Abstract
Gene transfer with recombinant murine leukemia viruses (MuLV) provides the potential to permanently correct inherited lung diseases, such as cystic fibrosis (CF). Several problems prevent the application of MuLV-based recombinant retroviruses to lung gene therapy: (i) the lack of cell proliferation in mature pulmonary epithelia, (ii) inefficient gene transfer with a vector applied to the apical surface, and (iii) low titers of many retroviral preparations. We found that keratinocyte growth factor (KGF) stimulated proliferation of differentiated human tracheal and bronchial epithelia. Approximately 50% of epithelia divided in response to KGF as assessed by bromodeoxyuridine histochemistry. In airway epithelia stimulated to divide with KGF, high-titer ampho- and xenotropic enveloped vectors preferentially infected cells from the basal side. However, treatment with hypotonic shock or EGTA transiently increased transepithelial permeability, enhancing gene transfer with the vector applied to the mucosal surfaces of KGF-stimulated epithelia. Up to 35% of cells expressed the transgene after gene transfer. By using this approach, cells throughout the epithelial sheet, including basal cells, were targeted. Moreover, the Cl− transport defect in differentiated CF airway epithelia was corrected. These findings suggest that barriers to apical infection with MuLV can be overcome.
Several vector systems for the treatment of cystic fibrosis (CF) are under investigation. One shortcoming of current nonviral vectors and recombinant adenovirus is the short duration of transgene expression (41, 52). Since CF is a chronic, progressive disease, it may be advantageous to develop strategies that result in transgene integration and persistent expression. Moloney murine leukemia virus (MuLV)-based retroviruses have received extensive use in gene transfer studies and are approved for human trials. However, there are limitations which prevent the practical application of retroviral gene transfer to airway epithelia.
The mature lung presents several potential barriers for efficient gene transfer with retroviral vectors. In vivo studies suggest that gene transfer efficiency is low in the absence of cell proliferation (13, 16). With the exception of disease states such as CF, epithelial injury, or tumors, pulmonary epithelia are mitotically quiescent (<1% of cells dividing) (3, 9, 24, 37). Also, the secreted products of airway epithelia may cause vector entrapment in mucus or vector inactivation by a number of antimicrobial factors. For example, phagocytes resident in the lung, including alveolar macrophages, inhibit gene transfer (28, 48). Finally, adequate titers of recombinant retrovirus preparations are required to achieve a useful multiplicity of infection (MOI) to impact the estimated 6 to 10% of epithelia required for correction of the Cl− transport defect associated with CF (20).
Recent studies have identified specific growth factors that stimulate proliferation of pulmonary epithelia, including keratinocyte growth factor (KGF) (18, 36, 44) and hepatocyte growth factor (25, 33, 36, 42, 51), suggesting a means to increase cell proliferation. Further advances in retroviral-vector design and concentration methods allow production of ampho- and xenotropic viruses with titers of 108 to 109 CFU/ml (8, 19, 21, 22). We investigated the feasibility of using growth factor stimulation and high-titer retrovirus to attain gene transfer to differentiated human airway epithelia in vitro. We also tested the ability of retrovirus to infect airway epithelia from the apical or basolateral surface.
MATERIALS AND METHODS
Primary culture of human airway epithelia.
Primary cultures of human airway epithelia were prepared from trachea and bronchi by enzymatic dispersion as previously described (23, 50, 54). Briefly, epithelial cells were dissociated and seeded onto collagen-coated, semipermeable membranes with a 0.4-μm pore size (Millicell-HA; surface area, 0.6 cm2; Millipore Corp., Bedford, Mass.). Twenty-four hours after seeding, the mucosal medium was removed and the cells were allowed to grow at the air-liquid interface as reported previously (50). The culture medium consisted of a 1:1 mixture of Dulbecco modified Eagle medium and Ham’s F-12 with 2% Ultroser G (Sepracor Inc., Marlborough, Mass.), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Representative preparations from all cultures were scanned by electron microscopy, and the presence of tight junctions was confirmed by transepithelial resistance measurements (resistance, >1,000 Ω · cm2). All preparations used in the study were well differentiated, and only well-differentiated cultures >2 weeks old were used in these studies. Previous studies showed that differentiated epithelia in this model are multilayered and consist of ciliated cells (cytokeratin 18 positive), secretory cells containing granules that are reactive to goblet cell- and mucous-cell-specific antibodies, and basal cells positive for cytokeratin 14 (50, 54). This study was approved by the Institutional Review Board of the University of Iowa.
Reagents. (i) Growth factors.
Recombinant human KGF was a gift from Amgen, Inc. (Thousand Oaks, Calif.). Except when stated otherwise, KGF was applied to the basal side of differentiated airway cells at a concentration of 50 ng/ml. KGF-containing medium was changed every 24 h.
(ii) Recombinant retrovirus and vector formulation.
High-titer recombinant amphotropic and xenotropic retroviruses were prepared at Chiron Technologies-Center for Gene Therapy, Inc. (San Diego, Calif.) as described previously (7, 28). Reporter viruses used included DA-βgal (β-galactosidase [β-Gal] reporter, amphotropic envelope) and DX-βgal (β-Gal reporter, xenotropic envelope) (19, 21). The β-Gal reporter gene was driven by retroviral long terminal repeat. The vector formulation buffer included 19.5 mM trimethamine at pH 7.4, 37.5 mM NaCl, and 40 mg of lactose per ml. The osmolality of the viral buffer was 105 mmol/kg, as measured with a vapor pressure osmometer (model 5500; Wescor, Inc., Logan, Utah). Polybrene was included in all infection mixtures at a concentration of 8 μg/ml.
A vector expressing the human CF transmembrane conductance regulator (CFTR) was prepared by cloning the human CFTR cDNA (40) into a retroviral vector plasmid with the viral long terminal repeat promoter (21). Producer clones were selected based on the ability of crude vector stocks to confer cyclic AMP (cAMP)-activated Cl− transport to undifferentiated CF epithelia in vitro, and a stable producer cell line was selected (21, 27). For gene transfer to differentiated CF airway epithelia, crude producer cell supernatants were concentrated by centrifugation and applied to epithelia with or without KGF stimulation. Epithelia were tested for the presence of CFTR Cl− currents in Ussing chambers 3 to 10 days after gene transfer as previously described (27).
In selected experiments transepithelial permeability was increased before or at the time of application of the vector to the apical membranes of cultured epithelia. Water and EGTA were used to treat the epithelia. For EGTA treatment, a solution of 1.5 mM EGTA in water (osmolality, 33 mmol/kg) was used to rinse the apical sides of cells for 20 min. An EGTA-virus mixture was obtained by mixing the viral preparation and 3 mM EGTA in water at a 1:1 ratio (osmolality, 48 mmol/kg). Gene transfer to the apical surface was performed by applying the vector in 100-μl volumes. For gene transfer to the basal side of the cell membrane, the Millicell culture insert was turned over and the vector was applied to the bottom of the membrane in a 100-μl volume.
Assessment of cell proliferation by BrdU immunohistochemistry.
Bromodeoxyuridine (BrdU) labeling and immunostaining were performed with a kit from Zymed Laboratories Inc. (South San Francisco, Calif.). Cells were treated with 50 ng of KGF per ml for 36 h. A 1:100 dilution of the BrdU labeling reagent was added to the culture medium, and cells were labeled for 4 h followed by fixation in 10% neutralized Formalin. BrdU histochemistry was performed by following the methods of the Zymed BrdU kit. Labeled nuclei stained brown under these conditions. Epithelial cell preparations were examined microscopically en face or in cross sections of paraffin-embedded membranes, and the percentage of brown-staining nuclei was determined. Hematoxylin or 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes, Eugene, Oreg.) was used for counterstaining.
β-Gal expression.
Epithelial cells were fixed with 2% paraformaldehyde–phosphate-buffered saline (PBS) solution for 20 min and rinsed with PBS twice for 5 min each. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining solution was added to the cells at 37°C for ≥4 h or overnight as previously described (26). Cell membranes were examined microscopically en face or in cross sections for β-Gal expression. The percentage of β-Gal-positive cells was determined by counting a minimum of 1,000 cells from cross sections of each treated cell culture insert.
Identification of amphotropic retroviral receptor (GLVR-2) in cultured human airway epithelia. (i) GLVR-2 antibody.
Affinity-purified polyclonal GLVR-2 antisera were prepared by immunizing rabbits with a synthetic peptide (GLVR-2A), an extracellular domain sequence that is conserved in rat Ram-1 and human GLVR-2 (31, 32, 44a). The peptide was coupled to keyhole limpet hemocyanin, and then the rabbits were immunized. Different postimmunization bleeds were tested by using enzyme-linked immunosorbent assay and immobilized free peptide. The resulting antipeptide antisera were pooled and affinity purified on columns of immobilized GLVR-2A peptides. These affinity-purified antibodies were then used for all GLVR-2 expression analyses.
(ii) Western blotting.
Airway epithelial cells were lysed in 10 mM Tris-HCl buffer (pH 7.4) containing 0.5% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride. Cell lysates were collected and protein concentrations were determined by the Lowry method. Thirty-five micrograms of protein in loading buffer was denatured at room temperature (not boiled) for 40 min and run on a 10% polyacrylamide–sodium dodecyl sulfate gel. Following electrophoresis, the proteins were transferred to a Nytran membrane (Schleicher & Schuell, Inc., Keene, N.H.) by electroblotting and blocked with 10% skim milk powder. Immunoblotting was performed with the polyclonal antisera at a 1:10,000 dilution. Goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase was used for the secondary antibody (Bio-Rad, Hercules, Calif.), and the proteins were identified by autoradiography by using the ECL system (Amersham, Arlington Heights, Ill.). The specificity of the antibody was confirmed by preincubating the antibody with 20 μM free synthetic peptide for 30 min in PBS–1% bovine serum albumin at room temperature prior to incubation with the blots.
Measurement of transepithelial resistance.
Differentiated epithelial cells were treated with 50 ng of KGF per ml for 24 h. Solutions of water or 1.5 mM EGTA in water were used to rinse the apical side for 20 min. The solution was then replaced with viral formulation buffer alone, or a 1:1 mixture of viral formulation buffer plus 3 mM EGTA water solution, and incubated for 4 h. Control cells received KGF treatment and PBS washes instead of water or EGTA washes. Transepithelial resistance was monitored with an ohmmeter (EVOM; World Precision Instruments, Inc., Sarasota, Fla.) over 16 to 18 h until resistance returned to baseline.
RESULTS
KGF induces proliferation of differentiated human airway epithelia.
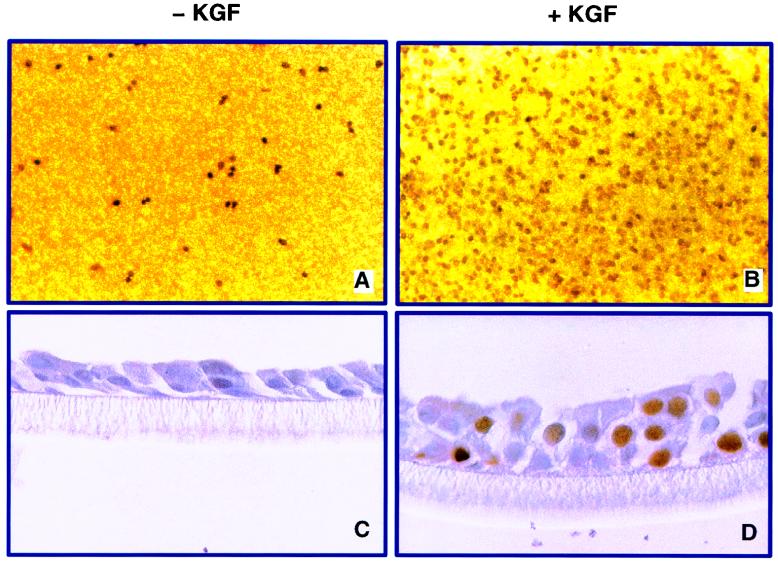
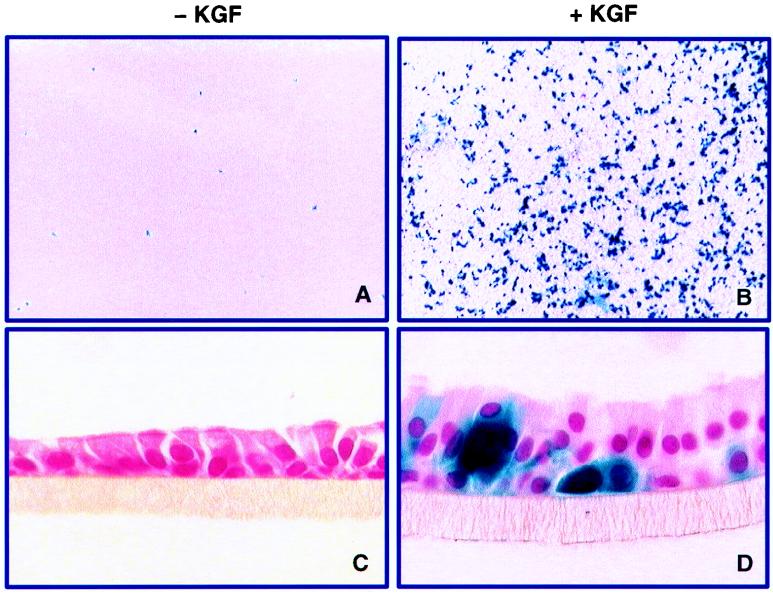
We first tested the hypothesis that KGF stimulates proliferation of human airway epithelia grown at the air-liquid interface (50). To determine the percentage of cells dividing and identify the cell types responsive to KGF, human airway epithelia were treated with 50 ng of KGF per ml in the basal medium for 36 h, followed by BrdU labeling and immunostaining. As shown in Fig. 1, 52% ± 8% (mean ± standard error [SE]) of the nuclei of KGF-treated airway epithelia were labeled, compared to only 7% ± 3% of control cells. Cross sections of the cell membranes demonstrated that the height of the epithelial sheets increased in KGF-treated samples and that basal cells, ciliated surface cells, and cells between the basal cell layer and the surface of the membrane divided in response to the growth factor. Apical application of KGF had the same effects as basolateral treatment (data not shown). These findings show conclusively that KGF stimulates a marked proliferative response in differentiated human airway epithelia.
FIG. 1.
KGF stimulates proliferation of differentiated human airway epithelia, as assessed by BrdU labeling. KGF treatment at 50 ng/ml caused substantial labeling of nuclei (B) and increased the height of the epithelial layer (D) compared with control epithelia (A and C). (E) Percentage of BrdU-positive nuclei for control and KGF-treated cells from three separate airway cell preparations.
Retroviral gene transfer via the apical surfaces of proliferating airway epithelia.
While KGF can stimulate epithelial cell proliferation, it is not clear whether cell division is sufficient to enhance retrovirus-mediated gene transfer to differentiated human airway epithelia. To address this question, airway epithelia were stimulated with 50 ng of KGF per ml for 24 h followed by application of DA-βgal amphotropic vector (MOI, ∼20) to the apical side of the membrane for 4 h. Three days after infection, X-Gal staining was performed to evaluate transgene expression. As shown in Fig. 2, when the vector was applied to the apical membranes of quiescent or KGF-stimulated cells, there was no gene transfer. When the same approach was used with xenotropic vector, we again saw no gene transfer with the vector applied to the mucosal surface (not shown). These results were unexpected and suggested that either the receptors for amphotropic and xenotropic viruses were not present or they were inaccessible to virus applied to the apical surface.
FIG. 2.
Gene transfer is inefficient with application of vector to the mucosal surface. Human airway epithelia were treated with KGF at 50 ng/ml (B and D) or basal medium (A and C) for 36 h. DA-βgal virus was then added to the apical surface. There was no gene transfer regardless of the proliferative state of the cells. Identical results were obtained with cells from three separate airway cell preparations.
Expression of the amphotropic retroviral receptor GLVR-2 in human airway epithelia.
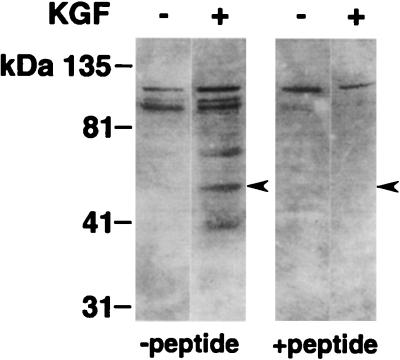
Retroviral transduction begins with the interaction and binding of viral envelope glycoproteins and cell surface receptors. In the case of amphotropic enveloped vectors, the receptor (Ram-1 or GLVR-2) has been cloned and identified as a sodium-dependent phosphate transporter (31, 45), a 656-amino-acid transmembrane protein. We used a rabbit polyclonal antibody raised against a synthetic peptide sequence from the extracellular domain of GLVR-2 to learn if the receptor is expressed and whether KGF influences receptor abundance. As shown in Fig. 3, treatment of human airway epithelia with KGF for 24 h increased the abundance of the GLVR-2 protein as determined by Western blot analysis in comparison to that in untreated controls. Thus, human airway epithelia express GLVR-2, and its expression is increased by KGF. We also performed immunohistochemistry on human airway epithelia with or without KGF stimulation by using the same polyclonal antisera used for the Western blot and an additional GLVR-2 antibody (10). However, the results were inconclusive because it was not possible to localize the protein by using these reagents. Together, these data suggested that the receptor was inaccessible to virus applied to the apical surface.
FIG. 3.
KGF upregulates expression of the amphotropic retroviral receptor GLVR-2 in human airway epithelia. Differentiated epithelia were grown for 24 h with or without KGF. The cells from four membranes were pooled for analysis for each condition. Cells from two different preparations were studied. Shown is a Western blot of GLVR-2 expression in the absence (−) or presence (+) of KGF. The left panels show results in the absence of preincubation of antibody with GLVR-2 peptide. A band of ∼70 kDa (indicated by arrowhead) was present in KGF-treated cells but barely perceptible in untreated cells. The experiment was repeated three times, with identical results.
Polarity of retroviral gene transfer to proliferating airway epithelia.
In marked contrast to the results with apically applied virus, vector application to the basal sides of KGF-treated epithelia resulted in efficient gene transfer, with 17% ± 1.5% (mean ± SE for six membranes from two preparations) of cells expressing the transgene. Cross sections of epithelia showed that most β-Gal expressing cells were located basally in the epithelial sheet (Fig. 4). Occasional β-Gal-positive cells were noted in the cell layer above the basal cells. Occasional β-Gal-positive cells were also noted among cells that received no KGF when the vector was applied to the basal surface, in agreement with the lower mitotic indices of cells grown under these conditions (Fig. 1). Similar results were obtained with xenotropic enveloped virus (data not shown). These data demonstrate that KGF-induced proliferation enhances retroviral gene transfer when the vector is applied to the basal cell surface.
FIG. 4.
KGF-induced proliferation facilitates gene transfer to the basal side of human airway epithelia. DA-βgal virus was added for 4 h to the basal surfaces of non-KGF-stimulated cells (A and C) and KGF-stimulated cells (B and D) (virus was added 24 h after stimulation). β-Gal expression was detected 72 h later. Gene transfer occurred only when the vector was applied to the basal side. The MOI was ∼20 for all experiments. Results are representative of experiments done with three different airway epithelium preparations.
Increasing transepithelial permeability facilitates gene transfer from the apical side.
The polarity of gene transfer to proliferating cells suggested that receptors may be accessible only from the basal side. However, the most direct means for in vivo application would be to deliver the vector to the apical surface. To address this issue, experiments were designed to test if increasing epithelial permeability would facilitate gene transfer to dividing cells with an apically applied vector. We reasoned that treatments expected to increase transepithelial permeability might allow access of virus to all epithelial cells, even when applied apically.
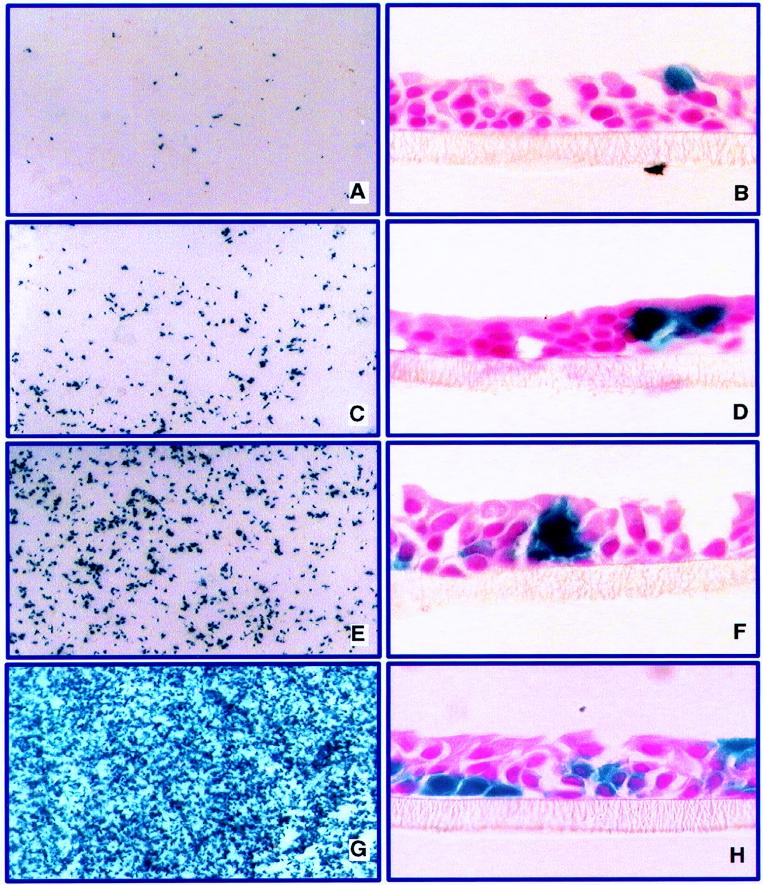
Two methods were tested to increase transepithelial permeability. The first was the application of water to the mucosal surface. Widdicombe and colleagues have shown that hypotonic solutions rapidly increase transepithelial permeability in cultured airway epithelia (47). The second method was the application of EGTA, a calcium chelator, under isotonic or hypotonic conditions. Since the tight junction complex is calcium dependent, EGTA treatment causes a reversible increase in paracellular permeability (1, 5, 15). Differentiated airway epithelia were first treated with 50 ng of KGF per ml for 24 h to induce proliferation. Then the apical surfaces of cells were exposed to 50 μl of water or 1.5 mM EGTA in water for 20 min. During this period, the basolateral surfaces of the cells remained in contact with the normal nutrient medium containing calcium. After the specified 20-min wash, DA-βgal vector was applied to the apical membrane at an MOI of ∼20 for 4 h in formulation buffer or buffer spiked with EGTA to a final concentration of 1.5 mM. β-Gal expression was detected by histochemical staining 3 days later. In contrast to the previous findings of inefficient gene transfer with the apically applied vector (Fig. 2), gene transfer was greatly enhanced for all treatment groups. As shown in Fig. 5A and B, 3% ± 0.5% of epithelia from preparations pretreated with water expressed β-Gal (mean ± SE for 13 membranes from three preparations). Treatment with water and then EGTA in water for 10 min each, followed by addition of vector, resulted in 8% ± 1.3% (mean ± SE) positive cells (Fig. 5C and D) (nine membranes from three preparations). A further incremental increase in expression was seen in cells pretreated with a combination of water and EGTA for 20 min followed by addition of vector (20.3% ± 2.5% cells positive [mean ± SE for nine membranes from two preparations] (Fig. 5E and F). Finally, cells pretreated with water and EGTA for 20 min followed by the addition of vector containing EGTA showed 34.3% ± 5.4% β-Gal-positive cells 3 days following vector delivery (mean ± SE for nine membranes from two preparations) (Fig. 5G and H). Application of vector solution, spiked 1:1 with 6 mM EGTA to obtain a final concentration of 3 mM EGTA in the formulation buffer, with no wash steps also resulted in ∼35% transduction (data not shown). Cells treated with the water or EGTA solution without vector showed no evidence of increased proliferation or endogenous β-Gal activity (not shown). Importantly, cross sections of epithelia demonstrated that these maneuvers facilitated gene transfer to cells at all levels of the epithelial sheet, including surface cells and basally located cells (Fig. 5). Gene transfer with apically applied xenotropic enveloped MuLV was also enhanced in a fashion similar to that seen with the amphotropic vector when epithelial permeability was increased by treatment with water or EGTA (not shown).
FIG. 5.
Transient disruption of epithelial tight junctions enhances gene transfer to airway epithelia via the apical surface. The airway epithelia were treated with KGF for 36 h prior to vector addition to the apical surface. Cells were pretreated with water or EGTA as described in Materials and Methods. An amphotropic vector was applied to the apical surface at an MOI of ∼20 (n = 3). Gene transfer was assessed 72 h later by X-Gal staining. Left panels show en face views of X-Gal-stained epithelial sheets; right panels show corresponding cross sections of epithelia. (A and B) Pretreatment with water for 20 min followed by addition of vector. (C and D) Pretreatment with water for 10 min and then with EGTA for 10 min followed by addition of vector. (E and F) Pretreatment with EGTA for 20 min followed by addition of vector. (G and H) Pretreatment with EGTA for 20 min followed by addition of vector spiked with EGTA. Results were replicated with two or three airway cell preparations.
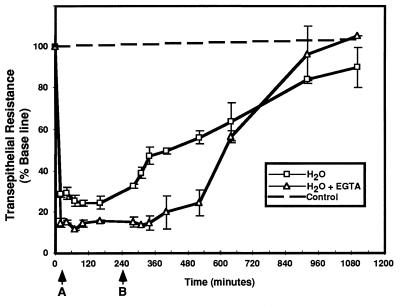
As shown in Fig. 6, treatment with water or EGTA caused a marked fall in transepithelial resistance across differentiated human airway epithelia, consistent with a loss of tight junctions and increased transepithelial permeability (5, 47). This effect was rapid, occurring in less than 60 s (data not shown). Interestingly, EGTA in isotonic PBS also decreased transepithelial resistance when applied to the mucosal surface, but the effects were slower in onset (not shown). When the EGTA-containing or hypotonic buffer was removed (Fig. 6), transepithelial resistance recovered gradually and reached pretreatment levels within 16 to 18 h. These data show that the effects of water and EGTA on epithelial permeability are transient.
FIG. 6.
Transepithelial resistance promptly decreases in response to the addition of water or 1.5 mM EGTA to the apical surface. Water or EGTA was applied for 20 min. Arrow A indicates addition of vector buffer to the apical surface after the 20-min treatment with water or EGTA. Arrow B indicates removal of apical solution at 4 h. The fall in resistance was reversible over a 16- to 18-h period. Control epithelia (dotted line) showed no change in transepithelial resistance over a similar time. Each point represents the mean ± SE for three epithelial cell membranes.
An additional study was performed to address the issue of whether gene transfer facilitated by hypotonic and EGTA vector solutions was receptor mediated or receptor independent. We reasoned that if the process was receptor independent, a vector might be able to infect cells which lack the receptor. For this experiment, an ecotropic vector expressing β-Gal was substituted for DA-βgal by using conditions similar to those described for Fig. 5G and H. While the ecotropic vector infected the control 3T3 mouse fibroblasts, no infection of KGF-stimulated airway epithelia occurred (data not shown).
Retrovirus-mediated gene transfer to differentiated CF epithelia corrects the Cl− transport defect.
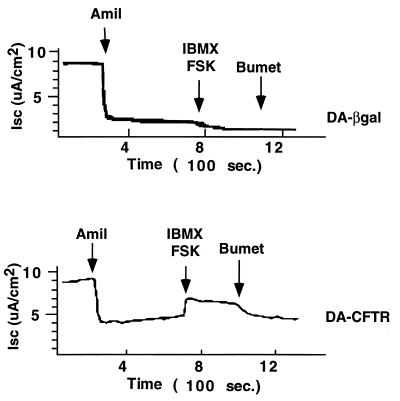
The feasibility of gene transfer to correct the Cl− transport defect in differentiated CF airway epithelia was also tested. As described above for non-CF epithelia, fully differentiated CF nasal-polyp epithelia were pretreated for 24 h with KGF to induce proliferation and then infected with the amphotropic retroviral vector expressing CFTR. The DA-CFTR vector was applied apically with hypotonic buffer and EGTA as described for Fig. 5G and H. Control cells received the DA-βgal vector. As shown in Fig. 7, when the vector was applied apically to KGF-stimulated CF epithelia treated with hypotonic shock and EGTA, cAMP-activated Cl− current was detectable 10 days following gene transfer. In control cells that received the DA-βgal vector, no correction of the CFTR transport defect was detected.
FIG. 7.
Retroviral gene transfer via the apical surfaces of differentiated CF airway epithelia corrects the CFTR defect. An amphotropic vector expressing human CFTR (DA-CFTR) was applied apically to KGF-stimulated CF epithelia treated with hypotonic shock and EGTA. In control cells that received DA-βgal vector after hypotonic shock and EGTA, cAMP agonists failed to stimulate Cl− secretion (top panel). In cells treated with DA-CFTR (bottom panel), cAMP-activated Cl− current was detectable 10 days following gene transfer. Amil, amiloride; IBMX, 3-isobutyl-1-methyl xanthine; FSK, forskolin; bumet, bumetanide; ISC, short-circuit current.
DISCUSSION
These novel findings demonstrate the feasibility of gene transfer to differentiated human airway epithelia by using MuLV-based retroviruses. First, KFG stimulated proliferation in more than 50% of cells. The use of this agent overcomes the previous limitation of low mitotic indices in differentiated airway epithelia. Second, we found that cell proliferation was required but not sufficient to support gene transfer to airway epithelia with the vector applied to the apical surface. Gene transfer was markedly polar, but the inefficiency of gene transfer from the apical surface was overcome by treatments which transiently decreased transepithelial resistance. Importantly, the percentage of transgene-expressing cells attained after increasing transepithelial permeability was within the range required for complementing defective ion and fluid transport in CF airway epithelia (20, 53).
Recombinant retroviruses were used in gene complementation studies following the identification of the CF gene (2, 11) and have been successfully applied in cell culture systems to transfer the CFTR cDNA and generate cAMP-activated Cl− secretion in a variety of cell types, including human airway epithelia (2, 11, 20, 34). In vitro studies of basal and secretory cells isolated from rabbit trachea showed that both cell populations were susceptible to gene transfer with amphotropic vectors while actively proliferating (16). Amphotropic MuLV vectors were also used to stably transfer genes to human and rat pulmonary epithelia in xenografts (12, 13). Studies of xenografts populated with human bronchial epithelial cells demonstrated that the efficiency of gene transfer to regenerating epithelia with 40% BrdU-positive cells was good, while well-differentiated grafts (1% of cells BrdU positive) showed poor infectivity with retrovirus (13). In vivo studies of gene transfer with retrovirus to the lung are limited but suggest that efficiency is low in the absence of injury to the epithelium (16, 29, 38). The present study indicates that in addition to overcoming the limitation of low mitotic indices, the low transduction efficiency of the vector applied to the mucosal surfaces of differentiated cells must be addressed.
The tight junction, also known as zonula occludens, is the apical-most component of the epithelial junctional complex (1). A variety of agents transiently increase epithelial permeability by disrupting tight junctions. Bhat and coworkers reported that lowering intra- or extracellular calcium levels or disrupting the cytoskeleton reversibly increased permeability in rabbit tracheal epithelium (5). Widdicombe and colleagues found that hypotonic shock from the application of water to the apical surface transiently increased the permeability of cultured bovine and human tracheal epithelia (47). Hypotonic shock reversibly increased both transcellular and paracellular permeability (47).
The efficiency of infection with retroviruses is determined in part by the availability of specific cellular receptors that mediate virus entry (30, 46). In the case of amphotropic enveloped vectors, the receptor (Ram-1 or GLVR-2) is a cell surface protein that functions as a sodium-dependent phosphate transporter (31, 32). In hematopoietic cells (35) and hepatocytes (17), levels of Ram-1 mRNA expression correlate directly with infection efficiencies. In some cases receptor abundance and infectivity are regulated by nutritional or hormonal conditions. For example, there is evidence for the regulation of Ram-1 mRNA expression by insulin, dexamethasone (49), or hypophosphatemia (10, 32, 39). Our findings suggest that, in addition to stimulating cell proliferation, KGF also increases the expression of the GLVR-2 amphotropic receptor protein.
The observation that retroviral gene transfer to proliferating airway epithelia is polar is quite striking. This may be due to (i) a polarized distribution of retroviral receptors to the basolateral membrane, (ii) inaccessibility or impaired function of apical receptors, or (iii) inhibition or inactivation of apically applied virus by secreted products of epithelia. Other than the observations that amphotropic (34) or gibbon ape leukemia virus (4) enveloped vectors can infect airway epithelia, there has been little research to characterize the abundance, cellular location, or regulation of receptor expression in airway epithelia. In support of the first explanation, conditions known to transiently disrupt the integrity of epithelial tight junctions (i.e., hypotonic shock and low Ca2+ levels) enhanced gene transfer efficiency with an amphotropic or xenotropic vector applied to the mucosal surface. Disruption of tight junctions may have also caused a transient loss of cell polarity and shifting of receptors to the apical pole. However, there is considerable precedence in epithelia for viral infection to occur in a polarized fashion. Studies with high-resistance MDCK cells showed that vesicular stomatitis virus infected them at least 100 times more efficiently when applied to the basal side than when applied to the apical surface (14). In contrast, cytomegalovirus (43) and measles virus (6) infect more efficiently from the apical surface. While virus may bind when applied apically, perhaps cytoplasmic elements involved in the internalization and translocation of the nucleocapsid are inoperative at the apical surface, thus limiting gene transfer efficiency. We found that washing the cells with PBS to remove mucus or inhibitory factors from the apical surface did not enhance gene transfer. This suggests that factors elaborated by airway epithelia, such as mucus or antimicrobial peptides, are not a barrier to gene transfer in this model.
These studies provide novel approaches to stimulate differentiated human airway epithelia to divide and to facilitate gene transfer to proliferating cells from the apical surface. Further studies of MuLV-based gene transfer and virus-receptor interactions in airway epithelia may advance the development of this integrating vector system for the treatment of lung disease.
ACKNOWLEDGMENTS
We thank Phil Karp and Pary Weber for culturing the human epithelial cells and Emily Appleton and David Lewis for technical assistance. We thank Phil Karp for assistance with electrophysiology studies. We especially thank Mike Welsh, Joe Zabner, John Engelhardt, and Mike Shasby for helpful discussions. We thank Tom Ulich at Amgen, Inc., for discussions and for providing recombinant human KGF.
This work was funded by Cystic Fibrosis Foundation PO96 (P.B.M. and B.L.D.) and the Children’s Miracle Network Telethon. We acknowledge the support of the Cell Culture Core, partially supported by the Cystic Fibrosis Foundation and NHLBI (PPG HL51670-05). P.B.M. is a recipient of a Career Investigator Award from the American Lung Association. B.L.D. is a fellow of the Roy J. Carver Trust.
REFERENCES
- 1.Anderson J M, Itallie C M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M P, Gregory R J, Thompson S, Souza D W, Paul S, Mulligan R C, Smith A E, Welsh M J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 3.Ayers M M, Jeffery P K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 4.Bayle J, Johnson L G, St. George J A, Boucher R C, Olsen J C. High-efficiency gene transfer to primary monkey airway epithelial cells with retrovirus vectors using the gibbon ape leukemia virus receptor. Hum Gene Ther. 1993;4:161–170. doi: 10.1089/hum.1993.4.2-161. [DOI] [PubMed] [Google Scholar]
- 5.Bhat M, Toledo-Velasquez D, Wang L, Malanga C J, Ma J K H, Rojanasakul Y. Regulation of tight junction permeability by calcium mediators and cell cytoskeleton in rabbit tracheal epithelium. Pharm Res. 1993;10:991–997. doi: 10.1023/a:1018906504944. [DOI] [PubMed] [Google Scholar]
- 6.Blan D M, Compans R W. Entry and release of measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- 7.Bosch A, McCray P B, Jr, Chang S M, Ulich T M, Simonet W S, Jolly D J, Davidson B L. Proliferation induced by keratinocyte growth factor enhances in vivo retroviral-mediated gene transfer to mouse hepatocytes. J Clin Investig. 1996;98:2683–2687. doi: 10.1172/JCI119091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles N E, Eisensmith R C, Mohuiddin R, Pyron M, Woo S L C. A simple and efficient method for the concentration and purification of recombinant retrovirus for increased hepatocyte transduction in vivo. Hum Gene Ther. 1996;7:1735–1742. doi: 10.1089/hum.1996.7.14-1735. [DOI] [PubMed] [Google Scholar]
- 9.Carey F A, Fabbroni G, Lamb D. Expression of proliferating cell nuclear antigen in lung cancer: a systematic study and correlation with DNA ploidy. Histopathology. 1992;20:499–503. doi: 10.1111/j.1365-2559.1992.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 10.Chien M-L, Foster J L, Douglas J L, Garcia J V. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J Virol. 1997;71:4564–4570. doi: 10.1128/jvi.71.6.4564-4570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drumm M L, Pope H A, Cliff W H, Rommens J M, Marvin S A, Tsui L-C, Collins F S, Frizzell R A, Wilson J M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt J F, Allen E D, Wilson J M. Reconstitution of tracheal grafts with a genetically modified epithelium. Proc Natl Acad Sci USA. 1991;88:11192–11196. doi: 10.1073/pnas.88.24.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelhardt J F, Yankaskas J R, Wilson J M. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Investig. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller S, von Bonsdorff C H, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 15.Gumbiner B M. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halbert C L, Aitken M L, Miller A D. Retroviral vectors efficiently transduce basal and secretory airway epithelial cells in vitro resulting in persistent gene expression in organotypic culture. Hum Gene Ther. 1996;7:1871–1881. doi: 10.1089/hum.1996.7.15-1871. [DOI] [PubMed] [Google Scholar]
- 17.Hatzoglou M, Moorman A, Lamers W. Persistent expression of genes transferred in the fetal rat liver via retroviruses. Somatic Cell Mol Genet. 1995;21:265–278. doi: 10.1007/BF02255781. [DOI] [PubMed] [Google Scholar]
- 18.Housley R M, Morris C F, Boyle W, Ring B, Biltz R, Tarpley J E, Aukerman S L, Devine P L, Pierce G F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Investig. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin M J, Laube L S, Lee V, Austin M, Chada S, Anderson C-G, Townsend K, Jolly D J, Warner J F. Direct injection of a recombinant retroviral vector induces human immunodeficiency virus-specific immune responses in mice and nonhuman primates. J Virol. 1994;68:5036–5044. doi: 10.1128/jvi.68.8.5036-5044.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson L G, Olsen J C, Sarkadi B, Moore K L, Swanstrom R, Boucher R C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 21.Jolly D. Viral vector systems for gene therapy. Can Gene Ther. 1994;1:51–64. [PubMed] [Google Scholar]
- 22.Kitten O, Cosset F-L, Ferry N. Highly efficient retrovirus-mediated gene transfer into rat hepatocytes in vivo. Hum Gene Ther. 1997;8:1491–1494. doi: 10.1089/hum.1997.8.12-1491. [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, Finkbeiner W E, Widdicombe J H. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am J Physiol. 1991;263:L105–L117. doi: 10.1152/ajplung.1991.261.2.L106. [DOI] [PubMed] [Google Scholar]
- 24.Leigh M W, Kylander J E, Yankaskas J R, Boucher R C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 25.Mason R J, Leslie C C, McCormic-Shannon K, Deterding R R, Nakamura T, Rubin J S, Shannon J M. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol. 1994;11:561–567. doi: 10.1165/ajrcmb.11.5.7524567. [DOI] [PubMed] [Google Scholar]
- 26.McCray P B, Jr, Armstrong K, Zabner J, Miller D W, Koretzky G A, Couture L, Robillard J E, Smith A E, Welsh M J. Adenoviral-mediated gene transfer to fetal pulmonary epithelia in vitro and in vivo. J Clin Investig. 1995;95:2620–2632. doi: 10.1172/JCI117964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCray P B, Jr, Bettencourt J D, Bastacky J, Denning G M, Welsh M J. Expression of CFTR and a cAMP-stimulated chloride secretory current in cultured human fetal alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993;9:578–585. doi: 10.1165/ajrcmb/9.6.578. [DOI] [PubMed] [Google Scholar]
- 28.McCray P B, Jr, Wang G, Kline J N, Zabner J, Chada S, Jolly D J, Chang S M W, Davidson B L. Alveolar macrophages inhibit retrovirus-mediated gene transfer to airway epithelia. Hum Gene Ther. 1997;8:1087–1093. doi: 10.1089/hum.1997.8.9-1087. [DOI] [PubMed] [Google Scholar]
- 29.McCray P B, Jr, Wang G, O’Brien L, Davidson B L, Thomas P. Proliferation indices of pulmonary epithelia during human and ovine lung development: gene transfer targets for integrating vectors. Cell Vision. 1997;4:1–8. [Google Scholar]
- 30.Miller A D. Cell-surface receptors for retroviruses and implications for gene transfer. Proc Natl Acad Sci USA. 1996;93:11407–11413. doi: 10.1073/pnas.93.21.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohmichi H, Matsumoto K, Nakamura T. In vivo mitogenic action of HGF on lung epithelial cells: pulmotrophic role in lung regeneration. Am J Physiol. 1996;270:L1031–L1039. doi: 10.1152/ajplung.1996.270.6.L1031. [DOI] [PubMed] [Google Scholar]
- 34.Olsen J C, Johnson L G, Wong-Sun M L, Moore K L, Swanstrom R, Boucher R C. Retrovirus-mediated gene transfer to cystic fibrosis airway epithelial cells: effect of selectable marker sequences on long-term expression. Nucleic Acids Res. 1993;21:663–669. doi: 10.1093/nar/21.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlic D, Girard L J, Jordan C T, Anderson S M, Cline A P, Bodine D M. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panos R J, Rubin J S, Aaronson S A, Mason R J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Investig. 1993;92:969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendleton N, Dixon G R, Burnett H E, Occleston N L, Myskow M W, Green J A. Expression of proliferating cell nuclear antigen (PCNA) in dysplasia of the bronchial epithelium. J Pathol. 1993;170:169–172. doi: 10.1002/path.1711700212. [DOI] [PubMed] [Google Scholar]
- 38.Pitt B R, Schwarz M A, Pilewski J M, Nakayama D, Mueller G M, Robbins P D, Watkins S A, Albertine K H, Bland R D. Retrovirus-mediated gene transfer in lungs of living fetal sheep. Gene Ther. 1995;2:344–350. [PubMed] [Google Scholar]
- 39.Richardson C, Bank A. Developmental-stage-specific expression and regulation of an amphotropic retroviral receptor in hematopoietic cells. Mol Cell Biol. 1996;16:4240–4247. doi: 10.1128/mcb.16.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rommens J M, Iannuzzi M C, Kerem B-S, Drumm M L, Melmer G, Dean M, Rozmahel R, Cole J L, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan J R, Tsui L-C, Collins F S. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 41.Scheule R K, George J A, Bagley R G, Marshall J, Kaplan J M, Akita G Y, Wang K X, Lee E R, Harris D J, Jiang C, Yew N S, Smith A E, Cheng S H. Basis of pulmonary toxicity associated with cationic lipid-mediated gene transfer to the mammalian lung. Hum Gene Ther. 1997;8:689–707. doi: 10.1089/hum.1997.8.6-689. [DOI] [PubMed] [Google Scholar]
- 42.Tsao M, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- 43.Tugizov S, Maidji E, Pereira I. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 44.Ulich T R, Yi E S, Longmuir K, Yin S, Biltz R, Morris C F, Housley R M, Pierce G F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Investig. 1994;93:1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.van Es, H. H. G. Unpublished data.
- 45.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O’Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss R A, Tailor C S. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 47.Widdicombe J H, Azizi F, Kang T, Pittet J F. Transient permeabilization of airway epithelium by mucosal water. J Appl Physiol. 1996;81:491–499. doi: 10.1152/jappl.1996.81.1.491. [DOI] [PubMed] [Google Scholar]
- 48.Worgall S, Leopold P L, Wolff G, Ferris B, Van Roijen N, Crystal R G. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- 49.Wu J Y, Robinson D, Kung H-J, Hatzoglou M. Hormonal regulation of the gene for the type C ecotropic retrovirus receptor in rat liver cells. J Virol. 1994;68:1615–1623. doi: 10.1128/jvi.68.3.1615-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 51.Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:21212–21217. [PubMed] [Google Scholar]
- 52.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabner J, Couture L A, Smith A E, Welsh M J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther. 1994;5:585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]
- 54.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]