Abstract
Certain types of chemokine receptors have been identified as coreceptors for HIV-1 infection. The process of viral entry is initiated by the interaction between an envelope protein gp120 of HIV-1, CD4, and one of the relevant coreceptors. To understand the precise mechanism of the Env-mediated fusion and entry of HIV-1, we examined whether the V3 region of gp120 of T-cell line tropic (T-tropic) virus directly interacts with the coreceptor, CXCR-4, by using five synthetic V3 peptides: two cyclized V3 peptides (V3-BH10 and V3-ELI) which correspond to the V3 regions of the T-tropic HIV-1 IIIB and HIV-1 ELI strains, respectively, a linear V3 peptide (CTR36) corresponding to that of HIV-1 IIIB strain; and cyclized V3 peptides corresponding to that of the macrophage-tropic (M-tropic) HIV-1 ADA strain (V3-ADA) or the dualtropic HIV-1 89.6 strain (V3-89.6). FACScan analysis with a CXCR-4+ human B-cell line, JY, showed that V3-BH10, V3-ELI, and V3-89.6 but not CTR36 or V3-ADA blocked the binding of IVR7, an anti-CXCR-4 monoclonal antibody (MAb), to CXCR-4 with different magnitudes in a dose-dependent manner, while none of the V3 peptides influenced binding of an anti-CD19 MAb at all. Next, the effects of the V3 peptides on SDF-1β-induced transient increases in intracellular Ca2+ were investigated. Three V3 peptides (V3-BH10, V3-ELI, and V3-89.6) prevented Ca2+ mobilization. Furthermore, the three peptides inhibited infection by T-tropic HIV-1 in a dose-dependent manner as revealed by an MTT assay and a reverse transcriptase assay, while the other peptides had no effects. These results present direct evidence that the V3 loop of gp120 of T-tropic HIV-1 can interact with its coreceptor CXCR-4 independently of the V1/V2 regions of gp120 or cellular CD4.
Infection with human immunodeficiency virus type 1 (HIV-1) results in a progressive deterioration of the immune system, mainly due to both quantitative and qualitative defects of CD4+ T lymphocytes (5, 17, 20, 29). It is now established that HIV-1 enters target cells through a set of at least two receptor molecules: CD4 and one of the coreceptors that are members of the seven-transmembrane-domain, G-protein-coupled receptor family. It is noted that usage of a coreceptor is related to the cell tropism of the HIV-1 strain. For example, CXCR-4 (fusin) serves as a coreceptor for T-cell line tropic (T-tropic) HIV-1 (14, 18, 19, 30) while CCR-5 supports infection of macrophage-tropic (M-tropic) HIV-1 (2, 10, 11, 15). In addition, CCR-3 and CCR2b have been reported to support infection of some dualtropic HIV-1 strains (10, 14). Furthermore, Bonzo/STRL33, Bob/GPR15, GPR1, and US28 have been identified as cofactors for simian immunodeficiency virus, HIV-1, or HIV-2 entry (12, 16, 36). However, it has been widely accepted that CXCR-4 and CCR-5 are the major coreceptors for T-tropic and M-tropic HIV-1 strains, respectively.
Thus, the cell tropism of HIV-1 is thought to be determined at the level of viral entry by the interaction of the viral envelope proteins with certain types of coreceptors that support either T-tropic or M-tropic HIV-1 infection. Recent studies have shown that the V3 region of gp120 is involved in this early step of the HIV-1 virion-cell interaction. In the case of M-tropic virus, a report has been published demonstrating the formation of a trimolecular complex of CD4, gp120, and CCR-5 (44). Binding experiments using mutant gp120 molecules with a deletion at the V3 region or anti-V3 loop neutralizing antibody have strongly suggested that the V3 region is crucial for interaction with CCR-5 (46). However, the possibility of the involvement of other domains of gp120, including the V1/V2 regions, cannot be excluded on the basis of such experiments. Indeed, it has been speculated that subsequent to the binding to the CD4 molecule, gp120 changes its conformation and exposes the cryptic V3 loop together with the V1/V2 loop embedded in the gp120 molecule (40, 48). Therefore, it remains to be explored whether the V3 region by itself has a binding affinity to a relevant coreceptor.
In the present study, we examined the direct binding of the V3 region of T-tropic HIV-1 to CXCR-4 by using synthesized V3 peptides: linear and cyclized V3 peptides of T-tropic virus (HIV-1 IIIB and HIV-1 ELI) and cyclized V3 peptides of M-tropic virus (HIV-1 ADA) and dualtropic virus (HIV-1 89.6). Furthermore, we investigated the effects of the V3 peptides on HIV-1 infection. Here, we show that only the cyclized V3 peptides of T-tropic and dualtropic HIV-1 specifically bind to CXCR-4 and inhibit infection of T-tropic HIV-1.
MATERIALS AND METHODS
Synthetic peptides.
Peptides were synthesized using an automated peptide synthesizer 430A (Applied Biosystems, Foster City, Calif.) as described previously (38). The amino terminal amino acids were protected by a t-butyloxycarbonyl group during synthesis. All chemicals and program cycles were supplied by the manufacturer. After synthesis, the protecting group was removed, and the peptides were cleaved from the supporting resin with trifluoromethanesulfonic acid. The crude peptides thus obtained were purified by high-performance liquid chromatography (HPLC) using a preparative reverse-phase column (YMC D-ODS-5, 20 by 250 mm; Yamamura Chemical Laboratories, Kyoto, Japan) and linear gradient elution with acetonitrile (25 to 70%) containing 0.1% trifluoroacetic acid at a flow rate of 5 ml/min. The amino acid sequences of the synthesized peptides and their codes are EINCTRPNNNTRKSIRIQRGPGRAFVTIGKIGNMRQAHCNIS (V3-BH10) and CTRPNNNTRKSIRIQRGPGRAFVTIGKIGNMRQAHC (CTR36), which correspond to the V3 region of the T-tropic HIV-1 III BH10 clone. A disulfide bond was made for V3-BH10. The purified peptide was neutralized with an ammonia solution and oxidized in air for 4 days with gentle stirring at 4°C. The formation of disulfide bonds was confirmed by the determination of thiol groups by using a fluorescent probe, ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate (Dojindo Laboratories, Kumamoto, Japan), and l-cysteine as a standard. The concentration of thiol was approximately 5% of that of the original reactant after 4 days of oxidation. The loop peptide with an intramolecular disulfide bond was desalted and purified by HPLC as described above. The fractions containing the peptide were collected and lyophilized. The purity of the peptides was established by HPLC and exceeded 95%. The binding of V3-BH10 to a neutralizing monoclonal antibody (MAb) directed against the native gp120 of the HIV-1 III BH10 clone, 0.5β, was evaluated by an enzyme-linked immunosorbent assay as described previously (31). The other cyclized V3 peptides corresponding to the V3 region of T-tropic, M-tropic, and dualtropic HIV-1 strains (V3-ELI, V3-ADA, and V3-89.6, respectively) were synthesized by the Peptide Institute Inc. (Osaka, Japan), and their amino acid sequences were ESVKITCARPYQNTRQRTPIGLGQSLYTTRSRSIIGQAHCNIS (V3-ELI), EINCTRPNNNTRKSIHIGPGRAFYTTGEIIGDIRQAHCNIS (V3-ADA), and ESVVINCTRPNNNTRRRLSIGPGRAFYARRNIIGDIRQAHCNIS (V3-89.6). Ion spray mass spectrum analysis of the five V3 peptides was kindly performed with an API IIIE biomolecular mass analyzer (Sciex, Toronto, Canada) by H. Tamamura (Faculty of Pharmaceutical Sciences, Kyoto University) before they were used in this study. The ion spray mass spectra indicated that these synthesized peptides had their theoretical molecular weights.
Cells and culture conditions.
JY is a human B-cell line (50), and SupT1 is a human T-cell line (42). MT-4 is a human T-cell leukemia virus type 1-infected human T-cell line (21). These cell lines were cultured in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum (Summit; Monfort, Fort Collins, Colo.) and 30 μg of tobramycin/ml. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation from heparinized venous blood taken from healthy donors as described previously (38).
MAbs and flow cytometric analysis.
IVR7, an anti-CXCR-4 MAb, was established in our laboratory as described previously (22). Fluorescein isothiocyanate (FITC)-conjugated MAbs, Leu12 (anti-CD19), and mouse control immunoglobulin G (IgG) were purchased from Becton Dickinson (San Jose, Calif.). IVR7 [IgG1(κ)] and control mouse IgG1 were biotinylated with EZ-Link Biotin-BMCC (Pierce Chemical, Rockford, Ill.) in accordance with the manufacturer’s instructions.
Direct or indirect immunofluorescence staining was performed as described previously (26). JY cells were incubated with 1 mg of human IgG/ml on ice for 15 min to block nonspecific binding. The cells were then preincubated with various concentrations (15, 5, 1.7, and 0 μM) of V3-BH10, V3-ELI, V3-89.6, CTR36, and V3-ADA or 5 μg of SDF-1β or MIP-1β/ml on ice for 30 min. Then, biotinylated anti-CXCR-4 MAb (biotinylated IVR7) or FITC-conjugated MAb (FITC-Leu12) was added to the cells, and they were incubated on ice for an additional 30 min. After being washed, the cells treated with biotinylated MAb were incubated with phycoerythrin (PE)-conjugated streptavidin (Becton Dickinson) on ice for 30 min, washed, and subjected to flow cytometric analysis using a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Reactivity was determined by comparing control staining with biotinylated or FITC-conjugated irrelevant mouse IgG.
Measurement of Ca2+ mobilization.
For Ca2+ mobilization studies, 107 SupT1 cells were incubated in 1 ml of loading buffer containing 136 mM NaCl, 48 mM KCl, 1 mM CaCl2, 1 mg of glucose/ml, and 20 mM HEPES (pH 7.4), with 5 μM Fura-2 AM (Molecular Probes, Eugene, Oreg.) for 20 min at 37°C as described previously (6). The loaded cells were centrifuged, resuspended in fresh loading buffer, incubated with each V3 peptide at 20 μΜ, added to a stirred cuvette (2.5 × 105 in 500 μl), and inserted into a model F2000 spectrometer (Hitachi, Tokyo, Japan). Human SDF-1β (R & D systems, Minneapolis, Minn.) was added to a volume of 5 μl for a final concentration of 1 μg/ml, and increases in intracellular Ca2+ were measured by using the Intracellular Calcium Measurement Program for the Hitachi F2000 spectrometer.
MTT assay.
An MTT assay was performed in 96-well flat-bottomed culture plates in a total volume of 200 μl in triplicate. Anti-HIV-1 (HIV-1 IIIB strain) activities in vitro of the five synthesized peptides were measured by inhibition of the virus-induced cell death as described previously (39). In brief, MT-4 cells were suspended in culture medium at 2.5 × 105/ml. One-hundred microliters of the cell suspension was added to each well. Then, 50 μl of solution of various concentrations of synthetic V3 peptides was put into each well and incubated for 30 min at 37°C. HIV-1 IIIB (50 μl) was then added to each well at a multiplicity of infection of 0.0008. After a 5-day incubation at 37°C, the cell viability was determined by the MTT method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
RT assay.
A reverse transcriptase (RT) assay using supernatants from 3-day culture of MT-4 cells and HIV-1 IIIB was carried out as described in a previous report (39). In brief, 10 μl of each sample was mixed with 90 μl of reaction mixture containing 50 mM Tris-HCl (pH 8.3), 150 mM KCl, 10 mM MgCl2, 0.1% Nonidet P-40, 10 mM dithiothreitol, 5 mg of poly(rA)/ml, 5 mg of (dT)12–18/ml, and 1 mCi of [3H]dTTP. After incubation at 37°C for 3 h, the reaction mixtures were chilled on ice and passed through a DEAE-Filtermat (LKB-Pharmacia, Turku, Finland) using a cell harvester. After the filters were washed with 5% Na2HPO4 and H2O, radioactivity on the filters was determined by LKB Beta Plate scintillation spectroscopy.
Detection and measurement of HIV-1 infection by using PBMC.
Wild-type NL432 strain of HIV-1 was produced by transfection of human colon carcinoma cell line SW480 with the pNL432 infectious clone (1). M8166 cells were then infected with the virus, and the culture supernatants were collected after the appearance of cytopathic effects, filtered, and stored frozen in aliquots at −80°C. These culture supernatants were used as T-tropic HIV-1. NL162 strain of HIV-1 was produced from a hybrid clone, pNL162, in which the region including env (EcoRI-BamHI) of the backbone pNL432 was replaced with that of SF162 (41). The culture supernatants containing NL162 virus were prepared as described above and used as M-tropic virus. HIV-1 infection was measured by RT activity of the culture supernatants with HIV-1 and PBMC as follows.
This assay was performed in triplicate in 96-well flat-bottomed culture plates in a total volume of 200 μl. PBMC were suspended in culture medium containing NL432 or NL162 strain and incubated for 2 h at room temperature. After being washed, cells were resuspended in fresh medium containing 200 ng of PHA (Wellcome)/ml at 107 cells/ml. One-hundred microliters of the cell suspension was added to each well. Then, 100 μl of solution of 80 μM V3 peptides was put into each well (final concentrations: PBMC, 106 cells/ml; PHA, 100 ng/ml; V3 peptides, 40 μM). After 5 days, RT activity of the culture supernatants was measured as described above.
RESULTS
Effects of human SDF-1β or the V3 peptides on the binding of anti-CXCR-4 MAb, IVR7, to CXCR-4.
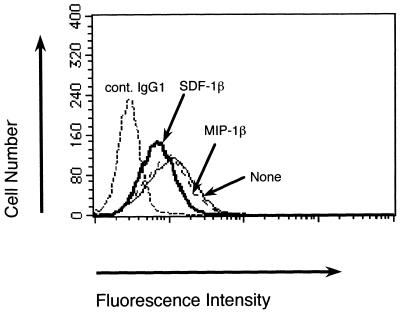
At present, the only known ligand for the CXCR-4 molecule is SDF-1. We first examined whether human recombinant SDF-1β or the V3 peptides could block the binding of anti-CXCR-4 MAb, IVR7, to JY cells. The FACScan analysis demonstrated that SDF-1β but not MIP-1β (which is a ligand of CCR-5) inhibited the binding of IVR7 (Fig. 1), suggesting that IVR7 recognizes an epitope on CXCR-4 close to the SDF-1β binding site.
FIG. 1.
Effect of SDF-1β on the staining of biotinylated IVR7. JY cells were incubated with human IgG on ice for 15 min to block nonspecific binding. After that, the cells were preincubated with SDF-1β or MIP-1β on ice for 30 min. Biotinylated IVR7 was added and the cells were incubated for additional 30 min. After being washed, cells treated with biotinylated IVR7 were incubated with PE-conjugated streptavidin, washed, and subjected to flow cytometric analysis. Reactivity was determined by comparing the results with those from a control staining with biotinylated irrelevant mouse IgG1 (cont. IgG1).
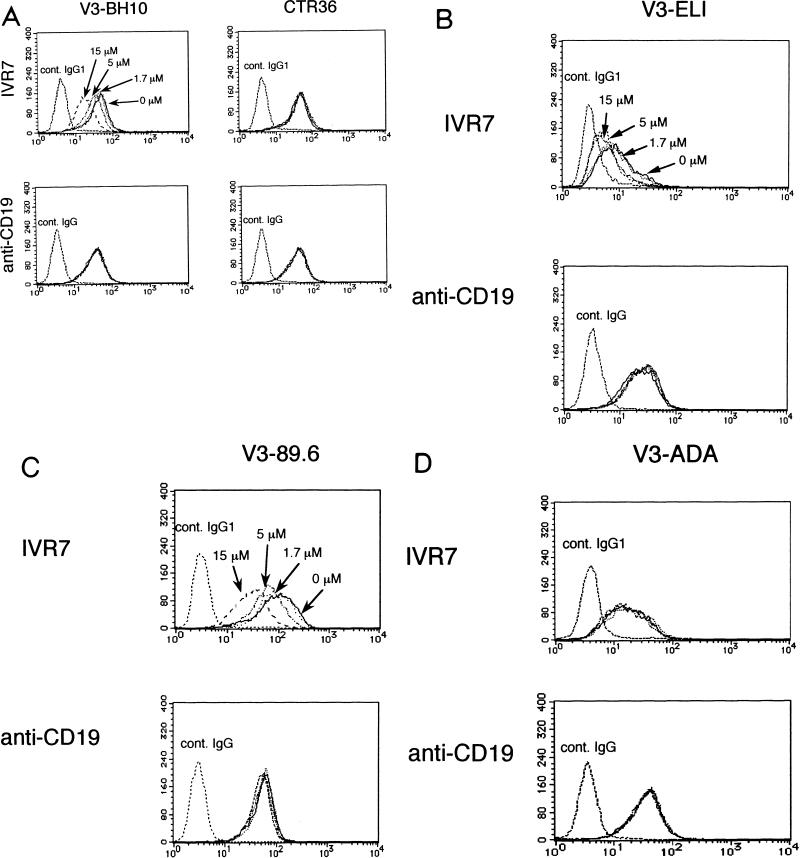
Among the five V3 peptides, three cyclized peptides corresponding to the V3 regions of T-tropic and dualtropic strains (V3-BH10, V3-ELI, and V3-89.6) blocked the binding of IVR7 in a dose-dependent manner, while CTR36, a T-tropic linear V3 peptide, and V3-ADA, a cyclized M-tropic V3 peptide, had no effects (Fig. 2). In contrast, binding of anti-CD19 (Leu12), an irrelevant MAb included as a control, was affected by none of these peptides.
FIG. 2.
Effects of the V3 peptides (V3-BH10 and CTR36) corresponding to the V3 region of HIV-1 IIIB (A), V3-ELI corresponding to that of T-tropic HIV-1 strain ELI (B), V3-89.6 corresponding to that of dualtropic HIV-1 strain 89.6 (C), or V3-ADA corresponding to that of M-tropic HIV-1 strain ADA (D) on the staining of biotinylated IVR7. JY cells were incubated with human IgG on ice for 15 min to block nonspecific binding. Cells were then preincubated with various concentrations (15 μM [dashed line], 5 μM [dashed and dotted line], 1.7 μM [dotted line], and 0 μM [solid line]) of V3-BH10, control linear V3 peptide (CTR36), V3-ELI, V3-89.6, or V3-ADA on ice for 30 min. Then, biotinylated IVR7 or FITC-Leu12 was added. After being washed, cells were further incubated with PE-conjugated streptavidin on ice, washed, and subjected to flow cytometric analysis. Reactivity was determined by comparison of results of the control staining with those with biotinylated irrelevant mouse IgG1 or FITC-conjugated irrelevant mouse IgG (cont. IgG1 or cont. IgG, respectively).
Effects of the V3 peptides on intracellular Ca2+ mobilization.
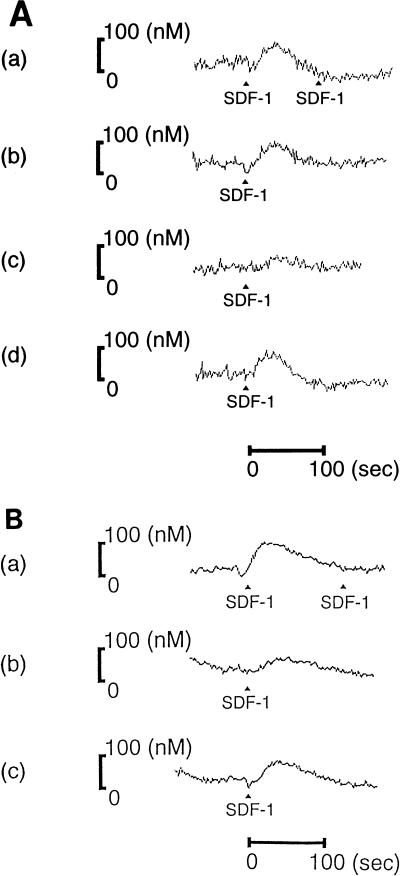
Next, in order to determine whether the V3 peptides had antagonistic activities against SDF-1β, the effects of the V3 peptides on the SDF-1-induced transient increases in intracellular Ca2+ were investigated. As shown in Fig. 3A(a) and B(a), the addition of 1 μg of SDF-1β/ml elicited rapid and transient Ca2+ mobilization within a few seconds in SupT1 cells. The first stimulation abrogated responsiveness to a subsequent stimulation with the same ligand, as has been reported in CXCR-4 and other chemokine receptors (6, 34). Pretreatment of V3-BH10 or V3-ELI effectively prevented the SDF-1β-induced Ca2+ mobilization by nearly 90% [Fig. 3A(c) and B(b)] and that of V3-89.6 did so by nearly 30% [Fig. 3B(c)], whereas CTR36 or V3-ADA had no inhibitory effects [Fig. 3A(b and d)].
The addition of the V3 peptides did not induce Ca2+ mobilization (data not shown), suggesting that they did not desensitize CXCR-4 to SDF-1β stimulation.
Effects of the V3 peptides on HIV-1 infection in MT-4 cells.
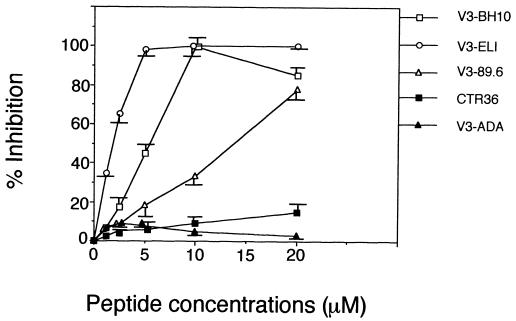
The Ca2+ mobilization experiments strongly suggested that the binding site of V3-BH10, V3-ELI, and V3-89.6 on CXCR-4 overlapped with the SDF-1β binding region. Based on the fact that SDF-1 has anti-HIV-1 activity, we subsequently investigated the effects of the V3 peptides on HIV-1 (IIIB strain) infection of MT-4 cells, which express both CD4 and CXCR-4 on the cell surface. No toxic effects were found with any peptide at concentrations up to 40 μM. Among the five V3 peptides, V3-BH10 and V3-ELI strongly inhibited the infection by HIV-1 IIIB of MT-4 cells at 10 μM. V3-89.6 inhibited infection by 80% at 20 μM, while CTR36 or V3-ADA had no inhibitory effects on the infection (Fig. 4).
FIG. 4.
Effects of the V3 peptides on HIV-1 infection in an MTT assay. One-hundred microliters of the cell suspension of MT-4 cells was added to each well. Then, various concentrations of the indicated synthetic peptides were added to each well and incubated for 30 min at 37°C. After that, HIV-1 solution (HIV-1 IIIB strain) was further added to each well at a multiplicity of infection of 0.0008. After a 5-day incubation, the cell viability was determined by the MTT method. Anti-HIV-1 activity in vitro of the five synthesized V3 peptides was analyzed by inhibition of virus-induced cell death. Values are means and are expressed as the arithmetic means ± standard deviations.
Effects of the V3 peptides on RT activity.
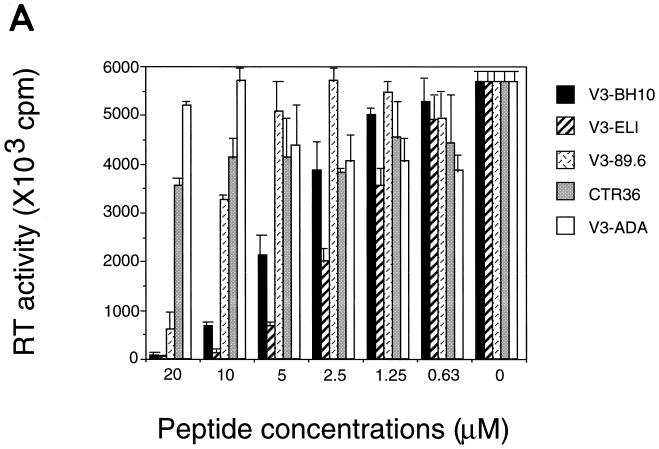
In order to confirm the inhibitory effects of the V3 peptides on HIV-1 infection, an RT assay was carried out. In the experiments using the culture supernatants from a 3-day culture of MT-4 cells infected with HIV-1 IIIB strain, V3-BH10, V3-ELI or V3-89.6, but not CTR36 or V3-ADA, dose-dependently inhibited the RT activity of the supernatants (Fig. 5A).
FIG. 5.
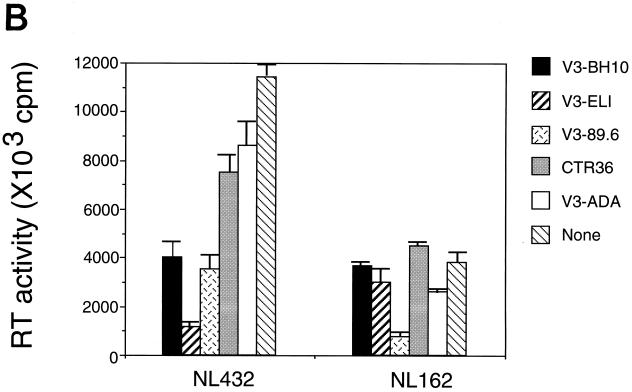
Effects of the V3 peptides on the RT activity of HIV-1. (A) Using supernatants from 3-day culture of V3 peptide-treated MT-4 cells and HIV-1 IIIB, the RT assay was carried out as described in Materials and Methods. The radioactivity on the filters was determined by LKB Beta Plate scintillation spectroscopy. Values are means and are expressed as the arithmetic means of [3H]dTTP incorporation ± standard deviations. (B) Using supernatants from 5-day culture of NL432 or NL162-treated PBMC and each of the V3 peptides (40 μM), the RT assay was carried out. Values are means and are expressed as the arithmetic means of [3H]dTTP incorporation ± standard deviations.
In the RT assay using the supernatants of the culture with HIV-1 and PBMC, V3-BH10 and V3-ELI inhibited T-tropic virus NL432-derived RT activity but not M-tropic virus NL162-derived RT activity. V3-89.6 inhibited not only NL432-derived but also NL162-derived activity. On the other hand, V3-ADA slightly prevented NL162-derived but not NL432-derived RT activity. Our preliminary experiments using the anti-CCR-5 MAb 2D7 (kindly provided by the NIH AIDS Research and Reference Reagent Program) (47) showed that V3-ADA weakly inhibited the binding of 2D7 as evaluated by FACScan analysis (data not shown), which seems to be consistent with the modest inhibitory effect of V3-ADA on NL162 infection. CTR36 had no effect on the infection of NL432 or NL162 (Fig. 5B).
DISCUSSION
HIV-1 viral entry is thought to be initiated by interaction of the envelope protein gp120 with the cellular receptors. Among several epitopes in gp120, the V3 loop region has drawn much attention for its close association with the cell tropism of HIV-1 (8, 9, 45). Recent discoveries of the coreceptors have presented an interpretation of cell tropism as the selection of one of the coreceptors that support either T-tropic or M-tropic HIV-1 infection. The next question is that of what defines the specific binding of gp120 to a certain type of coreceptor. With regard to this issue, by using mutant gp120 and neutralizing antibodies against V3 epitopes, Wu et al. showed that the V3 region of M-tropic virus gp120 was critical for interaction with CCR-5 (46). In the case of CXCR-4, the direct binding of CXCR-4 to the CD4-gp120 complex on the cell membrane has been reported previously (28). Bandres et al. recently showed that oligomeric gp160 purified from cell cultures infected with HIV451 bound to CXCR-4 independently of CD4 (4). The reports, however, did not clearly show which sites of the CD4-gp120 complex or the oligomeric gp160 were involved in the binding to CXCR-4. Moreover, some groups have reported that other domains within both gp120 and gp41 can also influence these specific envelope functions, resulting in a change of the virus tropism (23, 35, 41). Also, the V1/V2 regions have been shown to determine these biological phenotypes of HIV-1 (3, 24, 43). Therefore, it remains to be addressed whether the V3 loop of gp120 by itself can bind to the coreceptors.
In this study, we have presented evidence that the T-tropic or dual-tropic virus-derived synthetic cyclized V3 peptides (V3-BH10, V3-ELI, or V3-89.6) but not the linear V3 peptide (CTR36) or the M-tropic virus-derived cyclized V3 loop (V3-ADA) directly bind to CXCR-4, as revealed by competition with the anti-CXCR-4 MAb, IVR7, which has strong suppressive effects on T-tropic HIV-1 infection as described in our previous report (22). Murakami et al. reported the inhibition of T-tropic HIV-1 infection by a small-molecule CXCR-4 antagonist, T22, which has a loop structure with two disulfide bonds, but not by a linear control peptide, 4 Ala-T-I (32). Thus, it appears that the loop structure is important for the binding of the peptides to CXCR-4, resulting in the inhibition of HIV infection. With regard to the charge of these peptides, which are evaluated by computer analysis, all five V3 peptides are positively charged (isoelectric points: CTR36, 12.5; V3-BH10, 12.2; V3-ELI, 10.6; V3-89.6, 12.0; V3-ADA, 9.1) whereas the first and second extracellular loops of CXCR-4 are negatively charged. However, our data demonstrated that only three V3 peptides (V3-BH10, V3-ELI, and V3-89.6) among the five V3 peptides which are positively charged specifically bound to CXCR-4. These results suggest that the binding is, however, more likely to be due to interactions between the V3 loop and CXCR-4 other than the electrostatic one (positive charge-negative charge interaction).
After the submission of this manuscript, an analysis of the crystal structure of an HIV-1 gp120 core glycoprotein in complex with CD4 and a neutralizing antibody was published (27, 49). Based on this analysis and using various gp120 mutants, Rizzuto et al. suggested that a conserved gp120 structure adjacent to the V3 loop containing neutralization epitopes induced by CD4 binding (CD4i epitopes) is important for chemokine receptor binding (37). We think that their results and ours are complementary. The V3 loop and certain other regions in gp120, including those containing CD4i epitopes, are likely to be involved in interaction with chemokine receptors. Our study strongly suggests that the V3 loop can directly bind to the relevant chemokine receptor by itself and determine the coreceptor usage. The binding affinities of the cyclized V3 loop peptides for CXCR-4 seemed to be relatively low, which might be due to a lack of other regions, such as those containing CD4i epitopes. It remains to be elucidated, however, whether these regions directly bind to chemokine receptors or indirectly augment the binding of the V3 loop.
When SDF-1 binds to CXCR-4, a rapid and transient Ca2+ mobilization is elicited (6, 34). Data from our Ca2+ mobilization studies showed that increases in intracellular Ca2+ were detected within a few seconds after the addition of SDF-1β in SupT1 cells, and that V3-BH10, V3-ELI, and V3-89.6 prevented the SDF-1β-induced Ca2+ mobilization with different magnitudes (Fig. 3). No Ca2+ mobilization was detected following the addition of each V3 peptide alone (data not shown), indicating that the inhibitory effects of the three V3 peptides (V3-BH10, V3-ELI, and V3-89.6) on the intracellular Ca2+ increase were not due to the desensitization of CXCR-4 but rather that they inhibited the binding of SDF-1β to CXCR-4. As described in our previous report (22), IVR7 blocks the SDF-1-induced increase in intracellular Ca2+, and both IVR7 and SDF-1 inhibit T-tropic HIV-1 infection. Therefore, it seems that the binding sites on CXCR-4 for SDF-1 and IVR7 are located close to the region, which is important for HIV-1 entry. In accordance with this assumption, we have shown by MTT assay and RT assay that the three V3 peptides among the five we used could inhibit HIV-1 IIIB infection of MT-4 cells. Furthermore, the three V3 peptides also prevented the infection of PMBC by another T-tropic HIV-1, NL432. In contrast, two V3 peptides (V3-BH10 and V3-ELI) among the three peptides did not inhibit the infection of PMBC by M-tropic HIV-1 NL162. In consideration of these findings, it is likely that the epitope of IVR7 and the binding sites of the three V3 peptides locate close to each other such that they overlap the SDF-1 binding site and, therefore, the three V3 peptides as well as IVR7 or that SDF-1 suppresses T-tropic HIV-1 infection by preventing the interaction between the virus and its coreceptor CXCR-4.
FIG. 3.
Effects of V3 peptides on SDF-1-induced Ca2+ mobilization. After loading of Fura-2, cells were pretreated without peptides [A(a) and B(a)] or with 20 μM CTR36 [A(b)], V3-BH10 [A(c)], V3-ADA [A(d)], V3-ELI [B(b)], or V3-89.6 [B(c)] and were then stimulated with SDF-1β (indicated with solid triangles). Increases in cytosolic free Ca2+ concentration were measured with a fluorescence spectrophotometer.
The issue of whether V3 region-derived peptides inhibit or enhance HIV-1 infection has been controversial. Some groups have reported that synthetic V3 peptides corresponding to the V3 regions of MN and IIIB strains enhanced the infection of Molt-3 cells by different HIV-1 strains (7, 13). In contrast, Nehete et al. showed that V3 peptides inhibited HIV-1 infection (33). Koito et al. have reported that synthetic V3 peptides corresponding to the V3 region of HIV-1 strain IIIB inhibited syncytium formation by interacting with the cell surface (25). Our data are consistent with those of the latter two groups: in our hands, three of five cyclized V3 peptides clearly inhibited HIV-1 IIIB infection in MT-4 cells and NL432 infection in PBMC, and none of the synthesized V3 peptides tested enhanced the infection, as shown in Fig. 4 and 5. Although further studies are required to define the biological activity of the V3 loop peptides, the present study indicates that these peptides are useful tools to analyze the mechanism of HIV-1 entry into target cells and may serve as anti-HIV-1 reagents.
ACKNOWLEDGMENTS
We thank H. Tamamura for the ion spray mass spectrum analyses.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture and Scientific Research Expenses for Health and Welfare Programs from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkatib G, Combadiere C, Broder C C, Feng Y, Murphy P M, Berger E. CC-CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Andeweg A C, Leeflang P, Osterhaus A D M E, Bosch M L. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J Virol. 1993;67:3232–3239. doi: 10.1128/jvi.67.6.3232-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandres J C, Wang Q F, O’Leary J, Baleaux F, Amara A, Hoxie J A, Zolla-Pazner S, Gorney M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barré-Sinoussi F, Chermann J C, Ray P, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Carlo Z, Calderazzo F, Dettin M, Bello C D, Autiero M, Guardiola J, Chieco-Bianchi L, De Rossi A. Minimal sequence requirements for synthetic peptides derived from the V3 loop of the human immunodeficiency virus type 1 (HIV-1) to enhance HIV-1 binding to cells and infection. Virology. 1995;206:807–816. doi: 10.1006/viro.1995.1003. [DOI] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Quiroga M, Tung J W, Dina D, Levy J A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990;64:4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe H, Farzen M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolate. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Deng H K, Choe S, Ellmeier W, Liu R, Unutmaz D, Burkhart M, di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 13.De Rossi A, Pasti M, Mammano F, Panozzo M, Dettin M, Bello C D, Chieco-Bianchi L. Synthetic peptides from the principal neutralizing domain of human immunodeficiency virus type 1 (HIV-1) enhance HIV-1 infection through a CD4-dependent mechanism. Virology. 1991;184:187–196. doi: 10.1016/0042-6822(91)90835-y. [DOI] [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smith R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S, Fuang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moor J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Farzan M, Choe H, Martin K, Markon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-membrane segment receptors which expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauci A S. The immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 18.Federsppiel B, Melhado I, Duncan A, Delancy A, Schappert K, Clark-Lewis I, Jirik F. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seven-transmembrane segment (7-TMS) receptor isolated from human spleen. Genomics. 1993;16:707–712. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 20.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 21.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori T, Sakaida H, Sato A, Nakajima T, Shida H, Yoshie O, Uchiyama T. Detection and delineation of CXCR-4 (fusin) as an entry and fusion cofactor for T-tropic HIV-1 by three different monoclonal antibodies. J Immunol. 1998;160:180–188. [PubMed] [Google Scholar]
- 23.Kim F M, Kolson D L, Balliet J W, Srinivasan A, Collman R G. V3-independent determinants of macrophage tropism in a primary human immunodeficiency virus type 1 isolate. J Virol. 1995;69:1755–1761. doi: 10.1128/jvi.69.3.1755-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koito A, Stamatatos L, Cheng-Mayer C. Small amino acid sequence changes within the V2 domain can affect the function of a T-cell-line-tropic human immunodeficiency virus type 1 envelope gp120. Virology. 1995;206:878–884. doi: 10.1006/viro.1995.1010. [DOI] [PubMed] [Google Scholar]
- 25.Koito A, Hattori T, Murakami T, Matsushita S, Maeda Y, Yamamoto T, Takatsuki K. A neutralizing epitope of human immunodeficiency virus type 1 has homologous amino acid sequences with the active site of inter-alpha-tripsin inhibitor. Int Immunol. 1989;1:613–618. doi: 10.1093/intimm/1.6.613. [DOI] [PubMed] [Google Scholar]
- 26.Kondo A, Imada K, Hattori T, Yamabe H, Tanaka T, Miyasaka M, Okuma M, Uchiyama T. A model of in vivo cell proliferation of adult T cell leukemia. Blood. 1993;82:2501–2509. [PubMed] [Google Scholar]
- 27.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell line. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 29.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 31.Matsushita S, Robert-Guroff M, Rusche J, Koito A, Hattori T, Hoshino H, Javaherian K, Takatsuki K, Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988;62:2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehete P N, Arlinghaus R B, Sastry K J. Inhibition of human immunodeficiency virus type 1 infection and syncytium formation in human cells by V3 loop synthetic peptides from gp120. J Virol. 1993;67:6841–6846. doi: 10.1128/jvi.67.11.6841-6846.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwarz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien W A, Koyanagi Y, Namazie A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S Y. HIV-tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 36.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a coreceptor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 37.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 38.Sakaida H, Murakami T, Kawamata S, Hattori T, Uchiyama T. V3 loop of human immunodeficiency virus type 1 suppresses interleukin 2-induced T cell growth. AIDS Res Hum Retroviruses. 1997;13:151–159. doi: 10.1089/aid.1997.13.151. [DOI] [PubMed] [Google Scholar]
- 39.Sato A, Kodama M, Abe K, Miki S, Nishimura M, Suyama A, Ogata M, Toyoda T, Sugimoto H, Yoshie O, Fujiwara T. A simple and rapid method for preliminary evaluation of in vivo efficacy of anti-HIV compounds in mice. Antivir Res. 1995;27:151–163. doi: 10.1016/0166-3542(95)00004-6. [DOI] [PubMed] [Google Scholar]
- 40.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shioda T, Levy J A, Cheng-Meyer C. Macrophage and T-cell line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 42.Stephen D S, Shatsky M, Cohen P S, Warnke R, Link M P, Glader B E. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res. 1984;44:5657–5660. [PubMed] [Google Scholar]
- 43.Sullivan N, Thali M, Furman C, Ho D D, Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993;67:3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD-4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 45.Westervelt P, Gendelman H E, Ratner L. Identification of a determinant within the human immunodeficiency virus type 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Gerard N P, Wyatt R, Choe H, Oarolin C, Raffing N, Boysetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 47.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 50.Yssel H, deVries J E, Koken M, Blitterswijk W V, Spits H. A serum free medium for the generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]