherapy resistance in nasopharyngeal carcinoma. BMC Cancer. 2021;21:273. doi: 10.1186/s12885-021-08000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchana N., DiVincenzo M.J., Regan K., Abrams Z., Zhang X., Jacob N.K., Gru A.A., Fadda P., Markowitz J., Howard J.H., et al. Alterations in patient plasma microRNA expression profiles following resection of metastatic melanoma. Surg. Oncol. 2018;118:501–509. doi: 10.1002/jso.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao J., Wang Z., Cheng Y., Ma C., Zhong Y., Xiao Y., Gao X., Li Z. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. Transl. Med. 2021;19:99. doi: 10.1186/s12967-021-02766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borsos B.N., Pahi Z.G., Ujfalundi Z., Sukosd F., Nikoleny A., Banko S., Pankotai-Bodo G., Olah-Nemeth O., Pankotai T. BC-miR: Monitoring Breast Cancer-related miRNA Profile in Blood Sera-A Prosperous Approach for Tumor Detection. Cells. 2022;11:2721. doi: 10.3390/cells11172721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Halima M., Oberhoffer F.S., Wagner V., Abd El Rahman M., Jung A.M., Zemlin M., Rohrer T.R., Meese E., Abdul-Khaliq H. MicroRNA-126-3p/5p and Aortic Stiffness in Patients with Turner Syndrome. Children. 2022;9:1109. doi: 10.3390/children9081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y., Kotian S., Zeiger M.A., Zhang L., Kebebew E. MiR-126-3p Inhibits Thyroid Cancer Cell Growth and Metastasis, and Is Associated with Aggressive Thyroid Cancer. PLoS ONE. 2015;10:e0130496. doi: 10.1371/journal.pone.0130496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Ma G., Xing E., Xu M., Song X., Zhang Y. Expression and diagnostic value of miR-142-5p and miR-155-5p in the serum of children with allergic rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2023;165:111425. doi: 10.1016/j.ijporl.2022.111425. [DOI] [PubMed] [Google Scholar]
- 19.Bayomy N.R., Alfottoh W.M.A., Eldeep S.A.A., Mersal A.M.S.I.M., El-Bary H.M.A.A., El Gayed E.M.A. Mir-142-5p as an indicator of autoimmune processes in childhood idiopathic nephrotic syndrome and as a part of MicroRNAs expression panels for its diagnosis and prediction of response to steroid treatment. Mol. Immunol. 2022;141:21–32. doi: 10.1016/j.molimm.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Zheng A. MiR-142-5p promotes retinoblastoma cell proliferation, migration and invasion by targeting PTEN. J. Biochem. 2021;170:195–202. doi: 10.1093/jb/mvaa121. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho T.M., Brasil G.O., Jucoski T.S., Adamoski D., Silveira de Lima R., Spautz C.C., Anselmi K.F., Ozawa P.M., Cavalli I.J., De Oliveira J.C., et al. MicroRNAs miR-142-5p, miR-150-5p, miR-320a-3p, and miR-4433b-5p in Serum and Tissue: Potential Biomarkers in Sporadic Breast Cancer. Front. Genet. 2022;13:865472. doi: 10.3389/fgene.2022.865472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahanbani I., Al-Abdallah A., Ali R.H., Al-Brahim N., Mojiminiyi O. Discriminatory miRNAs for the Management of Papillary Thyroid Carcinoma and Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features. Thyroid. 2018;28:319–327. doi: 10.1089/thy.2017.0127. [DOI] [PubMed] [Google Scholar]
- 23.Eldosoky M.A., Hammad R., Elmadbouly A.A., Aglan R.B., AbdelHamid S.G., Alboraie M., Hassan D.A., Shaheen M.A., Rushdi A., Ahmed R.M., et al. Diagnostic Significance of hsa-miR-21-5p, hsa-miR-192-5p, hsa-miR-155-5p, hsa-miR-199a-5p Panel and Ratios in Hepatocellular Carcinoma on Top of Liver Cirrhosis in HCV-Infected Patients. Int. J. Mol. Sci. 2023;24:3157. doi: 10.3390/ijms24043157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Jin J., Liang Y., Zhang Y., Wu N., Fan M., Zeng F., Deng F. MiR-21-5p/PRKCE axis implicated in immune infiltration and poor prognosis of kidney renal clear cell carcinoma. Front. Genet. 2022;13:978840. doi: 10.3389/fgene.2022.978840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultana A., Alam S., Liu X., Sharma R., Singla R.K., Gundamaraju R., Shen B. Single-cell RNA-seq analysis to identify potential biomarkers for diagnosis, and prognosis of non-small cell lung cancer by using comprehensive bioinformatics approaches. Transl. Oncol. 2023;27:101571. doi: 10.1016/j.tranon.2022.101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Constantin A., Comarița I.K., Alexandru N., Filippi A., Bojin F., Gherghiceanu M., Vîlcu A., Nemecz M., Niculescu L.S., Păunescu V., et al. Stem cell-derived extracellular vesicles reduce the expression of molecules involved in cardiac hypertrophy-In a model of human-induced pluripotent stem cell-derived cardiomyocytes. Front. Pharmacol. 2022;13:1003684. doi: 10.3389/fphar.2022.1003684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trummer O., Foessl I., Schweighofer N., Arifi E., Haudum C.W., Reintar S., Pilz S., Theiler-Schwetz V., Trummer C., Zirlik A., et al. Expression Profiles of miR-22-5p and miR-142-3p Indicate Hashimoto’s Disease and Are related to Thyroid Antibodies. Genes. 2022;13:171. doi: 10.3390/genes13020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saberinia A., Alinezhad A., Jafari F., Soltany S., Sigari R.A. Oncogenic miRNAs and target therapies in colorectal cancer. Clin. Chim. Acta. 2020;508:77–91. doi: 10.1016/j.cca.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.-S., Park H. Machine learning on thyroid disease: A review. Front. Biosci. 2022;27:101. doi: 10.31083/j.fbl2703101. [DOI] [PubMed] [Google Scholar]
- 30.Hamidi A.A., Taghehchian N., Basirat Z., Zangouei A.S., Moghbeli M. MicroRNAs as the critical regulators of cell migration and invasion in thyroid cancer. Biomark. Res. 2022;10:40. doi: 10.1186/s40364-022-00382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aversa T., Corrias A., Salerno M., Tessaris D., Di Mase R., Valenzise M., Corica D., De Luca F., Wasniewska M. Five-Year Prospective Evaluation of Thyroid Function Test Evolution in Children with Hashimoto’s Thyroiditis Presenting with Either Euthyroidism or Subclinical Hypothyroidism. Thyroid. 2016;26:1450–1456. doi: 10.1089/thy.2016.0080. [DOI] [PubMed] [Google Scholar]
- 32.Wasniewska M., Aversa T., Salerno M., Corrias A., Messina M.F., Mussa A., Capalbo D., De Luca F., Valenzise M. Five-year prospective evaluation of thyroid function in girls with subclinical mild hypothyroidism of different etiology. Eur. J. Endocrinol. 2015;173:801–808. doi: 10.1530/EJE-15-0484. [DOI] [PubMed] [Google Scholar]
- 33.Lydersen S., Fagerland M.W., Laake P. Recommended tests for association in 2 × 2 tables. Statist. Med. 2009;28:1159–1175. doi: 10.1002/sim.3531. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 35.Lewontin R.C. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49:49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [(accessed on 15 March 2023)]. R version 4.2.3. Available online: http://www.R.-project.org/ [Google Scholar]
- 37.Zhu J., Zhang Y., Zhang W., Zhang W., Fan L., Wang L., Liu Y., Liu S., Guo Y., Wang Y., et al. MicroRNA-142-5p contributes to Hashimoto’s thyroiditis by targeting CLDN1. J. Transl. Med. 2016;14:166. doi: 10.1186/s12967-016-0917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao X., Wang Y., Wang L., Cao M., Chen A., Zhang X. Expression patterns of serum MicroRNAs related to endothelial dysfunction in patietns with subclinical hypothyroidism. Front. Endocrinol. 2022;13:981622. doi: 10.3389/fendo.2022.981622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin J., Zhang J., Xue Y., Luo L., Wang S., Tian H. MiRNA-15a regulates the proliferation and apoptosis of papillary thyroid carcinoma via regulating AKT pathway. OncoTargets Ther. 2019;12:6217–6226. doi: 10.2147/OTT.S213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J., Li C., Liu C., Zhao S., Wang Y., Fu Z. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the clinical characteristics of PTC. Cancer Biomark. 2017;18:87–94. doi: 10.3233/CBM-161723. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L., Wu Z., Meng X., Chu X., Huang H., Xu C. LncRNA HOXA-AS2 facilitates tumorigenesis and progression of papillary thyroid cancer by modulating the miR-15a-5p/HOXA3 axis. Hum. Gene Ther. 2019;30:618–631. doi: 10.1089/hum.2018.109. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., Cai Y., Zheng L., Zhang Z., Lin X., Jiang N. LncRNA BISPR promotes the progression of thyroid papillary carcinoma by regulating miR-21-5p. Int. J. Immunopathol. Pharmacol. 2018;32:2058738418772652. doi: 10.1177/2058738418772652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Wu L., Jin Y., Li S., Liu X. Identification of key miRNAs in papillary thyroid carcinoma based on data mining and bioinformatics methods. Biomed. Rep. 2020;12:11–16. doi: 10.3892/br.2019.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu F., Li F., Lin X., Xu F., Cui R.R., Zhong J.Y., Zhu T., Shan S.K., Liao X.B., Yuan L.Q., et al. Exsomes increased angiogensesis in papillary thyroid cancer microenvironment. Endocr. Relat. Cancer. 2019;26:525–538. doi: 10.1530/ERC-19-0008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.