Abstract
Purpose
Post-induction hypotension (PIH) is a common clinical phenomenon linked to increased morbidity and mortality in various non-cardiac surgeries. Patients with surgery in the afternoon may have preoperative hypovolemia caused by prolonged fasting and dehydration, which increases the risk of hypotension during the induction period. However, studies on the fluid therapy in early morning combating PIH remain inadequate. Therefore, we aimed to investigate the influence of prophylactic high-volume fluid in the early morning of the operation day on the incidence of PIH during non-cardiac surgery after noon.
Patients and Methods
We reviewed the medical records of patients who underwent non-cardiac surgery after noon between October 2021 and October 2022. The patients were divided into two groups based on whether they received a substantial volume of intravenous fluid (high-volume group) or not (low-volume group) in the early morning of the surgery day. We investigated the incidence of PIH and intraoperative hypotension (IOH) as well as the accumulated duration of PIH in the first 15 minutes. In total, 550 patients were included in the analysis.
Results
After propensity score matching, the incidence of PIH was 39.7% in the high-volume group and 54.1% in the low-volume group. Multivariate logistic regression analysis showed that patients in the high-volume group had lower incidence of hypotension after induction compared with the low-volume group (odds ratio, 0.55; 95% CI, 0.34–0.89; p = 0.016). The high-volume fluid infusion in the preoperative morning was significantly correlated with the decreased duration of PIH (p = 0.013), but no statistical difference was observed for the occurrence of IOH between the two groups (p = 0.075).
Conclusion
The fluid therapy of more than or equal to 1000 mL in the early morning of the surgery day was associated with a decreased incidence of PIH compared with the low-volume group in patients undergoing non-cardiac surgery after noon.
Keywords: anesthesia induction, hypotension, fluid optimization, preoperative rehydration, hemodynamic instability
Introduction
Post-induction hypotension (PIH) is known as a common clinical phenomenon of hemodynamic instability after general anesthesia. Intraoperative hypotension has been reported to be associated with an increased risk of acute kidney injury, myocardial injury, delirium, and cerebral infarction after non-cardiac surgery.1–4 It is worth pointing out that a considerable proportion of all hypotension occurs between anesthetic induction and surgical incision,3,5 which is a largely preventable consequence of anesthesia.
The mechanism of PIH is multifactorial and complex, such as decreased myocardial contractility, reduced preload from venous dilatation, baroreceptor inhibition, arterial dilation with reduced vascular resistance and positive pressure ventilation.6–9 Furthermore, patients may have preoperative hypovolemia caused by dehydration, fasting, and impaired compensatory responses, which increases the risk of hypotension during the induction period.10–12The primary approaches to PIH management include rapid fluid administration and timely vasopressor.13–15 However, in practice, it proves challenging to infuse a clinically meaningful amount of fluid in just a few available minutes to effect.
Additionally, compared to procedures of other periods, patients with non-cardiac surgery after noon are commonly involved with prolonged fasting, dehydration, and hypovolemia.16 However, there are currently limited clinical data regarding the effect of fluid therapy in the preoperative morning on post-induction hypotension after noon. Therefore, the purpose of this retrospective study was to investigate the influence of prophylactic high-volume fluid therapy in the early morning of the operation day on the incidence of PIH during non-cardiac surgery after noon. Preoperative fasting was generally reported to cause a fluid deficit of approximately 1 litre.17 As such, we hypothesized that more than or equal to 1000 mL fluid therapy in the early preoperative morning may reduce the incidence of PIH after noon.
Materials and Methods
Study Design
The study protocol had been reviewed and approved by the Ethics Committee of Affiliated Lianyungang Hospital of Xuzhou Medical University (ethics committee number, KY-20220905001-01; approval date, 1 September 2022). The ethics committee waived the requirement for informed consent due to the retrospective nature of the research. Meanwhile, this retrospective study was registered prior to enrollment at the Chinese Clinical Trial Registry (ChiCTR2200065787).
We reviewed and extracted data from the electronic medical records of patients who underwent elective non-cardiac surgery in the afternoon or evening between October 2021 and October 2022 in our anesthesia department (Department of Anesthesiology, The Affiliated Lianyungang Hospital of Xuzhou Medical University, China).
Anesthetic Management
All patients were required to fast after midnight before their procedure and received restricted pre-operative carbohydrate drink. Routine hemodynamic monitoring was performed including heart rate (HR), pulse oximetry, electrocardiograph, and arterial blood pressure. General anesthesia was induced after the institutional protocol with standard hemodynamic monitoring in all patients. Propofol and sufentanil were administered manually and titrated to effect intravenously. All patients received tracheal intubation, followed by maintenance with sevoflurane combined with remifentanil infusion. Norepinephrine and ephedrine were given to combat hypotension at the discretion of the attending anesthesiologist.
Inclusion and Exclusion Criteria
Eligible patients who met the following inclusion criteria: 1) aged between 18 and 75 years old; 2) American Society of Anesthesiologists (ASA) physical status ≤2; 3) preoperative systolic blood pressure <160 mmHg; 4) invasive blood pressure monitoring data available before the induction; 5) entering the operating room after noon were enrolled in this study.
Exclusion criteria included 1) patients with severe cardiovascular disease, severe hepatic or renal impairment; 2) patients who underwent cardiac surgery, emergency surgery, or pheochromocytoma surgery; 3) patients induced with inhalation anesthesia or high doses of opioids or benzodiazepines; 4) intraoperative blood pressure recording duration lost >5% of total anesthesia time; and 5) pre-induction systolic blood pressure <90 mmHg. For the subsequent analysis, we also excluded incomplete or missing medical records.
Data Collection
Demographic characteristics (age, sex, and body mass index); preoperative comorbidities (ASA class, hypertension, diabetes mellitus); operative records (surgery type, hospitalization frequency) and anesthesia data (the amount and type of fluid administration in the preoperative morning, the blood pressure measurement values in the ward before the operation and after entering the operating room, respective time of entering the operating room) were extracted in this retrospective observational study.
Outcomes
In our anesthesia department, PIH is defined as the minimum mean arterial pressure (MAP) of patients during the first 15 minutes after induction being below 55 mmHg or decreased by more than 30% from baseline,2 therefore requiring arterial catheters and pressure transducers to perform continuous invasive arterial blood pressure (ABP) measurement.18 The baseline MAP was defined as the mean value of the three measurements taken during the preoperative morning in the ward and after arriving at the operating room. The three MAP values were calculated from different blood pressure readings when they were as follows: (1) measured in the ward at 7 a.m. on the morning of the procedure, (2) taken within the first 5 minutes after entering the operating room, and (3) the first stable MAP measured after arterial line placement before induction. The incidence of PIH before the beginning of the operation was the primary objective of this retrospective analysis. We additionally investigated arterial hypotension between incision and completion of surgery and defined it as intraoperative hypotension (IOH) in this study. Secondary objective included the accumulated duration of PIH in the first 15 minutes after induction and incidence of IOH for assessing if there was a correlation between high-volume fluid therapy on the morning of the surgery day and the degree of hypotension.
Statistical Analysis
Continuous variables were presented as mean ± SD and median with 25th percentile to 75th percentile range if normality was not met. Categorical variables were shown as absolute frequencies with percentages. Differences between the two groups were evaluated using the t-test or Mann–Whitney U-test for continuous variables and chi-square test or Fisher’s exact test for categorical variables, as appropriate.
We performed propensity score matching to minimize the confounding effects and the risk of selection bias between groups. All eligible patients were matched at a 1:1 ratio following the nearest-neighbor method with match tolerance set to 0.02. Propensity scores were calculated from the logistic regression analysis, including covariates such as demographics, preoperative comorbidities, and surgical data. The balance between groups was evaluated by testing the standardized mean difference for each covariate. After conducting propensity score matching, Paired t-test, Wilcoxon signed-rank test, or McNemar’s test was used for analysis, as appropriate.
To determine the association between each factor and PIH, we performed a univariate logistic regression and included variables with p ≤ 0.10 in the subsequent multivariate logistic regression analysis. The same method was also performed for the incidence of IOH. We adopted the Mann–Whitney U-test and the Wilcoxon signed-rank test to analyze the duration of PIH in the first 15 minutes before and after the propensity score matching, respectively. We utilized SPSS version 25.0 software (IBM Corp., Armonk, NY, USA) for all statistical analysis. Statistical significance was considered for two-tailed p < 0.05.
Results
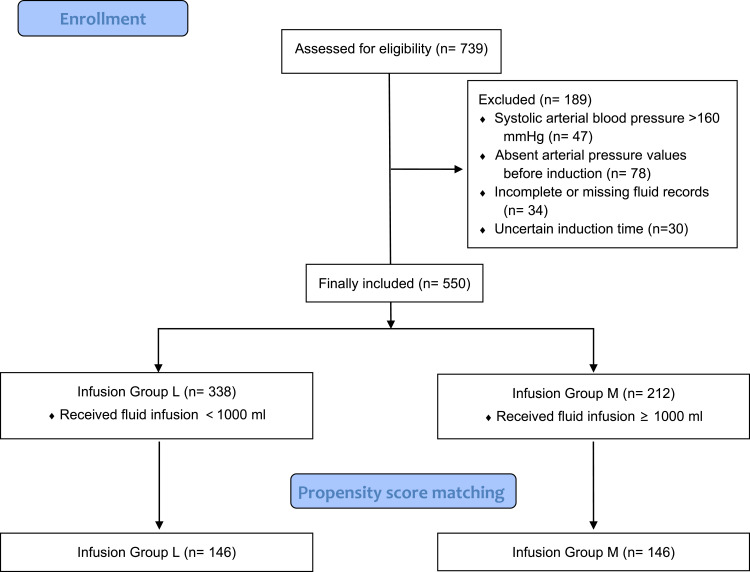
A total of 739 patients undergoing non-cardiac surgery in the afternoon or evening from October 2021 to October 2022 were screened, and 550 patients were finally enrolled in our analysis. More than or 1000 mL fluid boluses were preoperatively administered in 212 patients, and 338 patients received less than 1000 mL rehydration treatment on the morning of the surgery day. Then, 146 patients were allocated to each group following the propensity score matching (Figure 1). A comparison of baseline characteristics before propensity score matching is shown in Table 1. Baseline characteristics after propensity score matching were compared as follows (Table 2). The standardized mean differences of those covariates between groups were less than 0.1, which indicates a good balance and avoids selection bias effectively.
Figure 1.
Flowchart of patient selection.
Table 1.
Baseline Characteristics Before Propensity Score Matching
| L(n=338) | M(n=212) | SMD | P | |
|---|---|---|---|---|
| Age(y) | 50.5±13.3 | 49.9±13.6 | 0.087 | 0.272 |
| Sex, n(%) | ||||
| Male | 170(50.3) | 103(48.6) | 0.034 | 0.756 |
| Female | 168(49.7) | 109(51.4) | ||
| BMI(kg/m2) | 24.8±4.7 | 24.3±4.8 | 0.137 | 0.176 |
| ASA, n(%) | ||||
| I | 21(6.2) | 26(12.3) | 0.139 | 0.031 |
| II | 317(93.8) | 186(87.7) | ||
| HBP, n(%) | 64(18.9) | 46(21.7) | 0.067 | 0.220 |
| DM, n(%) | 20(5.9) | 9(4.2) | 0.083 | 0.747 |
| Ward MAP(mmHg) | 96.8±8.8 | 96.6±8.9 | – | 0.742 |
| NI-MAP(mmHg) | 96.9±8.4 | 96.3±7.6 | – | 0.426 |
| Invasive MAP(mmHg) | 99.1±10.1 | 99.2±8.3 | – | 0.937 |
| Baseline MAP(mmHg) | 97.6±7.9 | 97.4±7.2 | 0.078 | 0.737 |
| Surgery Type, n(%) | ||||
| Orthopedics | 215(63.6) | 65(30.7) | 0.569 | 0.779 |
| Urology | 21(6.2) | 46(21.7) | ||
| Gynecology | 26(7.7) | 6(2.8) | ||
| Hepatobiliary surgery | 58(17.2) | 42(19.8) | ||
| Thyroid surgery | 18(5.3) | 53(25.0) | ||
| Infusion(mL) | 500(0,500) | 1000(1000,1000) | – | <0.001 |
| Hospitalization times | 1.5±1.4 | 1.4±0.8 | 0.061 | 0.603 |
| Admission time(h) | 14.4±2.9 | 15.1±3.3 | 0.310 | 0.111 |
Notes: Values are presented as mean ± SD, median (P25, P75) or number (percentage). Admission time, time of entering the operation room.
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; HBP, Hypertension; DM, diabetes mellitus; MAP, mean arterial pressure; NI-MAP, non-invasive mean arterial pressure.
Table 2.
Baseline Characteristics After Propensity Score Matching
| L(n=146) | M(n=146) | SMD | P | |
|---|---|---|---|---|
| Age(y) | 50.3±13.1 | 50.0±13.8 | 0.018 | 0.894 |
| Sex, n(%) | ||||
| Male | 75(51.4) | 76(52.1) | <0.001 | 0.896 |
| Female | 71(48.6) | 70(47.9) | ||
| BMI(kg/m2) | 24.6±3.7 | 24.9±4.1 | 0.095 | 0.424 |
| ASA, n(%) | ||||
| I | 11(7.5) | 15(10.3) | 0.018 | 0.541 |
| II | 135(92.5) | 131(89.7) | ||
| HBP, n(%) | 29(19.9) | 28(19.2) | 0.063 | 0.684 |
| DM, n(%) | 8(5.5) | 6(4.1) | 0.096 | 0.791 |
| Ward MAP(mmHg) | 96.0±8.4 | 96.8±8.7 | – | 0.432 |
| NI-MAP(mmHg) | 96.4±7.9 | 96.1±7.5 | – | 0.755 |
| Invasive MAP(mmHg) | 98.9±9.9 | 99.3±8.2 | – | 0.699 |
| Baseline MAP(mmHg) | 97.1±7.6 | 97.4±6.9 | 0.054 | 0.736 |
| Surgery Type, n(%) | ||||
| Orthopedics | 74(50.7) | 60(41.1) | 0.048 | 0.385 |
| Urology | 9(6.2) | 35(24.0) | ||
| Gynecology | 10(6.8) | 4(2.7) | ||
| Hepatobiliary surgery | 40(27.4) | 23(15.8) | ||
| Thyroid surgery | 13(8.9) | 24(16.4) | ||
| Infusion(mL) | 500(0,500) | 1000(1000,1000) | – | <0.001 |
| Hospitalization times | 1.6±1.2 | 1.5±1.3 | 0.052 | 0.327 |
| Admission time(h) | 15.1±2.4 | 15.0±2.3 | 0.058 | 0.729 |
Notes: Values are presented as mean ± SD, median (P25, P75) or number (percentage). Admission time, time of entering the operation room.
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; HBP, Hypertension; DM, diabetes mellitus; MAP, mean arterial pressure; NI-MAP, non-invasive mean arterial pressure.
The results of the propensity score matching showed that there were significant differences in the incidence of PIH (p = 0.014) and the duration of PIH in the observed 15 min (p = 0.013) between high-volume fluid infusion group (Group M) and low-volume infusion group (Group L). The occurrence of PIH in Group M was significantly lower than that in Group L (Table 3). The high-volume fluid infusion group revealed less PIH duration in the first 15 min after induction than the low-volume infusion group, but there was no statistical difference in the incidence of IOH between groups (p = 0.075; Table 4).
Table 3.
Results of Univariate Analysis of Variables Associated with PIH
| Odds Ratio (95% CI) | p value | |
|---|---|---|
| Age | 1.04(1.02–1.06) | <0.001 |
| Sex | ||
| Male | 1 | |
| Female | 1.26(0.79–1.99) | 0.330 |
| BMI | 0.96(0.90–1.02) | 0.225 |
| ASA state | ||
| I | 1 | |
| II | 3.24(1.26–8.31) | 0.015 |
| HBP | 2.27(1.25–4.13) | 0.007 |
| DM | 16.15(2.08–25.12) | 0.008 |
| Baseline MAP | 1.02(0.99–1.06) | 0.151 |
| Admission time | 0.95(0.86–1.05) | 0.312 |
| Hospitalization times | 0.90(0.73–1.11) | 0.316 |
| Surgery Type | ||
| Orthopedics | 1.07(0.67–1.69) | 0.790 |
| Urology | 0.60(0.31–1.16) | 0.131 |
| Gynecology | 0.61(0.20–1.88) | 0.393 |
| Hepatobiliary surgery | 1.32(0.76–2.31) | 0.327 |
| Thyroid surgery | 1.23(0.62–2.44) | 0.564 |
| High-volume fluid infusion | 0.56(0.35–0.89) | 0.014 |
Note: Admission time, time of entering the operation room.
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; HBP, Hypertension; DM, diabetes mellitus; MAP, mean arterial pressure.
Table 4.
Accumulated Duration of PIH in the First 15 Min and Incidence of IOH, Before and After Propensity Score Matching
| Group L | Group M | Estimated Difference (95% CI) | p value | |
|---|---|---|---|---|
| Before matching | ||||
| Duration of PIH(min) | 0(0,5) | 0(0,4) | – | 0.208 |
| IOH, n(%) | 211(62.4) | 107(50.5) | 0.84(0.59–1.19) | 0.323 |
| After matching | ||||
| Duration of PIH(min) | 1(0,6) | 0(0,3) | – | 0.013 |
| IOH, n(%) | 94(64.4) | 79(54.1) | 0.65(0.41–1.04) | 0.075 |
Notes: Data are presented as median (P25, P75) or number (percentage).
Abbreviations: PIH, post-induction hypotension; IOH, intraoperative hypotension.
According to univariate regression analysis, preoperative morning high-volume fluid infusion (odds ratio, 0.56; 95% CI, 0.35–0.89; p = 0.014), age (odds ratio, 1.04; 95% CI, 1.02–1.06; p < 0.001) and the medical history of hypertension (odds ratio, 2.27; 95% CI, 1.25–4.13; p = 0.007) and diabetes mellitus (odds ratio, 16.15; 95% CI, 2.08–25.12; p = 0.008) were significantly related to the incidence of PIH (Table 3). The same was also true for multivariate logistic regression analysis, in which preoperative morning high-volume fluid infusion (odds ratio, 0.55; 95% CI, 0.34–0.89; p = 0.016), age (odds ratio, 1.03; 95% CI, 1.01–1.05; p = 0.005), and the medical history of hypertension (odds ratio, 1.22; 95% CI, 1.12–2.41; p=0.024) were significant factors associated with PIH (Table 5). The multivariate model showed proper goodness-of-fit as assessed with the Hosmer–Lemeshow test (p = 0.506).
Table 5.
Results of Multivariate Analysis of Variables Associated with PIH
| Odds Ratio (95% CI) | p value | |
|---|---|---|
| Age | 1.03(1.01–1.05) | 0.005 |
| ASA state | ||
| I | 1 | |
| II | 1.66(0.60–4.60) | 0.330 |
| HBP | 1.22(1.12–2.41) | 0.024 |
| DM | 10.90(0.86–18.15) | 0.566 |
| High-volume fluid infusion | 0.55(0.34–0.89) | 0.016 |
Abbreviations: ASA, American Society of Anesthesiologists; HBP, Hypertension; DM, diabetes mellitus.
Discussion
Our results demonstrated that intravenous administration of 1000 mL or more fluid in the early morning before surgery could significantly decrease the incidence of PIH during the non-cardiac surgery after noon. This retrospective study represents the first propensity-score-matched comparison of PIH after noon between patients receiving high- or low-volume fluid therapy in the preoperative early morning.
It has been reported previously that the prevalence rate of PIH during various surgeries ranges from 5% to 99%.19 A recent study showed that there seems to be no difference in the risk of myocardial infarction and acute kidney injury between relative and absolute pressure drops.1 Hence, we chose the definition as a decrease in MAP of more than 30% compared to baseline or being below 55 mmHg.20 Anxiety before anesthesia induction could lead to an increase in sympathetic tone, which might affect blood pressure values measured in the operating room. Therefore, we compared relative pressure drops dominantly and chose the mean value of MAP at three time points as the baseline to alleviate the interference of pre-operative “white coat hypertension”.6,20
In the present study, adequate fluid pre-loading in the preoperative morning was found to be capable of decreasing the incidence of PIH after noon. In contrast, Kahn et al have reported that pre-anesthetic fluid optimization did not significantly impact hemodynamic instability induced by general anesthesia, further questioning the plausibility of the association between PIH and volume status.20 This discrepancy might be caused by their unbalanced fluid intervention between groups with convenience sampling. Additionally, the operation time we observed is different. As the association of organ injury and perioperative morbidity rates with hypotension is time- and magnitude-dependent,1 the duration of hypotension was also taken into consideration. Given that the duration of PIH is subject to the interval from anesthesia induction to operation beginning, we merely accumulated the onset time of PIH in the first 15 minutes after general anesthesia. As a result, another notable corollary of our study was that high-volume fluid infusion group revealed a decreased PIH duration in the first 15 min after induction than low-volume infusion group. In our study, the patients with surgery after noon have been suffering from hypovolemia caused by dehydration and prolonged fasting, making prophylactic fluid therapy indispensable for patients’ perioperative volume management.
According to previous studies, age, ASA class, preoperative comorbidities (hypertension, diabetes mellitus), emergency surgery, anesthesia protocol, and long duration from induction to skin incision are known risk factors of PIH.5,21–23 Notably, we confirmed that age, ASA, and the presence of hypertension were significantly associated with the incidence of PIH. The occurrence of PIH was higher in the higher age class, being 32.5%, 56.7%, and 65.6% in the groups aged <50, 50–65, and ≥65 years, respectively. Historically, PIH has been attributed to the patients’ own physical state and vascular dilatation of certain induction agents.24 When faced with vasodilatation to such an extent, it is difficult for patients with long-term hypertension and the elderly to maintain hemodynamic stability due to their chronic dysregulation of vasoconstriction. What is more, elderly patients with hypertension may have difficulty in resisting the sharp fluctuations of blood pressure and, as its onset is delayed, inotrope therapy is usually difficult and futile. It is worth bearing in mind that the amount of rehydration in those population and the timing of fluid therapy should be paid with more attention.
Although the high-volume fluid infusion in the early morning was associated with decreased occurrence and duration of PIH, we failed to find statistically significant association between high-volume fluid and the incidence of IOH. In this study, IOH was narrowly defined as hypotension occurring merely during the operation period. Therefore, preoperative fluid infusion seemed to alleviate the risk of IOH through partial mechanism, which may be affected by complex surgical manipulation, vascular elasticity, or patient positioning rather than modified volume-related factors.5
However, is it necessary to use larger boluses of crystalloid in all patients via passive leg raise testing? Despite the relatively high incidence of PIH in the elderly and hypertensive patients, healthy individuals present acceptable hemodynamic fluctuation. Unnecessary “fluid preload” might lead to potential consequences such as cyclic overload and postoperative edema. Of note, determining the risk factors for PIH may help individualize perioperative volume management. Preoperative identification of patients with a high risk of PIH with taking corresponding actions to counteract PIH—such as administering more crystal fluid—may optimize rehydration strategies for non-cardiac surgery in the afternoon or evening.
Our study also suffers from several limitations. First of all, given the intrinsic restriction of observational study, this protocol was unable to assess causality definitively, but rather only to prove associations. Second, as the etiology of PIH is multifactorial and complex, there might have been some unknown confounding factors that affected our results despite the propensity score matching adjustment, such as usage of vasopressors and administration of regional anesthetic techniques. Third, exploration of the direct association between the preoperative fluid therapy and postoperative complications such as acute kidney injury and myocardial injury was beyond the scope of this study; hence, further prospective studies are required to detect the underlying mechanism. Finally, as a single-center study that did not include ASA III patients, the generalizability of our results may be limited.
Conclusion
Our findings demonstrated that a substantial intravenous fluid bolus in the early morning of the operation day could attenuate the risk of PIH in the surgery after noon. This retrospective study may provide an additional clue that adequate prophylactic intravenous fluids, with or without intraoperative inotropes, could improve intravascular volume to combat PIH and treat potential dehydration. Ultimately, a multicenter study with a larger sample and longer follow-up is still warranted to confirm our findings in the future.
Acknowledgments
All authors would like to express their gratitude to the Anesthesiology Department of the Affiliated Lianyungang Hospital of Xuzhou Medical University for their assistance and support.
Funding Statement
This research was supported by Lianyungang Science and Technology Project (JCYJ2305) and Clinical Research Fund of The Affiliated Lianyungang Hospital of Xuzhou Medical University (LC13).
Confidentiality of Data
The authors declare that they have followed the protocols of their center on the publication of patient data.
Data Sharing Statement
All data generated or analyzed during this study are included in the article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. The patient datasets anonymity will be preserved prior to distribution.
Ethical Disclosures Statement
Protection of human and animal subjects: The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Right to Privacy and Informed Consent
The authors have obtained approval from the Ethics Committee for analysis and publication of routinely acquired clinical data. In addition, informed consent was not required for this retrospective observational study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65. doi: 10.1097/ALN.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 2.Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–664. doi: 10.1097/ALN.0b013e3182472320 [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari K, Turan A, Mao G, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73(10):1223–1228. doi: 10.1111/anae.14416 [DOI] [PubMed] [Google Scholar]
- 4.Duan W, Zhou CM, Yang JJ, et al. A long duration of intraoperative hypotension is associated with postoperative delirium occurrence following thoracic and orthopedic surgery in elderly. J Clin Anesth. 2023;88:111125. doi: 10.1016/j.jclinane.2023.111125 [DOI] [PubMed] [Google Scholar]
- 5.Sudfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi: 10.1093/bja/aex127 [DOI] [PubMed] [Google Scholar]
- 6.Wong GTC, Irwin MG. Post‐induction hypotension: a fluid relationship? Anaesthesia. 2020;76(1):15–18. doi: 10.1111/anae.15065 [DOI] [PubMed] [Google Scholar]
- 7.Saugel B, Bebert EJ, Briesenick L, et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput. 2022;36(2):341–347. doi: 10.1007/s10877-021-00653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell DW, Casey JD, Gibbs KW, et al. Effect of fluid bolus administration on cardiovascular collapse among critically ill patients undergoing tracheal intubation: a randomized clinical trial. JAMA. 2022;328(3):270–279. doi: 10.1001/jama.2022.9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Cai G, Xu Z, et al. High positive end expiratory pressure levels affect hemodynamics in elderly patients with hypertension admitted to the intensive care unit: a prospective cohort study. BMC Pulm Med. 2019;19(1):224. doi: 10.1186/s12890-019-0965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juri T, Suehiro K, Tsujimoto S, et al. Pre-anesthetic stroke volume variation can predict cardiac output decrease and hypotension during induction of general anesthesia. J Clin Monit Comput. 2018;32(3):415–422. doi: 10.1007/s10877-017-0038-7 [DOI] [PubMed] [Google Scholar]
- 11.Morley AP, Nalla BP, Vamadevan S, et al. The influence of duration of fluid abstinence on hypotension during propofol induction. Anesth Analg. 2010;111(6):1373–1377. doi: 10.1213/ANE.0b013e3181f62a2b [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Critchley LA. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124(3):580–589. doi: 10.1097/ALN.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5 [DOI] [PubMed] [Google Scholar]
- 14.Lin FQ, Li C, Zhang LJ, et al. Effect of rapid plasma volume expansion during anesthesia induction on haemodynamics and oxygen balance in patients undergoing gastrointestinal surgery. Int J Med Sci. 2013;10(4):355–361. doi: 10.7150/ijms.5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L’hermite J, Muller L, Cuvillon P, et al. Stroke volume optimization after anaesthetic induction: an open randomized controlled trial comparing 0.9% NaCl versus 6% hydroxyethyl starch 130/0.4. Ann Fr Anesth Reanim. 2013;32(10):e121–7. doi: 10.1016/j.annfar.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Xie S, Chen Y, et al. Effect of preoperative oral saline administration on postoperative delirium in older persons: a randomized controlled trial. Clin Interv Aging. 2022;17:1539–1548. doi: 10.2147/CIA.S377360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holte K, Kehlet H. Compensatory fluid administration for preoperative dehydration--does it improve outcome? Acta Anaesthesiol Scand. 2002;46(9):1089–1093. doi: 10.1034/j.1399-6576.2002.460906.x [DOI] [PubMed] [Google Scholar]
- 18.Kouz K, Wegge M, Flick M, et al. Continuous intra-arterial versus intermittent oscillometric arterial pressure monitoring and hypotension during induction of anaesthesia: the AWAKE randomised trial. Br J Anaesth. 2022;129(4):478–486. doi: 10.1016/j.bja.2022.06.027 [DOI] [PubMed] [Google Scholar]
- 19.Bijker JB, Van Klei WA, Kappen TH, et al. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220. doi: 10.1097/01.anes.0000270724.40897.8e [DOI] [PubMed] [Google Scholar]
- 20.Khan AI, Fischer M, Pedoto AC, et al. The impact of fluid optimisation before induction of anaesthesia on hypotension after induction. Anaesthesia. 2020;75(5):634–641. doi: 10.1111/anae.14984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622–628. doi: 10.1213/01.ANE.0000175214.38450.91 [DOI] [PubMed] [Google Scholar]
- 22.Jor O, Maca J, Koutna J, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth. 2018;32(5):673–680. doi: 10.1007/s00540-018-2532-6 [DOI] [PubMed] [Google Scholar]
- 23.Smischney NJ, Demirci O, Diedrich DA, et al. Incidence of and risk factors for post-intubation hypotension in the critically ill. Med Sci Monit. 2016;22:346–355. doi: 10.12659/MSM.895919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Wit F, Van Vliet AL, de Wilde RB, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth. 2016;116(6):784–789. doi: 10.1093/bja/aew126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. The patient datasets anonymity will be preserved prior to distribution.