Coronavirus disease 2019 (COVID-19) can lead to various cardiovascular complications, including myocardial injury, vascular injury, and thromboembolism1. Arrhythmias are one of the most common cardiac manifestations in COVID-19. Although tachycardias such as atrial fibrillation are often seen in the acute phase of COVID-19 patients, bradycardias are also reported in the acute phase of COVID-192. More recently, long-term sequelae of COVID-19, known as Long-COVID, have been reported in various organs including the heart3–5. In the post-acute phase, some COVID-19 survivors suffer from palpitations due to dysregulated heart rates, atrial fibrillation, and postural orthostatic tachycardia syndrome.
In this issue of Circulation Research6, Han et al. demonstrated that sinoatrial node (SAN) damage caused by direct infection of SARS-CoV-2 into SAN pacemaker cells is a potential mechanism of COVID-19-induced bradycardia. First, using a hamster model intranasally infected with SARS-CoV-2, the authors detected evidence of viral infection in SAN pacemaker cells. Under immunostaining, SARS-CoV-2 Spike protein and dsRNA were detected in HCN4+ SAN cells in the heart of infected hamsters, suggesting that SARS-CoV-2 can infect SAN pacemaker cells directly in vivo. Second, they investigated the impact of SARS-CoV-2 infection on human SAN pacemaker cells in vitro using human embryonic stem cell-derived SAN-like pacemaker cells, which were generated with a newly established in-house protocol with inhibitors of FGFR, TGFβ, and STAT3. The hESC-SAN-like pacemaker cells were readily infected with SARS-CoV-2, leading to decreased expression of SAN marker genes, SAN dysfunction, and induction of ferroptosis, which was evidenced by the downregulation of GPX4. Third, they performed a drug screening using hESC-SAN-like pacemaker cells and identified deferoxamine and imatinib as potential candidate drugs to protect pacemaker cells from SARS-CoV-2 infection and ferroptosis.
An important aspect of this study is that SAN pacemaker cells were susceptible to SARS-CoV-2 infection in vivo. Although there are several reports showing that in vitro cardiomyocytes (CMs), especially stem cell-derived CMs, are highly susceptible to SARS-CoV-2 infection7–9, animal models and patients’ autopsy samples have not shown strong evidence of direct infection of SARS-CoV-2 for CMs10. Thus, COVID-19-induced myocardial injury, which is evidenced by increased serum troponin levels and one of the common cardiac representations of COVID-19, is likely to be an indirect consequence of systemic inflammation or cytokine storms rather than being caused by direct viral infection to patients’ CMs. The results in this article suggest that some CM subtypes could be more susceptible to SARS-CoV-2 than typical ventricular-type CMs and show phenotypes unique to the subtypes when infected. Given that even a small percentage of damaged CMs could cause serious arrhythmic problems in the heart, subtype-specific susceptibility to SARS-CoV-2 may warrant further attention. Another important point is the potential of the two hit compounds, imatinib and deferoxamine. Imatinib was identified as inhibiting SARS-CoV-2 infection consistently in their lung and colon organoid systems11. Deferoxamin, which is a known inhibitor of ferroptosis, decreased SARS-CoV-2 entry as well as ferroptosis with unknown mechanisms.
There are several remaining questions for further investigation. First, as the authors acknowledged, there is no clinical report documenting infected SAN in COVID-19 patients. Although preclinical animal models and stem cell-based platforms are undoubtedly useful tools as a surrogate to model diseases with limited availability to clinical samples, it is still important to confirm their findings in human samples such as post-mortem autopsies and imaging modalities. Second, sex differences in bradycardia of COVID-19 patients would be also important although only male hamsters were used in this study. Third, it is unknown whether the SAN damage caused by SARS-CoV-2 is reversible. If the viral infection leads to ferroptosis and eventually cell death in SAN pacemaker cells, the SAN damage is likely irreversible. Thus, the patients with SAN damage would suffer from bradycardia even in the post-acute or chronic phases. This may explain a part of the relevant mechanism of heart rate dysregulation in Long-COVID. Fourth, there are other possibilities that may cause SAN damage or bradycardia in COVID-19, including hypoxemia, cytokine storm, and damage in autonomous neurons.
In conclusion, this study by Han et al. demonstrated a potential mechanism of COVID-19-induced bradycardia, in which direct infection of SARS-CoV-2 into SAN pacemaker cells was found to cause dysfunction and ferroptosis of pacemaker cells. A drug screening identified two already-existing drugs, deferoxamine and imatinib as candidates to block this process. Further studies are needed to determine whether the SAN damage can be detected in COVID-19 patients and if the proposed mechanism contritubes to dysregulated heart rates after recovery from COVID-19, which is one of the common chronic-phase sequelae of COVID-19, known as Long-COVID.
Figure. Discovery of novel anti-COVID drugs with ESC-derived SAN-like pacemaker cells.
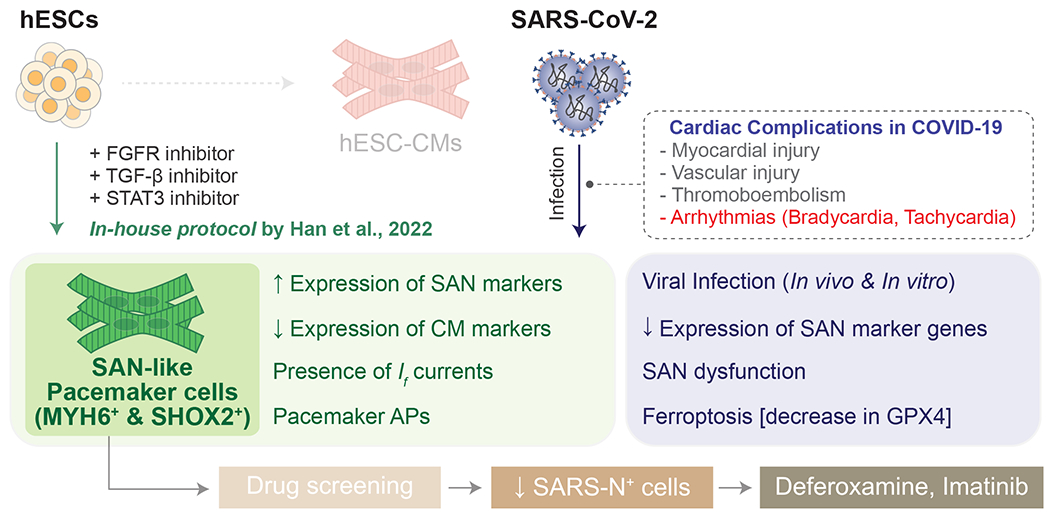
Modulating CM differentiation protocol with chemically defined in-house protocol by Han et al led to generating a pure population of SAN-like pacemaker cells (MYH6+, SHOX2+) with electrophysiological characteristics of pacemaker cells. SAN-like pacemaker cells were susceptible to SARS-CoV-2 infection, and ferroptosis was an underlying mechanism of arrhythmias in COVID-19. Screening >1,000 FDA-approved drugs led to Deferoxamine and Imatinib being identified as novel anti-COVID-19 drugs modulating ferroptosis in SAN-like pacemaker cells. hESC, human embryonic stem cell; CM, cardiomyocyte; SAN, sinoatrial node; AP, action potential;
Acknowledgement
This publication was supported by research grants from the National Institutes of Health R01 HL126527, R01 HL130020 and R01 HL141851 (J.C.W.), Tobacco-Related Disease Research Program (TRDRP) postdoctoral fellowship T31FT1758 (M.N.), the Translational Research Institute for Space Health (TRISH) through Cooperative Agreement NNX16AO69A (J.W.S.J).
Footnotes
Disclosure
None.
References
- 1.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, Kim J-H, Yeo I, Tracy C, Ayanian S, Akar J, Singh A, Jain S, Zimerman L, Pimentel M, Osswald S, Twerenbold R, Schaerli N, Crotti L, Fabbri D, Parati G, Li Y, Atienza F, Zatarain E, Tse G, Leung KSK, Guevara-Valdivia ME, Rivera-Santiago CA, Soejima K, De Filippo P, Ferrari P, Malanchini G, Kanagaratnam P, Khawaja S, Mikhail GW, Scanavacca M, Abrahão Hajjar L, Rizerio B, Sacilotto L, Mollazadeh R, Eslami M, Laleh Far V, Mattioli AV, Boriani G, Migliore F, Cipriani A, Donato F, Compagnucci P, Casella M, Dello Russo A, Coromilas J, Aboyme A, O’Brien CG, Rodriguez F, Wang PJ, Naniwadekar A, Moey M, Kow CS, Cheah WK, Auricchio A, Conte G, Hwang J, Han S, Lazzerini PE, Franchi F, Santoro A, Capecchi PL, Joglar JA, Rosenblatt AG, Zardini M, Bricoli S, Bonura R, Echarte-Morales J, Benito-González T, Minguito-Carazo C, Fernández-Vázquez F, Wan EY. Worldwide Survey of COVID-19-Associated Arrhythmias. Circ Arrhythm Electrophysiol. 2021;14:e009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Zhu J, Yang L, Nilsson-Payant BE, Hurtado R, Lacko L, Sun X, Gade AR, Higgins CA, Sisso WJ, Dong X, Wang M, Chen Z, Ho DD, Pitt GS, Schwartz RE, tenOever BR, Evans T, Chen S. SARS-CoV-2 Infection Induces Ferroptosis of Human Pluripotent Stem Cell-Derived Sinoatrial Nodal Cells. Circ Res. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen D-HT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125–136.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Garcia G, Wang Y, Plummer JT, Morizono K, Arumugaswami V, Svendsen CN. Human iPSC-Derived Cardiomyocytes Are Susceptible to SARS-CoV-2 Infection. Cell Rep Med. 2020;1:100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Bermejo JA, Kang S, Rockwood SJ, Simoneau CR, Joy DA, Silva AC, Ramadoss GN, Flanigan WR, Fozouni P, Li H, Chen P-Y, Nakamura K, Whitman JD, Hanson PJ, McManus BM, Ott M, Conklin BR, McDevitt TC. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci Transl Med. 2021;13:eabf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, Blankenberg S, Püschel K, Westermann D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S, Bram Y, Richardson C, Zhu J, Zhao Z, Redmond D, Houghton S, Nguyen D-HT, Xu D, Wang X, Jessurun J, Borczuk A, Huang Y, Johnson JL, Liu Y, Xiang J, Wang H, Cantley LC, tenOever BR, Ho DD, Pan FC, Evans T, Chen HJ, Schwartz RE, Chen S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]


