Abstract
Curcumae Rhizoma, a traditional Chinese medicine with a wide range of pharmacological activities, is obtained from the dried rhizomes of Curcuma phaeocaulis VaL., Curcuma kwangsiensis S. G. Lee et C. F. Liang, and Curcuma wenyujin Y. H. Chen et C. Ling. Sesquiterpenoids and curcuminoids are found to be the main constituents of Curcumae Rhizoma. Sesquiterpenoids are composed of three isoprene units and are susceptible to complex transformations, such as cyclization, rearrangement, and oxidation. They are the most structurally diverse class of plant-based natural products with a wide range of biological activities and are widely found in nature. In recent years, scholars have conducted abundant studies on the structures and pharmacological properties of components of Curcumae Rhizoma. This article elucidates the chemical structures, medicinal properties, and biological properties of the sesquiterpenoids (a total of 274 compounds) isolated from Curcumae Rhizoma. We summarized extraction and isolation methods for sesquiterpenoids, established a chemical component library of sesquiterpenoids in Curcumae Rhizoma, and analyzed structural variances among sesquiterpenoids sourced from Curcumae Rhizoma of diverse botanical origins. Furthermore, our investigation reveals a diverse array of sesquiterpenoid types, encompassing guaiane-type, germacrane-type, eudesmane-type, elemane-type, cadinane-type, carane-type, bisabolane-type, humulane-type, and other types, emphasizing the relationship between structural diversity and activity. We hope to provide a valuable reference for further research and exploitation and pave the way for the development of new drugs derived from medicinal plants.
Keywords: Curcumae Rhizoma, sesquiterpenoids, chemical constituents, pharmacological activity
1. Introduction
Natural products encompass secondary metabolites crafted by organisms over millions of years of natural evolution, showcasing a plethora of diverse chemical structures. Human life is intricately connected to natural products, serving as a primary source of numerous medicinal drugs or pivotal lead compounds. Sesquiterpenoids are a class of natural products consisting of three isoprene units with structurally diverse basic skeletons. They are derived from farnesyl pyrophosphate (FPP), formed from three molecules of isopentenyl pyrophosphate (IPP), through a series of complex transformations, including cyclization, rearrangement, and oxidation. Although the basic skeleton of sesquiterpenoids contains only 15 carbons, the number of sesquiterpenoids is the highest among terpenoids. Emerging evidence has shown that these compounds have multifaceted biological activities, including, but not limited to, anti-inflammatory, cytotoxic, antitumor, hepatoprotective, and cardiovascular disease-improving properties, both in vitro in cell models and in vivo in animal models [1,2,3,4].
Curcumae Rhizoma (Ezhu) is the dried rhizomes of Curcuma phaeocaulis VaL., Curcuma kwangsiensis S. G. Lee et C. F. Liang, and Curcuma wenyujin Y. H. Chen et C. Ling [5]. It is an important traditional Chinese medicine commonly used in clinical practice for treating dysmenorrhea, amenorrhea, irregular menstruation, stasis in the pelvis, tumors of the abdomen and epigastrium, arrhythmia, coronary heart disease, stroke, dyspepsia, and gastritis [6]. In the modern world, Curcumae Rhizoma attracts great interest because of its various pharmacological effects on gynecological-related, cancer-related, immune system-related, cardiovascular system-related, and hepatoprotective activities, which mainly overlap with its traditional applications [7,8,9,10,11,12,13,14]. The major bioactive compounds of Curcumae Rhizoma are sesquiterpenoids and curcuminoids [15].
To date, numerous experimental studies have been conducted on the sesquiterpenoids in Curcumae Rhizoma [2,16,17,18]. However, there are fewer reviews on the sesquiterpenoids and their bioactivities in Ezhu. Some reviews primarily focus on a specific activity, including its effects on cancer, hepatobiliary disease, and infectious diseases [9,10,19,20,21], while others concentrate on the differences between several herbs derived from the genus Curcuma (Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma) [6,7]. Accordingly, in this article, we review the sesquiterpenoids derived from the dried rhizomes of C. phaeocaulis, C. kwangsiensis, and C. wenyujin, three sources of Curcumae Rhizoma, and emphasize the structural variances among sesquiterpenoids sourced from diverse botanical origins. Additionally, we also summarize the structural features of different types of sesquiterpenoids and their pharmacological activities, revealing the relationship between structural diversity and activity. These discussions aim to serve as a reference and provide foundational knowledge for the prospective advancement and exploitation of Curcumae Rhizoma.
2. Medicinal Plants of Curcumae Rhizoma
Ezhu, a traditional Chinese medicine, comes from the genus Curcuma in the family Zingiberaceae. There are approximately 80 species of the genus Curcuma worldwide, mainly produced in Southeast Asia and from southeastern to southwestern regions in China [7]. Curcuma phaeocaulis, Curcuma kwangsiensis, Curcuma longa, Curcuma zanthorrhiza, Curcuma wenyujin, Curcuma aeruginosa, Curcuma zedoaria, and Curcuma caesia all belong to this genus [22]. The rhizomes are usually the main commercial sources of Curcumae Rhizoma, Curcumae Longae Rhizoma, or Wenyujin Rhizoma Concisum, while the tuberous roots are the main source of Curcumae Radix [7]. However, complicated relationships exist between these herbs, and there is confusion with respect to their application due to the similarity of their efficacy, the intersection of and variation in plant sources, and the overlap of herb and plant names. In addition, some plant sources are not included in the Pharmacopoeia of the people’s Republic of China, although they are widely used in folklore medicine. According to the Chinese Pharmacopoeia, Curcumae Rhizoma (Ezhu) only comes from the dried rhizomes of C. phaeocaulis, C. kwangsiensis, and C. wenyujin.
3. Chemical Composition of Curcumae Rhizoma
Through modern research, it has been discovered that volatile oil and curcuminoids are the main bioactive constituents of Curcumae Rhizoma, and the volatile oil predominantly comprises sesquiterpenoids [15]. These sesquiterpenoids are of various types, including guaiane-type, germacrane-type, eudesmane-type, elemane-type, cadinane-type, carane-type, bisabolane-type, humulane-type, and other types.
A wide range of published studies have revealed the isolation and identification of sesquiterpenoids with diverse structural skeletons. Considering operability in the laboratory, sesquiterpenoids are mainly obtained by organic solvent extraction and steam distillation. Nevertheless, since conventional extraction techniques have several drawbacks, such as long times of extraction or the use of large amounts of solvents, the use of green extraction techniques is suggested, without affecting the efficiency of the extraction. When employing steam distillation, some thermally unstable components are prone to degradation. Chemical compounds are purified primarily by repeated column chromatography, including silica gel column chromatography, reversed-phase C18 silica gel column chromatography, Sephadex LH-20 column chromatography, ODS column chromatography, HPLC, and preparative TLC. Compared to other compounds, sesquiterpenoids possess lower polarity, as well as differences in their affinity and solubility in organic phases. When using silica gel column chromatography, several solvent systems are generally used for elution, including petroleum ether–acetone, petroleum ether–EtOAc, CHCl3–MeOH, CH2Cl2–MeOH, CH2Cl2–EtOAc, and CH2Cl2–acetone. The structures of isolated compounds were established based on 1D and 2D NMR data, mass spectrometry, circular dichroism (CD), X-ray analysis, and chemical methods. In comparison with other types of sesquiterpenoids, guaiane-type, germacrane-type, and eudesmane-type sesquiterpenoids are prone to recrystallization, suggesting that recrystallization may be a consideration when the configuration is undetermined.
3.1. Guaiane-Type Sesquiterpenoids of Curcumae Rhizoma
Guaiane-type sesquiterpenoids are the most dominant type of sesquiterpenoids in Curcumae Rhizoma. They are characterized by a five-membered ring fused to a seven-membered ring. To date, 115 guaiane-type sesquiterpenoids have been isolated from three medicinal sources (C. phaeocaulis, C. kwangsiensis, and C. wenyujin) (Figure 1, Table 1). Roughly one-fourth of these compounds form a five-membered lactone ring between C-8 and C-12 (77–110, 115), and six additional compounds produce a furan ring at the C-8 and C-12 positions (73–76, 113, 114). This type of compounds tend to generate oxygen bridges at various positions, including C-5/C-8 (55–67), C-7/C-10 (68, 69), C-5/C-10 (102, 103), and C-1/C-8 (104–109); in addition, compound 110 features a peroxide bridge between C-1 and C-8. Distinctively, several seco-guaiane-type sesquiterpenoids exist, and compounds 112 and 115 are subjected to ring opening on the seven-membered ring, while compounds 113 and 114 are opened at C-3–C-4. It is noteworthy that these guaiane-type sesquiterpenoids tend to possess hydroxyl groups at C-4, C-5, C-8, and C-10. Moreover, they are readily oxidized to carbonyl groups at C-8 and easily generate double bonds and oxygen rings, which make them structurally diverse. These compounds feature multiple chiral carbons, which lead to various stereoisomers, diastereoisomers, enantiomers (10/11, 12/13, 36/37, 41/42, 68/69, 77/83), and epimers (29/30, 31/32, 61/62), among which all enantiomers originate from C. phaeocaulis.
Figure 1.
Guaiane-type sesquiterpenoids of Curcumae Rhizoma.
In a comparative analysis of the distribution of sesquiterpenoids across three plant sources, guaiane-type sesquiterpenoids exhibited a predominant presence in C. wenyujin and C. phaeocaulis, with a marked pre-eminence in abundance noted specifically in C. wenyujin. Conversely, bicyclic sesquiterpenoids demonstrated a significant association with C. wenyujin and C. phaeocaulis, while sesquiterpenoids with a furan ring or a lactone ring were mainly found in C. wenyujin and C. kwangsiensis. In addition, while each of the three botanical specimens shares certain chemical constituents, their contents exhibit notable divergence. Specifically, C. kwangsiensis demonstrates a notable enrichment in curcumol (55), while C. phaeocaulis showcases the highest proportions of isocurcumenol (60) and curcumenol (61) among the three plants. These findings highlight the nuanced variations in compound distribution across closely related plant species [23,24,25,26].
Table 1.
Guaiane-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 1 | epi-Guaidiol A | C. wenyujin | [27] |
| 2 | Phaeocaulisguatriol | C. phaeocaulis | [28] |
| 3 | Alismoxide | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,29,30] |
| 4 | 4α,10α,11-Trihydroxy-1βH,5βH-guai-7(8)-ene | C. phaeocaulis | [28] |
| 5 | Wenyujinol E | C. wenyujin | [27] |
| 6 | Guaianediol | C. phaeocaulis, C. wenyujin | [27,28] |
| 7 | 6-Guaiene-4α,10α-diol | C. wenyujin | [31] |
| 8 | 4α,10β,11-Trihydroxy-1,5-trans-guai-6-ene | C. phaeocaulis | [28] |
| 9 | Wenyujinol N | C. wenyujin | [32] |
| 10 | (+)-Phaeocauline A | C. phaeocaulis | [33] |
| 11 | (−)-Phaeocauline A | C. phaeocaulis | [33] |
| 12 | (+)-Phaeocauline B | C. phaeocaulis | [33] |
| 13 | (−)-Phaeocauline B | C. phaeocaulis | [33] |
| 14 | Phaeocaulisin Q | C. phaeocaulis | [34] |
| 15 | Wenyujinin A | C. wenyujin, C. kwangsiensis | [12,35] |
| 16 | Wenyujinin B | C. wenyujin | [27,35] |
| 17 | Wenyujinin Q | C. wenyujin | [36] |
| 18 | Zedoarondiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,27,31,37,38] |
| 19 | Neozedoarondiol | C. wenyujin | [10] |
| 20 | Isozedoarondiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,27,28,36,38] |
| 21 | Phaeocaulisin E | C. wenyujin, C. phaeocaulis | [16,31,37] |
| 22 | (1S,4S,5S,10R)-Zedoarondiol | C. phaeocaulis, C. wenyujin, C. kwangsiensis | [16,38,39] |
| 23 | (1S,4S,5S,10R)-Isozedoarondiol | C. wenyujin | [31] |
| 24 | Wenyujinin R | C. wenyujin | [36] |
| 25 | 4,10-Epizedoarondiol | C. kwangsiensis, C. wenyujin | [31,38] |
| 26 | 4-Hydroxy-10-methoxy-guai-7(11)-en-8-one | C. phaeocaulis | [28] |
| 27 | Methylzedoarondiol | C. wenyujin | [27] |
| 28 | Wenyujinol M | C. wenyujin | [32] |
| 29 | Procurcumenol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,27,29,31,37] |
| 30 | Epiprocurcumenol | C. wenyujin | [40] |
| 31 | Aerugidiol | C. wenyujin, C. kwangsiensis | [29,38,39] |
| 32 | 1-epi-Aerugidiol | C. phaeocaulis | [37] |
| 33 | Procurcumadiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,31,37,38] |
| 34 | Phaeocaulisin F | C. phaeocaulis | [16] |
| 35 | Neoprocurcumenol | C. wenyujin | [36] |
| 36 | (+)-Phaeocauline D | C. phaeocaulis | [33] |
| 37 | (−)-Phaeocauline D | C. phaeocaulis | [33] |
| 38 | Dihydroprocurcumenol | C. kwangsiensis | [12] |
| 39 | Wenyujinol D | C. wenyujin | [27] |
| 40 | Phaeocaulisin P | C. phaeocaulis | [34] |
| 41 | (+)-Phaeocauline E | C. phaeocaulis | [33] |
| 42 | (−)-Phaeocauline E | C. phaeocaulis | [33] |
| 43 | Isoprocurcumenol | C. wenyujin | [41] |
| 44 | Wenyujinol F | C. wenyujin | [27] |
| 45 | 9-Oxo-neoprocurcumenol | C. wenyujin | [27] |
| 46 | 7α,11α-Epoxy-5β-hydroxy-9-guaiane-8-one | C. wenyujin, C. phaeocaulis | [16,31] |
| 47 | 8,9-seco-4β-Hydroxy-1α,5βH-7(11)-guaen-8,10-olide | C. wenyujin | [29] |
| 48 | Phaeocaulisin L | C. phaeocaulis | [42] |
| 49 | Phaeocaulisin D | C. phaeocaulis | [16] |
| 50 | Phaeocaulisin R | C. phaeocaulis | [37] |
| 51 | Phaeocaulisin K | C. phaeocaulis | [42] |
| 52 | Phaeocaulisin J | C. phaeocaulis | [16,28] |
| 53 | 4α,10β-Dihydroxy-1βH,5αH-guai-6(7)-en-11-one | C. phaeocaulis | [34] |
| 54 | Phaeocaulisin N | C. phaeocaulis | [34] |
| 55 | Curcumol | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [26,31,36,43] |
| 56 | 4-Epicurcumol | C. wenyujin | [44] |
| 57 | 7β,8α-Dihydroxy-1α,4αH-guai-10(15)-en-5β,8β-endoxide | C. wenyujin | [29] |
| 58 | 10β-Hydroxy-9,10-dihydrocurcumenol | C. phaeocaulis | [28] |
| 59 | Wenyujinin I | C. wenyujin, C. kwangsiensis | [12,35] |
| 60 | Isocurcumenol | C. phaeocaulis, C. wenyujin, C. kwangsiensis | [16,26,45] |
| 61 | Curcumenol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,28,31,46] |
| 62 | 4-Epicurcumenol | C. wenyujin, C. phaeocaulis | [16,46] |
| 63 | 15-Hydroxycurcumenol | C. phaeocaulis | [28] |
| 64 | 12-Hydroxycurcumenol | C. wenyujin | [31] |
| 65 | Isocurcumol | C. wenyujin | [44] |
| 66 | 7β,8α-dihydroxy-1α,4αH-guai-9,11-dien-5β,8β-endoxide | C. wenyujin | [46] |
| 67 | Neocurcumenol | C. wenyujin | [46] |
| 68 | (+)-Phaeocauline C | C. phaeocaulis | [33] |
| 69 | (−)-Phaeocauline C | C. phaeocaulis | [33] |
| 70 | 4α,7α-Epoxyguaiane-10α,11-diol | C. wenyujin | [32] |
| 71 | (1R,4R,5S,7S)-Curwenyujinone | C. wenyujin | [47] |
| 72 | Wenyujinin H | C. wenyujin | [35] |
| 73 | Curcumafuranol | C. kwangsiensis | [48] |
| 74 | Zedoarol | C. kwangsiensis | [49] |
| 75 | Wenyujinin F | C. wenyujin | [35] |
| 76 | Linderazulene | C. kwangsiensis | [50] |
| 77 | (+)-Zedoalactone A | C. wenyujin, C. phaeocaulis | [28,51] |
| 78 | Zedoalactone C | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,52,53] |
| 79 | Zedoalactone E | C. wenyujin | [39,46] |
| 80 | Zedoalactone G | C. wenyujin, C. kwangsiensis | [51,52] |
| 81 | Zedoalactone H | C. wenyujin | [46] |
| 82 | Phaeocaulisin C | C. phaeocaulis, C. kwangsiensis | [16,52] |
| 83 | Zedoalactone A | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,28,31,52] |
| 84 | Phaeocaulisin B | C. phaeocaulis | [16] |
| 85 | Zedoarolide B | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,28,31,51] |
| 86 | Wenyujinol H | C. wenyujin | [27] |
| 87 | 8-O-Methylzedoarolide B | C. wenyujin | [32] |
| 88 | Zedoarolide A | C. phaeocaulis, C. wenyujin | [16,28,32] |
| 89 | Wenyujinol G | C. wenyujin | [27] |
| 90 | Phaeocaulisin I | C. phaeocaulis, C. kwangsiensis | [12,16] |
| 91 | Phaeocaulisin G | C. phaeocaulis | [16] |
| 92 | Phaeocaulisin H | C. phaeocaulis | [16] |
| 93 | Phaeocaulisin O | C. kwangsiensis, C. phaeocaulis | [34,52] |
| 94 | Zedoalactone B | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,27,51,52] |
| 95 | (1R,4R,5S,10S)-Zedoalactone B | C. wenyujin | [51] |
| 96 | Zedoalactone D | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,39,52] |
| 97 | (4S)-Hydroxy-(8)-methoxy-(5S)-(H)-guaia1(10),7(11)-dien-12,8-olide | C. kwangsiensis | [12] |
| 98 | Zedoalactone F | C. wenyujin, C. kwangsiensis | [38,39] |
| 99 | Gweicurculactone | C. kwangsiensis | [54] |
| 100 | (4S)-4-Hydroxy-gweicurculactone | C. wenyujin, C. kwangsiensis | [51,54] |
| 101 | 2-Oxoguaia-1(10),3,5,7(11),8-pentaen-12,8-olide | C. wenyujin, C. kwangsiensis | [27,54] |
| 102 | 4β-Methyl-8β,9β-dihydroxy-5α,10α-epoxy-guai-12,8-olide | C. kwangsiensis | [52] |
| 103 | 4α-Methyl-8β,9β-dihydroxy-5α,10α-epoxy-guai-12,8-olide | C. kwangsiensis | [52] |
| 104 | Phaeocaulisin A | C. phaeocaulis, C. kwangsiensis | [16,54] |
| 105 | (1R,4R,5S,8S,9Z)-4-Hydroxy-1,8-epoxy-5H-guaia-7(11),9-dien-12,8-olide | C. kwangsiensis | [54] |
| 106 | Wenyujinol A | C. wenyujin | [27] |
| 107 | Wenyujinol B | C. wenyujin | [27] |
| 108 | Wenyujinol C | C. wenyujin | [27] |
| 109 | Wenyujinin G | C. wenyujin | [35] |
| 110 | 1α,8α-Epidioxy-4α-hydroxy-5αH-guai-7(11),9-dien-12,8-olide | C. wenyujin, C. kwangsiensis | [12,29] |
| 111 | Phaeocaulisin M | C. phaeocaulis | [42] |
| 112 | Curcuzedoalide | C. wenyujin | [31] |
| 113 | Kwangsiensis A | C. kwangsiensis | [55] |
| 114 | Kwangsiensis B | C. kwangsiensis | [55] |
| 115 | 12-Dehydroxy-chloraniolide | C. phaeocaulis | [56] |
3.2. Germacrane-Type Sesquiterpenoids of Curcumae Rhizoma
Germacrane-type sesquiterpenoids, a notably abundant class among numerous sesquiterpenoids, can generate distinct sesquiterpenoids, including guaiane-, eudesmane-, and cadinane-type sesquiterpenoids. Members of this class of compounds typically contain one or more double bonds, which are formed at C-1/C-10 and C-4/C-5. These sesquiterpenoids can be separated into four different configurations due to the cis-trans isomerism of the double bonds. The readily deformable 10-membered rings inherent in germacrane sesquiterpenoids result in a diverse array of stereo structures. Currently, 54 germacrane sesquiterpenoids have been isolated from Curcumae Rhizoma (Figure 2, Table 2). These natural products frequently engage in the formation of a five-membered ring at C-8 and C-12, yielding diverse structural moieties, such as furan rings (139–145), lactone rings (146–158), and lactam rings (161–168). This type of compound is prone to oxidation, which can produce aldehydes, ketones, esters, or oxygen bridges. Specifically, the C-5, C-7, and C-8 positions are particularly susceptible to oxidation, resulting in carbonyl groups. Germacrane-type sesquiterpenoids containing a lactone ring are frequently substituted with hydroxyl groups at C-8 (147, 149–151, 154–156). Sesquiterpenoids are also prone to forming oxygen bridges, with tricyclic oxygen rings appearing frequently at C-1/C-10 (129–135), C-4/C-5 (126, 128, 143–145), or C-1/C-5 (136). It is noteworthy that multiple pairs of germacrane-type enantiomers (152/153, 155/156, 163/164, 165/166) have been isolated from Curcumae Rhizoma. All these enantiomers originate from C. phaeocaulis, implying the pervasive presence of germacrane-type enantiomers in C. phaeocaulis.
Figure 2.
Germacrane-type sesquiterpenoids of Curcumae Rhizoma.
Germacrane-type sesquiterpenoids are obtained from C. wenyujin and C. phaeocaulis, with a relatively low occurrence in C. kwangsiensis. Notably, compounds characterized by oxygen bridges exhibit an almost exclusive presence within C. wenyujin, while sesquiterpenoids featuring either a furan or a lactone ring manifest a consistent and uniform distribution across three plant species. The studies elucidate a commonality in the presence of certain compounds across all three plants. Among them, germacrone (116), curdione (117), neocurdione (118), germacrene D (125), and furanodiene (139) exhibit higher concentrations in C. wenyujin compared to the other two botanical specimens. Conversely, furanodienone (140) attains greater levels in C. phaeocaulis. Notably, dehydrocurdione (121) levels in C. kwangsiensis are relatively high. These findings underscore the nuanced variations in the phytochemical compositions among closely related plant species, accentuating the unique metabolic pathways shaping the distinctive chemical profiles of C. wenyujin, C. kwangsiensis, and C. phaeocaulis [23,24,25,26].
Table 2.
Germacrane-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 116 | Germacrone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,57] |
| 117 | Curdione | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [17,26,29,31,43] |
| 118 | Neocurdione | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [26,43,44,58] |
| 119 | (2R)-2β-Hydroxycurdione | C. wenyujin | [18] |
| 120 | Wenyujinone D | C. wenyujin | [18] |
| 121 | Dehydrocurdione | C. kwangsiensis | [12] |
| 122 | Heyneanone C | C. phaeocaulis | [59] |
| 123 | Heyneanone D | C. wenyujin | [18,40] |
| 124 | 13-Hydroxygermacrone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [31,56,60] |
| 125 | Germacrene D | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [26] |
| 126 | (4S,5S)-Germacrone-4,5-epoxide | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [49,57,58,61] |
| 127 | (+)-(4S,5S)-Germacrone-4,5-epoxide | C. wenyujin | [17,62] |
| 128 | (4S,5S)-13-Hydroxygermacrone-4,5-epoxide | C. phaeocaulis | [59] |
| 129 | Germacrone-1,10-epoxide | C. wenyujin, C. kwangsiensis | [49,58] |
| 130 | (1R,10R)-(−)-1,10-Dihydrocurdione | C. wenyujin | [63] |
| 131 | (1R,10R)-Epoxy-1,10-dihydrocurdione | C. wenyujin | [43] |
| 132 | (1S,10S),(4S,5S)-Germacrone-1(10),4(5)-diepoxide | C. wenyujin | [43,62] |
| 133 | (+)-(1S,4S,5S,10S)-Germacrone-1(10)-4-diepoxide | C. wenyujin | [17] |
| 134 | (1R,4S,5R,6R,7S,10R)-1(10),4(5)-Diepoxygermacran-11(12)-en-6-ol | C. phaeocaulis | [15] |
| 135 | Germacrone-1(10),4,7(11)-triepoxide | C. wenyujin | [62] |
| 136 | Wenyujinin J | C. wenyujin | [35] |
| 137 | Wenyujinol O | C. wenyujin | [32] |
| 138 | Phagermadiol | C. phaeocaulis | [42,59] |
| 139 | Furanodiene | C. kwangsiensis, C. wenyujin, C. phaeocaulis | [26] |
| 140 | Furanodienone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,56] |
| 141 | (1S)-1-Hydroxy-isofuranodienone | C. phaeocaulis | [37] |
| 142 | 1(10)Z,4Z-Furanodiene-6-one | C. wenyujin | [31] |
| 143 | Zederone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,56] |
| 144 | Wenyujinin K | C. wenyujin | [35] |
| 145 | (1R,4S,5R,9R,10S)-9-Hydroxy-zederone epoxide | C. phaeocaulis | [59] |
| 146 | Curdionolide B | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,17,44,59] |
| 147 | Curdionolide A | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [17,31,52,59] |
| 148 | Souliene A | C. kwangsiensis | [12] |
| 149 | Wenyujinone C | C. wenyujin | [18] |
| 150 | Aeruginolactone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,30,56] |
| 151 | Curcuminol G | C. wenyujin, C. kwangsiensis | [12,45] |
| 152 | (+)-Phaeocaulin C | C. phaeocaulis | [64] |
| 153 | (−)-Phaeocaulin C | C. phaeocaulis | [64] |
| 154 | (1E,4Z)-8-Hydroxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone | C. wenyujin, C. phaeocaulis | [17,30,56] |
| 155 | (+)-Phaeocaulin D | C. phaeocaulis | [64] |
| 156 | (−)-Phaeocaulin D | C. phaeocaulis | [64] |
| 157 | Wenyujinone A | C. wenyujin | [18] |
| 158 | 1,8-Epoxy-7(11)-germacren-5-one-12,8-olide | C. wenyujin | [18] |
| 159 | Curkwangsien A | C. kwangsiensis | [65] |
| 160 | Curkwangsien B | C. kwangsiensis | [65] |
| 161 | Curdionolide C | C. wenyujin | [17] |
| 162 | Wenyujinone B | C. wenyujin | [18] |
| 163 | (+)-Phaeocaulin B | C. phaeocaulis | [64] |
| 164 | (−)-Phaeocaulin B | C. phaeocaulis | [64] |
| 165 | (+)-Phaeocaulin A | C. phaeocaulis | [59] |
| 166 | (−)-Phaeocaulin A | C. phaeocaulis | [59] |
| 167 | (−)-Phaeocaulin E | C. phaeocaulis | [56] |
| 168 | (+)-Phaeocaulin F | C. phaeocaulis | [56] |
| 169 | Wenjine | C. wenyujin | [62] |
3.3. Eudesmane-Type Sesquiterpenoids of Curcumae Rhizoma
Eudesmane-type sesquiterpenoids are a common type of natural product, whose fundamental structure comprises two six-membered rings. Previous studies have identified 41 eudesmane sesquiterpenoids from Curcumae Rhizoma (Figure 3, Table 3). These natural products are likely to form furan rings (185–196), lactone rings (197–208), and lactam rings (209 and 210) at the C-8 and C-12 positions. They are highly prone to oxidation and dehydrogenation, resulting in hydroxyl and carbonyl groups and double bonds. Among these, hydroxyl substitutions often occur at the C-1, C-4, and C-11 positions, and carbonyl substitution occurs at the C-6 and C-8 positions. Some compounds are oxidized to carbonyl groups at C-1 and C-4, while those at C-3/C-4, C-4/C-5, C-7/C-8, C-8/C-9, C-7/C-11, C-11/C-12, and C-4/C-15 positions are often dehydrogenated to form double bonds. Eudesmane-type sesquiterpenoids typically possess three or more chiral carbons, resulting in a diverse range of conformations, among which a pair of enantiomers has been identified (188 and 189). Of these isolated compounds, the majority originated from C. phaeocaulis, with only three compounds from C. kwangsiensis.
Figure 3.
Eudesmane-type sesquiterpenoids of Curcumae Rhizoma.
Table 3.
Eudesmane-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 170 | Phaeocaulistriol A | C. phaeocaulis | [28] |
| 171 | Phaeocaulistriol B | C. phaeocaulis | [28] |
| 172 | 1α,4β-Dihydroxyeudesm-7(11)-en-8-one | C. phaeocaulis, C. kwangsiensis | [2,38] |
| 173 | 1-Hydroxyeudesma-4(14),7(11)-dien-8-one | C. phaeocaulis | [2] |
| 174 | 1-Hydroxyeudesma-3,7(11)-dien-8-one | C. phaeocaulis | [2] |
| 175 | 9-Hydroxyeudesma-3,7(11)-dien-6-one | C. phaeocaulis | [2] |
| 176 | Phaeusmane A | C. phaeocaulis | [2] |
| 177 | Phaeusmane B | C. phaeocaulis | [2] |
| 178 | Phaeusmane D | C. phaeocaulis | [2] |
| 179 | Phaeusmane E | C. phaeocaulis | [2] |
| 180 | Phaeusmane C | C. phaeocaulis | [2] |
| 181 | Eudesm-11-ene-4α,6α-diol | C. phaeocaulis, C. kwangsiensis | [2,12] |
| 182 | Capillosanane Z | C. wenyujin | [18] |
| 183 | Cyperusol C | C. wenyujin, C. phaeocaulis | [2,31] |
| 184 | 1β-Hydroxyeudesma-4,11-dien-3-one | C. phaeocaulis | [2] |
| 185 | Zedoarofuran | C. phaeocaulis | [37] |
| 186 | Curcolonol | C. wenyujin, C. phaeocaulis | [2,66] |
| 187 | 9α-Hydroxycurcolonol | C. phaeocaulis | [56] |
| 188 | (+)-Phaeocauline G | C. phaeocaulis | [33] |
| 189 | (−)-Phaeocauline G | C. phaeocaulis | [33] |
| 190 | Curcodione | C. wenyujin, C. phaeocaulis | [2,66] |
| 191 | 4α-Hydroxy-8,12-epoxyeudesma-7,11-diene-1,6-dione | C. phaeocaulis | [37] |
| 192 | Curcolone | C. phaeocaulis | [2] |
| 193 | 3α-Hydroxy-4-deoxy-5-dehydrocurcolonol | C. phaeocaulis | [56] |
| 194 | Chlorantene D | C. phaeocaulis | [28] |
| 195 | Chlomultin B | C. phaeocaulis | [2] |
| 196 | Myrrhterpenoid N | C. phaeocaulis | [2] |
| 197 | Phaeusmane F | C. phaeocaulis | [2] |
| 198 | Phaeusmane G | C. phaeocaulis | [2] |
| 199 | 1β,8β-Dihydroxyeudesma-4,7(11)-dien-8α,12-olide | C. phaeocaulis | [2] |
| 200 | (7Z)-1β,4α-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | C. phaeocaulis, C. wenyujin | [2,66] |
| 201 | (7Z)-1β,4β-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | C. phaeocaulis, C. wenyujin | [2,32] |
| 202 | Curcolide | C. wenyujin | [29,66] |
| 203 | Wenyujinlactone A | C. wenyujin | [67] |
| 204 | 1β,8β-Dihydroxyeudesma-3,7(11)-dien-8α,12-olide | C. phaeocaulis | [2] |
| 205 | Serralactone A | C. phaeocaulis | [2,56] |
| 206 | Hydroxyatractylolide | C. kwangsiensis | [60] |
| 207 | Butenolide III | C. wenyujin | [68] |
| 208 | Neolitacumone A | C. phaeocaulis, C. wenyujin | [2,37,67] |
| 209 | Phaeusmane I | C. phaeocaulis | [69] |
| 210 | Phaeusmane H | C. phaeocaulis | [2] |
3.4. Elemane-Type Sesquiterpenoids of Curcumae Rhizoma
At present, 14 elemane-type sesquiterpenoids have been reported from Curcumae Rhizoma (Figure 4, Table 4). Of these, some are monocyclic elemane-type sesquiterpenoids (211–213), and others form furan rings (214–216), five-membered lactone rings (217–222), or five-membered lactam rings (223 and 224) between C-8 and C-12. This class of compounds contains multiple double bonds, which often present at the C-1/C-2 or C-3/C-4 positions, while some compounds also exhibit these at C-6/C-7, C-7/C-8, C-7/C-11, C-8/C-9, or C-11/C-12.
Figure 4.
Elemane-type sesquiterpenoids of Curcumae Rhizoma.
Table 4.
Elemane-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 211 | β-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 212 | γ-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 213 | δ-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 214 | Curzerene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [26] |
| 215 | Curzerenone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [31,38,57] |
| 216 | Epicurzerenone | C. phaeocaulis | [73] |
| 217 | Isogermafurenolide | C. wenyujin | [44,46] |
| 218 | 5-Isopropenyl-3,6-dimethyl-6-vinyl-5,6,7,7α-tetrahydro-4H-benzofuran-2-one | C. wenyujin | [58] |
| 219 | 8β-Hydroxy-isogermafureolide | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,56,68] |
| 220 | Hydroxyisogermafurenolide | C. wenyujin, C. kwangsiensis | [44,46,52,58,66] |
| 221 | 5βH-Elema-1,3,7,8-tetraen-8,12-olide | C. wenyujin | [44] |
| 222 | 8β-Methoxy-isogermafurenolide | C. phaeocaulis | [69] |
| 223 | 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam | C. wenyujin, C. phaeocaulis | [36,46,69] |
| 224 | 8β(H)-Elema-1,3,7(11)-trien-8,12-lactam | C. phaeocaulis | [56] |
3.5. Cadinane-Type Sesquiterpenoids of Curcumae Rhizoma
In total, 14 cadinane-type sesquiterpenoids have been identified from Curcumae Rhizoma (Table 5, Figure 5). Within this group, compounds 231–235 and 238 exhibit a furan ring or a five-membered lactone ring at the C-8/C-12 positions, while compound 236 has a six-membered lactone ring at the C-5/C-12 positions. Compounds 237 and 238 are subject to A-ring opening, and in certain instances, the B-ring acquires a benzene ring structure (227, 230–238). These compounds are susceptible to oxidation at the C-5 position, leading to the generation of hydroxyl (231 and 232) or carbonyl groups (225–228, 230, 235, 237, 238). Furthermore, dehydrogenation readily occurs at C-4/C-5, giving rise to the formation of double bonds (229, 233, 234). Enantiomers are also present within the group of cadinane sesquiterpenoids (231 and 232).
Table 5.
Cadinane-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 225 | Wenyujinone F | C. wenyujin | [18] |
| 226 | Wenyujinone E | C. wenyujin | [18] |
| 227 | 7-Hydroxy-5(10),6,8-cadinatriene-4-one | C. wenyujin | [29] |
| 228 | Phacadinane B | C. phaeocaulis | [37,74] |
| 229 | Phacadinane A | C. phaeocaulis | [74] |
| 230 | Curcujinone B | C. wenyujin | [31] |
| 231 | (+)-Commyrrin A | C. wenyujin, C. kwangsiensis | [18,38] |
| 232 | (−)-Commyrrin A | C. wenyujin, C. kwangsiensis | [38] |
| 233 | Pyrocurzerenone | C. kwangsiensis | [38] |
| 234 | Furanocadalene | C. kwangsiensis | [38] |
| 235 | Curcujinone A | C. wenyujin | [31] |
| 236 | Phacadinane C | C. phaeocaulis | [74] |
| 237 | Phacadinane D | C. phaeocaulis, C. kwangsiensis | [12,56,74] |
| 238 | 4,5-Seco-pyrocurzerenone | C. kwangsiensis | [38] |
Figure 5.
Cadinane-type sesquiterpenoids of Curcumae Rhizoma.
3.6. Other-Type Sesquiterpenoids of Curcumae Rhizoma
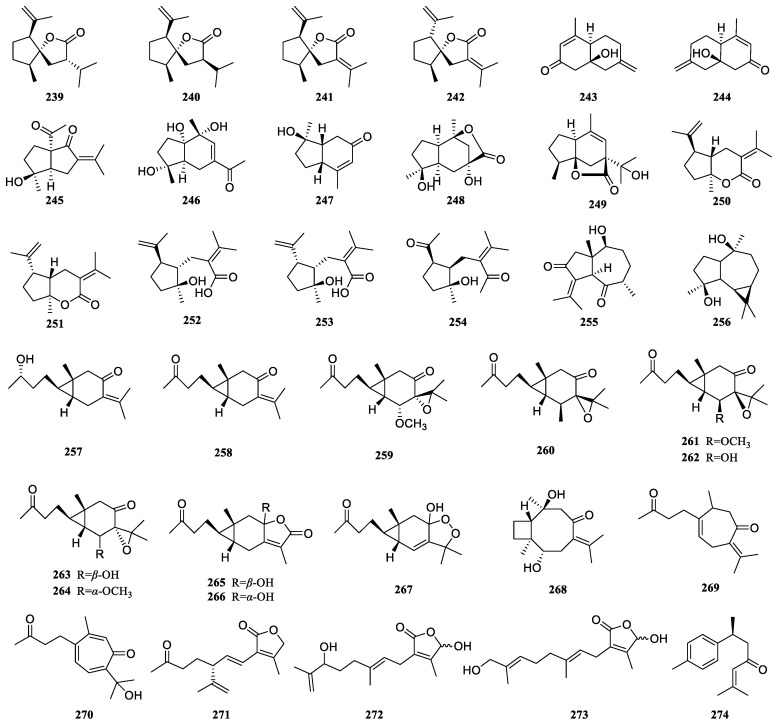
Presently, 36 distinct sesquiterpenoids of Curcumae Rhizoma have been reported (Table 6, Figure 6), encompassing spironolactone-type sesquiterpenoids (239–242), carane-type sesquiterpenoids (257–267), bisabolane-type sesquiterpenoids (274), xanthane-type sesquiterpenoids (269 and 270), and diverse other-type sesquiterpenoids. These compounds typically show hydroxyl and carbonyl substitutions, among which compound 267 features a distinctive peroxy pentacyclic ring and compounds 259–264 each possess a three-membered oxygen ring. Intriguingly, compounds 239/240, 241/242, 250/251, 252/253, and 265/266 are epimers, while compounds 243/244 are enantiomers.
Table 6.
Other-type sesquiterpenoids of Curcumae Rhizoma.
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 239 | Curcumalactone | C. wenyujin | [29,31,43] |
| 240 | 7-Epicurcumalactone | C. wenyujin | [68] |
| 241 | Curcumanolide A | C. wenyujin, C. phaeocaulis | [15,31,45] |
| 242 | Curcumanolide B | C. wenyujin | [31,45] |
| 243 | (+)-Phaeocauline F | C. phaeocaulis | [33] |
| 244 | (−)-Phaeocauline F | C. phaeocaulis | [33] |
| 245 | Phaeocaudione | C. phaeocaulis | [69] |
| 246 | Phaeocauone | C. phaeocaulis, wenyujin | [32,69] |
| 247 | Wenyujinin L | C. wenyujin, C. phaeocaulis | [35,37] |
| 248 | Wenyujinol P | C. wenyujin | [32] |
| 249 | Curcumolide | C. wenyujin, C. kwangsiensis | [12,45] |
| 250 | Gajutsulactone A | C. wenyujin | [31] |
| 251 | Gajutsulactone B | C. wenyujin | [31] |
| 252 | Wenyujinin C | C. wenyujin | [31,35] |
| 253 | Wenyujinin D | C. wenyujin | [35] |
| 254 | Wenyujinin E | C. wenyujin | [35,36] |
| 255 | Phasalvione | C. phaeocaulis, C. wenyujin | [28,69] |
| 256 | Acomadendrane-4β,10β-diol | C. kwangsiensis | [65] |
| 257 | (4S)-Dihydrocurcumenone | C. wenyujin | [66] |
| 258 | Curcumenone | C. wenyujin | [44,46,66] |
| 259 | 7α,11-Epoxy-6α-hydroxy-carabrane-4,8-dione | C. wenyujin | [44,68] |
| 260 | 4,8-Dioxo-6β-methoxyl-7α,11-epoxycarabrane | C. wenyujin | [40] |
| 261 | 4,8-Dioxo-6β-methoxyl-7β,11-epoxycarabrane | C. wenyujin | [40] |
| 262 | 4,8-Dioxo-6β-hydroxyl-7β,11-epoxycarabrane | C. wenyujin | [36] |
| 263 | 4,8-Dioxo-6β-hydroxyl-7α,11-epoxycarabrane | C. wenyujin | [36,40] |
| 264 | 7α,11-Epoxy-6α-methoxy-carabrane-4,8-dione | C. wenyujin | [44] |
| 265 | Curcumenolactone A | C. phaeocaulis | [37] |
| 266 | Curcumenolactone B | C. phaeocaulis | [37] |
| 267 | 8,11-Epidioxy-8-hydroxy-4-oxo-6-carabren | C. wenyujin | [44] |
| 268 | Wenyujindiol A | C. wenyujin | [61] |
| 269 | Curcumadione | C. wenyujin | [63] |
| 270 | Curcumadionol | C. wenyujin, C. phaeocaulis | [16,66] |
| 271 | (6R)-Dehydroxysipanolinolide | C. wenyujin | [66] |
| 272 | Wenyujinone H | C. wenyujin | [18] |
| 273 | Wenyujinone I | C. wenyujin | [18] |
| 274 | αr-Turmerone | C. phaeocaulis | [57] |
Figure 6.
Other-type sesquiterpenoids of Curcumae Rhizoma.
In summary, 74 sesquiterpenoids have been obtained from C. kwangsiensis, 160 from C. wenyujin, and 145 from C. phaeocaulis. As depicted in Figure 7, sesquiterpenoids originating from three distinct plants predominantly comprise guaiane- and germacrane-type sesquiterpenoids. Nevertheless, notable variations are also evident. For instance, eudesmane-type sesquiterpenoids are mainly found in C. phaeocaulis, while there is a higher abundance of sesquiterpenoids in C. wenyujin. Additionally, the proportion of guaiane-type sesquiterpenoids exhibit a markedly elevated level compared to other types of sesquiterpenoids in C. kwangsiensis (Figure 7). In a further analysis of the distribution patterns of guaiane- and germacrane-type sesquiterpenoids across the three medicinal plants, it can be observed that certain sesquiterpenoids are documented in two or three plants (Figure 8). The guaiane-type sesquiterpenoids shared among all three herbs include alismoxide (3), zedoarondiol (18), isozedoarondiol (20), (1S,4S,5S,10R)-zedoarondiol (22), procurcumenol (29), procurcumadiol (33), curcumol (55), isocurcumenol (60), curcumenol (61), zedoalactone C (78), zedoalactone A (83), zedoarolide B (85), zedoalactone B (94), and zedoalactone D (96); the germacrane-type sesquiterpenoids include germacrone (116), curdione (117), neocurdione (118), 13-hydroxygermacrone (124), germacrene D (125), (4S,5S)-germacrone-4,5-epoxide (126), furanodiene (139), furanodienone (140), zederone (143), curdionolide B (146), curdionolide A (147), and aeruginolactone (150). From the abovementioned results, it becomes apparent that the three medicinal plants exhibit similarities in sesquiterpenoids, featuring overlap. Notably, certain monomers are present in larger quantities in Curcumae Rhizoma, such as zedoarondiol (18), isozedoarondiol (20), procurcumenol (29), procurcumadiol (33), curcumol (55), isocurcumenol (60), curcumenol (61), germacrone (116), curdione (117), neocurdione (118), 13-hydroxygermacrone (124), furanodiene (139), and furanodienone (140), which indicates that all three sources can be utilized as substitutes for Curcumae Rhizoma, notwithstanding their diverse botanical origins. Additionally, in terms of the abundance of compounds, investigations into C. wenyujin exhibit greater depth, whereas research on C. kwangsiensis is relatively limited.
Figure 7.
Distribution of sesquiterpenoids in three plant species.
Figure 8.
Distribution of guaiane-type and germacrane-type sesquiterpenoids in three medicinal herbs.
4. Biological Activity
It has been demonstrated that sesquiterpenoids in Curcumae Rhizoma have a wide range of pharmacological activities, including anti-inflammatory, cytotoxic, antitumor, anti-platelet aggregation, anti-atherosclerotic, hypoglycemic, hepatoprotective, antibacterial, anti-viral, antioxidant, anti-aging, neuroprotective, and anti-sepsis effects, as well as protective effects against myocardial ischemia–reperfusion injury.
4.1. Anti-Inflammatory Activity
Research has demonstrated that sesquiterpenoids in Curcumae Rhizoma exhibit remarkable anti-inflammatory activity (Table 7). Currently, research on anti-inflammatory activity primarily employs three different models: the lipopolysaccharide (LPS)-induced RAW 246.7 cell inflammation model, the LPS-induced THP-1 cell inflammation model, and the neuro-inflammatory model of LPS-stimulated BV-2 cells [75,76,77]. Further studies have revealed that some compounds, such as isozedoarondiol (20), phaeocaulisin D (49), curcumenol (61), 15-hydroxycurcumenol (63), zedoalactone B (94), phaeocaulisin M (111), phaeusmane B (177), curcolide (202), and curzerenone (215) have significant anti-inflammatory activities, with IC50 values ranging from 0.8 to 9.6 μM [2,16,42,56,78]. Subsequent mechanistic investigations revealed that sesquiterpenoids derived from Curcumae Rhizoma manifest their anti-inflammatory effects primarily by modulating the NF-κB, MAPK, JAK2/STAT3, and ERK–MAPK signal pathways. In addition, some of the compounds can also play a role in other conditions triggered by inflammation, for example, by exerting anti-inflammatory and analgesic effects, ameliorating lung inflammation and inducing airway remodeling, treating the inflammation in bronchial asthma and rheumatoid arthritis, and others [12,79,80,81,82,83,84].
Comparing the structural types of the various anti-inflammatory components mentioned above, it can be observed that all types of sesquiterpenoids in Curcumae Rhizoma have certain anti-inflammatory activities, especially guaiane-type and eudesmane-type sesquiterpenoids, and their anti-inflammatory activities bear certain structure–activity relationships. The study by Xia [44] demonstrated that guaiane-type sesquiterpenoids are more effective than other types of sesquiterpenoids. The inhibitory effects of curcumalactone (239) are stronger than those of 7-epicurcumalactone (240), possibly because of the isopropyl group’s different spatial position at C-7 [68]. The anti-inflammatory activity of phaeocaulisin D (49) is stronger than that of phaeocaulisin L (48), and it is speculated that the hydroxyl group at C-4 can enhance the activity [85].
Table 7.
Anti-inflammatory activity of sesquiterpenoids in Curcumae Rhizoma.
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | IC50 (μM) | Positive Control IC50 (μM) | Reference |
|---|---|---|---|---|---|---|---|
| Isozedoarondiol (20) | Guaiane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 1.4 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] |
| Phaeocaulisin L (48) | 54.27 ± 4.23 | 58.66 ± 6.39 (Hydrocortisone) | [42] | ||||
| Phaeocaulisin D (49) | 5.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] | ||||
| Phaeocaulisin N (54) | 3.58 ± 0.17 | 58.79 ± 3.32 (Hydrocortisone) | [34] | ||||
| 4-Epicurcumol (56) | 17.26 ± 1.26 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| 15-Hydroxycurcumenol (63) | 6.44 ± 0.51 | 14.1 ± 0.69 (Indomethacin) | [78] | ||||
| 12-Hydroxycurcumenol (64) | 9.64 ± 0.47 | 14.1 ± 0.69 (Indomethacin) | [78] | ||||
| Isocurcumol (65) | 22.36 ± 1.32 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Zedoalactone A (83) | 1.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] | ||||
| Phaeocaulisin B (84) | 1.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] | ||||
| Zedoalactone B (94) | 1.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] | ||||
| Zedoalactone D (96) | 1.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[16] | ||||
| Phaeocaulisin A (104) | 8.5 | 51.4 (Hydrocortisone) | [35] | ||||
| Wenyujinin G (109) | 7.6 | 51.4 (Hydrocortisone) | [35] | ||||
| Phaeocaulisin M (111) | 6.05 ± 0.43 | 58.66 ± 6.39 (Hydrocortisone) | [42] | ||||
| Gweicurculactone (99) | Inhibit NO production and the expressions of iNOS and COX-2 mRNA | 27.3 | 5.6 ± 0.3 (CAPE) 26.3 ± 0.3 (Indomethacin) 65.0 ± 1.2 (L-NA) |
[86] | |||
| Curcuzedoalide (112) | Inhibit NO production and suppress pre-inflammatory protein expressions of iNOS and COX-2 | 12.21 ± 1.67 | 4.15 ± 1.35 (Quercetin) | [87] | |||
| 4α,10α,11-Trihydroxy-1βH,5βH-guai-7(8)-ene (4) | LPS-induced THP-1 cell inflammation model | Inhibit the release of inflammatory mediator (TNF-α) | [88] | ||||
| Zedoarondiol (18) | LPS-induced RAW 264.7 cell and mouse peritoneal macrophage cell models | Inhibit iNOS, COX-2, and pro-inflammatory cytokine (TNF-α, IL-1β, and IL-6) expressions by suppressing the phosphorylations of IKK and MAPKs, and inactivating the NF-κB pathway | [89] | ||||
| LPS-induced THP-1-blue cell inflammation model | Inhibit LPS-stimulated TLR4 activation | 22.5 ± 1.0 | 2.6 ± 0.8 (Luteolin) | [37] | |||
| Procurcumenol (29) | Anti neuro-inflammatory activity | LPS-induced BV-2 cell inflammation model | Inhibit LPS-induced NO production | 20.05 | 23.53 ± 4.70 (Minocycline) | [52] | |
| Dihydroprocurcumenol (38) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediator (COX-2) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 28.1% and 35.3% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rates of 46.9% | [12] | ||||
| Curcumol (55) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Suppress iNOS mRNA expression and protein level; inhibit the transcriptional and translational levels of TNF-α, IL-1β, and IL-6; interfere with the JNK-mediated AP-1 pathway | [90] | |||
| Alleviate psoriasis-like inflammation activity | NHEK cell model | Reduce proliferation and inflammatory gene expression in stimulated keratinocytes by inhibiting JAK1/STAT3 signaling | [83] | ||||
| Ameliorate lung Inflammation activity | Asthmatic mice model established by ovalbumin induction | Inhibit the abnormal activation of the Wnt/β-catenin pathway | [82] | ||||
| Curcumenol (61) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediators (COX-2, IL-1β, and TNF-α) | [12] | |||
| LPS-induced macrophage inflammation model | Inhibit LPS-induced NO production | 5.42 ± 0.64 | 14.1 ± 0.69 (Indomethacin) | [78] | |||
| Anti neuro-inflammatory activity | LPS-induced BV-2 cell inflammation model | Inhibit releases of the inflammatory mediators (COX-2, IL-1β, and TNF-α) and diminish the expression of the regulatory genes by inhibiting Akt-dependent NF-κB activation and downregulating Akt and p38 MAPK signaling | [91] | ||||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 29.5% and 30% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 32.7% | [12] | ||||
| Neocurdione (118) | Germacrane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 24.18 ± 1.66 | 64.34 ± 7.49 (Hydrocortisone) | [44] |
| Curdionolide B (146) | 14.50 ± 0.87 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Germacrone (116) | Anti-inflammatory activity; alleviate bronchial asthma and rheumatoid arthritis activity, etc. | Multiple inflammation models | Regulate the expressions of related genes and proteins by PI3K III/Beclin-1/Bcl-2 and PI3K/Akt/mTOR pathways; regulate the expression of pro-inflammatory cytokines (IL-6, TNF-α, TGF-β1, and IL-10); regulate Th1/Th2 balance and NF-κB activation; upregulate TLR8 expression in THP-1 cells, etc. | [80] | |||
| Alleviate rheumatoid arthritis activity | Collagen-induced arthritis (CIA) model | Alleviate the progression of arthritis through regulating Th1/Th2 balance and inactivating the NF-κB pathway | [84] | ||||
| Dehydrocurdione (121) | Analgesic activity; antipyretic activity; anti-inflammatory activity | Acetic acid-induced writhing method; baker’s yeast-treated rat model; carrageenan-induced paw edema model | Mitigate the writhing reflex induced by acetic acid and the fever elicited by baker’s yeast; inhibit the carrageenan-induced paw edema; reduce chronic adjuvant arthritis | [79] | |||
| Souliene A (148) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediator (COX-2) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 40.7% and 35.9% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 38.5% | [12] | ||||
| Curcuminol G (151) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediators (COX-2, IL-1β, and TNF-α) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 31.4% and 45.4% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 26.2% | [12] | ||||
| 1α,4β-Dihydroxy-eudesm-7(11)-en-8-one (172) | Eudesmane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 5.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] |
| 1-Hydroxyeudesma-4(14),7(11)-dien-8-one (173) | 1.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane A (176) | 3.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane B (177) | 9.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane D (178) | 14.4 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane C (180) | 19.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Eudesm-11-ene-4α,6α-diol (181) | 0.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| 1β-Hydroxyeudesma-4,11-dien-3-one (184) | 9.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Curcolonol (186) | 16.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Chlomultin B (195) | 18.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Myrrhterpenoid N (196) | 19.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane F (197) | 4.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| (7Z)-1β,4α-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide (200) | 15.3 | 53.8 (Hydrocortisone) | [66] | ||||
| (7Z)-1β,4β-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide (201) | 3.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Curcolide (202) | 0.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| 1β,8β-Dihydroxy-eudesma-3,7(11)-dien-8α,12-olide (204) | 8.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Phaeusmane H (210) | 20.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) |
[2] | ||||
| Hydroxyisogermafurenolide (220) | 26.0 | 53.8 (Hydrocortisone) | [66] | ||||
| 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam (223) | 9.4 ± 1.6 | 42.7 ± 3.1 (Hydrocortisone) | [46] | ||||
| Curzerenone (215) | IL-6-stimulated STAT-3 expression model | Inhibit STAT-3 expression stimulated by IL-6; suppress the mRNA expression levels of the proinflammatory genes IL-1β and CRP via blockade of the IL-6-activated and ERK-MAPK signaling pathways | 4.8 | [56] | |||
| Phacadinane B (228) | Cadinane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 2.25 ± 0.71 | 43.80 ± 6.79 (Hydrocortisone) | [74] |
| Phacadinane A (229) | 3.88 ± 0.58 | 43.80 ± 6.79 (Hydrocortisone) | [74] | ||||
| Curcumalactone (239) | Other-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 inflammation model | Inhibit LPS-induced NO production | 23.28 ± 1.47 | 64.34 ± 7.49 (Hydrocortisone) | [68] |
| 7-Epicurcumalactone (240) | 45.49 ± 2.96 | 64.34 ± 7.49 (Hydrocortisone) | [68] | ||||
| Phaeocauone (246) | 2.35 ± 0.17 | 58.79 ± 3.32 (Hydrocortisone) | [69] | ||||
| Phasalvione (255) | 7.46 ± 0.69 | 58.79 ± 3.32 (Hydrocortisone) | [69] | ||||
| 8,11-Epidioxy-8-hydroxy-4-oxo-6-carabren (267) | 25.36 ± 3.26 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Curcumolide (249) | Suppress LPS-induced NF-κB activation, including the nuclear translocation and DNA binding activity of NF-κB; decrease pro-inflammatory mediators (TNF-α, IL-6, and IL-1β); NO and ROS production | [92] |
4.2. Cancer-Related Activity
Sesquiterpenoids derived from Curcumae Rhizoma exhibit noteworthy efficacy against diverse tumor cell lines (Table 8), including ovarian, breast, cervical, gastric, leukemia, and various other malignancies. Ongoing research is particularly focused on exploring their impacts on breast cancer and hepatic cancer. Research on breast cancer has primarily focused on MCF-7 and MDA-MB-231 cell models. Studies have revealed the notable efficacy of furanodiene (139) in the context of breast cancer. This compound can inhibit the proliferation of breast cancer cells in multiple ways, including regulating cyclin D1, CDK2, pRb, and Bcl-2 family proteins, activating caspases and PARP in a mitochondria-mediated pathway, inhibiting cancer cell growth via the Akt pathway and the AMPK pathway, and inducing apoptosis via metabolic regulation [93,94]. In addition, sesquiterpenoids in Curcumae Rhizoma, including zedoarondiol (18), furanodiene (139), and δ-elemene (213), have cytotoxic activity against leukemia cells [95,96,97]. Furthermore, certain compounds, including curcumol (55), germacrone (116), furanodiene (139), and β-elemene (211), have been found to exhibit broad-spectrum cancer-related activity through various pathways [80,98,99,100,101].
In conclusion, all types of sesquiterpenoids in Curcumae Rhizoma exhibit cancer-related activities, and the main active substances are guaiane-type, germacrane-type, and elemane-type sesquiterpenoids. These compounds are evenly distributed among the three plants, with most of them being common to two or three of them. Some of the shared compounds have a high content and broad-spectrum cancer-related activity, inducing apoptosis in many types of cancer cells, and it is presumed that these compounds are the important material basis for Curcumae Rhizoma.
Table 8.
Cancer-related activity of sesquiterpenoids in Curcumae Rhizoma.
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | IC50 | Positive Control IC50 | Reference |
|---|---|---|---|---|---|---|---|
| Zedoarondiol (18) | Guaiane-type sesquiterpenoids | Cytotoxic activity against lung carcinoma | A-549 cell model | Exhibit cytotoxic activity | 3.64 ± 0.66 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] |
| Cytotoxic activity against breast cancer | MCF-7 cell model | 7.34 ± 0.94 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 7.51 ± 1.35 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against leukemia | HL-60 cell model | 7.35 ± 0.61 μM | 0.0776 ± 0.0082 μM (Doxorubicin) | [95] | |||
| Isozedoarondiol (20) | Cytotoxic activity against lung carcinoma | A-549 cell model | 4.21 ± 0.93 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 9.19 ± 0.79 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 9.40 ± 1.21 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Phaeocaulisin E (21) | Cytotoxic activity against lung carcinoma | A-549 cell model | 4.79 ± 0.81 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 9.85 ± 1.02 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 10.15 ± 1.43 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Procurcumenol (29) | Cytotoxic activity against lung carcinoma | A-549 cell model | 5.82 ± 0.91 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Aerugidiol (31) | Cytotoxic activity against breast cancer | MCF-7 cell model | 7.23 ± 1.01 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 7.40 ± 0.93 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Isoprocurcumenol (43) | Cytotoxic activity against lung carcinoma | A-549 cell model | 3.81 ± 0.65 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 8.13 ± 0.93 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 8.34 ± 1.14 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Phaeocaulisguatriol (2) | Cytotoxic activity against breast cancer | MCF-7 cell model | Induce cell apoptosis by activating the expressions of TP53 and caspase 3 proteins | 40.73 ± 0.42 μM | 9.86 ± 0.13 μM (Cisplatin) | [28] | |
| Curcumol (55) | Cytotoxic activity against lung carcinoma, breast cancer, nasopharyngeal carcinoma, etc.; antitumor activity against lung cancer, nasopharyngeal carcinoma, colorectal cancer, etc. | Multi-models | Arrest the cell cycle at G0/G1 or G2/M phases; induce apoptosis in numerous cancer cells via targeting key signaling pathways, such as MAPK/ERK, PI3K/Akt, and NF-κB; regulate various signaling cascades | [98] | |||
| Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MDA-MB-231 cell model; MDA-MB-231 cell xenograft model in nude mice | Trigger apoptosis of p53 mutant triple-negative human breast cancer cells via activation of p73 and PUMA | [102] | ||||
| Cytotoxic activity against hepatic cancer; antitumor activity against hepatic cancer | Hela, A549, HUVEC cell models; Hep3B cell xenograft model in murine | Inhibit the expression of PD-L1 through crosstalk between HIF-1α and p-STAT3 (T705) signaling pathways | [103] | ||||
| Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | LoVo and SW 480 cell models; LoVo cell xenograft model in nude mice | Inhibit growth and induce apoptosis via IGF-1R and p38 MAPK pathways | [104] | ||||
| Curcumenol (61) | Cytotoxic activity against breast cancer | MCF-7 cell model | Induce apoptosis by inhibiting the proliferation of the cancer cell | 9.3 ± 0.3 μg/mL | 0.1 ± 0.0 μg/mL (Doxorubicin) | [105] | |
| Cytotoxic activity against lung carcinoma; antitumor activity against lung carcinoma | CCD19, BEAS-2B, H1299, H460, and HEK293T cell models and mice xenograft model | Induce cell death, suppress cell proliferation, and trigger ferroptosis in lung cancer cells via the lncRNA H19/miR-19b-3p/FTH1 axis | [106] | ||||
| Curcuzedoalide (112) | Cytotoxic activity against gastric cancer | AGS cell model | Activate caspase-8, caspase-9, caspase-3, and PARP, inducing apoptosis | [107] | |||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Cytotoxic activity against colorectal cancer, gastric cancer, breast cancer, cervical cancer, prostate cancer, etc. | Multi-cell models | Regulate the expressions of Akt/MDM2/p53, JAK2/STAT3, AMPK, and Akt/mTOR pathways and related proteins; inhibit the proliferation of cancer cells, promote the apoptosis of cancer cells, promote autophagy; reverse the resistance of drugs, enhance the antitumor activity of drugs, and reduce the toxicity of chemotherapeutic drugs | [80] | ||
| Cytotoxic activity against gastric cancer | BGC823 cell model | Inhibit cell proliferation through the induction of G2/M-phase cell cycle arrest and promote cell apoptosis through modulations of cell cycle-associated protein expression and mitochondria-mediated apoptosis | [108] | ||||
| Cytotoxic activity against breast cancer | MCF-7 and MDA-MB-23 cell models | Induce cell cycle arrest and apoptosis through mitochondria-mediated caspase pathway | [109] | ||||
| Cytotoxic activity against hepatic carcinoma | HepG2 and Bel7402 cell models | Regulate the expression of proteins related to G2/M cell cycle and apoptosis; p53 and oxidative damage may be involved in the inhibition of human hepatoma cells’ growth | [3] | ||||
| Cytotoxic activity against esophageal squamous cell carcinoma | Esophageal squamous cell carcinoma (ESCC) cell models | Exert an anti-esophageal effect through intrinsic apoptotic signaling pathways and by inhibiting STAT3 activity | [110] | ||||
| Curdione (117) | Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | CRC cell model; CRC cell xenograft model in nude mice | Induce ferroptosis in CRC by virtue of m6A methylation | [111] | |||
| Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MCF-7 and MDA-MB-23 cell models; MCF-7 cell xenograft model in nude mice | Inhibit proliferation and induce apoptosis; exert a synergistically inhibitory effect with other chemotherapy drugs through MAPKs and PI3K/AKT pathways | [19] | ||||
| Cytotoxic activity against uterine leiomyosarcoma; antitumor activity against uterine leiomyosarcoma | SK-UT-1 and SK-LMS-1 cell models; SK-UT-1 cell xenograft model in nude mice | Decrease the viability and proliferation of SK-UT-1 and SK-LMS-1 cells, improve apoptosis and autophagic death, and exhibit an antitumor effect through indoleamine-2, 3-dioxygenase-1 | [112] | ||||
| Cytotoxic activity against hepatic carcinoma | HHSEC under the micro-environment of HepG2 cells | Inhibit the expressions of VEGF and VEGFR2 in HHSECs in HepG2 cell micro-environment | [113] | ||||
| Furanodiene (139) | Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MCF-7 and MDA-MB-231 cell models and MCF-7 cell xenograft model in nude mice | Inhibit cell proliferation through apoptosis in a mitochondria-mediated pathway by regulating cyclin D1, CDK2, pRb, and Bcl-2 family proteins; activating caspases and PARP; and the Akt pathway is also be involved | [93] | |||
| Cytotoxic activity against breast cancer | MCF-7 cell model | Inhibit cancer cell growth via the AMPK pathway and induce cell apoptosis via metabolic regulation in chemoresistant MCF-7 breast cancer cells | [94] | ||||
| Cytotoxic activity against leukemia | HL60 cell model | Activate bid protein (a substrate of caspase-8), upregulate TNFR1, promote the formation of the TNFR1 complex and the production of TNF-α through the activation of TNFR1 signaling pathway, inducing cell apoptosis | [96] | ||||
| Cytotoxic activity against lung cancer, breast cancer, leukemia, etc.; antitumor activity against breast cancer | Multi-models | Induce apoptosis in several cancer types by modulating MAPKs/ERK, NF-κB, Akt, and other pathways | [99] | ||||
| Furanodienone (140) | Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | RKO and HT-29 cell models and CRC cell xenograft model in nude mice | Induce G0/G1 arrest and cause apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway | [114] | |||
| Zederone (143) | Cytotoxic activity against ovarian cancer | SKOV-3 cell model | Inhibit mTOR/p70s6K signaling pathway | [115] | |||
| Curcolonol (186) | Eudesmane-type sesquiterpenoids | Cytotoxic activity against breast cancer | MDA-MB-231 cell model | Inhibit LIM kinase 1 to downregulate cofilin 1 phosphorylation | [116] | ||
| Serralactone A (205) | Cytotoxic activity against breast cancer | MDA-MB-231 and MDA-MB-468 cell models | Downregulate LIMK1 activation | [117] | |||
| β-Elemene (211) | Elemane-type sesquiterpenoids | Cytotoxic activity against gastric cancer, hepatocarcinoma, breast cancer, etc.; antitumor activity against hepatocarcinoma, lung cancer, etc. | Multi-models | Inhibit cell proliferation, arrest the cell cycle and induce cell apoptosis; enhance cell immune function associated with malignancy; activate cytoprotective autophagy; reverse multidrug resistance; prevent tumor angiogenesis; enhance the sensitivity of tumor cells to radiotherapy | [101] | ||
| Cytotoxic activity against lung cancer, hepatocarcinoma, breast cancer, etc.; antitumor activity against leukemia, esophageal cancer, gastric cancer, etc. | Multi-models | Inhibit and kill tumor cells through a variety of mechanisms; enhance the effect of radiotherapy or chemotherapy synergistically; regulate autoimmune activity in the treatment of tumors | [100] | ||||
| δ-Elemene (213) | Cytotoxic activity against leukemia | HL-60 cell model | Induce apoptosis by activating caspase-3 and interfering with the cell cycle at the G2/M phase | [97] | |||
| Curzerene (214) | Cytotoxic activity against lung carcinoma; antitumor activity against lung carcinoma | SPC-A1 cell model and SPC-A1 cell xenograft model in nude mice | Induce the downregulation of GSTA1 protein and mRNA expression in SPC-A1 cells | [118] | |||
| Curzerenone (215) | Cytotoxic activity against lung carcinoma | H69AR and MRC5 cell models | Mediate programmed cell death, loss of mitochondrial membrane potential, ROS; and block the ERK/MAPK and NF-κB signaling pathways |
[119] | |||
| Acomadendrane-4β,10β-diol (256) | Other-type sesquiterpenoids | Cytotoxic activity against colon cancer | RKO cell model | Exhibit antimigratory activity | [65] | ||
| Curcumenone (258) | Cytotoxic activity against breast cancer | MCF-7 cell model | Exhibit cytotoxic activity | 8.3 ± 1.0 μg/mL | 0.1 ± 0.0 μg/mL (Doxorubicin) | [105] |
4.3. Effects on Cardiovascular System
Some of the sesquiterpenoids in Curcumae Rhizoma can exert more prominent effects on cardiovascular disease (Table 9), such as anti-platelet aggregation, anti-thrombotic, vasodilation-inducing, and anti-atherosclerotic effects, as well as protective effects against myocardial ischemia–reperfusion injury.
Numerous compounds have been demonstrated to possess anti-thrombotic and anti-platelet activities, with curdione (117) emerging as the most potent among them. Fang et al. found that curdione can inhibit thrombin-induced platelet aggregation via regulating the AMP-activated protein kinase-vinculin/talin-integrin αIIbβ3 signaling pathway [120]. Furthermore, certain compounds exhibit a specific structure–activity relationship. For instance, the enantiomers (+)-phaeocauline A (10) and (−)-phaeocauline A (11) exhibited similar activity against arachidonic acid-induced abnormal platelet aggregation. However, their C-4 epimers (+)-phaeocauline B (12) and (−)-phaeocauline B (13) showed no activity. This indicates that the anti-platelet aggregation activity is stereoselective rather than enantioselective [33].
Beyond the anti-platelet aggregation effects, sesquiterpenoids potentially exert influences on various other aspects of cardiovascular health, including vasodilation, combating atherosclerosis, alleviating cerebral ischemia–reperfusion injury, mitigating myocardial ischemia–reperfusion injury, and intervening with restenosis [1,33,121,122,123,124,125].
In summary, the therapeutic effects on the cardiovascular system are mainly exerted by guaiane-type sesquiterpenoids, including anti-thrombotic, vasodilatory, and anti-atherosclerotic effects, as well as protective effects against cerebral ischemia–reperfusion injury. Certain germacrane-type sesquiterpenoids exhibit notable activity, primarily manifesting as anti-thrombotic effects, with curdione demonstrating particularly pronounced efficacy.
Table 9.
Effects on cardiovascular system of sesquiterpenoids in Curcumae Rhizoma.
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| (+)-Phaeocauline A (10) | Guaiane-type sesquiterpenoids | Anti-platelet effect | Abnormal platelet aggregation induced by arachidonic acid | Inhibit the platelet aggregation induced by AA | Inhibition rate: 27.78 ± 4.36% | Inhibition rate: 72.89 ± 7.65% (Aspirin) | [33] |
| (−)-Phaeocauline A (11) | Inhibition rate: 31.63 ± 7.10% | ||||||
| Procurcumenol (29) | Platelet aggregation induced by ADP | Inhibit the activity of the MAPK and PI3K/AKT pathways | Inhibitionmax: 76.3%; IC50: 0.2560 mg/mL | [8] | |||
| Isoprocurcumenol (43) | Inhibitionmax: 62.8%; IC50: 0.2680 mg/mL | ||||||
| (+)-Phaeocauline D (36) | Vasorelaxant effect | Contraction of rat aortic rings induced by KCl | Exhibit vasorelaxant effects against KCl-induced contraction | Vasorelaxation: 35.51 ± 3.65% | [33] | ||
| (−)-Phaeocauline D (37) | Maximal vasorelaxation: 38.96 ± 3.26% | ||||||
| (+)-Phaeocauline E (41) | Maximal vasorelaxation: 39.42 ± 4.63% | ||||||
| (−)-Phaeocauline E (42) | Maximal vasorelaxation: 40.93 ± 5.68% | ||||||
| (+)-Phaeocauline C (68) | Maximal vasorelaxation: 47.71 ± 4.35% | ||||||
| (−)-Phaeocauline C (69) | Maximal vasorelaxation: 45.64 ± 6.85% | ||||||
| Curcumol (55) | Protective effect against cardiac remodeling | Isoproterenol (ISO)-induced cardiac remodeling | Attenuate cardiac dysfunction, myocardial fibrosis, and hypertrophy; inhibit the inflammation and apoptosis induced by ISO and TGF-β1; inhibit the AKT/NF-κB pathway | [126] | |||
| Zedoarondiol (19) | Protective effect against ox-LDL-induced injury of endothelial cells | ox-LDL-induced endothelial cell injury | Inhibit oxidative stress and inflammation via the Nrf2/HO-1 pathway | [121] | |||
| Anti-atherosclerosis effect | Arteriosclerosis in apoE mice induced by high-fat diet; THP-1 monocyte migration and adhesion experience | Ameliorate AS plaque and inhibit monocyte migration and adhesion to endothelial cells via regulating the CXCL12/CXCR4 pathway | [122] | ||||
| Arteriosclerosis in apoE mice induced by high-fat diet | Inhibit aortic plaque, inhibit the expressions of HIF 1α and downstream protein VEGF, and alleviate oxidative stress injury | [123] | |||||
| Anti-atherosclerosis effect, intervene in-sent restenosis effect | PDGF-BB-induced VSMCs proliferation | Inhibit PDGF-BB-induced VSMCs proliferation via AMPK-mediated downregulation of the mTOR/p70S6K pathway and upregulation of the p53/p21 pathway | [124] | ||||
| Protective effect against coronary heart disease and cardiovascular events | RAW264.7 macrophage inflammation model | Regulate the expression of Sirt1 of the target gene of miRNA-34a and the downstream inflammatory pathway | [125] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Protective effect against cardiac remodeling | Isoproterenol-induced mouse model; isoproterenol-induced neonatal rat cardiomyocytes | Attenuate oxidative stress, inflammation, and apoptosis in cardiac remodeling by inhibiting the PI3K/AKT pathway | [127] | ||
| Protective effect against cerebral ischemia/reperfusion injury | Cerebral ischemia–reperfusion injury model in rats | Increase the levels of Bcl-2 and inhibit the levels of caspase-3 and Bax; induce Akt activation | [128] | ||||
| Curdione (117) | Neuroprotective effects against focal cerebral ischemia reperfusion injury in rats | Cerebral ischemia–reperfusion injury model in rats | Reduce infarct size and neurological deficits, promote cognitive function recovery and recover neuronal morphologic damage; block the increase in MDA content and elevate the activities of SOD, CAT, and GSH-PX; increase the Bcl-2/Bax ratio and decrease cellular apoptosis | [1] | |||
| Anti-platelet aggregation effect | Thrombin-induced platelet aggregation | Regulate the AMP-activated protein kinase-vinculin/talin-integrin αIIbβ3 signaling pathway | [120] | ||||
| Platelet aggregation induced by ADP | Inhibit the activity of MAPK and PI3K/AKT pathways | Inhibitionmax: 85.6%; IC50: 0.1611 mg/mL | [8] | ||||
| Platelet aggregation induced by thrombin, PAF, ADP, AA, and tail thrombosis models | Increase cAMP levels, inhibit intracellular Ca2+ mobilization, and increase vasodilation | [13] | |||||
| Neocurdione (118) | Platelet aggregation induced by ADP | Inhibit the activity of the MAPK and PI3K/AKT pathways | Inhibitionmax: 77.6%; IC50: 0.2290 mg/mL | [8] | |||
| (1R,4S,5R,9R,10S)-9-Hydroxy-zederone epoxide (145) | Platelet aggregation induced by ADP and AA | Inhibit the platelet aggregation induced by ADP and AA | Inhibitionmax: 21.07 ± 8.67%; 27.73 ± 6.42% | Inhibitionmax: 44.83 ± 1.24%; 72.74 ± 7.54% (Aspirin) | [59] | ||
| β-Elemene (211) | Elemane-type sesquiterpenoids | Anti-thrombotic effect | Anticoagulant experiment and plasma recalcificatic time in wistar rabbits, acute blood-stasis rat model made by using ice-cold water, platelet aggregation induced by ADP and AA | Dissolve the thrombus and blood clots, prolong prothrombin and thrombin times, inhibit platelet aggregation | [129] | ||
| Anti-atherosclerosis effect | Arteriosclerosis in apoE mice induced by high-fat diet; HUVEC cell model | Increase the levels of plasma NO2/NO3, increase the expression of phosphorylation-eNOS; upregulate the Akt/eNOS signaling pathway and NO production in HUVECs | [130] | ||||
| Curcumadione (269) | Other-type sesquiterpenoids | Anti-platelet effect | Platelet aggregation induced by ADP | Inhibit the activity of MAPK and PI3K/AKT pathways | Inhibitionmax: 76.3%; IC50: 0.2560 mg/mL | [8] |
4.4. Hepatoprotective Activity
Modern research has revealed that many compounds in Curcumae Rhizoma have hepatoprotective activity, which aligns with the traditional belief that Curcumae Rhizoma benefits the liver (Table 10). These compounds markedly attenuate the oxidative damage induced by H2O2 in LO2 cells and induce HepG2 apoptosis to play a hepatoprotective role [18]. In addition, certain compounds demonstrate a protective effect against acute liver injury induced by D-galactosamine (D-GalN)/LPS and inhibit D-GalN-induced cytotoxicity. Interestingly, several sesquiterpenoids are found to strengthen the cytotoxicity induced by D-GalN, even though they show little cytotoxic effect on the hepatocytes in the absence of D-GalN, such as zedoarondiol (18), aerugidiol (31), isocurcumenol (60), and curcumenone (258). Actually, this phenomenon exhibits structural relevance rather than concentration dependence, as germacrane-type sesquiterpenoids are prone to exert inhibition, while guaiane-type sesquiterpenoids tend to strengthen the effect [4,131]. Several compounds manifest hepatoprotective, anti-fibrotic, and anti-fatty liver effects through mechanisms encompassing cytotoxic activity, choleretic properties, and ameliorating liver fibrosis and the modulation of sinusoidal capillarization [3,9,80,132,133,134]. In particular, β-elemene (211) in the volatile oils of Ezhu has been developed into an injection, which has been approved by the state for antitumor drugs and has been widely applied in hepatoma treatment. Some studies have found that Ezhu exhibits certain hepatotoxicity; at a high dosage, Ezhu can obviously decrease hepatocytic activity, even aggravating liver injury. It has been shown that the maximum tolerable dose in experimental mice is 224 g crude drug/kg of Ezhu medicinal material. Moreover, several compounds of Ezhu, including germacrone (116), curdione (117), and furanodiene (139), are found to have both hepatoprotective and hepatocytotoxic effects, implying that the use of these drugs carries risks. Germacrone (116) exerts effects at non-toxic concentrations (30 μM) but leads to alterations in cholesterol and lipid metabolism at slightly toxic (100 μM) and toxic concentrations (250 μM) [9].
Table 10.
Hepatoprotective of sesquiterpenoids in Curcumae Rhizoma.
| Compound | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| Zedoarondiol (18) | Guaiane-type sesquiterpenoids | Hepatoprotective effect | D-GalN/LPS-induced liver injury | Show a potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 60.7 ± 10.5%, 54.7 ± 12.7% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] |
| Aerugidiol (31) | Liver injury: 88.0 ± 2.0%, 89.1 ± 0.7% | ||||||
| Isocurcumenol (60) | Liver injury: 77.3 ± 6.6%, 80.2 ± 5.5% | ||||||
| Curcumenol (61) | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show a potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 50.7 ± 13.8%, 53.4 ± 13.4%; hepatocytotoxicity: 25.1 ± 5.3% | ||||
| Curcumol (55) | Anti-liver fibrosis effect | LSECs accompanied by an abnormal angioarchitecture; liver fibrosis rats induced by CCl4 | Attenuate liver sinusoidal endothelial cell angiogenesis via regulating Glis-PROX1-HIF-1α in liver fibrosis | [132] | |||
| Liver fibrosis rats induced by CCl4; HSCs and LX-2 cell models | Target RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling for the treatment of hepatic fibrosis | [133] | |||||
| HSC cell model | Promote autophagy in HSCs, mediate the degradation of NCOA4 and FTH1 complexes, release iron ions, lead to iron overload, and induce ferroptosis | [134] | |||||
| Anti-hepatobiliary disease effect | Liver fibrosis rats induced by CCl4; HSCs, HepG2, and RBE cell models | Inhibit the activity of RhoROCK and MAPK signaling pathways, inhibit HSC migration and adhesion, and inhibit cell proliferation | [9] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Anti-liver fibrosis effect | LX-2 and LO2 cell models | Reduce ROS release to avoid liver injury-induced HSC activation; inhibit the activation and survival of HSCs by regulating TGF-beta/Smad and apoptosis pathways | [135] | ||
| Liver fibrosis rats induced by CCl4; LX-2 cell model | Attenuate hepatic fibrosis via the PI3K/AKT/mTOR signaling pathway | [136] | |||||
| Anti-hepatoma effect | HepG2 and Bel7402 cell models | Regulate the expression of proteins related to the G2/M cell cycle, apoptosis and p53; oxidative damage may be involved | [3] | ||||
| Hepatoprotective effect | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 82.9 ± 5.4%, 78.1 ± 6.8%; hepatocytotoxicity: 59.8 ± 6.3% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] | ||
| Curdione (117) | Hepatoprotective effect | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 76.6 ± 4,7%, 74.6 ± 4.7%; hepatocytotoxicity: 77.1 ± 5.8% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] | |
| Neocurdione (118) | Liver injury: 59.3 ± 10.6%, 58.4 ± 11.1%; hepatocytotoxicity: 44.6 ± 5.3% | ||||||
| Wenyujinone D (120) | Oxidative damage induced by H2O2 in LO2 cells | Weaken the oxidative damage induced by H2O2 in LO2 cells via strengthening cell viability | Cell viability: 63.6% (H2O2: 50.7%) | [18] | |||
| Wenyujinone B (162) | Cell viability: 86.0% (H2O2: 50.7%) | ||||||
| Furanodiene (139) | Hepatoprotective effect | Oxidative damage induced by H2O2 in LO2 cells | Weaken the oxidative damage induced via strengthening cell viability | Cell viability: 85.0% (H2O2: 50.7%) | [18] | ||
| D-GalN/LPS-induced liver injury | Show potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 72.9 ± 6.7%, 74.3 ± 5.7% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [131] | |||
| Anti-hepatoma effect | HepG2 cell model | Induce G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in HepG2 cells | [137] | ||||
| Anti-hepatobiliary disease effect | HepG2 cell model | Induce G2/M cell cycle arrest and apoptosis | [9] | ||||
| β-Elemene (211) | Elemane-type sesquiterpenoids | Hepatoprotective effect; anti-fibrotic effect; anti-hepatoma effect | Liver fibrosis rats induced by CCl4; HSC-T6, HepG2, BNL, and H22 cell models | Inhibit the biological effect of ANG II and delayed liver fibrosis; inhibit cell migration and invasion through TGF-β1/Smad, JNK1/2-ROS, NF-κB, and other pathways | [9] | ||
| Curcumenone (258) | Other-type sesquiterpenoids | Hepatoprotective effect | D-GalN/LPS-induced liver injury | Show potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 90.1 ± 0.5%, 88.0 ± 0.4% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [131] |
| Curcumenolactone A (265) | D-GalN-induced cytotoxicity | Inhibit D-GalN-induced cytotoxicity | Inhibition: 65.5 ± 5.7% (100 μM) | [4] | |||
| Curcumenolactone B (266) | Inhibition: 71.1 ± 4.3% (100 μM) |
In summary, the material basis of hepatoprotective activity mainly comprises guaiane-type, germacrane-type, as well as individual other types of sesquiterpenoids. Among them, β-elemene, which is an elemane-type sesquiterpenoid, mainly exerts its activity through protecting against liver injury, ameliorating hepatic fibrosis, exerting antitumor effects, and stimulating bile flow into the duodenum. Most of these active ingredients are shared by two or three medicinal sources.
4.5. Anti-Diabetic Activity
Diabetes is the third most prevalent chronic ailment in China, following cardiovascular disease and oncological conditions, with its incidence steadily rising each year. Sesquiterpenoids from Curcumae Rhizoma have been found to exert anti-diabetic effects by increasing glucose consumption [31], improving insulin signaling and glucose circulation [138], accelerating pre-adipocyte differentiation [139], and inhibiting fatty acid synthesis and uptake (Table 11) [80]. In addition, some of the compounds improve diabetic retinopathy and also reduce diabetic retinal vascular exudation and leakage [81,140].
Table 11.
Anti-diabetic effects of sesquiterpenoids in Curcumae Rhizoma.
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Ref. |
|---|---|---|---|---|---|---|---|
| 4,10-Epizedoarondiol (25) | Guaiane-type sesquiterpenoids | Anti-diabetic effect | PTP1B inhibitory assay | Inhibit the activity of PTP1B | IC50: 35.1 μM | IC50: 5.62 μM (RK-682); 2.75 μM (Ulsolic acid) | [138] |
| Procurcumenol (29) | IC50: 45.6 μM | ||||||
| Aerugidiol (31) | IC50: 35.7 μM | ||||||
| Alismoxide (3) | Type 2 diabetes mellitus mouse model induced by combined administration of streptozotocin and nicotinamide | Accelerate 3T3-L1 pre-adipocyte differentiation and possess a hypoglycemic property | [139] | ||||
| 7α,11α-Epoxy-5β-hydroxy-9-guaiane-8-one (46) | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 46.1% (10 μM) | [31] | |||
| Curcumenol (61) | 47.0% (10 μM) | [31] | |||||
| Curdione (117) | Germacrane-type sesquiterpenoids | Anti-diabetic effect | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 74.0% (10 μM) | [31] | |
| Zederone (143) | 57.0% (10 μM) | [31] | |||||
| Heyneanone C (122) | PTP1B Inhibitory Assay | Inhibit the activity of PTP1B | IC50: 35.2 μM | IC50: 5.62 μM (RK-682); 2.75 μM (Ulsolic acid) | [138] | ||
| Germacrone (116) | Regulation of glucose–lipid metabolism | Multi-models | Regulate adipogenesis, lipolysis, and AMPKα pathway; inhibit fatty acid synthesis and uptake by suppressing the activation of the SREBP signaling pathway to alleviate hyperlipidemia and stimulate FA-β oxidation to improve lipid metabolism | [80] | |||
| 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam (223) | Elemane-type sesquiterpenoids | Attenuate ischemia-induced retinal neovascularization effect | Diabetic retinopathy rat models | Exert anti-inflammatory and anti-angiogenic effects through inhibiting NF-κB and VEGFR2 signaling pathways; reduce retinal microvascular leakage; induce retinal neovascularization | [141] | ||
| Gajutsulactone B (251) | Other-type sesquiterpenoids | Anti-diabetic effect | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 47.2% (10 μM) | [31] | |
| Wenyujinin C (252) | 49.7% (10 μM) | ||||||
| Curcumolide (249) | Attenuate diabetic retinopathy effect | STZ-induced diabetic rat model and TNF-α-stimulated HUVECs | Reduce diabetic retinal vascular leukostasis and leakage partly via the inhibition of the p38MAPK/NF-κB signaling pathway | [81] | |||
| Attenuate ischemia-induced retinal neovascularization effect | HUVEC cell model; oxygen-induced mouse retinopathy model | Exert anti-angiogenic activity and attenuate ischemia-induced retinal neovascularization via the VEGFR2 signaling pathway | [140] |
In conclusion, germacrane-type, guaiane-type, and other types of sesquiterpenoids in Curcumae Rhizoma demonstrate predominant anti-diabetic properties. These sesquiterpenoids are evenly distributed across the three plants, with a significant proportion being shared compounds among two or three medicinal herbs.
4.6. Other Biological Activities
In addition to the above activities, it has been found that sesquiterpenoids in Curcumae Rhizoma have a variety of other biological activities, including antioxidant, anti-microbial, anti-viral, skin regeneration, anti-aging, neuroprotective, and anti-septic effects (Table 12). The research findings indicate that wenyujinin Q (17), zedoarondiol (18), isozedoarondiol (20), phaeocaulisin E (21), procurcumadiol (33), neoprocurcumenol (35), and various other compounds show extensive antibacterial effects and antifungal properties [36,40]. Notably, certain compounds exhibit noteworthy efficacy against both influenza A and influenza B viruses [29]. Regarding the antioxidant potential, a diverse array of sesquiterpenoids manifest noteworthy antioxidant properties and the efficacious scavenging of free radicals. Studies have elucidated the involvement of specific compounds, such as germacrone (116), in diverse oxidative stress models. These compounds actively diminish free radical concentrations within the organism, alleviate oxidative harm, and consequently hold promise in the prevention and treatment of associated diseases [80]. In the context of skin regeneration, it has been discovered that alismoxide (3), isozedoarondiol (20), isoprocurcumenol (43), germacrone (116), and 13-hydroxygermacrone (124) can activate the epidermal growth factor receptor, thereby promoting skin regeneration [142,143,144]. In relation to other activities, procurcumenol (29), germacrone (116), and dehydrocurdione (121) also exhibit neuroprotective effects [143]; curcumanolide A (241) has a relaxant effect on uterine smooth muscle tissue [15]; curdione (117) attenuates sepsis-induced lung injury [145]; curcumenol (61) reduces disc inflammation and improves disc catabolism [146]; zederone (143) has the potential to be a drug for the treatment of dementia [147]; and curcumenone (258) can exert a protective effect against intoxication [148]. These activities are primarily attributed to guaiane-type, germacrane-type, eudesmane-type, and elemane-type sesquiterpenoids.
Table 12.
Other biological activities of sesquiterpenoids in Curcumae Rhizoma.
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| Wenyujinin Q (17) | Guaiane-type sesquiterpenoids | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities |
A. brassicicola: 50 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 50 μg/mL; E. turcicum: 25 μg/mL; P. theae: 25 μg/mL; A. citri: 100 μg/mL |
A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) |
[36] |
| Phaeocaulisin E (21) |
A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 100 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL |
||||||
| Neoprocurcumenol (35) |
A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 25 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL |
||||||
| Procurcumadiol (33) | Antibacterial effect | Antibacterial activity against E. coli | Antibacterial activity against E. coli | E. coli: 1.25 μg/mL | E. coli: 0.3 μg/mL (Ciprofloxacin) | [40] | |
| 7β,8α-Dihydroxy-1α,4αH-guai-10(15)-en-5β,8β-endoxide (57) | Anti-viral activity | Influenza virus A | Show anti-viral activity against the influenza virus A | IC50: 9.18 ± 0.46 μM | IC50: 8.06 ± 0.64 μM (Ribavirin); 47.42 ± 1.96μM (Oseltamivir) | [29] | |
| 1α,8α-Epidioxy-4α-hydroxy-5αH-guai-7(11),9-dien-12,8-olide (110) | IC50: 6.80 ± 0.13 μM | IC50: 8.06 ± 0.64 μM (Ribavirin); 47.42 ± 1.96μM (Oseltamivir) | |||||
| 9-Oxo-neoprocurcumenol (45) | Antioxidant property | Nrf2-luciferase activity in HEK 293 cells | Exhibit antioxidant activity via the activation of the Nrf2-ARE pathway | [27] | |||
| Zedoarolide B (85) | Dual-luciferase reporter gene assay in 293 T cells | Activate the transcription of Nrf2 in 293 T cells | [32] | ||||
| Alismoxide (3) | Anti-aging effect | UVB-mediated HaCaT cell model | Inhibit the production of MMP-1 in UV-irradiated HaCaT cells | [149] | |||
| Zedoarondiol (18) | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities |
A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 100 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL |
A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) |
[36] | |
| Endothelial cell injury protective effect | ox-LDL-induced HUVEC injury | Attenuate ox-LDL-induced endothelial cell injury by inhibiting oxidative stress and inflammation via the Nrf2/HO-1 pathway | [121] | ||||
| Isozedoarondiol (20) | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities |
A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 100 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 50 μg/mL; A. citri: 50 μg/mL |
A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) |
[36] | |
| Anti-aging effect | UVB-mediated HaCaT cell model | Inhibit production of MMP-1 in UV-irradiated HaCaT cells | [149] | ||||
| Procurcumenol (29) | Antioxidant activity | Nrf2-luciferase activity in HEK 293 cells | Exhibit antioxidant activity via activation of the Nrf2-ARE pathway | [27] | |||
| Antibacterial effect | Antibacterial activity against S. albus | Antibacterial activity against S. albus | S. albus: 1.25 μg/mL | S. albus: 0.6 μg/mL (Ciprofloxacin) | [40] | ||
| Neuroprotective property | H2O2-induced oxidative stress in NG108-15 cells | Show moderate protection of NG108-15 cells | Neuroprotective: cell viability: 80.00 ± 0.71% (15 μM) (H2O2: 67.63 ± 0.86) | [143] | |||
| Isoprocurcumenol (43) | Skin function maintenance activity | UVB-induced cellular damage | Activate EGFR signaling, increase the phosphorylation of ERK and AKT, upregulate the expression of genes related to cell growth and proliferation, and induce the proliferation of keratinocytes | [142] | |||
| Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show moderate protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 80.96 ± 0.91% (4 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 26.43 ± 1.88 μM/100 μg |
[143] | |||
| Curcumenol (61) | Neuroprotective property | H2O2-induced oxidative stress in NG108-15 cells | Show moderate protection of NG108-15 cells | Neuroprotective: cell viability: 103.04 ± 2.17% (4 μM) (H2O2: 67.63 ± 0.86) | [143] | ||
| Improvement of intervertebral disc catabolism status | Lumbar spine-instability mouse model | Inhibit TNFα/NF-κB signaling pathway and mitigate the expression of the MMP family (MMP-3, MMP-9, and MMP-13) | [146] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show moderate protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 89.99 ± 2.01% (15 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 24.86 ± 2.33 μM/100 μg |
[143] | |
| Anti-aging effect | UVB-induced damage in HaCaT cells | Inhibit UVB-induced upregulation of mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 | [144] | ||||
| Curdione (117) | Effect on sepsis-induced lung injury | CLP surgery established mice sepsis model | Inhibit platelet-mediated neutrophil recruitment, infiltration, and NET formation; exert anti-inflammatory and antioxidant properties | [145] | |||
| Dehydrocurdione (121) | Ca(2+) channel blocker-like effect | Ca(2+) channel blocker-like model | Exhibit a Ca(2+) channel blocker-like effect on rodent intestinal and vascular smooth muscles | [150] | |||
| Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show obvious protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 100.60 ± 1.72% (10 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 26.18 ± 2.59 μM/100 μg |
[143] | |||
| Heyneanone D (123) | Antibacterial effect | Antibacterial activity against E. coli | Antibacterial activity against E. coli | E. coli: 1.25 μg/mL | E. coli: 0.3 μg/mL (Ciprofloxacin) | [40] | |
| 13-Hydroxygermacrone (124) | Anti-aging effect | UVB-induced damage in HaCaT cells | Inhibit UVB-induced upregulation of mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 | [144] | |||
| Zederone (143) | Alzheimer’s disease | Aluminium-induced dementia rat model | Improve fecal microbiological profiles; regulate gut bacterial ecological imbalances | [147] | |||
| Antioxidant activity | Oxygen radical antioxidant capacity assay | Antioxidant | TE: 27.78 ± 2.53 μM/100 μg | [143] | |||
| Curcolide (202) | Eudesmane-type sesquiterpenoids | Antioxidant property | Dual-luciferase reporter gene assay in 293 T cells | Activate the transcription of Nrf2 in 293 T cells | [32] | ||
| Curcumanolide A (241) | Other-type sesquiterpenoids | Relaxant effect on uterine smooth muscle | Oxytocin-induced contraction model of rat uterine smooth muscle | Show an inhibitory effect against oxytocin-induced rat uterine smooth muscle contraction | [15] | ||
| Curcumenone (258) | Protective effect on drunkenness | Alcohol-induced drunkenness model | Increase liver alcohol dehydrogenase activity and decrease the elevation of blood alcohol concentrations | [148] | |||
| Antioxidant activity | Oxygen radical antioxidant capacity assay | Antioxidant | TE: 21.16 ± 2.12 μM/100 μg | [143] |
5. Conclusions
Curcumae Rhizoma, a crucial medicinal herb, has a long history of medicinal use and exhibits remarkable therapeutic efficacy. Research on the chemical composition and pharmacological activities of this medicine has been extensively conducted both in China and internationally. The primary chemical constituents identified include curcumins and sesquiterpenoids. The traditional utilization of Curcumae Rhizoma among communities exhibits a lack of systematicity, with medicinal sources presenting notable diversity. Therefore, this article provides an extensive review of sesquiterpenoids isolated from the rhizomes of C. phaeocaulis, C. kwangsiensis, and C. wenyujin, primarily based on the Chinese Pharmacopeia. A total of 279 sesquiterpenoids have been reported in the relevant literature, showcasing an extensive structural diversity comprising many analogs, enantiomers, diastereomers, and geometric isomers. These compounds encompass diverse types, featuring guaiane-type, germacrane-type, eudesmane-type, elemane-type, and cadinane-type sesquiterpenoids. A total of 79 sesquiterpene compounds were obtained from C.kwangsiensis, 167 from C. wenyujin, and 143 from C. phaeocaulis. It was found that all three plants were dominated by guaiane-type and germacrane-type sesquiterpenoids, and some compounds were present in all three plants at the same time, with 14 shared compounds among the guaiane-type sesquiterpenoids and 12 among the germacrane-type sesquiterpenoids. These findings demonstrate that all three sources can be utilized as substitutes for Curcumae Rhizoma, notwithstanding their diverse botanical origins.
Pharmacological studies have revealed that all types of sesquiterpenoids possess some anti-inflammatory properties, while their cancer-related activity is concentrated in guaiane-type, germacrane-type, and elemane-type sesquiterpenoids. For the treatment of cardiovascular diseases, guaiane-type and germacrane-type sesquiterpenoids are mainly involved, of which zedoarondiol has a significant anti-atherosclerotic effect and curdione has a significant anti-thrombotic effect. The hepatoprotective and anti-diabetic effects are also predominantly concentrated in the guaiane-type and germacrane-type sesquiterpenoids. In terms of compound types, guaiane-type and germacrane-type sesquiterpenoids were found to include a variety of active substances. Eudesmane-type sesquiterpenoids are among the material bases for anti-inflammatory activity, while elemane-type sesquiterpenoids are mostly associated with significant cancer-related effects. Most of the active monomers in them are present in two or three medicinal herbs. Some of the compounds common to all three plants, such as zedoarondiol, isozedoarondiol, curcumol, curcumenol, curdione, furanodiene, zederone, β-elemene, curzerene, and curzerenone, are found at high levels in Curcumae Rhizoma, and they have a wide range of activities and high therapeutic efficacy, which also supports the scientific validity of the use of the three herbs together as Curcumae Rhizoma.
Various studies have shown that the sesquiterpenoids in Curcumae Rhizoma have immense potential as new drug sources, but there are still barriers to their use. To address these limitations, future research should focus on several areas: (1) at present, sesquiterpenoids are mainly extracted by means of organic solvent extraction and steam distillation, followed by further purification by column chromatography, including silica gel column chromatography, reversed-phase C18 silica gel column chromatography, Sephadex LH-20 column chromatography, ODS column chromatography, HPLC, and preparative TLC. Contemporary isolation methods are relatively mature, leading to a diverse array of compounds. However, due to the volatile and unstable nature of the sesquiterpenoids, the extant separation methodologies encounter certain constraints. Thus, the imperative arises to embrace innovative technological paradigms to surmount these challenges. For instance, separation can be conducted in a closed system at room temperature using HPLC-SPE, which gradually emerges as an indispensable solution to address these limitations. (2) Guaiane-type and germacrane-type sesquiterpenoids possess more prominent bioactivities compared to other sesquiterpenoids. However, the isolation methods currently in common use are not specific for obtaining different types of sesquiterpenoids. Therefore, it is crucial to implement novel methods, techniques, and strategies, including Global Natural Products Social (GNPS) molecular networking, Small Molecule Accurate Recognition Technology (SMART), and high-sensitivity LC-MS/MS, to achieve the precise and targeted identification of specific compounds. (3) The activity of sesquiterpenoids within Curcumae Rhizoma is rich and diverse, oriented towards anti-inflammatory, antitumor, cytotoxic, anti-cardiovascular disease, and hepatoprotective effects. Several compounds have been used for the development of new drugs, showing that this herb possesses great potential as a new drug source. Thus, there is a need to broaden the research on pharmacological activity and expand the study on the underlying mechanisms.
Author Contributions
All authors contributed to this work. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (NNSFC; Grant Nos. 82022072 and 82104371) and the Natural Science Foundation of Sichuan Province (Grant Nos. 2023NSFSC1773, 2022NSFSC1557 and 2022NSFSC1577).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Li X.J., Liang L., Shi H.X., Sun X.P., Wang J., Zhang L.S. Neuroprotective Effects of Curdione against Focal Cerebral Ischemia Reperfusion Injury in Rats. Neuropsychiatr. Dis. Treat. 2017;13:1733–1740. doi: 10.2147/NDT.S139362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Ma J.H., Wang Y., Donkor P.O., Li Q., Gao S.Y., Hou Y.G., Xu Y., Cui J.N., Ding L.Q., et al. Eudesmane-Type Sesquiterpenes from Curcuma phaeocaulis and Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Eur. J. Org. Chem. 2014;2014:5540–5548. doi: 10.1002/ejoc.201402465. [DOI] [Google Scholar]
- 3.Liu Y., Wang W., Fang B., Ma F., Zheng Q., Deng P., Zhao S., Chen M., Yang G., He G. Anti-Tumor Effect of Germacrone on Human Hepatoma Cell Lines through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Eur. J. Pharmacol. 2013;698:95–102. doi: 10.1016/j.ejphar.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda H., Morikawa T., Ninomiya K., Yoshikawa M. Hepatoprotective Constituents from Zedoariae Rhizoma: Absolute Stereostructures of Three New Carabrane-Type Sesquiterpenes, Curcumenolactones A, B, and C. Bioorg. Med. Chem. 2001;9:909–916. doi: 10.1016/S0968-0896(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 5.National PC . Pharmacopoeia of the People’s Republic of China (I) Chinese Medical Science and Technology Press; Beijing, China: 2020. pp. 286–287. [Google Scholar]
- 6.Zhou Y., Xie M., Song Y., Wang W.P., Zhao H.R., Tian Y.X., Wang Y., Bai S.J., Zhao Y.C., Chen X.Y., et al. Two Traditional Chinese Medicines Curcumae Radix and Curcumae Rhizoma: An Ethnopharmacology, Phytochemistry, and Pharmacology Review. Evid. Based Complement. Alternat. Med. 2016;2016:4973128. doi: 10.1155/2016/4973128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X., Quan Y.Y., Yin Z.J., Li M., Wang T., Zheng L.Y., Feng S.Q., Zhao J.N., Li L. Sources, Morphology, Phytochemistry, Pharmacology of Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma: A Review of the Literature. Front. Pharmacol. 2023;14:1229963. doi: 10.3389/fphar.2023.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong H.J., Yu M.T., Fei C.H., Ji D., Dong J.J., Su L.L., Gu W., Mao C.Q., Li L., Bian Z.H., et al. Bioactive Constituents and the Molecular Mechanism of Curcumae Rhizoma in the Treatment of Primary Dysmenorrhea Based on Network Pharmacology and Molecular Docking. Phytomedicine. 2021;86:153558. doi: 10.1016/j.phymed.2021.153558. [DOI] [PubMed] [Google Scholar]
- 9.Gao T.H., Liao W., Lin L.T., Zhu Z.P., Lu M.G., Fu C.M., Xie T. Curcumae Rhizoma and Its Major Constituents against Hepatobiliary Disease: Pharmacotherapeutic Properties and Potential Clinical Applications. Phytomedicine. 2022;102:154090. doi: 10.1016/j.phymed.2022.154090. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Zhu Z.-P., Chen J., Zheng Y.-F., Limsila B., Lu M.-G., Gao T.-H., Yang Q.-S., Fu C.-M., Liao W. Terpenoids from Curcumae Rhizoma: Their Anticancer Effects and Clinical Uses on Combination and Versus Drug Therapies. Biomed. Pharmacother. 2021;138:111350. doi: 10.1016/j.biopha.2021.111350. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.H., Wu Y.C., Li Y.M., Guo F.J. Review of the Traditional Uses, Phytochemistry, and Pharmacology of Curcuma wenyujin Y. H. Chen et C. Ling. J. Ethnopharmacol. 2021;269:113689. doi: 10.1016/j.jep.2020.113689. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H.L., Zhao Y.L., Ding C.F., Zhu P.F., Jin Q., Liu Y.P., Ding Z.T., Luo X.D. Anti-Inflammatory and Antinociceptive Effects of Curcuma kwangsiensis and its Bioactive Terpenoids in vivo and in vitro. J. Ethnopharmacol. 2020;259:112935. doi: 10.1016/j.jep.2020.112935. [DOI] [PubMed] [Google Scholar]
- 13.Xia Q., Wang X., Xu D.J., Chen X.H., Chen F.H. Inhibition of Platelet Aggregation by Curdione from Curcuma wenyujin Essential Oil. Thromb. Res. 2012;130:409–414. doi: 10.1016/j.thromres.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen X.P., Pei L.X., Zhong Z.F., Guo J.J., Zhang Q.W., Wang Y.T. Anti-Tumor Potential of Ethanol Extract of Curcuma phaeocaulis Valeton against Breast Cancer Cells. Phytomedicine. 2011;18:1238–1243. doi: 10.1016/j.phymed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Cui T., Ni H., Liu J., Peng C., Xiong L., Liu F. Sesquiterpenoids from Volatile Oil of Curcuma phaeocaulis and Relaxant Effects on Uterine Smooth Muscle. Chin. Tradit. Herb. Drugs. 2022;53:4265–4269. [Google Scholar]
- 16.Liu Y., Ma J.H., Zhao Q., Liao C.R., Ding L., Chen L.Q., Zhao F., Qiu F. Guaiane-Type Sesquiterpenes from Curcuma phaeocaulis and Their Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2013;76:1150–1156. doi: 10.1021/np400202f. [DOI] [PubMed] [Google Scholar]
- 17.Lou Y., Zhao F., Wu Z., Peng K.F., Wei X.C., Chen L.X., Qiu F. Germacrane-Type Sesquiterpenes from Curcuma wenyujin. Helv. Chim. Acta. 2009;92:1665–1672. doi: 10.1002/hlca.200900059. [DOI] [Google Scholar]
- 18.Li Y., Wang H., Wang H., Wu Y.C., Li Y.M., Guo F.J. Nine New Sesquiterpenes from Curcuma wenyujin Rhizomes. Fitoterapia. 2022;158:105167. doi: 10.1016/j.fitote.2022.105167. [DOI] [PubMed] [Google Scholar]
- 19.Zhao P., Qiu J.F., Pan C.L., Tang Y.Y., Chen M.J., Song H., Yang J., Hao X.J. Potential Roles and Molecular Mechanisms of Bioactive Ingredients in Curcumae Rhizoma against Breast Cancer. Phytomedicine. 2023;114:154810. doi: 10.1016/j.phymed.2023.154810. [DOI] [PubMed] [Google Scholar]
- 20.Lu J.-J., Dang Y.-Y., Huang M., Xu W.-S., Chen X.-P., Wang Y.-T. Anti-Cancer Properties of Terpenoids Isolated from Rhizoma Curcumae–A Review. J. Ethnopharmacol. 2012;143:406–411. doi: 10.1016/j.jep.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y.-Q., Tong T. Curcumae Rhizoma: A Botanical Drug against Infectious Diseases. Front. Pharmacol. 2023;13:1015098. doi: 10.3389/fphar.2022.1015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dosoky N., Setzer W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients. 2018;10:1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F.Q., Li S.P., Zhao J., Lao S.C., Wang Y.T. Optimization of GC–MS Conditions Based on Resolution and Stability of Analytes for Simultaneous Determination of Nine Sesquiterpenoids in Three Species of Curcuma rhizomes. J. Pharm. Biomed. Anal. 2007;43:73–82. doi: 10.1016/j.jpba.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Xiang Z., Wang X.Q., Cai X.J., Zeng S. Metabolomics Study on Quality Control and Discrimination of Three Curcuma Species Based on Gas Chromatograph–Mass Spectrometry. Phytochem. Anal. 2011;22:411–418. doi: 10.1002/pca.1296. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., He T., Wang J.J., Wang L., Ren X.Y., He S.H., Liu X.Y., Dong Y., Ma J.M., Song R.L., et al. High Performance Liquid Chromatography Fingerprint and Headspace Gas Chromatography-Mass Spectrometry Combined with Chemometrics for the Species Authentication of Curcumae rhizoma. J. Pharm. Biomed. Anal. 2021;202:114144. doi: 10.1016/j.jpba.2021.114144. [DOI] [PubMed] [Google Scholar]
- 26.Yang F.Q., Li S.P., Chen Y., Lao S.C., Wang Y.T., Dong T.T.X., Tsim K.W.K. Identification and Quantitation of Eleven Sesquiterpenes in Three Species of Curcuma Rhizomes by Pressurized Liquid Extraction and Gas Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2005;39:552–558. doi: 10.1016/j.jpba.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.H., Liu J.W., Wu Y.C., Li Y.M., Guo F.J. Guaiane-Type Sesquiterpenes from Curcuma wenyujin. Phytochemistry. 2022;198:113164. doi: 10.1016/j.phytochem.2022.113164. [DOI] [PubMed] [Google Scholar]
- 28.Zhong X.J., Yan X., Liu W.R., Tian Y.X., Song R.L., Dong Y., Ren X.Y., Zheng Y., Shan D.J., Lv F., et al. Sesquiterpenoids Isolated from the Rhizome of Curcuma phaeocaulis Valeton: Antitumor Activity, in Silico Molecular Docking and Molecular Dynamics Study. New J. Chem. 2023;47:7830–7839. doi: 10.1039/D2NJ06011F. [DOI] [Google Scholar]
- 29.Dong J.Y., Ma X.Y., Cai X.Q., Yan P.C., Yue L., Lin C., Shao W.W. Sesquiterpenoids from Curcuma wenyujin with Anti-Influenza Viral Activities. Phytochemistry. 2013;85:122–128. doi: 10.1016/j.phytochem.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Lou Y., He H., Wei X.C., Li X.G., Chen L.X., Qiu F. Sesquiterpenes from Curcuma wenyujin. J. Shenyang Pharm. Univ. 2010;27:195–199. [Google Scholar]
- 31.Zhou C.X., Zhang L.S., Chen F.F., Wu H.S., Mo J.X., Gan L.S. Terpenoids from Curcuma wenyujin Increased Glucose Consumption on HepG2 Cells. Fitoterapia. 2017;121:141–145. doi: 10.1016/j.fitote.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Chen L.J., Liu J.W., Wang H., Li Y.H., Li Y.M., Guo F.J. Four New Sesquiterpenes from Curcuma wenyujin. Fitoterapia. 2022;163:105344. doi: 10.1016/j.fitote.2022.105344. [DOI] [PubMed] [Google Scholar]
- 33.Liu F., Chen J.F., Qiao M.M., Zhao H.Y., Zhou Q.M., Guo L., Peng C., Xiong L. Seven Pairs of New Enantiomeric Sesquiterpenoids from Curcuma phaeocaulis. Bioorg. Chem. 2020;99:103820. doi: 10.1016/j.bioorg.2020.103820. [DOI] [PubMed] [Google Scholar]
- 34.Ma J.H., Zhao F., Wang Y., Liu Y., Gao S.Y., Ding L.Q., Chen L.X., Qiu F. Four New Sesquiterpenoids as Natural Nitric Oxide (NO) Inhibitors from the Rhizomes of Curcuma phaeocaulis. Phytochem. Lett. 2015;14:221–225. doi: 10.1016/j.phytol.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Yin G.P., Li L.C., Zhang Q.Z., An Y.W., Zhu J.J., Wang Z.M., Chou G.X., Wang Z.T. iNOS Inhibitory Activity of Sesquiterpenoids and a Monoterpenoid from the Rhizomes of Curcuma wenyujin. J. Nat. Prod. 2014;77:2161–2169. doi: 10.1021/np400984c. [DOI] [PubMed] [Google Scholar]
- 36.Huang H.F., Zheng C.J., Chen G.Y., Yin W.Q., Huang X., Mo Z.R. Sesquiterpenoids from Curcuma wenyujin Dreg and their Biological Activities. Chin. Chem. Lett. 2016;27:1612–1616. doi: 10.1016/j.cclet.2016.03.043. [DOI] [Google Scholar]
- 37.Jang H.J., Kim J.H., Oh H.M., Kim M.S., Jo J.H., Jung K., Lee S., Kim Y.H., Lee W.S., Lee S.W., et al. Sesquiterpenoids from the Rhizomes of Curcuma phaeocaulis and Their Inhibitory Effects on LPS-Induced TLR4 Activation. Chem. Pharm. Bull. 2016;64:1062–1066. doi: 10.1248/cpb.c16-00086. [DOI] [PubMed] [Google Scholar]
- 38.Dai W.F., Zhang L.L., Liu Y.F., Zhang M. A New 4,5-Secofurancadinene from the Rhizome of Curcuma kwangsiensis. Rec. Nat. Prod. 2020;14:297–300. doi: 10.25135/rnp.169.20.01.1520. [DOI] [Google Scholar]
- 39.Lou Y., Zhao F., He H., Peng K.F., Zhou X.H., Chen L.X., Qiu F. Guaiane-type Sesquiterpenes from Curcuma wenyujin and Their Inhibitory Effects on Nitric Oxide Production. J. Asian Nat. Prod. Res. 2009;11:737–747. doi: 10.1080/10286020903042358. [DOI] [PubMed] [Google Scholar]
- 40.Huang H.F., Zheng C.J., Mo Z.R., Yin W.Q., Chen G.Y., Han C.R., Huang X. Antibacterial Sesquiterpenoids from the Petroleum Ether Extract of Curcuma wenyujin Dreg. Chem. Nat. Compd. 2016;52:527–530. doi: 10.1007/s10600-016-1699-z. [DOI] [Google Scholar]
- 41.Zhan X.R., Zeng Z.W., Meng F.L., Wang S.L., Xie T. Pharmaceutical Researches on Zedoary Turmeric Oil. J. Hangzhou Norm. Univ. Nat. Sci. Ed. 2011;10:454–458. [Google Scholar]
- 42.Ma J.H., Wang Y., Liu Y., Gao S.Y., Ding L.Q., Zhao F., Chen L.X., Qiu F. Four New Sesquiterpenes from the Rhizomes of Curcuma phaeocaulis and Their iNOS Inhibitory Activities. J. Asian Nat. Prod. Res. 2015;17:532–540. doi: 10.1080/10286020.2015.1046449. [DOI] [PubMed] [Google Scholar]
- 43.Harimaya K., Gao J.F., Ohkura T., Kawamata T., Irraka Y., Guo Y.T., Inayama S. A Series of Sesquiterpenes with a 7α-isopropyl Side Chain and Related Compounds Isolated from Curcuma wenyujin. Chem. Pharm. Bull. 1991;39:843–853. doi: 10.1248/cpb.39.843. [DOI] [Google Scholar]
- 44.Xia G.Y., Zhou L., Ma J.H., Wang Y., Ding L.Q., Zhao F., Chen L.X., Qiu F. Sesquiterpenes from the Essential oil of Curcuma wenyujin and their Inhibitory Effects on Nitric Oxide Production. Fitoterapia. 2015;103:143–148. doi: 10.1016/j.fitote.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H.Y., Zhang L.S., Zhang J., Zhang Y.Y., Pan J.R. Chemical Constituents from Curcuma wenyujin. Chin. Tradit. Pat. Med. 2016;38:1534–1537. [Google Scholar]
- 46.Qiu G.G., Yan P.C., Shao W.W., Zhou J., Lin W.W., Fang L.L., Zhao X.W., Dong J.Y. Two New Sesquiterpenoids Including a Sesquiterpenoid Lactam from Curcuma wenyujin. Chem. Pharm. Bull. 2013;61:983–986. doi: 10.1248/cpb.c13-00405. [DOI] [PubMed] [Google Scholar]
- 47.Wu H.-H., Zheng H.-H., Xu Y.-T., Zhang P., Chen G., Zhu Y. Two New Sesquiterpenes from a Kind of TCM Pieces, Curcumae Radix. Rec. Nat. Prod. 2014;8:334. [Google Scholar]
- 48.Cai Y. A New Sesquiterpene Compound—Curcumafuranol. J. Beijing Med. Univ. 1998;30:49–52. [Google Scholar]
- 49.Zhu K. Master’s Thesis. Shenyang Pharmaceutical University; Shenyang, China: 2008. Studies on the Chemical Constituents from Curcuma kwangsiensis. [Google Scholar]
- 50.Jiang D.Q., Pu J.L., Huang P., Huang Y.M., He Y.Z., He C.H., Zheng Q.T. Studies on the Chemical Composition of Curcuma kwangsiensis. Chin. Pharm. J. 1989;24:42. [PubMed] [Google Scholar]
- 51.Yin G.P., An Y.W., Hu G., Zhu J.J., Chen L.M., Li L.C., Wang Z.M. Three New Guaiane Sesquiterpene Lactones from Rhizomes of Curcuma wenyujin. J. Asian Nat. Prod. Res. 2013;15:723–730. doi: 10.1080/10286020.2013.796936. [DOI] [PubMed] [Google Scholar]
- 52.Liao H.B., Feng W.Y., Wang H.S., Liang D. Sesquiterpenoid Compounds from Curcuma kwangsiensis (Thunb.) Sweet. Chem. Biodivers. 2019;16:e1900123. doi: 10.1002/cbdv.201900123. [DOI] [PubMed] [Google Scholar]
- 53.Hu D., Ma N., Lou Y., Qu G.X., Qiu F. Guaiane Sesquiterpenes of Curcuma wenyujin. J. Shenyang Pharm. Univ. 2008;25:188–190. [Google Scholar]
- 54.Phan M.G., Tran T.T.N., Phan T.S., Matsunami K., Otsuka H. Guaianolides from Curcuma kwangsiensis. Phytochem. Lett. 2014;9:137–140. doi: 10.1016/j.phytol.2014.05.009. [DOI] [Google Scholar]
- 55.Xiang F.F., He J.W., Liu Z.X., Peng Q.Z., Wei H. Two New Guaiane-Type Sesquiterpenes from Curcuma kwangsiensis and Their Inhibitory Activity of Nitric Oxide Production in Lipopolysaccharide-Stimulated Macrophages. Nat. Prod. Res. 2018;32:2670–2675. doi: 10.1080/14786419.2017.1378203. [DOI] [PubMed] [Google Scholar]
- 56.Jang H.J., Lim H.J., Park E.J., Lee S.J., Lee S., Lee S.W., Rho M.C. STAT3-Inhibitory Activity of Sesquiterpenoids and Diterpenoids from Curcuma phaeocaulis. Bioorg. Chem. 2019;93:103267. doi: 10.1016/j.bioorg.2019.103267. [DOI] [PubMed] [Google Scholar]
- 57.Oh S., Han A.R., Park H.R., Jang E.J., Kim H.K., Jeong M.G., Song H., Park G.H., Seo E.K., Hwang E.S. Suppression of Inflammatory Cytokine Production by ar-Turmerone Isolated from Curcuma phaeocaulis. Chem. Biodiversity. 2014;11:1034–1041. doi: 10.1002/cbdv.201300397. [DOI] [PubMed] [Google Scholar]
- 58.Liu X.Y., Lou Y., Hu D., Chen L.X., Bu G.M., Qiu F. Chemical Constituents of the Essential Oil from Curcuma wenyujin Y. H. Chen et C. Ling. J. Shenyang Pharm. Univ. 2007;24:686. [Google Scholar]
- 59.Li X.C., Chen J.F., Xiong L., Peng C., Guo L., Liu F. Study on Germacrane-Type Sesquiterpenoids from Curcuma phaeocaulis. Chin. Tradit. Herb. Drugs. 2021;52:28–34. [Google Scholar]
- 60.Li J. Ph.D. Thesis. Shenyang Pharmaceutical University; Shenyang, China: 2010. Studies on Chemical Substances of Curcuma kwangsiensis and Metabolites of Natural Curcuminoids in Rats. [Google Scholar]
- 61.Yin G.P., Yang D., Zhu T., Zhang Z.L., Xie W., Hu C.H., Zhu J.J., Wang Z.M. Wenyujindiol A, A New Sesquiterpene from the Rhizomes of Curcuma wenyujin. Tetrahedron Lett. 2020;61:152448. doi: 10.1016/j.tetlet.2020.152448. [DOI] [Google Scholar]
- 62.Gao J.F., Xie J.H., Harimaya K., Kawamata T., Iitaka Y., Inayama S. The Absolute Structure and Synthesis of Wenjine Isolated from Curcuma wenyujin. Chem. Pharm. Bull. 1991;39:854–856. doi: 10.1248/cpb.39.854. [DOI] [Google Scholar]
- 63.Niu Z.G., Chen H.H., Gao C.W., Chen G.Y., Li G.N. Chemical Constituents from the Dregs of Curcuma wenyujin. Guang Dong Chem. 2014;16:22–23. [Google Scholar]
- 64.Xia G.Y., Sun D.J., Ma J.H., Liu Y., Zhao F., Donkor P.O., Ding L.Q., Chen L.X., Qiu F. (+)/(−)-Phaeocaulin A-D, Four Pairs of New Enantiomeric Germacrane-Type Sesquiterpenes from Curcuma phaeocaulis as Natural Nitric Oxide Inhibitors. Sci. Rep. 2017;7:43576. doi: 10.1038/srep43576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J.T., Ge D., Qu H.F., Wang G.K., Wang G. Chemical Constituents of Curcuma kwangsiensis and Their Antimigratory Activities in RKO Cells. Natl. Prod. Res. 2019;33:3493–3499. doi: 10.1080/14786419.2018.1484463. [DOI] [PubMed] [Google Scholar]
- 66.Lou Y., Zhao F., He H., Peng K.F., Chen L.X., Qiu F. Four New Sesquiterpenes from Curcuma wenyujin and Their Inhibitory Effects on Nitric-Oxide Production. Chem. Biodivers. 2010;7:1245–1253. doi: 10.1002/cbdv.200900160. [DOI] [PubMed] [Google Scholar]
- 67.Wang S.S., Zhang J.M., Guo X.H., Song Q.L., Zhao W.J. A New Eudesmane Sesquiterpene Lactone from Curcuma wenyujin. Acta Pharm. Sin. B. 2007;42:1062–1065. [PubMed] [Google Scholar]
- 68.Gao S.Y., Xia G.Y., Wang L.Q., Zhou L., Zhao F., Huang J., Chen L.X. Sesquiterpenes from Curcuma wenyujin with Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Nat. Prod. Res. 2017;31:548–554. doi: 10.1080/14786419.2016.1205053. [DOI] [PubMed] [Google Scholar]
- 69.Ma J.H., Zhao F., Wang Y., Liu Y., Gao S.Y., Ding L.Q., Chen L.X., Qiu F. Natural Nitric Oxide (NO) Inhibitors from the Rhizomes of Curcuma phaeocaulis. Org. Biomol. Chem. 2015;13:8349–8358. doi: 10.1039/C5OB00964B. [DOI] [PubMed] [Google Scholar]
- 70.Song G.Q., Wu P., Dong X.M., Cheng L.H., Lu H.Q., Lin Y.Y., Tang W.Y., Xie T., Zhou J.L. Elemene Induces Cell Apoptosis via Inhibiting Glutathione Synthesis in Lung Adenocarcinoma. J. Ethnopharmacol. 2023;311:116409. doi: 10.1016/j.jep.2023.116409. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J.J., Lower-Nedza A.D., Hong M., Jie S., Wang Z.M., Dong Y.M., Tschiggerl C., Bucar F., Brantner A.H. Chemical Composition and Antimicrobial Activity of Three Essential Oils from Curcuma wenyujin. Nat. Prod. Commun. 2013;8:523–526. doi: 10.1177/1934578X1300800430. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L.Y., Yang Z.W., Huang Z.B., Zhao M.C., Li P.H., Zhou W., Zhang K., Zheng X., Lin L., Tang J., et al. Variation in Essential Oil and Bioactive Compounds of Curcuma kwangsiensis Collected from Natural Habitats. Chem. Biodivers. 2017;14:e1700020. doi: 10.1002/cbdv.201700020. [DOI] [PubMed] [Google Scholar]
- 73.Liang H., Wang Q., Ding C.B., Zhang L., Yang R.W. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oil of Curcuma phaeocaulis Valeton. Bangladesh J. Bot. 2020;49:531–540. doi: 10.3329/bjb.v49i3.49621. [DOI] [Google Scholar]
- 74.Ma J.H., Wang Y., Liu Y., Gao S.Y., Ding L.Q., Zhao F., Chen L.X., Qiu F. Cadinane Sesquiterpenes from Curcuma phaeocaulis with Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Fitoterapia. 2015;103:90–96. doi: 10.1016/j.fitote.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 75.Zuo J., Zhang T.-H., Peng C., Xu B.-J., Dai O., Lu Y., Zhou Q.-M., Xiong L. Essential Oil from Ligusticum chuanxiong Hort. Alleviates Lipopolysaccharide-Induced Neuroinflammation: Integrating Network Pharmacology and Molecular Mechanism Evaluation. J. Ethnopharmacol. 2024;319:117337. doi: 10.1016/j.jep.2023.117337. [DOI] [PubMed] [Google Scholar]
- 76.Xu J., Zhao Y., Aisa H.A. Anti-Inflammatory Effect of Pomegranate Flower in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Macrophages. Pharm. Biol. 2017;55:2095–2101. doi: 10.1080/13880209.2017.1357737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao W., Ma L., Cai C., Gong X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019;15:1571–1581. doi: 10.7150/ijbs.34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M., Zhao Q., Liang Y.Y., Ma J.H., Chen L.X., Zhang X., Ding L.Q., Zhao F., Qiu F. Stereo- and Regiospecific Biotransformation of Curcumenol by Four Fungal Strains. J. Mol. Catal. B Enzym. 2015;115:13–19. doi: 10.1016/j.molcatb.2015.01.005. [DOI] [Google Scholar]
- 79.Yoshioka T., Fujii E., Endo M., Wada K., Tokunaga Y., Shiba N., Hohsho H., Shibuya H., Muraki T. Antiinflammatory Potency of Dehydrocurdione, A Zedoary-Derived Sesquiterpene. Inflamm Res. 1998;47:476–481. doi: 10.1007/s000110050361. [DOI] [PubMed] [Google Scholar]
- 80.Li Z.Y., Hao E.W., Cao R., Du Z.C., Liang L.L., Shen Y.B., Hou X.T., Deng J.G. Research Progress on Pharmacological Action and Mechanism of Germacrone. Drugs Clin. 2022;37:644–652. [Google Scholar]
- 81.Cai Y., Li W.C., Tu H.F., Chen N.M., Zhong Z.P., Yan P.C., Dong J.Y. Curcumolide Reduces Diabetic Retinal Vascular Leukostasis and Leakage Partly via Inhibition of the p38MAPK/NF-κB Signaling. Bioorg. Med. Chem. Lett. 2017;27:1835–1839. doi: 10.1016/j.bmcl.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 82.Jia S.S., Guo P., Lu J.H., Huang X.J., Deng L.M., Jin Y., Zhao L.Y., Fan X.F. Curcumol Ameliorates Lung Inflammation and Airway Remodeling via Inhibiting the Abnormal Activation of the Wnt/β-Catenin Pathway in Chronic Asthmatic Mice. Drug Des. Devel. Ther. 2021;15:2641–2651. doi: 10.2147/DDDT.S292642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lv M.F., Shao J.Y., Jiang F., Liu J.J. Curcumol may Alleviate Psoriasis-Like Inflammation by Inhibiting Keratinocyte ProliferAtion and Inflammatory Gene Expression via JAK1/STAT3 Signaling. Aging. 2021;13:18392–18403. doi: 10.18632/aging.203287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z.R., Zhuo F., Chu P.G., Yang X.L., Zhao G. Germacrone Alleviates Collagen-Induced Arthritis via Regulating Th1/Th2 Balance and NF-κB Activation. Biochem. Biophys. Res. Commun. 2019;518:560–564. doi: 10.1016/j.bbrc.2019.08.084. [DOI] [PubMed] [Google Scholar]
- 85.Li Y.Q., Li G.Z., Dong Y., Ma X., Dong H.J., Wu Q.Q., Zhao W.J. Orobanone Analogues from Acid-Promoted Aromatization Rearrangement of Curcumol Inhibit Hypoxia-Inducible Factor-1 (HIF-1) in Cell-Based Reporter Assays. Bioorg. Chem. 2019;85:357–363. doi: 10.1016/j.bioorg.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Tungcharoen P., Wattanapiromsakul C., Tansakul P., Nakamura S., Matsuda H., Tewtrakul S. Antiinflammation Constituents from Curcuma zedoaroides. Phytother. Res. 2018;32:2312–2320. doi: 10.1002/ptr.6173. [DOI] [PubMed] [Google Scholar]
- 87.Lee T.K., Trinh T.A., Lee S.R., Kim S., So H.M., Moon E., Hwang G.S., Kang K.S., Kim J.H., Yamabe N., et al. Bioactivity-Based Analysis and Chemical Characterization of Anti-Inflammatory Compounds from Curcuma zedoaria Rhizomes Using LPS-Stimulated RAW264.7 Cells. Bioorg. Chem. 2019;82:26–32. doi: 10.1016/j.bioorg.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 88.Wang G.K., Zhang N., Yao J.N., Yu Y., Wang G., Hung C.C., Cheng Y.Y., Morris-Natschke S.L., Zhou Z.Y., Liu J.S., et al. Kalshinoids A–F, Anti-Inflammatory Sesquiterpenes from Kalimeris shimadae. J. Nat. Prod. 2019;82:3372–3378. doi: 10.1021/acs.jnatprod.9b00693. [DOI] [PubMed] [Google Scholar]
- 89.Cho W., Nam J.W., Kang H.J., Windono T., Seo E.K., Lee K.T. Zedoarondiol Isolated from the Rhizoma of Curcuma heyneana is Involved in the Inhibition of iNOS, COX-2 and Pro-Inflammatory Cytokines via the Downregulation of NF-kappa B Pathway in LPS-Stimulated Murine Macrophages. Int. Immunopharmacol. 2009;9:1049–1057. doi: 10.1016/j.intimp.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 90.Chen X., Zong C.J., Gao Y., Cai R.L., Fang L., Lu J., Liu F., Qi Y. Curcumol Exhibits Anti-Inflammatory Properties by Interfering with the JNK-Mediated AP-1 Pathway in Lipopolysaccharide-Activated RAW264.7 Cells. Eur. J. Pharmacol. 2014;723:339–345. doi: 10.1016/j.ejphar.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Lo J.Y., Kamarudin M.N.A., Hamdi O.A.A., Awang K., Kadir H.A. Curcumenol Isolated from Curcuma zedoaria Suppresses Akt-Mediated NF-κB Activation and p38 MAPK Signaling Pathway in LPS-Stimulated BV-2 Microglial Cells. Food Funct. 2015;6:3550–3559. doi: 10.1039/C5FO00607D. [DOI] [PubMed] [Google Scholar]
- 92.Dong J.Y., Shao W.W., Yan P.C., Cai X.Q., Fang L.L., Zhao X.W., Lin W.W., Cai Y. Curcumolide, A Unique Sesquiterpenoid with Anti-Inflammatory Properties from Curcuma wenyujin. Bioorg. Med. Chem. Lett. 2015;25:198–202. doi: 10.1016/j.bmcl.2014.11.075. [DOI] [PubMed] [Google Scholar]
- 93.Zhong Z.F., Dang Y.Y., Yuan X., Guo W., Li Y.B., Tan W., Cui J.R., Lu J.J., Zhang Q.W., Chen X.P., et al. Furanodiene, A Natural Product, Inhibits Breast Cancer Growth Both in vitro and in vivo. Cell. Physiol. Biochem. 2012;30:778–790. doi: 10.1159/000341457. [DOI] [PubMed] [Google Scholar]
- 94.Zhong Z.F., Tan W., Qiang W.W., Scofield V.L., Tian K., Wang C.M., Qiang W.A., Wang Y.T. Furanodiene Alters Mitochondrial Function in Doxorubicin-Resistant MCF-7 Human Breast Cancer Cells in an AMPK-Dependent Manner. Mol. Biosyst. 2016;12:1626–1637. doi: 10.1039/C6MB00003G. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen T.T., Tran T.H., Nguyen T.H., Do T.H. Cytotoxic Sesquiterpenes and Diterpenes from the Rhizomes of Curcuma zedoaroides Chaveer. & Tanee. Biochem. Syst. Ecol. 2024;112:104781 [Google Scholar]
- 96.Ma E., Wang X.L., Li Y.C., Sun X.Y., Tai W.J., Li T., Guo T. Induction of Apoptosis by Furanodiene in HL60 Leukemia Cells through Activation of TNFR1 Signaling Pathway. Cancer Lett. 2008;271:158–166. doi: 10.1016/j.canlet.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 97.Ying J., Yang W., Xie C.Y., Ni Q.C., Pan X.D., Dong J.H., Liu Z.M., Wang X.S. Induction of Caspase-3-Dependent Apoptosis in Human Leukemia HL-60 Cells by δ-Elemene. J. Pharm. Soc. Jpn. 2011;131:1383–1394. doi: 10.1248/yakushi.131.1383. [DOI] [PubMed] [Google Scholar]
- 98.Wei W., Rasul A., Sadiqa A., Sarfraz I., Hussain G., Nageen B., Liu X., Watanabe N., Selamoglu Z., Ali M., et al. Curcumol: From Plant Roots to Cancer Roots. Int. J. Biol. Sci. 2019;15:1600–1609. doi: 10.7150/ijbs.34716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batool R., Rasul A., Hussain G., Shah M.A., Nageen B., Sarfraz I., Zahoor M.K., Riaz A., Ajaz A., Adem Ş. Furanodiene: A Novel, Potent, and Multitarget Cancer-Fighting Terpenoid. Curr. Pharm. Des. 2021;27:2628–2634. doi: 10.2174/1381612827666210211125304. [DOI] [PubMed] [Google Scholar]
- 100.Tong H.X., Liu Y.H., Jiang L.J., Wang J.S. Multi-Targeting by β-Elemene and Its Anticancer Properties: A Good Choice for Oncotherapy and Radiochemotherapy Sensitization. Nutr. Cancer. 2019;72:554–567. doi: 10.1080/01635581.2019.1648694. [DOI] [PubMed] [Google Scholar]
- 101.Zhai B.T., Zhang N.N., Han X.M., Li Q.J., Zhang M.M., Chen X.Y., Li G.H., Zhang R.N., Chen P., Wang W.G., et al. Molecular Targets of β-elemene, A Herbal Extract used in Traditional Chinese Medicine, and its Potential Role in Cancer Therapy: A Review. Biomed. Pharmacother. 2019;114:108812. doi: 10.1016/j.biopha.2019.108812. [DOI] [PubMed] [Google Scholar]
- 102.Huang L.Z., Li A., Liao G.Z., Yang F.C., Yang J., Chen X., Jiang X.S. Curcumol Triggers Apoptosis of p53 Mutant Triple-Negative Human Breast Cancer MDA-MB 231 Cells via Activation of p73 and PUMA. Oncol. Lett. 2017;14:1080–1088. doi: 10.3892/ol.2017.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zuo H.X., Jin Y., Wang Z., Li M.Y., Zhang Z.H., Wang J.Y., Xing Y., Ri M.H., Jin C.H., Xu G.H., et al. Curcumol Inhibits the Expression of Programmed Cell Death-Ligand 1 through Crosstalk between Hypoxia-Inducible Factor-1α and STAT3 (T705) Signaling Pathways in Hepatic Cancer. J. Ethnopharmacol. 2020;257:112835. doi: 10.1016/j.jep.2020.112835. [DOI] [PubMed] [Google Scholar]
- 104.Wang J., Huang F.X., Bai Z., Chi B.X., Wu J.C., Chen X. Curcumol Inhibits Growth and Induces Apoptosis of Colorectal Cancer LoVo Cell Line via IGF-1R and p38 MAPK Pathway. Int. J. Mol. Sci. 2015;16:19851–19867. doi: 10.3390/ijms160819851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamdi O.A.A., Rahman S.N.S.A., Awang K., Wahab N.A., Looi C.Y., Thomas N.F., Malek S.N.A. Cytotoxic Constituents from the Rhizomes of Curcuma zedoaria. Sci. World J. 2014;2014:321943. doi: 10.1155/2014/321943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang R.N., Pan T., Xiang Y., Zhang M.M., Xie H., Liang Z.M., Chen B., Xu C., Wang J., Huang X.X., et al. Curcumenol Triggered Ferroptosis in Lung Cancer Cells via LncRNA H19/miR-19b-3p/FTH1 Axis. Bioact. Mater. 2021;13:23–36. doi: 10.1016/j.bioactmat.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung E.B., Trinh T.A., Lee T.K., Yamabe N., Kang K.S., Song J.H., Choi S., Lee S., Jang T.S., Kim K.H., et al. Curcuzedoalide Contributes to the Cytotoxicity of Curcuma zedoaria Rhizomes against Human Gastric Cancer AGS Cells through Induction of Apoptosis. J. Ethnopharmacol. 2018;213:48–55. doi: 10.1016/j.jep.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 108.Wu L., Wang L.F., Tian X.G., Zhang J.Y., Feng H. Germacrone Exerts Anti-Cancer Effects on Gastric Cancer Through Induction of Cell Cycle Arrest and Promotion of Apoptosis. BMC Complement. Med. Ther. 2020;20:21. doi: 10.1186/s12906-019-2810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhong Z.F., Chen X.P., Tan W., Xu Z.T., Zhou K.Y., Wu T., Cui L., Wang Y.T. Germacrone Inhibits the Proliferation of Breast Cancer Cell Lines by Inducing Cell Cycle Arrest and Promoting Apoptosis. Eur. J. Pharmacol. 2011;667:50–55. doi: 10.1016/j.ejphar.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 110.Zhang R., Hao J., Guo K.W., Liu W.X., Yao F., Wu Q.M., Liu C., Wang Q., Yang X.Z. Germacrone Inhibits Cell Proliferation and Induces Apoptosis in Human Esophageal Squamous Cell Carcinoma Cells. BioMed Res. Int. 2020;2020:7643248. doi: 10.1155/2020/7643248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang F., Sun Z., Zhang Q.Y., Yang H., Yang G., Yang Q., Zhu Y.M., Wu W.Y., Xu W.W., Wu X.Y. Curdione Induces Ferroptosis Mediated by m6A Methylation via METTL14 and YTHDF2 in Colorectal Cancer. Chin. Med. 2023;18:122. doi: 10.1186/s13020-023-00820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei C., Li D.H., Liu Y., Wang W.N., Qiu T.T. Curdione Induces Antiproliferation Effect on Human Uterine Leiomyosarcoma via Targeting IDO1. Front. Oncol. 2021;11:637024. doi: 10.3389/fonc.2021.637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao R.R., Zhou J., Wang Q.M., Zhang X., Wang R.R., Chen M. The Effect of the Curdione on the Proliferation of HHSEC Under the Microenvironment of HepG2 Cells via VEGF/VEGFR2 Signaling Pathway. J. Hunan Univ. Chin. Med. 2021;41:1835–1839. [Google Scholar]
- 114.Jiang Y., Wang X.Q., Hu D.D. Furanodienone Induces G0/G1 Arrest and Causes Apoptosis via the ROS/MAPKs-Mediated Caspase-Dependent Pathway in Human Colorectal Cancer Cells: A Study in vitro and in vivo. Cell Death Dis. 2017;8:e2815. doi: 10.1038/cddis.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z.L., Li L.Y., Wang J.M., Liang X., Wang Y.Y., Wang X.F., Qiao Y.H., Zhao B. A Study of Zederone for the Inhibition on Ovarian Cancer Cell Proliferation through mTOR/p70s6K Signaling Pathway. J. BUON. 2020;25:785–791. [PubMed] [Google Scholar]
- 116.Lu H., Chen J., Luo Y.M., Xu H.J., Xiong L., Fu J.J. Curcolonol Suppresses the Motility of Breast Cancer Cells by Inhibiting LIM Kinase 1 to Downregulate Cofilin 1 Phosphorylation. Int. J. Oncol. 2018;53:2695–2704. doi: 10.3892/ijo.2018.4592. [DOI] [PubMed] [Google Scholar]
- 117.Fu J.J., Yu J.J., Chen J., Xu H.J., Luo Y.M., Lu H. In vitro Inhibitory Properties of Sesquiterpenes from Chloranthus serratus on Cell Motility via Down-Regulation of LIMK1 Activation in Human Breast Cancer. Phytomedicine. 2018;49:23–31. doi: 10.1016/j.phymed.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y.D., Li J.H., Guo J.Q., Wang Q.Y., Zhu S.G., Gao S.Y., Yang C., Wei M., Pan X.D., Zhu W., et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017;83:23–29. doi: 10.1055/s-0042-107083. [DOI] [PubMed] [Google Scholar]
- 119.Zheng T.T., Xiao H.T., Shen Y.H., Zhang X., Jiang K.K., Liu L., Bai X.H., Peng J., Chen Y. Anticancer Effects of Curzerenone against Drug-Resistant Human Lung Carcinoma Cells are Mediated via Programmed Cell Death, Loss of Mitochondrial Membrane Potential, ROS, and Blocking the ERK/MAPK and NF-κB Signaling Pathway. J. BUON. 2019;24:907–912. [PubMed] [Google Scholar]
- 120.Fang H., Gao B.B., Zhao Y.L., Fang X., Bian M.H., Xia Q. Curdione Inhibits Thrombin-Induced Platelet Aggregation via Regulating the AMP-Activated Protein Kinase-Vinculin/Talin-Integrin αIIbβ3 Sign Pathway. Phytomedicine. 2019;61:152859. doi: 10.1016/j.phymed.2019.152859. [DOI] [PubMed] [Google Scholar]
- 121.Mao H.M., Tao T.Q., Wang X.R., Liu M., Song D.D., Liu X.H., Shi D.Z. Zedoarondiol Attenuates Endothelial Cells Injury Induced by Oxidized Low-Density Lipoprotein via Nrf2 Activation. Cell Physiol. Biochem. 2018;48:1468–1479. doi: 10.1159/000492257. [DOI] [PubMed] [Google Scholar]
- 122.Chai H., Qu H., He S., Song L., Yang Y., Huang H.B., Shi D.Z. Zedoarondiol Inhibits Atherosclerosis by Regulating Monocyte Migration and Adhesion via CXCL12/CXCR4 Pathway. Pharmacol. Res. 2022;182:106328. doi: 10.1016/j.phrs.2022.106328. [DOI] [PubMed] [Google Scholar]
- 123.Chen X.J. Master’s Thesis. China Academy of Chinese Medical Sciences; Beijing, China: 2021. Research on Anti-Atherosclerosis of the Zedoarondiol an Active Ingredients of Curcuma by Mediating Hif-1α Signaling Pathway. [Google Scholar]
- 124.Mao H.M., Tao T.Q., Song D.D., Liu M., Wang X.R., Liu X.H., Shi D.Z. Zedoarondiol Inhibits Platelet-Derived Growth Factor-Induced Vascular Smooth Muscle Cells Proliferation via Regulating AMP-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2016;40:1506–1520. doi: 10.1159/000453201. [DOI] [PubMed] [Google Scholar]
- 125.Liu J. Master’s Thesis. China Academy of Chinese Medical Sciences; Beijing, China: 2018. Analysis of Risk Factors Related to Prethrombotic State and Effect of Zedoarondiol Regulation Target miRNA on Inflammatory Response. [Google Scholar]
- 126.Fang Z., Li S., Yushanjiang F., Feng G.K., Cui S.Y., Hu S., Jiang X.J., Liu C.Y. Curcumol Alleviates Cardiac Remodeling via the AKT/NF-κB Pathway. Int. Immunopharmacol. 2023;122:110527. doi: 10.1016/j.intimp.2023.110527. [DOI] [PubMed] [Google Scholar]
- 127.Fang Z., Yushanjiang F., Wang G.J., Zheng X.X., Jiang X.J. Germacrone Mitigates Cardiac Remodeling by Regulating PI3K/AKT-Mediated Oxidative Stress, Inflammation, and Apoptosis. Int. Immunopharmacol. 2023;124:110876. doi: 10.1016/j.intimp.2023.110876. [DOI] [PubMed] [Google Scholar]
- 128.Wu T.H., Yin F., Kong H.M., Peng J. Germacrone Attenuates Cerebral Ischemia/Reperfusion Injury in Rats via Antioxidative and Antiapoptotic Mechanisms. J. Cell. Biochem. 2019;120:18901–18909. doi: 10.1002/jcb.29210. [DOI] [PubMed] [Google Scholar]
- 129.Huo W.M., Duan W.L., Liu J., Shang J. Studies on the Anticoagulant and Antithromboticm Effects of β-elemene. Asia Pac. Tradit. Med. 2013;9:30–33. [Google Scholar]
- 130.Liu M., Chen X.T., Ma J., Hassan W., Wu H.L., Ling J.W., Shang J. β-Elemene Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice via Restoring NO Levels and Alleviating Oxidative Stress. Biomed. Pharmacother. 2017;95:1789–1798. doi: 10.1016/j.biopha.2017.08.092. [DOI] [PubMed] [Google Scholar]
- 131.Matsuda H., Ninomiya K., Morikawa T., Yoshikawa M. Inhibitory Effect and Action Mechanism of Sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/ Lipopolysaccharide-Induced Liver Injury. Bioorg. Med. Chem. Lett. 1998;8:339–344. doi: 10.1016/S0960-894X(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 132.Yang X., Wang Z.M., Kai J., Wang F.X., Jia Y., Wang S.J., Tan S.Z., Shen X.K., Chen A.P., Shao J.J., et al. Curcumol Attenuates Liver Sinusoidal Endothelial Cell Angiogenesis via Regulating Glis-PROX1-HIF-1α in Liver Fibrosis. Cell Prolif. 2020;53:e12762. doi: 10.1111/cpr.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jia Y., Wang F.X., Guo Q., Li M.M., Wang L., Zhang Z.L., Jiang S.Y., Jin H.H., Chen A.P., Tan S.Z., et al. Curcumol Induces RIPK1/RIPK3 Complex-Dependent Necroptosis via JNK1/2-ROS Signaling in Hepatic Stellate Cells. Redox Biol. 2018;19:375–387. doi: 10.1016/j.redox.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng Y., Zhao T.J., Wang J.-R., Jiang R.Z., Huang J.B., Li W.M., Wang J.H. Curcumol Alleviates Liver Fibrosis through Inducing Autophagy and Ferroptosis in Hepatic Stellate Cells. FASEB J. 2022;36:e22665. doi: 10.1096/fj.202200933RR. [DOI] [PubMed] [Google Scholar]
- 135.Li Z.Y., Wang Z.L., Dong F., Shi W., Dai W.Z., Zhao J., Li Q., Fang Z.-E., Ren L.T., Liu T.T., et al. Germacrone Attenuates Hepatic Stellate Cells Activation and Liver Fibrosis via Regulating Multiple Signaling Pathways. Front. Pharmacol. 2021;12:745561. doi: 10.3389/fphar.2021.745561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ji D., Zhao Q., Qin Y.W., Tong H.J., Wang Q.H., Yu M.T., Mao C.Q., Lu T.L., Qiu J.C., Jiang C.X. Germacrone Improves Liver Fibrosis by Regulating the PI3K/AKT/mTOR Signaling Pathway. Cell Biol. Int. 2021;45:1866–1875. doi: 10.1002/cbin.11607. [DOI] [PubMed] [Google Scholar]
- 137.Xiao Y., Yang F.Q., Li S.P., Gao J.L., Hu G., Lao S.C., Conceição L.E., Fung K.P., Wang Y.T., Lee M.Y. Furanodiene Induces G2/M Cell Cycle Arrest and Apoptosis through MAPK Signaling and Mitochondria-Caspase Pathway in Human Hepatocellular Carcinoma Cells. Cancer Biol. Ther. 2007;6:1044–1050. doi: 10.4161/cbt.6.7.4317. [DOI] [PubMed] [Google Scholar]
- 138.Saifudin A., Tanaka K., Kadota S., Tezuka Y. Sesquiterpenes from the Rhizomes of Curcuma heyneana. J. Nat. Prod. 2013;76:223–229. doi: 10.1021/np300694a. [DOI] [PubMed] [Google Scholar]
- 139.Zhang W.Y., Wang M.J., Liu H.X., Wang Q., Chen Q.C. Hypoglycemic Effect of Alismoxide in Type 2 Diabetic Mice. Chin. Pharmacol. Bull. 2019;35:1240–1244. [Google Scholar]
- 140.Lin W.W., Tu H.F., Zhu Y., Guan Y.J., Liu H., Ling W., Yan P.C., Dong J.Y. Curcumolide, A Unique Sesquiterpenoid from Curcuma wenyujin Displays Anti-Angiogenic Activity and Attenuates Ischemia-Induced Retinal Neovascularization. Phytomedicine. 2019;64:152923. doi: 10.1016/j.phymed.2019.152923. [DOI] [PubMed] [Google Scholar]
- 141.Cai Y., Tu H.F., Wu C.M., Liu T., Chen S.S., Shen L.L., Xiao Q.W., Zhao S.M., Xu S.Y., Lin W.W., et al. Therapeutic Potential of Elema-1,3,7(11),8-tetraen-8,12-lactam from Curcuma wenyujin on Diabetic Retinopathy via Anti-Inflammatory and Anti-Angiogenic Pathways. J. Ethnopharmacol. 2024;318:116843. doi: 10.1016/j.jep.2023.116843. [DOI] [PubMed] [Google Scholar]
- 142.Kwon P.K., Kim S.W., De R., Jeong S.W., Kim K.T. Isoprocurcumenol Supports Keratinocyte Growth and Survival through Epidermal Growth Factor Receptor Activation. Int. J. Mol. Sci. 2021;22:12579. doi: 10.3390/ijms222212579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamdi O.A.A., Ye L.J., Kamarudin M.N.A., Hazni H., Paydar M., Looi C.Y., Shilpi L.A., Kadir H.A., Awang K. Neuroprotective and Antioxidant Constituents from Curcuma zedoaria Rhizomes. Rec. Nat. Prod. 2015;9:349–355. [Google Scholar]
- 144.Park J.H., Mohamed M.A.A., Thi N.N., Seo K.H., Jung Y.J., Shrestha S., Lee T.H., Kim J., Baek N.I. Guaiane Sesquiterpenes from the Rhizome of Curcuma xanthorrhiza and Their Inhibitory Effects on UVB-Induced MMP-1 Expression in Human Keratinocytes. Nat. Prod. Commun. 2017;12:1535–1538. doi: 10.1177/1934578X1701201003. [DOI] [Google Scholar]
- 145.Yang K., Wu B., Wei W., Li C., Li L., Cong Z., Xiang Q. Curdione Ameliorates Sepsis-Induced Lung Injury by Inhibiting Platelet-Mediated Neutrophil Extracellular Trap Formation. Int. Immunopharmacol. 2023;118:110082. doi: 10.1016/j.intimp.2023.110082. [DOI] [PubMed] [Google Scholar]
- 146.Yang X., Li B.X., Tian H.J., Cheng X.F., Zhou T.J., Zhao J. Curcumenol Mitigates the Inflammation and Ameliorates the Catabolism Status of the Intervertebral Discs in vivo and in vitro via Inhibiting the TNFα/NFκB Pathway. Front. Pharmacol. 2022;13:905966. doi: 10.3389/fphar.2022.905966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borah S., Sarkar P., Sharma H.K. Zederone Improves the Fecal Microbial Profile in Dementia Induced Rat Model: A First Report. CNS Neurol. Disord. Drug Targets. 2022;21:335–342. doi: 10.2174/1871527320666210827114227. [DOI] [PubMed] [Google Scholar]
- 148.Kimura Y., Sumiyoshi M., Tamaki T. Effects of the Extracts and an Active Compound Curcumenone Isolated from Curcuma zedoaria Rhizomes on Alcohol-Induced Drunkenness in Mice. Fitoterapia. 2013;84:163–169. doi: 10.1016/j.fitote.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 149.Park J.-H., Mohamed M.A.A., Jung Y.J., Shrestha S., Lee T.H., Lee C.H., Han D., Kim J., Baek N.I. Germacrane Sesquiterpenes Isolated from the Rhizome of Curcuma xanthorrhiza Roxb. Inhibit UVB-Induced Upregulation of MMP-1, -2, and -3 Expression in Human Keratinocytes. Arch. Pharm. Res. 2015;38:1752–1760. doi: 10.1007/s12272-014-0525-z. [DOI] [PubMed] [Google Scholar]
- 150.Irie K., Yoshioka T., Nakai A., Ochiai K., Nishikori T., Wu G.R., Shibuya H., Muraki T. A Ca(2+) Channel Blocker-Like Effect of Dehydrocurdione on Rodent Intestinal and Vascular Smooth Muscle. Eur. J. Pharmacol. 2000;403:235–242. doi: 10.1016/S0014-2999(00)00445-3. [DOI] [PubMed] [Google Scholar]