Abstract
Here we report that severe combined immunodeficient (SCID) mice engrafted with human K562 cells (K562-SCID mice) can be used as an animal model to study dengue virus (DEN) infection. After intratumor injection into K562 cell masses of PL046, a Taiwanese DEN-2 human isolate, the K562-SCID mice showed neurological signs of paralysis and died at approximately 2 weeks postinfection. In addition to being detected in the tumor masses, high virus titers were detected in the peripheral blood and the brain tissues, indicating that DEN had replicated in the infected K562-SCID mice. In contrast, the SCID mice were resistant to DEN infection and the mock-infected K562-SCID mice survived for over 3 months. These data illustrate that DEN infection contributed directly to the deaths of the infected K562-SCID mice. Other serotypes of DEN were also used to infect the K562-SCID mice, and the mortality rates of the infected mice varied with different challenge strains, suggesting the existence of diverse degrees of virulence among DENs. To determine whether a neutralizing antibody against DEN in vitro was also protective in vivo, the K562-SCID mice were challenged with DEN-2 and received antibody administration at the same time or 1 day earlier. Our results revealed that the antibody-treated mice exhibited a reduction in mortality and a delay of paralysis onset after DEN infection. In contrast to K562-SCID, the persistently DEN-infected K562 cells generated in vitro invariably failed to be implanted in the mice. It seems that in the early stage of implantation, a gamma interferon activated, nitric oxide-mediated anti-DEN effect might play a role in the innate immunity against DEN-infected cells. The system described herein offers an opportunity to explore DEN replication in vivo and to test various antiviral protocols in infected hosts.
Dengue viruses (DENs) are a group of mosquito-borne flaviviruses that cause public health problems worldwide, especially in tropical and subtropical areas (30, 43). There are four antigenically related but distinct serotypes of DENs (DEN-1, DEN-2, DEN-3, and DEN-4) identified based on the plaque reduction neutralization test results (40). The DEN genome is a single-stranded, positive-sense RNA of approximately 11 kb in length which contains a single open reading frame (ORF) encoding a polyprotein. In the infected cells, this viral polyprotein is proteolytically cleaved into at least 11 proteins. The virus structural proteins, including the capsid, membrane (M), precursor M, and envelope (E) proteins, are encoded by the 5′ one-third of the ORF and the nonstructural (NS) proteins, designated NS1 through NS5, are encoded in the remainder of the ORF (reviewed in references 8 and 38).
These viruses generally cause a mild febrile illness, dengue fever (DF), and infrequently cause a much more severe disease, dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). There is still considerable controversy about the pathogenesis of DHF/DSS, though most investigators suppose that the severity of DEN infection is related to the magnitude of viral replication. The primary site of DEN replication after injection into humans by the feeding mosquito is believed to be phagocytic monocytes (15). DENs were shown to replicate to high titers in human mononuclear cells, especially in the presence of cross-reactive nonneutralizing dengue antibodies (15). This phenomenon, known as antibody-dependent enhancement (ADE) of viral infectivity (37), is probably caused by the infectious complexes of virion and antibody gaining access to monocytic cells via their Fcγ receptors. It has been hypothesized that DHF/DSS may be the consequence of enhanced viral replication and immunopathological processes evoked by monocyte dysfunction and detrimental reaction caused by activated T lymphocytes (19, 25; reviewed in reference 31). This may explain in part why sequential infection with different serotypes of DEN predisposes the infected host towards DHF/DSS. In addition, since all four DEN serotypes can cause DHF/DSS, the sequence of infecting serotypes, the interval between infections, and strain differences in virulence have all been suggested to be important determinants for clinical outcomes (reviewed in reference 31).
So far there are only three known natural hosts for DEN infections: mosquitoes, humans, and lower primates (reviewed in reference 13). Viremia in humans may last 2 to 12 days (average, 4 to 5 days), with titers ranging from undetectable to high. Several species of lower primates, including chimpanzees, gibbons, and macaques, have been experimentally infected; they were able to develop a high-titer viremia sufficient to infect feeding mosquitoes (42). Nevertheless, DENs are known to cause only clinical illness and disease in humans. After intracerebral challenge, small animals such as mice are often used as models for DEN infections. In baby mice, unadapted virus strains usually produce subclinical infections and, sporadically, illnesses with paralysis and death, whereas the mice develop disease status when challenged with some mouse-brain-adapted strains. On the other hand, adult mice inoculated with unadapted DENs produced no symptoms at all, although challenge by highly adapted DEN strains sometimes caused the symptom of overt encephalitis in the host (5).
Development of a small-animal model that can be infected with DEN by peripheral inoculation will greatly facilitate the study of DEN infection. The C.B.-17 mouse with homozygous mutation for the SCID phenotype (6) can support multiple tissue xenografts, and this system has been used for studies of infections by pathogenic viruses that grow in lymphocytes or other hematopoietic cells (32). SCID mice reconstituted with adult peripheral blood mononuclear cells (hu-PBL-SCID mice) have also been employed to explore primary infection with human immunodeficiency virus and reactivation of latent infection with Epstein-Barr virus (reviewed in reference 33). Moreover, some, but not all, hu-PBL-SCID mice have also been shown to be susceptible to DEN-1 infection (45). In this study, we have improved the infection frequency for SCID mice by engrafting DEN-susceptible human cell lines and rectified the challenging route by selecting a peripheral path. Our results demonstrate that SCID mice implanted with human K562 cells (K562-SCID mice) appear to be a suitable animal model for studying DEN infection in vivo.
MATERIALS AND METHODS
Viruses and cell lines.
Local Taiwanese strains of DENs, DEN-2 PL046 and DEN-1 766733A, which were isolated from patients with DF, and DEN-4 466088A, isolated from a patient with DHF, were generously provided by the National Institute of Preventive Medicine, Taiwan, Republic of China. DEN-1 prototype Hawaii strain, DEN-2 prototype New Guinea C (NGC) strain, and DEN-3 prototype H87 strain were kindly provided by D. J. Gubler of the Centers for Disease Control and Prevention, Fort Collins, Colo. DEN-2 07587, derived from a Malaysian patient with DHF, was a kind gift from S. K. Lam in Kuala Lumpur, Malaysia. All of the above-listed viruses, which have not been adapted in mouse brain, were used in this animal study. The DEN-1 Hawaii-N strain, obtained from C. J. Lai of the National Institutes of Health, Bethesda, Md., and DEN-2 PL046 were used to establish the persistently infected K562 cells. Virus propagation was carried out in C6/36 cells utilizing RPMI 1640 medium containing 5% fetal calf serum (FCS) (GIBCO). Virus titers were determined by a plaque-forming assay on BHK-21 cells. K562 (ATCC CCL-243), U937 (ATCC CRL-1593), HL-60 (ATCC CCL-240), THP-1 (ATCC TIB-202), and N18 cells, a mouse neuroblastoma cell line (2), were all grown in RPMI 1640 medium containing 10% FCS (GIBCO). The mouse monocyte/macrophage cell line RAW 264.7 (ATCC TIB-71) was cultured in Dulbecco’s modified Eagle’s medium with 10% FCS.
DEN infection of SCID mice engrafted with human K562 cells.
Groups of 3- to 4-week-old female SCID mice, purchased from the animal facility of National Taiwan University, Taipei, Taiwan, were engrafted by intraperitoneal (i.p.) injection with 107 K562 cells, and approximately 5 weeks postimplantation, the mice were inoculated with 107 PFU of DEN directly into the peritoneal tumor mass. To determine virus yields from sera, blood samples were diluted with an equal volume of phosphate-buffered saline (PBS) and centrifuged immediately after being removed from the infected mice. To examine the distribution profiles of viruses, different organs were individually collected and processed to make tissue suspensions as previously described (10). The virus titers expressed as PFU/mouse for some experiments reflect the total amounts of virus detected in each tissue sample from the animals examined.
Generation and characterization of DEN-specific MAbs.
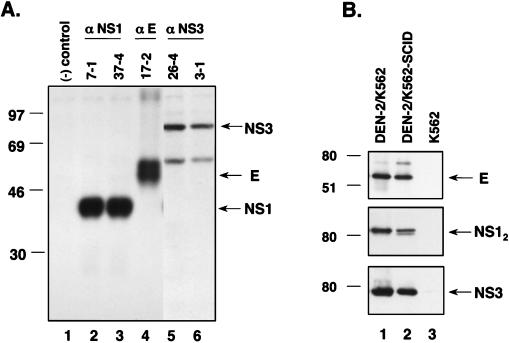
DEN-specific monoclonal antibodies (MAbs) were generated and analyzed as previously described (9). Briefly, 6- to 8-week-old BALB/c mice were immunized i.p. with DEN-2 PL046-infected suckling mouse brain suspensions mixed with an equal volume of complete Freund’s adjuvant for the first inoculation and mixed with incomplete Freund’s adjuvant for the subsequent boosting. Splenocytes were fused with NS-1 myeloma cells and selected as described by Kohler and Milstein (22). The hybridoma-secreted specific antibodies were identified by enzyme-linked immunosorbent assay and immunoprecipitation assays with DEN-2-infected C6/36 cell lysates as the antigen source as previously described (9). The specificities of several MAbs demonstrated by immunoprecipitation are shown in Fig. 1A: MAbs 7-1 (lane 2) and 37-4 (lane 3) were specific for NS1 protein, MAb 17-2 (lane 4) was specific for E protein, and MAbs 26-4 (lane 5) and 3-1 (lane 6) were specific for NS3 protein. Single-cell clones of hybridoma were generated by limiting dilution, and for ascitic fluid production the cells were injected into incomplete Freund’s adjuvant-primed BALB/c mice.
FIG. 1.
(A) Demonstration of the specificity of anti-DEN MAbs used in this study. Lysates from 35S-labeled DEN-2 PL046-infected C6/36 cells were immunoprecipitated with MAbs as indicated on the top of the gel. Culture supernatant collected from NS-1 myeloma cells was used as the negative control (lane 1). The numbers on the left side denote the positions of molecular mass standards. Positions of E, NS1 (monomer), and NS3 proteins are indicated by arrows on the right side of the gel. (B) Detection of DEN antigens from different K562 cells by Western immunoblotting assay. The lysates of DEN-2 PL046-infected K562 cells (lane 1), DEN-2 PL046-inoculated K562 tumor mass of the SCID mouse (lane 2), and K562 cells alone (lane 3) were immunoblotted with MAbs specific for DEN E, NS1, or NS3 protein as described in the Materials and Methods. The numbers on the left side denote the positions of molecular mass standards. Positions of E, NS1 (dimer) (NS12), and NS3 proteins are indicated by arrows on the right side of the gel.
Western immunoblot analysis.
Cell monolayers were rinsed and lysed by lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA) containing a cocktail of protease inhibitors, 20 μg of phenylmethylsulfonyl fluoride per ml, 2 μg of leupeptin per ml, and 2 μg of aprotinin per ml. Cell lysates were mixed with an equal volume of sample buffer (without β-mercaptoethanol), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane (Hybond-C Super; Amersham). The nonspecific antibody-binding sites were blocked with 5% skim milk in PBS and reacted with monoclonal anti-DEN E, NS1, and NS3 protein antibodies. The blots were then treated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Cappel) and developed with an ECL kit system (Amersham).
ADE assay.
MAb-containing ascitic fluid was preheated at 56°C for 30 min to inactivate the complement, and the resulting MAb was serially diluted and incubated with 2.5 × 106 PFU of DEN-2 PL046 at 37°C for 60 min. The immunocomplexes were then added to 5 × 105 cells per well to incubate (at a multiplicity of infection of about 5) at 37°C for 2.5 h, and the infected cells were washed three times with RPMI 1640 medium with 2% FCS. Four days (for U937 cells) or 6 days (for K562 cells) postinfection (p.i.), the culture supernatants were collected for virus titration on BHK-21 cells.
Coculture of DEN-infected cells with IFN-γ treated murine macrophages.
Cocultures were performed by the published method (20) with minor modifications as described earlier (26). Briefly, 106 RAW 264.7 murine macrophages (in a six-well plate) were first treated with various amounts of gamma interferon (IFN-γ) (Genzyme, Cambridge, Mass.) in the presence or absence of the nitric oxide synthase inhibitor N-monomethyl-l-arginine acetate (l-NMA) (Biomol Research Laboratories) at 500 μM for 24 h, and the resulting cells were cocultured with DEN-2 PL046-infected N18 or K562 cells (106 cells per well in a six-well plate). In the presence or absence of 500 μM l-NMA, these cocultured cells were incubated for another 48 h with medium containing fresh IFN-γ, and the supernatants were harvested for virus titration. The amount of nitric oxide (NO) produced in the media was determined by assaying its stable end-product, NO2− (nitrite), as previously described (4).
RESULTS
Establishment of SCID mice engrafted with human K562 cells that support DEN infection.
In a previous study, hu-PBL-SCID mice were used as an animal model for DEN-1 infection (45). In that study, the PBL from 10 different human donors were employed to individually reconstitute SCID mice, and all of the DEN-infected hu-PBL-SCID mice were derived from the same donor. Furthermore, only 5 of 19 hu-PBL-SCID mice derived from this donor were infected with DEN-1 (strain Western Pacific 74). A scanty number of appropriate human target cells in the reconstituted mice was suggested to be the main reason for the low infection rate. In the present study, we endeavored to improve the infection rate of the SCID mouse model by engrafting the mice with DEN-susceptible human cell lines. Four human cell lines were first tested for their permissiveness to DEN-2 PL046 infection: K562, an erythroleukemia cell line; U937, a monocyte-like cell line; HL-60, a promyelocytic leukemia cell line; and THP-1, a monocyte cell line. As characterized by virus yields, immunofluorescent staining of target cells (Table 1), and Western blotting with MAbs against DEN structural (E protein) and nonstructural (NS1 and NS3 proteins) proteins (Fig. 1B), only K562 cells were shown to be susceptible to DEN-2 PL046 infection, which is consistent with a previous report (24).
TABLE 1.
Cell susceptibility to DEN-2 PL046 infection
| Cell line and no. of days p.i. | Virus titer (PFU/ml) | Immunofluorescence assaya | Immunoblotting |
|---|---|---|---|
| K562 | |||
| 2 | 1.0 × 105 | + | + |
| 4 | 9.5 × 106 | + | + |
| 6 | 2.0 × 106 | + | + |
| U937 | |||
| 2 | 1.0 × 104 | +/− | − |
| 4 | 1.0 × 104 | +/− | − |
| 6 | <50 | +/− | − |
| HL-60 | |||
| 2 | <50 | − | − |
| 4 | <50 | − | − |
| 6 | <50 | − | − |
| THP-1 | |||
| 2 | <50 | − | − |
| 4 | <50 | − | − |
| 6 | <50 | − | − |
+/−, approximately 5% of the cells were positively stained by DEN-specific MAbs against E, NS1, and NS3 proteins.
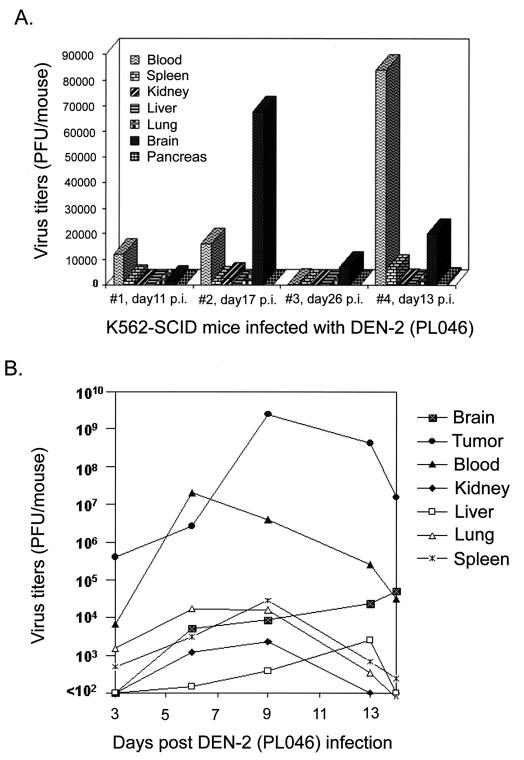
Next, we engrafted the SCID mice with 107 K562 cells (K562-SCID mice) by i.p. injection; about 3 to 4 weeks postengraftment, noticeable tumor masses in the peritoneal cavity of the mice were observed. To ensure that DEN would infect the human K562 cells inside the mice, we injected 107 PFU of DEN-2 (PL046) into the peritoneal tumor masses (intratumor [i.t.]) of four K562-SCID mice. The mice started exhibiting signs of limb weakness and paralysis at 1 to 2 weeks p.i., and the mice died at approximately 2 to 4 weeks p.i. In contrast, all of the three mock-infected K562-SCID mice survived for over 3 months without any neurological symptoms. As a control, two SCID mice infected with 107 PFU of PL046 by i.p. challenge showed no signs of disease, nor could virus be recovered from the mice, indicating that by peripheral injection the SCID mice per se were not permissive hosts for DEN-2 (PL046) replication. The cells recovered from the peritoneal tumor masses of these four DEN-2-infected K562-SCID mice were strongly positive for DEN antigens according to results from immunofluorescent staining (data not shown) and immunoblotting (Fig. 1B, lane 2). The presence of the nonstructural proteins NS1 and NS3 in the cells derived from such mice clearly illustrates that the productive replication of DEN-2 PL046 had occurred in these animals. Since K562 cells were reported to be able to differentiate into recognizable progenitors of various cell types (28), it remains to be studied further whether K562 cells implanted in mice can undergo differentiation and which cell populations can be infected by dengue virus in vivo. To study the organ distribution of virus, four moribund mice were sacrificed for the detection of DEN-2 PL046. As shown in Fig. 2A, the major sites with high titer of DEN-2 PL046 were the brain and the peripheral blood of the mice, which appeared to correlate well with the encephalopathy progression of the infected mice. Together, these data demonstrate that DEN infection contributed directly to the death of infected K562-SCID mice.
FIG. 2.
(A) Distribution of DEN-2 PL046 in blood and different organs of K562-SCID mice after infection. Various organs and blood from four DEN-2 PL046-inoculated K562-SCID mice were individually obtained for virus titration when the animals showed signs of disease at the times postinfection indicated in the figure. Virus titers were determined by a standard plaque assay on BHK-21 cells as described in Materials and Methods. (B) Kinetics of DEN-2 PL046 replication in different organs of the infected K562-SCID mice. Two mice were sacrificed at the specified days postinfection for virus titration.
The time course of virus replication in different organs was further studied in infected K562-SCID mice, and the results are shown in Fig. 2B. Two mice were sacrificed at each indicated time point, and individual organs as well as tumor masses from the mice were pooled and processed together for virus titration. The growth curve with the highest virus titers, which peaked at day 9 p.i. and then slowly declined, was exhibited in the tumor masses. The second highest curve, which paralleled the curve from the tumor masses, was observed in the serum samples. The virus replication in the brain tissues seemed to increase as the infection proceeded, although not as prominently as in the tumor and the blood samples. By contrast, transient and low virus titers were detected in other organs, such as the kidneys, livers, lungs, and spleens. It is unclear whether the low virus titers in these various organs were due to reduced levels of virus replication or merely due to blood infiltration in the sites examined. Taken together, these results suggest that in the infected K562-SCID mice, DEN-2 PL046 first replicated primarily in K562 cells; afterward, the free virus or possibly the infected K562 cells might have entered the circulation, disseminated to various organs, penetrated the blood-brain barrier to infect the central nervous system, and as a result killed the mice.
Infections of K562-SCID mice with different strains of DEN result in distinct mortality rates.
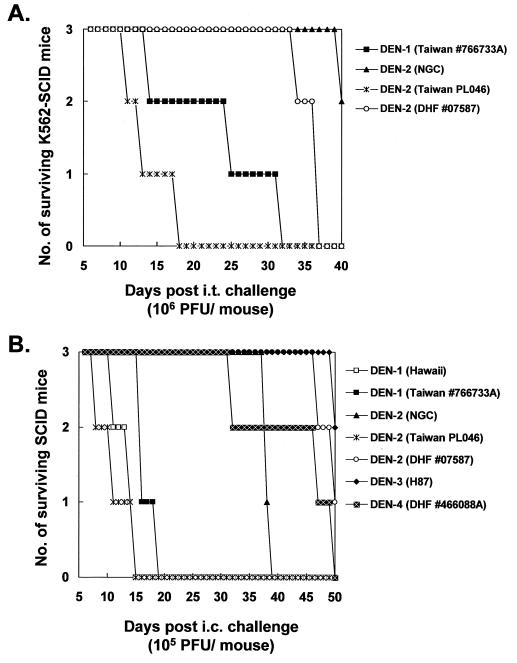
By peripheral challenge, the K562-SCID mice appeared to be an ideal animal model with which to study DEN infection of different human isolates. To test this notion, in addition to DEN-2 PL046 we examined the mice for infectivity resulting from other DEN strains, including a prototype DEN-2 strain (NGC), a DEN-2 strain (07587) isolated from a Malaysian patient with DHF, and a Taiwanese DEN-1 strain (766733A). As the representative result in Fig. 3A shows, DEN-1 766733A killed all the mice by day 32 p.i.—slightly later than the control, DEN-2 PL046, which killed all the mice by day 18 p.i. These data suggest that the infection of K562-SCID mice with DEN was not restricted to a particular serotype. In addition, infection with other DEN-2 strains, such as NGC, did not result in disease symptoms as apparent as those produced by DEN-2 PL046 (Fig. 3A), in spite of the measurable levels of DEN structural and nonstructural antigens in these inoculated tumor masses (data not shown). This suggested that differences in virulence in K562-SCID mice exist among various DEN strains.
FIG. 3.
(A) Survival patterns of K562-SCID mice following i.t. challenge with different DEN strains. (B) Neurovirulence of different DEN strains examined in SCID mice by i.c. inoculation (B).
To determine the neurovirulence of these seemingly avirulent strains administered by peripheral challenge, we inoculated SCID mice with virus by direct intracerebral (i.c.) injection. The infected mice showed survival patterns (representative data are shown in Fig. 3B) comparable with the results from their counterparts, shown in Fig. 3A. DEN-2 NGC was consistently less virulent than DEN-2 PL046 and DEN-1 766733A by either challenge method. These data imply that the avirulence of DEN strains tested through the peripheral route was due to not only their lack of the ability to penetrate the central nervous system but likely also their replications being innocuous to the brain tissues of the mice. Moreover, the neurovirulence of a DEN-1 prototype strain (Hawaii), a DEN-3 prototype strain (H87), and a Taiwanese DEN-4 strain (DHF 466088A) isolated from a patient with DHF was also examined in SCID mice by i.c. challenge; again, the survival results, shown in Fig. 3B, demonstrated that virulence variation among the DEN strains tested was present. The results shown in Fig. 3 also document that the DEN strains derived from patients with DHF (07587 and 466088A) were not more neurovirulent in the K562-SCID mice than those isolated from patients with DF. These results suggest the involvement of a very complicated and as yet unknown mechanism for DHF pathogenesis, whereby such mice do not respond as expected to the infections. Collectively, the data illustrate that by peripheral inoculation, K562-SCID mice are permissive for infections of different serotypes of DEN and that such infections usually result in distinct mortality rates that probably reflect virulence differences among diverse virus strains.
Neutralizing antibody protects K562-SCID mice from DEN challenge.
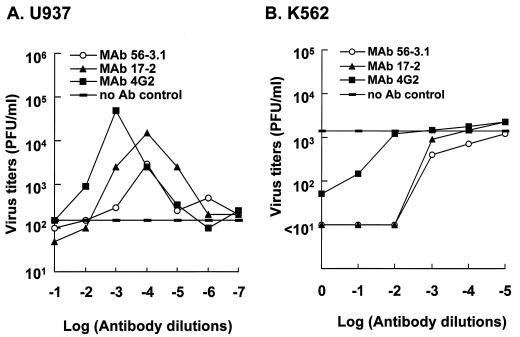
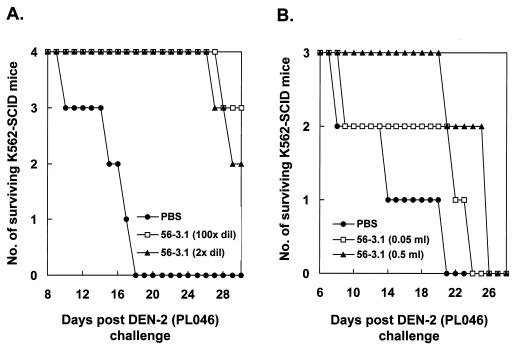
To determine whether a neutralizing antibody against DEN in vitro could also be protective in vivo, we tested the effects of antibodies in K562-SCID mice upon DEN-2 challenge. We first characterized two MAbs (17-2 and 56-3.1) generated in our laboratory, both of which were specific for DEN E protein and showed neutralizing activity by plaque reduction neutralization tests on BHK-21 cells (data not shown). Similar to the control antibody 4G2 (ATCC HB-112), which is a well-known ADE MAb (17), in U937 cells MAbs 17-2 and 56-3.1 exhibited an apparent ADE phenomenon for DEN infection. Beginning at an antibody dilution of 1:102, the ADE phenomenon peaked at an antibody dilution of 1:104 and then declined as antibody was further diluted (Fig. 4A). In addition to an augmentation of virus yields, the inclusion of MAbs 17-2 and 56-3.1 appeared to increase the proportion of DEN antigen-positive U937 cells from <5% to about 20% according to immunofluorescence analysis (data not shown). To ensure that these two MAbs were incapable of generating ADE in K562 cells resulting from DEN infections, we performed an experiment similar to that described in the legend for Fig. 4A for these cells. As Fig. 4B shows, no ADE effect in K562 cells was noted for MAbs 17-2 and 56-3.1, nor for 4G2; in contrast, good neutralizing activity was observed at antibody dilutions ranging from 1:1 to 1:100. The protective properties of MAb 56-3.1 were further characterized in K562-SCID mice infected with DEN. Pretreatment of 107 PFU of DEN-2 PL046 with the diluted MAb 56-3.1 at 37°C for 1 h greatly prolonged the life spans of infected mice, whereas the PBS-treated virus killed all mice by day 18 p.i. (Fig. 5A). Although not as effective as the pretreatment described in the legend for Fig. 5A, a protective effect was still observed when the MAb was applied to the mice 1 day before DEN challenge (Fig. 5B). Furthermore, this protection seemed to be MAb dose-dependent; the average survival times for the groups of mice inoculated were as follows: PBS, 13.3 days; 0.05 ml of MAb 56-3.1, 17.3 days; and 0.5 ml of MAb 56-3.1, 23.3 days. These results indicate that an in vitro neutralizing antibody also defended K562-SCID mice against DEN-2 infection.
FIG. 4.
In vitro effects of the addition of antibody specific for viral E protein on DEN infection. U937 cells (A) or K562 cells (B) were infected with DEN-2 PL046 which had been preincubated with serially diluted MAb-containing ascitic fluid at 37°C for 60 min. Virus titers in the culture supernatants were determined at day 4 (for U937 cells) or day 6 (for K562 cells) p.i. Ab, antibody.
FIG. 5.
In vivo effects of a neutralizing antibody on DEN infection in K562-SCID mice. (A) K562-SCID mice were infected by 107 PFU of DEN-2 PL046 that was pretreated with either PBS or 100-fold- or 2-fold-diluted ascitic fluid containing MAb 56-3.1 at 37°C for 60 min. (B) K562-SCID mice were passively transferred with either 0.5 ml of PBS or undiluted or 10-fold diluted MAb 56-3.1 1 day prior to the challenge of 107 PFU of DEN-2 PL046. The survival of mice was checked daily up to 30 days p.i.
An innate immunity against DEN may exist in SCID mice.
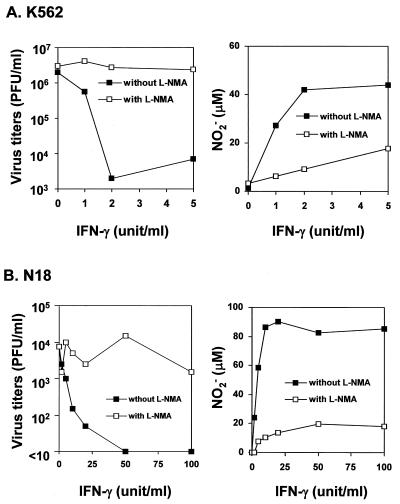
Persistent DEN infection was readily established in K562 cells infected with DEN-1 Hawaii-N or DEN-2 PL046, in which the cells constantly produced virus without obvious cytopathic effects (data not shown). By omitting the step of direct i.t. challenge, we next modified the previous challenge protocol and explored if such persistently infected cells could be implanted into SCID mice. Data from two independent experiments (involving a total of eight mice) revealed that all the persistently infected cells failed to colonize the SCID mice, which survived as long as did normal SCID mice (data not shown). The exact mechanism for the growth failure of persistently DEN-infected K562 cells, but not of K562 cells, in the SCID mice remains unclear. However, the inability of DEN to kill the SCID mice engrafted with persistently infected K562 cells seems to suggest that an innate immunity might play a role in protection against DEN during the early stages of implantation. One possible mechanism to confer the innate immunity and defensive power is the generation of NO. Previously, our group demonstrated that NO could mediate an antiviral effect against both primary and persistent infections with Japanese encephalitis virus in vitro and in vivo (26). To test if an NO-mediated antiviral mechanism might operate against DEN replication, we performed a coculture assay by mixing DEN-infected target cells with activated macrophages capable of producing a large quantity of NO (26). As shown in Fig. 6, when cocultured with the DEN-2 PL046-infected K562 cells (Fig. 6A) or murine neuroblastoma N18 cells (Fig. 6B), IFN-γ-activated murine macrophage RAW 264.7 cells efficiently hindered the virus replication in these contiguous bystanders, and this antiviral effect could be reversed by an NO-synthase inhibitor, l-NMA. Moreover, the inhibition as well as the reversal featured in Fig. 6 appeared to correlate closely with the amounts of nitrite generated in the cultures, i.e., IFN-γ induced NO production in a dose-dependent manner, while the addition of 500 μM l-NMA abrogated the IFN-γ-induced NO production to nearly the basal level. The combined results imply that an antiviral effect of NO, if properly triggered, may play a crucial role in SCID mice to restrict DEN replication, especially at the early phase of cell implantation.
FIG. 6.
Inhibition of DEN-2 PL046 replication in human K562 cells (A) or murine neuronal N18 cells (B) by coculture with IFN-γ-activated murine RAW 264.7 cells in the presence or absence of the NO synthase inhibitor l-NMA. The virus titers in the culture supernatants were measured by plaque assays. The amounts of NO2− production in culture media from the tested cells were measured by Griess assay as described in Materials and Methods.
DISCUSSION
Lack of a suitable animal model has hampered the study of DEN, especially in the areas of viral pathogenesis, virus-host interaction, and vaccine development against DEN infection. In the present study, we have demonstrated the feasibility of K562-SCID mice as an animal model for DEN infections. By contrast, without manipulation the SCID mice themselves appear to resist peripheral infections with DEN. Thus, implantation of DEN-susceptible human cell lines into SCID mice can potentially be used as a powerful protocol to construct a variety of hosts suited for a variety of DEN studies. In addition, the infection frequency of K562-SCID mice for DEN shown here appeared to be higher than that of hu-PBL-SCID mice previously documented by Wu et al. (45). This is particularly crucial for some DEN research studies, such as evaluation of an antivirus maneuver that may require the study of a large number of animals to answer specific statistical questions.
In recent years, there have been many reports of DEN infection accompanied by encephalitis or encephalitic manifestations in humans (29, 34, 35, 39). The universal neurotropism of flaviviruses in both rodents and arthropod vectors (31) may somewhat parallel the clinical findings in DEN-infected patients. Similarly, not long before they were killed by virus infection, symptoms with overt encephalopathy were also observed in DEN-infected K562-SCID mice. Since the K562-SCID mice were engineered to accommodate an extracerebral site for DEN replication, the course of DEN infection in these animals was, to some extent, similar to that of other flavivirus infections, in which after inoculation, the virus first replicated in local tissues and regional lymph nodes and was then carried via lymphatics into the thoracic duct and the bloodstream. Thus, the K562-SCID mice seem to be a suitable animal model system to study DEN-associated encephalopathy. On the other hand, the failure of K562-SCID mice to manifest DHF/DSS in response to DEN infections supports the notion that the pathogenesis of DHF/DSS may involve a complicated immunopathological mechanism requiring further exploration.
Lack of an extraneural replication site appears to explain why DEN infections are avirulent by peripheral challenge for both immunocompetent and SCID mice. Contrary to DEN infection, both Semliki Forest virus (3) and West Nile virus (14) can kill infected SCID mice without any prior manipulation. By peripheral inoculation, the K562-SCID mice, but not the SCID mice themselves, succumbed to infections by different strains of DEN (Fig. 3A), whereas the mock-infected K562-SCID mice healthily survived as long as normal SCID mice. These data strongly suggest that DEN infection plays a decisive role in leading to the ailment status and the eventual death of the mice tested, though we cannot rule out the possible involvement of infected K562 cells in the killing process. Moreover, the severity of illness and the mortality rate of the infected K562-SCID mice varied when they were challenged by different DEN strains (Fig. 3), suggesting the existence of diverse degrees of virulence in the engrafted mice among DENs isolated from humans. Thus, this animal system might potentially be utilized to define the virulence of various human DEN isolates and to characterize the molecular determinant(s) for such viral virulence. Yet, there are limitations to use of this animal model to interpret DEN pathogenesis in humans. For instance, the results from several previous studies (41, 44) have demonstrated that the so-called neurovirulent strains of mouse-adapted viruses were actually shown to be attenuated for humans. Further studies are apparently needed to elucidate whether any correlation exists between neurovirulence in SCID mice and virus-specified factors determining DEN pathogenesis in humans. Nevertheless, although the disease presentation in K562/SCID mice may differ from that in humans following DEN infections, this animal model is considered suitable for, at least, studying some aspects of DEN infection by a peripheral route. In addition, when subjected to adaptive transfer of different kinds of immune components from immunocompetent hosts, this animal model may also facilitate our understanding regarding how immune responses control dengue virus replication in vivo.
ADE has been suggested to play a major role in DEN pathogenesis and has been demonstrated to enhance DEN infection in a number of in vitro experiments (18). For example, a human monocytic cell line, U937, has been widely employed for in vitro ADE studies (7). However, except for that in rhesus monkeys (16), no experimental systems have been able to mimic the ADE phenomenon in vivo. To study ADE in vivo, we have tried to establish an SCID mouse model implanted with human U937 cells (U937-SCID mice). These cells apparently consist of high affinity Fc receptors that are presumably important for the occurrence of ADE. However, we were unable to engraft SCID mice with U937 cells; these cells grew vigorously and quickly killed the mice within 1 month postimplantation even without the presence of DEN infections. Thus, SCID mice implanted with other human cells require further investigation in order to perform in vivo ADE experiments.
The major envelope protein, E protein, of flaviviruses can elicit neutralizing antibodies and induce a protective immune response in the infected host (reviewed in reference 31). At low concentrations in vitro, the DEN E-protein-specific antibodies, both neutralizing and nonneutralizing, can mediate an ADE phenomenon implicated in the pathogenesis of DHF/DSS. Among the three classes of human Fcγ receptors, K562 cells express only the low-affinity RII that has been shown to moderately enhance mouse antibody with subclass 1 of immunoglobulin G (IgG1) in ADE experiments for DEN infection (27). The MAbs 56-3.1 and 17-2 used in this study are neutralizing antibodies and belong to the IgG1 subclass (data not shown). Yet, for unknown reasons they failed to augment DEN infection in K562 cells either in vitro or in vivo (Fig. 4B). Nevertheless, we have demonstrated that in DEN-infected K562-SCID mice, passive transfers of MAb 56-3.1 significantly reduced the mortality and delayed the onset of paralysis symptoms (Fig. 5), indicating that an in vitro neutralizing antibody could also efficiently protect the mice from lethal challenge. Conceivably, this animal system may be suitable for exploring the effects of new antiviral drugs or immune-modulating cytokines on DEN infection in vivo.
In contrast to the case of SCID mice engrafted with K562 cells, inoculation of persistently DEN-1 Hawaii-N- or DEN-2 PL046-infected K562 cells invariably failed to result in tumor mass formation in the peritoneal cavities of the mice. It has been demonstrated that prior immunosuppression of SCID mice, either by whole-body irradiation or by treatment with an antiserum against natural killer (NK) cells (anti-asialo GM1), was necessary to establish the growth of tumors derived from human T-cell leukemia-transformed human cell lines (12), indicating the direct involvement of NK cells in innate immunity for SCID mice. Similarly, human NK cells have been shown to lyse DEN-infected cells to a greater degree than uninfected cells (23), suggesting that human NK cells may also play a role against primary DEN infection. Conceivably, the failure of implantation of the persistently DEN-infected K562 cell line into SCID mice is likely due to activated mouse NK cells that kill infected cells at an early stage of engraftment. However, it has been reported that when assayed against human K562 cells, the NK cells derived from interleukin-2 activated SCID mice exhibited no cytotoxicity across the species barrier (36). Recently, the generation of NO has been demonstrated to hinder the productive infection of several animal viruses, including herpes simplex virus type 1, ectromelia virus, vaccinia virus (11, 21), vesicular stomatitis virus (4), murine Friend leukemia retrovirus (1), and Japanese encephalitis virus (26). In addition, we showed here that DEN replication in K652 and murine neuronal N18 cells was inhibited, in an NO-dependent manner, by IFN-γ-activated mouse macrophages (Fig. 6), implying that NO could be one of the vital factors that enable the host’s innate immunity to control DEN infections. The combined data illustrate that the establishment of K562-SCID mice may provide a convenient system able to facilitate not only the study of DEN pathogenesis but also the evaluation of anti-DEN agents as well as vaccine development.
ACKNOWLEDGMENTS
We thank the National Institute of Preventive Medicine, Taiwan, Republic of China, for providing the DEN-2 PL046, DEN-1 766733A, and DEN-4 466088A. We also wish to thank D. J. Gubler for the DEN-2 prototype strain New Guinea C, DEN-1 prototype strain Hawaii, and DEN-3 prototype strain H87, K. Lam for DEN-2 07587, and C. J. Lai for the DEN-1 strain Hawaii-N. We also thank D. E. Griffin for N18 cells.
This work was supported by the National Health Research Institute, Republic of China (85-CNT-CR-01-P, 86-CNT-CR-501-P, and DD01-86IX-CR-501P).
REFERENCES
- 1.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo M A. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor S, Scallan M F, Morris M M, Dyson H, Fazakerley J K. Role of immune responses in protection and pathogenesis during Semliki Forest virus encephalitis. J Gen Virol. 1996;77:281–291. doi: 10.1099/0022-1317-77-2-281. [DOI] [PubMed] [Google Scholar]
- 4.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonpucknavig S, Vuttiviroj O, Boonpucknavig V. Infection of young adult mice with dengue virus type 2. Trans R Soc Trop Med Hyg. 1981;75:647–653. doi: 10.1016/0035-9203(81)90142-5. [DOI] [PubMed] [Google Scholar]
- 6.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 7.Brandt W E, McCown J M, Gentry M K, Russell P K. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect Immun. 1982;36:1036–1041. doi: 10.1128/iai.36.3.1036-1041.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome, organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 9.Chen L-K, Liao C-L, Lin C-G, Lai S-C, Liu C-I, Ma S-H, Huang Y-Y, Lin Y-L. Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology. 1996;217:220–229. doi: 10.1006/viro.1996.0109. [DOI] [PubMed] [Google Scholar]
- 10.Chen L-K, Lin Y-L, Liao C-L, Lin C-G, Huang Y-L, Yeh C-T, Lai S-C, Jan J-T, Chin C. Generation and characterization of organ-tropism mutants of Japanese encephalitis virus in vivo and in vitro. Virology. 1996;223:79–88. doi: 10.1006/viro.1996.0457. [DOI] [PubMed] [Google Scholar]
- 11.Croen K D. Evidence for antiviral effect of nitric oxide: inhibition of herpes simplex virus type-1 replication. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuer G, Stewart S A, Baird S M, Lee F, Feuer R, Chen I S. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J Virol. 1995;69:1328–1333. doi: 10.1128/jvi.69.2.1328-1333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubler D J. Dengue viruses. In: Webster R G, Granoff A, editors. Encyclopedia of virology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 324–331. [Google Scholar]
- 14.Halevy M, Akov Y, Ben-Nathan D, Kobiler D, Lachmi B, Lustig S. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch Virol. 1994;137:355–370. doi: 10.1007/BF01309481. [DOI] [PubMed] [Google Scholar]
- 15.Halstead S B, O’Rourke E J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead S B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 17.Halstead S B, Venkateshan C N, Gentry M K, Larsen L K. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol. 1984;132:1529–1532. [PubMed] [Google Scholar]
- 18.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 19.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 20.Harris N, Buller R M, Karupiah G. Gamma interferon-induced nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 22.Kohler G, Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976;6:511–519. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 23.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Rothman A L, Livingston P G, Janus J, Ennis F A. Human immune responses to dengue viruses. Southeast Asian J Trop Med Public Health. 1990;21:658–662. [PubMed] [Google Scholar]
- 24.Kurane I, Kontny U, Janus J, Ennis F A. Dengue-2 virus infection of human mononuclear cell lines and establishment of persistent infection. Arch Virol. 1990;110:91–101. doi: 10.1007/BF01310705. [DOI] [PubMed] [Google Scholar]
- 25.Kurane I, Ennis F A. Immunity and immunopathology in dengue virus infections. Semin Immunol. 1992;4:121–127. [PubMed] [Google Scholar]
- 26.Lin Y-L, Huang Y-L, Ma S-H, Yeh C-T, Chiou S-Y, Chen L-K, Liao C-L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littaua R, Kurane I, Ennis F A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 28.Lozzio B B, Lozzio C B, Bamberger E G, Feliu A S. A multipotential leukemia cell line (K-562) of human origin. Proc Soc Exp Biol Med. 1981;166:546–550. doi: 10.3181/00379727-166-41106. [DOI] [PubMed] [Google Scholar]
- 29.Lum L C, Lam S K, Choy Y S, George R, Harum F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 30.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 961–1034. [Google Scholar]
- 32.Mosier D E. Immunodeficient mice xenografted with human lymphoid cells: new models for in vivo studies of human immunobiology and infectious diseases. J Clin Immunol. 1990;10:185–191. doi: 10.1007/BF00918650. [DOI] [PubMed] [Google Scholar]
- 33.Mosier D E. Viral pathogenesis in hu-PBL-SCID mice. Semin Immunol. 1996;8:255–262. doi: 10.1006/smim.1996.0032. [DOI] [PubMed] [Google Scholar]
- 34.Nimmannitya S, Thisyakorn U, Hemsrichart V. Dengue haemorrhagic fever with unusual manifestations. Southeast Asian J Trop Med Public Health. 1987;18:398–406. [PubMed] [Google Scholar]
- 35.Patey O, Ollivaud L, Breuil J, Lafaix C. Unusual neurologic manifestations occurring during dengue fever infection. Am J Trop Med Hyg. 1993;48:793–802. doi: 10.4269/ajtmh.1993.48.793. [DOI] [PubMed] [Google Scholar]
- 36.Pisa P, Sitnicka E, Hansson M. Activated natural killer cells suppress myelopoiesis in mice with severe combined immunodeficiency. Scand J Immunol. 1993;37:529–532. doi: 10.1111/j.1365-3083.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 37.Porterfield J S. Antibody-dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- 38.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 39.Row D, Weinstein P, Murray-Smith S. Dengue fever with encephalopathy in Australia. Am J Trop Med Hyg. 1996;54:253–255. doi: 10.4269/ajtmh.1996.54.253. [DOI] [PubMed] [Google Scholar]
- 40.Russel P K, Nisalak A. Dengue virus identification by the plaque reduction neutralisation test. J Immunol. 1967;99:291–296. [PubMed] [Google Scholar]
- 41.Sabin A B, Schlesinger R W. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger R W. Dengue viruses. New York, N.Y: Springer-Verlag; 1977. pp. 72–73. [Google Scholar]
- 43.Sinniah M, Igarashi A. Dengue haemorrhagic fever. Rev Med Virol. 1995;5:193–203. [Google Scholar]
- 44.Wissman C L, Jr, Sweet B H, Rosenzweig E C, Eylar O R. Attenuated living type 1 dengue vaccines. Am J Trop Med Hyg. 1963;12:620–623. [Google Scholar]
- 45.Wu S-J L, Hayes C G, Dubois D R, Windheuser M G, Kang Y-H, Watts D M, Sieckmann D G. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am J Trop Med Hyg. 1995;52:468–476. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]