Abstract
Recombinant adenoviruses (Ad) have become the vector system of choice for a variety of gene therapy applications. However, the utility of Ad vectors is limited due to the low efficiency of Ad-mediated gene transfer to cells expressing marginal levels of the coxsackievirus and adenovirus receptor (CAR). In order to achieve CAR-independent gene transfer by Ad vectors in clinically important contexts, we proposed modification of viral tropism via genetic alterations to the viral fiber protein. We have shown that incorporation of an Arg-Gly-Asp (RGD)-containing peptide in the HI loop of the fiber knob domain results in the ability of the virus to utilize an alternative receptor during the cell entry process. We have also demonstrated that due to its expanded tissue tropism, this novel vector is capable of efficient transduction of primary tumor cells. An increase in gene transfer to ovarian cancer cells of 2 to 3 orders of magnitude was demonstrated by the vector, suggesting that recombinant Ad containing fibers with an incorporated RGD peptide may be of great utility for treatment of neoplasms characterized by deficiency of the primary Ad type 5 receptor.
Adenovirus (Ad) vectors are useful in a wide variety of gene therapy applications. One of the principal attributes recommending the employment of these vectors is their unparalleled efficacy in accomplishing gene transfer in vivo. This property has been noted in a variety of different organs.
There are, however, some limitations associated with the use of recombinant Ad for gene therapy. One such disadvantage is related to the reliance of the virus on the presence of the coxsackievirus and adenovirus receptor (CAR) to achieve high levels of gene transfer. In certain settings, this may result in sequestration of recombinant virions by nontarget, yet high-CAR-expressing cells, whereas the true target cells, if low in CAR, are poorly transduced. In order to compensate for this sequestration, significant escalation in the dose of administered vector is needed, which increases the risk of inducing both direct toxicity and immune responses against the vector, thus further compromising the overall efficacy of the therapy. Therefore, the utility of the present generation of Ad vectors for gene therapy may be significantly improved by achieving targeted transduction of specific cell types by the virus.
In this regard, the initial steps of Ad infection involve at least two sequential virus-cell interactions, each being mediated by a specific protein component of Ad capsid. The primary binding of the virus to the cell surface receptor, CAR (9, 10, 38), is mediated by the knob domain of the fiber protein (23). This is followed by the internalization of the virus within a clathrin-coated endosome (39). The virus then escapes from the endosome by triggering its acidification via a secondary interaction of the argininine-glycine-aspartic acid (RGD) motif of Ad penton base protein with cellular integrins αvβ3 and αvβ5 (4, 5, 41, 42). Following the endosome escape, partially dismantled virus translocates to the nuclear pore complex and releases its genome into the nucleoplasm where subsequent steps of viral replication take place.
As the fiber and the penton base are key mediators of the cell entry mechanism developed by Ad, targeting of recombinant Ad vectors may be achieved via genetic modifications of these capsid proteins. In order to overcome the limitations imposed by the CAR dependence of Ad infection, Michael et al. (27) proposed the incorporation of small peptide motifs possessing receptor binding specificities into the carboxy terminus of Ad fiber protein, thus enabling the virus to attach and infect via a novel cell surface receptor. This concept has been further developed by Wickham et al. (43, 44), who have proved the feasibility of this approach by generating several recombinant Ad containing fibers with targeting ligands positioned at the carboxy terminus of the fiber molecule.
Although in some cases genetic modification of the carboxy terminus of Ad fiber has proved its utility with respect to vector retargeting, it failed in others (44), thereby suggesting that this locale in the fiber molecule is not the optimal site for incorporation of targeting protein moieties. In this regard, published findings (15, 44) strongly suggest that the addition of more than 25 to 30 amino acid residues of heterologous protein sequence to the carboxy terminus of the fiber molecule has dramatic negative effect on the stability of the fiber trimer and, therefore, is incompatible with the fiber functions. In addition, the three-dimensional structure of the fiber knob (45) clearly indicates that the carboxy terminus of the fiber points toward the virion, that is, away from the cell surface, thereby providing a suboptimal environment for the incorporation of targeting ligands.
With these findings in mind, we recently reported that another locale within the fiber molecule, the HI loop of the fiber knob domain, could be used as a convenient site for incorporation of heterologous ligands (21). As the next logical step, we explored the utility of the HI loop for incorporation of targeting ligands to allow modification of Ad tropism. Specifically, we sought to capitalize on the recently published reports on phage biopanning (3, 31) by choosing an RGD motif proven to have in vivo targeting capabilities. We have shown that incorporation of this peptide into the fiber knob allowed the virus to utilize the RGD-integrin interactions as an alternative infection pathway, thereby dramatically improving the ability of the virus to transduce several types of cells, which normally are refractory to Ad infection. In order to show the utility of the modified virion for application which may have immediate clinical translation, we employed this viral vector as a means for efficient gene transfer to primary ovarian cancer cells. Specifically, we have shown that recombinant Ad vector containing fibers with RGD motif in the HI loop is capable of dramatically augmenting gene delivery to target cells via a CAR-independent cell entry mechanism.
MATERIALS AND METHODS
Cells and tissues.
The 293 human kidney cell line transformed with Ad type 5 (Ad5) DNA was purchased from Microbix (Toronto, Ontario, Canada). Human ovarian carcinoma cell lines SKOV3.ip1 and OV-4 were obtained from Janet Price (M. D. Anderson Cancer Center, Houston, Tex.) and Timothy J. Eberlein (Brigham and Women’s Hospital, Harvard Medical School, Boston, Mass.), respectively. Human umbilical vein endothelial cells (HUVEC) and human embryonal rhabdomyosarcoma (RD) cells were from American Type Culture Collection (Rockville, Md.). All cell lines were grown at 37°C in media recommended by the suppliers in a humidified atmosphere of 5% CO2.
Ascitic fluid samples from patients with epithelial ovarian carcinoma were obtained at the Hospital of the University of Alabama at Birmingham (UAB), Division of Gynecologic Oncology. All samples were classified by pathologists at UAB Hospital, Department of Pathology. The samples were processed immediately once received or stored at −70°C until needed.
Enzymes.
Restriction endonucleases, Klenow enzyme, T4 DNA ligase, T4 polynucleotide kinase, and proteinase K were from either New England Biolabs (Beverly, Mass.) or Boehringer Mannheim (Indianapolis, Ind.).
Monoclonal antibodies.
Anti-αvβ3 integrin monoclonal antibody LM609 and anti-αvβ5 integrin monoclonal antibody P1F6 were purchased from Chemicon International, Inc. (Temecula, Calif.) and Gibco-BRL (Gaithersburg, Md.), respectively. Anti-CAR monoclonal antibody RmcB (9) was obtained from R. W. Finberg (Dana-Farber Cancer Institute, Harvard Medical School, Boston, Mass.).
Viruses.
A recombinant Ad5 vector, AdCMVLuc, containing a firefly luciferase-expressing cassette in place of the E1 region of the Ad genome, was obtained from R. D. Gerard (University of Texas Southwestern Medical Center, Dallas). Ad vector, Ad5lucRGD, containing recombinant fiber-RGD protein and expressing the firefly luciferase was generated by transfection of 293 cells with PacI-digested pVK703 by the method described previously (12). Ad were propagated on 293 cells and purified by centrifugation in CsCl gradients by a standard protocol. Determination of virus particle titer was accomplished spectrophotometrically by the method of Maizel et al. (24), using a conversion factor of 1.1 × 1012 viral particles per absorbance unit at 260 nm. To determine the titer of infectious viral particles, the plaque assay on 293 cells was performed by the method of Mittereder et al. (28) was used.
Construction of recombinant plasmids.
In order to generate a recombinant Ad5 fiber gene encoding the fiber with the RGD-4C peptide within the HI loop of the knob domain, a duplex made of oligonucleotides CAC ACT AAA CGG TAC ACA GGA AAC AGG AGA CAC AAC TTG TGA CTG CCG CGG AGA CTG TTT CTG CCC and GGG CAG AAA CAG TCT CCG CGG CAG TCA CAA GTT GTG TCT CCT GTT TCC TGT GTA CCG TTT AGT GTG was cloned into the EcoRV site of previously designed plasmid pQE.KNOBΔHI (21), thereby generating pQE.KNOB.RGDHI.
To make a shuttle vector suitable for the generation of the viral genome of interest, a BstXI-MunI fragment of the modified fiber gene containing RGD-4C coding sequence was subcloned from pQE.KNOB.RGDHI into the fiber shuttle vector pNEB.PK3.6 (22) cleaved with BstXI and MunI. In order to obtain Ad5 genome containing the fiber-RGD gene, the resultant plasmid, pNEB.PK.FHIRGD, was then utilized for homologous DNA recombination with SwaI-digested pVK50 (21) in Escherichia coli BJ5183 as previously described (12). The plasmid obtained as a result of this recombination was designated pVK503.
Firefly luciferase gene was excised from plasmid pGEMR-luc (Promega, Madison, Wis.) as a 1.7-kb BamHI-XhoI fragment and cloned into BamHI-XhoI-digested pcDNA3 (Invitrogen, Carlsbad, Calif.), resulting in pcDNA.Luc. To destroy PacI and ClaI sites in the luciferase open reading frame, a synthetic duplex consisting of oligonucleotides CAA ATA CAA AGG ATA TCA GGT GGC CCC CGC TGA ATT GGA GT and CGA CTC CAA TTC AGC GGG GGC CAC CTG ATA TCC TTT GTA TTT GAT was used to replace the 41-bp PacI-ClaI fragment in pcDNA.Luc, thereby generating pcLucPC1.
In order to make a shuttle vector containing this modified luciferase gene in the context of expression cassette, the gene was cloned in pACCMVpLpA (8) as follows. Plasmid pcLucPC1 was cleaved with BamHI, treated with Klenow enzyme to fill in the ends, and then cut with XhoI. The cloning vector, pACCMVpLpA, was cut with EcoRI, treated with Klenow enzyme, and then cleaved with SalI. The ligation of these two DNA molecules resulted in pACCMV.LucΔPC. This plasmid was then used for homologous DNA recombination with ClaI-linearized pVK503 in order to generate pVK703, containing the genome of Ad5lucRGD.
To derive a recombinant baculovirus expressing fiber-RGD, previously made transfer vector pFB.F5HIFLAG (21) was modified in the following way. First, EcoRI linker, CGG CGA ATT CGC, was incorporated into the ClaI site of pFB.F5HIFLAG, resulting in pFB.F5.RI. Then, the NcoI-MunI fragment of pNEB.PK.FHIRGD containing the 3′ portion of the fiber-RGD gene was used to replace an NcoI-MunI fragment in pFB.F5.RI, generating pFB.F5HIRGD. This plasmid was then used to generate recombinant baculovirus genome via site-specific transposition by utilizing a Bac-to-Bac kit (Gibco-BRL) according to the manufacturer’s recommendations.
Flow cytometry.
Cells grown in T75 flasks were released from the flasks by the addition of EDTA and resuspended in SM buffer (HEPES-buffered saline, 0.1% sodium azide, 1% bovine serum albumin [BSA]) at 2 × 106 cells/ml. Two hundred thousand cells were incubated with 5 μg of LM609, P1F6, RmcB, or no primary monoclonal antibody (negative control) per ml in 200 μl of SM for 2 h at 4°C. Cells were then washed with SM and incubated with secondary fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G serum (Jackson Labs, West Grove, Pa.) (5 μg/ml) for 1 h at 4°C. After the cells were washed with SM, 104 cells per sample were analyzed by flow cytometry at the UAB FACS Core Facility.
Recombinant proteins.
Recombinant Ad5 fiber knob protein was expressed in E. coli and purified by immobilized metal ion affinity chromatography (IMAC) on Ni-nitrilotriacetic acid (NTA)–Sepharose (Qiagen, Valencia, Calif.) as recommended by the manufacturer.
Human Ad2 penton base protein was expressed in Spodoptera frugiperda Sf9 cells by recombinant baculovirus AcNPV-PbWT (18) provided by P. Boulanger (Institute of Biology, Montpellier, France). The penton base protein was purified from baculovirus-infected cells by two-step ion-exchange chromatography utilizing a DEAE-Sepharose FF column (Pharmacia, Piscataway, N.J.) followed by purification on POROS HQ column (PerSeptive Biosystems, Framingham, Mass.).
Recombinant fiber proteins expressed in baculovirus-infected Sf9 cells were purified by chromatography on Ni-NTA-Sepharose as previously described (21).
The protein concentrations were determined by the Bradford protein assay (Bio-Rad, Hercules, Calif.) with bovine gamma globulin as the standard.
ELISA.
Solid-phase binding assay was performed by a method previously described by Sharma et al. (36). Briefly, purified fiber proteins or Ad virions were diluted in 50 mM carbonate-bicarbonate buffer, pH 9.6, to a concentration of 10 μg of protein per ml, and 100-μl aliquots were added to the wells of a 96-well Nunc-Maxisorp enzyme-linked immunosorbent assay (ELISA) plate. Plates were incubated overnight at 4°C and then blocked for 2 h at room temperature by the addition of 200 μl of blocking buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% casein) to each well. Wells were then washed three times with the washing buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl). Purified integrin αvβ3 (Chemicon) diluted in binding buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 0.5% casein) to concentrations ranging from 0.04 to 0.5 μg/ml was added to the wells in 100-μl aliquots. After overnight incubation at 4°C, the wells were washed three times with washing buffer containing 2 mM CaCl2, 1 mM MgCl2, and 1 mM MnCl2. Bound integrin was detected with mouse monoclonal anti-human integrin αv subunit antibody VNR139 (Gibco-BRL). VNR139 antibody diluted 1:3,000 in binding buffer was added to the wells in 100-μl aliquots, incubation was continued for 1 h at room temperature, and then the wells were washed again. The ELISA plate was then developed with VECTASTAIN kit (Vector Laboratories, Burlingame, Calif.) as recommended by the manufacturer. Color development was stopped by the addition of 1 N H2SO4, and plates were read in a microtiter plate reader set at 490 nm.
Ad-mediated gene transfer assay.
Ad-mediated transduction experiments utilizing cell lines were performed as described previously (22).
Primary cells from ascitic fluid samples obtained from ovarian cancer patients were prepared for this analysis as follows. First, the erythrocytes present in the samples were lysed by the addition of buffer containing 150 mM NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA. Then, the cell debris and dead cells were separated from the live cells by slow-speed centrifugation on a step gradient of Ficoll-Hypaque (Media Preparation Shared Facility, UAB Comprehensive Cancer Center, Birmingham, Ala.). The cells were washed twice with Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) (Cellgro, Herndon, Va.) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, Utah), 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Ad binding assay.
Binding of 125I-labeled Ad to 293, HUVEC, or RD cells was assayed in a procedure described previously (21).
RESULTS
Fiber-RGD protein efficiently interacts with integrins via the RGD tripeptide. Recently we demonstrated that the FLAG octapeptide incorporated in the HI loop of Ad5 fiber does not interfere with correct folding of the cell-binding site localized in the knob and is available for binding to FLAG-specific antibody in immunoprecipitation assay (21). To utilize these findings for the purposes of Ad retargeting, we chose to introduce in the HI loop of the fiber knob an RGD-4C peptide, CDCGRDCFC, which is known to bind with high affinities to several types of integrins present on the surfaces of mammalian cells. This effort was undertaken in an attempt to generate an Ad vector, which would be able to bind to cells by utilizing fiber-RGD–integrin interaction. Therefore, the infection by such virus would not be dependent on the presence of CAR on a cell membrane.
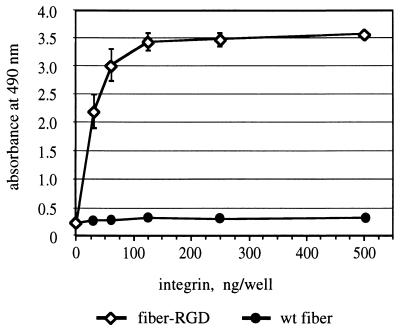
We first chose to express an RGD-4C-containing fiber protein, fiber-RGD, in a baculovirus expression system in order to characterize the protein with respect to its ability to perform the targeting functions. The sequence encoding the amino-terminal six-His tag was incorporated in the fiber-RGD gene in order to facilitate downstream purification of the product. As anticipated from our previous experiments done with fiber-FLAG protein, electrophoresis of IMAC-purified fiber-RGD protein showed that the fiber retains its native trimeric structure (data not shown), which is known to be crucial for association of the fiber with the penton base during virion assembly. In order to assess the ability of the fiber-RGD protein to bind to integrins, we employed this fiber protein for an ELISA utilizing purified integrin αvβ3. This assay showed that, in contrast to the wild-type fiber protein used as a negative control, the fiber-RGD protein binds αvβ3 integrin very efficiently (Fig. 1). Therefore, these experiments confirmed the functional utility of the modified fiber and provided a rationale for generation of recombinant Ad containing such fibers.
FIG. 1.
Analysis of interaction between recombinant fiber proteins and αvβ3 integrin. Baculovirus-expressed fiber proteins absorbed on an ELISA plate were incubated with various concentrations of purified integrin αvβ3. Integrin bound to fiber proteins was then detected with anti-α subunit monoclonal antibody VNR139. Each point represents a mean of three readings obtained in one experiment. Some error bars depicting standard deviations are smaller than the symbols. wt, wild type.
The virus was derived by the method described by Chartier et al. (12). To simplify the downstream gene transfer assays, an expression cassette containing the firefly luciferase gene driven by cytomegalovirus promoter was introduced in place of the E1 region of the Ad genome. The genome of the new virus designated Ad5lucRGD was generated in E. coli via a two-step protocol utilizing homologous DNA recombination between the previously designed plasmid pVK50 (21) and fragments of DNA isolated from two shuttle vectors, pNEB.PK.FHIRGD and pACCMV.LucΔPC, which contain the fiber gene and the luciferase expression cassette flanked by Ad DNA sequences, respectively. Utilization of this method requires the digestion of the resultant recombinant plasmid containing the newly generated Ad genome with restriction endonuclease PacI to release inverted terminal repeats of Ad5 DNA from the plasmid backbone. In order to be able to use the firefly luciferase gene, which contains an internal PacI site, in the context of this method, we eliminated this site by introducing a silent mutation into the gene. The plasmid obtained as a result of aforementioned DNA recombinations, pVK703, was then utilized for transfection of 293 cells to rescue Ad5lucRGD. The identity of the virus was confirmed by PCR as well as by cycle sequencing of viral DNA isolated from CsCl-purified virions of Ad5lucRGD.
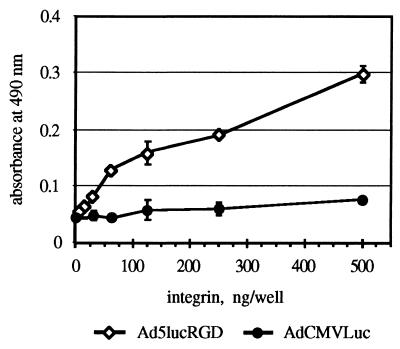
To demonstrate the accessibility of the RGD tripeptide incorporated in the fiber of Ad5lucRGD, we utilized this virus in an ELISA analogous to the one used previously for purified fiber protein. This analysis clearly showed efficient binding of the αvβ3 integrin to immobilized particles of Ad5lucRGD, while binding of αvβ3 to a control virus was at the background level at all concentrations of integrin used (Fig. 2). Based on these results, we hypothesized that Ad5lucRGD is able to interact in vitro and in vivo with various types of RGD-binding integrins, thereby utilizing this interaction at early steps of infection in order to attach to target cells.
FIG. 2.
ELISA of αvβ3 integrin binding to immobilized AdCMVLuc and Ad5lucRGD virions. CsCl-purified virions of AdCMVLuc and Ad5lucRGD immobilized in the wells of an ELISA plate were incubated with affinity-purified αvβ3 integrin, followed by incubation with monoclonal antibody VNR139. Data shown are means ± standard deviations from an experiment performed in triplicate.
Ad5lucRGD is capable of mediating a CAR-independent gene delivery.
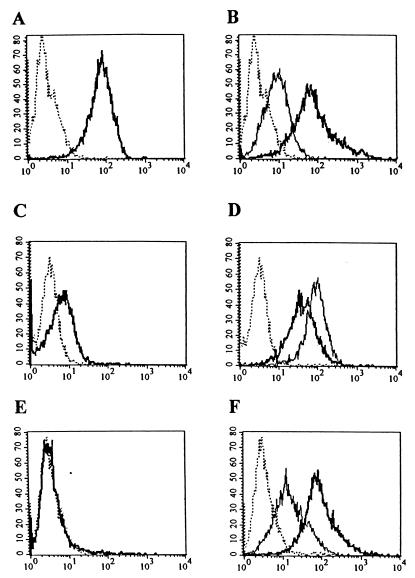
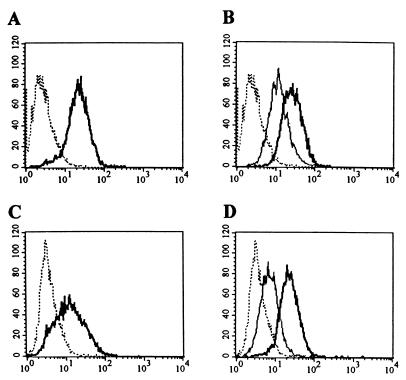
Our next goal was to examine whether introduction of the RGD motif in the fiber of Ad5lucRGD resulted in any changes with respect to the ability of this virus to infect cells. In order to investigate the infection pathway utilized by Ad5lucRGD, we sought to employ this virus for gene transfer to several cell lines, expressing various levels of CAR as well as integrins αvβ3 and αvβ5. To achieve this goal, a panel of the cell lines, including the 293 human kidney cells, human umbilical cord endothelial cells, HUVECs, and human embryonal RD cells, was employed for a series of flow cytometry assays. While 293 cells readily support Ad infection, HUVECs have been shown to bind Ad poorly (43), whereas CAR expression in RD cells was reported to be passage dependent (35). Our flow cytometry assay showed that 293 cells express high levels of CAR (Fig. 3A) and αvβ5 integrin, while expression of αvβ3 is moderate (Fig. 3B). HUVECs demonstrated moderate levels of CAR expression (Fig. 3C), whereas both integrins were present at the cell surface in rather large amounts (Fig. 3D). RD cells were CAR negative (Fig. 3E), while being high αvβ5 and moderate αvβ3 expressors (Fig. 3F). Therefore, for our subsequent gene transfer experiments, we established a set of cell lines covering a full range of CAR expression profiles and with moderate to high levels of integrins αvβ3 and αvβ5 present on their cytoplasmic membranes.
FIG. 3.
Flow cytometric analysis of CAR and integrin expression in 293, HUVEC, and RD cells. Cells were incubated with anti-CAR (RmcB), anti-αvβ3 (LM609), or anti-αvβ5 (P1F6) integrin monoclonal antibodies, washed with SM to remove unbound monoclonal antibodies, and incubated with secondary FITC-labeled goat anti-mouse immunoglobulin G serum as described in Materials and Methods. After removal of the FITC-labeled antibodies, aliquots of 104 cells were analyzed by flow cytometry. Expression of CAR in 293 (A), HUVEC (C), and RD (E) cells and of αvβ3 (thin line) and αvβ5 (heavy line) integrins in 293 (B), HUVEC (D), and RD (F) cells is shown. The dotted line shows the results for the negative control.
Ad5lucRGD was then utilized for an assay based on competitive inhibition of Ad-mediated gene delivery by recombinant Ad5 fiber knob protein, known to efficiently block virus binding to CAR.
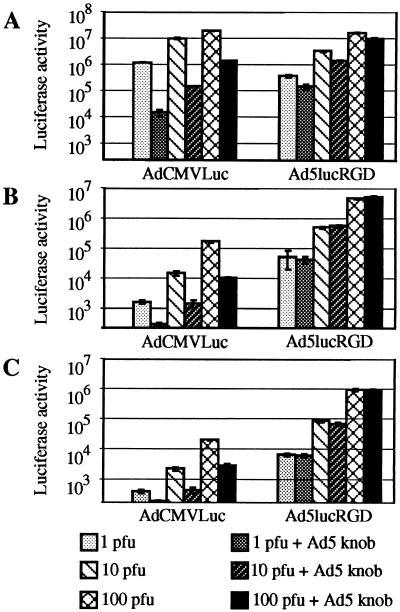
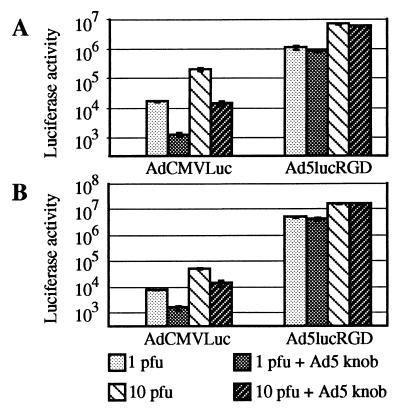
As shown in Fig. 4A, luciferase expression in 293 cells mediated by our control virus, AdCMVLuc, was efficiently blocked by recombinant knob protein. Depending on the multiplicity of infection (MOI) used, knob protein blocked 85 to 93% of luciferase activity in AdCMVLuc-transduced cells.
FIG. 4.
Ad-mediated gene transfer to various human cell lines. 293 (A), HUVEC (B), or RD (C) cells preincubated for 10 min at room temperature in either DMEM/F12 or DMEM/F12 containing recombinant Ad5 fiber knob at 100 μg/ml were then exposed for 30 min at room temperature to AdCMVLuc or Ad5lucRGD in DMEM/F12 at 1, 10, or 100 PFU/cell. The unbound virus was aspirated, and complete medium was added. After incubation at 37°C for 30 h, the cells were lysed and the luciferase activity (in relative light units) was determined. Background luciferase activities detected in mock-infected cells were 261, 223, and 163 relative light units for 293, HUVEC, and RD cells, respectively. These activities were subtracted from all readings obtained with the corresponding cell line. Each point represents the mean ± standard deviation of three determinations.
In marked contrast, the same concentration of knob was able to block only 40 to 60% of Ad5lucRGD-mediated gene expression in 293 cells, thereby indicating that in addition to well-characterized fiber-CAR interaction utilized by the wild-type Ad5, Ad5lucRGD is capable of using an alternative, CAR-independent cell entry pathway. The contribution of that alternative mechanism of cell binding was quite significant, providing 40 to 60% of overall gene transfer to 293 cells.
To further investigate the phenomenon of Ad5lucRGD-directed gene delivery, we utilized the same strategy to look into transduction of HUVECs. It has been shown that these cells are relatively difficult to transduce with Ad vectors containing wild-type fibers (43, 44). These findings were corroborated with our flow cytometry data, which showed modest levels of CAR expression in HUVECs. Importantly, rather high levels of αvβ3 and αvβ5 integrins detected in these cells suggested that HUVECs should be readily transduced with Ad5lucRGD. Although the levels of luciferase activity in HUVECs mediated by either virus were considerably lower than those in 293 cells, our experiment revealed striking differences between the transduction profiles demonstrated by these two viruses (Fig. 4B). First, luciferase expression in the Ad5lucRGD-transduced cells was about 30-fold higher than in the cells transduced with AdCMVLuc. Second, the effect of the Ad5 fiber knob on AdCMVLuc-mediated transduction was less dramatic than in our experiments with 293 cells, consistent with a relative lack of CAR in the HUVECs. Most importantly, recombinant knob protein had no inhibition effect on the levels of luciferase expression directed by Ad5lucRGD.
Very similar results were then generated on RD cells, which do not express CAR. The luciferase activity detected in the lysates of AdCMVLuc-transduced RD cells was extremely low: at an MOI of 1 PFU/cell, it was almost equal to background readings obtained in mock-infected cells (Fig. 4C). Once again, Ad5lucRGD was capable of directing the levels of transgene expression 16- to 47-fold higher than those mediated by AdCMVLuc. This expression was not responsive to inhibition by the fiber knob.
These experiments clearly showed that incorporation of the RGD-4C peptide into the fiber of Ad5lucRGD resulted in dramatic changes in the initial steps of virus-cell interaction, presumably by creating an alternative cell attachment pathway.
Ad5lucRGD demonstrates increased efficiencies of cell binding due to utilization of RGD-integrin interaction.
Having established that AdCMVLuc and Ad5lucRGD demonstrate different efficiencies of gene delivery as well as different profiles of fiber knob-mediated inhibition of transduction, our next task was to compare the cell binding profiles of these two viruses. To address this issue, we labeled both viruses with 125I and employed them in the virus binding assay on 293, HUVEC, and RD cells. This assay was performed under conditions (4°C) allowing the viruses to bind the cells, but preventing virus internalization.
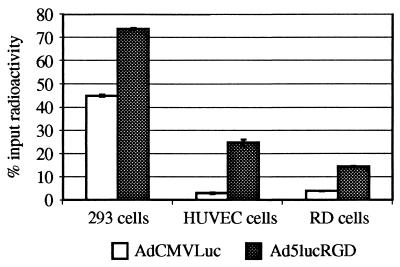
As shown in Fig. 5, binding efficiencies demonstrated by Ad5lucRGD and AdCMVLuc on CAR-positive 293 cells were similar, while the percentages of labeled Ad5lucRGD virions bound to HUVEC and RD cells were significantly higher than those of AdCMVLuc virions.
FIG. 5.
Comparison of binding of 125I-labeled adenoviruses to 293, HUVEC, or RD cells. One-hundred-microliter aliquots of cells in DMEM-Ad medium (DMEM, 20 mM HEPES, 0.5% BSA), 106 cells per aliquot, were incubated for 1 h at 4°C with 50 μl of 125I-labeled Ad (105 cpm per sample). The samples were then diluted with 4 ml of phosphate-buffered saline containing 0.1% BSA, and the cells were pelleted by centrifugation. Radioactivities of cell pellets were determined with a gamma counter. Data shown are means ± standard deviations from an experiment performed in triplicate.
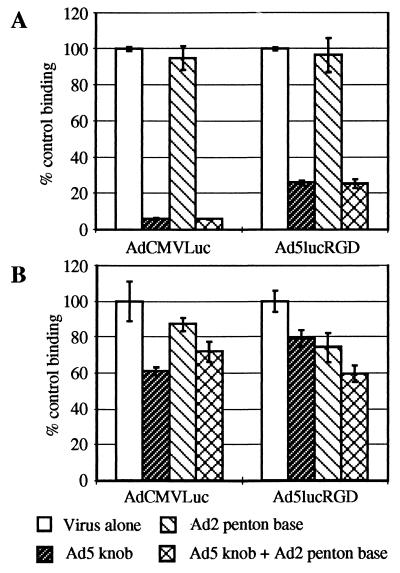
Since the ultimate goal of incorporating the RGD-containing peptide within the fiber molecule was to allow the virus to utilize cellular integrins as alternative receptors, we conducted an assay in which binding of radiolabeled viruses to the cells was accomplished in the presence of recombinant Ad2 penton base protein. Due to the presence of RGD motif in the highly mobile loop protrusion identified within its molecule (37), the penton base is able to bind αvβ3 and αvβ5 integrins and therefore competes for binding to these cellular receptors with other molecules or macromolecular complexes containing an RGD motif.
When binding of the viruses to 293 cells was assayed (Fig. 6A), the penton base protein failed to inhibit cell binding of either virus, whereas the fiber knob protein, alone as well as together with the penton base, blocked 94% of AdCMVLuc and 75% of Ad5lucRGD binding.
FIG. 6.
Inhibition of binding of labeled AdCMVLuc and Ad5lucRGD to 293 and HUVEC cells. 293 (A) or HUVEC (B) cells were preincubated with DMEM-Ad or DMEM-Ad containing either Ad5 fiber knob (100 μg/ml) or Ad2 penton base (100 μg/ml) or both for 1 h at 4°C. Fifty-microliter aliquots of 125I-labeled viruses were then added to the samples. The rest of the procedure was as described in the legend for Fig. 5.
The same experiment performed with HUVECs showed that, once again, the knob protein inhibited binding of AdCMVLuc particles to a greater extent than that of Ad5lucRGD virions (Fig. 6B). In addition, penton base was capable of decreasing Ad5lucRGD-associated radioactivity bound to these cells by 25%, while its effect on AdCMVLuc binding was marginal. When used together, both blocking agents caused 40% decrease in Ad5lucRGD binding. Similar results were obtained when these viruses were employed for binding assay on RD cells (data not shown). Although the penton base did not block binding of Ad5lucRGD to HUVECs as efficiently as the knob protein blocked binding of our control virus, its utilization as an integrin-specific inhibitor showed that Ad5lucRGD is capable of using cellular integrins as alternative receptors during the infection process.
Ad5lucRGD mediates enhanced gene transfer to human ovarian cancer cells.
Since a number of clinical trials utilizing Ad vectors to treat cancer patients via direct in vivo gene delivery are currently under way, we chose to investigate whether the expanded tropism of Ad5lucRGD would render it useful for this type of clinical application.
First, we looked into the ability of this recombinant vector to deliver genes to cultured human ovarian cancer cells. Characterization of two cell lines, SKOV3.ip1 and OV-4, by flow cytometry showed that they both express moderate to high levels of integrins αvβ3 and αvβ5 (Fig. 7B and D), SKOV3.ip1 expresses a high level of CAR (Fig. 7A), whereas OV-4 is modest CAR expressor (Fig. 7C).
FIG. 7.
Flow cytometric analysis of human ovarian cancer cells. Expression of CAR, αvβ3, and αvβ5 integrins in SKOV3.ip1 or OV-4 cells was analyzed by flow cytometry essentially as described in the legend for Fig. 3. Expression of CAR in SKOV3.ip1 (A) and OV-4 cells (C) and of αvβ3 (thin line) and αvβ5 (heavy line) integrins in SKOV3.ip1 (B) and OV-4 (D) cells is shown. The results for the negative control are shown by the dotted line.
Gene transfer experiments utilizing SKOV3.ip1 and OV-4 showed that incorporation of recombinant RGD-containing fiber protein into Ad5lucRGD virion dramatically improved the ability of the virus to efficiently transduce these cells (Fig. 8A). At different MOIs tested, Ad5lucRGD-transduced cultures of SKOV3.ip1 cells showed 30- to 60-fold increases in luciferase activity over that of cells transduced with control virus. Interestingly, while the fiber knob blocked more than 90% of AdCMVLuc-mediated gene transfer, it could block only 15 to 20% of luciferase activity in Ad5lucRGD-treated cells.
FIG. 8.
Comparison of the gene transfer efficiencies to cultured ovarian cancer cells mediated by AdCMVLuc and Ad5lucRGD. Human ovarian cancer cells SKOV3.ip1 (A) and OV-4 (B) were transduced with AdCMVLuc or Ad5lucRGD at an MOI of 1 or 10 PFU/cell essentially as described for 293, HUVEC, and RD cells. Recombinant Ad5 fiber knob protein was added to cells prior to infection with the virus. Each datum point is the average of three independent measurements obtained in one experiment.
The difference in transduction efficiencies demonstrated by these two viral vectors was even greater, 300- to 600-fold, when OV-4 cells were employed (Fig. 8B). As before, the fiber knob used as an inhibitor of CAR-mediated cell entry did not have a significant effect on Ad5lucRGD-mediated gene delivery, strongly suggesting that this virus primarily utilizes RGD-integrin interaction in order to bind to OV-4 cells.
We next evaluated the utility of the Ad5lucRGD vector in the context of human ovarian cancer primary cells. In this regard, recent human clinical trials have highlighted the disparity between the efficacy of Ad vectors in various model systems and in the clinical context, where rather low transduction efficiencies have been noted (1, 2). These findings suggest the need to improve vector design as a general approach to augment the therapeutic index of the cancer gene therapy strategies. As integrins have been shown to be frequently overexpressed by various epithelial tumors (for reviews, see references 17 and 40), vector targeting to these cell surface receptors can provide a means to achieve CAR-independent gene transfer.
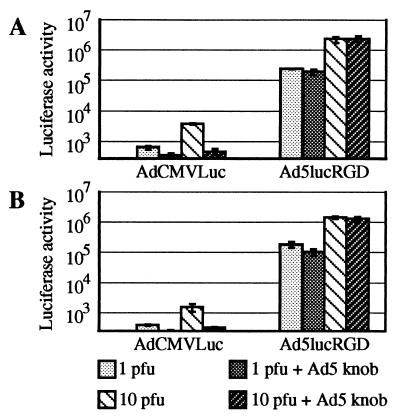
In our experiments, ovarian cancer cells obtained from two patients were treated with both Ad5lucRGD and AdCMVLuc in the presence or absence of blocking knob protein. The results obtained corroborated our previous findings generated with cultured cells. Note that luciferase readings in the lysates of cells treated with AdCMVLuc were extremely low (Fig. 9A and B), thereby indicating the inability of Ad vector containing unmodified fibers to efficiently infect ovarian cancer cells. Strong inhibition by the fiber knob on AdCMVLuc-mediated luciferase expression suggests that the fiber-CAR interaction is the only pathway this virus can use to infect this type of cell. In marked contrast, Ad5lucRGD directed levels of transgene expression 2 to 3 orders of magnitude higher than those detected in AdCMVLuc-transduced cells. The knob blocked 20% of the gene transfer at an MOI of 1 PFU/cell. No effect was observed at an MOI of 10 PFU/cell. Thus, the ability to achieve significant enhancement of gene delivery via CAR-independent pathway suggests the general utility of genetic retargeting of Ad vectors for efficient tumor transduction.
FIG. 9.
Transduction of primary cells isolated from ascitic fluid samples obtained from ovarian cancer patients. Cells isolated from ascitic fluid samples from two (A and B) ovarian cancer patients as described in Materials and Methods were transduced with AdCMVLuc or Ad5lucRGD at an MOI of 1 or 10 in the presence or absence of blocking Ad5 fiber knob protein. The datum points represent the means ± standard deviations of three independent determinations.
DISCUSSION
In this report, we describe the generation and characterization of recombinant Ad vector containing fibers with an RGD-4C sequence genetically incorporated within the HI loop of the carboxy-terminal knob domain. An effort to create such a virus was undertaken in order to demonstrate the utility of the HI loop of the fiber knob as an optimal site for incorporation of short peptide ligands, which would allow the virus to bind to ligand-specific cellular receptors, thereby resulting in altered or expanded tropism of the vector.
The interaction between cellular integrins and various proteins containing an RGD tripeptide is one of the best characterized interactions between macromolecules. This interaction plays an important role in a variety of fundamental biological processes, including cell adhesion and viral infection. In this regard, it has been shown that the RGD motif contained in adhesive proteins, such as fibrinectin, vitronectin, collagen, osteopontin, thrombospondin, fibrinogen, laminin, and von Willebrand factor (16, 29), allows efficient and specific interaction between these proteins and integrin molecules. It is also known that an RGD motif is present in some viral proteins including the VP1 proteins of coxsackievirus (32–34) and foot-and-mouth disease virus (13), the penton base protein of the majority of known Ad (25), the VP7 proteins of the African horsesickness virus and bluetongue virus (7), the Tat protein of human immunodeficiency virus (6), and the glycoprotein H of herpes simplex virus (14). In some of these instances, this tripeptide has been shown to play an important role in the process of viral infection by mediating primary or secondary interactions between the virion and cell surface-localized integrins. Furthermore, recent studies showed that genetic incorporation of the RGD-containing sequences into chimeric hepatitis B cores (11, 36), poliovirus particles (30), bacteriophage fd (19, 20), and human Ad virions (44) allows specific interaction of these viral particles with cellular integrins, thereby resulting in binding of aforementioned structures to cell surface.
We utilized a similar genetic strategy in order to expand the tropism of recombinant Ad vector with respect to cell types which normally are refractory to Ad infection. Based on our previous findings on accessibility of the HI loop-localized FLAG peptide (21), we hypothesized that positioning of the RGD-4C peptide in close proximity to the putative cell binding domain localized within the knob of Ad5 fiber protein (45) should make this ligand available for efficient interaction with integrins on the cell membrane. By using an ELISA-based binding assay, we have been able to show direct interaction between the RGD motif of the fiber-RGD protein with purified integrin αvβ3. This key finding provided a rationale for the generation of recombinant Ad vector, Ad5lucRGD, containing such fiber-RGD proteins. The data generated with Ad5lucRGD on several cell lines showed that this virus demonstrates profiles of gene transfer significantly different from those by the virus with unmodified fibers. This difference was especially dramatic when CAR-negative cells were utilized for the gene delivery experiments. Investigation of radiolabeled virus binding to the cells in vitro paralleled our gene transfer experiments, thereby supporting the concept of augmented efficiency of transgene expression as a result of more-efficient primary interaction between the virus and the target cell.
In order to demonstrate the utility of the newly generated viral vector for clinical applications in the context of gene therapy, we employed Ad5lucRGD for gene delivery to cells isolated from ascitic fluid samples obtained from ovarian cancer patients. Our experiments showed that in this model Ad5lucRGD was able to direct levels of transgene expression 2 to 3 orders of magnitude higher that those mediated by control virion containing unmodified fibers. These results strongly suggest that recombinant Ad vectors containing fibers with genetically incorporated RGD peptides may be of great utility in the context of cancer gene therapy approaches based on in vivo gene delivery. In addition, well-documented overexpression of several types of integrins in tumor vasculature (26) suggests that derivatives of Ad5lucRGD expressing therapeutic genes may be utilized for eradication of tumors via abrogation of their blood supply.
Successful utilization of the RGD tripeptide incorporated into HI loop of Ad fiber protein for the purposes of vector retargeting suggests that other peptide ligands may work just as well in a context of the fiber molecule. In this regard, the rapidly emerging technology of phage display libraries has proved its utility as a means to identify peptides, which demonstrate the ability to specifically bind to certain molecules on a cell surface in vivo. This high-throughput method is based on the capability of small peptide ligands to target a bacteriophage particle to previously characterized as well as to unknown structures on a cell membrane. Recent successes in phage biopanning in an in vivo context (3) strongly suggest that this novel technology may provide an ideal source of targeting peptides to be used for modification of endogenous tropism of recombinant Ad vectors.
Whereas we have demonstrated the utility of small peptides to be incorporated into the HI loop of the fiber knob, the size restrictions of this locale have not been fully defined. In this regard, the compatibility of the HI loop structure with protein ligands of a larger size, such as, for example, single-chain antibodies (scFv), would significantly expand the range of potential targeting approaches. Furthermore, incorporation of large polypeptide ligands into the HI loop, which connects β-strands H and I involved in the formation of the cell-binding site, may create a steric hindrance, thereby preventing direct interaction of the fiber knob with CAR and resulting in elimination of endogenous tropism of the virus. This, in turn, would result in a new generation of truly retargeted Ad vectors, capable of cell-specific gene delivery exclusively via CAR-independent mechanisms.
ACKNOWLEDGMENTS
This work was supported by the following grants: NIH grants RO1 CA-74242, CA-68245, and RO1 HL-50255; a grant from the American Lung Association; and a grant from the U.S. Department of Defense, DAMD 17-94-J4398.
We are grateful to Pierre Boulanger for making the recombinant baculovirus AcNPV-PbWT available and Robert Gerard for providing the plasmid pACCMVpLpA. We are indebted to Robert Finberg for anti-CAR monoclonal antibody RmcB. We thank Paul Reynolds and Alex Pereboev for fruitful discussions and proofreading of the manuscript.
REFERENCES
- 1.Alvarez R D, Curiel D T. A phase I study of recombinant adenovirus vector-mediated delivery of an anti-erbB-2 single-chain (sFv) antibody gene for previously treated ovarian and extraovarian cancer patients. Hum Gene Ther. 1997;8:229–242. doi: 10.1089/hum.1997.8.2-229. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez R D, Curiel D T. A phase I study of recombinant adenovirus vector-mediated intraperitoneal delivery of herpes simplex virus thymidine kinase (HSV-TK) gene and intravenous ganciclovir for previously treated ovarian and extraovarian cancer patients. Hum Gene Ther. 1997;8:597–613. doi: 10.1089/hum.1997.8.5-597. [DOI] [PubMed] [Google Scholar]
- 3.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 4.Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barillari G, Gendelman R, Gallo R C, Ensoli B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basak A K, Gouet P, Grimes J, Roy P, Stuart D. Crystal structure of the top domain of African horse sickness virus VP7: comparisons with bluetongue virus VP7. J Virol. 1996;70:3797–3806. doi: 10.1128/jvi.70.6.3797-3806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 9.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 10.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers M A, Dougan G, Newman J, Brown F, Crowther J, Mould A P, Humphries M J, Francis M J, Clarke B, Brown A L, Rowlands D. Chimeric hepatitis B virus core particles as probes for studying peptide-integrin interactions. J Virol. 1996;70:4045–4052. doi: 10.1128/jvi.70.6.4045-4052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox G, Parry N R, Barnett P V, McGinn B, Rowlands D J, Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid) J Gen Virol. 1989;70:625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- 14.Galdiero M, Whiteley A, Bruun B, Bell S, Minson T, Browne H. Site-directed and linker insertion mutagenesis of herpes simplex virus type 1 glycoprotein H. J Virol. 1997;71:2163–2170. doi: 10.1128/jvi.71.3.2163-2170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J S, Engler J A. Domains required for assembly of adenovirus type 2 fiber trimers. J Virol. 1996;70:7071–7078. doi: 10.1128/jvi.70.10.7071-7078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 17.Juliano R L, Varner J A. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 18.Karayan L, Gay B, Gerfaux J, Boulanger P A. Oligomerization of recombinant penton base of adenovirus type 2 and its assembly with fiber in baculovirus-infected cells. Virology. 1994;202:782–795. doi: 10.1006/viro.1994.1400. [DOI] [PubMed] [Google Scholar]
- 19.Koivunen E, Gay D A, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993;268:20205–20210. [PubMed] [Google Scholar]
- 20.Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J Cell Biol. 1994;124:373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasnykh V, Dmitriev I, Mikheeva G, Miller C R, Belousova N, Curiel D T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasnykh V N, Mikheeva G V, Douglas J T, Curiel D T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis N, Fender P, Barge A, Kitts P, Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994;68:4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizel J V J, White D O, Scharff M D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 25.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Max R, Gerritsen R R, Nooijen P T, Goodman S L, Sutter A, Keilholz U, Ruiter D J, De Waal R M. Immunohistochemical analysis of integrin alpha v beta 3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Michael S I, Hong J S, Curiel D T, Engler J A. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995;2:660–668. [PubMed] [Google Scholar]
- 28.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery A M, Becker J C, Siu C H, Lemmon V P, Cheresh D A, Pancook J D, Zhao X, Reisfeld R A. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin alpha v beta 3. J Cell Biol. 1996;132:475–485. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow, C. Personal communication.
- 31.Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 32.Roivainen M, Hyypia T, Piirainen L, Kalkkinen N, Stanway G, Hovi T. RGD-dependent entry of coxsackievirus A9 into host cells and its bypass after cleavage of VP1 protein by intestinal proteases. J Virol. 1991;65:4735–4740. doi: 10.1128/jvi.65.9.4735-4740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roivainen M, Piirainen L, Hovi T. Efficient RGD-independent entry process of coxsackievirus A9. Arch Virol. 1996;141:1909–1919. doi: 10.1007/BF01718203. [DOI] [PubMed] [Google Scholar]
- 34.Roivainen M, Piirainen L, Hovi T, Virtanen I, Riikonen T, Heino J, Hyypia T. Entry of coxsackievirus A9 into host cells: specific interactions with alpha v beta 3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 35.Shafren D R, Williams D T, Barry R D. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J Virol. 1997;71:9844–9848. doi: 10.1128/jvi.71.12.9844-9848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Rao Z, Fry E, Booth T, Jones E Y, Rowlands D J, Simmons D L, Stuart D I. Specific interactions between human integrin alpha v beta 3 and chimeric hepatitis B virus core particles bearing the receptor-binding epitope of foot-and-mouth disease virus. Virology. 1997;239:150–157. doi: 10.1006/viro.1997.8833. [DOI] [PubMed] [Google Scholar]
- 37.Stewart P L, Chiu C Y, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow G R. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varner J A, Cheresh D A. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 41.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 43.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 44.Wickham T J, Tzeng E, Shears L L N, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia D, Henry L J, Gerard R D, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994;2:1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]