Abstract
Borna disease virus (BDV) uses a unique strategy of replication and transcription which takes place in the nucleus, unlike other known, nonsegmented, negative-stranded RNA viruses of animal origin. In this process, viral constituents necessary for replication must be transported to the nucleus from the cytoplasm. We report here the evidence that BDV P protein, which may play an important role in viral replication and transcription, is transported into the nucleus in the absence of other viral constituents. This transportation is accomplished by its own nuclear localization signals (NLSs), which are present in both N-terminal (29PRPRKIPR36) and C-terminal (181PPRIYPQLPSAPT193) regions of the protein. These two NLSs can function independently and both have several Pro residues as key amino acids.
Borna disease (BD) is a naturally occurring progressive encephalopathy of horses and sheep caused by infection with a neurotropic enveloped virus, BD virus (BDV) (16, 26). Molecular biological analyses have shown that BDV has a nonsegmented, negative-stranded RNA genome of 8.9 kb, which is present in the nucleus of BDV-infected cells (3–6, 22). The BDV genome consists of at least six open reading frames (ORFs). ORF I encodes nucleoproteins (N) of 38 or 40 kDa (15, 17, 20). ORF II encodes a phosphoprotein (P) of 23 to 24 kDa (12, 15, 28). ORF III encodes an 18-kDa glycoprotein (M, gp18) (11). ORF IV encodes a protein of 56 or 64 kDa which is glycosylated to give a glycoprotein of 84 or 94 kDa (8, 23). ORF V encodes a predicted RNA-dependent RNA polymerase (L) of 170 to 180 kDa (3, 5). Recently, ORF ×1, which overlaps ORF II, was reported to encode a protein (p10, or X) of 10 kDa (30). The genomic organization of BDV is similar to those of animal rhabdoviruses such as vesicular stomatitis virus and rabies virus except that the BDV genome has an additional ORF ×1 which overlaps with ORF II but uses a different reading frame. Another major difference from the animal rhabdoviruses is that the BDV genome replicates in the nucleus of infected cells (4–6, 22).
The function of the BDV P protein is not known, although it is generally assumed that the BDV P associates and cooperates with the L protein to play a pivotal role in viral transcription and replication. P proteins with such a role have been identified in animal rhabdoviruses (29). If BDV P is a cofactor of L polymerase, it should migrate to the nucleus, where viral transcription and replication take place. Here, we report that BDV P is transported into the nucleus in the absence of other viral constituents and that the transportation is accomplished by virtue of BDV P protein’s own nuclear localization signals (NLSs), which are present in both N-terminal and C-terminal regions. The NLSs of BDV P are unique in that both can function independently and both have several proline residues as key amino acids.
MATERIALS AND METHODS
BDV and cells.
Madin-Darby canine kidney (MDCK) cells persistently infected with BDV (MDCK/BDV) (9) were kindly provided by R. Rott, Justus-Liebig-Universität Giessen. MDCK cells and COS-7 cells (7) were cultured as described previously (13).
Plasmid construction.
The eukaryotic expression plasmid pP-Wild encoding BDV P (amino acids 1 to 201) was constructed as follows. To obtain cDNA corresponding to the products of the entire BDV genome, total RNA extracted from MDCK/BDV cells was reverse transcribed by using a BDV-specific primer pair as described previously (25). BDV P cDNA was amplified by PCR from a BDV cDNA with BDV P-specific primers 1 and 2 (Tables 1 and 2) and then cloned into the EcoRI-KpnI sites immediately downstream of and in frame with the influenza virus hemagglutinin (HA) 12CA5 epitope tag in the eukaryotic expression vector pcDL-HA (13). Eukaryotic expression plasmids encoding a series of deletion mutants of BDV P were constructed as described above with the sets of primers listed in Tables 1 and 2. The resultant constructs, pP-del.N18, pP-del.N40, pP-del.N82, pP-del.C117, pP-del.C148, and pP-del.C182, encoded BDV P lacking the N-terminal 18, 40, and 82 amino acid residues and the C-terminal 85, 54, and 20 amino acid residues, respectively. pRSV-1/41, pRSV-41/181, pRSV-172/201, pRSV-18/36, pRSV-172/193, and pRSV-179/201, eukaryotic expression plasmids encoding Escherichia coli lacZ fused with relatively large polypeptide chains from BDV P, were constructed as described for pRSV-N.LacZ (13). Briefly, cDNA fragments encoding various polypeptides from BDV P were amplified by PCR with pairs of primers (Tables 1 and 2) with BDV P cDNA as a template and then cloned into the KpnI site between the gpt and trpS sequences, in frame with the lacZ gene in RSV-LacZ (14). Plasmids pRSV-18/28, pRSV-29/36, pRSV-181/193, and pRSV-192/201, expressing β-galactosidase fused with relatively short polypeptides from BDV P, were constructed similarly except for the use of adapters (Tables 1 and 2). pRSV-sub.29, pRSV-sub.31, pRSV-sub.35, and pRSV-sub.32/33, encoding amino acid-substituted fusion proteins of pRSV-29, -31, -35, and -32/33, respectively, and pRSV-sub.182, pRSV-sub.186, pRSV-sub.189, and pRSV-sub.192, encoding amino acid-substituted fusion proteins of pRSV-182, -186, -189, and -192, respectively, were constructed similarly with adapters (Tables 1 and 2). The nucleotide numbering used here follows that used previously for a horse-derived BDV, strain V (the EMBL databank, accession number U04608). The nucleotide sequences of recombinant constructs were confirmed by using a 373A automatic DNA sequencer (Applied Biosystems).
TABLE 1.
Primers and adapters used to construct expression plasmids
| Oligonucleotide | Amino acid position | Nucleotide sequencea |
|---|---|---|
| Primer | ||
| 1 | 1272–1292 | 5′-tgtgaattcATGGCAACGCGACCATCGAGT-3′ |
| 2 | 1877–1855 | 5′-tccggtaccTTATGGTATGATGTCCCATTCAT-3′ |
| 3 | 1325–1346 | 5′-tgtgaattCCAGACACTACGACGGGAACGA-3′ |
| 4 | 1391–1412 | 5′-tgtgaattCCAACCAGTCGACCAGCTCCTG-3′ |
| 5 | 1518–1539 | 5′-tgcgaattcGCCGAGAATAGCATGATCGAGG-3′ |
| 6 | 1619–1599 | 5′-tatggtaccttaCACTTGGAGGGCGGACAGGGA-3′ |
| 7 | 1712–1693 | 5′-tgcggtaccttaTGTCTTCATGGAGCGATCCA-3′ |
| 8 | 1814–1793 | 5′-tatggtaccttaAGGTGCAGGATGGGAGGGCAAC-3′ |
| 9 | 1272–1289 | 5′-tctggtacctATGGCAACGGGACCATCG-3′ |
| 10 | 1393–1376 | 5′-tgcggtaccTGGGTCAATGCATTCCTT-3′ |
| 11 | 1392–1411 | 5′-tctggtacctCAACCAGTCGACCAGCTCCT-3′ |
| 12 | 1815–1800 | 5′-ataggtacctGAGGTGCAGGATGGGA-3′ |
| 13 | 1785–1805 | 5′-tacggtacctGCACCCATGTTGCCCTCCCAT-3′ |
| 14 | 1874–1857 | 5′-tctggtacctgTGGTATGATGTCCCACTC-3′ |
| 15 | 1326–1346 | 5′-actggtacctCAGACACTACGACGGGAACGA-3′ |
| 16 | 1379–1360 | 5′-actggtacctgCCTTGGGATCTTCCGTGGTC-3′ |
| 17 | 1850–1833 | 5′-tatggtacctgTGTCGGGGCACTTGGGAG-3′ |
| 18 | 1806–1826 | 5′-tctggtaCCTGCACCTCCGCGCATTTAT-3′ |
| Adapter | ||
| 1 | 1323–1355 | 5′-CCCAGACACTACGACGGGAACGATCGGGGTCAcaggtac |
| 3′-catGGGGTCTGTGATGCTGCCCTTGCTAGCCCCAGTgtc | ||
| 2 | 1356–1379 | 5′-caCCAAGACCACGGAAGATCCCAAGGcaggtac |
| 3′-catggtGGTTCTGGTGCCTTCTAGGGTTCCgtc | ||
| 3 | 1813–1850 | 5′-CTCCGCGCATTTATCCCCAGCTCCCAAGTGCCCCGACAcaggtac |
| 3′-catgGAGGCGCGTAAATAGGGGTCGAGGGTTCACGGGGCTGTgtc | ||
| 4 | 1874–1845 | 5′-CGACAACGGATGAGTGGGACATCATACCAcaggtac |
| 3′-catgGCTCTTGCCTACTCACCCTGTAGTATGGTgtc | ||
| 5 | 1356–1379 | 5′-caGCAAGACCACGGAAGATCCCAAGGcaggtac |
| 3′-catggtCGTTCTGGTGCCTTCTAGGGTTCCgtc | ||
| 6 | 1356–1379 | 5′-caCCAAGAGCACGGAAGATCCCAAGGcaggtac |
| 3′-catggtGGTTCTCGTGCCTTCTAGGGTTCCgtc | ||
| 7 | 1356–1379 | 5′-caCCAAGACCACGGAAGATCGCAAGGcaggtac |
| 3′-catggtGGTTCTGGTGCCTTCTAGCGTTCCgtc | ||
| 8 | 1356–1379 | 5′-caCCAAGACCACAGCAGATCCCAAGGcaggtac |
| 3′-catggtGGTTCTGGTGTCGTCTAGGGTTCCgtc | ||
| 9 | 1813–1850 | 5′-CTGCGCGCATTTATCCCCAGCTCCCAAGTGCCCCGACAcaggtac |
| 3′-catgGACGCGCGTAAATAGGGGTCGAGGGTTCACGGGGCTGTgtc | ||
| 10 | 1813–1850 | 5′-CTCCGCGCATTTATGCCCAGCTCCCAAGTGCCCCGACAcaggtac |
| 3′-catgGAGGCGCGTAAATACGGGTCGAGGGTTCACGGGGCTGTgtc | ||
| 11 | 1813–1850 | 5′-CTCCGCGCATTTATCCCCAGCTCGCAAGTGCCCCGACAcaggtac |
| 3′-catgGAGGCGCGTAAATAGGGGTCGAGCGTTCACGGGGCTGTgtc | ||
| 12 | 1813–1850 | 5′-CTCCGCGCATTTATCCCCAGCTCCCAAGTGCCGCGACAcaggtac |
| 3′-catgGAGGCGCGTAAATAGGGGTCGAGGGTTCACGGCGCTGTgtc |
Uppercase letters represent the nucleotides derived from BDV P, and the lowercase letters indicate what was added to generate the restriction site. The restriction sites are underlined. The boldface letters represent the nucleotide substituted from the BDV P sequence.
TABLE 2.
Primer pairs and adapters used to amplify BDV P and its mutant fragments inserted into eukaryotic expression plasmids
| Construct | Primer pair
|
Adapter | |
|---|---|---|---|
| Sense | Antisense | ||
| pP-Wild | Primer 1 | Primer 2 | |
| pP-del.N18 | Primer 3 | Primer 2 | |
| pP-del.N40 | Primer 4 | Primer 2 | |
| pP-del.N82 | Primer 5 | Primer 2 | |
| pP-del.C117 | Primer 1 | Primer 6 | |
| pP-del.C148 | Primer 1 | Primer 7 | |
| pP-del.C182 | Primer 1 | Primer 8 | |
| pRSV-1/41 | Primer 9 | Primer 10 | |
| pRSV-41/181 | Primer 11 | Primer 12 | |
| pRSV-172/201 | Primer 13 | Primer 14 | |
| pRSV-18/36 | Primer 15 | Primer 16 | |
| pRSV-172/193 | Primer 13 | Primer 17 | |
| pRSV-179/201 | Primer 18 | Primer 14 | |
| pRSV-18/28 | Adapter 1 | ||
| pRSV-29/36 | Adapter 2 | ||
| pRSV-181/193 | Adapter 3 | ||
| pRSV-192/201 | Adapter 4 | ||
| pRSV-sub.29 | Adapter 5 | ||
| pRSV-sub.31 | Adapter 6 | ||
| pRSV-sub.35 | Adapter 7 | ||
| pRSV-sub.32/33 | Adapter 8 | ||
| pRSV-sub.182 | Adapter 9 | ||
| pRSV-sub.186 | Adapter 10 | ||
| pRSV-sub.189 | Adapter 11 | ||
| pRSV-sub.192 | Adapter 12 | ||
Antibodies.
Anti-BDV P polyclonal antiserum was prepared by immunization of a rabbit with glutathione column-purified glutathione S-transferase (GST)–BDV-P fusion protein expressed in E. coli. Mouse monoclonal antibody (MAb) to the 12CA5 epitope of influenza virus HA was obtained from Boehringer Mannheim. Mouse anti-β-galactosidase MAb was from Gibco BRL. Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin G (IgG) antibody was from Amersham. Dichlorotriazinyl amino fluorescein (DTAF)-conjugated goat anti-rabbit IgG antibody was from Immunotech. HRP-conjugated sheep anti-mouse IgG antibody was from Amersham. DTAF-conjugated goat anti-mouse IgG antibody was from Immunotech.
Transfection, Western blotting, and immunofluorescence analysis.
For eukaryotic expression of BDV P, COS-7 cells were seeded at 2.0 × 105 cells per 35-mm-diameter glass-bottom culture dish, and on the next day the cells were transfected with 2 μg of plasmid DNA with Lipofectamine (Gibco BRL). After cultivation for 48 h, the transfected cells were harvested for detection of the expressed BDV P by Western blotting with rabbit anti-BDV P serum (1:500 dilution) as the first antibody and HRP-conjugated sheep anti-mouse IgG antibody (1:5,000 dilution) as the secondary antibody. To detect the β-galactosidase fusion proteins, the cultured cells were subjected to Western blotting analysis with the anti-β-galactosidase MAb (1:500 dilution) as the first antibody and HRP-conjugated sheep anti-mouse IgG antibody (1:5,000 dilution) as the secondary antibody. An ECL Western blotting kit (Amersham) was used to visualize the expressed protein.
The transfected cells were also subjected to indirect immunofluorescence assay (IFA). After culture, the cells were fixed with 4% paraformaldehyde prior to treatment with 0.4% Triton X-100 (32). To detect BDV P and its deletion mutants, anti-BDV P antiserum (1:500 dilution) was used as the first antibody, and DTAF-conjugated goat anti-mouse IgG antibody (1:50 dilution) was used as the secondary antibody. Similarly, for the detection of BDV P-fused β-galactosidase proteins, mouse anti-β-galactosidase MAb (1:100 dilution) and DTAF-conjugated goat anti-mouse IgG antibody (1:50 dilution) were used. To detect the 12CA5 epitope of HA, mouse anti-12CA5 MAb (1:40 dilution) and DTAF-conjugated goat anti-mouse IgG antibody (1:50 dilution) were used. After being stained, the cells were analyzed with a confocal laser scanning microscope (Meridian Instruments). MDCK/BDV cells were used as a positive control, and noninfected MDCK cells or nontransfected COS-7 cells served as negative controls. The results shown in Fig. 1C, 2C, 3C, 4C, 5C, and 6C are representative examples of the average expression pattern found in each experiment.
FIG. 1.
Expression and subcellular localization of deletion mutants of BDVP. (A) The cDNA fragments of BDV P inserted into pcDL-HA are shown. The numbers indicate amino acid positions of BDV P. (B) Expression of wild-type and deletion mutants of BDV P in COS-7 cells as detected by Western blotting with rabbit antiserum. (C) IFA staining of COS-7 cells expressing wild-type and deletion mutants of BDV P with rabbit anti-BDV P. Noninfected MDCK cells (MDCK/−) served as a negative control (a), and MDCK/BDV cells served as a positive control of BDV P in persistently infected cells (b). COS-7 cells were transfected with pCDL-HA (c), pP-Wild (e), pP-del.N18 (f), pP-del.N40 (g), pP-del.N82 (h), pP-del.C117 (i), pP-del.C148 (j), or pP-del.C182 (k). Nontransfected COS-7 cells (COS7/−) served as a negative transfection control (d).
FIG. 2.
Expression and subcellular localization of β-galactosidase fused with the N-terminal, C-terminal, and middle regions of BDV P. (A) Fragments of BDV P fused to β-galactosidase are shown schematically. The numbers indicate the amino acid positions of BDV P. (B) Expression of the fusion protein in COS-7 cells as detected by Western blotting with mouse anti-β-galactosidase MAb. (C) IFA staining of COS-7 cells expressing fusion proteins with anti-β-galactosidase MAb. COS-7 cells were transfected with pRSV-LacZ (a), pRSV-HA (b), pRSV-1/41 (d), pRSV-41/181 (e), or pRSV-172/201 (f). Nontransfected COS-7 cells (COS7/−) served as a negative control (c).
FIG. 3.
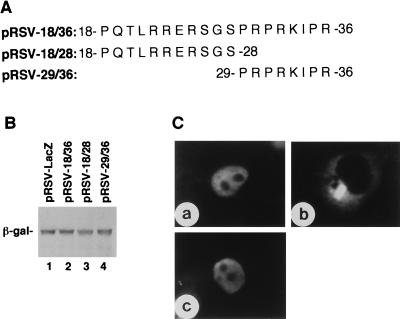
Expression and subcellular localization of β-galactosidase fused with short peptides from the N-terminal region of BDV P. (A) Amino acid sequences of short peptides fused to β-galactosidase. (B) Expression of the fusion proteins in COS-7 cells as detected by Western blotting with mouse anti-β-galactosidase MAb. (C) IFA staining of COS-7 cells expressing fusion proteins with anti-β-galactosidase MAb. COS-7 cells were transfected with pRSV-18/36 (a), pRSV-18/28 (b), or pRSV-29/36 (c).
FIG. 4.
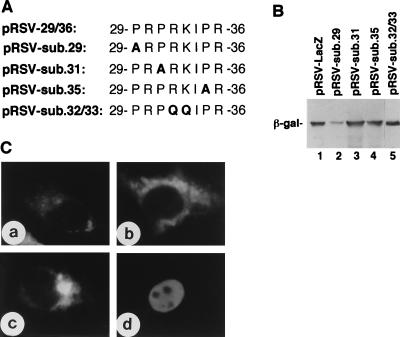
Expression and subcellular localization of β-galactosidase fused with substitution mutants of 29PRPRKIPR36 derived from BDV P. (A) The mutant peptides fused to β-galactosidase are shown. The Pro residue (P) at position 29, 31, or 35 was replaced with Ala (A, boldface), and Arg and Lys residues at positions 32 and 33 were replaced with Gln (Q, boldface). (B) Expression of fusion proteins in COS-7 cells as detected by Western blotting with mouse anti-β-galactosidase MAb. (C) IFA staining of COS-7 cells expressing fusion proteins with anti-β-galactosidase. COS-7 cells were transfected with pRSV-sub.29 (a), pRSV-sub.31 (b), pRSV-sub.35 (c), or pRSV-sub.32/33 (d).
FIG. 5.
Expression and subcellular localization of β-galactosidase fused with short peptides from the C-terminal region of BDV P. (A) Amino acid sequences of short peptides fused to β-galactosidase. (B) Expression of the fusion proteins in COS-7 cells as detected by Western blotting with mouse anti-β-galactosidase MAb. (C) IFA staining of COS-7 cells expressing fusion proteins with anti-β-galactosidase MAb. COS-7 cells were transfected with pRSV-172/193 (a), pRSV-179/201 (b), pRSV-181/193 (c), or pRSV-192/201 (d).
FIG. 6.
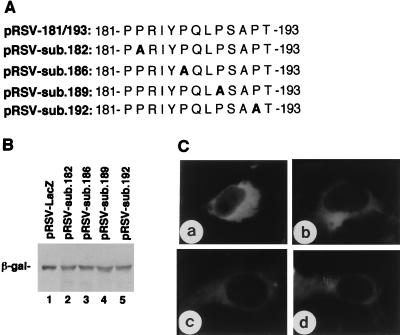
Expression and subcellular localization of β-galactosidase fused with substitution mutants of 181PPRIYPQLPSAPT193 derived from the C-terminal region of BDV P. (A) Mutant peptides fused to β-galactosidase. The Pro residue (P) at position 182, 186, 189, or 192 was replaced with Ala (A, boldface). (B) Expression of fusion proteins in COS-7 cells as detected by Western blotting with mouse anti-β-galactosidase MAb. (C) IFA staining of COS-7 cells expressing fusion proteins with anti-β-galactosidase. COS-7 cells were transfected with pRSV-sub.182 (a), pRSV-sub.186 (b), pRSV-sub.189 (c), or pRSV-sub.192 (d).
RESULTS
Intracellular localization of BDV P in cells infected with BDV and transfected with BDV-P expression plasmids.
The specificity of rabbit anti-BDV P antiserum was verified by Western blotting of the lysates of MDCK cells noninfected and persistently infected with BDV. As shown in Fig. 1B, a protein of approximately 23 to 24 kDa was detected with the anti-BDV P antiserum in the MDCK/BDV cell lysate (lane 2) but not in the noninfected MDCK lysate (lane 1). Anti-BDV P antiserum preabsorbed with a GST-BDV P fusion protein expressed in E. coli did not detect this protein in MDCK/BDV cells (data not shown), confirming that this antiserum specifically recognized BDV P. IFA with the anti-BDV P antiserum allowed visualization of the intracellular localization of the BDV P. The staining pattern of MDCK/BDV cells was compatible with the previously reported observations (9) of intense spot-like staining in the nucleus with relatively weak and diffuse staining in the cytoplasm (Fig. 1C, panel b). No staining was detected in noninfected MDCK cells (Fig. 1C, panel a). Neither the preabsorbed antiserum nor the preimmune rabbit serum stained the MDCK/BDV cells (data not shown).
To examine whether BDV P is transported into the nucleus in the absence of other viral constituents, the eukaryotic expression plasmid pP-Wild (Fig. 1A) encoding the entire BDV P was constructed and transfected into COS-7 cells. Transient expression of BDV P was examined by Western blotting with anti-BDV P antiserum. A protein of approximately 23 to 24 kDa was detected in the lysate of cells transfected with pP-Wild (Fig. 1B, lane 5). Similar results were obtained by Western blotting with a mouse MAb to the HA epitope (data not shown). The anti-BDV P antiserum did not react with the HA 12CA5 epitope in the lysate of cells transfected with pcDL-HA (Fig. 1B, lane 3). Thus, the recombinant BDV P protein which was detectable with anti-BDV P antiserum was transiently expressed in COS-7 cells after transfection with pP-Wild.
The subcellular localization of BDV P in COS-7 cells transfected with pP-Wild was examined by IFA with anti-BDV P antiserum. In contrast to the IFA pattern observed in MDCK/BDV cells, the transfected COS-7 cells showed diffuse and intense fluorescent staining mostly in the nucleus (Fig. 1C, panel e). This antiserum did not react with cells transfected with pcDL-HA as a control (Fig. 1C, panel c). Neither the preabsorbed antiserum nor the preimmune rabbit serum stained pP-Wild-transfected cells (data not shown). IFA staining of the pP-Wild-transfected cells with a mouse MAb to the HA epitope gave essentially the same results (data not shown). These results indicated that BDV P protein is transported into the nucleus in the absence of other viral constituents.
Subcellular localization of a series of deletion mutants of BDV P.
To map the regions involved in the nuclear targeting activity within BDV P, eukaryotic expression plasmids encoding a series of deletion mutants of BDV P, i.e., pP-del.N18, pP-del.N40, pP-del.N82, pP-del.C117, pP-del.C148, and pP-del.C182, were constructed (Fig. 1A) and transfected into COS-7 cells. Transient expression of the P deletion mutants was confirmed by Western blotting (Fig. 1B, lanes 6 to 11). On IFA, all of the deletion mutants of BDV P tested were localized to the nucleus (Fig. 1C, panels f to k). These results suggested that the nuclear targeting activity of BDV P is associated with the central region comprised of amino acid residues 83 to 116. An alternative possibility was that BDV P has more than one NLS, one of which is located in the N-terminal region and the other is located in the C-terminal region.
Nuclear targeting activity associated with the N- and C-terminal regions of BDV P.
To discriminate between the two alternative possible explanations for the localization of the NLS(s) in BDV P, eukaryotic expression plasmids encoding β-galactosidase fused with the N-terminal region (pRSV-1/41), the middle region (pRSV-41/181), or the C-terminal region (pRSV-172/201) of BDV P were constructed (Fig. 2A), and their nuclear targeting activities were examined. Western blotting analysis with anti-β-galactosidase MAb showed that the recombinant proteins of expected sizes were expressed in lysates of cells transfected with each plasmid construct (Fig. 2B, lanes 4, 5, and 6). No protein reactive with the MAb was detected in lysates of nontransfected cells (Fig. 2B, lane 3) or those transfected with pRSV-HA (Fig. 2B, lane 2). COS-7 cells transfected with pRSV-LacZ served as a positive control for staining with the MAb used (Fig. 2B, lane 1). On IFA with the anti-β-galactosidase MAb (Fig. 2C), the cells transfected with pRSV-41/181 showed cytoplasmic staining (panel e), while those transfected with either pRSV-1/41 (panel d) or pRSV-172/201 (panel f) showed nuclear staining. β-Galactosidase, by itself, was resident in the cytoplasm (Fig. 2C, panel a). Thus, BDV P may have two independent NLSs located in the N-terminal as well as the C-terminal region.
Analysis of the NLS in the N-terminal region of BDV P.
The N-terminal region of BDV P contains a basic amino-acid-rich sequence, 22RRERSGSPRPRK33, which resembles the bipartite NLS, for example, KRKIEEPEPEPKKAK found in Xenopus laevis protein factor xnf7 (18). In view of these facts, we constructed pRSV-18/36, which expressed β-galactosidase fused with a peptide, including the previously suggested NLS (peptide 22/33) of BDV P (28) and tested for nuclear targeting activity of the expressed fusion protein. The fused protein was observed as a single protein band on Western blotting with anti-β-galactosidase MAb (Fig. 3B, lane 2) and was targeted to the nucleus (Fig. 3C, panel a). To map the NLS within this region in more detail, pRSV-18/28 expressing β-galactosidase fused with peptide 18/28 and pRSV-29/36 expressing β-galactosidase fused with peptide 29/36 of BDV P were transfected into COS-7 cells. Western blotting analysis confirmed the expression of these fusion proteins in transfected COS-7 cells (Fig. 3B, lanes 3 and 4). On IFA with anti-β-galactosidase MAb (Fig. 3C), the protein expressed from pRSV-18/28 was found solely in the cytoplasm (panel b), but the β-galactosidase fused with peptide 29/36 of BDV P expressed from pRSV-29/36 was targeted to the nucleus (panel c). Thus, the NLS present in the N-terminal region of BDV P was not a bipartite NLS, but rather the short sequence 29PRPRKIPR36. As the N-terminal NLS, 29PRPRKIPR36, was rich in Pro residues, we constructed the substitution mutants pRSV-sub.29, pRSV-sub.31, and pRSV-sub.35 from pRSV-29/36 and transfected them into COS-7 cells in which each of the Pro residues in 29PRPRKIPR36 was replaced by Ala (Fig. 4A). Expression of these mutant proteins was confirmed by Western blotting with anti-β-galactosidase MAb (Fig. 4B, lanes 2, 3, and 4). On IFA with anti-β-galactosidase MAb (Fig. 4C), all mutant fusion proteins transiently expressed by transfection with pRSV-sub.29, pRSV-sub.31, and pRSV-sub.35 were found in the cytoplasm (panels a, b, and c). These results indicated that all three Pro residues in 29PRPRKIPR36 are indispensable for the nuclear targeting activity. In contrast, a mutant sequence, 29PRPQQIPR36 in which 32RK33 was replaced with 32QQ33, retained nuclear targeting activity (Fig. 4C, panel d).
Analysis of NLS in the C-terminal region of BDV P.
According to the experiments presented in Fig. 2, another NLS, peptide 172/201, may be present in the C-terminal region of BDV P. To map the candidate NLS in this region, four recombinant plasmids (pRSV-172/193, pRSV-179/201, pRSV-181/193, and pRSV-192/201) were constructed and transfected into COS-7 cells. These plasmids encoded β-galactosidase fused with peptides 172/193, 179/201, 181/193, and 192/201 of BDV P, respectively (Fig. 5B). On IFA (Fig. 5C), β-galactosidase fused with peptides 172/193 (panel a), 179/201 (panel b), or 181/193 (panel c) was targeted to the nucleus, whereas the fusion protein with the peptide 192/201 was retained in the cytoplasm (panel d). These results indicated that the NLS activity was associated with the amino acid sequence 181PPRIYPQLPSAPT193 of BDV P. To analyze the features of the NLS present in the C-terminal region of BDV P, four substitution mutants of pRSV-181/193—pRSV-sub.182, pRSV-sub.186, pRSV-sub.189, and pRSV-sub.192—were constructed (Fig. 6A). Each plasmid encoded a Pro-Ala substitution at the indicated position. As shown in Fig. 6B, fusion proteins of the expected sizes were expressed in the transfected COS-7 cells. IFA showed that all four substitution mutant proteins were retained in the cytoplasm (Fig. 6C). Therefore, Pro residues also play an important role in the C-terminal NLS of BDV P.
DISCUSSION
Transcription and replication of the BDV genome take place in the nucleus of BDV-infected cells. Both N and P proteins have been found in the nucleus of BDV-infected cells (3). Recently, an additional viral protein X was also found in the nucleus of infected cells (24, 30). The BDV L polymerase is thought to be a constituent of a viral ribonucleoprotein complex required for the replication and transcription of BDV genome in the nucleus (3, 5). The mechanisms by which these proteins are imported from the cytoplasm into the nucleus are not well understood. L polymerase has basic amino acid clusters which are good candidates as NLS (3, 5), but the activities of these sequences have not been tested as yet. Protein X may pass through nuclear pores due to its low molecular weight or by association with P (24). N protein has an NLS consisting of a basic amino acid cluster in the N-terminal region, the activity of which has been established experimentally (13, 19). An NLS candidate in P protein was suggested by Thierer et al. (28), but the activity of this sequence has not been studied. Since P associates with N, P (self-association), X (24), and probably with L, P may play a key role in viral replication and transcription.
We have identified two NLSs within the P protein: 29PRPRKIPR36 in the N-terminal region and 181PPRIYPQLPSAPT193 in the C-terminal region. Each NLS was concluded to function independently based on the following observations. The deletion mutants lacking either N-terminal or C-terminal regions were still targeted to the nucleus (Fig. 1). On recombinant protein analysis, either the sequence from the N-terminal region or the sequence from the C-terminal region conferred nuclear targeting activity on β-galactosidase, which is normally resident in the cytoplasm (Fig. 2). In contrast, the sequence derived from the middle part of BDV P protein did not cause β-galactosidase to move to the nucleus. The reason why BDV P protein has two independent NLSs is not clear at present.
A cluster of basic amino acid residues of BDV P 22RRKRSGSPRPRK33 has been proposed as an NLS (28), but its activity has not yet been confirmed experimentally. Although the BDV P clone in that study was derived from MDCK/BDV, the sequence was different from those of many other clones reported in the literature. The BDV P clone used in the present study, which was also cloned from MDCK/BDV, has the sequence 22RRERSGSPRPRK33; i.e., the Lys at position 24 was replaced by Glu. Horse-derived strains, including strains V (GenBank accession number U04608; see also reference 1) and C6BV (5), also had Glu at position 24. It remains to be determined whether the 22RRKRSGS28 sequence of Thierer’s BDV P has NLS activity. Irrespective of this issue, however, the 22RRERSGS28 sequence that is present in many BDV P proteins is not an NLS, but the neighboring 29PRPRKIPR36 sequence did show NLS activity.
The most intriguing findings of the present investigation are the unique features of the NLSs present in the BDV P protein. Unlike those of many nucleoproteins, the NLSs of the BDV P protein lack basic amino acids. In particular, the C-terminal NLS, 181PPRIYPQLPSAPT193, contained only one basic amino acid residue. Instead, both NLSs are rich in Pro residues. Pro has been shown to conform unfolded structures and has been reported to be important for the conformation of NLS in combination with several successive basic amino acid residues (2). For example, PKKKRK in simian virus 40 large T antigen (10), PNKKKRK in simian virus 40 VP2 (31), and PKKARED in the polyomavirus large T antigen (21) are all composed of a Pro succeeded by basic-amino-acid-rich sequences. In these NLSs, the roles of basic-amino acids have been analyzed extensively, leaving Pro unchanged. At a glance, the N-terminal NLS of BDV P, 29PRPRKIPR36, resembles the NLSs of viral proteins as cited above. However, substitution of any Pro residue within this sequence abolished the nuclear targeting activity (Fig. 4). In contrast, replacement of 32RK33 with 32QQ33 within 29PRPRKIPR36 did not abrogate the NLS activity (Fig. 4C, panel d). The NLS present in the C-terminal region of BDV P was also a Pro-rich sequence. Again, the replacement of any of these Pro residues with Ala abrogated the NLS activity (Fig. 6). NLS composed of Pro-rich residues may therefore represent a novel functional element.
According to a computer simulation model of BDV P (12), both N and C termini are extruded from the folded compact structure, presumably forming random coils due to their high contents of Pro residues. The NLSs found in the present study are located in these random-coiled structures.
Recently, the regions of BDV P critical for interactions with N, P, and X proteins were mapped by a two-hybrid system by using deletion mutants of BDV P (24). The sites for interactions with X, P, and N proteins are present in the regions 33 to 115, 135 to 172, and 197 to 201, respectively. The two NLSs identified here do not overlap with these binding sites, except that the 33-to-36 region of the N-terminal NLS was included in the border of the X-binding region. As the X-binding site has not been narrowed, the N-terminal NLS of BDV P may be distinct from the X-binding site. Even if the N-terminal NLS overlapped the X-binding site, N-X heterodimers may target the nucleus by virtue of the NLS in the C-terminal region of BDV P.
The results presented here were obtained with COS-7 cells, which are resistant to BDV infection. In addition, interactions between BDV P and other viral constituents and/or cellular factors have not been studied in the context of the nuclear targeting activity of BDV P. Despite these limitations, the presence of two potential NLSs in BDV P would provide a fundamental basis for elucidation of the biology of BDV infection.
ACKNOWLEDGMENTS
We thank Rudolf Rott (Justus-Liebig-Universität Giessen, Giessen, Germany) for providing the MDCK/BDV cells.
This work was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture and by The Akiyama Foundation.
REFERENCES
- 1.Binz T, Lebelt J, Niemann H, Hagenau K. Sequence analysis of the p24 gene of Borna disease virus in naturally infected horse, donkey and sheep. Virus Res. 1994;34:281–289. doi: 10.1016/0168-1702(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 2.Boulikas T. Nuclear localization signals (NLS) Crit Rev Eukaryot Gene Expr. 1993;3:193–227. [PubMed] [Google Scholar]
- 3.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubitt B, Oldstone C, de la Torre J C. Sequence and genomic organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Dunia D, Cubitt B, Grässer F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzog S, Rott R. Replication of Borna disease virus in cell culture. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 10.Kalderon D, Richardson W D, Markham A F, Smith A E. Sequence requirements for nuclear location of simian virus large-T antigen. Nature. 1994;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 11.Kliche S, Briese T, Henschen A, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliche S, Stitz L, Mangalam H, Shi L, Binz T, Niemann H, Briese T, Lipkin W I. Characterization of the Borna disease virus phosphoprotein, p23. J Virol. 1996;70:8133–8137. doi: 10.1128/jvi.70.11.8133-8137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Shoya Y, Koda T, Takashima I, Lai P K, Ikuta K, Kakinuma M, Kishi M. Nuclear targeting activity associated with Borna disease virus nucleoprotein. Virology. 1998;243:188–197. doi: 10.1006/viro.1998.9049. [DOI] [PubMed] [Google Scholar]
- 14.Koda T, Hasan S, Sasaki A, Arimura Y, Kakinuma M. Regulatory sequences required for hst-1 expression in embryonal carcinoma cells. FEBS Lett. 1994;342:71–75. doi: 10.1016/0014-5793(94)80587-3. [DOI] [PubMed] [Google Scholar]
- 15.Lipkin W I, Travis G H, Carbone K M, Wilson M C. Isolation and characterization of Borna disease cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 17.McClure M A, Thibault K J, Hatalski C G, Lipkin W I. Sequence similarity between Borna disease virus p40 and a duplicated domain within the paramyxovirus and rhabdovirus polymerase proteins. J Virol. 1992;66:6572–6577. doi: 10.1128/jvi.66.11.6572-6577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M, Reddy B A, Kloc M, Li X X, Dreyer C, Etkin L D. The nuclear-cytoplasmic distribution of the Xenopus nuclear factor, xnf7, coincides with its state of phosphorylation during early development. Development. 1991;113:569–575. doi: 10.1242/dev.113.2.569. [DOI] [PubMed] [Google Scholar]
- 19.Pyper J M, Gartner A E. Molecular basis for the differential subcellular localization of the 38- and 39-kilodalton structural proteins of Borna disease virus. J Virol. 1997;71:5133–5139. doi: 10.1128/jvi.71.7.5133-5139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyper J M, Richt J A, Brown L, Rott R, Narayan O, Clements J E. Genomic organization of the structural proteins of Borna disease virus revealed by a cDNA clone encoding the 38-kDa protein. Virology. 1993;195:229–238. doi: 10.1006/viro.1993.1364. [DOI] [PubMed] [Google Scholar]
- 21.Richardson W D, Roberts B L, Smith A E. Nuclear localization signals in polyomavirus large-T. Cell. 1986;44:77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- 22.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 23.Schneider P A, Hatalski C G, Lewis A J, Lipkin W I. Biochemical and functional analysis of the Borna disease virus G protein. J Virol. 1997;71:331–336. doi: 10.1128/jvi.71.1.331-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwemmle M, Salvatore M, Shi L, Richt J, Lee C H, Lipkin W I. Interaction of the Borna disease virus P, N, and X proteins and their functional implications. J Biol Chem. 1998;273:9007–9012. doi: 10.1074/jbc.273.15.9007. [DOI] [PubMed] [Google Scholar]
- 25.Shoya Y, Kobayashi T, Koda T, Lai P K, Tanaka H, Koyama T, Ikuta K, Kakinuma M, Kishi M. Amplification of a full-length Borna disease virus (BDV) cDNA from total RNA of cells persistently infected with BDV. Microbiol Immunol. 1997;41:481–486. doi: 10.1111/j.1348-0421.1997.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 26.Stitz, L., T. Bilzer, J. A. Richt, and R. Rott. 1993. Pathogenesis of Borna disease. Arch. Virol. 7(Suppl.):135–151. [DOI] [PubMed]
- 27.Thiedemann N, Presek P, Rott R, Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992;73:1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- 28.Thierer J, Reihle H, Grebenstein O, Binz T, Herzog S, Thiedemann N, Stitz L, Rott R, Lottspeich F, Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992;73:413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- 29.Wagner R R. Rhabdoviridae and their replication. In: Fields B N, et al., editors. Fields virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 867–882. [Google Scholar]
- 30.Wehner T, Ruppert A, Herden C, Frese K, Becht H, Richt J A. Detection of a novel Borna disease virus encoded 10 kilodalton protein in infected cells and tissues. J Gen Virol. 1997;78:2459–2466. doi: 10.1099/0022-1317-78-10-2459. [DOI] [PubMed] [Google Scholar]
- 31.Wychowski C, Benichou D, Girard M. The intranuclear location of simian virus 40 polypeptides VP2 and VP3 depends on a specific amino acid sequence. J Virol. 1987;61:3862–3869. doi: 10.1128/jvi.61.12.3862-3869.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Z, Robinson D, Wagner R R. Nucleus-targeting domain of the matrix protein (M1) of influenza virus. J Virol. 1995;69:1964–1970. doi: 10.1128/jvi.69.3.1964-1970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]