Abstract
Prevention of the initial infection of mucosal dendritic cells (DC) and interruption of the subsequent transmission of HIV-1 from DC to T cells are likely to be important attributes of an effective human immunodeficiency virus type 1 (HIV-1) vaccine. While anti-HIV-1 neutralizing antibodies have been difficult to elicit by immunization, there are several human monoclonal antibodies (MAbs) that effectively neutralize virus infection of activated T cells. We investigated the ability of three well-characterized neutralizing MAbs (IgG1b12, 2F5, and 2G12) to block HIV-1 infection of human DC. DC were generated from CD14+ blood cells or obtained from cadaveric human skin. The MAbs prevented viral entry into purified DC and the ensuing productive infection in DC/T-cell cultures. When DC were first pulsed with HIV-1, MAbs blocked the subsequent transmission to unstimulated CD3+ T cells. Thus, neutralizing antibodies can block HIV-1 infection of DC and the cell-to-cell transmission of virus from infected DC to T cells. These data suggest that neutralizing antibodies could interrupt the initial events associated with mucosal transmission and regional spread of HIV-1.
Dendritic cells (DC) at the mucosal surface of the genital tract are likely to be the initial target of human immunodeficiency type 1 (HIV-1) infection (19, 26, 33, 36). Intraepithelial DC express CD4 and the beta chemokine receptor CCR5, which serve as coreceptors for HIV-1 cell entry (46). During their normal function of immunosurveillance, DC capture antigen, traffic to draining lymph nodes, and present antigen to T cells. During migration to regional lymph nodes, the maturing DC upregulates expression of immunostimulatory molecules such as B7.1 (CD80) and B7.2 (CD86). The mature DC then presents antigen to T cells to initiate immunity (3, 8, 35, 37). While the exact mechanism of mucosal transmission is unknown, HIV-1 appears to exploit the normal DC pathway of antigen uptake and presentation to gain access to the CD4+ T cells in the lymphoid tissue. In humans, active replication of HIV-1 has been shown to occur in DC/T-cell syncytia at the lymphoepithelial surface of tonsils (14), adenoids (14, 15), and parotid glands and colon (13a). In macaques, intravaginal inoculation with the simian immunodeficiency virus appears to target DC in the lamina propria of the cervicovaginal mucosa (36), and in vitro studies have provided evidence for viral entry into DC (1, 7, 17, 18, 28) and for viral replication in immature DC (5, 43).
Immature DC selectively replicate macrophage-tropic isolates of HIV-1 (16, 33, 46), while mature DC do not support HIV-1 replication until they are cocultured with CD4+ T cells (1, 2, 7, 16, 17, 30–32, 44). In the mature DC/T-cell culture system, robust HIV-1 replication occurs without mitogen stimulation of T cells and without the addition of exogenous cytokines (17, 32, 44). The DC/T-cell syncytia in these cocultures are phenotypically similar to those observed in vivo (15, 30, 32). Recent studies have shown that freshly isolated (immature) epidermal Langerhans cells expressed CCR5 but not CXCR4 on their surface and that these cells fused with CCR5 (R5) but not CXCR4 (X4) using HIV-1 envelopes (46). In addition, when HIV-1 strains were applied to the abraded epidermal surface of skin organ cultures, it was found that R5 viruses were selectively captured by DC that emigrated from the explanted skin (33). These findings for cultured DC indicate that DC may initiate HIV-1 transmission in vivo, by capturing R5 HIV-1 and subsequently initiating viral replication in T cells.
Neutralization of HIV-1 at the initial stages of virus entry might be a critical determinant of vaccine efficacy. Most neutralization studies have measured HIV-1 infection in mitogen- and interleukin-2 (IL-2)-stimulated T cells (11), a target cell type that may not participate in the initial events of infection. It is not known if anti-HIV-1 antibody can prevent infection of mucosal DC or the subsequent transmission of HIV-1 from DC to T cells. Since anti-HIV-1 antibodies that neutralize infection by primary HIV-1 isolates are uncommon and are not readily elicited by most HIV-1 immunogens (23), we studied three human monoclonal antibodies (MAbs) (IgG1b12, 2F5, and 2G12) that potently neutralize infection of activated T cells by primary HIV-1 isolates (6, 21, 39). As a model for the interaction of HIV-1 with DC at mucosal surfaces and in draining lymph nodes, we sought to determine if these MAbs could prevent HIV-1 infection of pure DC as well as transmission of HIV-1 from infected DC to unstimulated T cells. Using blood and skin DC, we found that anti-HIV-1 neutralizing MAbs can block both virus entry into DC and the transmission of HIV-1 from infected DCs to T cells. These data suggest that anti-HIV-1 neutralizing antibodies could interrupt the mucosal transmission and regional spread of HIV-1 and that a vaccine eliciting neutralizing antibody could prevent mucosal transmission of HIV-1.
MATERIALS AND METHODS
Preparation of blood DC.
DC were generated from the blood of normal donors by using protocols similar to those previously reported (4, 34). Peripheral blood mononuclear cells (PBMC) obtained by Ficoll-Hypaque sedimentation were depleted of CD3+ T cells by two incubations with anti-CD3 immunomagnetic beads (Dynal Inc., Great Neck, N.Y.). The T-cell-depleted leukocytes were adhered to immunoglobulin G (IgG)-coated plastic flasks, and nonadherent cells were vigorously removed by four washes with phosphate-buffered saline. Adherent cells were maintained for 7 days in culture medium (RPMI 1640 medium supplemented with 15% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine) containing 1,000 U each of recombinant human IL-4 (R&D Systems, Minneapolis, Minn.) and granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, Wash.) per ml. Cytokines were replenished on alternate days. On day 7, culture medium was replaced with macrophage conditioned medium (MCM) that was also supplemented with 1,000 U each of granulocyte-macrophage colony-stimulating factor and IL-4 per ml. To prepare MCM, PBMC were adhered to IgG-coated plastic flasks in culture medium containing 10% heat-inactivated normal human serum. After removal of nonadherent cells, the MCM supernatant was collected daily for 5 days. Cell-free supernatant was analyzed for the level of tumor necrosis factor alpha by enzyme-linked immunosorbent assay (R&D Systems), and medium containing 50 IU or more was used as MCM. Mature DC were harvested for use on day 11 and resuspended in culture medium without supplemental cytokines. Purified CD3+ T cells were obtained by positive selection from PBMC as described above, using anti-CD3 immunomagnetic beads according to manufacturer’s instructions. Viability of cryopreserved purified CD3+ T cells was routinely ≥90%; the cells did not proliferate when returned to culture.
Skin-derived DC.
Skin-derived DC and T cells were obtained from split-thickness skin harvested from cadavers within 12 h of death (Lifenet, Virginia Beach, Va.) as previously described (29–31). Briefly, skin explants were placed dermal side down in 150-ml tissue culture dishes (Corning Plastics, Corning, N.Y.) in 45 ml of culture medium. The skin was cultured for 2 to 4 days, and the migrated cells were treated with collagenase D (Boehringer Mannheim), washed, and resuspended in culture medium. Skin leukocytes from this organ culture method contain a mixture of DC and CD3+ T cells and are permissive for HIV-1 replication (29, 30, 32). To obtain pure skin DC, skin leukocytes were depleted of CD3+ T cells by using anti-CD3-coated immunomagnetic beads as described above.
Flow cytometry.
DC were characterized by two-color staining with fluorescein isothiocyanate- and phycoerythrin-conjugated mouse MAbs, using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). The MAbs used were anti-HLA-DR, anti-CD80, anti-CD86, anti-CD3, anti-CD14, anti-CD19, and anti-CD45 (Becton Dickinson); anti-CD83 (Immunotech, Miami, Fla.); and anti-CD1a (PharMingen, San Diego, Calif.) and isotype-matched controls. CD45-negative events were excluded from the fluorescence-activated cell sorting analysis.
Immunohistochemical staining of cytospin preparations.
Cell cytospins were prepared as previously described (14, 31, 32). The primary antibodies were anti-HLA-DR, anti-CD3, anti-CD4, anti-CD8, anti-CD20, anti-CD68, anti-S-100, and anti-HIV-1 p24gag (DAKO, Carpinteria, Calif.) and anti-CD1a, anti-CD80, and anti-CD83 (Immunotech). Cytospins of infected DC/T-cell cultures were prepared between 2 and 11 days after exposure to HIV-1.
Virus stocks.
HIV-1 isolates BaL, 89.6, and IIIB were obtained from the NIH AIDS Reference and Reagent Repository, and isolates US1 and SG365 were from the repository of the U.S. Military HIV Research Program. Virus stocks were prepared by infection of phytohemagglutinin (PHA)-stimulated PBMC as previously described (22). BaL, US1, and SG365 are primary R5 viruses. HIV-1 89.6 is known to use both CCR5 and CXCR4 (10), and IIIB is a T-cell line-adapted (TCLA) virus (12). BaL, US1, and IIIB have been previously characterized as genetic subtype B, and SG365 has been characterized as genetic subtype C (20). SG365 is resistant to neutralization by the MAbs that we tested (unpublished data).
Antibodies.
Anti-HIV-1 human MAbs IgG1b12, 2F5, and 2G12 were chosen because each can block infection of PHA-stimulated PBMC target cells by primary isolates of HIV-1 (6, 39, 40). MAb 2F5 recognizes the gp41 sequence ELDKWA (9, 27); MAbs 2G12 and IgG1b12 bind to different conformationally sensitive epitopes on gp120 (6, 40). A control nonneutralizing MAb (4.8D) was obtained from the NIH AIDS Reference and Reagent Repository (donated by James Robinson). MAb 4.8D binds to a conformational epitope on gp120 but does not neutralize primary HIV-1 isolates at concentrations of up to 50 μg/ml (11). All four MAbs are isotype IgG1.
DC infection and antibody neutralization assays.
Virus infection and neutralizing antibody experiments with DC were performed by using methodologies similar to those previously described for PHA/IL-2-stimulated PBMC (21, 22). Blood DC harvested on day 11 were washed, resuspended in culture medium, and exposed to 500 50% tissue culture infectious doses (TCID50) of virus per 5 × 104 cells (multiplicity of infection [MOI] = 0.01) in triplicate wells. DC were exposed to virus overnight, or for 90 min as noted, and then washed. For neutralization experiments, virus and antibody were preincubated for 30 min at 37°C prior to the addition of DC. Since mature DC replicate HIV-1 only in the presence of T cells, positively selected CD3+ cells were added back to the DC (2:1 ratio of CD3+ cells to DC). Virus replication was monitored by enzyme-linked immunosorbent assay measurement of p24 antigen produced in culture supernatants. Neutralization was assessed at days 6 to 8 for DC/T cells, or days 4 to 6 for PHA/IL-2-stimulated PBMC, representing the early kinetics of virus growth. Neutralization experiments with skin DC (depleted of CD3+ T cells) were performed in a similar manner.
Molecular detection of viral entry and proviral formation.
A semiquantitative PCR measurement of early and late HIV-1 reverse transcripts in cell lysates was used to assess viral entry and proviral formation, respectively, based on a modification of published techniques (17, 45). Briefly, viral supernatant was centrifuged at 900 × g for 10 min, filtered through a 0.22-μm-pore-size filter, and treated with RNase-free DNase (50 U/ml; Boehringer Mannheim Corp., Indianapolis, Ind.). DC were exposed to virus (MOI = 0.01) for 90 min at 37°C, washed to remove unabsorbed virus, and then cultured alone or with CD3+ T cells. Cell lysates from approximately 25,000 cells were separately amplified with 32P-end-labeled sense oligonucleotides to generate 108- and 204-bp products representing early and late viral reverse transcripts, respectively. Equivalent amounts of lysate were subjected to amplification with 32P-end-labeled primers specific for the single-copy gene CCR5 (25). PCR products were resolved on 8% nondenaturing polyacrylamide gels and quantitated by using storage phosphor technology. Copy numbers were computed by comparison to a calibrated series of ACH-2 cell lysates which contain a single provirus per cell (13) and then normalized for cell input by reference to CCR5 signal intensity.
RESULTS
Purified mature DC do not become productively infected with HIV-1 but can transmit HIV-1 to unstimulated T lymphocytes.
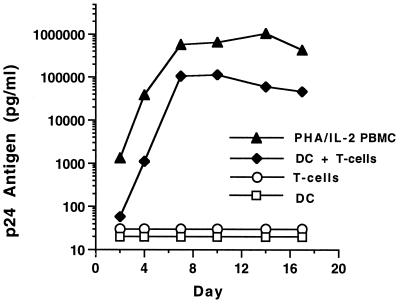
We generated mature blood DC from adherent CD14+ PBMC. Since our methods were slightly different than those described (4, 34), we reconfirmed that mixtures of mature DC and T cells, but not pure DC or T cells alone, are permissive for HIV-1 replication (17, 30–32). After culture in cytokines, the DC developed into a uniform population of large cells with typical processes, with both veils and dendrites. They maintained this morphology despite the withdrawal of exogenous cytokines on day 11. The population was homogeneous by cytofluorometry and by immunocytochemical stains on cytospins. Less than 1% of the cells expressed CD14, CD19, CD56, or CD3. The cells expressed high levels of HLA-DR, the immunostimulatory molecules CD80 and CD86, and the DC marker CD83. Functionally, the DC were potent stimulators of an allogeneic mixed leukocyte response, inducing strong lymphoproliferative responses with 100- to 1,000-fold-fewer cells than PBMC stimulators (data not shown). Thus, these DC fulfill the phenotypic and functional criteria for mature DC (35). When exposed to HIV-1 BaL, neither mature DC alone nor CD3+ T cells alone became productively infected. However, robust HIV-1 replication was observed if CD3+ T cells were added to DC that had been pulsed with HIV-1 and washed (Fig. 1).
FIG. 1.
Infection kinetics of purified DC cultured in the presence and absence of T cells. The DC were exposed to HIV-1 BaL (500 TCID50/105 DC) overnight at 37°C, thoroughly washed to remove free virus, and cultured alone or with positively selected CD3+ T cells. PHA/IL-2-stimulated PBMC and unstimulated CD3+ cells were infected in a similar manner. No p24 antigen expression was detected in purified DC or CD3+ T cells alone. Robust infection, close to that seen with activated PBMC, was detected when T cells were added to infected DC. During infection, DC and CD3+ cells were cultured in medium without exogenous cytokines. PBMC were maintained in culture medium supplemented with 20 U of IL-2 per ml.
Neutralizing MAbs inhibit productive infection of DC/T-cell cocultures.
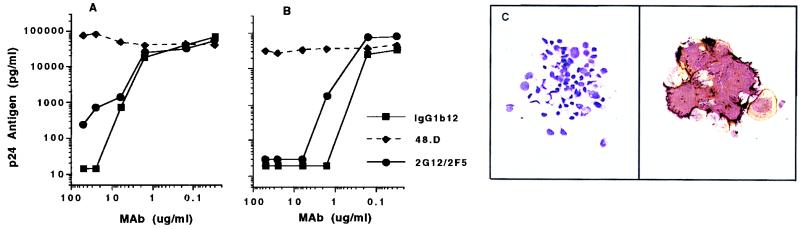
Preliminary data showed that HIV-1 BaL infection of PBMC targets was abrogated (95 to 99% neutralization) by MAb IgG1b12 (25 to 50 μg/ml) or by the combination of MAbs 2G12 and 2F5 (each at 25 μg/ml). MAbs 2F5 and 2G12 were less potent individually than in combination. Thus, MAb IgG1b12 and the combination 2F5/2G12 were used for DC neutralization experiments. The neutralization dose-effect curves for IgG1b12, 2F5/2G12, and the nonneutralizing MAb 4.8D, using PBMC or DC targets, are shown in Fig. 2A and B, respectively. No productive infection was seen in DC/T-cell cultures when MAbs IgG1b12 and 2F5/2G12 were preincubated with BaL at concentrations of >10 μg/ml. These experiments were repeated several times with similar results. The 90 and 99% inhibitory concentrations for MAbs were approximately 5- to 10-fold lower when DC were use as targets than when activated PBMC were used. Antibody-mediated virus neutralization in DC/T-cell cultures was confirmed by immunocytochemical analysis of cultured cells. The p24 antigen found almost entirely in small and large syncytia in the cultures was uniformly absent in cytospins of DC/T-cell cultures when BaL had been preincubated with MAb IgG1b12 (Fig. 2C).
FIG. 2.
(A and B) Antibody-mediated neutralization of HIV-1 BaL infection of PHA/IL-2 stimulated PBMC (A) and DC (B), with CD3+ cells added on day 1 after exposure of DC to HIV-1. MAb IgG1b12, or the combination 2F5/2G12, was preincubated with virus at a starting concentration (for each MAb) of 50 μg/ml prior to addition of PBMC or DC. Extracellular p24 antigen was measured in the early viral growth phase for PBMC (day 4) and DC/T cells (day 7). Identical concentrations of the nonneutralizing MAb 4.8D did not inhibit viral infection of PBMC or DC targets. (C) Immunocytochemical staining of DC/T-cell cytospins for p24 antigen expression (brown) and HLA-DR (purple). The right-hand panel shows a p24-positive syncytium on day 7 after DC exposure to BaL (magnification, ×200). No HIV-1-infected cells were seen when BaL was preincubated with 25 μg of MAb IgG1b12 per ml (left, p24 antigen stain only; ×160).
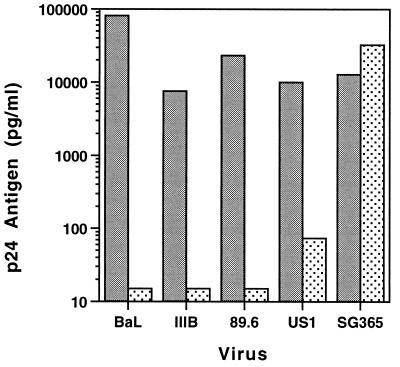
To evaluate neutralization of other HIV-1 strains, experiments were performed with the TCLA virus IIIB, the dualtropic strain 89.6, and a primary R5 isolate, US1. With the MAb combination 2F5/2G12, greater than 99% neutralization of all four viruses was seen, although infection of DC by US1 was not completely prevented. As a control for possible nonspecific MAb effects on cell growth, we used a clade C virus (SG365) previously shown to be insensitive to neutralization by 2F5/2G12. SG365 infection of DC/T-cell cultures was not neutralized by 2F5/2G12 (Fig. 3).
FIG. 3.
Neutralization of four clade B HIV-1 isolates by the combination 2F5/2G12 (each at 25 μg/ml). Target cells were DC with T cells added back as in Fig. 1. Stippled bars indicate preincubation of DC with MAbs; gray bars show virus growth without antibody. The clade C isolate (SG365) was not neutralized by 2F5/2G12.
Neutralizing MAbs block entry of HIV-1 into DC.
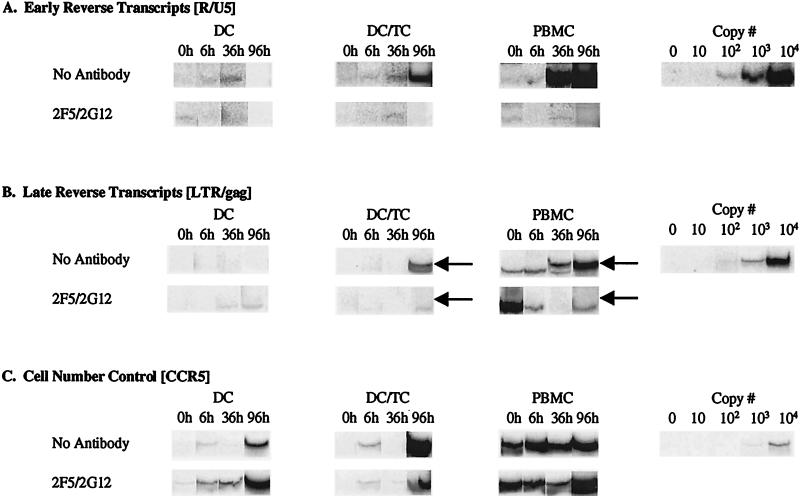
To determine if neutralizing MAbs block entry of HIV-1 into DC, we quantitated early and late HIV-1 reverse transcripts present in cell lysates by PCR. As shown in Fig. 4, HIV-1 BaL was incubated with DC or PBMC, and early and late transcripts were amplified at 0, 6, 36, and 96 h after infection. Similar to findings in a previous report (17), only early transcripts were detected in pure cultures of mature DC, whereas both early and late reverse transcripts were detected in DC/T-cell cultures and activated PBMC. Preincubation of BaL with the MAb combination 2F5/2G12 (each at 25 μg/ml) blocked the formation of early reverse transcripts in pure DC (Fig. 4A). The weak or undetectable early transcript signal at 6 and 36 h after DC infection indicates the lack of effective viral entry. As expected, 2F5/2G12 also blocked the formation of late transcripts in DC/T-cell and PBMC cultures. In a separate experiment, similar data were obtained with MAb IgG1b12 (25 μg/ml) used in place of 2F5/2G12 (data not shown).
FIG. 4.
Neutralizing MAbs block virus entry into DC. Purified blood DC were exposed to a DNase-treated, cell-free virus stock (HIV-1 BaL; MOI = 0.01) in the presence or absence of MAb combination 2F5/2G12 (each at 25 μg/ml) and subsequently incubated alone (DC) or in the presence of purified CD3+ T cells (DC/TC). Parallel control infections of PHA/IL-2-stimulated PBMC were also conducted. Cell lysates were prepared at the times indicated postinfection and amplified with 32P-labeled primers specific for early and late HIV-1 reverse transcripts and for CCR5. Parallel amplification of known numbers of ACH-2 cells (which contain a single integrated HIV-1 provirus per cell) were performed with each primer set to provide a copy number quantitation curve. (A) Early reverse transcripts (RU5 long terminal repeat [LTR] region sequences). (B) Late reverse transcripts (LTR/gag). Arrows indicate the positions of specific signals; the remaining visible bands are nonspecific (i.e., no late reverse transcripts were seen in PBMC until the 36-h time point). (C) CCR5 gene amplification (cell number control).
Neutralizing MAbs block transmission of HIV-1 from infected DC to unstimulated T cells.
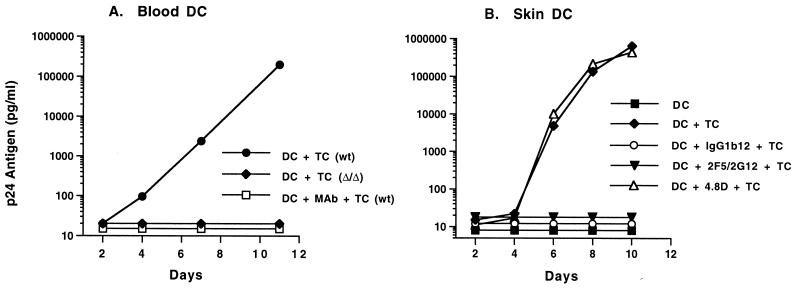
It might be difficult for immunization to elicit an effective concentration of neutralizing antibody at mucosal surfaces where HIV-1 first interacts with DC. Thus, we sought to determine if neutralizing MAbs could interfere with transmission of HIV-1 from infected DC to unstimulated T cells, an event which may be relevant to the initial transmission of HIV-1 in draining lymph nodes. Blood or skin DC were pulsed with HIV-1 BaL for 90 min at 37°C, extensively washed, and returned to culture. MAb was added to the culture 24 h later, and T cells were added 30 min after antibody. On blood DC (Fig. 5A), MAb IgG1b12 blocked the transmission of BaL from purified, infected DC (which contained early reverse transcripts) to T cells. In the absence of neutralizing antibody, these same DC established a vigorous infection when combined with T cells. When T cells were obtained from a donor homozygous for the 32-bp deletion in the CCR5 gene, a coreceptor defect known to abrogate infection with CCR5-using HIV-1 strains, there was no propagation of HIV-1 infection. This finding demonstrates that transmission of infection from DC to T cells was CCR5 dependent. Prior work has shown that functional CCR5 is needed on both DC and T cells to obtain a productive HIV-1 infection with R5 viruses (16). To test the ability of MAbs to prevent HIV-1 transmission from skin DC to T cells, we used skin DC depleted of CD3+ cells. Similar to results of experiments with blood DC, pure skin DC replicated virus only upon addition of CD3+ cells to the culture (Fig. 5B). Neutralizing MAbs completely blocked transmission of HIV-1 infection to T cells by skin DC that had been pulsed with HIV-1.
FIG. 5.
Neutralizing MAbs prevent the transmission of HIV-1 from infected DC to T cells. Purified blood (A) or skin (B) DC were exposed to HIV-1 BaL for 90 min and washed to remove free virus. MAbs were added 24 h later. After a 30-min incubation, CD3+ T cells were added to the culture. (A) MAb IgGb12 was used at 25 μg/ml. As a control, CD3+ cells from a known homozygous CCR5 (Δ/Δ) donor were also used. Transmission of infection from DC to T cells was dependent on CCR5 and could be blocked by neutralizing MAbs. wt, T cells wild type for the CCR5 gene. (B) MAbs were used at a concentration of 25 μg/ml.
DISCUSSION
The protective mechanisms of immunoglobulin at mucosal surfaces are likely to be complex. Both locally produced IgG and IgA and transudative IgG could play a role in protective immunity (24). In this study, we used IgG MAbs along with blood and skin DC to model the interaction of antibody, HIV-1, and DC at mucosal surfaces and in lymphoid tissue. Three HIV-1 envelope-specific human IgG MAbs were studied because they were previously shown to neutralize HIV-1 infection of activated T cells. Our data show that these MAbs prevent HIV-1 infection of pure DC and block the transmission of HIV-1 from infected DC to unstimulated T cells. Neutralization dose-response data demonstrated potent antibody-mediated virus neutralization when pure DC were used as targets of infection. MAb concentrations of ≥10 μg/ml resulted in complete neutralization of virus infection in the DC cultures. The reason that inhibition of HIV-1 infection of DC occurred at antibody concentrations lower than required to block infection of mitogen-stimulated T cells is not clear. Additional experiments are in progress to carefully monitor replication kinetics in each culture system, using virus titered on each target cell (47). When DC were exposed to HIV-1 in the presence of neutralizing MAb, immunocytochemical staining of DC/T-cell cultures showed no p24-positive DC/T-cell syncytia. In comparison, and as previously described (30–32), multiple DC/T-cell syncytia were seen on cytospins from cultures of DC exposed to HIV-1 in the absence of MAb. We also tested the neutralizing activity of MAbs against HIV-1 strains other than HIV-1 BaL. Potent neutralization was demonstrated for a TCLA strain, a dualtropic (R5 and X4) virus, and a clinical R5 isolate (IIIB, 89.6, and US1, respectively). Thus, these MAbs appear to show broad anti-HIV neutralizing activity on DC targets, as they do on PBMC targets. Prior studies have shown that HIV-1 efficiently enters mature DC, but only early products of reverse transcription are produced until T cells are added to the culture (16, 17). We confirm these data and show that neutralizing MAbs block viral entry, as assessed by the absence of early reverse transcripts in pure DC cultures.
While our initial experiments focused on the ability of antibody to block virus entry into purified DC, it may be more relevant to mucosal HIV-1 infection to determine if antibody can block transmission of HIV-1 from an infected DC to a resting T cell. We modeled this interaction by treating HIV-1-pulsed DC with MAb prior to the addition of unstimulated T cells and observed no virus replication. Results were similar for blood-derived and skin DC. These data suggest that if luminal antibodies fail to prevent the initial interaction of HIV-1 with DC, antibodies present at draining lymph nodes could interrupt DC-to-T-cell transmission. Several groups have studied the mechanism of DC-to-T-cell HIV-1 transmission (5, 16, 17, 41, 44). Granelli-Piperno and colleagues recently showed that functional CCR5 on both DC and T cells was required for DC to infect T cells (16), and Weissman et al. reported that transmission from HIV-1-pulsed DC to T cells could be blocked by anti-CD4 antibody (44). Our data confirm that transmission to T cells is dependent on CCR5 and are the first to show that HIV-1 transmission to DC can be blocked by HIV-1 envelope-specific antibody. Taken together, these results suggest that neutralizing MAbs are blocking DC-to-T-cell transmission via established mechanisms, i.e., by blocking virus binding to CD4 or CCR5 or by inhibiting gp41-mediated cell fusion (38, 42). Our results are limited to IgG HIV-1 envelope-specific MAbs and do not address the role of IgA at mucosal surfaces (24).
In summary, our data demonstrate that anti-HIV-1 neutralizing antibodies block HIV-1 entry into DC, the likely first cells infected at the mucosal surface. In addition, neutralizing antibodies block the in vitro transmission from HIV-1-infected DC to unstimulated T cells, indicating that antibody could interrupt the initial spread of HIV-1 in draining lymph nodes. These data suggest that both mucosal and systemic immunoglobulin could play a role in prevention of sexual transmission of HIV-1, thus supporting the rationale that generation of a neutralizing antibody response will be an important component of an effective HIV-1 vaccine.
ACKNOWLEDGMENTS
We thank Thach Bui, Marc Martin-ez, Poonam Mannan, and Robert McLinden for excellent technical assistance, Lifenet (Virginia Beach, Va.) for providing skin, and John McNeil, John Moore, Mark Lewis, and Francine McCutchan for helpful discussions.
This work was supported in part by NIH grants HL59718 (J.R.M.), AI33292 (D.R.B.), and AI40874 (R.M.S.) and by cooperative agreement DAMD17-93-V 3004, between the U.S. Army Medical Research and Materiel Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
REFERENCES
- 1.Ayehunie S, Garcia-Zepeda E A, Hoxie J A, Horuk R, Kupper T S, Luster A D, Ruprecht R M. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. [PubMed] [Google Scholar]
- 2.Ayehunie S, Groves R W, Bruzzese A-M, Ruprecht R M, Kupper T S, Langhoff E. Acutely infected Langerhans cells are more efficient than T cells in disseminating HIV type 1 to activated T cells following a short cell-cell contact. AIDS Res Hum Retroviruses. 1995;11:877–884. doi: 10.1089/aid.1995.11.877. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Asada H, Saville M W, Klaus-Kovtun V, Altman D J, Yarchoan R, Katz S I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Investig. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton D R, Pyati J, Koduri R, Sharpe S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 7.Cameron P U, Lowe M G, Sotzik F, Coughlan A F, Crowe S M, Shortman K. The interaction of macrophage and non-macrophage tropic isolates of HIV-1 with thymic and tonsillar dendritic cells in vitro. J Exp Med. 1996;183:1851–1856. doi: 10.1084/jem.183.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 9.Conley A J, Kessler II J A, Boots L J, Tung J-S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doranz B J, Rucker J, Yi Y J, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza M P, Milman G, Bradac J A, McPhee D, Hanson C V, Hendry R M, Corcoran T, Stott J, Fung M, Hanson C, Laman J, Mascola J, Rasheed S, Richman D, Schuitemaker H, Thiriart C, Wainberg M, Weber J, Beddows S, Tilley S, Robinson J, Zolla-Pazner S, Katinger H. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS. 1995;9:867–874. doi: 10.1097/00002030-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13.Folks T, Powell D M, Lightfoote M M, Benn S, Martin M A, Fauci A S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986;231:600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- 13a.Frankel, S. S. Unpublished data.
- 14.Frankel S S, Tenner-Racz K, Racz P, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D R, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 16.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langhoff E, Kalland K H, Haseltine W A. Early molecular replication of human immunodeficiency virus type 1 in cultured-blood-derived T helper dendritic cells. J Clin Investig. 1993;91:2721–2726. doi: 10.1172/JCI116512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner T, Hussain L, Wilson J, Chapman M. Mucosal transmission of HIV. Nature. 1991;353:709. doi: 10.1038/353709c0. [DOI] [PubMed] [Google Scholar]
- 20.Louwagie J, Janssens W, Mascola J, Heyndrickx L, Hegerich P, van der Groen G, McCutchan F E, Burke D S. Genetic diversity of the envelope glycoprotein from human immunodeficiency virus type 1 isolates of African origin. J Virol. 1995;69:263–271. doi: 10.1128/jvi.69.1.263-271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 23.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 24.Mazzoli S, Trabattoni D, Caputo S L, Piconi S, Blé C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 25.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 26.Miller C J, McChesney M, Moore P F. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab Investig. 1992;67:628–634. [PubMed] [Google Scholar]
- 27.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson S, Roberts M S, English N R, Macatonia S E, Gompels M N, Pinching A J, Knight S C. Detection of HIV DNA in peripheral blood dendritic cells of HIV-infected individuals. Res Virol. 1994;145:171–176. doi: 10.1016/s0923-2516(07)80019-7. [DOI] [PubMed] [Google Scholar]
- 29.Pope M, Betjes M G H, Hirmand H, Hoffman L, Steinman R. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J Investig Dermatol. 1995;104:11–17. doi: 10.1111/1523-1747.ep12613452. [DOI] [PubMed] [Google Scholar]
- 30.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 31.Pope M, Frankel S S, Mascola J R, Trkola A, Isdell F, Birx D L, Burke D S, Ho D D, Moore J P. Human immunodeficiency virus type 1 strains of subtypes B and E replicate in cutaneous dendritic cell T-cell mixtures without displaying subtype-specific tropism. J Virol. 1997;71:8001–8007. doi: 10.1128/jvi.71.10.8001-8007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low level of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reece J C, Handley A J, Anstee E J, Morrison W A, Crowe S M, Cameron P U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 35.Schuler G, Steinman R M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 38.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 39.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsunetsugu-Yokota Y, Yasuda S, Sugimoto A, Yagi T, Azuma M, Tagita H, Akagawa K, Takemori T. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology. 1997;239:259–268. doi: 10.1006/viro.1997.8895. [DOI] [PubMed] [Google Scholar]
- 42.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S A, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissman D, Li Y, Orenstein J M, Fauci A S. Both a precursor and a mature population of dendritic cells can bind HIV. J Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]
- 45.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 46.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]